Abstract
Melanosomes are melanocyte-specific organelles that protect cells from ultraviolet (UV)-induced deoxyribonucleic acid damage through the production and accumulation of melanin and are transferred from melanocytes to keratinocytes. The relatively well-known process by which melanin is synthesized from melanocytes is known as melanogenesis. The relationship between melanogenesis and autophagy is attracting the attention of researchers because proteins associated with autophagy, such as WD repeat domain phosphoinositide-interacting protein 1, microtubule-associated protein 1 light chain 3, autophagy-related (ATG)7, ATG4, beclin-1, and UV-radiation resistance-associated gene, contribute to the melanogenesis signaling pathway. Additionally, there are reports that some compounds used as whitening cosmetics materials induce skin depigmentation through autophagy. Thus, the possibility that autophagy is involved in the removal of melanin has been suggested. To date, however, there is a lack of data on melanosome autophagy and its underlying mechanism. This review highlights the importance of autophagy in melanin homeostasis by providing an overview of melanogenesis, autophagy, the autophagy machinery involved in melanogenesis, and natural compounds that induce autophagy-mediated depigmentation.
Keywords: autophagy, melanogenesis, melanin
1. Introduction
The process by which melanin, the pigment of the skin, is synthesized in melanocytes is known as melanogenesis [1,2]. Melanogenesis is induced via various internal or external factors, such as aging, hormonal changes, and ultraviolet (UV) B-mediated skin irritation, and uncontrolled melanogenesis causes hyperpigmentation of the skin, which leads to skin effects such as melasma, freckles, age spots, and dark spots [3,4]. The melanosome is a melanocyte-specific lysosome-related organelle in which melanin pigment is synthesized and stored [5], and melanosomes are transferred from melanocytes to keratinocytes [6]. Conversely, the hypopigmentation that occurs in vitiligo is usually a result of inflammation caused by skin stress or due to other causes, including innervation, microvascular malformation, degeneration of melanocytes by oxidative stress, adhesion defects of melanocytes, somatic mosaic, and genetic influences [5,7,8,9,10,11,12,13].
Particularly, researchers have proposed that autophagy may play a role in redox stress-related vitiligo [14,15]. Impairment of autophagy may disrupt the antioxidant defense system, causing oxidative damage to melanocytes. Autophagy is a highly conserved cellular degradation and recycling process in all eukaryotes, and three types of autophagy occur in mammalian cells: microautophagy, macroautophagy, and chaperone-mediated autophagy. Although each type is morphologically distinct, all three have in common the delivery of cargo to lysosomes for degradation and recycling [16]. Among these three autophagy types, macroautophagy has been well-studied and is known to play an important role in maintaining intracellular homeostasis by inducing the degradation of cytoplasmic substances or metabolites under stress conditions, e.g., nutrient or energy deprivation, and decomposition of damaged or unnecessary organelles [16,17]. Interestingly, some research has shown that autophagy is an important factor in determining skin color. Caucasian skin-derived keratinocytes exhibit higher autophagic activity than those derived from African-American skin, and the accumulation of melanosomes is known to be accelerated via treatment with lysosomal inhibitors or small interfering ribonucleic acids specific to autophagy-associated proteins [18]. Additionally, various studies have provided evidence that the autophagy machinery may regulate melanogenesis.
In this review, we discuss the signal transduction pathways that induce melanogenesis, the relationship between the autophagy machinery and melanogenesis, and autophagy-inducing skin whitening materials.
2. Signal Transduction Pathways That Induce Melanogenesis
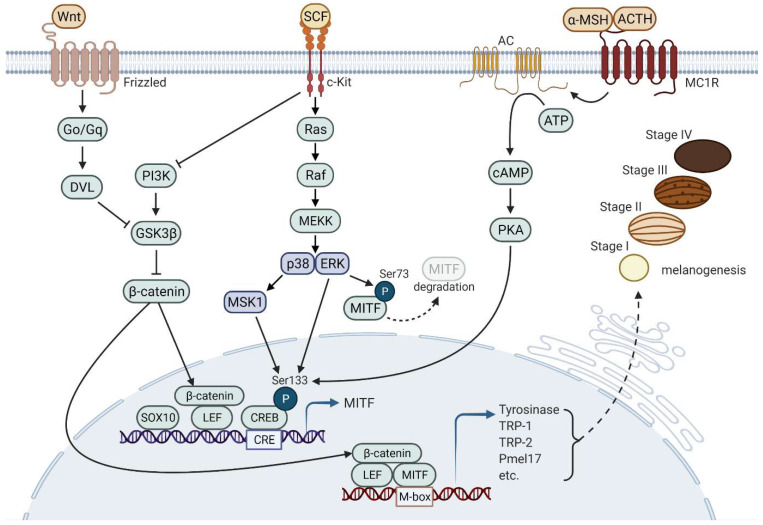
The synthesis of melanin in melanosomes is the result of complex pathways involving enzyme reactions. Tyrosinase (TYR), tyrosine-related protein-1 (TRP-1), and TRP-2 are mainly involved in enzyme reactions that transform tyrosine to melanin pigments [19]. Figure 1 illustrates common signaling pathways inducing melanogenesis.
Figure 1.
Signaling pathways that induce melanogenesis. Three representative signaling pathways, including MC1R-mediated signaling, SCF/c-KIT signaling, and Wnt signaling, are involved in melanogenesis. Expression and activation of MITF induce the expression of various proteins that play important roles in the formation and maturation of melanosomes as well as melanin synthesis. The continuous reaction of enzymatic proteins (e.g., TYR and TRP-1/2) and structural proteins (e.g., Pmel17) leads to melanogenesis in melanosomes wherein melanin pigments are synthesized and stored. DVL, Disheveled; Go/Gq, main families of G proteins.
Melanogenesis can be induced by various factors, including adrenocorticotropic hormone (ACTH) [20], α-melanocyte-stimulating hormone (α-MSH) [21,22], and stem cell factor (SCF) [23,24]. These factors induce melanogenesis through microphthalmia-associated transcription factor (MITF) expression and activation, which in turn induces the expression of pigment-related genes, such as TYR, TRP-1, TRP-2, and premelanosome protein (PMEL) [25]. Melanocortin 1 receptor (MC1R) is expressed in melanocytes in the plasma membrane, and ACTH and α-MSH are the ligands of MC1R [26]. MC1R-mediated signaling induces adenosine 3′,5′-cyclic monophosphate (cAMP), which activates PKA [27,28,29]. Activated PKA translocates into the nucleus and phosphorylates cAMP-response element-binding protein (CREB) [30]. CREB co-operates with SOX10 to induce MITF expression, resulting in the expression of pigment-related genes [31,32]. The binding of SCF to its receptor, tyrosine-protein kinase kit (c-KIT), initiates mitogen-activated protein kinase (MAPK) cascades that induce melanogenesis [33]. Autophosphorylated c-KIT activates p38 MAPK, resulting in CREB phosphorylation and sequential MITF activation [34,35,36]. The SCF/c-KIT pathway also activates extracellular signal-regulated kinase (ERK), inducing CREB phosphorylation for melanogenesis, whereas Ser73 phosphorylation of MITF via ERK leads to proteasomal degradation of MITF [37,38]. Furthermore, the SCF/c-KIT pathway is associated with phosphoinositide 3-kinase (PI3K) signaling that leads to glycogen synthase kinase-3 β (GSK3β) inactivation, which contributes to increasing the stability of β-catenin operating as a cofactor for MITF [39,40]. Wnt signaling is also a representative signaling pathway that contributes to β-catenin stability and plays a role in melanogenesis [41,42,43,44,45]. Frizzled-1 as a receptor for Wnt couples via G proteins, Go and Gq, and Dvl to activate β-catenin [46].
3. Autophagy
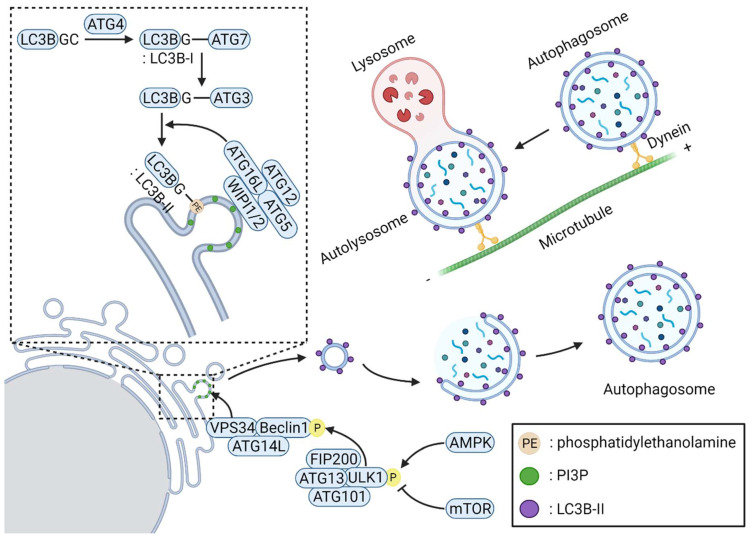
Starvation is a representative type of stress that induces macroautophagy, which occurs through sequential events involving initiation, nucleation, elongation and substrate selection, fusion of the autophagosome and lysosome, and lysosomal degradation [47,48]. During cell starvation, the lack of nutrients increases cellular 5′-adenosine monophosphate (AMP) levels. The ratio of AMP to ATP leads to AMP-activated protein kinase (AMPK) activation and inactivation of the target of the rapamycin complex 1 (mTORC1) [16,49], resulting in the activation of an autophagy-initiation complex containing FIP200, ULK1, autophagy-related (ATG)101, and ATG13 [50]. Under rapamycin treatment or starvation, mTORC1 is dissociated from the initiation complex, ATG13 and ULK1/2 become partially dephosphorylated, and autophagy is induced [51,52]. Phagophore nucleation is triggered upon phosphatidylinositol 3-phosphate (PI3P) generation by a complex with class III PI3K activity consisting of VPS34, VPS15, beclin-1, AMBRA1, and/or UV-radiation resistance-associated gene (UVRAG) along with the recruitment of vesicles containing ATG9 [53,54,55,56,57]. Elongation of phagophores formed with the support of WD repeat domain phosphoinositide-interacting protein (WIPI) includes two ubiquitin-like conjugation systems. ATG7 and ATG10 operate sequentially to catalyze the formation of the ATG12–ATG5:ATG16L1 complex. ATG4, ATG7, and ATG3 function together to cut the precursors of microtubule-associated protein 1 light chain 3 (LC3)-like proteins into their mature forms, after which they bond to phosphatidylethanolamine to generate LC3II-B, which is recruited and integrated into growing phagophores in an Atg5–Atg12:ATG16L1-dependent manner [58,59,60,61,62]. Cargo and/or cargo-selective proteins allow the formation of autophagosomes by binding to LC3 and LC3 homologs. Selective autophagy that degrades specific cargoes has also been reported, although autophagy induced by starvation is nonselective. Various cargo-selective proteins, also known as autophagy receptors, recognize the ubiquitinated cargoes and mediate autophagosome formation by surrounding the cargoes through LC3II-B binding on phagophores [63]. After complete fusion of the extended ends of the phagophore membrane, the formed autophagosomes fuse with lysosomes to form autolysosomes, in which substrate degradation is mediated through luminal acidification and lysosomal hydrolases. Figure 2 illustrates the representative common autophagy process.
Figure 2.
The most common autophagy process. Under nutrient-deficient conditions, increased AMP levels induce AMPK activation and AMPK-mediated activation of ULK1 complex (FIP200–ULK1–ATG13-ATG101). The ULK1 complex phosphorylates beclin-1, enabling the formation of the class III PI3K complex (VPS34–beclin-1–ATG14L). WIPI1/2 are recruited at the phagophore nucleation site by binding with PI3P, which is generated by the class III PI3K complex. ATG4 protease cleaves the C-terminal end of LC3B to expose glycine (LC3B-I). LC3B-I is then incorporated into the phagophore nucleation membrane through lipidation with phosphatidylethanolamine (LC3B-II) via sequential interactions with ATG7, ATG3, and the ATG12–ATG5:ATG16L complex. The LC3B-II-positive phagophore is then elongated and forms an autophagosome. While elongated, the random cytosolic contents are captured in the autophagosome and degraded after the autophagosome matures to an autolysosome.
4. Autophagy Machinery That Regulates Melanogenesis
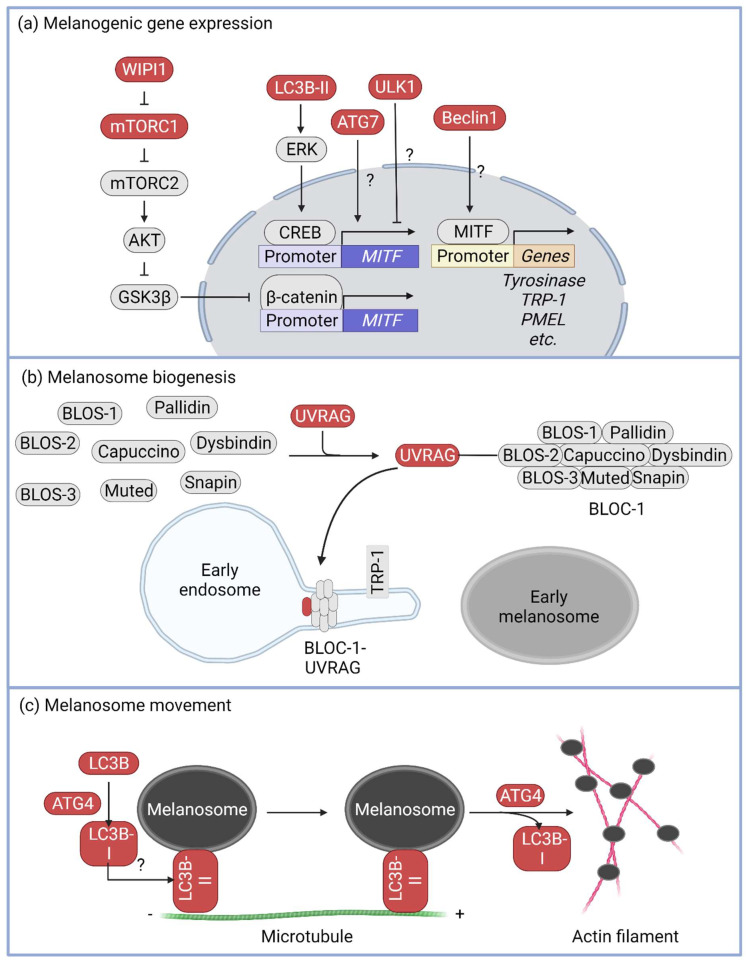
Melanogenesis in pigment cells proceeds in three stages: (1) melanogenic gene expression, (2) melanosome biogenesis and maturation, and (3) melanosome migration to the cell tip. Studies have indicated that autophagy machinery proteins may be involved in melanogenesis regulation (Figure 3) [64].
Figure 3.
Autophagy machinery in melanogenesis. Autophagy machinery is related to melanogenesis in various steps. (a) In step 1, the melanogenesis-related gene expression step, MITF expression is regulated by WIPI1, LC3B-II, and ATG7. WIPI1 increases MITF expression through upregulation of β-catenin stability via GSK3β inhibition. LC3B-II induces ERK activation, and ERK increases MITF expression via phosphorylating CREB, whereas ATG7 and beclin-1 are positively related to MITF expression and MITF transcription activity, respectively, and ULK1 plays a negative role in melanogenesis. However, the precise mechanisms underlying these processes remain unclear. (b) In step 2, the melanosome biogenesis, UVRAG interacts with the BLOC-1 complex and upregulates its protein stability. (c) In step 3, the melanosome movement, LC3B is incorporated into the melanosomal membrane via cleavage through ATG4. LC3B on the melanosomal membrane mediates melanosome–microtubule interactions to facilitate melanosome movement to the cell tip. Before the transfer of the melanosome to an actin filament, lipidated LC3B on the melanosomal membrane is removed via ATG4 protease.
MITF, a master regulator of melanogenesis, plays an important role in melanogenesis stage 1 (i.e., the melanogenesis gene expression stage). MITF induces the expression of various genes involved in melanogenesis, such as TYR, TRP-1, TRP-2, and PMEL [65,66,67]. ATG7, a critical gene associated with LC3 lipidation, might also be involved in melanogenesis [68]. Knockdown of ATG7 in natural human epidermal melanocytes decreased the MITF expression level and reduced melanin accumulation in the cells, whereas overexpression of ATG7 increased MITF expression [69]. Additionally, tail skin pigmentation in ATG7f/fTyr:Cre mice was consistently lower than that in ATG7f/f mice [70]. In Melan-a cells, knockdown of LC3 decreased ERK activity, which suppressed α-MSH-mediated melanogenesis by attenuating phosphorylation of CREB and MITF expression [71]. Moreover, mice heterozygous for beclin-1, a scaffold protein in the class III PI3K complex, showed mislocalization of MITF-nucleus, resulting in depigmentation in MNT-1 cells in relation to coat color [64], and the embryos of beclin-1-depleted zebrafish showed almost 50% lower melanin levels compared with those in control embryos. Transactional downregulation of both TYR and TRP-1 was also shown in beclin-1-depleted zebrafish [72]. WIPI1 has been reported to bind with phosphoatidylinositol-3 phosphate in the early stage autophagosome to recruit the ATG12–ATG5:ATG16L complex and elongate the autophagosome membrane [73]. In MNT-1 cells, WIPI1 induces AKT activation through activation of mTORC2, which results in the inactivation of GSK3β. WIPI1-mediated GSK3β inactivation increases β-catenin stability, which in turn induces MITF expression [74]. However, it has been suggested that ULK1, which contributes to autophagy through the activation of the class III PI3K complex, plays a role in inhibiting melanin synthesis. In one study, ULK1 knockdown in MNT-1 cells increased MITF expression, resulting in upregulation of melanogenesis (Figure 3a) [75].
Numerous proteins are involved in melanogenesis stage II, i.e., the melanosome biogenesis and maturation stage, including adaptor protein (AP)-1, AP-2, biogenesis of lysosome-related organelle complex (BLOC)-1, BLOC-2, BLOC-3, and various Rab GTPases [76,77,78,79,80]. UVRAG, identified as a beclin-1-binding autophagy-associated protein [81,82], has specialized functions in melanosome biosynthesis through its interaction with BLOC-1. UVRAG facilitates the classification and delivery of melanogenic cargoes by maintaining the localization and stability of BLOC-1. When UVRAG levels are reduced, cells do not respond to UVR–α-MSH–MITF signaling and melanocyte development becomes defective in vivo (Figure 3b) [83].
At melanogenesis stage III (i.e., melanosome movement toward the cell tip), the melanosome should move from the perinucleus to the tip of melanocytes and transfer to keratinocytes, after which the transferred melanosome plays a protective role against UV-mediated DNA damage [6]. It has been proposed that autophagic proteins, such as LC3B and ATG4, mediate melanosome trafficking in the cytoskeletal track [84]. In LC3B-knockdown B16 cells, melanosomes do not interact with microtubules and remain at the perinuclear site. LC3B lipidation and delipidation were mediated by ATG4B, and the LC3BII delipidation activity of ATG4B was critical for melanosome separation from microtubules to actin filaments (Figure 3c).
5. Autophagy Inducers That Induce Skin Depigmentation
While autophagy-associated proteins have been reported to play critical roles in melanogenesis in many studies, some reports suggest that autophagy-inducing agents are involved in skin depigmentation. Autophagy activity in Caucasian melanocytes was higher than that in African-American melanocytes, and decreased autophagy was shown in hyperpigmented skin, such as that in senile lentigo [18,85]. A lysosomal protease, cathepsin L, was found to be involved in melanosome degradation in melanocytes through autophagosome–lysosome fusion [86]. Some studies suggest that autophagy inducers might induce melanosome degradation in an autophagy-dependent manner (Table 1). The listed autophagy inducer-mediated depigmentation is inhibited by knockdown of autophagy essential genes, such as ATG5 [87,88,89,90], ATG7 [91], and LC3 [92], or treatment with autophagy inhibitors, such as 3-MA [88,92,93,94,95], hydroxychloroquine [96], bafilomycin A1 [89], and chloroquine [97]. For example, β-mangostin cannot induce autophagy in B16F10 cells, but it induces depigmentation through autophagy-mediated melanosome degradation in pigmented B16F10 cells via α-MSH stimulation, and the depigmentation is inhibited by ATG5 knockdown or 3-MA treatment [88]. Although many autophagy machinery components are associated with melanogenesis, the degradation of existing melanosomes might be induced by autophagy. To date, the studies on agents that induce autophagy-associated skin discoloration are limited to the inhibition of depigmentation by either autophagy-associated gene knockdown or the use of autophagy inhibitors, and the molecular mechanisms underlying melanosome-targeted autophagy are yet to be clarified.
Table 1.
Agents that induce skin depigmentation in an autophagy-dependent manner.
| Agent/Stimulation | Reported Finding | Ref. |
|---|---|---|
| ARP101 | ATG5 knockdown inhibited the antimelanogenic effect of ARP101. Electron microscopy analysis showed that autophagosomes engulf melanosomes. |
[87] |
| Ellagic acid (EA) | 3-MA treatment or LC3 silencing significantly reduced EA-induced antimelanogenic activity in B16F10 cells. | [92] |
| 3-O-Glyceryl-2-O-hexyl ascorbate (VC-HG) |
VC-HG activates autophagy, and VC-HG-mediated depigmentation is partially inhibited by autophagy inhibitors, namely hydroxychloroquine or pepstatin A, in B16 cells. | [96] |
| 3′-Hydroxydaidzein (3′-ODI) | 3′-ODI significantly reduced α-MSH-mediated melanogenesis, and the inhibition of autophagy significantly reduced the antimelanogenic effects of 3′-ODI in α-MSH-stimulated melanoma cells. | [93] |
| Isoliquiritigenin | Autophagy inhibition via si-ATG7 or 3-MA treatment decreased LC3 II protein levels and increased PMEL17, p62, and melanin levels in HaCaT cells. | [91] |
| β-mangostin | Melanosome-engulfing autophagosomes were observed via transmission electron microscopy. Previously formed melanin could be degraded effectively in an autophagy-dependent manner, which was inhibited by ATG5 knockdown or 3-MA treatment in β-mangostin-treated B16F10 cells. | [88] |
| Melasolv | Melasolv suppressed the accumulation of melanin content and induced autophagy. ATG5 knockdown or bafilomycin A1 treatment suppressed melasolv-mediated depigmentation in B16F1 cells. |
[89] |
| 5-Methyl-3-tetradecylidene-dihydro-furan-2-one (DMF02) | DMF02 induced melanosome degradation via autophagy in vitro, and this degradation was inhibited by a lysosomal inhibitor, chloroquine, in B16F10 cells. | [97] |
| Patrinia villosa (Thunb.) Juss extract (Pv-EE) | Pv-EE induced autophagy, and the Pv-EE-mediated antimelanogenic effect was inhibited by 3-MA in B16F10 cells. | [94] |
| PTPD-12 | PTPD-12 induced melanosome degradation through stimulation of autophagic flux in human melanocytes and keratinocytes. | [98] |
| Radiofrequency (RF) irradiation | RF irradiation upregulated autophagy-initiation factors, such as FIP200, ULK1, ULK2, ATG13, and ATG101, in the skin. Beclin-1 expression and the expression ratio of LC3-I to LC3-II increased with UV-B/RF irradiation, and melanin-containing autophagosome levels increased with RF irradiation. | [99] |
| Resveratrol (RSV) | ATG5 knockdown significantly suppressed RSV-mediated antimelanogenesis as well as RSV-induced autophagy in Melan-A cells. | [90] |
| Schaftoside | Schaftoside treatment had an antimelanogenic effect and induced autophagy activation in B16F1 cells, and 3-MA treatment reduced the antimelanogenic effect via schaftoside in B16F1 cells. | [95] |
| Tranexamic acid (TXA) | TXA reduced melanin accumulation by activating ERK signaling and the autophagy system. | [100] |
6. Conclusions and Outlook
In skin pigmentation homeostasis, the balance between melanogenesis and melanosome degradation is likely important. Although the melanogenesis pathway has been well-studied, and proteins that play roles in autophagy are known to be involved in melanogenesis, autophagy by starvation does not induce melanogenesis [74], and ATG7-dependent autophagy activity has little effect on melanogenesis [70]. Melanogenesis is a process leading to the synthesis of melanin in melanosome including melanosome formation, and autophagy is a process degrading cellular components. Strangely, proteins essential for autophagy degrading cellular components including melanosome are involved in melanogenesis for de novo synthesis of melanin and melanosome. Although the mechanism to clarify the relationship between the two processes has not been elucidated so far, there is no direct evidence that the autophagy process is essential for melanogenesis. Another possibility is that proteins involved in autophagy may play a role in the signaling for melanogenesis independent of the autophagy process. For example, although knockdown or knockout of ATG7, an essential gene for autophagy, inhibits melanin synthesis through reduction of MITF expression [69,70], knockdown of ULK1, an essential kinase forming autophagy initiation complex, induces melanin synthesis by increasing the expression of MITF [75]. Therefore, the precise role of autophagy in melanogenesis regulation must be determined as well as how the autophagy pathway cross-talks with the melanosome pathway and how the roles of several factors are balanced in various physiological processes.
It has been proposed that the autophagy process is involved in melanin degradation, particularly in skin whitening material research. Ho et al. suggested that, under stress conditions, including starvation or defective melanosomes, autophagy could be activated to form autophagosomes that engulf and degrade melanosomes [101]. Selective autophagy is an important cellular event that maintains cellular physiological homeostasis through the degradation of specific cellular compartments, such as aggregated proteins, damaged organelles, and pathogens. Selective autophagy is mediated using autophagy receptors that recognize target cargo via binding to ubiquitinated organelles. For example, membrane proteins of damaged mitochondria are recognized by PINK/PARKIN and sequentially phosphorylated and ubiquitinated. The autophagy receptor optineurin recognizes and binds to membrane proteins and links to the LC3-embedded phagophore [102]. Melanosomes are also known as specific organelles of melanocytes. The results of several studies suggest that autophagy-mediated degradation of melanosomes exists, but direct evidence and the underlying molecular mechanism have not been reported. We suggest that future research should be focused on the following: (1) how melanosomes induce the autophagy-initiation signal, (2) identification of the E3-ligase that ubiquitinates melanosome membrane proteins, and (3) identification of melanosome-targeted autophagy receptors and molecular mechanisms.
Author Contributions
K.D.K. drafted the manuscript outline; K.D.K., K.W.L., M.K. and S.H.L. conceived the ideas and prepared the figures. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the BioGreen21 Agri-Tech Innovation Program (PJ015731), Rural Development Administration, Korea, and the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (2021R1A2C1004847 and 2021R1A5A8029490).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lin J.Y., Fisher D.E. Melanocyte biology and skin pigmentation. Nature. 2007;445:843–850. doi: 10.1038/nature05660. [DOI] [PubMed] [Google Scholar]
- 2.Delevoye C. Melanin transfer: The keratinocytes are more than gluttons. J. Investig. Dermatol. 2014;134:877–879. doi: 10.1038/jid.2013.487. [DOI] [PubMed] [Google Scholar]
- 3.Lee A.Y. Recent progress in melasma pathogenesis. Pigment Cell Melanoma Res. 2015;28:648–660. doi: 10.1111/pcmr.12404. [DOI] [PubMed] [Google Scholar]
- 4.Fu C., Chen J., Lu J., Yi L., Tong X., Kang L., Pei S., Ouyang Y., Jiang L., Ding Y., et al. Roles of inflammation factors in melanogenesis (Review) Mol. Med. Rep. 2020;21:1421–1430. doi: 10.3892/mmr.2020.10950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marks M.S., Seabra M.C. The melanosome: Membrane dynamics in black and white. Nat. Rev. Mol. Cell Biol. 2001;2:738–748. doi: 10.1038/35096009. [DOI] [PubMed] [Google Scholar]
- 6.Boissy R.E. Melanosome transfer to and translocation in the keratinocyte. Exp. Dermatol. 2003;12((Suppl. 2)):5–12. doi: 10.1034/j.1600-0625.12.s2.1.x. [DOI] [PubMed] [Google Scholar]
- 7.Frisoli M.L., Essien K., Harris J.E. Vitiligo: Mechanisms of Pathogenesis and Treatment. Annu. Rev. Immunol. 2020;38:621–648. doi: 10.1146/annurev-immunol-100919-023531. [DOI] [PubMed] [Google Scholar]
- 8.Taieb A., Morice-Picard F., Jouary T., Ezzedine K., Cario-Andre M., Gauthier Y. Segmental vitiligo as the possible expression of cutaneous somatic mosaicism: Implications for common non-segmental vitiligo. Pigment Cell Melanoma Res. 2008;21:646–652. doi: 10.1111/j.1755-148X.2008.00511.x. [DOI] [PubMed] [Google Scholar]
- 9.van Geel N., Speeckaert R., Melsens E., Toelle S.P., Speeckaert M., De Schepper S., Lambert J., Brochez L. The distribution pattern of segmental vitiligo: Clues for somatic mosaicism. Br. J. Dermatol. 2013;168:56–64. doi: 10.1111/bjd.12013. [DOI] [PubMed] [Google Scholar]
- 10.van Geel N., Mollet I., Brochez L., Dutre M., De Schepper S., Verhaeghe E., Lambert J., Speeckaert R. New insights in segmental vitiligo: Case report and review of theories. Br. J. Dermatol. 2012;166:240–246. doi: 10.1111/j.1365-2133.2011.10650.x. [DOI] [PubMed] [Google Scholar]
- 11.Westerhof W., d’Ischia M. Vitiligo puzzle: The pieces fall in place. Pigment Cell Res. 2007;20:345–359. doi: 10.1111/j.1600-0749.2007.00399.x. [DOI] [PubMed] [Google Scholar]
- 12.Gauthier Y., Cario Andre M., Taieb A. A critical appraisal of vitiligo etiologic theories. Is melanocyte loss a melanocytorrhagy? Pigment Cell Res. 2003;16:322–332. doi: 10.1034/j.1600-0749.2003.00070.x. [DOI] [PubMed] [Google Scholar]
- 13.Speeckaert R., Dugardin J., Lambert J., Lapeere H., Verhaeghe E., Speeckaert M.M., van Geel N. Critical appraisal of the oxidative stress pathway in vitiligo: A systematic review and meta-analysis. J. Eur. Acad. Dermatol. Venereol. 2018;32:1089–1098. doi: 10.1111/jdv.14792. [DOI] [PubMed] [Google Scholar]
- 14.Qiao Z., Wang X., Xiang L., Zhang C. Dysfunction of Autophagy: A Possible Mechanism Involved in the Pathogenesis of Vitiligo by Breaking the Redox Balance of Melanocytes. Oxid. Med. Cell. Longev. 2016;2016:3401570. doi: 10.1155/2016/3401570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He Y., Li S., Zhang W., Dai W., Cui T., Wang G., Gao T., Li C. Dysregulated autophagy increased melanocyte sensitivity to H2O2-induced oxidative stress in vitiligo. Sci. Rep. 2017;7:42394. doi: 10.1038/srep42394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Z., Klionsky D.J. Mammalian autophagy: Core molecular machinery and signaling regulation. Curr. Opin. Cell Biol. 2010;22:124–131. doi: 10.1016/j.ceb.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yorimitsu T., Klionsky D.J. Autophagy: Molecular machinery for self-eating. Cell Death Differ. 2005;12((Suppl. 2)):1542–1552. doi: 10.1038/sj.cdd.4401765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murase D., Hachiya A., Takano K., Hicks R., Visscher M.O., Kitahara T., Hase T., Takema Y., Yoshimori T. Autophagy has a significant role in determining skin color by regulating melanosome degradation in keratinocytes. J. Investig. Dermatol. 2013;133:2416–2424. doi: 10.1038/jid.2013.165. [DOI] [PubMed] [Google Scholar]
- 19.Yamaguchi Y., Brenner M., Hearing V.J. The regulation of skin pigmentation. J. Biol. Chem. 2007;282:27557–27561. doi: 10.1074/jbc.R700026200. [DOI] [PubMed] [Google Scholar]
- 20.Hunt G., Todd C., Kyne S., Thody A.J. ACTH stimulates melanogenesis in cultured human melanocytes. J. Endocrinol. 1994;140:R1–R3. doi: 10.1677/joe.0.140R001. [DOI] [PubMed] [Google Scholar]
- 21.Hunt G., Todd C., Cresswell J.E., Thody A.J. Alpha-melanocyte stimulating hormone and its analogue Nle4DPhe7 alpha-MSH affect morphology, tyrosinase activity and melanogenesis in cultured human melanocytes. Pt 1J. Cell Sci. 1994;107:205–211. doi: 10.1242/jcs.107.1.205. [DOI] [PubMed] [Google Scholar]
- 22.Hill S.E., Buffey J., Thody A.J., Oliver I., Bleehen S.S., Mac Neil S. Investigation of the regulation of pigmentation in alpha-melanocyte-stimulating hormone responsive and unresponsive cultured B16 melanoma cells. Pigment Cell Res. 1989;2:161–166. doi: 10.1111/j.1600-0749.1989.tb00181.x. [DOI] [PubMed] [Google Scholar]
- 23.Kim M., Shibata T., Kwon S., Park T.J., Kang H.Y. Ultraviolet-irradiated endothelial cells secrete stem cell factor and induce epidermal pigmentation. Sci. Rep. 2018;8:4235. doi: 10.1038/s41598-018-22608-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vanover J.C., Spry M.L., Hamilton L., Wakamatsu K., Ito S., D’Orazio J.A. Stem cell factor rescues tyrosinase expression and pigmentation in discreet anatomic locations in albino mice. Pigment Cell Melanoma Res. 2009;22:827–838. doi: 10.1111/j.1755-148X.2009.00617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vachtenheim J., Borovansky J. “Transcription physiology” of pigment formation in melanocytes: Central role of MITF. Exp. Dermatol. 2010;19:617–627. doi: 10.1111/j.1600-0625.2009.01053.x. [DOI] [PubMed] [Google Scholar]
- 26.Millington G.W. Proopiomelanocortin (POMC): The cutaneous roles of its melanocortin products and receptors. Clin. Exp. Dermatol. 2006;31:407–412. doi: 10.1111/j.1365-2230.2006.02128.x. [DOI] [PubMed] [Google Scholar]
- 27.D’Orazio J., Fisher D.E. Central role for cAMP signaling in pigmentation and UV resistance. Cell Cycle. 2011;10:8–9. doi: 10.4161/cc.10.1.14292. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez C.I., Setaluri V. Cyclic AMP (cAMP) signaling in melanocytes and melanoma. Arch. Biochem. Biophys. 2014;563:22–27. doi: 10.1016/j.abb.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 29.Saha B., Singh S.K., Sarkar C., Bera R., Ratha J., Tobin D.J., Bhadra R. Activation of the Mitf promoter by lipid-stimulated activation of p38-stress signalling to CREB. Pigment Cell Res. 2006;19:595–605. doi: 10.1111/j.1600-0749.2006.00348.x. [DOI] [PubMed] [Google Scholar]
- 30.Gonzalez G.A., Montminy M.R. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989;59:675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- 31.Bertolotto C., Abbe P., Hemesath T.J., Bille K., Fisher D.E., Ortonne J.P., Ballotti R. Microphthalmia gene product as a signal transducer in cAMP-induced differentiation of melanocytes. J. Cell Biol. 1998;142:827–835. doi: 10.1083/jcb.142.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huber W.E., Price E.R., Widlund H.R., Du J., Davis I.J., Wegner M., Fisher D.E. A tissue-restricted cAMP transcriptional response: SOX10 modulates alpha-melanocyte-stimulating hormone-triggered expression of microphthalmia-associated transcription factor in melanocytes. J. Biol. Chem. 2003;278:45224–45230. doi: 10.1074/jbc.M309036200. [DOI] [PubMed] [Google Scholar]
- 33.Li P.H., Liu L.H., Chang C.C., Gao R., Leung C.H., Ma D.L., David Wang H.M. Silencing Stem Cell Factor Gene in Fibroblasts to Regulate Paracrine Factor Productions and Enhance c-Kit Expression in Melanocytes on Melanogenesis. Int. J. Mol. Sci. 2018;19:1475. doi: 10.3390/ijms19051475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flaherty K.T., Hodi F.S., Fisher D.E. From genes to drugs: Targeted strategies for melanoma. Nat. Rev. Cancer. 2012;12:349–361. doi: 10.1038/nrc3218. [DOI] [PubMed] [Google Scholar]
- 35.Bonaventure J., Domingues M.J., Larue L. Cellular and molecular mechanisms controlling the migration of melanocytes and melanoma cells. Pigment Cell Melanoma Res. 2013;26:316–325. doi: 10.1111/pcmr.12080. [DOI] [PubMed] [Google Scholar]
- 36.Ahn J.H., Jin S.H., Kang H.Y. LPS induces melanogenesis through p38 MAPK activation in human melanocytes. Arch. Dermatol. Res. 2008;300:325–329. doi: 10.1007/s00403-008-0863-0. [DOI] [PubMed] [Google Scholar]
- 37.D’Mello S.A., Finlay G.J., Baguley B.C., Askarian-Amiri M.E. Signaling Pathways in Melanogenesis. Int. J. Mol. Sci. 2016;17:1144. doi: 10.3390/ijms17071144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim J.Y., Lee E.J., Ahn Y., Park S., Kim S.H., Oh S.H. A chemical compound from fruit extract of Juglans mandshurica inhibits melanogenesis through p-ERK-associated MITF degradation. Phytomedicine. 2019;57:57–64. doi: 10.1016/j.phymed.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 39.Hwang E., Lee T.H., Lee W.J., Shim W.S., Yeo E.J., Kim S., Kim S.Y. A novel synthetic Piper amide derivative NED-180 inhibits hyperpigmentation by activating the PI3K and ERK pathways and by regulating Ca2+ influx via TRPM1 channels. Pigment Cell Melanoma Res. 2016;29:81–91. doi: 10.1111/pcmr.12430. [DOI] [PubMed] [Google Scholar]
- 40.Widlund H.R., Horstmann M.A., Price E.R., Cui J., Lessnick S.L., Wu M., He X., Fisher D.E. Beta-catenin-induced melanoma growth requires the downstream target Microphthalmia-associated transcription factor. J. Cell Biol. 2002;158:1079–1087. doi: 10.1083/jcb.200202049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu P.Y., Yin W.H., Wang M.R., Dang Y.Y., Ye X.Y. Andrographolide suppresses melanin synthesis through Akt/GSK3beta/beta-catenin signal pathway. J. Dermatol. Sci. 2015;79:74–83. doi: 10.1016/j.jdermsci.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 42.Hwang I., Park J.H., Park H.S., Choi K.A., Seol K.C., Oh S.I., Kang S., Hong S. Neural stem cells inhibit melanin production by activation of Wnt inhibitors. J. Dermatol. Sci. 2013;72:274–283. doi: 10.1016/j.jdermsci.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 43.Stamos J.L., Weis W.I. The beta-catenin destruction complex. Cold Spring Harb. Perspect. Biol. 2013;5:a007898. doi: 10.1101/cshperspect.a007898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takeda K., Yasumoto K., Takada R., Takada S., Watanabe K., Udono T., Saito H., Takahashi K., Shibahara S. Induction of melanocyte-specific microphthalmia-associated transcription factor by Wnt-3a. J. Biol. Chem. 2000;275:14013–14016. doi: 10.1074/jbc.C000113200. [DOI] [PubMed] [Google Scholar]
- 45.Pillaiyar T., Namasivayam V., Manickam M., Jung S.H. Inhibitors of Melanogenesis: An Updated Review. J. Med. Chem. 2018;61:7395–7418. doi: 10.1021/acs.jmedchem.7b00967. [DOI] [PubMed] [Google Scholar]
- 46.Liu T., DeCostanzo A.J., Liu X., Wang H., Hallagan S., Moon R.T., Malbon C.C. G protein signaling from activated rat frizzled-1 to the beta-catenin-Lef-Tcf pathway. Science. 2001;292:1718–1722. doi: 10.1126/science.1060100. [DOI] [PubMed] [Google Scholar]
- 47.Galluzzi L., Bravo-San Pedro J.M., Levine B., Green D.R., Kroemer G. Pharmacological modulation of autophagy: Therapeutic potential and persisting obstacles. Nat. Rev. Drug Discov. 2017;16:487–511. doi: 10.1038/nrd.2017.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsuboyama K., Koyama-Honda I., Sakamaki Y., Koike M., Morishita H., Mizushima N. The ATG conjugation systems are important for degradation of the inner autophagosomal membrane. Science. 2016;354:1036–1041. doi: 10.1126/science.aaf6136. [DOI] [PubMed] [Google Scholar]
- 49.Gwinn D.M., Shackelford D.B., Egan D.F., Mihaylova M.M., Mery A., Vasquez D.S., Turk B.E., Shaw R.J. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zachari M., Ganley I.G. The mammalian ULK1 complex and autophagy initiation. Essays Biochem. 2017;61:585–596. doi: 10.1042/EBC20170021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hosokawa N., Hara T., Kaizuka T., Kishi C., Takamura A., Miura Y., Iemura S., Natsume T., Takehana K., Yamada N., et al. Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol. Biol. Cell. 2009;20:1981–1991. doi: 10.1091/mbc.e08-12-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jung C.H., Jun C.B., Ro S.H., Kim Y.M., Otto N.M., Cao J., Kundu M., Kim D.H. ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol. Biol. Cell. 2009;20:1992–2003. doi: 10.1091/mbc.e08-12-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Furuya N., Yu J., Byfield M., Pattingre S., Levine B. The evolutionarily conserved domain of Beclin 1 is required for Vps34 binding, autophagy and tumor suppressor function. Autophagy. 2005;1:46–52. doi: 10.4161/auto.1.1.1542. [DOI] [PubMed] [Google Scholar]
- 54.Itakura E., Kishi C., Inoue K., Mizushima N. Beclin 1 forms two distinct phosphatidylinositol 3-kinase complexes with mammalian Atg14 and UVRAG. Mol. Biol. Cell. 2008;19:5360–5372. doi: 10.1091/mbc.e08-01-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kihara A., Noda T., Ishihara N., Ohsumi Y. Two distinct Vps34 phosphatidylinositol 3-kinase complexes function in autophagy and carboxypeptidase Y sorting in Saccharomyces cerevisiae. J. Cell Biol. 2001;152:519–530. doi: 10.1083/jcb.152.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liang C., Feng P., Ku B., Dotan I., Canaani D., Oh B.H., Jung J.U. Autophagic and tumour suppressor activity of a novel Beclin1-binding protein UVRAG. Nat. Cell Biol. 2006;8:688–699. doi: 10.1038/ncb1426. [DOI] [PubMed] [Google Scholar]
- 57.Fimia G.M., Stoykova A., Romagnoli A., Giunta L., Di Bartolomeo S., Nardacci R., Corazzari M., Fuoco C., Ucar A., Schwartz P., et al. Ambra1 regulates autophagy and development of the nervous system. Nature. 2007;447:1121–1125. doi: 10.1038/nature05925. [DOI] [PubMed] [Google Scholar]
- 58.Ohsumi Y. Molecular dissection of autophagy: Two ubiquitin-like systems. Nat. Rev. Mol. Cell Biol. 2001;2:211–216. doi: 10.1038/35056522. [DOI] [PubMed] [Google Scholar]
- 59.Kim J., Dalton V.M., Eggerton K.P., Scott S.V., Klionsky D.J. Apg7p/Cvt2p is required for the cytoplasm-to-vacuole targeting, macroautophagy, and peroxisome degradation pathways. Mol. Biol. Cell. 1999;10:1337–1351. doi: 10.1091/mbc.10.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kuma A., Mizushima N., Ishihara N., Ohsumi Y. Formation of the approximately 350-kDa Apg12-Apg5.Apg16 multimeric complex, mediated by Apg16 oligomerization, is essential for autophagy in yeast. J. Biol. Chem. 2002;277:18619–18625. doi: 10.1074/jbc.M111889200. [DOI] [PubMed] [Google Scholar]
- 61.Satoo K., Noda N.N., Kumeta H., Fujioka Y., Mizushima N., Ohsumi Y., Inagaki F. The structure of Atg4B-LC3 complex reveals the mechanism of LC3 processing and delipidation during autophagy. EMBO J. 2009;28:1341–1350. doi: 10.1038/emboj.2009.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Murrow L., Malhotra R., Debnath J. ATG12-ATG3 interacts with Alix to promote basal autophagic flux and late endosome function. Nat. Cell Biol. 2015;17:300–310. doi: 10.1038/ncb3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gatica D., Lahiri V., Klionsky D.J. Cargo recognition and degradation by selective autophagy. Nat. Cell Biol. 2018;20:233–242. doi: 10.1038/s41556-018-0037-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ganesan A.K., Ho H., Bodemann B., Petersen S., Aruri J., Koshy S., Richardson Z., Le L.Q., Krasieva T., Roth M.G., et al. Genome-wide siRNA-based functional genomics of pigmentation identifies novel genes and pathways that impact melanogenesis in human cells. PLoS Genet. 2008;4:e1000298. doi: 10.1371/journal.pgen.1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yasumoto K., Yokoyama K., Shibata K., Tomita Y., Shibahara S. Microphthalmia-associated transcription factor as a regulator for melanocyte-specific transcription of the human tyrosinase gene. Mol. Cell. Biol. 1995;15:1833. doi: 10.1128/MCB.15.3.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bertolotto C., Busca R., Abbe P., Bille K., Aberdam E., Ortonne J.P., Ballotti R. Different cis-acting elements are involved in the regulation of TRP1 and TRP2 promoter activities by cyclic AMP: Pivotal role of M boxes (GTCATGTGCT) and of microphthalmia. Mol. Cell. Biol. 1998;18:694–702. doi: 10.1128/MCB.18.2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Du J., Miller A.J., Widlund H.R., Horstmann M.A., Ramaswamy S., Fisher D.E. MLANA/MART1 and SILV/PMEL17/GP100 are transcriptionally regulated by MITF in melanocytes and melanoma. Am. J. Pathol. 2003;163:333–343. doi: 10.1016/S0002-9440(10)63657-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Taherbhoy A.M., Tait S.W., Kaiser S.E., Williams A.H., Deng A., Nourse A., Hammel M., Kurinov I., Rock C.O., Green D.R., et al. Atg8 transfer from Atg7 to Atg3: A distinctive E1–E2 architecture and mechanism in the autophagy pathway. Mol. Cell. 2011;44:451–461. doi: 10.1016/j.molcel.2011.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qiao Z., Xu Z., Xiao Q., Yang Y., Ying J., Xiang L., Zhang C. Dysfunction of ATG7-dependent autophagy dysregulates the antioxidant response and contributes to oxidative stress-induced biological impairments in human epidermal melanocytes. Cell Death Discov. 2020;6:31. doi: 10.1038/s41420-020-0266-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang C.F., Gruber F., Ni C., Mildner M., Koenig U., Karner S., Barresi C., Rossiter H., Narzt M.S., Nagelreiter I.M., et al. Suppression of autophagy dysregulates the antioxidant response and causes premature senescence of melanocytes. J. Investig. Dermatol. 2015;135:1348–1357. doi: 10.1038/jid.2014.439. [DOI] [PubMed] [Google Scholar]
- 71.Yun W.J., Kim E.Y., Park J.E., Jo S.Y., Bang S.H., Chang E.J., Chang S.E. Microtubule-associated protein light chain 3 is involved in melanogenesis via regulation of MITF expression in melanocytes. Sci. Rep. 2016;6:19914. doi: 10.1038/srep19914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rai A., Chatterjee B., Bhowmick S., Sagar S., Roy S.S. Beclin 1 controls pigmentation by changing the nuclear localization of melanogenic factor MITF. Biochem. Biophys. Res. Commun. 2020;528:719–725. doi: 10.1016/j.bbrc.2020.05.118. [DOI] [PubMed] [Google Scholar]
- 73.Grimmel M., Backhaus C., Proikas-Cezanne T. WIPI-Mediated Autophagy and Longevity. Cells. 2015;4:202–217. doi: 10.3390/cells4020202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ho H., Kapadia R., Al-Tahan S., Ahmad S., Ganesan A.K. WIPI1 coordinates melanogenic gene transcription and melanosome formation via TORC1 inhibition. J. Biol. Chem. 2011;286:12509–12523. doi: 10.1074/jbc.M110.200543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kalie E., Razi M., Tooze S.A. ULK1 regulates melanin levels in MNT-1 cells independently of mTORC1. PLoS ONE. 2013;8:e75313. doi: 10.1371/journal.pone.0075313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Theos A.C., Tenza D., Martina J.A., Hurbain I., Peden A.A., Sviderskaya E.V., Stewart A., Robinson M.S., Bennett D.C., Cutler D.F., et al. Functions of adaptor protein (AP)-3 and AP-1 in tyrosinase sorting from endosomes to melanosomes. Mol. Biol. Cell. 2005;16:5356–5372. doi: 10.1091/mbc.e05-07-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Setty S.R., Tenza D., Truschel S.T., Chou E., Sviderskaya E.V., Theos A.C., Lamoreux M.L., Di Pietro S.M., Starcevic M., Bennett D.C., et al. BLOC-1 is required for cargo-specific sorting from vacuolar early endosomes toward lysosome-related organelles. Mol. Biol. Cell. 2007;18:768–780. doi: 10.1091/mbc.e06-12-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dennis M.K., Mantegazza A.R., Snir O.L., Tenza D., Acosta-Ruiz A., Delevoye C., Zorger R., Sitaram A., de Jesus-Rojas W., Ravichandran K., et al. BLOC-2 targets recycling endosomal tubules to melanosomes for cargo delivery. J. Cell Biol. 2015;209:563–577. doi: 10.1083/jcb.201410026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dennis M.K., Delevoye C., Acosta-Ruiz A., Hurbain I., Romao M., Hesketh G.G., Goff P.S., Sviderskaya E.V., Bennett D.C., Luzio J.P., et al. BLOC-1 and BLOC-3 regulate VAMP7 cycling to and from melanosomes via distinct tubular transport carriers. J. Cell Biol. 2016;214:293–308. doi: 10.1083/jcb.201605090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fukuda M. Rab GTPases: Key players in melanosome biogenesis, transport, and transfer. Pigment Cell Melanoma Res. 2021;34:222–235. doi: 10.1111/pcmr.12931. [DOI] [PubMed] [Google Scholar]
- 81.Takahashi Y., Coppola D., Matsushita N., Cualing H.D., Sun M., Sato Y., Liang C., Jung J.U., Cheng J.Q., Mule J.J., et al. Bif-1 interacts with Beclin 1 through UVRAG and regulates autophagy and tumorigenesis. Nat. Cell Biol. 2007;9:1142–1151. doi: 10.1038/ncb1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wu S., He Y., Qiu X., Yang W., Liu W., Li X., Li Y., Shen H.M., Wang R., Yue Z., et al. Targeting the potent Beclin 1-UVRAG coiled-coil interaction with designed peptides enhances autophagy and endolysosomal trafficking. Proc. Natl. Acad. Sci. USA. 2018;115:E5669–E5678. doi: 10.1073/pnas.1721173115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang Y., Jang G.B., Yang X., Wang Q., He S., Li S., Quach C., Zhao S., Li F., Yuan Z., et al. Central role of autophagic UVRAG in melanogenesis and the suntan response. Proc. Natl. Acad. Sci. USA. 2018;115:E7728–E7737. doi: 10.1073/pnas.1803303115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ramkumar A., Murthy D., Raja D.A., Singh A., Krishnan A., Khanna S., Vats A., Thukral L., Sharma P., Sivasubbu S., et al. Classical autophagy proteins LC3B and ATG4B facilitate melanosome movement on cytoskeletal tracks. Autophagy. 2017;13:1331–1347. doi: 10.1080/15548627.2017.1327509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Murase D., Kusaka-Kikushima A., Hachiya A., Fullenkamp R., Stepp A., Imai A., Ueno M., Kawabata K., Takahashi Y., Hase T., et al. Autophagy Declines with Premature Skin Aging resulting in Dynamic Alterations in Skin Pigmentation and Epidermal Differentiation. Int. J. Mol. Sci. 2020;21:5708. doi: 10.3390/ijms21165708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim J.Y., Lee E.J., Ahn Y., Park S., Bae Y.J., Kim T.G., Oh S.H. Cathepsin L, a Target of Hypoxia-Inducible Factor-1-alpha, Is Involved in Melanosome Degradation in Melanocytes. Int. J. Mol. Sci. 2021;22:8596. doi: 10.3390/ijms22168596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim E.S., Jo Y.K., Park S.J., Chang H., Shin J.H., Choi E.S., Kim J.B., Seok S.H., Kim J.S., Oh J.S., et al. ARP101 inhibits alpha-MSH-stimulated melanogenesis by regulation of autophagy in melanocytes. FEBS Lett. 2013;587:3955–3960. doi: 10.1016/j.febslet.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 88.Lee K.W., Ryu H.W., Oh S.S., Park S., Madhi H., Yoo J., Park K.H., Kim K.D. Depigmentation of alpha-melanocyte-stimulating hormone-treated melanoma cells by beta-mangostin is mediated by selective autophagy. Exp. Dermatol. 2017;26:585–591. doi: 10.1111/exd.13233. [DOI] [PubMed] [Google Scholar]
- 89.Park H.J., Jo D.S., Choi H., Bae J.E., Park N.Y., Kim J.B., Choi J.Y., Kim Y.H., Oh G.S., Chang J.H., et al. Melasolv induces melanosome autophagy to inhibit pigmentation in B16F1 cells. PLoS ONE. 2020;15:e0239019. doi: 10.1371/journal.pone.0239019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim E.S., Chang H., Choi H., Shin J.H., Park S.J., Jo Y.K., Choi E.S., Baek S.Y., Kim B.G., Chang J.W., et al. Autophagy induced by resveratrol suppresses alpha-MSH-induced melanogenesis. Exp. Dermatol. 2014;23:204–206. doi: 10.1111/exd.12337. [DOI] [PubMed] [Google Scholar]
- 91.Yang Z., Zeng B., Pan Y., Huang P., Wang C. Autophagy participates in isoliquiritigenin-induced melanin degradation in human epidermal keratinocytes through PI3K/AKT/mTOR signaling. Biomed. Pharmacother. 2018;97:248–254. doi: 10.1016/j.biopha.2017.10.070. [DOI] [PubMed] [Google Scholar]
- 92.Yang H.L., Lin C.P., Vudhya Gowrisankar Y., Huang P.J., Chang W.L., Shrestha S., Hseu Y.C. The anti-melanogenic effects of ellagic acid through induction of autophagy in melanocytes and suppression of UVA-activated alpha-MSH pathways via Nrf2 activation in keratinocytes. Biochem. Pharmacol. 2021;185:114454. doi: 10.1016/j.bcp.2021.114454. [DOI] [PubMed] [Google Scholar]
- 93.Kim E.S., Shin J.H., Seok S.H., Kim J.B., Chang H., Park S.J., Jo Y.K., Choi E.S., Park J.S., Yeom M.H., et al. Autophagy mediates anti-melanogenic activity of 3′-ODI in B16F1 melanoma cells. Biochem. Biophys. Res. Commun. 2013;442:165–170. doi: 10.1016/j.bbrc.2013.11.048. [DOI] [PubMed] [Google Scholar]
- 94.Jeong D., Park S.H., Kim M.H., Lee S., Cho Y.K., Kim Y.A., Park B.J., Lee J., Kang H., Cho J.Y. Anti-Melanogenic Effects of Ethanol Extracts of the Leaves and Roots of Patrinia villosa (Thunb.) Juss through Their Inhibition of CREB and Induction of ERK and Autophagy. Molecules. 2020;25:5375. doi: 10.3390/molecules25225375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kim P.S., Shin J.H., Jo D.S., Shin D.W., Choi D.H., Kim W.J., Park K., Kim J.K., Joo C.G., Lee J.S., et al. Anti-melanogenic activity of schaftoside in Rhizoma Arisaematis by increasing autophagy in B16F1 cells. Biochem. Biophys. Res. Commun. 2018;503:309–315. doi: 10.1016/j.bbrc.2018.06.021. [DOI] [PubMed] [Google Scholar]
- 96.Katsuyama Y., Taira N., Yoshioka M., Okano Y., Masaki H. 3-O-Glyceryl-2-O-hexyl Ascorbate Suppresses Melanogenesis through Activation of the Autophagy System. Biol. Pharm. Bull. 2018;41:824–827. doi: 10.1248/bpb.b17-01042. [DOI] [PubMed] [Google Scholar]
- 97.Yun C.Y., Choi N., Lee J.U., Lee E.J., Kim J.Y., Choi W.J., Oh S.H., Sung J.H. Marliolide Derivative Induces Melanosome Degradation via Nrf2/p62-Mediated Autophagy. Int. J. Mol. Sci. 2021;22:3995. doi: 10.3390/ijms22083995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim J.Y., Kim J., Ahn Y., Lee E.J., Hwang S., Almurayshid A., Park K., Chung H.J., Kim H.J., Lee S.H., et al. Autophagy induction can regulate skin pigmentation by causing melanosome degradation in keratinocytes and melanocytes. Pigment Cell Melanoma Res. 2020;33:403–415. doi: 10.1111/pcmr.12838. [DOI] [PubMed] [Google Scholar]
- 99.Kim H.M., Oh S., Yang J.Y., Sun H.J., Jang M., Kang D., Son K.H., Byun K. Evaluating Whether Radiofrequency Irradiation Attenuated UV-B-Induced Skin Pigmentation by Increasing Melanosomal Autophagy and Decreasing Melanin Synthesis. Int. J. Mol. Sci. 2021;22:10724. doi: 10.3390/ijms221910724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cho Y.H., Park J.E., Lim D.S., Lee J.S. Tranexamic acid inhibits melanogenesis by activating the autophagy system in cultured melanoma cells. J. Dermatol. Sci. 2017;88:96–102. doi: 10.1016/j.jdermsci.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 101.Ho H., Ganesan A.K. The pleiotropic roles of autophagy regulators in melanogenesis. Pigment Cell Melanoma Res. 2011;24:595–604. doi: 10.1111/j.1755-148X.2011.00889.x. [DOI] [PubMed] [Google Scholar]
- 102.Rogov V., Dotsch V., Johansen T., Kirkin V. Interactions between autophagy receptors and ubiquitin-like proteins form the molecular basis for selective autophagy. Mol. Cell. 2014;53:167–178. doi: 10.1016/j.molcel.2013.12.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.