Abstract
A gene encoding a protein homologous to known bacterial N-acetyl-muramidases has been cloned from Leuconostoc citreum by a PCR-based approach. The encoded protein, Mur, consists of 209 amino acid residues with a calculated molecular mass of 23,821 Da including a 31-amino-acid putative signal peptide. In contrast to most of the other known peptidoglycan hydrolases, L. citreum Mur protein does not contain amino acid repeats involved in cell wall binding. The purified L. citreum Mur protein was shown to exhibit peptidoglycan-hydrolyzing activity by renaturing sodium dodecyl sulfate-polyacrylamide gel electrophoresis. An active chimeric protein was constructed by fusion of L. citreum Mur to the C-terminal repeat-containing domain (cA) of AcmA, the major autolysin of Lactococcus lactis. Expression of the Mur-cA fusion protein was able to complement an acmA mutation in L. lactis; normal cell separation after cell division was restored by Mur-cA expression.
Bacteria produce one or several peptidoglycan hydrolases (PGHs), which are capable of hydrolyzing covalent bonds in the peptidoglycan of their own cell envelope (for reviews, see references 46 and 49). Some of them, named autolysins, are able to trigger cell autolysis. PGHs are located in the cell wall and are involved in various cellular functions, including cell wall expansion, cell wall turnover, or cell separation. On the basis of their cleavage site in the peptidoglycan, four types of PGHs are defined: (i) N-acetyl-muramidases, (ii) N-acetyl-glucosaminidases, (iii) N-acetyl-muramoyl-l-alanine amidases, and (iv) peptidases. Most of the PGHs characterized so far have a modular structural organization with two domains: a catalytic domain containing the enzyme active site and a cell wall binding domain composed of several amino acid repeats (22, 30).
Autolysis of lactic acid bacteria (LAB) used as starters for cheese manufacturing plays an important role in flavor development during ripening (for reviews, see references 13 and 18). It has been shown that lysis of Lactococcus lactis starter strains leads to the release of intracellular peptidases in the cheese curd, and as a result more free amino acids (which are aroma precursors) are produced and hydrophobic bitter peptides are degraded (12, 35, 52).
The PGH activities present in L. lactis were studied by renaturing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), which allowed their detection after renaturation in a substrate-containing gel. Several activity bands were evidenced by this technique (9, 36, 40, 44). The major autolysin, AcmA, was characterized at the genetic level. It is an N-acetyl-muramidase that is required for proper cell separation after cell division (9) and is involved in autolysis observed during stationary phase after growth in liquid medium (10).
Leuconostocs are heterofermentative LAB used as cheese starters in association with lactococci. They contribute to the development of cheese organoleptic properties by metabolizing citrate to diacetyl, an important flavor compound, and to CO2, which is responsible for eye formation in some Dutch cheeses (17). Like lactococci, leuconostocs contain a diverse pool of peptidases (23). Thus, autolysis of Leuconostoc starter strains could contribute to peptidolysis during cheese ripening. Recently, researchers have demonstrated several PGH activities in dairy leuconostocs (15). In order to understand and control autolysis, a first step is to identify and to characterize at the molecular level the enzymes involved in this phenomenon.
In the present study, we report the cloning, sequencing, and expression of a PGH-encoding gene, named mur, from Leuconostoc citreum 22R. The L. citreum Mur protein shows sequence homology to the N-terminal catalytic domain of several known bacterial muramidases. However, in contrast to these muramidases, L. citreum Mur is devoid of a specific cell wall binding domain. We constructed an active chimeric protein by fusion of L. citreum Mur and the L. lactis AcmA cell wall binding domain and showed that it complements an AcmA deficiency in L. lactis.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The strains and plasmids used in this study are listed in Table 1. Leuconostoc strains and L. lactis strains were grown at 30°C in MRS medium (20) and M17 medium containing 0.5% glucose (M17-glu) (50), respectively. Escherichia coli strains were grown in Luria-Bertani (LB) medium at 37°C under vigorous shaking conditions. When required, antibiotics were added at the following concentrations, except where otherwise stated: ampicillin, 50 μg/ml; chloramphenicol, 25 μg/ml; tetracycline, 10 μg/ml; and erythromycin, 5 μg/ml.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s) | Reference or source |
|---|---|---|
| Strains | ||
| E. coli | ||
| TG1 | supE hsdΔ5 thi Δ(lac proAB) F′ [traD36proAB+lacIqlacZΔM15] | 25 |
| XL1-Blue | supE44 hsdR17 recA1 endA1 gyrA46 thi relA1 lac− F′ [proAB+ lacIqlacZΔM15 Tn10(tetr)] | Stratagene |
| L. citreum 22R | Wild-type strain | CNRZ collection |
| L. mesenteroides 50M | Wild-type strain | CNRZ collection |
| L. lactis | ||
| MG1363 acmAΔ1 | Derivative of MG1363 carrying a 701-bp SacI-SpeI deletion in acmA | 9 |
| NZ9000 | Derivative of NZ3000; pepN::nisRnisK | O. Kuipersa |
| NCDO763 | Wild-type strain | CNRZ collection |
| Plasmids | ||
| pBluescript SK+ (pBSK+) | Apr | Stratagene |
| pQE30 | Apr, expression vector, six-His tag at the N terminus of the protein | Qiagen |
| pCYT1 | Cmr, pNZ8010 derivative carrying a transcriptional fusion of the nisA promoter, the usp45 RBS, and the S. aureus nucA gene | P. Langellab |
| pNZ9520 | Emr, nisRK genes cloned in high-copy-number cloning vector pIL253 (48) | 31 |
| pNZ9530 | Emr, nisRK genes cloned in low-copy-number cloning vector pIL252 (48) | 31 |
| pTIL340 | Apr, pBSK+ carrying the 552-bp BamHI-HindIII fragment encoding L. citreum Mur amplified with the primers LnP5 and LnP6 | This study |
| pTIL341 | Apr, pBSK+ carrying the 636-bp PstI-ClaI fragment encoding L. citreum Mur amplified with the primers LnP7 and LnP8 | This study |
| pTIL342 | Apr, pBSK+ carrying the 740-bp ClaI-XhoI fragment encoding the amino acid repeats of acmA (cA) amplified with the primers LnP9 and LnP10 | This study |
| pTIL343 | pQE30 derivative with the 552-bp BamHI-HindIII insert of pTIL340 | This study |
| pTIL344 | Cmr, pCYT1 derivative carrying the 1,370-bp chimeric mur-cA gene under control of the nisA promoter | This study |
| pTIL345 | Apr, pBSK+ carrying the 648-bp PstI-HindIII fragment encoding L. citreum Mur amplified with the primers LnP6 and LnP7 | This study |
| pTIL346 | Cmr, pCYT1 derivative carrying the 648-bp PstI-HindIII insert of pTIL345 under control of the nisA promoter | This study |
NIZO, Ede, The Netherlands.
URLGA, INRA, 78352 Jouy-en-Josas, France.
General DNA techniques, PCR, and transformation.
Molecular cloning techniques were performed essentially as described previously (45). Total DNA was isolated from L. citreum 22R according to de los Reyes-Gavilan et al. (19) except that 10 IU of mutanolysin (Sigma Chemicals, St. Louis, Mo.) was added to TES buffer (25% sucrose, 1 mM EDTA, 50 mM Tris-HCl [pH 8.0]). Plasmid DNA was isolated essentially as described previously (6); for L. lactis, cells were incubated in TES buffer containing 10 mg of lysozyme per ml at 37°C for 10 min before alkaline lysis. Restriction enzymes, DNA ligase, T4 DNA polymerase, and Klenow enzyme were obtained from Gibco BRL or New England Biolabs and used according to the suppliers' instructions. PCR was performed with a Perkin-Elmer Cetus (Norwalk, Conn.) thermocycler. Electroporation of L. lactis was performed as described before (33), and transformants were plated on M17-glu agar plates containing the required antibiotic. DNA sequencing was performed by the dideoxy-chain termination method with the Dye Terminator ABI Prism cycle sequencing kit (Perkin-Elmer). DNA sequence was determined with an automated Applied Biosystems 373 DNA sequencer (Perkin-Elmer). Protein homology searches were carried out with the Blast program (1).
Southern hybridization was performed according to standard protocol (45). Total DNA of Leuconostoc strains was digested with HindIII or EcoRI, electrophoresed in an agarose gel, and blotted onto a Hybond-N+ nylon membrane. Two primers (5′-GCGCAGGCTATTTTAG-3′, 465–480 forward, and 5′-ATGCATTAGCTGCTGC-3′, 740–724 reverse) were used to amplify a 276-bp DNA fragment corresponding to the internal region of L. citreum mur. This fragment, labeled with [α-32P]dCTP with a random primed DNA labeling kit, was used as a probe in a hybridization experiment under low-stringency conditions (20% formamide).
Cloning of the L. citreum mur gene.
Two conserved stretches of amino acids were selected from the alignment of the N-terminal regions of the N-acetylmuramidases of L. lactis (8), Enterococcus faecalis (5), and Enterococcus hirae (13). These were used to design the degenerate primers LnP1 and LnP2 (Table 2). A PCR with these primers on L. citreum 22R total DNA gave rise to a single DNA fragment with the expected size. The nucleotide sequence of this 323-bp fragment was determined, and the deduced amino acid sequence revealed similarity with the N-terminal region of AcmA, the muramidase of L. lactis. The entire gene was cloned by reverse PCR as previously described (47) with the divergent primers LnP3 and LnP4 (Table 2), which correspond to internal sequences of the 323-bp fragment. Total DNA from L. citreum 22R was digested with HindIII, and the resulting fragments were self ligated and used as the template for a PCR with the divergent primers. A 3.6-kb DNA fragment was amplified and sequenced.
TABLE 2.
Oligonucleotides used in this study
| Primera | Sequenceb | Restriction site | Positionsc |
|---|---|---|---|
| LnP1 | GTNATGATNGCNCARGC | Conserved region; aa 86-91d | |
| LnP2(r) | GGRTCNGTNGCRTANC | Conserved region; aa 190-195d | |
| LnP3(r) | CATTCCAACTCGTGCCATGA | nt 683 to 664 of the sequenced fragment | |
| LnP4 | CGGCCCAATATCAGCATGTT | nt 684 to 703 of the sequenced fragment | |
| LnP5 | CGCGGATCCACTTTTTGGTCGCAAACAC | BamHI | nt 305 to 323 of the sequenced fragment |
| LnP6(r) | GGAAGCTTCGGAACATTTCCGTT | HindIII | nt 857 to 843 of the sequenced fragment |
| LnP7 | GCCTGCAGCAAAAAGAAAAAGACGT | PstI | nt 216 to 232 of the sequenced fragment |
| LnP8(r) | CCATCGATTTTTTGTGGTACATCAAAGCG | ClaI | nt 838 to 818 of the sequenced fragment |
| LnP9 | CCATCGATGCTGGTACTTCTAATTCCGGT | ClaI | nt 652 to 675 of acmAe |
| LnP10(r) | CCGCTCGAGTCATCTTCAGTCTGTTTTAAAAG | XhoI | nt 1382 to 1359 of the 3′ end of the transcription terminator downstream acmAe |
Expression and purification of the six-His-tagged Mur protein in E. coli.
The expression vector pQE30 (Qiagen) was used for overproduction of the L. citreum Mur protein in E. coli. A DNA fragment encoding L. citreum Mur without its putative signal peptide was amplified with the primers LnP5 and LnP6 (Table 2) and fused in frame downstream of the N-terminal six-His box sequence in pQE30. The resulting plasmid (pTIL343) was used to transform E. coli XL1-Blue competent cells. Isopropyl-β-d-thiogalactopyranoside (IPTG) was added at a final concentration of 1 mM to the culture at an optical density at 650 nm of 0.6 to 0.8 to induce the expression of the six-His-tagged Mur protein. The culture was further incubated at 37°C for 4 h. The cells were harvested by centrifugation and broken by a freezing and thawing process followed by sonication. The inclusion bodies containing the recombinant protein were collected by centrifugation at 15,000 × g for 10 min at 4°C. The recombinant protein was solubilized in 8 M urea and purified on a nickel-nitriloacetic acid (Ni-NTA) spin column (Qiagen) as recommended by the supplier. Protein concentration was determined with the Coomassie protein assay kit (Pierce).
Mass spectrometry.
Protein molecular mass was determined by mass spectrometry with a matrix-assisted laser desorption ionization–time of flight system (LD-TOF G 2025A; Hewlett-Packard).
Construction of a chimeric protein between L. citreum Mur and the C-terminal domain of L. lactis AcmA and expression in L. lactis
A chimeric protein was constructed by fusion of L. citreum Mur with the C-terminal domain (cA) of L. lactis AcmA containing the amino acid repeats involved in cell wall binding (11). The Mur-cA chimeric protein was expressed in L. lactis using the nisin-inducible expression system (21) with the plasmid vector pCYT1 (P. Langella, personal communication). pCYT1 is a derivative of pNZ8010 (21), in which the gusA gene was replaced by a DNA fragment carrying the usp45 ribosome binding site (RBS) (51) fused to the Staphylococcus aureus nuc gene (34). pCYT1 was digested with NsiI and XhoI to remove nuc and to place subsequently the mur-cA gene fusion under control of the nisA promoter and to express it via the usp45 RBS. The oligonucleotides used in the study are listed in Table 2.
The L. citreum mur gene was amplified by PCR (with primers LnP7 and LnP8), and the 3′ end of the acmA gene (cA) was amplified by PCR from L. lactis IL1403 total DNA (with primers LnP9 and LnP10). The blunted PCR products were cloned in the EcoRV site of pBSK+ to yield plasmids pTIL341 (for L. citreum mur) and pTIL342 (for cA). pTIL341 and pTIL342 were subsequently digested with PstI and ClaI and with ClaI and XhoI, respectively, to recover the inserts. The 636-bp PstI-ClaI fragment carrying the L. citreum mur gene plus the 740-bp ClaI-XhoI fragment carrying the 3′ end of acmA were mixed with pCYT1 vector digested with NsiI and XhoI, and the mixture was ligated. Plasmid pTIL344 containing both fragments, allowing in-frame fusion of L. citreum mur and the 3′ end of acmA, was selected. It was used to transform L. lactis NZ9000 (kindly provided by Oscar Kuipers, Netherlands Institute for Dairy Research, (NIZO), Ede, The Netherlands), which contains the regulatory nisRK genes integrated in its chromosome and the acmA-negative mutant, L. lactis MG1363acmAΔ1 (9). In the latter case, MG1363acmAΔ1 harboring pTIL344 was then transformed with pNZ9520 or pNZ9530 plasmid (31), which carries the regulatory nisRK genes.
A similar construction was made with L. citreum mur. The L. citreum mur gene was amplified by PCR from total DNA from L. citreum 22R with the primers LnP6 and LnP7. The amplified DNA fragment was blunt ended and first cloned in the EcoRV site of plasmid pBSK+. The resulting plasmid, pTIL345, was subsequently digested with HindIII and PstI, and the 648-bp insert was transferred into the plasmid vector pCYT1 predigested with NsiI and HindIII. The resulting plasmid, pTIL346, was used to transform L. lactis NZ9000 or MG1363acmAΔ1 as described above.
For induction of the nisA promoter, strains were grown until an optical density at 650 nm (OD650) of 0.5 was reached and nisin was added at a final concentration of 2.5 ng/ml. Growth was continued for 5 h, and cells were harvested. SDS cell extracts were prepared as described below and submitted to SDS-PAGE or tested for bacteriolytic activity by renaturing SDS-PAGE.
SDS-PAGE and detection of bacteriolytic activity by renaturing SDS-PAGE.
SDS-PAGE was performed as described by Laemmli (32) with 10 to 15% (wt/vol) polyacrylamide separating gels. Gels were stained with Coomassie brilliant blue R250 (Sigma).
For renaturing SDS-PAGE, autoclaved cells of Micrococcus lysodeikticus ATCC 4698 (0.2% [wt/vol]) (Sigma), of Leuconostoc mesenteroides subsp. dextranicum 50 M (0.4% [wt/vol]), or of L. lactis subsp. lactis NCDO763 (0.4% [wt/vol]) were incorporated into polyacrylamide gels as the substrate. Preparation of samples and detection of bacteriolytic activity were performed essentially as described previously (36, 42). For the preparation of L. citreum SDS cell extract, 4 ml of culture was centrifuged and the cell pellet was resuspended in 40 μl of SDS-PAGE sample buffer. The suspension was boiled at 100°C for 3 min and centrifuged at 13,000 × g for 10 min, and the supernatant (SDS cell extract) was loaded on the gel. The culture supernatant was concentrated 25 times with a Centriplus concentrator (Amicon, Beverly, Mass.) with a 10,000-Da molecular mass cutoff. Following electrophoresis, the gels were soaked in 250 ml of distilled water for 30 min at room temperature under gentle agitation. They were transferred to 200 ml of renaturation buffer consisting of 50 mM potassium phosphate (pH 6.5) buffer with 1% (vol/vol) Triton X-100 and incubated at 37°C for an additional 6 h with gentle shaking. The gels were then stained with 0.1% methylene blue in 0.01% KOH and subsequently destained with distilled water. Bacteriolytic activity bands appeared as clear zones in the opaque background. Molecular masses were determined with standards run on the same gel.
Fluorescent in situ hybridization.
Fluorescent in situ hybridization was performed as described by Amann et al. (3). Cells were treated with lysozyme, and a 16S RNA-targeted probe (EUB338) (2) labeled with fluorescein or rhodamine was used for hybridization. Photographs of cells were taken with an epifluorescence microscope (Nikon).
Nucleotide sequence accession number.
The nucleotide sequence described in this paper is deposited in the EMBL/NCBI/DDBJ sequence databases under accession number AF176553.
RESULTS
Cloning and sequencing of the mur gene from L. citreum 22R.
Using the PCR-based strategy described in Materials and Methods, we cloned a 3.6-kb DNA fragment from L. citreum 22R total DNA. Analysis of the nucleotide sequence of this fragment revealed the presence of a 630-bp open reading frame (ORF) (mur) showing homology with L. lactis acmA (Fig. 1). L. citreum mur is preceded by a putative RBS as well as by consensus −10 and −35 regions corresponding to a putative promoter. A potential transcription terminator is present downstream of the ORF.
FIG. 1.
Nucleotide sequence (nucleotides 1 to 869) and deduced amino acid sequences of the L. citreum DNA fragment encoding Mur (Lnmur) (accession number AF176553). The putative −10 and −35 sequences are double underlined, and the putative RBS is indicated with a dotted line. The stop codon is indicated with an asterisk, and a putative transcription terminator is underlined. The putative peptide signal cleavage site is indicated with an arrow.
L. citreum mur specifies a 209-residue polypeptide (Mur), with a calculated molecular mass of 23,821 Da. The first 31 amino acids are predicted to serve as a signal peptide (39). The protein has a predicted isoelectric point (pI) of 9.6. Cleavage of the signal peptide would yield a 178-residue mature protein with a calculated molecular mass of 20,171 Da and a predicted pI of 5.2.
Sequence homology searches revealed that L. citreum Mur has sequence identity with the N-terminal regions of the L. lactis muramidase AcmA (37%) (9), the E. hirae muramidase-2 (37%) (14), and the E. faecalis autolysin (38%) (5) (Fig. 2). A significant level of identity was also found with the C-terminal region of the flagellar protein FlgJ of Salmonella enterica serovar Typhimurium (37%), which possesses peptidoglycan-hydrolyzing activity (28, 38), and the E. coli FlgJ homolog (37%) (7) (Fig. 2). These proteins have a modular structural organization with a catalytic domain fused to a cell wall binding domain (30). Surprisingly, L. citreum Mur comprises the catalytic domain of these proteins but lacks a domain containing amino acid repeats. L. citreum Mur contains several acidic residues separated by 13 to 33 residues, which could be involved in the catalytic site of the enzyme as proposed for other muramidases (30, 38). For example, E120 and D137 are separated by 16 amino acids.
FIG. 2.
Alignment of the amino acid sequences of the Mur protein of L. citreum 22R (Lnmur) and the catalytic domains of AcmA of L. lactis MG1363, autolysin (EfAutol) of E. faecalis, muramidase-2 (Mur2) of E. hirae, and flagellar protein (FlgJ) of serovar Typhimurium. Alignment was made using the ClustalW program. Identical residues present in all the sequences are indicated with an asterisk, and similar residues are indicated with a point. Putative acidic residues present in the catalytic site are in bold letters.
Part of an ORF (ORFB) was found upstream of L. citreum mur (Fig. 1), but no homology was found with sequences present in the databases. Downstream of L. citreum mur, another complete ORF was identified (accession number AF176554); it encodes a 750-residue polypeptide with high sequence similarity with Staphylococcus aureus DNA helicase PcrA (51%) (27) and the Bacillus subtilis homolog (55%) (41).
Distribution of the L. citreum mur gene.
Southern hybridization was carried out with a 276-bp probe derived from the L. citreum mur sequence with the DNA of several Leuconostoc strains (16) under low-stringency conditions. One hybridization band was detected in L. citreum 22R and in L. citreum 50A as well as in L. mesenteroides subsp. dextranicum 19S and L. mesenteroides subsp. mesenteroides 10L under the conditions used. Besides, a gene homologous to L. citreum mur, named mur1, was isolated from Streptococcus thermophilus (26), and a homologous one was identified in the complete sequence of L. lactis IL1403 (A. Bolotin and A. Sorokin, personal communication). All these data suggest a wide distribution of the gene, both in Leuconostoc spp. and in other LAB.
L. citreum Mur has peptidoglycan-hydrolyzing activity.
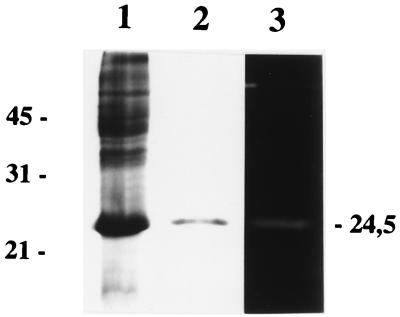
The L. citreum Mur protein devoid of its putative signal sequence was overproduced in E. coli as a six-His N-terminally tagged protein. It was purified from inclusion bodies by metal chelation affinity chromatography on a Ni-nitrilotriacetic acid spin column. The eluted fraction was analyzed by SDS-PAGE, and a single band with an apparent molecular mass of 24.5 kDa was detected after Coomassie blue staining (Fig. 3, lanes 1 and 2). The apparent molecular mass of the protein was higher than that calculated (21,569 Da). Nevertheless, the molecular mass of the purified protein determined by mass spectrometry (21,680 ± 100 Da) fits the calculated mass, thus indicating that the electrophoretic mobility of the protein may be altered by the six-His tag.
FIG. 3.
SDS-PAGE and renaturing SDS-PAGE analysis of the purified six-His-tagged L. citreum Mur protein. Coomassie blue staining was used for lanes 1 and 2. Lane 1, whole SDS cell extract of E. coli XL1-Blue harboring pTIL343 induced for 4 h with isopropyl-β-d-thiogalactopyranoside; lane 2, recombinant L. citreum Mur purified on Ni-nitrilotriacetic acid resin; lane 3, activity of the purified recombinant protein by renaturing SDS-PAGE containing 0.2% autoclaved M. lysodeikticus cells. The molecular masses (in kilodaltons) of standard proteins are indicated on the left.
The peptidoglycan-hydrolyzing activity of the purified protein was assayed by renaturing SDS-PAGE with autoclaved cells of M. lysodeikticus as the substrate. Activity was observed as a clear band at a molecular mass of around 24 kDa on the opaque background after incubation in renaturation buffer (Fig. 3, lane 3), thus indicating that L. citreum Mur has peptidoglycan-hydrolyzing activity. Also, L. citreum Mur exhibits hydrolyzing activity on L. lactis and L. mesenteroides substrates (results not shown). Several activity bands can be detected in L. citreum 22R cell extracts by renaturing SDS-PAGE (15). In order to determine whether the activity corresponding to that of L. citreum Mur could be detected in L. citreum 22R, whole SDS cell extract and concentrated culture supernatant were tested under the same conditions as the purified L. citreum Mur. However, even after 2 days of incubation of the gel in renaturation buffer, no activity at the expected molecular mass could be detected (data not shown). This suggests that the quantity of enzyme produced is too low to be detected in these conditions.
A chimeric fusion protein between L. citreum Mur and the L. lactis AcmA C-terminal domain is able to complement AcmA deficiency in L. lactis.
L. citreum Mur is devoid of a specific cell wall binding domain. Thus, a chimeric protein was constructed by fusion of L. citreum Mur with the L. lactis AcmA C-terminal domain (cA),which contains 3 amino acid repeats (11). The mur-cA chimeric gene and the L. citreum mur gene were then expressed in the acmA deletion mutant, L. lactis MG1363acmAΔ1 (9), in order to investigate whether they could complement an acmA mutation.
For expression purposes, the nisin-inducible expression system was used (21). The mur-cA and L. citreum mur genes were cloned under the control of the nisA promoter in pCYT1 (Table 1). The resulting plasmids carrying the fusion mur-cA or mur gene were first transformed in L. lactis NZ9000, which carries the regulatory nisRK genes integrated in its chromosome. Production of Mur-cA fusion protein and L. citreum Mur after nisin induction was checked by renaturing SDS-PAGE with M. lysodeikticus as the substrate. Activity bands at 44 and 24.5 kDa were revealed in NZ9000 harboring pTIL344 and pTIL346, respectively, which correspond to the expected molecular masses of the fusion proteins Mur-cA and L. citreum Mur, respectively (data not shown). This indicated that the genetic constructions were functional. The plasmids pTIL344 and pTIL346 were then transferred in L. lactis MG1363acmAΔ1. Since strain MG1363 does not contain the regulatory nisRK genes, plasmid pNZ9520 (Table 1) carrying nisRK was also introduced into MG1363acmAΔ1 harboring pTIL344 or pTIL346.
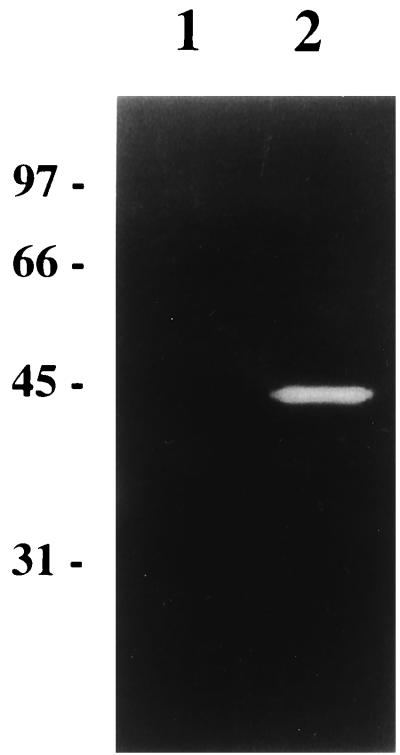
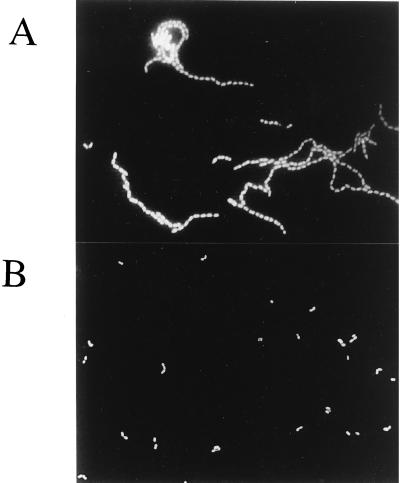
L. lactis MG1363acmAΔ1 is characterized by the presence of long bacterial chains and the absence of any activity band in renaturing SDS-PAGE with M. lysodeikticus as the substrate. However, both of these phenotypes are reversed in the MG1363acmAΔ1 strain harboring plasmids pTIL344 and pNZ9520 even in the absence of nisin. A 44-kDa activity band was observed using either M. lysodeiktikus (Fig. 4, lane 2), L. mesenteroides, or L. lactis cells as substrates (data not shown). This activity was absent from MG1363acmAΔ1 harboring pTIL344 alone (Fig. 4, lane 1). In addition, we observed that L. lactis MG1363acmAΔ1 harboring plasmids pTIL344 and pNZ9520 lost its sedimentation properties and formed short chains similar to those of wild-type MG1363 (Fig. 5). These results indicate that the chimeric protein Lnmur-cA is functional and able to complement an AcmA deficiency. In contrast, MG1363acmAΔ1 harboring plasmids pTIL346 and pNZ9520 still formed long chains even after nisin induction. It is worth noting that, although we checked that the construction was functional in strain NZ9000, L. citreum Mur was not detected in MG1363acmAΔ1 harboring plasmids pTIL346 and pNZ9520, most probably due to the low expression level and a lower specific activity of L. citreum Mur compared to those of Mur-cA. Nevertheless, the results suggest that unlike Mur-cA, L. citreum Mur alone was not able to complement the AcmA deficiency.
FIG. 4.
Renaturing SDS-PAGE analysis of the chimeric Mur-cA protein expressed in L. lactis MG1363acmAΔ1. Lane 1, L. lactis MG1363acmAΔ1 harboring plasmid pTIL344; lane 2, L. lactis MG1363acmAΔ1 harboring pTIL344 and pNZ9520 carrying nisRK genes. SDS cell extract of each strain was prepared from a noninduced culture and loaded onto polyacrylamide gel containing 0.2% autoclaved M. lysodeikticus cells. The molecular masses (in kilodaltons) of standard proteins are indicated on the left of the gel.
FIG. 5.
Epifluorescent micrograph of L. lactis MG1363acmAΔ1 (A) and L. lactis MG1363acmAΔ1 harboring pTIL344 and pNZ9520 (B). Bacteria were grown in M17-glu medium and were not induced with nisin. Micrographs were taken after in situ hybridization of the lysozyme-treated bacteria with the universal probe EUB338.
We were surprised to detect Mur-cA activity in the absence of nisin inducer, as well as a lack of induction after nisin addition. Previously, Kleerebezem et al. (31) also reported a significant level of transcription from the nisA promoter in the absence of nisin, when the nisRK genes were carried by the high-copy-number pNZ9520, as well as a reduced or abolished inducibility of the nisA promoter. They overcome this problem by the use of the low-copy-number plasmid pNZ9530 (Table 1) carrying the nisRK genes. In our case, even with pNZ9530 instead of pNZ9520 and regardless of the nisin concentration, nisin-inducible expression of the mur-cA and L. citreum mur genes was not obtained.
DISCUSSION
To our knowledge, this is the first report concerning the identification of a PGH gene from a Leuconostoc species. The L. citreum mur gene isolated from L. citreum encodes a PGH homologous to bacterial N-acetyl-muramidases.
In contrast to most of the other previously described bacterial muramidases, L. citreum Mur contains only the catalytic domain and is devoid of the cell wall binding domain that typically consists of repeated sequences (30). Very recently, a similar PGH, exhibiting 35% sequence identity with L. citreum Mur, was identified in the lactic acid bacterium Streptococcus thermophilus (26). Despite the lack of amino acid repeats, L. citreum Mur is endowed with peptidoglycan-hydrolyzing activity, as detected in vitro (Fig. 3) and in an overexpression system in L. lactis (data not shown). These results are in agreement with previous data showing that the AcmA N-terminal domain (10) or the FlgJ C-terminal domain (38) without amino acid repeats retains enzymatic activity. In addition, we constructed an active fusion protein between L. citreum Mur and the AcmA C-terminal domain containing amino acid repeats. The resulting chimeric protein was able to play the role of AcmA in cell separation after cell division in the L. lactis acmA deletion mutant, thus indicating that L. citreum Mur is also functional on the cell wall in vivo.
Nisin-inducible expression of the mur-cA fusion gene was not obtained in the MG1363acmAΔ1 deletion mutant, in which nisRK genes required for nisin-mediated signal transduction were plasmid carried. However, since inducible expression was observed in L. lactis NZ9000 with the nisRK genes integrated into its chromosome, this suggests that the problems encountered are most probably linked to the strain used, that is, the acmA deletion mutant. This observation could be due to a modification of the cell surface of the mutant devoid of AcmA, which could alter the interaction of nisin with the NisK sensor protein located in the cell cytoplasmic membrane.
No activity band migrating at the molecular mass expected for L. citreum Mur could be revealed in L. citreum 22R extract or in culture supernatant by renaturing SDS-PAGE either in cell extract or in culture supernatant. This is most probably due to a low expression level of the protein since the expression consensus sequences, putative promoter, and RBS sequences were identified upstream of the ORF encoding L. citreum Mur.
As discussed above, L. citreum Mur does not contain amino acid repeats involved in cell wall attachment. In the case of the S. thermophilus homolog, the protein was shown to be cell associated (26). The structural similarity between these proteins leads us to suggest that the Mur protein in L. citreum is thus also cell associated. Nevertheless, L. citreum Mur does not possess the characteristics described for surface proteins, such as an LPXTG motif (24) or a region rich in Pro-Gly and Ser-Thr (43). Other means of protein association with the cell wall are (i) via membrane association, i.e., by a transmembrane segment on the protein or indirectly by protein interactions with a membrane protein or (ii) via protein interactions with a cell wall component, such as teichoic acids or lipoteichoic acids (8, 29). It is worth noting that the PGH amino acid repeats have been proposed to direct the enzyme to the cell division site (4). The absence of these repeats could allow a more homogeneous distribution of the enzyme in the cell wall.
ACKNOWLEDGMENTS
We thank G. Buist for providing L. lactis MG1363acmAΔ and O. P. Kuipers for L. lactis NZ9000 and plasmids pNZ9520 and pNZ9530. We thank J. Bardowski and C. Husson-Kao for helpful discussions and O. Firmesse, C. Huard, J. Tremblay, J. Commissaire for technical assistance, and Christian Beauvallet for mass determination. We are very grateful to A. Gruss for critically reading the manuscript.
R.C. was recipient of a fellowship from the Turkish High Education Council.
REFERENCES
- 1.Altschul S F, Madden T L, Schaffer A, Zang J, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amann R I, Binder B J, Olson R J, Chisholm S W, Devereux R, Stahl D A. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl Environ Microbiol. 1990;56:1919–1925. doi: 10.1128/aem.56.6.1919-1925.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amann R I, Ludwig W, Schleifer K-H. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol Rev. 1995;59:143–159. doi: 10.1128/mr.59.1.143-169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baba T, Schneewind O. Targeting of muralytic enzymes to cell division site of gram-positive bacteria: repeat domains direct autolysin to the equatorial surface ring of Staphylococcus aureus. EMBO J. 1998;17:4639–4646. doi: 10.1093/emboj/17.16.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Béliveau C, Potvin C, Trudel J, Asselin A, Bellemare G. Cloning, sequencing, and expression in Escherichia coli of a Streptococcus faecalis autolysin. J Bacteriol. 1991;173:5619–5623. doi: 10.1128/jb.173.18.5619-5623.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birnboim H, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979;7:1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blattner F R, Plunkett G, Bloch C A, Perna N T, Burland V, Riley M, Collado-Vides J, Glasner F D, Rode C K, Mayhew G F, Gregor J, Davis N W, Kirkpatrick H A M, Goeden A, Rose D J, Mau B, Shao Y. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1474. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 8.Briese T, Hakenbeck R. Interaction of the pneumococcal amidase with lipoteichoic acid and choline. Eur J Biochem. 1985;146:417–427. doi: 10.1111/j.1432-1033.1985.tb08668.x. [DOI] [PubMed] [Google Scholar]
- 9.Buist G, Kok J, Leenhouts K J, Dabrowska M, Venema G, Haandrikman A J. Molecular cloning and nucleotide sequence of the gene encoding the major peptidoglycan hydrolase of Lactococcus lactis, a muramidase needed for cell separation. J Bacteriol. 1995;177:1554–1563. doi: 10.1128/jb.177.6.1554-1563.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buist G, Karsens H, Nauta A, van Sinderen D, Venema G, Kok J. Autolysis of Lactococcus lactis caused by overproduction of its major autolysin. Appl Environ Microbiol. 1997;63:2722–2728. doi: 10.1128/aem.63.7.2722-2728.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buist G. Ph.D. thesis. Groningen, The Netherlands: University of Groningen; 1997. [Google Scholar]
- 12.Chapot-Chartier M-P, Deniel C, Rousseau M, Vassal L, Gripon J C. Autolysis of two strains of Lactococcus lactis during cheese ripening. Int Dairy J. 1994;4:251–269. [Google Scholar]
- 13.Chapot-Chartier M-P. Les autolysines des bactéries lactiques. Lait. 1996;76:91–109. [Google Scholar]
- 14.Chu C-P, Kariyama R, Daneo-Moore L, Shockman G D. Cloning and sequence analysis of the muramidase-2 gene from Enterococcus hirae. J Bacteriol. 1992;174:1619–1625. doi: 10.1128/jb.174.5.1619-1625.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cibik R, Chapot-Chartier M-P. Autolysis of dairy leuconostocs and detection of peptidoglycan hydrolases by renaturing SDS-PAGE. J Appl Microbiol. 2000;89:1–9. doi: 10.1046/j.1365-2672.2000.01191.x. [DOI] [PubMed] [Google Scholar]
- 16.Cibik R, Lepage E, Tailliez P. Molecular diversity of Leuconostoc mesenteroides and Leuconostoc citreum isolated from traditional French cheeses as revealed by RAPD fingerprinting, 16S rDNA sequencing and 16rDNA fragment amplification. Syst Appl Microbiol. 2000;23:267–278. doi: 10.1016/s0723-2020(00)80014-4. [DOI] [PubMed] [Google Scholar]
- 17.Cogan T M, Jordan K N. Metabolism of Leuconostoc bacteria. J Dairy Sci. 1994;77:2704–2717. [Google Scholar]
- 18.Crow V L, Coolbear P K, Gopal P K, Martley F G, McKay L L, Riepe H. The role of autolysis of lactic acid bacteria in ripening of cheese. Int Dairy J. 1995;5:855–875. [Google Scholar]
- 19.de los Reyes-Gavilan C G, Limsowtin G K Y, Tailliez P, Séchaud L, Accolas J-P. A Lactobacillus helveticus-specific DNA probe detects restriction fragment length polymorphism in this species. Appl Environ Microbiol. 1992;58:3429–3432. doi: 10.1128/aem.58.10.3429-3432.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Man J C, Rogosa M, Sharpe M E. A medium for the cultivation of lactobacilli. J Appl Bacteriol. 1960;23:130–135. [Google Scholar]
- 21.de Ruyter P G, Kuipers O P, de Vos W M. Controlled gene expression systems for Lactococcus lactis with the food-grade inducer nisin. Appl Environ Microbiol. 1996;62:3662–3667. doi: 10.1128/aem.62.10.3662-3667.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Díaz E, López R, García J L. Chimeric pneumococcal cell wall lytic enzymes reveal important physiological and evolutionary traits. J Biol Chem. 1991;266:5464–5471. [PubMed] [Google Scholar]
- 23.El-Shafei H, El-Soda M, Ezzat N. The peptide hydrolase system of the Leuconostoc. J Food Prot. 1990;53:165–169. doi: 10.4315/0362-028X-53.2.165. [DOI] [PubMed] [Google Scholar]
- 24.Fischetti V A, Pancholi V, Schneewind O. Conservation of a hexapeptide sequence in the anchor region of surface proteins from gram-positive cocci. Mol Microbiol. 1990;4:1603–1605. doi: 10.1111/j.1365-2958.1990.tb02072.x. [DOI] [PubMed] [Google Scholar]
- 25.Gibson T J. Ph.D. thesis. Cambridge, United Kingdom: Cambridge University; 1984. [Google Scholar]
- 26.Husson-Kao C, Mengaud J, Benbadis L, Chapot-Chartier M-P. Mur1, a Streptococcus thermophilus peptidoglycan hydrolase devoid of a specific cell wall binding domain. FEMS Microbiol Lett. 2000;187:69–76. doi: 10.1111/j.1574-6968.2000.tb09139.x. [DOI] [PubMed] [Google Scholar]
- 27.Iordanescu I. Characterization of the Staphylococcus aureus chromosomal gene pcrA, identified by mutations affecting plasmid pT181 replication. Mol Gen Genet. 1993;241:185–192. doi: 10.1007/BF00280216. [DOI] [PubMed] [Google Scholar]
- 28.Jones C J, Homma M, Macnab R M. L-, P-, and M-ring proteins of the flagellar basal body of Salmonella typhimurium: gene sequences and deduced protein sequences. J Bacteriol. 1989;171:3890–3900. doi: 10.1128/jb.171.7.3890-3900.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jonquières R, Bierne H, Fiedler F, Gounon P, Cossart P. Interaction between InlB of Listeria monocytogenes and lipoteichoic acid: a novel mechanism of protein association at the surface of gram positive bacteria. Mol Microbiol. 1999;34:902–914. doi: 10.1046/j.1365-2958.1999.01652.x. [DOI] [PubMed] [Google Scholar]
- 30.Joris B, Englebert S, Chu C-P, Kariyama R, Daneo-Moore L, Shockman G D, Ghuysen J-M. Modular design of the Enterococcus hirae muramidase-2 and Streptococcus faecalis autolysin. FEMS Microbiol Lett. 1992;91:257–264. doi: 10.1016/0378-1097(92)90707-u. [DOI] [PubMed] [Google Scholar]
- 31.Kleerebezem M, Beerthuyzen M M, Vaughan E E, de Vos W M, Kuipers O P. Controlled gene expression systems for lactic acid bacteria: transferable nisin-inducible expression cassettes for Lactococcus, Leuconostoc, and Lactobacillus spp. Appl Environ Microbiol. 1997;63:4581–4584. doi: 10.1128/aem.63.11.4581-4584.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;277:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 33.Langella P, Le Loir Y, Ehrlich S D, Gruss A. Efficient plasmid mobilization by pIP501 in Lactococcus lactis subsp. lactis. J Bacteriol. 1993;175:5806–5813. doi: 10.1128/jb.175.18.5806-5813.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le Loir Y, Gruss A, Ehrlich S D, Langella P. Direct screening of recombinant gram-positive bacteria using the secreted staphylococcal nuclease as a reporter. J Bacteriol. 1994;176:5135–5139. doi: 10.1128/jb.176.16.5135-5139.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lepeuple A-S, Vassal L, Cesselin B, Delacroix A, Gripon J-C, Chapot-Chartier M-P. Involvement of a prophage in the lysis of Lactococcus lactis subsp. cremoris AM2 during cheese ripening. Int Dairy J. 1998;8:667–674. [Google Scholar]
- 36.Lepeuple A-S, van Gemert E, Chapot-Chartier M-P. Analysis of the bacteriolytic enzymes of the autolytic Lactococcus lactis subsp. cremoris strain AM2 by renaturing polyacrylamide gel electrophoresis: identification of a prophage-encoded enzyme. Appl Environ Microbiol. 1998;64:4142–4148. doi: 10.1128/aem.64.11.4142-4148.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lepeuple A-S. Ph.D. thesis. Paris, France: University Paris 7; 1998. [Google Scholar]
- 38.Nambu T, Minamino T, Macnab R M, Kutsukake K. Peptidoglycan-hydrolyzing activity of the FlgJ protein, essential for flagellar rod formation in Salmonella typhimurium. J Bacteriol. 1999;181:1555–1561. doi: 10.1128/jb.181.5.1555-1561.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nielsen H, Engelbrecht J, Brunak S, von Heijne G. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 1997;10:1–6. doi: 10.1093/protein/10.1.1. [DOI] [PubMed] [Google Scholar]
- 40.Østlie H M, Vegarud G, Langsrud T. Autolysis of lactococci: detection of lytic enzymes by polyacrylamide gel electrophoresis and characterization in buffer systems. Appl Environ Microbiol. 1995;61:3598–3603. doi: 10.1128/aem.61.10.3598-3603.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petit M-A, Dervyn E, Rose M, Entian K D, McGovern S, Ehrlich S D, Bruand C. PcrA is an essential DNA helicase of Bacillus subtilis fulfilling functions both in repair and rolling-circle replication. Mol Microbiol. 1998;29:261–273. doi: 10.1046/j.1365-2958.1998.00927.x. [DOI] [PubMed] [Google Scholar]
- 42.Potvin C, Leclerc D, Tremblay G, Asselin A, Bellemare G. Cloning, sequencing and expression of a Bacillus bacteriolytic enzyme in Escherichia coli. Mol Gen Genet. 1988;314:241–248. doi: 10.1007/BF00337717. [DOI] [PubMed] [Google Scholar]
- 43.Rathsam C, Jacques N A. Role of the C-terminal domains in surface attachment of the fructosyltransferase of Streptococcus salivarius ATCC 25975. J Bacteriol. 1998;180:6400–6413. doi: 10.1128/jb.180.23.6400-6403.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Riepe H R, Pillidge C J, Gopal P K, McKay L L. Characterization of the highly autolytic Lactococcus lactis subsp. cremoris strains CO and 2250. Appl Environ Microbiol. 1997;63:3757–3763. doi: 10.1128/aem.63.10.3757-3763.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 46.Shockman G D, Höltje J-V. Microbial peptidoglycan (murein) hydrolases. In: Ghuysen J-M, Hackenbeck R, editors. Bacterial cell wall. New York, N.Y: Elsevier; 1994. pp. 131–166. [Google Scholar]
- 47.Silver J. Inverse polymerase chain reaction. In: McPherson M J, Quirke P, Taylor G R, editors. PCR: a practical approach. Oxford, United Kingdom: Oxford University Press; 1991. pp. 137–146. [Google Scholar]
- 48.Simon D, Chopin A. Construction of a plasmid family and its use for molecular cloning in Streptococcus lactis. Biochimie. 1988;70:559–566. doi: 10.1016/0300-9084(88)90093-4. [DOI] [PubMed] [Google Scholar]
- 49.Smith T J, Blackman S A, Foster S J. Autolysins of Bacillus subtilis: multiple enzymes with multiple functions. Microbiology. 2000;146:249–262. doi: 10.1099/00221287-146-2-249. [DOI] [PubMed] [Google Scholar]
- 50.Terzaghi B E, Sandine W E. Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol. 1975;29:807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Asseldonk M, Rutten G, Oteman M, Siezen R J, de Vos W M, Simons G. Cloning, expression in Escherichia coli and characterization of usp45, a gene encoding a highly secreted protein from Lactococcus lactis MG1363. Gene. 1990;95:155–160. doi: 10.1016/0378-1119(90)90428-t. [DOI] [PubMed] [Google Scholar]
- 52.Wilkinson G M, Guinee T P, O'Callaghan D M, Fox P F. Autolysis and proteolysis in different strains of starter bacteria during cheddar cheese ripening. J Dairy Res. 1994;61:249–262. [Google Scholar]