Abstract
Pancreatic cancer is a leading cause of cancer death worldwide and its global burden has more than doubled over the past 25 years. The highest incidence regions for pancreatic cancer include North America, Europe and Australia, and although much of this increase is due to ageing worldwide populations, there are key modifiable risk factors for pancreatic cancer such as cigarette smoking, obesity, diabetes and alcohol intake. The prevalence of these risk factors is increasing in many global regions, resulting in increasing age-adjusted incidence rates for pancreatic cancer, but the relative contribution from these risk factors varies globally due to variation in the underlying prevalence and prevention strategies. Inherited genetic factors, although not directly modifiable, are an important component of pancreatic cancer risk, and include pathogenic variants in hereditary cancer genes, genes associated with hereditary pancreatitis, as well as common variants identified in genome-wide association studies. Identification of the genetic changes that underlie pancreatic cancer not only provides insight into the aetiology of this cancer but also provides an opportunity to guide early detection strategies. The goal of this Review is to provide an up-to-date overview of the established modifiable and inherited risk factors for pancreatic cancer.
The global burden of pancreatic cancer has increased dramatically over the past few decades and is expected to continue to represent a leading cause of cancer-related mortality (see Surveillance, Epidemiology, and End Results Program (SEER))1. The shifting age structure of the global population, particularly in developing regions2, as well as changes in established modifiable risk factors account for many of the observed trends. Genetic factors and modifiable exposures, either independently or acting jointly, play an important part in pancreatic cancer risk. As our ability to detect pancreatic cancer and its precursor lesions improves, understanding the underlying risk factors and their interactions will enable prevention efforts, including primary prevention strategies, to reduce exposures and to identify individuals most at risk of this often fatal cancer. This approach will help reduce the growing incidence of this disease. This Review outlines the current knowledge of established pancreatic cancer risk factors including lifestyle and disease-specific risks followed by inherited genetic risks. The underlying mechanisms of risk and risk factors with inconclusive associations with pancreatic cancer risk are not discussed.
Global trends in pancreatic cancer
The past two decades have seen a doubling in the global annual number of pancreatic cancers diagnosed. In 2017, there were 441,000 pancreatic cancers worldwide compared with 196,000 in 1990 (REF.1). Given that pancreatic cancer is a disease in which risk increases with age and rarely occurs before the age of 40 years1, the shifting age structure of the global population together with improved diagnosis account for much of the increased incidence (number of cases in the population in a year) of pancreatic cancer, particularly in high-income nations1. Incidence rates in low-income nations have remained low, and there is a lack of high-quality data on mortality in these regions due to poor access to advanced imaging and expertise in pathology1.
The risk of death from pancreatic cancer rises dramatically with age from <2 deaths per 100,000 person-years for individuals in the USA aged 35–39 years to >90 deaths per 100,000 person-years for individuals aged >80 years. As global health improves, resulting in lifespan increases, the overall incidence of pancreatic cancer is also likely to increase. An international report noted that in 2012, 8% of the global population was older than 65 years, the age group at highest risk of pancreatic cancer2. In the next 30 years, this proportion is expected to double to 16.7%, particularly in all regions in Asia, Australia, Europe, and many regions in Latin America2, regions with the some of the highest age-standardized death rates of pancreatic cancer1. Even in Africa, where incidence rates of pancreatic cancer remain low, in part due to reduced access to care, the proportion of the population above age 65 years is projected to double in the next several decades2. Thus, the incidence of pancreatic cancer will continue to grow over the next few decades.
The increased prevalence of key risk factors, especially in high-income nations, is leading to increased age-adjusted incidence rates of pancreatic cancer1,3 (number of cases in a population in a year standardized to the age distribution of a ‘standard’ population). The role of the changing prevalence of specific risk factors on age-adjusted incidence rates of pancreatic cancer risk is discussed later. Globally, age standardized incidence rates increased from 5.0 per 100,000 person-years in 1990 to 5.7 (5.6–5.8) per 100,000 person-years in 2017 (REF.1). As shown in FIG. 1, incidence is highest in North America, Europe and Argentina followed by East Asia and Australia4–6. In addition, in the USA, the 2017 age-adjusted incidence rates were higher in Black individuals (15.9 per 100,000 person-years) than white individuals (13.4 per 100,000 person years), SEER-defined Hispanic individuals (11.7 per 100,000 person-years) and Asian individuals (10.2 per 100,000 person-years) (see Related links). Although under-studied, the increased risk in Black individuals has been suggested to be primarily due to differences in the prevalence of the established risk factors such as smoking, high BMI and diabetes7. Additional studies are needed to understand differences in risk across racial and ethnic groups. Globally, incidence rates for pancreatic cancer tend to be somewhat higher in men than in women, particularly in those below the age of 75 years1.
Fig. 1 |. Incidence of pancreatic cancer.
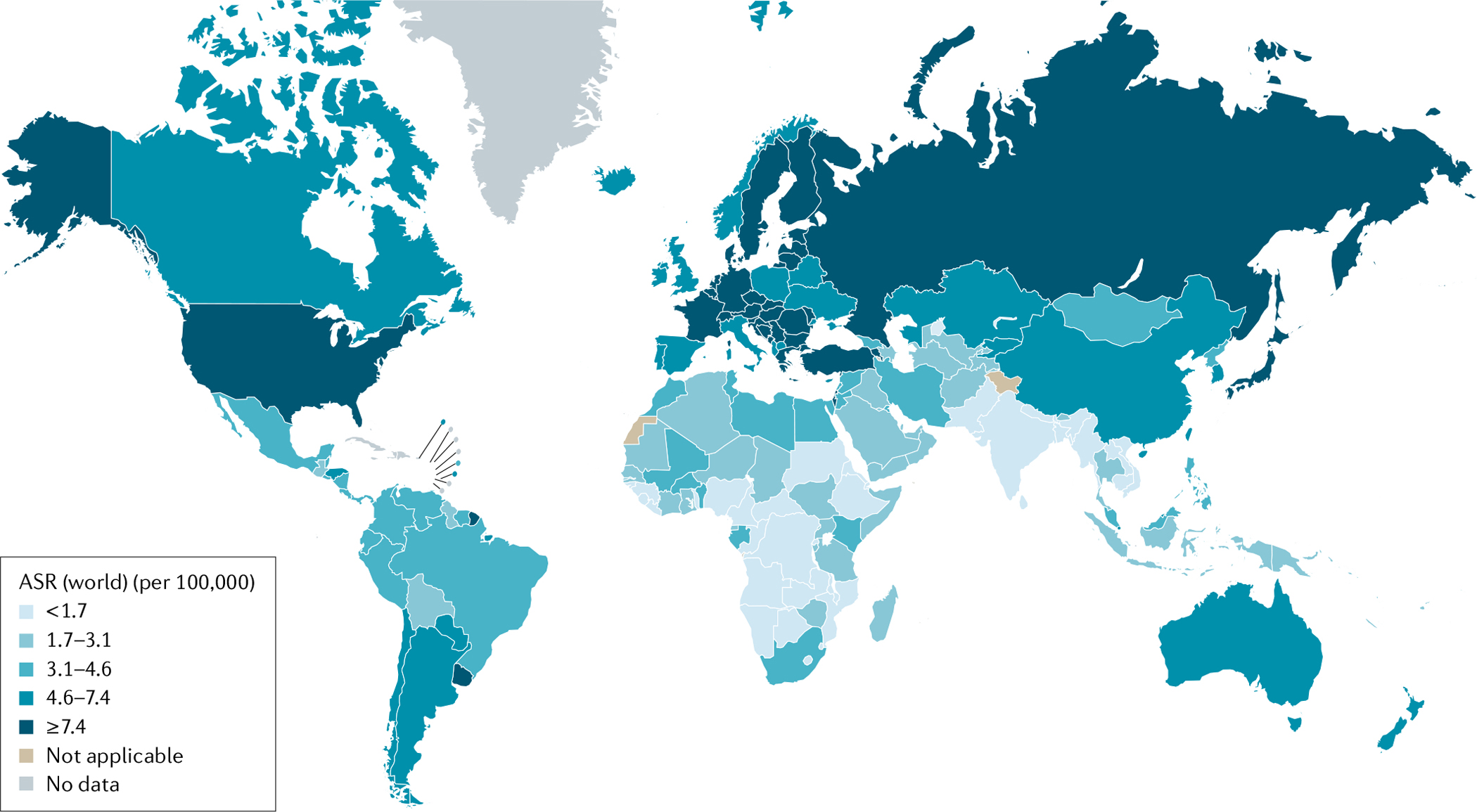
Age-standardized incidence rates (ASR) of pancreatic cancer across the globe in 2020. Figure adapted with permission from REF.5, International Agency for Research on Cancer (accessed 14 April 2021).
RELATED LINKS.
National Cancer Institute. SEER*Explorer: An interactive website for SEER cancer statistics [Internet]. Surveillance Research Program: https://seer.cancer.gov/explorer/
Survival rates for pancreatic cancer remain low, despite improvements in overall 5-year survival from <5% in the 1990s to as high as 9% in the USA and Europe in 2019 (REFS8,9). Low survival rates are, in part, attributed to the advanced stage at diagnosis in most cases, with only ~20% of patients presenting with early-stage, surgically resectable disease. Among patients who undergo surgical resection, the 5-year survival rate is ~15–25%10 and in the USA the survival rate for stage 1A disease is >80%11. The majority of cancers in the pancreas are pancreatic ductal adenocarcinomas (>90%)12, and the risk factor studies described in the following sections and summarized in TABLE 1 are focused on this tumour type.
Table 1 |.
Pancreatic cancer risk factors
| Risk factor | Associated risk of pancreatic cancer | Refs |
|---|---|---|
| Cigarette smoking | ∼1.7-fold increased risk compared with never smokers | 13–16 |
| Obesity | ∼1.6-fold increased risk in individuals with obesity compared with those with normal weight | 39,40,42 |
| Alcohol use | 1.6-fold increased risk in those consuming >6 drinks per day compared with those consuming >1 drink per day | 45–49 |
| New-onset diabetes | <0.3–0.8% of patients with new-onset diabetes develop pancreatic ductal adenocarcinoma within 3 years of diabetes diagnosis | 31–33 |
| Long-standing diabetes | 1.5–2-fold increased risk of pancreatic cancer for individuals with diabetes of >3 years in duration | 26–30 |
| Family history of pancreatic cancer | Twofold increased risk in individuals with a single family member with pancreatic cancer compared with the general population; sevenfold increased risk in individuals with multiple family members with pancreatic cancer compared with the general population | 78 |
| Pancreatitis | Twofold to threefold increased risk in individuals with long-standing chronic pancreatitis | 50–52 |
| Allergy | 25% lower risk of developing pancreatic cancer | 53–56 |
Risk factors
Cigarette smoking.
Cigarette smoking is a well-established risk factor for pancreatic cancer13–15. A meta-analysis of the effect of smoking on the risk of pancreatic cancer found an odds ratio of 1.74 (95% CI 1.61–1.87) for current smokers compared with never smokers12. The risk is highest among those who smoke the greatest number of cigarettes per day, with smokers of more than 35 cigarettes per day having an odds ratio for pancreatic cancer of 3.0 (95% CI 2.2–4.1) compared with never smokers12. Quitting smoking results in a reduction of this risk, such that the odds ratio of pancreatic cancer in former smokers is 1.2 (95% CI 1.11–1.29) compared with never smokers12. Interestingly, the risk of pancreatic cancer in former smokers decreases as the years since cessation of smoking increases, such that 10–20 years after smoking cessation14,15 the risk of pancreatic cancer in former smokers returns to that in never smokers. Although the majority of the studies in this meta-analysis were European, a pooled analysis of Japanese cohort studies demonstrated similar findings16. Large-scale studies have failed to show an association between passive smoking or parental smoking and pancreatic cancer risk in non-smoking individuals17,18. Some studies have found an increased risk of pancreatic cancer in non-cigarette smoking individuals who consume non-cigarette tobacco products, specifically cigars19, but other studies have failed to detect an association in this group20,21.
Over the past several decades there has been a decrease in the prevalence of cigarette smoking in much of Europe and North America22; however, prevalence rates in Asia (specifically, China and India)23 and in other parts of the world remain high. In areas where smoking prevalence has decreased, so too has the fraction of pancreatic cancers attributable to smoking. The use of the population attributable risk (PAR; also known as population attributable fraction) is a useful metric to plan population interventional strategies as it denotes the amount by which risk will decrease if a risk factor is eliminated. By definition, as based upon effect size and prevalence of the risk factor in the population, it is population-specific and affected by temporal trends in prevalence and, therefore, not generalizable across populations. A summary of meta-analyses indicates that the PAR of pancreatic cancer due to smoking ranges across studies and across global regions from 11% to 32%24, with a PAR of 13.6% (6.3–20.8%) reported in Italy, for example25.
Diabetes mellitus.
Diabetes mellitus is both a risk factor for pancreatic cancer as well as a consequence of the cancer, with many patients with newly diagnosed pancreatic cancer reporting onset of diabetes or, among those with diabetes, a worsening of the disease. Long-standing diabetes (>3 years) has been associated with a 1.5–2.4-fold increased risk of pancreatic cancer26–29. Some studies have suggested that there might be no increase in risk among individuals diagnosed with diabetes of >15–20 years duration29,30, whereas other studies have indicated that individuals with diabetes for 20 years or more28 remain at an elevated risk of pancreatic cancer.
Many patients with pancreatic cancer report developing diabetes in the months preceding diagnosis and these patients with new-onset diabetes who undergo surgical resection of their pancreatic cancer often see their diabetes resolve after removal of the pancreatic cancer31. New-onset of diabetes might be indicative of an underlying pancreatic cancer. Focused studies of 2,122 individuals in the Mayo Clinic population initially indicated that up to 1% of patients with newly diagnosed diabetes develop pancreatic cancer within 3 years of their diabetes diagnosis31. However, larger studies conducted with the US Department of Veteran Affairs system have shown a lower risk of <0.3% for developing pancreatic cancer within 3 years of a diabetes diagnosis32,33 compared with a risk of pancreatic cancer of ~0.11% in patients without diabetes. Ongoing efforts, including the US New-Onset Diabetes cohort (which aims to identify the subgroup with new-onset diabetes with underlying pancreatic cancer), are underway to improve the early detection of pancreatic cancer34.
The prevalence of diabetes worldwide has increased considerably over the past several decades from 4.3% in 1980 to 9.0% in 2014 in men, and from 5.0% to 7.9% in women. The number of adults with diabetes in the world increased from 108 million in 1980 to 422 million in 2014 (REF.35). The increasing prevalence of diabetes is probably one reason why the age-adjusted incidence rates of pancreatic cancer are increasing in many nations despite a decreasing prevalence of cigarette smoking. In addition to a diagnosis of diabetes, biomarkers of diabetes risk have also been shown to be associated with pancreatic cancer risk, including fasting glucose, insulin and insulin resistance levels in both European and Chinese populations36,37. A nested case–control study of 466 matched pairs within the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort demonstrated that patients with pancreatic cancer had higher levels of HbA1C, a marker of excessive blood sugar used to diagnose diabetes, prior to their pancreatic cancer diagnosis than cancer-free controls38.
Body mass index.
Increased weight or BMI has been shown to increase the risk of pancreatic cancer. In 2001, with data from 46,648 men and 117,041 women in the USA within the Health Professional follow-up study and Nurse’s Health study, Michaud et al. reported a 1.72 (95% CI 1.19–2.4) relative risk of pancreatic cancer in individuals with a BMI of >30 kg/m2 compared with individuals with a BMI of <23 kg/m2 after controlling for the effects of age, smoking and diabetes39. A pooled analysis of 2,170 cases and 2,209 control individuals (restricted to never smokers) from 13 US and European prospective cohort studies compared the odds ratios for pancreatic cancer in individuals with overweight (BMI 25–29.9 kg/m2), obesity (BMI 30–34.9 kg/m2) and severe obesity (BMI >35 kg/m2) with those in individuals with normal weight and found odds ratios of 1.19 (95% CI 1.02–1.40), 1.25 (95% CI 1.02–1.55) and 1.62 (95% CI 1.19–2.21), respectively (trend test P < 0.01), controlling for the effects of age40. The fraction of pancreatic cancer attributable to an increased BMI will vary globally and longitudinally as the prevalence of obesity shifts. The global prevalence of obesity has tripled since 1975 (REF.41), which will probably lead to an increasing burden of pancreatic cancer. Thus, it is expected that rates of pancreatic cancer will increase with the increasing obesity rates.
The association between changes in BMI in adulthood and pancreatic cancer risk has also been examined. However, the findings have been inconsistent across studies, or replication studies have not been reported. A study of just over 500,000 individuals, of whom 2,122 developed pancreatic cancer, in the AARP (American Association of Retired Persons) cohort suggested that weight gain after age 50 years is associated with an increased risk of pancreatic cancer42, with the hazard ratio increasing with increasing weight gain. A record linkage study of 293,209 individuals in the Danish population of whom 1,268 had pancreatic cancer suggested that BMI in early life is important, with high childhood BMI associated with an increased risk of pancreatic cancer before age 70 years43. Interestingly, in a study in 2020 of 112,818 women enrolled in the Nurse’s Health study and 46,207 men in the Health Professionals Follow-up study (of whom 1,116 developed pancreatic cancer), weight loss in adulthood was associated with an increased risk of pancreatic cancer compared with individuals who gained or maintained weight. Pancreatic cancer risk increased with increasing weight loss, with a weight loss of >8 lb associated with an adjusted hazard ratio of 1.92 (95% CI 1.58–2.32). This risk was even higher in individuals who lost weight and had new onset of diabetes44. Although weight loss might be an indicator of early precancerous changes, a better understanding of how changes in weight in adulthood affect the risk of pancreatic cancer is needed.
Alcohol.
Although some studies have found an increased risk of pancreatic cancer among heavy drinkers of alcohol, other studies have not found a statistically significant association. A pooled analysis of data of 5,585 cases and 11,827 controls from the Pancreatic Cancer Case Control Consortium (PanC4) found a statistically significant increased risk (OR 1.6, 95% CI 1.2–2.2) of pancreatic cancer among heavy alcohol drinkers (nine or more drinks per day) compared with consumers of less than one drink per day45. Pooled case–control data nested within prospective cohorts in North America, Australia and Europe showed a relative risk of 1.22 (95% CI 1.03–1.45) of pancreatic cancer when comparing individuals who drank >30 g of alcohol per day to those who drank 0 g per day. There was no significant evidence of interaction by sex46. Analysis of the NIH-AARP Diet and Health study (of >430,000 individuals of whom 1,149 were diagnosed with pancreatic cancer) showed a relative risk of 1.45 (95% CI 1.17–1.80) of pancreatic cancer among consumers of more than three drinks per day47. A focused analysis of never smokers from the American Cancer Society Cancer Prevention Study II showed a relative risk of 1.32 (95% CI 1.10–1.57) of pancreatic cancer in individuals who consumed more than three liquor drinks per day compared with non-drinkers48. Studies including 1,283 individuals who developed pancreatic cancer and 476,106 cancer-free participants within the EPIC cohort population also identified a significant association between heavy alcohol use and pancreatic cancer (HR 1.77, 95% CI 1.06–2.95) in men who consumed >60 g per day of alcohol compared with moderate drinkers (0.1–4.9 g per day), but the risk in women who drank >30 g per day was not significantly elevated49. In summary, evidence is growing that alcohol intake, particularly heavy alcohol intake, is associated with pancreatic cancer risk. As such, it is expected that the increasing prevalence of heavy alcohol use in some regions will translate to increased rates of pancreatic cancer. Notably, heavy alcohol consumption is associated with pancreatitis, an established risk factor for pancreatic cancer50.
Pancreatitis.
Like diabetes, pancreatitis is a risk factor for pancreatic cancer as inflammation and damage resulting from pancreatitis can lead to the development of pancreatic cancer; however, pancreatitis can also develop as a result of an underlying pancreatic cancer50. A pooled analysis within the PanC4 consortium showed that 6% of patients with pancreatic cancer reported a history of pancreatitis compared with 1% of control individuals51. When examining this association by the duration of pancreatitis, a recent diagnosis of pancreatitis (<1 year) was associated with an odds ratio of 21.35 (95% CI 12.03–37.86) for pancreatic cancer51, whereas individuals diagnosed with pancreatitis >2 years previously had an odds ratio for pancreatic cancer of 2.71 (95% CI 1.96–3.74)51. Similar results were found in a large nested population-based case–control study of pancreatitis from a Danish cancer registry. Unlike the PanC4 study, this study relied upon in-patient diagnosis of pancreatic cancer and compared 41,669 patients diagnosed with incident acute pancreatitis with 208,340 individuals from the general population without acute pancreatitis. The relative hazard of pancreatic cancer in individuals with acute pancreatitis was 19.28 (95% CI 14.62–25.41) within the 2 years following the pancreatitis diagnosis. Pancreatic cancer risk decreased with time since pancreatitis diagnosis but there continued to be an elevated adjusted relative hazard of 2.02 (95% CI 1.57–2.61) for pancreatic cancer in patients with pancreatitis followed beyond 5 years52. Fortunately, pancreatitis is relatively rare, being found in only 1% of controls in pooled case–control studies51.
Allergy.
The role of the immune system in the development of pancreatic cancer is of increasing interest. Individuals with a personal history of allergies have been shown to be protected against pancreatic cancer with the underlying hypothesis that individuals with an active immune system might have increased antitumour immunity53. Studies have demonstrated that individuals with allergy have a decreased risk of cancer and improved survival compared to those without an allergy53. A 2005 meta-analysis of 14 studies demonstrated a relative risk of 0.83 (95% CI 0.68–0.80) for pancreatic cancer in individuals with a history of allergy compared to those without53. This protective effect was only observed among individuals with atopy and not among patients with asthma. A pooled analysis of data from the PanC4 consortium showed lower pancreatic cancer risk in individuals with a history of hay fever and animal allergies (OR 0.74 (95% CI 0.56–0.96) and 0.62 (95% CI 0.41–0.94), respectively)54. A similar result was found when examining 345 patients with pancreatic cancer and 1,285 population controls matched for age and sex from the Ontario Cancer Registry55. Interestingly, the PanGenEU study of 1,297 patients with pancreatic cancer and 1,024 control individuals found a protective effect of both long-standing asthma (OR 0.39, 95% CI 0.24–0.64) and nasal allergies (OR 0.66, 95% CI 0.52–0.83)56. Although one can hypothesize that individuals with allergy might have a reduced risk of pancreatic cancer due to residual confounding of smoking, the protective effect of allergy seems to be even stronger in smokers than never smokers53,55.
Role of the microbiota.
In the past decade there has been an increased interest in the role of the microbiota in pancreatic cancer risk. Notably, studies have examined the role of the microbiota, including the tumour microbiota, on survival and treatment response57, but the focus in this Review is on associations between the microbiota and the risk of pancreatic cancer. A meta-analysis demonstrated that periodontal disease and tooth loss is associated with a 50–70% increased risk of pancreatic cancer58; the oral microbiota, particularly Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans, has been associated with future risk of pancreatic cancer in 361 individuals who developed pancreatic cancer and 371 matched controls from the American Cancer Society Cancer Prevention Study II and the National Cancer Institute Prostate, Lung, Colorectal and Ovarian Cancer Study59. This finding is supported by a study in 405 patients with pancreatic cancer and 416 matched controls in the EPIC cohort, which demonstrated that antigens to P. gingivalis in prediagnostic blood samples were more common among individuals who developed pancreatic cancer than in control individuals60. The mechanism underlying this association is unclear and questions remain as to whether it represented a direct causal correlation or whether it reflected a common underlying cause and was therefore not directly causal.
Infection with Helicobacter pylori has also been associated with pancreatic cancer in some studies. A meta-analysis found no overall association between H. pylori seropositivity and pancreatic cancer risk (OR 1.13, 95 % CI 0.86–1.50)61. However, there was some evidence that CagA-positive strains of H. pylori were associated with a low risk of pancreatic cancer (OR 0.78, 95% CI 0.67–0.91) and CagA-negative strains of H. pylori were associated with an increased risk of pancreatic cancer (OR 1.30, 95% CI 1.02–1.65)61. Further studies are needed to determine if inconsistency in the results was due to strain-specific associations and to understand the underlying mechanism.
Environmental and occupational causes
Numerous studies have investigated the relationship between environmental exposures and the risk of pancreatic cancer, often with inconsistent findings (reviewed elsewhere62,63). Accurate assessment of exposure to common environmental and occupational compounds is challenging, particularly in non-occupational settings, which often do not have detailed exposure measurements and rely on self-report64. Analysis of a large-scale case–control study including 2,092 patients with pancreatic cancer seen at the Mayo Clinic and 2,353 primary care Mayo Clinic patients as controls frequency-matched on the basis of age, race, sex and state or region of residence showed that patients with pancreatic cancer were more often regularly exposed to pesticides (OR 1.21, 95% CI 1.02–1.44), asbestos (OR 1.54, 95% CI 1.23–1.92), benzene (OR 1.70, 95% CI 1.23–2.35) and chlorinated hydrocarbons (OR 1.63, 95% CI 1.32–2.02)65. However, this study was based on retrospective self-reported lifetime exposures and differential recall between cases and controls might have led to biased associations. A series of studies examined the association between reported occupational history exposure in 116 patients with pancreatic cancer in eastern Spain and the concentration of 12 trace elements in the toenails and organochlorine compounds in blood66,67. Correlations with pancreatic cancer were reported between several compounds and self-reported occupation. These studies did not have a control group and, as such, the association between occupational history and pancreatic cancer risk is unclear66,67. However, in a study including 118 patients with pancreatic cancer from the same Spanish population and 339 hospital-based controls, an association was found between the risk of pancreatic cancer and the levels of lead, nickel, selenium, cadmium and arsenic in toenail samples68. Further work is needed to understand the role of environmental exposure in pancreatic cancer risk.
Familial history of cancers
Inherited genetic factors also play an important part in pancreatic cancer risk. The first lines of evidence supporting a role for inherited factors in pancreatic cancer were case reports followed by observational studies. These studies demonstrated that having a close blood relative with pancreatic cancer is associated with a 1.5–13-fold increased risk of pancreatic cancer69–78. Prospective studies have demonstrated that first-degree relatives of patients with familial pancreatic cancer (individuals with at least two close family members with pancreatic cancer) have a 6.79-fold (95% CI 4.54–9.75) increased risk of pancreatic cancer78. Unlike other cancers in which individuals with a family history develop cancer at a younger age, there is, at most, a 6-year difference in the average age of pancreatic cancer among those with and without a family history75,79–81. Pancreatic cancer in families also clusters with other cancers. Increased risks of breast, ovarian, prostate, colon, bile duct and liver cancer77,82,83 have all been reported among the relatives of patients with pancreatic cancer.
The pancreatic cancers that develop in patients with a family history of pancreatic cancer are quite similar to those that develop in individuals without a family history, as shown by a large-scale histopathological comparison that showed no statistically significant differences between familial and sporadic pancreatic cancers84. Furthermore, the somatic genetic mutational profile of pancreatic cancers that develop in those with a family history is also quite similar to that of apparently sporadic pancreatic cancers79. Although the cancers themselves are similar, patients with a family history of pancreatic cancer have a higher prevalence of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasia (IPMN) in the normal pancreas adjacent to their cancer than patients without a family history85. These studies suggest that the genetic basis of familial and sporadic pancreatic cancer is very similar; however, individuals with a family history are more likely to develop precursor lesions, some of which might progress to pancreatic cancer.
Inherited predisposition genes
The clustering of pancreatic cancer in families can be attributed to both shared environmental factors as well an underlying genetic predisposition. The proportion of pancreatic cancers due to inherited genetic factors (heritability) has been estimated to be 21.4–36%, with the higher estimates from family-based studies that might have better captured the contribution of rare genetic variation86,87. Over the past 15 years, as genomic technology has improved, both high-penetrance rare variants and low-penetrance common variants associated with an increased risk of pancreatic cancer have been identified88–95. However, the identified genetic changes explain only 20–25% of the heritability (4–5% of all pancreatic cancers)86, leaving much that still needs to be learned. For the 20% of familial pancreatic cancers for which a causative mutation has been identified, knowledge of the precise genetic mutation can help guide therapeutic decisions for those who develop pancreatic cancer and prompt early detection screening choices for at-risk relatives. For example, early detection screening or clinical trials using imaging techniques might be used in patients who have a strong family history of pancreatic cancer and/or who carry germline pathogenic variants in established familial pancreatic cancer genes96–100 (TABLE 2); furthermore, BRCA2-deficient or PALB2-deficient cancers have increased sensitivity to PARP inhibitors or mitomycin C101–104, microsatellite instability-high pancreatic cancers can be treated with anti-PD1 immunotherapy105, and patients with pancreatic cancer with a family history of breast, ovarian or pancreatic cancer have improved survival when treated with platinum-containing agents106.
Table 2 |.
High-risk pancreatic cancer inherited susceptibility genes
In the sections below, the associations between pancreatic cancer and specific syndromes or specific hereditary cancer genes (including the prevalence of pathogenic variants in these genes among patients with pancreatic cancer and the risk of pancreatic cancer among variant carriers) are discussed.
BRCA2.
Pathogenic variants in BRCA2 account for the largest proportion of patients with pancreatic cancer found to have a high-risk genetic variant107, with prevalence rates ranging from 1.4% to 7% in case series of unselected patients108–111. Prevalence rates are up to 16% among patients with a family history of pancreatic, ovarian or breast cancer (particularly cancer of early onset)112–114. Studies of the estimated lifetime risk of pancreatic cancer among BRCA2 pathogenic variant carriers are limited to studies of families ascertained on a history of breast or ovarian cancer, which suggest a 3.5–5.8-fold increased risk of pancreatic cancer compared with individuals without pathogenic BRCA2 variants109,115,116.
BRCA1.
Individuals who carry deleterious mutations in BRCA1 have also been shown to be at an increased risk of pancreatic cancer; however, studies of the prevalence and penetrance of BRCA1 mutations have demonstrated that the risk is probably lower than that for BRCA2 carriers. Estimates of the risk of pancreatic cancer in those with pathogenic variants in the BRCA1 gene suggest a 2.7–4.1-fold higher risk compared with individuals without pathogenic BRCA1 variants109,116,117. The prevalence of BRCA1 pathogenic variants in patients with pancreatic cancer unselected for family history range from 0.35% to 1.0%109–111 and, as with BRCA2, prevalence rates are at least about twofold higher among patients with pancreatic cancer with a family history of pancreatic or ovarian cancer107.
PALB2.
Pathogenic variants in the PALB2 gene, encoding a binding partner of BRCA2, are also associated with an increased risk of pancreatic cancer. Although initial reports suggested that up to 3% of patients with a family history of pancreatic cancer carry pathogenic variants in PALB2 (REF.88), subsequent studies suggested that the prevalence might be closer to 1% among those with a family history107,118,119 and 0.2–0.4% in unselected patients109–111.
ATM.
Pathogenic variants in the DNA repair gene ATM have also been associated with an increased risk of pancreatic cancer. The prevalence of ATM pathogenic variants is 0.5–2.3% in unselected patients with pancreatic cancer109–111,120. Among patients with pancreatic cancer with a family history of pancreatic cancer, the prevalence of pathogenic ATM variants is higher, ranging from 2.6% to 3.4%89,90.
CDKN2A.
Pathogenic variants in the CDKN2A gene, first associated with an increased risk of melanoma, are also associated with an increased risk of pancreatic cancer, which is the second leading cause of cancer death in individuals with pathogenic CDKN2A variants120. Pathogenic variants in CDKN2A have been reported in <1% of unselected patients with pancreatic cancer and up to 2.5% of patients with a family history of pancreatic cancer107. Individuals with pathogenic variants in CDKN2A have a 12–38-fold increased risk of pancreatic cancer compared with individuals without pathogenic variants in CDKN2A109,121 or a lifetime risk of pancreatic cancer of 17%122,123. Among individuals with a specific pathogenic variant in CDKN2A (the Leiden founder variant (c.225_243del19)) undergoing early detection screening for pancreatic cancer using MRI or magnetic resonance cholangiopancreatography, 7.3% were found to have pancreatic cancer124.
Lynch syndrome.
Lynch syndrome is associated with an increased risk of colorectal, endometrial, stomach, breast and pancreatic cancer125. Pathogenic variants in MLH1, MSH2, MSH6, PMS2 and EPCAM underlie Lynch syndrome. Individuals with Lynch syndrome have been shown to have a 3.68% (95% CI 1.45–5.88%) risk of pancreatic cancer by age 70 years which is 8.6-fold higher than in the general population125. Pathogenic variants in several genes associated with Lynch syndrome (such as MLH1, MSH2 and MSH6) have been shown to be more common in patients with pancreatic cancer, but these variants are still uncommon, collectively occurring in ~0.5–1.0% of unselected patients with pancreatic cancer109–111.
Peutz–Jeghers syndrome.
Individuals with Peutz–Jeghers syndrome, which is caused by pathogenic variants in the STK11 gene, develop hamartomatous polyps of the gastrointestinal tract126. These individuals have a remarkably high risk of any cancer (40% by age 40 years and 76% by age 70 years126) and a remarkably high lifetime risk of pancreatic cancer (11–32%)126–128.
Hereditary pancreatitis.
Pancreatitis, as discussed above, is a risk factor for pancreatic cancer and both common and rare genetic variants have been associated with an increased risk of pancreatitis and possible subsequent pancreatic cancer. The risk of developing pancreatic cancer is extremely high among individuals with hereditary pancreatitis (30–40% by age 70 years)129,130 and it has been suggested that the age of onset of pancreatic cancer is about 20 years younger in smokers with hereditary pancreatitis than in non-smoking individuals with hereditary pancreatitis130. A proportion of patients with hereditary pancreatitis have inherited pathogenic variants in the PRSS1 gene131–133 as well as specific pathogenic variants in CTFR and SPINK1. However, unlike pathogenic variants in PRSS1, evidence supporting a role for CFTR and SPINK1 variants in pancreatic cancer risk is limited134,135. In addition, one study has suggested that ER-stress-inducing variants in the pancreatic secretory enzyme genes CPA1 and CPB1 are more common in patients with pancreatic cancer, occurring in up to 1% of individuals with pancreatic cancer compared with <0.001% in population controls90,136.
Common genetic variants.
In addition to pathogenic variants in the above-mentioned genes (which lead to a moderate-to-high lifetime risk of pancreatic cancer), large-scale population-based genome-wide association studies (GWAS) have identified common variants in several genomic regions as significantly associated with pancreatic cancer risk. In European ancestry populations, associated regions include 1q32.1 (NR5A2), 1p36.33 (NOC2L), 2p13.3 (ETAA1), 3q29 (TP63), 5p15.33 (CLPTM1L, TERT), 7p14.1 (INHBA), 8q21.11 (HNF4G) 8q24.21 (MYC), 9q34.2 (ABO), 13q12.2 (PDX1), 13q22.1 (KLF5), 16q23.1 (BCAR1), 17q12 (HNF1B), 17q25.1 (LINC00673), 18q21.32 (GRP) and 22q12.1 (ZNRF)94,95,137,138. GWAS have been conducted both in Chinese and Japanese populations, with five loci (21q21.3, 5p13.1, 21q22.3, 22q13.32 and 10q26.11) found to be associated with pancreatic cancer in the Chinese population, and six loci (6p25.3, 12p11.21, 7q36.2, 13q12.2, 13q221 and 16p12.3) found in the Japanese population139–141. Many of the loci are distinct and have not been replicated across ancestral populations. However, a GWAS in the Japanese population found evidence of replication (P < 0.01) for 1q36.33, 9q24.2, 13.q12.2, 17q24.2 and 13q22.1 (REF.141), which are loci first reported to be associated with pancreatic cancer in European populations95. Individually, these variants have only a small effect on pancreatic cancer risk but each additional copy of a risk allele is associated with a 10–30% increase in the risk of pancreatic cancer95. The effects of these common variants are similar in patients with pancreatic cancer with a family history of pancreatic cancer142. In addition, secondary analyses of GWAS data, focusing on pathway-based associations or transcriptome-wide associations, have suggested several other candidate regions requiring further replication and follow-up137,143. Studies are underway to fully understand the mechanisms underlying both the primary and secondary associations and to increase the diversity of genomic studies of pancreatic cancer.
Pancreatic cancer screening
Currently, screening for pancreatic cancer is not recommended for the general population in the USA. However, individuals who have several close relatives with pancreatic cancer, individuals with a pathogenic variant in one of the high-risk pancreatic cancer susceptibility genes or individuals at high risk owing to a personal history of pancreatic cysts might consider screening144. The International Cancer of the Pancreas Screening Consortium have agreed that screening be conducted as part of a clinical trial, or if that is not possible, at a centre with experience in pancreatic cancer screening145. The consensus statements support screening using endoscopic ultrasonography (EUS) or MRI, with less support for CT scanning, which has lower sensitivity145. The results from EUS-based screening trials in both the USA and Europe have demonstrated that asymptomatic precursor lesions including IPMNs, as well as cancers, can be detected146. When compared with the US population Surveillance, Epidemiology, and End Results Program (SEER), there is an indication that the pancreatic cancers detected through screening are diagnosed at an earlier stage, yet additional work is needed to definitively show that early detection screening improves outcomes11,124,146–148. In addition, there is considerable effort underway to develop additional early detection screening tests and/or improve the ability of current technologies to identify early pancreatic cancer, for example with minimally invasive blood-based tests149,150 and improved imaging through radiomics151,152.
Conclusions
Pancreatic cancer is increasing, in part due to the ageing of the world’s population but also due to increases in the prevalence of modifiable pancreatic risk factors including obesity, diabetes and cigarette smoking. Population-based interventions such as smoking cessation, obesity prevention and weight loss studies can be implemented to reduce the burden of many diseases including pancreatic cancer. Inherited genetic changes also play a key part in pancreatic cancer risk; identifying high-risk individuals, together with improvements in screening technologies, might provide opportunities for earlier detection of a growing cause of cancer mortality.
Key points.
Smoking continues to be a leading cause of pancreatic cancer worldwide.
Increasing rates of diabetes and obesity will probably result in increased rates of pancreatic cancer.
Growing evidence indicates that high alcohol intake contributes to pancreatic cancer risk.
Knowledge of inherited genetic factors in pancreatic cancer continues to grow and probably explains 22–33% of pancreatic cancer risk.
Acknowledgements
The work of the author is supported by NCI RO1CA154823, U01CA247283, NCI P50 CA62924 and P30CA006973, and the Sol Goldman Pancreatic Cancer Research Center.
Footnotes
Competing interests
The author declares no competing interests.
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks the anonymous reviewers for their contribution to the peer review of this work.
References
- 1.GBD 2017 Pancreatic Cancer Collaborators. The global, regional, and national burden of pancreatic cancer and its attributable risk factors in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 4, 934–947 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He W, Goodkind D & Kowal P An Aging World: 2015. US Census Bureau Report Number P95/16–1 (US Government Publishing Office, 2016). [Google Scholar]
- 3.Klein AP Pancreatic cancer: a growing burden. Lancet Gastroenterol. Hepatol. 4, 895–896 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferlay J et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 144, 1941–1953 (2019). [DOI] [PubMed] [Google Scholar]
- 5.International Agency for Research on Cancer. Cancer Today https://gco.iarc.fr/today/online-analysis-map?v=2020&mode=population&mode_population=continents&population=900&populations=900&key=asr&sex=0&cancer=13&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&nb_items=10&group_cancer=1&include_nmsc=1&include_nmsc_other=1&projection=natural-earth&color_palette=default&map_scale=quantile&map_nb_colors=5&continent=0&show_ranking=0&rotate=%255B10%252C0%255D (2020).
- 6.Bray F et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Silverman DT et al. Why do Black Americans have a higher risk of pancreatic cancer than White Americans? Epidemiology 14, 45–54 (2003). [DOI] [PubMed] [Google Scholar]
- 8.Siegel RL, Miller KD & Jemal A Cancer statistics, 2020. CA Cancer J. Clin. 70, 7–30 (2020). [DOI] [PubMed] [Google Scholar]
- 9.Jemal A et al. Cancer statistics, 2006. CA Cancer J. Clin 56, 106–130 (2006). [DOI] [PubMed] [Google Scholar]
- 10.He J et al. 2564 resected periampullary adenocarcinomas at a single institution: trends over three decades. HPB 16, 83–90 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blackford AL, Canto MI, Klein AP, Hruban RH & Goggins M Recent trends in the incidence and survival of stage 1A pancreatic cancer: a Surveillance, Epidemiology, and End Results analysis. J. Natl Cancer Inst. 112, 1162–1169 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wood LD & Hruban RH Pathology and molecular genetics of pancreatic neoplasms. Cancer J. 18, 492–501 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iodice S, Gandini S, Maisonneuve P & Lowenfels AB Tobacco and the risk of pancreatic cancer: a review and meta-analysis. Langenbecks Arch. Surg. 393, 535–545 (2008). [DOI] [PubMed] [Google Scholar]
- 14.Bosetti C et al. Cigarette smoking and pancreatic cancer: an analysis from the International Pancreatic Cancer Case-Control Consortium (Panc4). Ann. Oncol. 23, 1880–1888 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lynch SM et al. Cigarette smoking and pancreatic cancer: a pooled analysis from the Pancreatic Cancer Cohort Consortium. Am. J. Epidemiol. 170, 403–413 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koyanagi YN et al. Smoking and pancreatic cancer incidence: a pooled analysis of 10 population-based cohort studies in Japan. Cancer Epidemiol. Biomarkers Prev. 28, 1370–1378 (2019). [DOI] [PubMed] [Google Scholar]
- 17.Bao Y, Giovannucci E, Fuchs CS & Michaud DS Passive smoking and pancreatic cancer in women: a prospective cohort study. Cancer Epidemiol. Biomarkers Prev. 18, 2292–2296 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou J, Wellenius GA & Michaud DS Environmental tobacco smoke and the risk of pancreatic cancer among non-smokers: a meta-analysis. Occup. Env. Med. 69, 853–857 (2012). [DOI] [PubMed] [Google Scholar]
- 19.Bertuccio P et al. Cigar and pipe smoking, smokeless tobacco use and pancreatic cancer: an analysis from the International Pancreatic Cancer Case-Control Consortium (PanC4). Ann. Oncol. 22, 1420–1426 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Araghi M et al. Use of moist oral snuff (snus) and pancreatic cancer: pooled analysis of nine prospective observational studies. Int. J. Cancer 141, 687–693 (2017). [DOI] [PubMed] [Google Scholar]
- 21.Tranah GJ, Holly EA, Wang F & Bracci PM Cigarette, cigar and pipe smoking, passive smoke exposure, and risk of pancreatic cancer: a population-based study in the San Francisco Bay area. BMC Cancer 11, 138 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marcon A et al. Trends in smoking initiation in Europe over 40 years: a retrospective cohort study. PLoS ONE 13, e0201881 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang JJ et al. Tobacco smoking and mortality in Asia: a pooled meta-analysis. JAMA Netw. Open 2, e191474 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maisonneuve P & Lowenfels AB Risk factors for pancreatic cancer: a summary review of meta-analytical studies. Int. J. Epidemiol. 44, 186–198 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Rosato V et al. Population attributable risk for pancreatic cancer in Northern Italy. Pancreas 44, 216–220 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Everhart J & Wright D Diabetes mellitus as a risk factor for pancreatic cancer. A meta-analysis. JAMA 273, 1605–1609 (1995). [PubMed] [Google Scholar]
- 27.Huxley R, Ansary-Moghaddam A, Berrington de Gonzalez A, Barzi F & Woodward M Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br. J. Cancer 92, 2076–2083 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bosetti C et al. Diabetes, antidiabetic medications, and pancreatic cancer risk: an analysis from the International Pancreatic Cancer Case-Control Consortium. Ann. Oncol. 25, 2065–2072 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elena JW et al. Diabetes and risk of pancreatic cancer: a pooled analysis from the Pancreatic Cancer Cohort Consortium. Cancer Causes Control. 24, 13–25 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li D et al. Diabetes and risk of pancreatic cancer: a pooled analysis of three large case-control studies. Cancer Causes Control. 22, 189–197 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chari ST et al. Probability of pancreatic cancer following diabetes: a population-based study. Gastroenterology 129, 504–511 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gupta S et al. New-onset diabetes and pancreatic cancer. Clin. Gastroenterol. Hepatol. 4, 1366–1372 (2006). [DOI] [PubMed] [Google Scholar]
- 33.Munigala S, Singh A, Gelrud A & Agarwal B Predictors for pancreatic cancer diagnosis following new-onset diabetes mellitus. Clin. Transl. Gastroenterol. 6, e118 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maitra A et al. A prospective study to establish a new-onset diabetes cohort: from the Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer. Pancreas 47, 1244–1248 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 387, 1513–1530 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stolzenberg-Solomon RZ et al. Insulin, glucose, insulin resistance, and pancreatic cancer in male smokers. JAMA 294, 2872–2878 (2005). [DOI] [PubMed] [Google Scholar]
- 37.Pang Y et al. Diabetes, plasma glucose and incidence of pancreatic cancer: a prospective study of 0.5 million Chinese adults and a meta-analysis of 22 cohort studies. Int. J. Cancer 140, 1781–1788 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grote VA et al. Diabetes mellitus, glycated haemoglobin and C-peptide levels in relation to pancreatic cancer risk: a study within the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Diabetologia 54, 3037–3046 (2011). [DOI] [PubMed] [Google Scholar]
- 39.Michaud DS et al. Physical activity, obesity, height, and the risk of pancreatic cancer. JAMA 286, 921–929 (2001). [DOI] [PubMed] [Google Scholar]
- 40.Arslan AA et al. Anthropometric measures, body mass index, and pancreatic cancer: a pooled analysis from the Pancreatic Cancer Cohort Consortium (PanScan). Arch. Intern. Med. 170, 791–802 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.World Health Organization. Obesity and overweight. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (2020).
- 42.Stolzenberg-Solomon RZ, Schairer C, Moore S, Hollenbeck A & Silverman DT Lifetime adiposity and risk of pancreatic cancer in the NIH-AARP Diet and Health Study cohort. Am. J. Clin. Nutr. 98, 1057–1065 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nogueira L, Stolzenberg-Solomon R, Gamborg M, Sorensen TIA & Baker JL Childhood body mass index and risk of adult pancreatic cancer. Curr. Dev. Nutr. 1, e001362 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yuan C et al. Diabetes, weight change, and pancreatic cancer risk. JAMA Oncol. 6, e202948 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lucenteforte E et al. Alcohol consumption and pancreatic cancer: a pooled analysis in the International Pancreatic Cancer Case-Control Consortium (PanC4). Ann. Oncol. 23, 374–382 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Genkinger JM et al. Alcohol intake and pancreatic cancer risk: a pooled analysis of fourteen cohort studies. Cancer Epidemiol. Biomarkers Prev. 18, 765–776 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiao L et al. Alcohol use and risk of pancreatic cancer: the NIH-AARP Diet and Health Study. Am. J. Epidemiol. 169, 1043–1051 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gapstur SM et al. Association of alcohol intake with pancreatic cancer mortality in never smokers. Arch. Intern. Med. 171, 444–451 (2011). [DOI] [PubMed] [Google Scholar]
- 49.Naudin S et al. Lifetime and baseline alcohol intakes and risk of pancreatic cancer in the European Prospective Investigation into Cancer and Nutrition study. Int. J. Cancer 143, 801–812 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yadav D & Lowenfels AB The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology 144, 1252–1261 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duell EJ et al. Pancreatitis and pancreatic cancer risk: a pooled analysis in the International Pancreatic Cancer Case-Control Consortium (PanC4). Ann. Oncol. 23, 2964–2970 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kirkegard J, Cronin-Fenton D, Heide-Jorgensen U & Mortensen FV Acute pancreatitis and pancreatic cancer risk: a nationwide matched-cohort study in Denmark. Gastroenterology 154, 1729–1736 (2018). [DOI] [PubMed] [Google Scholar]
- 53.Gandini S, Lowenfels AB, Jaffee EM, Armstrong TD & Maisonneuve P Allergies and the risk of pancreatic cancer: a meta-analysis with review of epidemiology and biological mechanisms. Cancer Epidemiol. Biomarkers Prev. 14, 1908–1916 (2005). [DOI] [PubMed] [Google Scholar]
- 54.Olson SH et al. Allergies and risk of pancreatic cancer: a pooled analysis from the Pancreatic Cancer Case-Control Consortium. Am. J. Epidemiol. 178, 691–700 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cotterchio M, Lowcock E, Hudson TJ, Greenwood C & Gallinger S Association between allergies and risk of pancreatic cancer. Cancer Epidemiol. Biomarkers Prev. 23, 469–480 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gomez-Rubio P et al. Reduced risk of pancreatic cancer associated with asthma and nasal allergies. Gut 66, 314–322 (2017). [DOI] [PubMed] [Google Scholar]
- 57.Riquelme E et al. Tumor microbiome diversity and composition influence pancreatic cancer outcomes. Cell 178, 795–806.e12 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maisonneuve P, Amar S & Lowenfels AB Periodontal disease, edentulism, and pancreatic cancer: a meta-analysis. Ann. Oncol. 28, 985–995 (2017). [DOI] [PubMed] [Google Scholar]
- 59.Fan X et al. Human oral microbiome and prospective risk for pancreatic cancer: a population-based nested case-control study. Gut 67, 120–127 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Michaud DS et al. Plasma antibodies to oral bacteria and risk of pancreatic cancer in a large European prospective cohort study. Gut 62, 1764–1770 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schulte A et al. Association between Helicobacter pylori and pancreatic cancer risk: a meta-analysis. Cancer Causes Control. 26, 1027–1035 (2015). [DOI] [PubMed] [Google Scholar]
- 62.Andreotti G & Silverman DT Occupational risk factors and pancreatic cancer: a review of recent findings. Mol. Carcinog. 51, 98–108 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barone E, Corrado A, Gemignani F & Landi S Environmental risk factors for pancreatic cancer: an update. Arch. Toxicol. 90, 2617–2642 (2016). [DOI] [PubMed] [Google Scholar]
- 64.Gasull M et al. Methodological issues in a prospective study on plasma concentrations of persistent organic pollutants and pancreatic cancer risk within the EPIC cohort. Env. Res. 169, 417–433 (2019). [DOI] [PubMed] [Google Scholar]
- 65.Antwi SO et al. Exposure to environmental chemicals and heavy metals, and risk of pancreatic cancer. Cancer Causes Control. 26, 1583–1591 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Camargo J et al. Toenail concentrations of trace elements and occupational history in pancreatic cancer. Env. Int. 127, 216–225 (2019). [DOI] [PubMed] [Google Scholar]
- 67.Bosch de Basea M et al. Relationships between occupational history and serum concentrations of organochlorine compounds in exocrine pancreatic cancer. Occup. Env. Med. 68, 332–338 (2011). [DOI] [PubMed] [Google Scholar]
- 68.Amaral AF et al. Pancreatic cancer risk and levels of trace elements. Gut 61, 1583–1588 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Falk RT, Pickle LW, Fontham ET, Correa P & Fraumeni JF Life-style risk factors for pancreatic cancer in Louisiana: a case-control study. Am. J. Epidemiol. 128, 324–336 (1988). [DOI] [PubMed] [Google Scholar]
- 70.Friedman GD & Van Den Eeden SK Risk factors for pancreatic cancer: an exploratory study. Int. J. Epidemiol. 22, 30–37 (1993). [DOI] [PubMed] [Google Scholar]
- 71.Fernandez E, La Vecchia C, d’Avanzo B, Negri E & Franceschi S Family history and the risk of liver, gallbladder, and pancreatic cancer. Cancer Epidemiol. Biomarkers Prev. 3, 209–212 (1994). [PubMed] [Google Scholar]
- 72.Price TF, Payne RL & Oberleitner MG Familial pancreatic cancer in south Louisiana. Cancer Nurs. 19, 275–282 (1996). [DOI] [PubMed] [Google Scholar]
- 73.Ghadirian P et al. Reported family aggregation of pancreatic cancer within a population-based case-control study in the francophone community in Montreal, Canada. Int. J. Pancreatol. 10, 183–196 (1991). [DOI] [PubMed] [Google Scholar]
- 74.Coughlin SS, Calle EE, Patel AV & Thun MJ Predictors of pancreatic cancer mortality among a large cohort of United States adults. Cancer Causes Control. 11, 915–923 (2000). [DOI] [PubMed] [Google Scholar]
- 75.Schenk M et al. Familial risk of pancreatic cancer. J. Natl Cancer Inst. 93, 640–644 (2001). [DOI] [PubMed] [Google Scholar]
- 76.Silverman DT Risk factors for pancreatic cancer: a case-control study based on direct interviews. Teratog. Carcinog. Mutagen. 21, 7–25 (2001). [DOI] [PubMed] [Google Scholar]
- 77.Jacobs EJ et al. Family history of cancer and risk of pancreatic cancer: a pooled analysis from the Pancreatic Cancer Cohort Consortium (PanScan). Int. J. Cancer 127, 1421–1428 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brune KA et al. Importance of age of onset in pancreatic cancer kindreds. J. Natl Cancer Inst. 102, 119–126 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Norris AL et al. Familial and sporadic pancreatic cancer share the same molecular pathogenesis. Fam. Cancer 14, 95–103 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Petersen GM et al. Pancreatic Cancer Genetic Epidemiology Consortium. Cancer Epidemiol. Biomarkers Prev. 15, 704–710 (2006). [DOI] [PubMed] [Google Scholar]
- 81.Silverman DT et al. Diabetes mellitus, other medical conditions and familial history of cancer as risk factors for pancreatic cancer. Br. J. Cancer 80, 1830–1837 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang L et al. Elevated cancer mortality in the relatives of patients with pancreatic cancer. Cancer Epidemiol. Biomarkers Prev. 18, 2829–2834 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McWilliams RR et al. Association of family history of specific cancers with a younger age of onset of pancreatic adenocarcinoma. Clin. Gastroenterol. Hepatol. 4, 1143–1147 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Singhi AD et al. A histomorphologic comparison of familial and sporadic pancreatic cancers. Pancreatology 15, 387–391 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shi C et al. Increased prevalence of precursor lesions in familial pancreatic cancer patients. Clin. Cancer Res. 15, 7737–7743 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen F et al. Analysis of heritability and genetic architecture of pancreatic cancer: a PanC4 study. Cancer Epidemiol. Biomarkers Prev. 28, 1238–1245 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lichtenstein P et al. Environmental and heritable factors in the causation of cancer–analyses of cohorts of twins from Sweden, Denmark, and Finland. N. Engl. J. Med. 343, 78–85 (2000). [DOI] [PubMed] [Google Scholar]
- 88.Jones S et al. Exomic sequencing identifies PALB2 as a pancreatic cancer susceptibility gene. Science 324, 217 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Roberts NJ et al. ATM mutations in patients with hereditary pancreatic cancer. Cancer Discov. 2, 41–46 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Roberts NJ et al. Whole genome sequencing defines the genetic heterogeneity of familial pancreatic cancer. Cancer Discov. 6, 166–175 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Amundadottir L et al. Genome-wide association study identifies variants in the ABO locus associated with susceptibility to pancreatic cancer. Nat. Genet. 41, 986–990 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Petersen GM et al. A genome-wide association study identifies pancreatic cancer susceptibility loci on chromosomes 13q22.1, 1q32.1 and 5p15.33. Nat. Genet. 42, 224–228 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wolpin BM et al. Genome-wide association study identifies multiple susceptibility loci for pancreatic cancer. Nat. Genet. 46, 994–1000 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Childs EJ et al. Common variation at 2p13.3, 3q29, 7p13 and 17q25.1 associated with susceptibility to pancreatic cancer. Nat. Genet. 47, 911–916 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Klein AP et al. Genome-wide meta-analysis identifies five new susceptibility loci for pancreatic cancer. Nat. Commun. 9, 556 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brentnall TA, Bronner MP, Byrd DR, Haggitt RC & Kimmey MB Early diagnosis and treatment of pancreatic dysplasia in patients with a family history of pancreatic cancer. Ann. Intern. Med. 131, 247–255 (1999). [DOI] [PubMed] [Google Scholar]
- 97.Canto MI et al. Screening for early pancreatic neoplasia in high-risk individuals: a prospective controlled study. Clin. Gastroenterol. Hepatol. 4, 766–781 (2006). [DOI] [PubMed] [Google Scholar]
- 98.Canto MI et al. International Cancer of the Pancreas Screening (CAPS) Consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut 62, 339–347 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Canto MI et al. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology 142, 796–804 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vitone LJ, Greenhalf W, McFaul CD, Ghaneh P & Neoptolemos JP The inherited genetics of pancreatic cancer and prospects for secondary screening. Best. Pract. Res. Clin. Gastroenterol. 20, 253–283 (2006). [DOI] [PubMed] [Google Scholar]
- 101.Bryant HE et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 434, 913–917 (2005). [DOI] [PubMed] [Google Scholar]
- 102.McCabe N et al. BRCA2-deficient CAPAN-1 cells are extremely sensitive to the inhibition of poly (ADP-ribose) polymerase: an issue of potency. Cancer Biol. Ther. 4, 934–936 (2005). [DOI] [PubMed] [Google Scholar]
- 103.van der Heijden MS et al. In vivo therapeutic responses contingent on Fanconi anemia/BRCA2 status of the tumor. Clin. Cancer Res. 11, 7508–7515 (2005). [DOI] [PubMed] [Google Scholar]
- 104.Villarroel MC et al. Personalizing cancer treatment in the age of global genomic analyses: PALB2 gene mutations and the response to DNA damaging agents in pancreatic cancer. Mol. Cancer Ther. 10, 3–8 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Le DT et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 357, 409–413 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fogelman D et al. Family history as a marker of platinum sensitivity in pancreatic adenocarcinoma. Cancer Chemother. Pharmacol. 76, 489–498 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhen DB et al. BRCA1, BRCA2, PALB2, and CDKN2A mutations in familial pancreatic cancer: a PACGENE study. Genet. Med. 17, 569–577 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Goggins M et al. Germline BRCA2 gene mutations in patients with apparently sporadic pancreatic carcinomas. Cancer Res. 56, 5360–5364 (1996). [PubMed] [Google Scholar]
- 109.Hu C et al. Association between inherited germline mutations in cancer predisposition genes and risk of pancreatic cancer. JAMA 319, 2401–2409 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yurgelun MB et al. Germline cancer susceptibility gene variants, somatic second hits, and survival outcomes in patients with resected pancreatic cancer. Genet. Med. 21, 213–223 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shindo K et al. Deleterious germline mutations in patients with apparently sporadic pancreatic adenocarcinoma. J. Clin. Oncol. 35, 3382–3390 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Murphy KM et al. Evaluation of candidate genes MAP2K4, MADH4, ACVR1B, and BRCA2 in familial pancreatic cancer: deleterious BRCA2 mutations in 17%. Cancer Res. 62, 3789–3793 (2002). [PubMed] [Google Scholar]
- 113.Hahn SA et al. BRCA2 germline mutations in familial pancreatic carcinoma. J. Natl Cancer Inst. 95, 214–221 (2003). [DOI] [PubMed] [Google Scholar]
- 114.Couch FJ et al. The prevalence of BRCA2 mutations in familial pancreatic cancer. Cancer Epidemiol. Biomarkers Prev. 16, 342–346 (2007). [DOI] [PubMed] [Google Scholar]
- 115.Breast Cancer Linkage Consortium. Cancer risks in BRCA2 mutation carriers. J. Natl Cancer Inst. 91, 1310–1316 (1999). [DOI] [PubMed] [Google Scholar]
- 116.Mocci E et al. Risk of pancreatic cancer in breast cancer families from the Breast Cancer Family Registry. Cancer Epidemiol. Biomarkers Prev. 22, 803–811 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Thompson D & Easton DF Cancer incidence in BRCA1 mutation carriers. J. Natl Cancer Inst. 94, 1358–1365 (2002). [DOI] [PubMed] [Google Scholar]
- 118.Tischkowitz MD et al. Analysis of the gene coding for the BRCA2-interacting protein PALB2 in familial and sporadic pancreatic cancer. Gastroenterology 137, 1183–1186 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Slater EP et al. PALB2 mutations in European familial pancreatic cancer families. Clin. Genet 78, 490–494 (2010). [DOI] [PubMed] [Google Scholar]
- 120.Grant RC et al. Prevalence of germline mutations in cancer predisposition genes in patients with pancreatic cancer. Gastroenterology 148, 556–564 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Goldstein AM, Struewing JP, Fraser MC, Smith MW & Tucker MA Prospective risk of cancer in CDKN2A germline mutation carriers. J. Med. Genet. 41, 421–424 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rutter JL et al. Heterogeneity of risk for melanoma and pancreatic and digestive malignancies: a melanoma case-control study. Cancer 101, 2809–2816 (2004). [DOI] [PubMed] [Google Scholar]
- 123.Vasen HF et al. Risk of developing pancreatic cancer in families with familial atypical multiple mole melanoma associated with a specific 19 deletion of p16 (p16-Leiden). Int. J. Cancer 87, 809–811 (2000). [PubMed] [Google Scholar]
- 124.Vasen H et al. Benefit of surveillance for pancreatic cancer in high-risk individuals: outcome of long-term prospective follow-up studies from three European Expert Centers. J. Clin. Oncol. 34, 2010–2019 (2016). [DOI] [PubMed] [Google Scholar]
- 125.Kastrinos F et al. Risk of pancreatic cancer in families with Lynch syndrome. JAMA 302, 1790–1795 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.van Lier MG et al. High cancer risk in Peutz-Jeghers syndrome: a systematic review and surveillance recommendations. Am. J. Gastroenterol. 105, 1258–1264 (2009). [DOI] [PubMed] [Google Scholar]
- 127.Giardiello FM et al. Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology 119, 1447–1453 (2000). [DOI] [PubMed] [Google Scholar]
- 128.Giardiello FM et al. Increased risk of cancer in the Peutz-Jeghers syndrome. N. Engl. J. Med. 316, 1511–1514 (1987). [DOI] [PubMed] [Google Scholar]
- 129.Lowenfels AB et al. Hereditary pancreatitis and the risk of pancreatic cancer. International Hereditary Pancreatitis Study Group. J. Natl Cancer Inst. 89, 442–446 (1997). [DOI] [PubMed] [Google Scholar]
- 130.Lowenfels AB, Maisonneuve P, Whitcomb DC, Lerch MM & DiMagno EP Cigarette smoking as a risk factor for pancreatic cancer in patients with hereditary pancreatitis. JAMA 286, 169–170 (2001). [DOI] [PubMed] [Google Scholar]
- 131.Vitone LJ, Greenhalf W, Howes NR, Raraty MG & Neoptolemos JP Trypsinogen mutations in pancreatic disorders. Endocrinol. Metab. Clin. North. Am. 35, 271–287 (2006). [DOI] [PubMed] [Google Scholar]
- 132.Whitcomb DC et al. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat. Genet. 14, 141–145 (1996). [DOI] [PubMed] [Google Scholar]
- 133.Whitcomb DC et al. A gene for hereditary pancreatitis maps to chromosome 7q35. Gastroenterology 110, 1975–1980 (1996). [DOI] [PubMed] [Google Scholar]
- 134.Schubert S et al. CFTR, SPINK1, PRSS1, and CTRC mutations are not associated with pancreatic cancer in German patients. Pancreas 43, 1078–1082 (2014). [DOI] [PubMed] [Google Scholar]
- 135.Matsubayashi H et al. Polymorphisms of SPINK1 N34S and CFTR in patients with sporadic and familial pancreatic cancer. Cancer Biol. Ther. 2, 652–655 (2003). [PubMed] [Google Scholar]
- 136.Tamura K et al. Mutations in the pancreatic secretory enzymes CPA1 and CPB1 are associated with pancreatic cancer. Proc. Natl Acad. Sci. USA 115, 4767–4772 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhong J et al. A transcriptome-wide association study identifies novel candidate susceptibility genes for pancreatic cancer. J. Natl Cancer Inst. 112, 1003–1012 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhang M et al. Three new pancreatic cancer susceptibility signals identified on chromosomes 1q32.1, 5p15.33 and 8q24.21. Oncotarget 7, 66328–66343 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wu C et al. Genome-wide association study identifies five loci associated with susceptibility to pancreatic cancer in Chinese populations. Nat. Genet. 44, 62–66 (2011). [DOI] [PubMed] [Google Scholar]
- 140.Low SK et al. Genome-wide association study of pancreatic cancer in Japanese population. PLoS ONE 5, e11824 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lin Y et al. Genome-wide association meta-analysis identifies GP2 gene risk variants for pancreatic cancer. Nat. Commun. 11, 3175 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Childs EJ et al. Association of common susceptibility variants of pancreatic cancer in higher-risk patients: a PACGENE Study. Cancer Epidemiol. Biomarkers Prev. 25, 1185–1191 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Walsh N et al. Agnostic pathway/gene set analysis of genome-wide association data identifies associations for pancreatic cancer. J. Natl Cancer Inst. 111, 557–567 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.US Preventive Services Task Force. Screening for pancreatic cancer: recommendation statement. Am. Fam. Physician 100, 770 (2019). [PubMed] [Google Scholar]
- 145.Goggins M et al. Management of patients with increased risk for familial pancreatic cancer: updated recommendations from the International Cancer of the Pancreas Screening (CAPS) Consortium. Gut 69, 7–17 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Canto MI et al. Risk of neoplastic progression in individuals at high risk for pancreatic cancer undergoing long-term surveillance. Gastroenterology 155, 740–751.e2 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Overbeek KA, Cahen DL, Canto MI & Bruno MJ Surveillance for neoplasia in the pancreas. Best Pract. Res. Clin. Gastroenterol. 30, 971–986 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Konings I et al. Surveillance for pancreatic cancer in high-risk individuals. BJS Open. 3, 656–665 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cohen JD et al. Combined circulating tumor DNA and protein biomarker-based liquid biopsy for the earlier detection of pancreatic cancers. Proc. Natl Acad. Sci. USA 114, 10202–10207 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cohen JD et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science 359, 926–930 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bartoli M et al. CT and MRI of pancreatic tumors: an update in the era of radiomics. Jpn. J. Radiol. 38, 1111–1124 (2020). [DOI] [PubMed] [Google Scholar]
- 152.Chu LC et al. Utility of CT radiomics features in differentiation of pancreatic ductal adenocarcinoma from normal pancreatic tissue. AJR Am. J. Roentgenol. 213, 349–357 (2019). [DOI] [PubMed] [Google Scholar]


