Abstract
Batch culture experiments were performed with 32 different sulfate-reducing prokaryotes to explore the diversity in sulfur isotope fractionation during dissimilatory sulfate reduction by pure cultures. The selected strains reflect the phylogenetic and physiologic diversity of presently known sulfate reducers and cover a broad range of natural marine and freshwater habitats. Experimental conditions were designed to achieve optimum growth conditions with respect to electron donors, salinity, temperature, and pH. Under these optimized conditions, experimental fractionation factors ranged from 2.0 to 42.0‰. Salinity, incubation temperature, pH, and phylogeny had no systematic effect on the sulfur isotope fractionation. There was no correlation between isotope fractionation and sulfate reduction rate. The type of dissimilatory bisulfite reductase also had no effect on fractionation. Sulfate reducers that oxidized the carbon source completely to CO2 showed greater fractionations than sulfate reducers that released acetate as the final product of carbon oxidation. Different metabolic pathways and variable regulation of sulfate transport across the cell membrane all potentially affect isotope fractionation. Previous models that explained fractionation only in terms of sulfate reduction rates appear to be oversimplified. The species-specific physiology of each sulfate reducer thus needs to be taken into account to understand the regulation of sulfur isotope fractionation during dissimilatory sulfate reduction.
The stable sulfur isotope ratio between 32S and 34S of solid and dissolved sulfur compounds is widely used as a marker for bacterial sulfate reduction and bacterial processes associated with the recycling of sulfide (5, 8, 18). The reduction of sulfate by sulfate reducers is coupled to a pronounced enrichment of 32S in the produced sulfide. However, the extent of the isotope enrichment remains a matter of ongoing debate. Results from batch-culture, continuous-culture, and resting-cell experiments suggested that the isotope enrichment is inversely proportional to sulfate reduction rates (9, 22, 23). Furthermore, below a threshold concentration of sulfate, the discrimination against 34S apparently decreases (21). Previous experimental studies of the isotope fractionation were conducted with only a few selected species that were known at that time, mainly Desulfovibrio spp. and two Desulfotomaculum spp. (9, 15, 22, 28). Moreover, since most of these species were isolated from freshwater environments, they are not necessarily of ecological importance in marine environments.
The different electron donors used in these early pure-culture studies included ethanol, lactate, acetate, pyruvate, glucose, yeast extract, and hydrogen (22, 23). Today, a number of sulfate reducers are known that can metabolize a wide range of substrates including long-chain fatty acids, alcohols, and even aromatic compounds that represent relevant substrates for natural environments (33, 45, 47). Hydrogen, propionate, butyrate, and acetate appear to be the most important electron donors for sulfate reducers in natural marine environments (31, 40), but propionate and butyrate have never been used as electron donors in sulfur isotope fractionation experiments. There is a need to expand the existing database of sulfur isotope fractionations by sulfate reducers with organisms that are important in natural environments and to conduct experiments with additional relevant electron donors. For this reason, we included microorganisms that cover the total temperature range of environments from which sulfate reducers have been isolated. Furthermore, we used a variety of likely natural substrates and conducted experiments at different salinities and pHs to cover as broad a range of natural conditions as possible.
MATERIALS AND METHODS
Cultures, growth conditions, and sampling.
The investigated microorganisms (Table 1) were obtained from the German Collection of Microorganisms and Cell Cultures (Deutsche Sammlung von Mikroorganismen und Zellkulturen [DSMZ], Braunschweig, Germany) or are recently isolated strains (Desulfobacter sp. ASv20; Desulfovibrio sp. strain X). The environmental sources for all organisms are listed in Table 1. In order to ensure reproducible growth conditions, all strains were transferred into fresh medium two times before an experiment was started. Cells were grown in strictly anoxic, carbonate-buffered mineral medium containing a single carbon source (see Table 2) and sodium sulfate in concentrations between 15 and 28 mM (46). Anoxic conditions were maintained by the addition of a 1 M sodium sulfide solution to a final concentration of 1 mM. The electron acceptor was not limiting. Strain-specific additives to the media (vitamins, trace metals, fatty acids) were prepared as described elsewhere in detail for each culture (DSMZ; http://www.dsmz.de/species/bacteria.htm). Growth experiments were performed in screw-cap glass bottles (56-ml volume) without headspace. To avoid cracking of the culture bottles from expanding hot media, the thermophilic strains were incubated in 125-ml butyl rubber-stoppered glass bottles containing 50 ml of growth media. The headspace was completely replaced by N2-CO2 (80/20 [vol/vol]). A similar incubation procedure was necessary for hydrogen-oxidizing Desulfomicrobium autotrophicum. For this culture, the headspace was replaced by H2-CO2 (80/20 [vol/vol]) at 105 Pa overpressure. The gas was replenished several times during the incubation. All strains were incubated in the dark without agitation. Bottles were shaken manually every second day for approximately 10 s to prevent biofilm formation.
TABLE 1.
List of investigated strains
| Microorganism | DSMZa | Nucleotideb | Incubation (°C) | NaCl (g/liter) |
|---|---|---|---|---|
| Archaeoglobus fulgidus strain Z | 4139 | Y00275 | 80 | 18 |
| “Desulfarculus baarsii” | 2075 | M34403 | 28 | 1 |
| Desulfobacca acetoxidans | 11109 | AF002671 | 37 | 1 |
| Desulfobacter sp. ASv20 | 20 | 20 | ||
| Desulfobacterium autotrophicum | 3382 | M34409 | 28 | 20 |
| Desulfobacula phenolica | 3384 | AJ237606 | 28 | 20 |
| Desulfobacula toluolica | 7467 | X70953 | 28 | 20 |
| “Desulfobotulus sapovorans” | 2055 | M34402 | 28 | 1 |
| Desulfobulbus elongatus | 2908 | X95180 | 28 | 1 |
| Desulfobulbus “marinus” | 2058 | M34411 | 28 | 20 |
| Desulfocella halophila | 11763 | AF022936 | 28 | 40 |
| Desulfococcus sp. | 8541 | 28 | 20 | |
| Desulfofrigus oceanense | 12341 | AF099064 | 9 | 20 |
| Desulfohalobium redbaense | 5692 | X99235 | 37 | 100 |
| Desulfomicrobium baculatum | 4028 | M37311 | 28 | 1 |
| Desulfonatronovibrio hydrogenovorans | 9292 | X99234 | 28 | 11 |
| Desulfonatronum lacustre | 10312 | Y14594 | 28 | 1 |
| Desulfonema magnum | 2077 | U45989 | 28 | 20 |
| Desulfosarcina variabilis | 2060 | M34407 | 28 | 14 |
| Desulfospira joergensenii | 10085 | X99637 | 28 | 20 |
| Desulfotalea arctica | 12342 | AF099061 | 20 | 20 |
| Desulfotalea psychrophila | 12343 | AF099062 | 9 | 20 |
| Desulfotignum balticum | 7044 | AF233370 | 28 | 20 |
| Desulfotomaculum geothermicum | 3669 | X80789 | 50 | 20 |
| Desulfotomaculum gibsoniae | 7213 | Y11576 | 28 | 1 |
| Desulfotomaculum thermocisternum | 10259 | U33455 | 60 | 21 |
| Desulfovibrio halophilus | 5663 | U48243 | 28 | 70 |
| “Desulfovibrio oxyclinae” | 11498 | U33316 | 28 | 70 |
| Desulfovibrio profundus | 11384 | U90726 | 28 | 20 |
| Desulfovibrio sp. strain X | 28 | 20 | ||
| Thermodesulfobacterium commune | 2178 | L10662 | 60 | 0 |
| Thermodesulfovibrio yellowstonii | 11347 | L14619 | 60 | 0 |
DSMZ strain number.
Nucleotide sequence accession number.
TABLE 2.
Cell-specific sulfate reduction rates and fractionation factors of investigated sulfate-reducing prokaryotes
| Microorganisma | Isolated from: | Electron donor (mM) | csSRR (fmol cell−1 day−1) | ɛ (‰) |
|---|---|---|---|---|
| Complete oxidizing | ||||
| Desulfonema magnum | Marine mud | Benzoate (3) | 5.9 | 42.0 |
| Desulfobacula phenolica | Marine mud | Benzoate (3) | 125.0 | 36.7 |
| Desulfobacterium autotrophicum | Marine mud | Butyrate (20) | 64.8 | 32.7 |
| Desulfobacula toluolica | Marine mud | Benzoate (3) | 40.3 | 28.5 |
| Desulfotomaculum gibsoniae | Freshwater mud | Butyrate (20) | 13.0 | 27.8 |
| Desulfospira joergensenii | Marine mud | Pyruvate (20) | 0.9 | 25.7 |
| “Desulfarculus baarsii” | Lake mud | Butyrate (20) | 11.5 | 23.2 |
| Desulfotignum balticum | Marine mud | Butyrate (20) | 4.2 | 23.1 |
| Desulfofrigus oceanense | Arctic sediment | Acetate (20) | 7.6 | 22.0 |
| Desulfobacter sp. ASv20 | Arctic sediment | Acetate (20) | 6.6 | 18.8 |
| Desulfobacca acetoxidans | Anaerobic sludge | Acetate (20) | 17.0 | 18.0 |
| Desulfococcus sp. | Marine mud | Pyruvate (20) | 41.8 | 16.1 |
| Desulfosarcina variabilis | Marine mud | Benzoate (3) | 11.1 | 15.0 |
| Incomplete oxidizing | ||||
| Desulfonatronum lacustre∗ | Alkaline lake mud | Ethanol (20) | 16.2 | 18.7 |
| Archaeoglobus fulgidus strain Z∗ | Submarine hot spring | Lactate (20) | 63.8 | 17.0 |
| Thermodesulfovibrio yellowstonii | Thermal vent water | Lactate (20) | 24.0 | 17.0 |
| “Desulfobotulus sapovorans” | Freshwater mud | Lactate (20) | 26.0 | 16.5 |
| Desulfotomaculum thermocisternum∗ | Oil reservoir | Lactate (20) | 310.0 | 15.0 |
| Desulfomicrobium baculatum | Manganese ore | Lactate (20) | 4.8 | 12.7 |
| Desulfotomaculum geothermicum∗ | Aquifer | Lactate (20) | 8.1 | 12.5 |
| Desulfohalobium redbaense | Saline sediment | Lactate (20) | 434.0 | 10.6 |
| Desulfocella halophila | Great salt lake | Pyruvate (20) | 10.2 | 8.1 |
| Desulfobulbus “marinus” | Marine mud | Propionate (20) | 28.9 | 6.8 |
| Desulfotalea arctica | Arctic sediment | Lactate (20) | 4.2 | 6.1 |
| Desulfobulbus elongatus | Digester | Propionate (20) | 35.3 | 5.5 |
| Desulfovibrio sp. strain X | Hydrothermal vent | Lactate (20) | 36.0 | 5.4 |
| Thermodesulfobacterium commune | Thermal spring | Lactate (20) | 45.4 | 5.0 |
| “Desulfovibrio oxyclinae” | Hypersaline mat | Lactate (20) | 69.1 | 4.5 |
| Desulfotalea psychrophila | Arctic sediment | Lactate (20) | 6.3 | 4.3 |
| Desulfovibrio profundus | Deep sea sediment | Lactate (20) | 17.0 | 4.1 |
| Desulfovibrio halophilus | Hypersaline microbial mat | Lactate (20) | 33.1 | 2.0 |
| Hydrogen and formate | ||||
| Desulfobacterium autotrophicum | Marine mud | H2 (105 Pa) | 34.8 | 14.0 |
| Desulfonatronovibrio hydrogenovorans | Lake mud | Formate (20) | 12.7 | 5.5 |
Under experimental conditions, strains followed by an asterisk are considered incomplete oxidizers.
For every experiment, a set of 10 bottles was inoculated with a culture grown to the mid-exponential growth phase. Measurements were made on individual culture bottles immediately after inoculation (T0) and in the early exponential (T1), mid-exponential (T2), late exponential (T3), and early stationary (T4) phases. At each time point, a screw-cap bottle was opened to withdraw aliquots of the cultures to determine the concentrations of dissolved sulfate, dissolved sulfide, δ34Ssulfate, δ34Ssulfide, and cell numbers. The aliquots were withdrawn in less than 30 s to minimize loss or oxidation of hydrogen sulfide. For the serum vials, aliquots for the determination of cell numbers and sulfate/sulfide concentrations were withdrawn with a syringe through the septum. The remaining volume was used for sulfur isotope analysis.
Determination of sulfate and sulfide.
A 50-μl aliquot of the culture was added to 300 μl of 20% zinc acetate to precipitate dissolved sulfide. This procedure guaranteed that loss or oxidation of dissolved sulfide was negligible. Sulfate was determined after further dilution by nonsuppressed anion chromatography and conductivity detection. The eluent was 1 mM isophthalic acid in 10% methanol adjusted to a pH of 4.7 with sodium tetraborate. The flow rate was 1 ml/min. Sulfide was determined spectrophotometrically by the methylene blue method (11).
csSRR.
A 500-μl aliquot of each culture was used for cell counting using an Axioplan phase-contrast microscope (Carl Zeiss, Jena, Germany) and a modified Neubauer grid (0.0025 mm2 by 0.02 mm). Cells were fixed in 2% glutaraldehyde and stained with 4′,6′-diamidino-2-phenylindole (32). Cell-specific sulfate reduction rates (csSRRs, in moles cell−1 day−1) were calculated for the exponential phase using the change in concentration of sulfate and cell number (cn) between time points (T1) and (T2) according to the following equation:
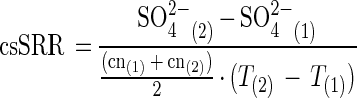 |
We prefer to use this measure of metabolic activity because it can be directly compared to the change in isotopic composition of sulfate during cell growth. The equation is not valid for the lag and stationary phases.
Determination of stable sulfur isotopes.
For the screw-cap bottles, 40 ml of the remaining culture was added to 10 ml of 20% zinc acetate to terminate microbial activity and to precipitate all dissolved sulfide. For the butyl rubber-stoppered serum vials, 10 ml of 20% zinc acetate was directly added though the septum. Dissolved sulfate and precipitated zinc sulfide were separated by filtration through 0.45-μm-pore-size Millipore filters. The filter was washed three times and the wash was added to the filtrate. Dissolved sulfate was precipitated as BaSO4 with 1 M BaCl2 at pH 4.0. For sulfur isotope determination, 300 to 400 μg of BaSO4 was weighed into tin cups that contained a 10-fold excess of V2O5. The isotopic composition of BaSO4 was determined by continuous-flow isotope-ratio-monitoring gas chromatography-mass spectrometry according to methods described elsewhere (16). The sulfur isotopic composition is expressed in the standard δ-notation given by δ34S = (Rsample/Rstandard − 1) · 1,000, where R = 34S/32S. Values are expressed on a per mille (‰) basis using the VCDT scale (37). The mean and standard deviation for the international reference standard NBS 127 (20.0‰) was 20.0‰ ± 0.3‰ versus VCDT.
Determination of isotope fractionation factors.
Microbial reduction of sulfate by the culture occurred in sealed serum vials without loss of product. These conditions are analogous to closed systems, allowing calculation of the isotope fractionation according to a Rayleigh fractionation model (27). Isotope fractionation factors (ɛ) were determined after non-linear regression to determine the function best reflecting the isotopic composition of dissolved sulfate (δ34S) at each time point (T0 to T4) on the basis of the isotopic composition of sulfate and the fraction of remaining sulfate (SO42−), according to the following equation: δ34ST1SO4 = −ɛ ln (SO42−) + δ34ST0(SO4)2−. In dual experiments, the standard deviation of ɛ usually was smaller than 1‰.
Comparative analysis of 16S rRNA sequences.
The sequences of the 16S rRNA genes were determined as described previously (29). Sequences that were not included in the 16S rRNA sequence database of the Technical University Munich in the program package ARB (41) were added from other databases. All sequences contained at least 1,200 bases. The tool ARB_ALIGN was used for sequence alignment. The alignment was checked visually and corrected manually. Tree topologies were evaluated by performing maximum parsimony, neighbor joining, and maximum likelihood analysis. Alignment positions at which less than 50% of the sequences of the entire data set shared the same residues were excluded from the calculations.
RESULTS
Variability of isotope fractionation.
All of the 32 sulfate-reducing bacteria discriminated against 34S during sulfate reduction. Desulfonema magnum showed the largest fractionation (ɛ = 42.0‰), and Desulfovibrio halophilus showed the smallest (ɛ = 2.0‰) (Table 2). Complete-oxidizing sulfate reducers fractionated sulfate between 15.0‰ (Desulfosarcina variabilis) and 42.0‰ (Desulfonema magnum), whereas the acetate-excreting incomplete oxidizers showed fractionations between 2.0‰ (Desulfovibrio halophilus) and 18.7‰ (Desulfonatrunum lacustre) (Table 2). The average isotope fractionation of the complete oxidizers (ɛ = 25‰) was more than 15‰ greater than that of the incomplete oxidizers (ɛ = 9.5‰). When the electron donor for Desulfobacterium autotrophicum was changed from butyrate to hydrogen, the fractionation decreased from 32.7 to 14.0‰. The oxidation of formate by Desulfonatronovibrio hydrogenovorans yielded a fractionation of 5.5‰.
Phylogeny.
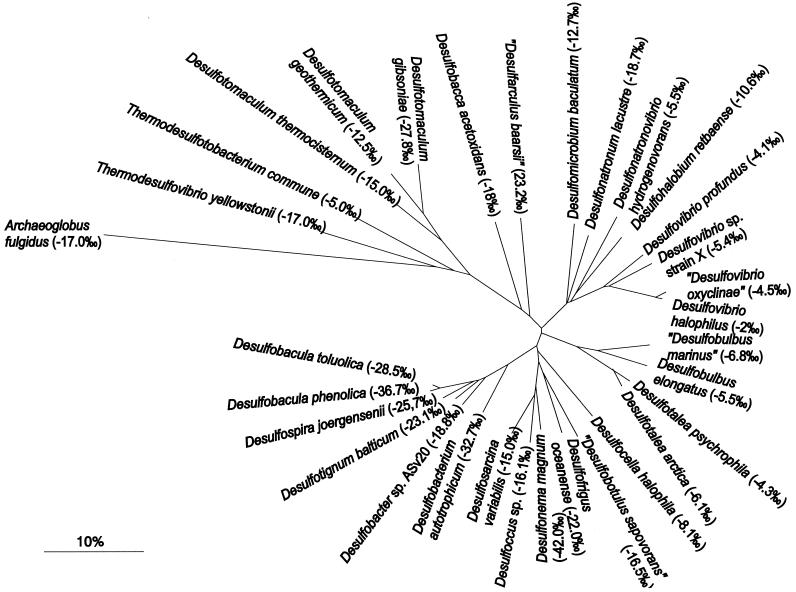
In order to cover the known phylogenetic diversity of sulfate reducers, we investigated Archaea (Archeaoglobus fulgidus), members of the deep-branching Thermodesulfovibrio (T. yellowstonii) and Nitrospira (Thermodesulfobacterium commune) subgroups, the low G+C subgroup (Desulfotomaculum spp.), and the δ subclass of the Proteobacteria (δ-Proteobacteria) (Fig. 1). Strains of all three orders of the δ-Proteobacteria with dissimilatory sulfate-reducing activity (Desulfovibrionales, Desulfobacterales, and Synthrophobacterales) were selected for isotopic characterization. However, there was no relationship between the fractionation and the phylogeny of the investigated strains (Fig. 1). For example, the distant species Desulfarculus baarsii and Desulfotignun balticum yielded similar fractionations of 23.2 and 23.1‰, whereas Desulfocella halophila (8.1‰), which is closely related to Desulfarculus baarsii, showed a very different fractionation. On the other hand, very closely related strains such as Desulfotalea arctica and Desulfotalea psychrophila fractionated similarly (4.6 and 6.5‰, respectively).
FIG. 1.
Phylogenetic affiliation and sulfur isotope fractionation factors of investigated sulfate-reducing microorganisms. Neighbor-joining tree based on 1,308 positions of nearly full-length 16S rRNA sequences from 30 bacteria. Archaeoglobus fulgidus was taken to root the tree. Trees constructed with other tree reconstruction algorithms (maximum likelihood and parsimony) resulted in general in the same overall tree topology. The bar indicates 10% sequence divergence.
Strain-specific factors.
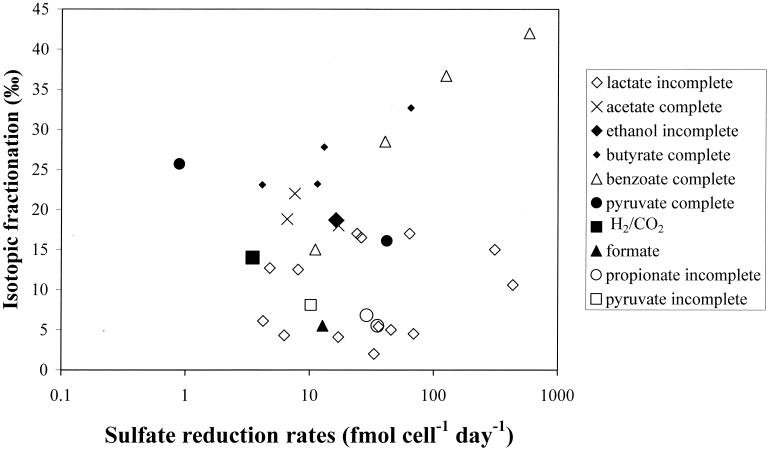
The sulfur isotope fractionation was independent of the sulfate reduction rates when the specific optimum growth conditions for each organism were used (Fig. 2). These rates can be considered as the maximum possible sulfate reduction rates for each organism under batch culture conditions. Nevertheless, the rates varied by more than 2 orders of magnitude. The scatter in Fig. 2 indicates that no uniform relationship exists between isotope fractionation and sulfate reduction rate that would be valid for all sulfate reducers. Furthermore, the lowest and highest rates measured, 0.9 fmol cell−1 day−1 for Desulfospira joergensenii and 4,340 fmol cell−1 day−1 for Desulfohalobium redbaense, yielded only intermediate fractionations of 25.7 and 10.6‰, respectively (Table 2). Conversely, despite similar sulfate reduction rates of 64.8 and 69.1 fmol cell−1 day−1 for Desulfobacterium autotrophicum and Desulfovibrio oxyclinae, the fractionations were very different, with values of 32.7‰ for Desulfobacterium autotrophicum when growing on butyrate and 4.5‰ for Desulfovibrio oxyclinae when growing on lactate.
FIG. 2.
Relationship between sulfate reduction rates in the mid-exponential growth phase (femtomoles of sulfate reduced per cell per day) and isotope fractionation. Each data point represents a different culture. The different substrates used are shown by the different symbols. Growth conditions were optimized for each culture so that the sulfate reduction rates were presumably close to the maximum potential rates for each organism.
We determined the isotope fractionation of 26 mesophilic sulfate reducers which had not been characterized previously with respect to their fractionation behavior. In addition, we also investigated psychrophilic sulfate reducers (Desulfofrigus oceanense, Desulfotalea psychrophila) and psychrotolerant (Desulfotalea arctica), thermophilic (Desulfotomaculum geothermicum, Desulfotomaculum thermocisternum, Thermodesulfobacterium commune, Thermodesulfovibrio yellowstonii), and hyperthermophilic (Archeaoglobus fulgidus) organisms. All organisms were incubated at or very close to their temperature optimum. A comparison of the incubation temperature and fractionation behavior also indicated no correlation (Tables 1 and 2).
The strains were isolated from freshwater, brackish, marine, and hypersaline environments (Table 1), and their pH optima are between 6.7 (Desulfotomaculum thermocisternum) (30) and 9.6 (Desulfonatronovibrio hydrogenovorans) (48). All strains were grown under their optimal conditions with respect to salinity and pH. Comparison with the isotope fractionation also indicated no systematic relationship. Other strain-specific characteristics such as cell size, capability for spore formation, or oxygen sensitivity also did not affect the isotope fractionation.
DISCUSSION
Diversity of isotope fractionation.
The overall range in sulfur isotope fractionation (ɛ = 2.0 to 42.0‰) for this diverse group of sulfate-reducing prokaryotes is very large and spans the full range of fractionations previously observed (4, 15, 22, 23, 28). In previous studies, very high fractionations (greater than 40‰) were obtained by growing cultures under physiologically stressed conditions, e.g., by determining fractionations with Desulfovibrio desulfuricans below the minimum temperature for growth (22). In contrast, our experimental conditions were optimized for each strain to permit a comparison of isotope fractionations.
There is no relationship between phylogenetic distance on the basis of 16S rRNA sequences and differences in isotope fractionation (Fig. 1). This is particularly apparent for the Archaeon Archaeoglobus fulgidus, whose isotope fractionation (ɛ = 17.0‰) was similar to that for a variety of incomplete-oxidizing sulfate reducers from the δ-Proteobacteria subgroup (Table 2). Thus, different isotope fractionation patterns do not reflect 16S rRNA-based phylogenetic relationships. Phylogenetic trees based on gene sequences that encode the dissimilatory bisulfite reductase (DSR) are not significantly different from 16S rRNA-based trees (44). Although the presently available data set is small, a comparison of DSR gene-based relative sequence dissimilarity with differences in isotope fractionation yields results similar to the 16S rRNA-based comparison.
Various attempts have been made to develop models for the sulfur isotope fractionation during dissimilatory sulfate reduction (10, 21, 22, 36), but none of these models take the physiological diversity of sulfate reducers into account. While it is clear that isotope fractionation most likely occurs when a sulfur-oxygen bond is broken, dissimilatory sulfate reduction proceeds in multiple steps (20). Isotope fractionation can occur at the adenosine phosphosulfate reductase (APSR) and the DSR (21, 22, 36), but too little is known about the structural differences between these enzymes among different sulfate reducers to assess their effect on isotope fractionation. It may be of interest, however, that the amino acid sequences important for gene function of the DSR are highly conserved (Bauer, personal communication). This may suggest a similarity in the structure of the reactive center and, possibly, similar isotope fractionation at these enzymes.
Sulfur isotope fractionation can also be modulated through a rate-limiting step that occurs either during sulfate uptake, at the APSR, or at the DSR (22, 23, 36). This rate-limiting step determines whether isotope fractionation can occur in successive reduction steps, but it may occur at different locations for the different sulfate reducers. Some sulfate reducers, in particular freshwater strains such as Desulfobulbus propionicus, have been shown to concentrate sulfate in their cells up to 2,500-fold (24). For these sulfate reducers, sulfate uptake is probably not the rate-limiting step. By contrast, marine species may not require a comparable preconcentration mechanism, and the regulation of sulfate uptake may take place by a different mechanism.
Complete versus incomplete electron donor oxidation are the only physiological characteristics that are consistently distinguished by sulfur isotope fractionation. In general, complete oxidizers fractionate more strongly (>15.0‰) than do incomplete oxidizers (<18.7‰). Only a small overlap exists between these two types. These results may be rationalized in terms of the energy conserved during electron donor oxidation for complete- and incomplete-oxidizing sulfate reducers (20, 47). In general, during incomplete oxidation of a substrate more energy is conserved per mole of sulfate reduced (Table 3). For example, the incomplete oxidation of lactate to acetate by sulfate yields more than three times as much energy as the complete oxidation of acetate to CO2 (−160.1 versus −47.6 kJ mol−1 sulfate). All incomplete-lactate-oxidizing sulfate reducers fractionated between 2.0 and 17.0‰, whereas all examined acetate-oxidizing species fractionated between 18.0 and 22.0‰. The underlying causes for the correlation between a thermodynamic property such as energy yield and a kinetic property such as isotope fractionation remain unclear. A possible explanation could be that for a reaction yielding more free energy the redox potential difference (ΔE0′) is also higher. In this case, the reaction equilibria for the partial reactions during sulfate reduction are shifted toward the product side and the potential for isotope discrimination between APS at the APSR and bisulfite at the DSR is minimized. A test of this hypothesis requires determination of the redox potential of each enzyme participating in the electron transport chain during dissimilatory sulfate reduction.
TABLE 3.
Free energy changes at standard state (ΔG0′) and corresponding range of isotope fractionations (ɛ) during dissimilatory sulfate reduction with various electron donors for complete and incomplete oxidation
| Electron donor (type of oxidation) | Stoichiometry | ΔG0′ [kJ mol−1 (SO4)2−]a | ɛ (‰) |
|---|---|---|---|
| Pyruvate (incomplete) | 4 CH3COCOO− + 4 H2O + SO42− → 4 CH3COO− + 4 HCO3− + HS− + 3 H+ | −340.9 | 8.1 |
| Lactate (incomplete) | 2 CH3CHOHCOO− + SO42− → 2 CH3COO− + 2 HCO3− + HS− + H+ | −160.1 | 2.0–17.0 |
| Hydrogen | 4 H2 + SO42− + H+ → 4 H2O + HS− | −152.2 | 14.0 |
| Formate | 4 HCOO− + SO42− + H+ → 4 HCO3− + HS− | −146.9 | 5.5 |
| Ethanol (incomplete) | 2 CH3CH2OH + SO42− → 2 CH3COO− + HS− + 2 H2O + H+ | −146.6 | 18.7 |
| Pyruvate (complete) | 4 CH3COCOO− + 4 H2O + 5 SO42− → 12 HCO3− + 5 HS− + 3 H+ | −106.3 | 16.1; 25.7 |
| Propionate (incomplete) | 4 CH3CH2COO− + 3 SO42− → 4 CH3COO− + 4 HCO3− + 3 HS− + H+ | −50.2 | 5.5; 6.8 |
| Benzoate (complete) | C7H5O2− + 3.75 SO42− + 4 H2O → 7 HCO3− + 3.75 HS− + 2.25 H+ | −49.7 | 15.0–42.0 |
| Butyrate (complete) | CH3CH2CH2COO− + 2.5 SO42− → 4 HCO3− + 2.5 HS− + 0.5 H+ | −49.2 | 23.1–32.7 |
| Acetate (complete) | CH3COO− + SO42− → 2HCO3− + HS− | −47.6 | 18.0–22.0 |
Electron donor effects on isotope fractionation were already suggested by Kaplan and Rittenberg (22), who observed an increasing fractionation for Desulfovibrio desulfuricans in the sequence of lactate, acetate, and ethanol and significantly lower fractionations for autotrophic growth on H2-CO2. In these studies, the increase in fractionation always coincided with a decrease in sulfate reduction rates. Therefore, these two authors postulated that changes in substrate affected isotope fractionation only insofar as they affected sulfate reduction rates. A correlation between sulfate reduction rates and fractionation is also supported by continuous culture experiments (9). Our data do not support a single relationship between the type of substrate, the sulfur isotope fractionation, and sulfate reduction rates because isotope fractionation was independent of the sulfate reduction rate (Fig. 2). The dependence of isotope fractionation on electron donor oxidation appears to be more important. Possibly, a correlation between sulfate reduction rate and isotope fractionation could be found if various substrates were tested for each organism. However, our data require a relationship between isotope fractionation and sulfate reduction rate that is characteristic for each organism. At the present time, we have sufficient information on the sulfate transport mechanisms, as well as the electron donor and electron acceptor pathways, for only a few sulfate reducers (12, 13, 20, 46, 47). This prevents us from relating the observed isotope fractionations to the specific physiology of each species. Further studies are required to understand sulfur isotope fractionation during dissimilatory sulfate reduction at the biochemical level.
Abundance of the investigated microorganisms in natural environments.
Most of the reported isotope fractionations by sulfate reducers published before this study were derived from experiments with Desulfovibrio spp. and Desulfotomaculum spp. (9, 15, 22, 23, 28). Although Desulfovibrio spp. were detected in marine sediments (1, 2, 38) and Desulfotomaculum spp. were encountered in aquifers (3, 14), these sulfate reducers are not abundant in many other environmental settings. Therefore, overall fractionations in sulfate-reducing environments may often be influenced by organisms other than Desulfovibrio spp. and Desulfotomaculum spp. Furthermore, all of the previously investigated strains were incomplete-oxidizing sulfate reducers. In some marine sediments, however, complete-oxidizing species represent more than 70% of the identifiable sulfate reducers (34). Some of the sulfate reducers investigated here are of quantitative importance in their natural habitats. For example, Desulfococcus spp. and Desulfotalea spp. were the most abundant sulfate reducers in marine arctic sediments (34, 35). In near-shore sediments of the Wadden Sea in northern Germany and in hypersaline mats, Desulfonema spp. accounted for an important fraction of the total bacterial biomass (26, 42). Desulfobulbus spp. were the most abundant sulfate reducers in a freshwater lake (25).
Biogeochemical implications for interpretation of the sulfur cycle from isotope abundances.
The present study is the first one to demonstrate that sulfate-reducing prokaryotes can produce widely different sulfur isotope fractionations during sulfate reduction. However, not the phylogenetic differences between the organisms but the physiological differences appear to be decisive for the isotope fractionation. Natural environments commonly contain a mixture of sulfate-reducing prokaryotes (26, 34). Thus, the characteristic community in a particular marine habitat can affect the isotope fractionations during bacterial sulfate reduction. Which sulfate reducers are present in a particular environment and which specific substrates are utilized thus become relevant controlling parameters for the isotope fractionation. There is no general agreement about the dominant substrates for sulfate reducers in marine environments. Acetate is generally regarded as an important terminal substrate in marine environments, but hydrogen can be an important substrate in syntrophic bacterial communities (31). Since the composition of the organic matter varies from place to place it is likely that the anaerobic food chain and the microbial community of sulfate reducers in different habitats varies as well. There is now clear molecular genetic evidence for the presence of different sulfate-reducing communities in different marine habitats (26, 34, 35, 39). Consequently, the overall isotope fractionations by sulfate-reducing communities in different environments may vary because different sulfate reducers are present.
Isotopic differences between sulfate and sulfide in marine sediments and porewaters are generally much greater than the experimentally determined isotope fractionations for pure cultures (5, 6, 18). In the natural environment prokaryotes are generally limited by the availability of organic substrate (32). General substrate limitation may also increase the isotope fractionation. Furthermore, in the natural environment additional isotope effects exist in the oxidative part of the sedimentary sulfur cycle through disproportionation of thiosulfate, elemental sulfur, or sulfite (7, 17). Therefore, in addition to considering variations in the microbial community structure of sulfate reducers, interpretation of isotope signals preserved in sediments and porewaters also have to take into account isotope effects in the oxidative part of the sulfur cycle.
ACKNOWLEDGMENTS
We thank Birte Meyer and Peter Søhoft for their assistance in the lab, Marga Bauer for helpful discussions on structural similarities between DSR and APSR, and Natasha Staats for helpful comments on an earlier version of the manuscript. We also thank Jon Fong at Indiana University for his help with the sulfur isotope analysis.
Volker Brüchert, Jan Detmers, and Jan Kuever were supported by the Max-Planck-Society. Kirsten S. Habicht was supported by the Danish National Research Foundation and the Madam Curie Training Program of the EU.
Footnotes
This paper is publication no. 139 of the Priority Program 546 “Geochemical processes with long-term effects in anthropogenically-affected seepage and groundwater” by the Deutsche Forschungsgemeinschaft.
REFERENCES
- 1.Bale S J, Goodman K, Rochelle P A, Marchesi J R, Fry J C, Weightman A J, Parkes R J. Desulfovibrio profundis sp. nov., a novel barophilic sulfate-reducing bacterium from deep sediment layers in the Japan Sea. Int J Syst Bacteriol. 1997;47:515–521. doi: 10.1099/00207713-47-2-515. [DOI] [PubMed] [Google Scholar]
- 2.Barnes S P, Bradbrook S D, Cragg B A, Marchesi J R, Weightman A J, Fry J C, Parkes R J. Isolation of sulfate-reducing bacteria from deep sediment layers of the Pacific ocean. Geomicrobiol J. 1998;15:67–83. [Google Scholar]
- 3.Boivin-Jahns V, Ruimy R, Bianchi A, Daumas S, Christen R. Bacterial diversity in a deep-subsurface clay environment. Appl Environ Microbiol. 1996;62:3405–3412. doi: 10.1128/aem.62.9.3405-3412.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Böttcher M, Sievert S, Kuever J. Fractionation of sulfur isotopes during dissimilatory reduction of sulfate by a thermophilic gram-negative bacterium at 60°C. Arch Microbiol. 1999;172:125–128. doi: 10.1007/s002030050749. [DOI] [PubMed] [Google Scholar]
- 5.Brüchert V, Pratt L M. Stable sulfur isotopic evidence for historical changes of sulfur cycling in estuarine sediments from northern Florida. Aquat Geochem. 1999;5:249–268. [Google Scholar]
- 6.Brüchert V, Pérez M E, Lange C B. Coupled primary production, benthic formainiferal assemblage, and sulfur diagenesis in organic-rich sediments of the Benguela upwelling system. Mar Geol. 2000;163:27–40. [Google Scholar]
- 7.Canfield D E, Thamdrup B, Fleischer S. Isotope fractionation and sulfur metabolism by pure and enrichment cultures of elemental sulfur-disproportionating bacteria. Limnol Oceanogr. 1998;43:253–264. [Google Scholar]
- 8.Canfield D E, Thamdrup B T. The production of 34S-depleted sulfide during disproportionation of elemental sulfur. Science. 1994;266:1973–1975. doi: 10.1126/science.11540246. [DOI] [PubMed] [Google Scholar]
- 9.Chambers L A, Trudinger P A, Smith J W, Burns M S. Fractionation of sulfur isotopes by continuous cultures of Desulvovibrio desulfuricans. Can J Microbiol. 1975;21:1602–1607. doi: 10.1139/m75-234. [DOI] [PubMed] [Google Scholar]
- 10.Chambers L A, Trudinger P A. Microbiological fractionation of stable sulfur isotopes. Geomicrobiol J. 1979;1:249–293. [Google Scholar]
- 11.Cline J D. Spectrophotometric determination of hydrogen sulfide in natural waters. Limnol Oceanogr. 1969;14:454–459. [Google Scholar]
- 12.Cypionka H. Characterization of sulfate transport in Desulvovibrio desulfuricans. Arch Microbiol. 1989;152:237–243. doi: 10.1007/BF00409657. [DOI] [PubMed] [Google Scholar]
- 13.Cypionka H. Sulfate transport. Methods Enzymol. 1994;243:3–14. [Google Scholar]
- 14.Daumas S, Cord-Ruwisch R, Garcia J L. Desulfotomaculum geothermicum sp. nov., a thermophilic, fatty acid-degrading, sulfate-reducing bacterium isolated with H2 from geothermal ground water. Antonie Leeuwenhoek. 1988;54:165–178. doi: 10.1007/BF00419203. [DOI] [PubMed] [Google Scholar]
- 15.Fry B, Gest H, Hayes J M. 34S/32S fractionation in sulfur cycles catalyzed by anaerobic bacteria. Appl Environ Microbiol. 1988;54:250–256. doi: 10.1128/aem.54.1.250-256.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giesemann A, Jaeger H J, Norman A L, Krouse H R, Brand W A. On-line sulfur isotope determination using an elemental analyzer coupled to a mass spectrometer. Anal Chem. 1994;66:2816–2819. [Google Scholar]
- 17.Habicht K N, Canfield D E, Rethmeier J. Sulfur isotope fractionation during bacterial reduction and disproportionation of thiosulfate and sulfite. Geochim Cosmochim Acta. 1998;62:2585–2595. [Google Scholar]
- 18.Habicht K S, Canfield D E. Sulfur isotope fractionation during bacterial sulfate reduction in organic-rich sediments. Geochim Cosmochim Acta. 1997;61:5351–5361. doi: 10.1016/s0016-7037(97)00311-6. [DOI] [PubMed] [Google Scholar]
- 19.Hanselmann K W. Microbial energetics applied to waste repositories. Experientia. 1991;47:645–687. [Google Scholar]
- 20.Hansen T A. Metabolism of sulfate-reducing bacteria. Antonie Leeuwenhoek. 1994;66:165–185. doi: 10.1007/BF00871638. [DOI] [PubMed] [Google Scholar]
- 21.Harrison A G, Thode H G. Mechanism of the bacterial reduction of sulphate from isotope fractionation studies. Trans Faraday Soc. 1958;54:84–92. [Google Scholar]
- 22.Kaplan I R, Rittenberg S C. Microbiological fractionation of sulphur isotopes. J Gen Microbiol. 1964;34:195–212. doi: 10.1099/00221287-34-2-195. [DOI] [PubMed] [Google Scholar]
- 23.Kemp A L W, Thode H G. The mechanism of the bacterial reduction of sulphate and sulphite from isotope fractionation studies. Geochim Cosmochim Acta. 1968;32:71–91. [Google Scholar]
- 24.Kreke B, Cypionka H. Proton motive force in freshwater sulfate-reducing bacteria, and its role in sulfate accumulation in Desulfobulbus propionicus. Arch Microbiol. 1992;158:183–187. doi: 10.1007/BF00290814. [DOI] [PubMed] [Google Scholar]
- 25.Li J, Purdy K, Takii S, Hayashi H. Seasonal changes in ribosomal RNA of sulfate-reducing bacteria and sulfate-reducing activity in a fresh water lake sediment. FEMS Microbiol Ecol. 1999;28:31–39. [Google Scholar]
- 26.Llobet-Brossa E, Rosselló-Mora R, Amann R. Microbial community composition of Wadden Sea sediments as revealed by fluorescence in situ hybridization. Appl Environ Microbiol. 1998;64:2691–2696. doi: 10.1128/aem.64.7.2691-2696.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mariotti A, Germon J C, Hubert P, Kaiser P, Letolle R, Tardieux A, Tardieux P. Experimental determination of nitrogen kinetic isotope fractionation: some principles. Illustration for the denitrification and nitrification processes. Plant Soil. 1981;62:413–430. [Google Scholar]
- 28.McCready R G L. Sulphur isotope fractionation by Desulvovibrio and Desulfotomaculum species. Geochim Cosmochim Acta. 1975;39:1395–1401. [Google Scholar]
- 29.Muyzer G, Teske A, Wirsen C O, Jannasch H W. Phylogenetic relationships of Thiomicrospira species and their identification in deep-sea hydrothermal vent samples by denaturating gradient gel electrophoresis of 16S rRNA fragments. Arch Microbiol. 1995;164:165–172. doi: 10.1007/BF02529967. [DOI] [PubMed] [Google Scholar]
- 30.Nilsen K R, Torsvik T, Lien T. Desulfotomaculum thermocisternum sp. nov., a sulfate reducer isolated from a hot North Sea oil reservoir. Int J Syst Bacteriol. 1996;46:397–402. [Google Scholar]
- 31.Parkes R J, Gibson G R, Mueller-Harvey I, Buckingham W J, Herbert R A. Determination of the substrates for sulphate-reducing bacteria within marine and estuarine sediments with different rates of sulphate reduction. J Gen Microbiol. 1989;135:175–187. [Google Scholar]
- 32.Porter K G, Feig Y S. The use of DAPI for identifying and counting aquatic microflora. Limnol Oceanogr. 1980;25:943–948. [Google Scholar]
- 33.Rabus R, Widdel F. Anaerobic degradation of ethylbenzene and other aromatic hydrocarbons by new denitrifying bacteria. Arch Microbiol. 1995;163:96–103. doi: 10.1007/BF00381782. [DOI] [PubMed] [Google Scholar]
- 34.Ravenschlag K, Sahm K, Knoblauch C, Jørgensen B, Amann R. Community structure, cellular rRNA content and activity of sulfate-reducing bacteria in marine arctic sediments. Appl Environ Microbiol. 2000;66:3592–3602. doi: 10.1128/aem.66.8.3592-3602.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ravenschlag K, Sahm K, Pernthaler J, Amann R. High bacterial diversity in permanently cold sediments. Appl Environ Microbiol. 1999;65:3982–3989. doi: 10.1128/aem.65.9.3982-3989.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rees C E. A steady state model for sulphur isotope fractionation in bacterial reduction processes. Geochim Cosmochim Acta. 1973;37:1141–1162. [Google Scholar]
- 37.Robinson B W. Reference and intercomparison materials for stable isotopes of light elements. TECDOC-825. Vienna, Austria: International Atomic Energy Agency; 1995. [Google Scholar]
- 38.Sahm K, Knoblauch C, Amann R. Phylogenetic affiliation and quantification of psychrophilic sulfate-reducing isolates in marine arctic sediments. Appl Environ Microbiol. 1999;65:3976–3981. doi: 10.1128/aem.65.9.3976-3981.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sahm K, MacGregor B J, Jørgensen B B, Stahl D A. Sulphate reduction and vertical distribution of sulphate-reducing bacteria quantified by rRNA slot-blot hybridization in a coastal marine sediment. Environ Microbiol. 1999;1:65–74. doi: 10.1046/j.1462-2920.1999.00007.x. [DOI] [PubMed] [Google Scholar]
- 40.Sørensen J, Christensen D, Jørgensen B B. Volatile fatty acids and hydrogen as substrates for sulfate-reducing bacteria in anaerobic marine sediment. Appl Environ Microbiol. 1981;42:5–11. doi: 10.1128/aem.42.1.5-11.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strunk O, Gross O, Reichel B, May M, Hermann S, Stuckmann N, Nonhoff M, Lenke M, Ginhart A, Vilbig A, Ludwig T, Bode A, Schleifer K-H, Ludwig W. ARB: a software environment for sequence data. Munich, Germany: Department of Microbiology, Technische Universität München; 1999. [Google Scholar]
- 42.Teske A, Ramsing N B, Habicht K, Fukui M, Küver J, Jørgensen B B, Cohen Y. Sulfate-reducing bacteria and their activities in cyanobacterial mats of Solar Lake (Sinai, Egypt) Appl Environ Microbiol. 1998;64:2943–2951. doi: 10.1128/aem.64.8.2943-2951.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thauer R K, Jungermann K, Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977;41:100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wagner M, Roger A J, Flax J L, Brusseau G A, Stahl D A. Phylogeny of dissimilatory sulfite reductase supports an early origin of sulfate respiration. J Bacteriol. 1998;180:2975–2982. doi: 10.1128/jb.180.11.2975-2982.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Widdel F. Ph.D. thesis. Göttingen, Germany: University of Göttingen; 1980. [Google Scholar]
- 46.Widdel F, Bak F. Gram-negative mesophilic sulfate reducing bacteria. In: Balows A, Trüper H G, Dworkin M, Harder W, Schleifer K H, editors. The prokaryotes. 2nd ed. IV. New York, N.Y: Springer; 1992. pp. 3352–3378. [Google Scholar]
- 47.Widdel F, Hansen T A. The dissimilatory sulfate- and sulfur-reducing bacteria. In: Balows A, Trüper H G, Dworkin M, Harder W, Schleifer K H, editors. The prokaryotes. 2nd ed. I. New York, N.Y: Springer; 1992. pp. 583–624. [Google Scholar]
- 48.Zhilina T, Zavarsin G, Rainy F, Pikuta E, Osipov G, Kostrikina N. Desulfonatronovibrio hydrogenovorans gen. nov., sp nov., an alkaliphilic, sulfate-reducing bacterium. Int J Syst Bacteriol. 1997;47:144–149. doi: 10.1099/00207713-47-1-144. [DOI] [PubMed] [Google Scholar]