Abstract
Following previous surveys to assess the incidence of Listeria monocytogenes in raw milk and nondairy foods processed in Northern Ireland, isolates were characterized as recurrent or sporadic on the basis of multilocus enzyme electrophoresis (MEE) analysis and restriction fragment length polymorphism typing. In the present study, 45 representative recurrent and sporadic electrophoretic types (ETs) previously identified by MEE were subjected to pulsed-field gel electrophoresis (PFGE) of genomic DNA macrorestriction fragments, monocin typing, plasmid profiling, and an examination of resistance to cadmium and nine different antibiotics. Although PFGE proved to be capable of subdividing a number of recurrent and sporadic ETs, the grouping of strains arrived at by PFGE and MEE were in broad agreement, and previous conclusions regarding the designation of L. monocytogenes strains as recurrent or sporadic remained unaltered. It is considered that PFGE was able to detect minor genetic changes in recurrent ETs which occurred during the time period in which food surveys were carried out. Production of type E monocin (Types A to E were found among the 45 strains), plasmid carriage, and resistance to cadmium occurred more frequently in recurrent than in sporadic strains and may be important with regard to the ability of L. monocytogenes to persist in food and food-processing environments. Only 2 of 45 strains showed resistance to any of the nine antibiotics tested: two sporadic strains were resistant to tetracycline (MIC, 64 μg ml−1).
The involvement of food as a vector for the transmission of listeriosis is clearly established in relation to both epidemic (18) and sporadic (15, 19) disease. Consequently, food industries, health agencies, and government bodies have an obligation to detect and control infections caused by the presence of Listeria monocytogenes in food. The Department of Agriculture and Rural Development in Northern Ireland has carried out several surveys (9, 10) to determine the incidence of L. monocytogenes in raw milk, dairy products, and nondairy foods produced in Northern Ireland. The samples examined in these surveys were obtained on successive monthly visits to selected farms (bulk tanks), milk-processing centers (balance tanks), and food factories (point of dispatch). The results of typing L. monocytogenes isolates from these surveys by means of multilocus enzyme electrophoresis (MEE) analysis and restriction fragment length polymorphism typing were in substantial agreement (11). The recognition of recurrent L. monocytogenes types in samples from certain processors led to the conclusion that L. monocytogenes strains frequently persist within food-processing environments and may subsequently contaminate processed foods. After these investigations were completed, a coordinated evaluation of MEE by the World Health Organization (WHO) Multicentre L. monocytogenes Subtyping Study (4) came to the conclusion that to ascertain immediate epidemiological relationships of L. monocytogenes strains, it is necessary to supplement MEE with other methods providing further discrimination. A concurrent WHO study (3) validated genomic fingerprinting via pulsed-field gel electrophoresis (PFGE) as a highly discriminating and reproducible method for subtyping L. monocytogenes. In addition, PFGE has an advantage over restriction fragment length polymorphism in that simplified chromosomal restriction fragment patterns suitable for computer analysis are generated by the former technique without the need to resort to probe hybridization methods. As a result of these considerations, it was decided to type recurrent and sporadic L. monocytogenes strains from our previous studies using PFGE and to compare the results with those previously obtained using MEE. Additionally, as a second aim of the present study, characteristics of recurrent and sporadic L. monocytogenes strains which might relate to the ability of this microorganism to persist in food-processing environments were examined. Recurrent and sporadic strains were examined for: (i) production of monocins, (ii) plasmid carriage, (iii) plasmid size, (iv) resistance to cadmium, and (v) resistance to antibiotics.
MATERIALS AND METHODS
Bacterial strains.
A total of 45 L. monocytogenes isolates (see Tables 2 and 3) were examined. The strains had been isolated, assigned to serogroups, and subjected to MEE analysis as described previously (9, 10, 11). MEE analysis required the determination, for each strain, of the electrophoretic mobilities of 11 commonly occurring cellular enzymes, the assignment of a number to each mobility variant (electromorph), and the designation of each unique combination of electromorphs as an electrophoretic type (ET). Prior to the commencement of the study, the strains were stored on beads in cryopreservative fluid at −80°C.
TABLE 2.
Identification of recurrent and sporadic L. monocytogenes isolates found in raw-milk samples from four different processors
| Source processor | Isolation date | Serogroup | MEE type | PFGE type (AscI) | Status |
|---|---|---|---|---|---|
| A | Feb. 1989 | 4 | ET 18 | XVIII | Sporadic |
| May 1989 | 1 | ET 27 | IV | Recurrent | |
| June 1989 | 1 | ET 27 | IV | Recurrent | |
| July 1989 | 1 | ET 37 | XII | Sporadic | |
| July 1989 | 4 | ET 13 | XVIII | Sporadic | |
| B | Dec. 1988 | 1 | ET 35 | XI | Recurrent |
| Apr. 1989 | 4 | ET 07 | I | Sporadic | |
| May–early 1989 | 1 | ET 35 | XIII | Recurrent | |
| May–late 1989 | 1 | ET 35 | XIII | Recurrent | |
| June 1989 | 1 | ET 35 | XIII | Recurrent | |
| Sept. 1989 | 1 | ET 36 | X | Sporadic | |
| C | Dec. 1988 | 1 | ET 26 | III | Sporadic |
| May 1989 | 4 | ET 20 | XVII | Sporadic | |
| July 1989 | 1 | ET 30 | V | Sporadic | |
| Aug. 1989 | 1 | ET 47 | VIII | Sporadic | |
| Sept. 1989 | 1 | ET 45 | V | Sporadic | |
| Oct.–early 1989 | 1 | ET 47 | IX | Sporadic | |
| Oct.–late 1989 | 1 | ET 19 | II | Sporadic | |
| Nov. 1989 | 1 | ET 46 | VII | Sporadic | |
| D | May 1989 | 1 | ET 40 | XIV | Recurrent |
| June 1989 | 1 | ET 40 | XV | Recurrent | |
| Aug. 1989 | 1 | ET 40 | XVI | Recurrent | |
| Nov. 1989 | 1 | ET 15 | VI | Sporadic |
TABLE 3.
Identification of recurrent and sporadic L. monocytogenes isolates found in nondairy food samples from four different processors
| Source processor | Isolation date | Serogroup | MEE type | PFGE type (AscI) | Status |
|---|---|---|---|---|---|
| J | July 1990 | 4 | ET 09 | XXI | Recurrent |
| Aug. 1990 | 4 | ET 09 | XXI | Recurrent | |
| Oct. 1990 | 1 | ET 41 | XXVI | Sporadic | |
| Oct. 1990 | 1 | ET 48 | XXIV | Sporadic | |
| Nov. 1990 | 1 | ET 24 | XXVIII | Sporadic | |
| Jan. 1991 | 4 | ET 09 | XXI | Recurrent | |
| Jan. 1991 | 1 | ET 38 | XXVII | Sporadic | |
| Feb. 1991 | 4 | ET 09 | XXI | Recurrent | |
| Feb. 1991 | 1 | ET 10 | XXIII | Sporadic | |
| Apr. 1991 | 4 | ET 09 | XXI | Recurrent | |
| K | May 1990 | 1 | ET 42 | XXX | Recurrent |
| June 1990 | 1 | ET 42 | XXX | Recurrent | |
| Sept. 1990 | 1 | ET 13 | XX | Sporadic | |
| Nov. 1990 | 1 | ET 39 | XXX | Recurrent | |
| Feb. 1991 | 1 | ET 42 | XXX | Recurrent | |
| Mar. 1991 | 1 | ET 14 | XX | Sporadic | |
| M | June 1990 | 1 | ET 42 | XXXI | Recurrent |
| July 1990 | 1 | ET 42 | XXIX | Recurrent | |
| Sept. 1990 | 1 | ET 42 | XXXII | Recurrent | |
| N | May 1990 | 1 | ET 22 | XXV | Sporadic |
| Aug. 1990 | 1 | ET 43 | XXII | Sporadic | |
| Nov. 1990 | 1 | ET 25 | XIX | Sporadic |
Preparation of genomic DNA and digestion with restriction enzymes.
Original or modified previously described protocols were used (3). The strains were grown overnight at 37°C in brain heart infusion broth, pelleted by centrifugation, washed once, and resuspended in TE buffer (10 mM Tris, 1 mM EDTA, pH 8.0). The cell suspensions were standardized by adjusting the optical density to 1.3 at 610 nm with TE buffer and mixed with an equal volume of 1.6% chromosomal-grade agarose (Bio-Rad) in TE buffer, and approximately 100 μl of the mixture was dispensed into disposable plastic molds (Bio-Rad). The solidified agarose plugs were incubated in a lysis solution containing 0.2 M EDTA, pH 8.0, 2 mg of deoxycholic acid ml−1, 3.0 mg of lysozyme (Sigma) ml−1, and 0.5% N-lauroyl sarcosine (Sigma) for 24 h at 37°C with gentle shaking. The plugs were then deproteinized by incubation in a solution containing 0.5 M EDTA, pH 8.0, 0.5% N-lauroyl sarcosine, and 2.0 mg of proteinase K (Boehringer Mannheim) ml−1 for 48 h at 55°C in a shaking water bath. Following deproteinization, the plugs were washed in 0.2 mM phenylmethylsulfonyl fluoride (Sigma) in TE buffer, rinsed in TE buffer, and then digested for 16 h with 100 U of ApaI (Boehringer Mannheim) or 25 U of AscI (New England Biolabs) in 200 μl of the respective buffer and at the temperature recommended by the manufacturer.
PFGE procedures.
Restriction fragments were resolved in 1% pulsed-field certified agarose (Bio-Rad) in TBE buffer (45 mM Tris, 45 mM boric acid, 1 mM EDTA, pH 8.0) at 200 V/h with pulse times ramped from 4 to 40 s over 22 h using a CHEF-DR III System (Bio-Rad). After electrophoresis, the gels were immersed for 1 h in a 1-μg ml−1 ethidium bromide solution followed by destaining for 1 h in distilled water.
Determination of DNA relatedness.
The gels were photographed under UV transillumination, and the images were digitized and analyzed with software (1D Advanced and 1D Database) supplied by Nonlinear Dynamics, Newcastle upon Tyne, United Kingdom. For band detection, the peak detection parameters used were as follows: minimum slope, 20; noise reduction, 11%; maximum peak, 9. The edge detection parameter was set to automatic. Rf (retardation factor) lines were applied to each gel to counteract distortions within individual gels. To allow comparisons between band patterns in different gels, Rf calibration was carried out and common bands were assigned the same Rf values. For background subtraction, the rolling-disk method with a radius setting of 50 was used. The matching of band patterns was based on the DICE coefficient, with a vector setting of 0.5. Dendrograms were created by the neighbor-joining method (17) algorithm for comparison of strain profiles within a single gel, and the unweighted pair group method using arithmetic average (UPGMA) (20) algorithm was used when strain profiles were compared between gels. Strains were considered to be indistinguishable and were assigned to the same PFGE group when the dendrogram derived from the UPGMA algorithm indicated an index of relatedness of ⩾99 verified by visual examination of the band patterns (see Fig. 2 and 3).
FIG. 2.
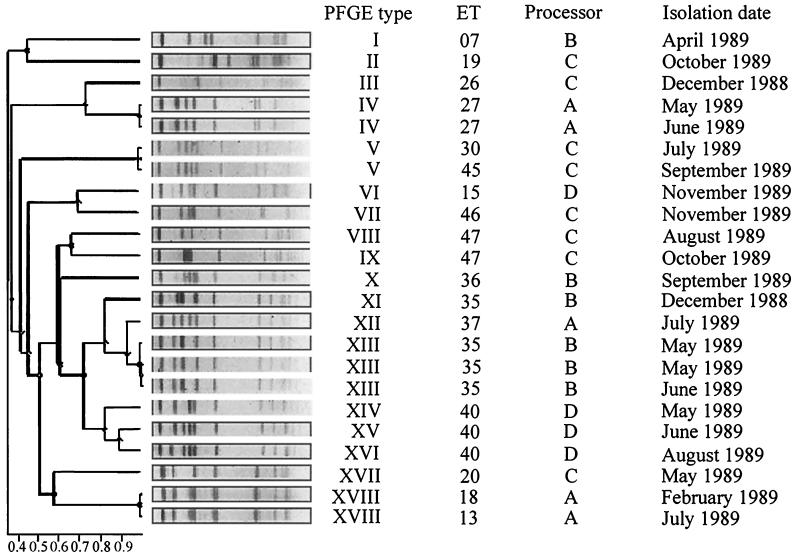
Dendrogram showing the relatedness of band patterns derived from PFGE of AscI-restricted genomic DNA from 23 L. monocytogenes strains isolated sporadically and recurrently in successive raw-milk samples obtained from processors A to D. PFGE was performed at 200 V with pulse times ramped from 4 to 40 s over 22 h. When the same ET was recovered on successive visits to a processor (ET 27, ET 35, and ET 40), these were regarded as recurrent strains. The dendrograms and depiction of band patterns were created using software supplied by Nonlinear Dynamics. The software uses the UPGMA algorithm of Sneath and Sokal (20) to compare profiles between gels.
FIG. 3.
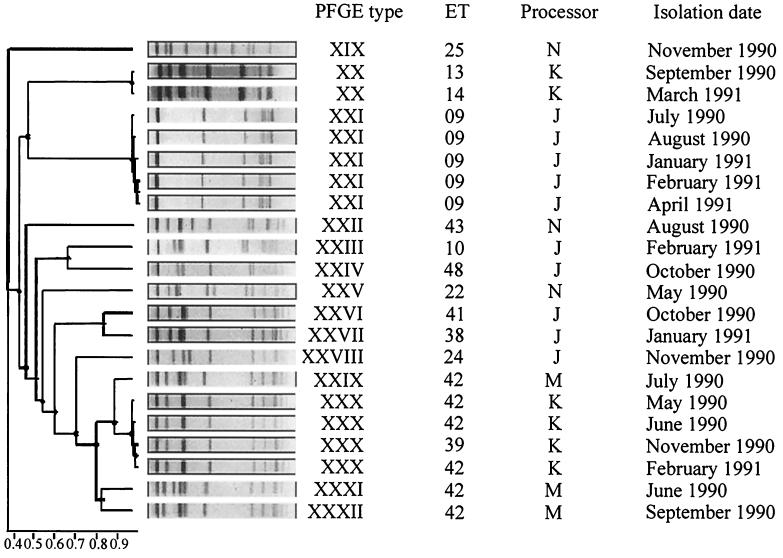
Dendrogram showing the relatedness of band patterns derived from PFGE of AscI-restricted genomic DNA from 22 L. monocytogenes strains isolated sporadically and recurrently in nondairy food samples obtained from processors J, K, M, and N. PFGE was performed at 200 V with pulse times ramped from 4 to 40 s over 22 h. When the same ET was recovered on successive visits to a processor (ET 09 and ET 42), these were regarded as recurrent strains. The dendrograms and depiction of band patterns were created using software supplied by Nonlinear Dynamics. The software uses the UPGMA algorithm of Sneath and Sokal (20) to compare profiles between gels.
Monocin production.
Strains were tested for monocin production using the method described by Lebek et al. (12). Blood agar cultures of L. monocytogenes strains were inoculated onto predetermined spots on brain heart infusion agar plates to which 10-μl drops of mitomycin C (2 μg ml−1) (catalog no. M 0503; Sigma) had previously been added and allowed to dry. The inoculated brain heart infusion agar plates were incubated for 48 h at 30°C and then exposed to chloroform vapor for 1 h. Subsequently, the chloroform was allowed to evaporate for 15 min, and the plates were flooded with suspensions of different indicator strains (Table 1). The cell suspensions were decanted, and following overnight incubation at 30°C, the plates were examined for zones of growth inhibition indicative of monocin activity. The monocins were classified according to their activities against each indicator strain (Table 1).
TABLE 1.
Designation of monocin types A to E produced by L. monocytogenes isolates according to their inhibitory actions against selected indicator strains
| Indicator straina | Inhibitory actionb
|
||||
|---|---|---|---|---|---|
| A | B | C | D | E | |
| BCH 20 (L. monocytogenes serotype 4b) | − | − | + | − | + |
| FV 481 (L. monocytogenes serotype 4b) | − | − | − | + | + |
| LP566 (L. monocytogenes serotype 4b) | − | − | + | + | + |
| FV 44 (L. monocytogenes serotype 1/2a) | − | − | − | − | − |
| FMT 1750 (L. monocytogenes serotype 1/2b) | − | − | − | − | − |
| MQ2708 (L. monocytogenes serotype 1/2c) | − | − | − | − | − |
| NCTC 5214 (L. monocytogenes serotype 4a) | − | + | − | + | + |
| NCTC 19116 (L. monocytogenes serotype 4c) | − | + | + | + | + |
| NCTC 11846 (L. ivanovii) | − | + | + | + | + |
Selected indicator strains. NCTC, National Collection of Type Cultures; BCH, FV, LP, FMT, and MQ are laboratory strain designations.
A to E are monocin types. +, growth of indicator strain inhibited; −, growth not inhibited.
Plasmid analysis.
The strains were grown overnight at 37°C in brain heart infusion broth and pelleted by centrifugation, and plasmid DNA was extracted from the cells as described by Anderson and McKay (2). The plasmids were analyzed in 0.7% agarose gels (catalog no. A6013; Sigma) in Tris acetate buffer (40 mM Tris, 20 mM acetic acid, and 1 mM EDTA) at 70 V for 3 h. Plasmid preparations from three Escherichia coli strains (NCTC 50001, NCTC 50192, and NCTC 50193) were used for molecular size markers. The gels were stained with 1 μg ml−1 ethidium bromide and photographed under UV transillumination.
Determination of heavy metal and antibiotic susceptibilities.
The agar dilution method (16) was used to determine the MICs of cadmium sulfate (Sigma) and the following antibiotics: ampicillin, gentamicin, tetracycline, streptomycin, kanamycin, erythromycin, chloramphenicol, cephalothin, and rifampin (all from Sigma).
RESULTS
MEE and PFGE typing of 45 L. monocytogenes strains.
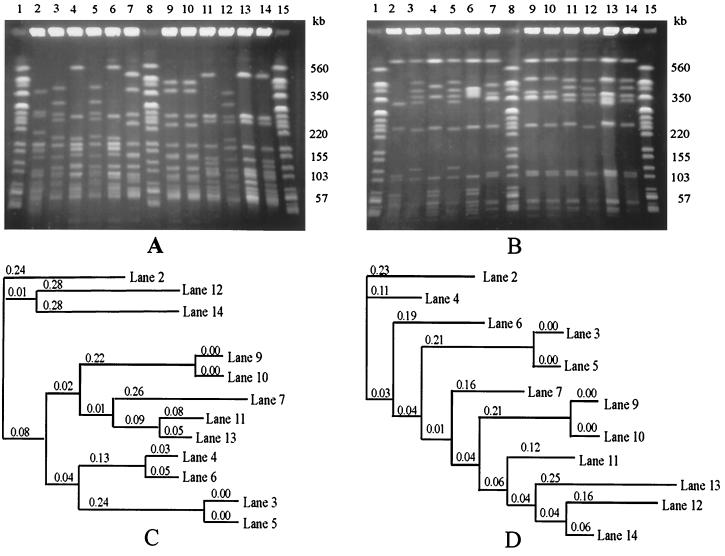
PFGE of both ApaI and AscI restriction fragments produced band patterns suitable for typing L. monocytogenes (Fig. 1). The AscI band patterns were simpler and more clearly resolved and so were used for the purpose of the present study. While MEE analysis had previously distinguished 29 ETs among the 45 L. monocytogenes strains used in the present study, PFGE of AscI restriction fragments revealed 32 PFGE types (Fig. 2 and 3). Comparison of MEE and PFGE typing results (see Table 4) showed that 23 strains were assigned to a single ET and a single PFGE type and a further 10 strains were assigned to eight ETs and comprised only 4 PFGE types, while 12 strains assigned to only four ETs comprised 10 PFGE types.
FIG. 1.
PFGE resolution of ApaI (A) and AscI (B) restriction fragments of genomic DNA from 12 L. monocytogenes strains (lanes 2 to 7 and 9 to 14). Lanes 1, 8, and 15 of each gel contained XbaI restriction fragments of genomic DNA from an E. coli reference strain (G5244) as molecular size markers. PFGE was performed at 200 V with pulse times ramped from 4 to 40 s over 22 h. The dendrograms showing the relationships between ApaI (C) and AscI (D) profiles for the 12 strains were created using software supplied by Nonlinear Dynamics, Newcastle upon Tyne, United Kingdom. The software uses the neighbor-joining method of Saitou and Nei (17) to compare profiles on the same gel. The numbers on the branches of the dendrograms denote the lengths of the branches. The shorter the distance, the more similar the lanes.
TABLE 4.
Comparison of MEE and PFGE typing for 45 L. monocytogenes strains
| MEE type | PFGE type (AscI) | No. of strains | Status |
|---|---|---|---|
| ET 07 | I | 1 | Sporadic |
| ET 19 | II | 1 | Sporadic |
| ET 26 | III | 1 | Sporadic |
| ET 27 | IV | 2 | Recurrent |
| ET 15 | VI | 1 | Sporadic |
| ET 46 | VII | 1 | Sporadic |
| ET 36 | X | 1 | Sporadic |
| ET 37 | XII | 1 | Sporadic |
| ET 20 | XVII | 1 | Sporadic |
| ET 25 | XIX | 1 | Sporadic |
| ET 09 | XXI | 5 | Recurrent |
| ET 43 | XXII | 1 | Sporadic |
| ET 10 | XXIII | 1 | Sporadic |
| ET 48 | XXIV | 1 | Sporadic |
| ET 22 | XXV | 1 | Sporadic |
| ET 41 | XXVI | 1 | Sporadic |
| ET 38 | XXVII | 1 | Sporadic |
| ET 24 | XXVIII | 1 | Sporadic |
| ET 30, 45 | V | 2 | Sporadic |
| ET 13, 18 | XVIII | 2 | Sporadic |
| ET 13, 14 | XX | 2 | Sporadic |
| ET 42 (3 strains), 39 (1 strain) | XXX | 4 | Recurrent |
| ET 47 | VIII, IX | 2 | Sporadic |
| ET 35 | XI (1 strain), XIII (3 strains) | 4 | Recurrent |
| ET 40 | XIV, XV, XVI | 3 | Recurrent |
| ET 42 | XXIX, XXXI, XXXII | 3 | Recurrent |
Recognition of recurrent strains.
In the present study, L. monocytogenes isolates were considered to represent recurrent strains when the same ET was found in successive samples obtained from a particular processor. From the results shown in Tables 2 and 3, it can be seen that ET 27, ET 35, ET 40, and ET 09 recurred at processors A, B, D, and J, respectively, while ET 42 recurred at processors K and M.
Subtyping of recurrent ETs by PFGE.
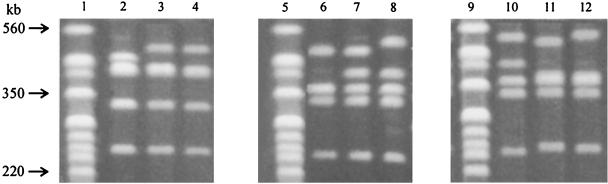
From Tables 2 and 3, it can be seen that recurrent ET 27 (two strains, both PFGE type IV), ET 09 (five strains, all PFGE type XXI), and ET 42 at processor K (three strains, all PFGE type XXX) were each specified by a single PFGE type. However, as shown in Fig. 4, recurrent ET 35 (three strains; PFGE types XI [one strain] and XIII [two strains]), ET 40 (three strains; PFGE types XIV, XV, and XVI), and ET 42 at processor M (three strains; PFGE types XXIX, XXXI, and XXXII) were not confined to a single PFGE type. Figure 4 shows the different band patterns obtained for ET 35 isolated in December 1988, May 1989, and June 1989 from processor B (lanes 2, 3, and 4), ET 40 isolated in May 1989, June 1989, and August 1989 from processor D (lanes 6, 7, and 8), and ET 42 isolated in June 1990, July 1990, and September 1990 from processor M (lanes 10, 11, and 12). The ET 35-PFGE type XI strain isolated in December 1988 differs from the other ET 35-PFGE type XIII isolates with regard to the position of a single band. The band pattern for the former strain has a fragment of approximately 450 kb, whereas the band patterns for the other strains have no fragment at this position but do have a fragment at approximately 500 kb. For ET 40, the band pattern of the May 1989 isolate (PFGE type XIV [Fig. 4, lane 6]) differs from the other two ET 40 isolates (PFGE type XV [lane 7] and PFGE type XVI [lane 8]) in not having a fragment of approximately 400 kb, while the last two isolates differ in the position of a single large fragment (approximately 500 kb changes to approximately 540 kb). For ET 42 from processor M, the PFGE patterns of strains isolated in June 1990, July 1990, and September 1990 differed by either the presence or absence of a single large fragment (approximately 400 kb) or of a positional change of a single band (approximately 500 kb changes to approximately 540 kb).
FIG. 4.
Subtyping of recurrent L. monocytogenes ETs by PFGE of AscI restriction fragments of genomic DNA. PFGE was performed at 200 V with pulse times ramped from 4 to 40 s over 22 h. The recurrent strains ET 35 (lanes 2 to 4), ET 40 (lanes 6 to 8), and ET 42 (lanes 10 to 12) were isolated from food samples obtained on successive visits to processors B, D (milk), and M (nondairy food), respectively. XbaI restriction fragments of genomic DNA from an E. coli reference strain (G5244) were used as molecular size markers (lanes 1, 5, and 9).
Subtyping of PFGE types by MEE.
There were four instances of a PFGE type comprising more than one ET: (i) PFGE type V at processor C contained ET 30 and ET 45, (ii) PFGE type XVIII at processor A contained ET 18 and ET 13, (iii) PFGE type XXX at processor K contained ET 39 and ET 42, and (iv) PFGE type XX at processor K contained ET 13 and ET 14 (Table 4).
Monocin types.
From Table 5, it can be seen that serogroup 4 L. monocytogenes strains all produced monocin type B, while for serogroup 1 L. monocytogenes strains, all 16 recurrent strains produced monocin type E whereas 20 sporadic strains produced monocin types A (2 strains), B (2 strains), C (2 strains), D (1 strain), and E (13 strains).
TABLE 5.
Comparison of L. monocytogenes isolates found recurrently and sporadically in raw-milk and nondairy food samples from eight different processors with regard to susceptibility to monocins, plasmid carriage, and resistance to cadmium
| Source | Serogroup | Status | No. of strains | Monocin type
|
Detection and mass of plasmidsa | Cadmium resistanceb
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | 16 | 32 | 64 | 128 | 256 | 512 | |||||
| Raw milk | 1 | Recurrent | 9 | 9 | 5/9 (29 [1], 50 [4]) | 3 | 2 | 4 | |||||||
| 1 | Sporadic | 10 | 2 | 1 | 1 | 6 | 1/10 (50) | 2 | 5 | 2 | 1 | ||||
| 4 | Sporadic | 4 | 4 | 0 | 2 | 2 | |||||||||
| Nondairy foods | 1 | Recurrent | 7 | 7 | 7/7 (29 [4], 29 and 31 [1], 50 [2]) | 7 | |||||||||
| 1 | Sporadic | 10 | 2 | 1 | 7 | 6/10 (13 [1], 29 [2], 50 [1], 55 [2]) | 3 | 1 | 6 | ||||||
| 4 | Recurrent | 5 | 5 | 0 | 5 | ||||||||||
Plasmid masses (in megadaltons) are shown in parentheses after the frequency (number of plasmids/total isolates) of plasmid detection; the number of plasmids with each mass is in brackets.
Number of isolates for which the MIC of cadmium was as indicated above each column.
Plasmid carriage.
Plasmid DNA was not detected in any serogroup 4 L. monocytogenes strains. For serogroup 1 strains, plasmid DNA was detected in 12 of 16 (75%) recurrent strains compared to 7 of 20 (35%) sporadic strains, with the sizes of the plasmids detected ranging from 13 to 55 mDa (Table 5).
Cadmium resistance.
No serogroup 4 L. monocytogenes strains were resistant to high levels of cadmium (MICs, ≤128 μg/ml), whereas for serogroup 1 strains, resistance to high levels of cadmium (MICs, ≥256 μg/ml) was detected in 13 of 16 (81.25%) recurrent strains compared to 8 of 20 (40%) sporadic strains (Table 5).
Antibiotic resistance.
The sensitivities of all 45 L. monocytogenes strains to nine antibiotics are shown in Table 6. Little antibiotic resistance was observed, so comparison between recurrent and sporadic strains was not possible. Only two L. monocytogenes strains displayed significant resistance to any of the nine antibiotics used, namely, two sporadic milk isolates from processor C (ETs 30 and 45; PFGE V), which were resistant to tetracycline (MIC, 64 μg/ml).
TABLE 6.
Antibiotic sensitivities of recurrent and sporadic strains from raw milk and nondairy foods
| Antibiotic | Sensitivitya
|
|||
|---|---|---|---|---|
| Raw-milk strains
|
Food strains
|
|||
| Recurrent | Sporadic | Recurrent | Sporadic | |
| Ampicillin | <0.125–0.25 | 0.125–0.5 | 0.125–0.5 | 0.125–0.5 |
| Gentamicin | <0.25–0.5 | <0.25–1.0 | 0.25–0.5 | 0.25–2.0 |
| Tetracycline | 0.5–1.0 | 0.5–64.0 | 0.5–1.0 | 0.5–1.0 |
| Streptomycin | 4.0 | 2.0–4.0 | 4.0 | 2.0–4.0 |
| Kanamycin | 1.0–2.0 | <1.0–4.0 | 1.0–2.0 | 1.0–8.0 |
| Erythromycin | 0.125–0.25 | 0.25 | <0.125–0.25 | 0.25 |
| Chloramphenicol | 4.0 | 4.0 | 4.0 | 4.0 |
| Cephalothin | 2.0–4.0 | 2.0–4.0 | 2.0–4.0 | 2.0–4.0 |
| Rifampin | <0.125 | <0.125–0.25 | <0.125 | <0.125 |
Range of MICs (micrograms per milliliter).
DISCUSSION
MEE and PFGE typing results in the present study were in broad agreement with regard to the groupings achieved for L. monocytogenes isolates obtained from raw milk and nondairy food samples. The software package used to analyze the restriction fragment length patterns generated by PFGE proved to be a useful tool in indicating the relatedness of individual strains. Visual examination of band profiles confirmed the relatedness indicated by the computer-generated dendograms (7).
Despite the large measure of concordance between results obtained using the two typing methods considered here, some differences were observed. Several strains found to be distinguishable by MEE (ETs 39 and 42, 13 and 14, and 30 and 45) were found to be indistinguishable by PFGE, and in these instances, MEE is apparently more discriminatory than PFGE. However, reexamination of these strains by MEE seems advisable. Graves et al. (8) commented on variations in reproducibility and discrimination observed among laboratories using MEE and attributed this to less-than-optimal activity of the enzyme in the cytoplasmic extracts applied to gels or to characteristics of particular strains. On the other hand, ET 35, ET 40, ET 42, and ET 47 were divisible by PFGE. Brosch et al. (3) in a WHO multicenter L. monocytogenes subtyping study to evaluate PFGE, validated previous claims for the typing method and found it to be highly discriminating and reproducible. ET 35, ET 40, and ET 42 are recurrent strains, and PFGE was apparently able to detect minor genetic changes which occurred in these strains over the period in which the food surveys were carried out. These genetic alterations may not have been detected previously by MEE due to an insufficient number of enzymes being analyzed or due to one or more of the causes noted above, which may affect the reproducibility and discrimination of MEE. Despite these differences in typing results, the same conclusions were reached as to which strains were recurrent or sporadic.
Most of the recurrent strains in the present study belong to serogroup 1. This accords with reports by many authors on the predominance of serogroup 1 L. monocytogenes found in foods and environmental sources. It is therefore interesting to note (Table 1) that serogroup 4 strains (five recurrent and four sporadic) all produced monocin type B, which was active only against L. monocytogenes serotypes 4a and 4c and Listeria ivanovii, whereas L. monocytogenes serogroup 1 strains produced monocin types C, D, and E, which were active against L. monocytogenes serotype 4b strains in addition to serotypes 4a and 4c and L. ivanovii. Two serogroup 1 strains also produced monocin type B, but these strains are known to belong to serotype 1/2b and so could be expected to resemble serotype 4b strains. There is a progressive increase in the number of indicator strains lysed by each of the monocin types (A to E), with monocin type E active against the greatest number of indicator strains. All recurrent serogroup 1 L. monocytogenes strains produced monocin type E, which supports the suggestion (5) that bacteriocins give the producing organism a competitive advantage over other bacteria existing in the same ecological niche.
Comparison of plasmid carriage by recurrent and sporadic L. monocytogenes strains in the present study was only possible for serogroup 1 strains, since no plasmid DNA was detected in the nine serogroup 4 strains examined. Lebrun et al. (13) have also reported greater predominance of plasmid carriage in serogroup 1 than in serogroup 4 L. monocytogenes. Overall, plasmid DNA was detected in 52.7% of serogroup 1 strains, with plasmids being detected less frequently in L. monocytogenes isolated from milk (6 of 19) than in strains isolated from nondairy foods (13 of 17). The frequency of their occurrence in recurrent serogroup 1 strains (75%) was over twice that found in sporadic serogroup 1 strains (35%). The range of plasmid sizes observed in the present study is consistent with the findings of Lebrun et al. (14), who demonstrated the presence of a transposon (Tn5422) in plasmid-mediated cadmium-resistant L. monocytogenes strains and attributed the observed size diversity of the plasmids to the process of intramolecular replicative transposition, which generates deletions leading to plasmids of decreasing size. Although in the present study size diversity of plasmids was apparent in L. monocytogenes isolates from different sources, the sizes of plasmids in strains recurrent at certain processors remained remarkably stable over the period of the surveys. For example, ET 35, recurrent in milk samples from processor B during a 7-month period (December 1988 to June 1989), always carried a 50-MDa plasmid, while ET 42, recurrent in cooked-meat samples from processor K during a 10-month period (May 1990 to February 1991), always carried a 29-MDa plasmid.
Plasmid carriage in serogroup 1 strains was not correlated with monocin activity or with the type of monocin produced. However, all 19 serogroup 1 strains in which plasmid DNA was detected were cadmium resistant (MIC, ≥256 μg/ml), whereas only 3 of 17 strains in which plasmid DNA was not detected were cadmium resistant. Detection of cadmium resistance in 81.25% of recurrent serogroup 1 strains compared to only 40% of sporadic strains and the high correlation of plasmid carriage with cadmium resistance are consistent with the suggestions of Lebrun et al. (14) that extensive industrial use of cadmium over the last century has led to widespread cadmium contamination of the environment and necessitated acquisition by bacteria of cadmium resistance mechanisms. These workers have shown that in the case of L. monocytogenes, resistance to cadmium is achieved by an energy-dependent efflux mechanism encoded by genes most often carried on plasmids and less frequently on the chromosome, which prevents accumulation of cadmium in the cell.
A general lack of antibiotic resistance in both plasmid-carrying and non-plasmid-carrying L. monocytogenes strains is not surprising and has been previously noted (1, 6). Espaze and Reynaud (6) considered that this general lack of antibiotic resistance may change if the numbers of L. monocytogenes in food-processing environments and foods increase, thereby facilitating genetic transfers among genera and species. In this context, the resistance to tetracycline of two sporadic isolates from raw milk at processor C is interesting. Neither plasmid DNA nor cadmium resistance was detected in either of these strains, and uniquely among the 45 strains examined, they produced no monocin activity against any indicator strain (type A) (Table 5), thus suggesting a relative disadvantage for survival of these strains in a food-processing environment. However, given the ability of L. monocytogenes strains to adapt to particular habitats or niches, there is a possibility that antibiotic-resistant strains could adapt and persist in foods and food-processing environments and that genetic transfers to other microorganisms might be facilitated, as predicted by Espaze and Reynaud (6).
In the present study, the conclusions reached regarding the recurrence of L. monocytogenes isolates in raw milk and nondairy foods on the basis of MEE typing results have been confirmed by PFGE. In addition, PFGE, when applied to recurrent strains, detected genetic changes in the strains during the period that food surveys were carried out. Production of monocin type E and plasmid-borne cadmium resistance were found more frequently in recurrent than in sporadic L. monocytogenes strains from raw milk and nondairy foods. The persistence of microbial pathogens within a food-processing environment obviously raises food safety concerns and emphasizes the requirement for implementation of concepts such as hazard analysis of critical control points. Such concepts will require a detailed knowledge of the behavior and characteristics of microbial pathogens, such as those described here for L. monocytogenes, if their presence in foods and food-processing environments is to be successfully tracked, monitored, and controlled.
REFERENCES
- 1.Abuin C M F, Fernandez E J Q, Sampayo C F, Otero J L R, Rodriguez L D, Saez A C. Susceptibilities of Listeria species isolated from food to nine antimicrobial agents. Antimicrob Agents Chemother. 1994;38:1655–1657. doi: 10.1128/aac.38.7.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson D G, McKay L L. Simple and rapid method for isolating large plasmid DNA from lactic streptococci. Appl Environ Microbiol. 1983;46:549–552. doi: 10.1128/aem.46.3.549-552.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brosch R, Brett M, Catimel B, Luchansky J B, Ojeniyi B, Rocourt J. Genomic fingerprinting of 80 strains from the WHO multicentre international typing study of Listeria monocytogenes via pulsed-field gel electrophoresis (PFGE) Int J Food Microbiol. 1996;32:343–355. doi: 10.1016/s0168-1605(96)01147-6. [DOI] [PubMed] [Google Scholar]
- 4.Caugant D A, Ashton F E, Bibb W F, Boerlin P, Donachie W, Low C, Gilmour A, Harvey J, Norrung B. Multilocus enzyme electrophoresis for characterization of Listeria monocytogenes isolates: results of an international comparative study. Int J Food Microbiol. 1996;32:301–311. doi: 10.1016/s0168-1605(96)01144-0. [DOI] [PubMed] [Google Scholar]
- 5.Dykes G A. Bacteriocins: ecological and evolutionary significance. Trends Ecol Evol. 1995;10:186–189. doi: 10.1016/s0169-5347(00)89049-7. [DOI] [PubMed] [Google Scholar]
- 6.Espaze E P, Reynaud A E. Antibiotic susceptibilities of Listeria: in vitro studies. Infection. 1988;16:S160–S164. doi: 10.1007/BF01639741. [DOI] [PubMed] [Google Scholar]
- 7.Gerner-Smidt P, Graves L M, Hunter S, Swaminathan B. Computerised analysis of restriction fragment length polymorphism patterns: comparative evaluation of two commercial software packages. J Clin Microbiol. 1998;36:1318–1323. doi: 10.1128/jcm.36.5.1318-1323.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graves L M, Swaminathan B, Hunter S B. Subtyping Listeria monocytogenes. In: Ryser E T, Marth E H, editors. Listeria, listeriosis and food safety. New York, N.Y: Marcel Dekker, Inc; 1999. pp. 279–297. [Google Scholar]
- 9.Harvey J, Gilmour A. Occurrence of Listeria species in raw milk and dairy products produced in Northern Ireland. J Appl Bacteriol. 1992;72:119–125. doi: 10.1111/j.1365-2672.1992.tb01812.x. [DOI] [PubMed] [Google Scholar]
- 10.Harvey J, Gilmour A. Occurrence and characteristics of Listeria in foods produced in Northern Ireland. Int J Food Microbiol. 1993;19:193–205. doi: 10.1016/0168-1605(93)90077-t. [DOI] [PubMed] [Google Scholar]
- 11.Harvey J, Gilmour A. Application of multilocus enzyme electrophoresis and restriction fragment length polymorphism analysis to the typing of Listeria monocytogenes strains isolated from raw milk, nondairy foods, and clinical and veterinary sources. Appl Environ Microbiol. 1994;60:1547–1553. doi: 10.1128/aem.60.5.1547-1553.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lebek G, Teysseire P, Baumgartner A. A method for typing Listeria monocytogenes strains by classification of listeriocins and phage receptors. Zentbl Bakteriol. 1993;278:58–68. doi: 10.1016/s0934-8840(11)80279-3. [DOI] [PubMed] [Google Scholar]
- 13.Lebrun M, Loulergue J, Chaslus-Dancla E, Audurier A. Plasmids in Listeria monocytogenes in relation to cadmium resistance. Appl Environ Microbiol. 1992;58:3183–3186. doi: 10.1128/aem.58.9.3183-3186.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lebrun M, Audurier A, Cossart P. Plasmid-borne cadmium resistance genes in Listeria monocytogenes are present on Tn5422, a novel transposon closely related to Tn917. J Bacteriol. 1994;176:3049–3061. doi: 10.1128/jb.176.10.3049-3061.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinner R W, Schuchat A, Swaminathan B, Hayes P S, Deaver K A, Weaver R E, Plikaytis B D, Reeves M, Broome C V, Wenger J D the Listeria Study Group. Role of foods in sporadic listeriosis. JAMA. 1992;267:2046–2050. [PubMed] [Google Scholar]
- 16.Sahm D F, Washington J A. Antimicrobial susceptibility tests: dilution methods. In: Balows A, Hausler W J Jr, Herrmann K L, Isenberg H D, Shadomy H J, editors. Manual of clinical microbiology. 5th ed. Washington, D.C.: American Society for Microbiology; 1991. pp. 1105–1116. [Google Scholar]
- 17.Saitou N, Nei N. The neighbour-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 18.Schuchat A, Swaminathan B, Broome C. Epidemiology of human listeriosis. Clin Microbiol Rev. 1991;4:169–183. doi: 10.1128/cmr.4.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schuchat A, Deaver K A, Wenger J D, Plikaytis B D, Mascola L, Pinner R W, Reingold A L, Broome C V the Listeria Study Group. Role of foods in sporadic listeriosis. JAMA. 1992;267:2041–2045. [PubMed] [Google Scholar]
- 20.Sneath P H A, Sokal R R. Numerical taxonomy. W. H. San Francisco, Calif: Freeman and Company; 1973. [Google Scholar]