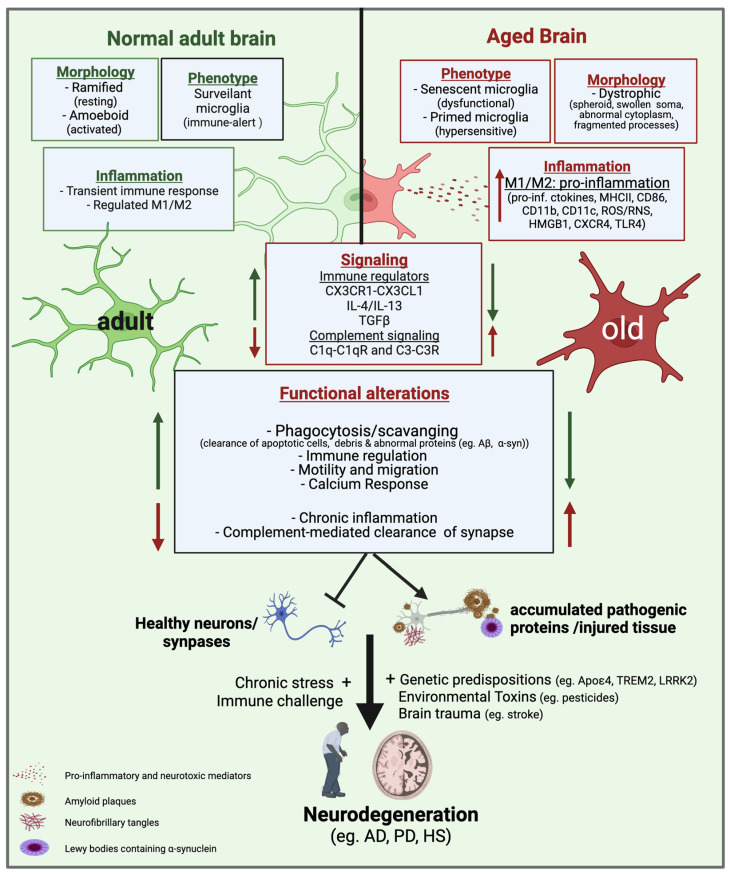
Figure 2.
Age-dependent microglial changes implicated in neurodegenerative diseases. During aging, microglia exhibit alterations in their morphology, phenotypes, inflammatory response, and overall functional response. Healthy adult microglia with normal branched processes can effectively survey the brain microenvironment, and upon encountering infections, injury, and inflammatory agents, they change morphology and execute effector functions by becoming transiently activated. In comparison, old microglia have a dystrophic morphology associated with cellular senescence and exhibit a primed phenotype in which they are hypersensitive to environmental changes and produce large amounts of M1-like pro-inflammatory and neurotoxic mediators which contribute to chronic neuroinflammation. Age-associated loss of endogenous microglial regulatory pathways, such as CX3CR1-CX3CL1 signaling and TGF-β signaling, impairs the ability of microglia to regulate inflammatory responses. Aging is also associated with a decline in calcium response, motility, and phagocytosis functions, all of which impair microglial clearance of injured cells, tissue debris, and pathogenic proteins. However, complement-mediated phagocytosis mechanisms are upregulated in aged microglia and contribute to the loss of healthy neurons. These age-related microglial dysfunctions, combined with a chronic stress environment, brain trauma, or genetic predisposing factors, can contribute to the progressive onset of neurodegenerative diseases. AD: Alzheimer’s disease; PD: Parkinson’s disease; HS: Hemorrhagic stroke. Figure created using BioRender.com (accessed on 14 April 2022).