Abstract
Purpose
Dehydroepiandrosterone sulfate (DHEAS) is observed to be decreased in sepsis and inflammatory conditions. In the present study, we assessed the levels of DHEAS and cortisol and the DHEAS/cortisol ratio and their association with inflammatory markers in patients with COVID-19.
Methods
The study recruited 76 RT-PCR-positive COVID-19-positive patients and 79 healthy controls. The blood samples were collected and were analyzed for cortisol and DHEAS.
Results
We observed decreased levels of DHEAS and DHEAS/cortisol ratio and increased levels of cortisol in cases when compared with controls. DHEAS and DHEAS/cortisol ratio showed a decreasing trend with the increase in disease severity.
Conclusion
The present study is the first of its kind comparing DHEAS levels and DHEAS/cortisol ratio in COVID-19 patients and control subjects. DHEAS, with its inhibitory effect on IL6 and activation of Tregs, may play a crucial role in immune defense mechanisms against COVID-19.
Supplementary Information
The online version contains supplementary material available at 10.1007/s42000-022-00382-x.
Keywords: COVID-19, DHEA, DHEAS, Cortisol
Introduction
Dehydroepiandrosterone sulfate (DHEAS) has been associated with inflammatory conditions in multiple studies. DHEAS was found to be decreased in severely ill patients, and the severity of inflammation was reported to be associated with a low DHEAS/cortisol ratio [1]. Irrespective of the outcome, severe sepsis patients were observed to have significantly decreased DHEAS/cortisol ratio [2]. Dehydroepiandrosterone (DHEA) blunts Th-2 immunological responses and the expression of various proinflammatory cytokines [3]. In viral infections, DHEAS plays a significant role in bringing about immune modulation. A decrease in DHEAS level aggravates the proinflammatory state and hampers the ability of the host to mount an immune defense response against infections. To our knowledge, no study has to date been undertaken to explore the role of DHEAS in coronavirus disease 2019 (COVID-19). The association between DHEAS, cortisol, and DHEAS/cortisol ratio and inflammatory markers in patients with COVID-19 will help to better understand the anti‐inflammatory role of DHEAS in COVID-19 patients. Hence, the study aimed to assess the levels of DHEAS and cortisol and the DHEAS/cortisol ratio and their association with inflammatory markers in patients with COVID-19.
Methods
This analytical cross-sectional study was conducted from April 2020 to December 2020 in the Department of Biochemistry, All India Institute of Medical Sciences, Jodhpur, India, after obtaining approval from Institutional Ethics Committee, and followed the principles of the Declaration of Helsinki. RT-PCR-positive COVID-19 patients who were admitted to our tertiary care center in Western Rajasthan were recruited for the study after informed consent. This being a pilot study, 76 COVID-19-positive patients, and 79 healthy controls were recruited. Control subjects were those with no apparent illness who accompanied the patients visiting the hospital. Blood samples were collected at the time of admission and were subjected to centrifugation, and serum was analyzed on Beckman AU680 Clinical Chemistry Analyzer (Beckman Coulter, Inc., Brea, CA, USA) for highly sensitive C-reactive protein (hs-CRP). The serum was analyzed on Siemens Advia Centaur immunoanalyzer for interleukin-6 (IL-6) and Diasorin XL for cortisol and DHEAS.
The severity of COVID-19 was assessed by the Guidelines of the Ministry of Health & Family Welfare, India (ICMR score). The severity of COVID-19 was graded as follows: uncomplicated illness, mild pneumonia, moderate pneumonia, and severe pneumonia [4]. The results were described as medians and inter-quartile range (quantitative variables) or by frequency and percentiles (qualitative variables). A chi-square test was used to compare non-continuous variables. The Wilcoxon rank-sum test was used to compare the levels of continuous variables, including DHEAS, cortisol, and DHEAS/cortisol ratio, between the two groups. Correlation between DHEAS, cortisol, and DHEAS/cortisol ratio and ferritin, IL-6, and CRP was evaluated by Spearman’s correlation coefficient as the parameters had non-parametric distribution. A p value of < 0.05 was considered statistically significant.
Results
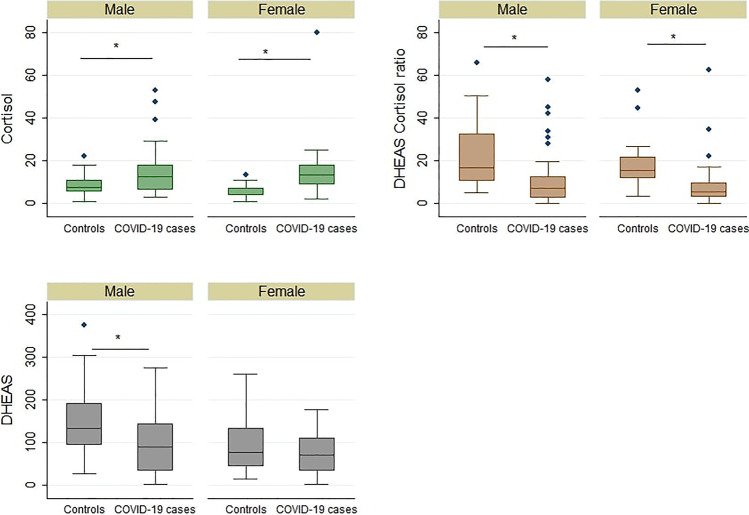
A total of 76 COVID-19 RT-PCR-positive patients as cases and 79 healthy individuals as controls were assessed. The epidemiological characteristics of the subjects recruited are listed in Table 1. DHEAS (p value: < 0.001) and DHEAS/cortisol ratio (p value: < 0.001) were found to be significantly decreased in cases when compared with controls. Cortisol was found to be significantly increased (p value: < 0.001) in cases when compared with controls (see Supplemental Data Table S1). The same trend was observed in the groups classified based on age (see Supplemental Data Table S2). In both males and females, we observed cortisol (p value (males): 0.0103; p value (females): < 0.001) and DHEAS/cortisol (p value (males): < 0.001; p value (females): < 0.001) to differ significantly in COVID-19 patients when compared with controls. However, DHEAS level, when compared with controls, was observed to differ significantly in male COVID-19 patients only (p value (males): 0.0021; p value (females): 0.4545) (Fig. 1). Cortisol levels were found to increase progressively with the severity of the disease. DHEAS and DHEAS/cortisol ratios showed a decreasing trend with the increase in severity (see Supplemental Data Fig. S1). The change in cortisol levels (p value: 0.0140) and DHEAS/cortisol ratio (p value: 0.0377) between the different grades of severity was found to be statistically significant, while a decreasing trend was observed in the case of DHEAS (p value: 0.3395) (Table 2). Comparing non-survivors with survivors, cortisol levels (p value: 0.0233) were significantly elevated in non-survivors. However, although the DHEAS and DHEAS/cortisol ratio showed a decreasing trend in non-survivors when compared with survivors, the trend was not statistically significant (see Supplemental Data Table S3). The correlation analysis of inflammatory parameters such as hsCRP and IL-6 revealed no significant correlation with cortisol and DHEAS levels and DHEAS/cortisol ratio in COVID patients (see Supplemental Data Table S4). ROC analysis of cortisol, DHEAS, and DHEAS/cortisol ratio between different grades of severity of the disease is presented in Supplemental Data Table S5 (see Supplemental Data Table S5).
Table 1.
Epidemiological characteristics of the subjects recruited
| Characteristics | COVID-19 cases median (IQR) | Controls median (IQR) | |
|---|---|---|---|
| Age | 48 (31–61.5) | 42 (34–46) | |
| Gender | Male | 40 (52.6%) | 54 (68.3%) |
| Female | 36 (47.3%) | 25 (31.6%) | |
| Comorbidities | Diabetes | 6 (7.8%) | - |
| Hypertension | 18 (23.6%) | - | |
| Coronary artery disease | 8 (10.5%) | - | |
| Hypothyroidism | 5 (6.5%) | - |
Fig. 1.
Box plots of gender-wise serum levels of cortisol, DHEAS, and DHEAS/cortisol ratio in cases and controls. * indicates p value < 0.05
Table 2.
Serum levels of cortisol, DHEAS, and DHEAS/cortisol ratio in patients with different ICMR severity scores
| Parameter | ICMR score | N | Median | IQR | p value |
|---|---|---|---|---|---|
| Cortisol | 0 | 35 | 9.52 | 6.3–14.98 | 0.0140 |
| 1 | 32 | 13.51 | 7.12–19.41 | ||
| 2 | 1 | 17.97 | |||
| 3 | 7 | 18.32 | 12.26–52.79 | ||
| DHEAS | 0 | 35 | 86.59 | 27.98–126 | 0.3395 |
| 1 | 32 | 94.335 | 55.72–132.15 | ||
| 2 | 1 | 46.41 | |||
| 3 | 8 | 46.62 | 14.54–92.25 | ||
| DHEAS/cortisol ratio | 0 | 35 | 7.233 | 2.64–12.50 | 0.0377 |
| 1 | 32 | 6.60 | 2.99–10.17 | ||
| 2 | 1 | 2.58 | |||
| 3 | 7 | 1.21 | 0.759–4.913 |
Statistical test used: Kruskal–Wallis test; p value < 0.05 considered statistically significant
DHEAS, dehydroepiandrosterone sulfate; ICMR, Indian Council of Medical Research
ICMR score 0, uncomplicated illness; ICMR score 1, mild pneumonia; ICMR score 2, moderate pneumonia; and ICMR score 3, severe pneumonia
Discussion
The zona reticularis of the adrenal cortex synthesizes DHEA from 17 α-hydroxypregnenolone and is sulfated to form DHEAS in the adrenals and peripheral tissues. The present study demonstrates that DHEAS and the DHEAS/cortisol ratio are significantly decreased in COVID-19 patients when compared with controls. COVID-19 pneumonia with ARDS is a hyperinflammatory state in which the proinflammatory cytokines, especially IL6, are elevated [5]. Serum levels of IL-6 in COVID-19 aid in assessing disease severity and predicting outcomes [2]. DHEA has been demonstrated to have an inhibitory effect on IL-6 secretion [2].
ACTH stimulates the zona reticularis of the adrenal cortex to produce dehydroepiandrosterone from 17 α-hydroxypregnenolone. DHEAS, the sulfated form of dehydroepiandrosterone, opposes the effects of cortisol by exerting immunostimulatory and antiglucocorticoid activity. As both DHEAS and cortisol are secreted from the adrenal cortex and have antagonistic actions, it has been proposed that their ratio more accurately reflects the degree of inflammatory response in critically ill patients when compared with isolated cortisol estimation [1]. The ratio was also shown to be an independent predictor for determining prognosis in septic patients [6]. DHEAS and DHEAS/cortisol ratios have been used in assessing prognoses in various diseases. For instance, the DHEAS/cortisol ratio has a prognostic role in cirrhotic patients with concurrent septic shock [1]. Moreover, patients in septic shock have demonstrated an extremely low level of DHEAS, and the cortisol/DHEAS ratio has been considered as a predictor of long-term mortality in septic patients [6]. Apart from the aforementioned associations, low DHEAS levels have also been observed to correlate with low CD4 T cell counts in HIV patients [7]. Interestingly, the latter low CD4 T cell counts did not show any correlation with cortisol levels in HIV patients. In the present study, we observed DHEAS to be decreased in COVID-19 patients when compared with controls. However, the derepression of IL-6 as a result of the decreased DHEAS and its interaction with the pathophysiology of COVID-19 warrant further investigation in order to better understand the role of DHEAS in COVID-19. Another interesting finding observed in the present study is the lack of any significant difference in DHEAS levels in female COVID-19 patients when compared with female controls. Comparing COVID-19 patients with controls, the magnitude of decrease in DHEAS levels was observed to be higher in males than in females. This accounts for the increased severity and mortality observed in male COVID-19 patients when compared with female patients [8].
Animal model studies have demonstrated the beneficial effects of DHEA supplementation in sepsis. In the latter studies, DHEA supplementation has been shown to decrease proinflammatory cytokines and increase Treg cells [9]. Furthermore, in vitro studies have demonstrated an additional benefit of DHEA, namely, in decreasing mechanical ventilation associated with cellular injuries [10]. In humans, DHEA supplementation has been assessed, with positive results, in asthma, in vitro fertilization, and schizophrenia [11]. However, few human studies have explored the effects of DHEA supplementation in sepsis. The decrease in DHEAS in COVID-19 patients opens up the possibility of exploring DHEA as adjuvant therapy in COVID-19, as previously illustrated by the authors [12].
To our knowledge, the present study is the first of its kind comparing DHEAS levels and the DHEAS/cortisol ratio in COVID-19 patients and control subjects. The absence of diurnal variation in DHEAS estimation makes it a better marker when compared with cortisol. DHEAS, due to its inhibitory effect on IL6 and activation of Tregs, may play a crucial role in immune defense mechanisms against COVID-19. Further studies are required in order to delineate the role of DHEAS in the immune response pathways in COVID-19, thereby opening up the possibility of repurposing DHEA for the treatment of COVID-19 pneumonia.
Supplementary Information
Below is the link to the electronic supplementary material.
Data availability
Not applicable.
Code availability
Not applicable.
Declarations
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The study was approved by the Bioethics Committee of the All India Institute of Medical Sciences, Jodhpur, India.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tsai MH, Huang HC, Peng YS, Chen YC, Tian YC, Yang CW, Lien JM, Fang JT, Wu CS, Hsieh SY, Lee FY. Dehydroepiandrosterone sulfate and dehydroepiandrosterone sulfate/cortisol ratio in cirrhotic patients with septic shock: another sign of hepatoadrenal syndrome? Crit Care. 2017;21(1):214. doi: 10.1186/s13054-017-1768-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marx C, Petros S, Bornstein SR, Weise M, Wendt M, Menschikowski M, Engelmann L, Höffken G. Adrenocortical hormones in survivors and nonsurvivors of severe sepsis: diverse time course of dehydroepiandrosterone, dehydroepiandrosterone-sulfate, and cortisol. Crit Care Med. 2003;31(5):1382–1388. doi: 10.1097/01.CCM.0000063282.83188.3D. [DOI] [PubMed] [Google Scholar]
- 3.Fernández RDV, Díaz A, Bongiovanni B, Gallucci G, Bértola D, Gardeñez W, Lioi S, Bertolin Y, Galliano R, Bay ML, Bottasso O, D'Attilio L. Evidence for a more disrupted immune-endocrine relation and cortisol immunologic influences in the context of tuberculosis and type 2 diabetes comorbidity. Front Endocrinol (Lausanne) 2020;11:126. doi: 10.3389/fendo.2020.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clinical management protocol: COVID-19. Ministry of health & family welfare, government of India directorate general of health services (EMR division), Available online at: https://www.mohfw.gov.in/pdf/ClinicalManagementProtocolforCOVID19.pdf (accessed February 14, 2022)
- 5.Liu F, Li L, Xu M, Wu J, Luo D, Zhu Y, Li B, Song X, Zhou X. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. 2020;127:104370. doi: 10.1016/j.jcv.2020.104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Castro R, Ruiz D, Lavín BA, Lamsfus JÁ, Vázquez L, Montalban C, Marcano G, Sarabia R, Paz-Zulueta M, Blanco C, Santibáñez M. Cortisol and adrenal androgens as independent predictors of mortality in septic patients. PLoS One. 2019;14(4):e0214312. doi: 10.1371/journal.pone.0214312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de la Torre B, von Krogh G, Svensson M, Holmberg V. Blood cortisol and dehydroepiandrosterone sulphate (DHEAS) levels and CD4 T cell counts in HIV infection. Clin Exp Rheumatol. 1997;15(1):87–90. [PubMed] [Google Scholar]
- 8.Jin JM, Bai P, He W, Wu F, Liu XF, Han DM, Liu S, Yang JK. Gender differences in patients with COVID-19: focus on severity and mortality. Front Public Health. 2020;8:152. doi: 10.3389/fpubh.2020.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Auci D, Kaler L, Subramanian S, Huang Y, Frincke J, Reading C, Offner H. A new orally bioavailable synthetic androstene inhibits collagen-induced arthritis in the mouse: androstene hormones as regulators of regulatory T cells. Ann N Y Acad Sci. 2007;1110:630–640. doi: 10.1196/annals.1423.066. [DOI] [PubMed] [Google Scholar]
- 10.Al-Ruweidi MKAA, Ali FH, Shurbaji S, Popelka A, Yalcin HC. Dexamethasone and transdehydroandrosterone significantly reduce pulmonary epithelial cell injuries associated with mechanical ventilation. J Appl Physiol. 2021;130(4):1143–1151. doi: 10.1152/japplphysiol.00574.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marozkina N, Zein J, DeBoer MD, Logan L, Veri L, Ross K, Gaston B. Dehydroepiandrosterone supplementation may benefit women with asthma who have low androgen levels: a pilot study. Pulm Ther. 2019;5(2):213–220. doi: 10.1007/s41030-019-00101-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tomo S, Banerjee M, Sharma P, Garg M. Does dehydroepiandrosterone sulfate have a role in COVID-19 prognosis and treatment? Endocr Regul. 2021;55(3):174–181. doi: 10.2478/enr-2021-0019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.
Not applicable.