Abstract
KYNAs, a compound with endogenous neuroprotective functions and an indole that is a building block of many biologically active compounds, such as a variety of neurotransmitters, are reacted in a transformation building upon Mannich bases. The reaction yields triarylmethane derivatives containing two biologically potent skeletons, and it may contribute to the synthesis of new, specialised neuroprotective compounds. The synthesis has been investigated via two procedures and the results were compared to those of previous studies. A possible alternative reaction route through acid catalysis has been established.
Keywords: kynurenic acid, indole, Mannich base, Mannich reaction, triarylmethanes, bioconjugates
1. Introduction
Indole derivatives are widely distributed in nature and many of them display important biological activities; moreover, a vast number of natural and synthetic indoles have found applications as pharmaceuticals [1,2,3]. Therefore, the synthesis [4,5] and functionalization [6,7] of indoles have become hot topics of organic synthesis in recent decades [8,9] with a novel emphasis on bisindole derivatives. These compounds, be it symmetrical [10,11,12,13] or unsymmetrical compounds [14,15,16,17], have highly promising biological activities such as antitumor, arylhydrocarbon receptor modifying, and AChE inhibitor activity.
Because of their biological potential, DIMs have received much attention in organic synthesis, and several highly efficient methods have been developed for their production [18,19,20,21,22].
Another compound with biological relevance is kynurenic acid (KYNA). Among the important features of KYNA, it is known to be one of the few known endogenous excitatory amino acid receptor blockers with a broad spectrum of antagonistic properties in supraphysiological concentrations. It is well established that KYNA has high affinity toward N-methyl-D-aspartate (NMDA) receptors. Moreover, it has recently been disclosed that KYNA shows an even higher affinity towards the positive modulatory binding site at the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor [23].
Since KYNA is a neuroprotective agent that is able to prevent neuronal loss following excitotoxic, ischemia-induced, and infectious neuronal injuries [24,25], there has recently been increasing interest in the synthesis and pharmacological studies of KYNA derivatives. The substitutions of KYNA at positions 5–8 were achieved by starting from the corresponding aniline via the modified Conrad–Limpach method [26,27,28]. The hydroxy group at position 4 was transformed to ether [28,29,30] or amine functions [31,32,33], while the carboxylic function at position 2 was mostly modified into the corresponding esters [28,29,30] or amides [34,35,36,37,38,39].
Taking in consideration of previous works based on the Mannich bases of naphthol derivatives and the ortho-quinone methides (oQm) derived from them [40], an extension of the synthetic procedures applying a possible special oQm—derived from KYNA through its naphthol analogy and its Mannich bases [41]—was planned. As a base for these transformations, the syntheses of indole- and 2-naphthol-containing triarylmethane (TRAM) derivatives [42,43,44] were chosen. These TRAM derivatives could be synthesized through two similar pathways: (i) through the reaction of 2-naphthol and the Mannich base of indole or (ii) using the reaction of indole and the Mannich base of 2-naphthol. We hypothesized that similar reactions can be carried out in the case of KYNA as well, possibly yielding special bioconjugates.
2. Materials and Methods
1H and 13C-NMR spectra were recorded in DMSO-d6 and CDCl3 solutions in 5 mm tubes at room temperature (RT), on a Bruker DRX-500 spectrometer (Bruker Biospin, Karlsruhe, Baden Württemberg, Germany) at 500 (1H) and 125 (13C) MHz, with the deuterium signal of the solvent as the lock and TMS as internal standard (1H, 13C).
Melting points were determined on a Hinotek X-4 melting point apparatus. Merck Kieselgel 60F254 plates were used for TLC.
HRMS flow injection analysis was performed with Thermo Scientific Q Exactive Plus hybrid quadrupole-Orbitrap (Thermo Fisher Scientific, Waltham, MA, USA) mass spectrometer coupled to a Waters Acquity I-Class UPLC™ (Waters, Manchester, UK).
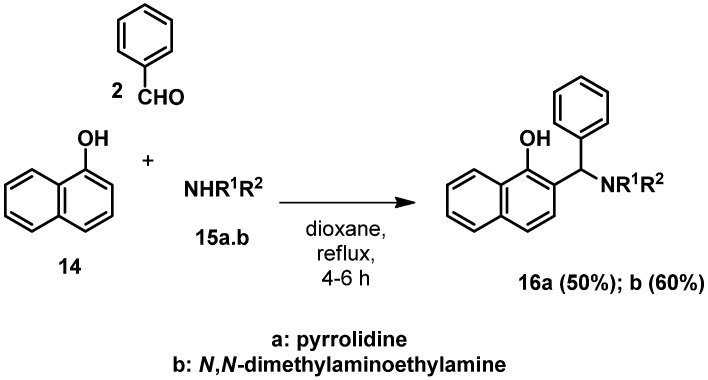
The synthesis of compounds 16a was carried out according to literature method [45].
2.1. General Procedure for the Synthesis of 7, 20a–h
-
(A)
4-Oxo-1,4-dihydroquinoline-2-carboxylic acid ethyl ester (6) or its derivatives (19a–h, 0.1 mmol) and 4 (0.15 mmol) were placed in a pressure-resistant 10 mL vessel with toluene (5 mL). The mixture was kept at 160 °C for 3 h in a CEM Discover SP microwave reactor (300 W). Work-up is similar to method B) but lower conversions and yields have been achieved with method A).
-
(B)
4-Oxo-1,4-dihydroquinoline-2-carboxylic acid ethyl ester (6) or its derivatives (19a–h, 0.5 mmol) and 4 (0.75 mmol) were placed in a 50 mL round-bottom flask. The mixture was heated at reflux temperature in toluene (25 mL) for 1.5–18 h. Work-up is described separately.
2.1.1. Ethyl 3-((1H-indol-3-yl)(phenyl)methyl)-4-oxo-1,4-dihydroquinoline-2-carboxylate (7)
Preparation according to general procedure, using 6; reflux time: 1.5 h. Work-up: crystals formed after cooling the reaction, filtered, and washed with 10 mL toluene. Yield: 131 mg (62%); M.p. 275–277 °C. HRMS calcd for [M + H+] m/z = 423.1708, found m/z = 423.1702; 1H NMR (DMSO-d6); 0.99 (3H, t, J = 7.2 Hz); 3.79–3,98 (2H, m); 6.11 (1H, s); 6.85–6.90 (2H, m); 7.03 (2H, s); 7.12–7.18 (2H, m); 7.73 (1H, t, J = 8.1 Hz); 7.22 (2H, t, J = 7.6 Hz); 7.26–7.30 (2H, m); 7.31–7.38 (2H, m); 7.61 (1H, d, J = 8.3 Hz); 7.68 (1H, t, J = 7.6 Hz); 8.08 (1H, d, J = 8.2 Hz); 10.80 (1H, s); 11.94 (1H, s); 13C NMR (DMSO-d6); 13.8; 62.5; 111.8; 115.5; 118.6; 118.8; 121.2; 122.1; 124.0; 124.7; 125.6; 125.7; 126.0; 127.8; 128.0; 129.2; 132.7; 136.5; 139.4; 140.5; 143.2; 163.8; 176.4; (Figures S1 and S2); FTIR in Figure S3.
2.1.2. Ethyl 3-((1H-indol-3-yl)(phenyl)methyl)-5-chloro-4-oxo-1,4-dihydroquinoline-2-carboxylate (20a)
Preparation according to general procedure, using 19a; reflux time: 8 h. Work-up: after evaporation of solvent purified by column chromatography (eluent = n-hexane:EtOAc 1:2), crystallized from 10 mL Et2O, filtered, and washed with 10 mL Et2O. Yield: 208 mg (91%); M.p. 245–247 °C. HRMS calcd for [M + H+] m/z = 457.1318, found m/z = 457.1315; 1H NMR (DMSO-d6); 0.95 (3H, t, J = 7.2 Hz); 3.71–3,80 (1H, m); 3.81–3,91 (1H, m); 6.10 (1H, s); 6.83 (1H, s); 6.89 (1H, t, J = 7.5 Hz); 7.04 (1H, t, J = 7.5 Hz); 7.12–7.19 (2H, m); 7.20–7.31 (5H, m); 7.35 (1H, d, J = 8.1 Hz); 7.53–7.59 (2H, m); 10.81 (1H, s); 11.92 (1H, s); 13C NMR (DMSO-d6); 13.7; 62.5; 111.8; 115.3; 118.3; 118.6; 118.9; 120.6; 121.3; 123.8; 125.8; 126.1; 126.5; 127.8; 128.0; 129.2; 132.4; 132.6; 136.5; 139.7; 142.0; 143.0; 163.4; 175.6; (Figures S4 and S5); FTIR in Figure S6.
2.1.3. Ethyl 3-((1H-indol-3-yl)(phenyl)methyl)-6-chloro-4-oxo-1,4-dihydroquinoline-2-carboxylate (20b)
Preparation according to general procedure, using 19b; reflux time: 8 h. Work-up: after evaporation of solvent purified by column chromatography (eluent = n-hexane:EtOAc 1:2), crystallized from 10 mL Et2O, filtered, and washed with 10 mL Et2O. Yield: 189 mg (83%); M.p. decomposition over 260 °C. HRMS calcd for [M + H+] m/z = 457.1318, found m/z = 457.1316; 1H NMR (DMSO-d6); 0.99 (3H, t, J = 7.2 Hz); 3.81–3,90 (1H, m); 3.92–4.00 (1H, m); 6.09 (1H, s); 6.85–6.91 (2H, m); 7.04 (1H, t, J = 7.5 Hz); 7.11–7.19 (2H, m); 7.22 (2H, t, J = 7.5 Hz); 7.27 (2H, d, J = 8.4 Hz); 7.34 (1H, d, J = 8.4 Hz); 7.66 (1H, d, J = 8.9 Hz); 7.73 (1H, d, J = 9.1 Hz); 8.01 (1H, s); 10.82 (1H, s); 12.15 (1H, s); 13C NMR (DMSO-d6); 13,8; 62.7; 111.8; 115.2; 118.6; 118.8; 121.3; 121.4; 122.6; 124.6; 125.6; 125.7; 126.1; 127.7; 128.0; 128.7; 129.1; 132.9; 136.5; 138.0; 140.6; 143.0; 163.6; 175.4; (Figures S7 and S8) FTIR in Figure S9.
2.1.4. Ethyl 3-((1H-indol-3-yl)(phenyl)methyl)-7-chloro-4-oxo-1,4-dihydroquinoline-2-carboxylate (20c)
Preparation according to general procedure, using 19c; reflux time: 8 h. Work-up: after evaporation of solvent purified by column chromatography (eluent = n-hexane:EtOAc 1:2), crystallized from 10 mL Et2O, filtered, and washed with 10 mL Et2O. Yield: 174 mg (76%); M.p. 262–264 °C. HRMS calcd for [M + H+] m/z = 457.1318, found m/z = 457.1314; 1H NMR (DMSO-d6); 1.00 (3H, t, J = 7.2 Hz); 3.81–4.01 (2H, m); 6.11 (1H, s); 6.85–6.91 (2H, m); 7.04 (1H, t, J = 7.5 Hz); 7.12–7.18 (2H, m); 7.22 (1H, t, J = 7.5 Hz); 7.27 (1H, d, J = 7.4 Hz); 7.32–7.38 (2H, m); 7.66 (1H, s); 8.07 (1H, d, J = 8.8 Hz); 10.82 (1H, s); 12.01 (1H, s); 13C NMR (DMSO-d6); 13.8; 39.0; 62.7; 111.8; 115.2; 117.9; 118.6; 118.8; 121.3; 123.1; 123.3; 124.4; 125.7; 126.1; 127.7; 128.0; 128.1; 129.1; 129.2; 136.5; 137.4; 140.1; 140.6; 143.0; 163.6; 176.0; (Figures S10 and S11) FTIR in Figure S12.
2.1.5. Ethyl 3-((1H-indol-3-yl)(phenyl)methyl)-8-chloro-4-oxo-1,4-dihydroquinoline-2-carboxylate (20d)
Preparation according to general procedure, using 19d; reflux time: 8 h. Work-up: after evaporation of solvent purified by column chromatography (eluent = n-hexane:EtOAc 1:2), crystallized from 10 mL Et2O, filtered, and washed with 10 mL Et2O. Yield: 87 mg (38%); M.p. 219–221 °C. HRMS calcd for [M + H+] m/z = 457.1318, found m/z = 457.1309; 1H NMR (DMSO-d6); 0.98 (3H, t, J = 7.5 Hz); 3.85–4.02 (2H, m); 5.93 (1H, s); 6.88 (1H, t, J = 7.0 Hz); 6.92 (1H, s); 7.04 (1H, t, J = 7.5 Hz); 7.12–7.19 (2H, m); 7.22 (1H, t, J = 7.5 Hz); 7.29 (1H, d, J = 7.6 Hz); 7.32–7.38 (2H, m); 7.88 (1H, d, J = 7.6 Hz); 8.07 (1H, d, J = 8.8 Hz); 10.82 (1H, s); 11.34 (1H, s); 13C NMR (DMSO-d6); 13.7; 62.6; 111.8; 111.9; 114.9; 118.6; 118.8; 121.2; 121.9; 122.3; 124.3; 125.2; 125.7; 126.2; 126.3; 127.7; 128.0; 129.1; 132.9; 136.0; 136.5; 141.6; 142.9; 163.1; 176.0; (Figures S13 and S14) FTIR in Figure S15.
2.1.6. Ethyl 3-((1H-indol-3-yl)(phenyl)methyl)-5-methoxy-4-oxo-1,4-dihydroquinoline-2-carboxylate (20e)
Preparation according to general procedure, using 19e; reflux time: 8 h. Work-up: after evaporation of solvent purified by column chromatography (eluent = n-hexane:EtOAc 1:2), crystallized from 10 mL Et2O, filtered, and washed with 10 mL Et2O. Yield: 185 mg (81%); M.p. 160–163 °C. HRMS calcd for [M + H+] m/z = 453.1814, found m/z = 453.1804; 1H NMR (CDCl3); 1.04 (3H, t, J = 7.1 Hz); 3.91–4.01 (2H, m); 4.03 (1H, s); 6.28 (1H, s); 6.83 (1H, d, J = 7.9 Hz); 6.97 (1H, s); 7.01 (1H, t, J = 7.3 Hz); 7.16 (1H, t, J = 7.5 Hz); 7.20 (1H, d, J = 7.3 Hz); 7.23–7.25 (1H, m); 7.30–7.39 (1H, m); 7.53 (1H, t, J = 8.3 Hz); 7.70 (1H, d, J = 8.5 Hz); 8.01 (1H, s); 10.21 (1H, s); 13C NMR (DMSO-d6); 13.7; 38.8; 56.0; 62.3; 104.5; 110.3; 111.8; 115.3; 115.6; 118.6; 118.8; 121.2; 123.6; 125.7; 125.9; 127.8; 127.9; 129.1; 133.0; 136.5; 138.8; 142.2; 143.4; 160.0; 163.7; 175.9; (Figures S16 and S17) FTIR in Figure S18.
2.1.7. Ethyl 3-((1H-indol-3-yl)(phenyl)methyl)-6-methoxy-4-oxo-1,4-dihydroquinoline-2-carboxylate (20f)
Preparation according to general procedure, using 19f; reflux time: 5 h. Work-up: after evaporation of solvent purified by column chromatography (eluent = DCM:EtOH 100:2.5), crystallized from 10 mL Et2O, filtered, and washed with 10 mL Et2O. Yield: 133 mg (58%); M.p. decomposition over 180 °C. HRMS calcd for [M + H+] m/z = 453.1814, found m/z = 453.1805; 1H NMR (DMSO-d6); 1.01 (3H, t, J = 7.1 Hz); 3.82 (1H, s); 3.85–4.03 (2H, m); 6.13 (1H, s); 6.87 (1H, t, J = 7.4 Hz); 6.90 (1H, s); 7.03 (1H, t, J = 7.3 Hz); 7.15 (1H, d, J = 7.9 Hz); 7.21 (1H, t, J = 7.7 Hz); 7.29 (1H, d, J = 7.9 Hz); 7.31–7.36 (2H, m); 7.48 (1H, s); 7.59 (1H, d, J = 9.1 Hz); 10.79 (1H, s); 11.94 (1H, s); 13C NMR (DMSO-d6); 13.8; 55.8; 62.5; 104.6; 111.8; 115.6; 118.6; 118.8; 120.7; 121.2; 123.5; 125.6; 125.9; 125.9; 127.8; 127.9; 129.1; 134.0; 136.5; 139.4; 143.4; 156.3; 164.0; 175.7; (Figures S19 and S20) FTIR in Figure S21.
2.1.8. Ethyl 3-((1H-indol-3-yl)(phenyl)methyl)-7-methoxy-4-oxo-1,4-dihydroquinoline-2-carboxylate (20g)
Preparation according to general procedure, using 19g; reflux time: 18 h. Work-up: after evaporation of solvent purified by column chromatography (eluent = n-hexane:EtOAc 1:2), crystallized from 10 mL Et2O, filtered, and washed with 10 mL Et2O. Yield: 217 mg (95%); M.p. decomposition over 260 °C. HRMS calcd for [M + H+] m/z = 453.1814, found m/z = 453.1807; 1H NMR (DMSO-d6); 1.00 (3H, t, J = 7.0 Hz); 3.81–3.99 (5H, m); 6.12 (1H, s); 6.85–6.90 (2H, m); 6.94 (1H, d, J = 8.9 Hz); 7.00 (1H, s); 7.03 (1H, t, J = 7.7 Hz); 7.11–7.17 (2H, m); 7.21 (1H, t, J = 7.6 Hz); 7.27 (1H, d, J = 7.8 Hz); 7.34 (1H, d, J = 8.1 Hz); 7.97 (1H, d, J = 9.0 Hz); 10.79 (1H, s); 11.70 (1H, s); 13C NMR (DMSO-d6); 13.8; 38.9; 56.0; 62.4; 99.3; 111.8; 114.4; 115.6; 118.7; 118.8; 119.2; 121.2; 122.2; 125.6; 126.0; 127.6; 127.8; 127.9; 129.1; 136.5; 139.9; 141.2; 143.4; 162.7; 163.8; 175.9; (Figures S22 and S23) FTIR in Figure S24.
2.1.9. Ethyl 3-((1H-indol-3-yl)(phenyl)methyl)-8-methoxy-4-oxo-1,4-dihydroquinoline-2-carboxylate (20h)
Preparation according to general procedure, using 19h; reflux time: 8 h. Work-up: after evaporation of solvent purified by column chromatography (eluent = n-hexane:EtOAc 1:2), crystallized from 10 mL Et2O, filtered, and washed with 10 mL Et2O. Yield: 23 mg (10%); M.p. 244–246 °C. HRMS calcd for [M + H+] m/z = 453.1814, found m/z = 453.1804; 1H NMR (DMSO-d6); 0.95 (3H, t, J = 7.1 Hz); 3.79–3.96 (2H, m); 3.99 (3H, s); 5.95 (1H, s); 6.84–6.91 (2H, m); 7.02 (1H, t, J = 7.3 Hz); 7.11–7.17 (2H, m); 7.21 (2H, t, J = 7.8 Hz); 7.24–7.32 (4H, m); 7.34 (1H, d, J = 8.1 Hz); 7.61–7.66 (1H, m); 10.79 (1H, s); 11.45 (1H, s); 13C NMR (DMSO-d6); 13.7; 56.7; 62.3; 111.8; 111.8; 115.3; 116.9; 118.6; 118.7; 121.2; 121.5; 123.7; 125.6; 125.7; 126.1; 127.8; 128.0; 129.2; 130.2; 136.5; 140.9; 143.2; 149.0; 163.5; 176.0; (Figures S25 and S26) FTIR in Figure S27.
2.2. Procedure for the Synthesis of 2-(((2-(dimethylamino)ethyl)amino)(phenyl)methyl)naphthalen-1-ol (16b)
1-Naphthol (14, 1.0 mmol) N,N-dimethylethane-1,2-diamine (1.0 mmol) and benzaldehyde (1.0 mmol) were placed in a 50 mL round-bottom flask. The mixture was heated at reflux temperature in toluene (25 mL) for 30 min. After the evaporation of the solvent the residue was purified using silica column chromatography with an eluent of n-hexane:MeOH:DCM 3:1:1 mixture collecting the last compound. The solvent was evaporated leaving behind a brownish yellow viscous liquid. Yield: 192 mg (60%). HRMS calcd for [M + H+] m/z = 321.1967, found m/z = 321.1957; 1H NMR (CDCl3); 2.20 (6H, s); 2.36–2.43 (1H, m); 2.57–2.67 (1H, m); 2.79–2.87 (2H, m); 5.01 (1H, s); 6.97 (1H, d, J = 8.3 Hz); 7.19–7.27 (2H, m); 7.27–7.35 (3H, m); 7.35–7.41 (2H, m); 7.41–7,49 (2H, m); 7.71 (1H, d, J = 7.3 Hz); 8.30 (1H, d, J = 8.2 Hz); 13C NMR (CDCl3); 45.3; 45.5; 58.1; 68.4; 117.1; 118.3; 122.4; 124.8; 125.6; 126.1; 126.9; 127.3; 127.5; 127.8; 128.9; 142.2; 153.6; (Figures S28 and S29) FTIR in Figure S30.
2.3. Procedures for the synthesis of 2-((1H-indol-3-yl)(phenyl)methyl)naphthalen-1-ol (18)
1-Naphthol (14, 0.5 mmol) and 4 (0.75 mmol) were placed in a 50 mL round-bottom flask. The mixture was heated at reflux temperature in toluene (25 mL) for 8 h. After the evaporation of the solvent, the residue was purified by column chromatography (eluent = n-hexane:EtOAc, 4:1). Crystallization from 10 mL Et2O, washed with 10 mL Et2O. Yield: 122 mg (70%); M.p. decomposition over 160 °C [46]. HRMS calcd for [M - H+] m/z = 348.1388, found m/z = 348.1385; 1H NMR (DMSO-d6); 6.36 (1H, s); 6.59 (1H, s); 6.79 (1H, d, J = 7.8 Hz); 6.86–6.93 (2H, m); 7.09 (1H, t, J = 7.6 Hz); 7.17 (1H, d, J = 7.5 Hz); 7.21–7.27 (1H, m); 7.28–7.36 (4H, m); 7.41 (1H, d, J = 8.1 Hz); 7.45–7,49 (2H, m); 8.09–8.14 (1H, m); 8.20–8.25 (1H, m); 10.01 (1H, brs); 10.87 (1H, brs); 13C NMR (DMSO-d6); 23.3; 43.9; 67.8; 107.7; 112.0; 118.7; 118.8; 119.4; 121.5; 123.0; 124.4; 124.5; 125.1; 125.5; 126.4; 126.5; 127.0; 127.0; 128.3; 128.6; 129.2; 130.4; 132.8; 137.2; 144.9; 152.3; (Figures S31 and S32) FTIR in Figure S33.
3. Results and Discussion
The work of Baruah et al. [42,43,44] includes both pathways of TRAM (3) synthesis starting either from indole-based Mannich products (Scheme 1, route i) or naphthol-/phenol-based Mannich products (route ii). Thus, the investigation of similar reaction routes is outlined for the ethyl ester of KYNA as well, starting from the Mannich base of indole (route iii) or the Mannich base of KYNA (route iv).
Scheme 1.
The synthesis of indole and 1-naphthol TRAMs (i) from indole-based Mannich products, (ii) naphthol-/phenol-based Mannich products and indole and KYNA TRAMs (iii) from the Mannich base of indole (iv) or the Mannich base of KYNA.
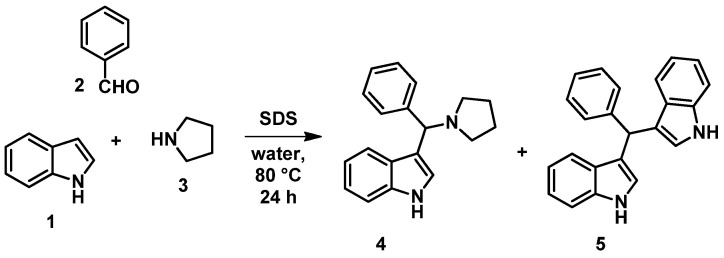
First, based on route (iii), a reaction between the Mannich base of indole and KYNA ethyl ester was planned. To synthesize the Mannich base of indole (4), several literature methods have been explored. Reactions utilizing catalysts, such as ferric phosphate [47] and iodine [48], or protic solvents, such as ethanol, methanol [49], and ethylene glycol [50], resulted mainly in bisindole derivative 5 mentioned in the corresponding literature as a byproduct. However, Mannich base 4 could be synthesized under neat conditions applying only indole, benzaldehyde, and pyrrolidine (with or without L-proline as catalyst [51,52]). Surprisingly, the highest yield was achieved by using the application of the surfactant sodium dodecyl sulfate (SDS, Scheme 2) [53].
Scheme 2.
The synthesis of aminoalkylated indole derivatives.
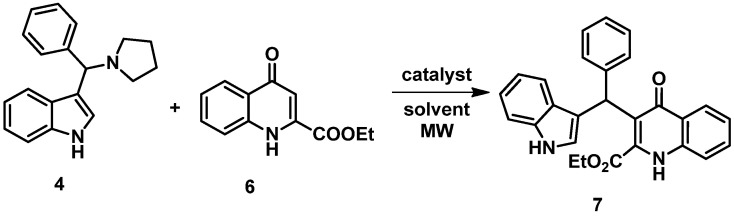
With the starting material in our hands, in the first C–C bond-forming reactions, 4 was reacted with the ethyl ester of KYNA (6) in MeCN at 100 °C (under MW conditions) with thiourea as the catalyst, based on the work of Baruah et al. [42] (Scheme 3). The conversion determined by NMR spectrometry was low; thus, the best conditions used for the alternative reaction route [43] were investigated, showing promising results (Table 1). Fortunately, the still low yield of 7 could be further improved by raising the reaction temperature to 160 °C. However, any further increase caused a decomposition of the starting materials.
Scheme 3.
Synthesis of KYNA TRAMs through indole-based Mannich products.
Table 1.
Optimization of the synthesis of 7.
|
Solvent | Catalyst | Temperature (°C) |
Time (min.) |
Conversion (%) a |
|---|---|---|---|---|---|
|
MeCN | thiourea | 100 | 10 | 5 |
|
toluene | pTsOH | 100 | 180 | 20 |
|
toluene | pTsOH | 130 | 180 | 60 |
|
toluene | pTsOH | 160 | 90 | 80 b |
|
toluene | - | 160 | 90 | 5 |
|
toluene | thiourea | 160 | 90 | 40 |
|
toluene | L-proline | 160 | 90 | 65 |
|
toluene | TEA | 160 | 90 | 70 |
|
1,2-dichlorobenzene | pTsOH | 160 | 90 | 0 |
|
MeCN | pTsOH | 160 | 90 | 10 |
|
anisole | pTsOH | 160 | 90 | 5 |
|
EtOH | pTsOH | 160 | 90 | 0 |
|
water | SDS | 160 | 60 | 0 |
a = determined from crude NMR spectra. b = work-up performed to isolate 12 (yield: 62%).
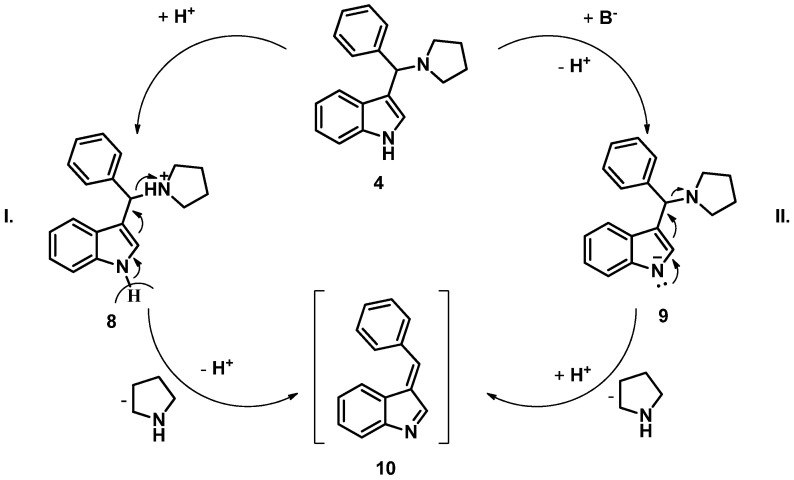
The use of a catalyst was crucial, as the desired TRAM did not form without the use of a base or an acid catalyst. It is interesting to mention that although acid catalysis resulted in somewhat higher conversion, both acid and base catalysis could enhance the synthesis of 7. Baruah et al. hypothesized that the reaction taking place between the indole derivative and varied electron-rich aromatic structures involves the formation of intermediate 10 [42,43]. In their proposed elimination–addition pathway starting from a Mannich base of indole, thiourea activates the amine moiety of the aminoalkyl function through double hydrogen bonding and converts it into a better leaving group (Scheme 4, I). Concerning triethylamine (TEA) used in our reactions, a hydrogen bond is unable to form; therefore, a more direct form of catalysis is proposed. Through the application of high temperature and TEA, the deprotonation of the indole moiety takes place followed by a subsequent rearrangement of the indole anion into benzylidene intermediate 10. Then, the latter is attacked by a molecule of the electron-rich KYNA yielding compound 7. It is also surmised that the C–N bond cleavage of the indole derivative could also take place through the elimination of pyrrolidine via the protonation of the amine moiety, making it a better leaving group and leading to intermediate 10 (Scheme 4, II).
Scheme 4.
Proposed mechanisms in the case of acid (I) or base (II) catalysis.
Further optimization of the reaction involved the change of solvent from the aprotic and apolar toluene to solvents representing a wider range of the aprotic–protic and apolar–polar scale (Table 1). It is hypothesized that toluene may be the best solvent because of the lack of H-bridge bonds and polarity of the solvent can contribute to a more unstable, and thus more reactive, intermediate [54].
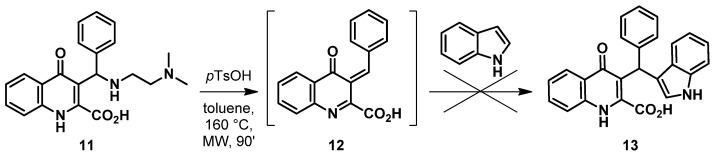
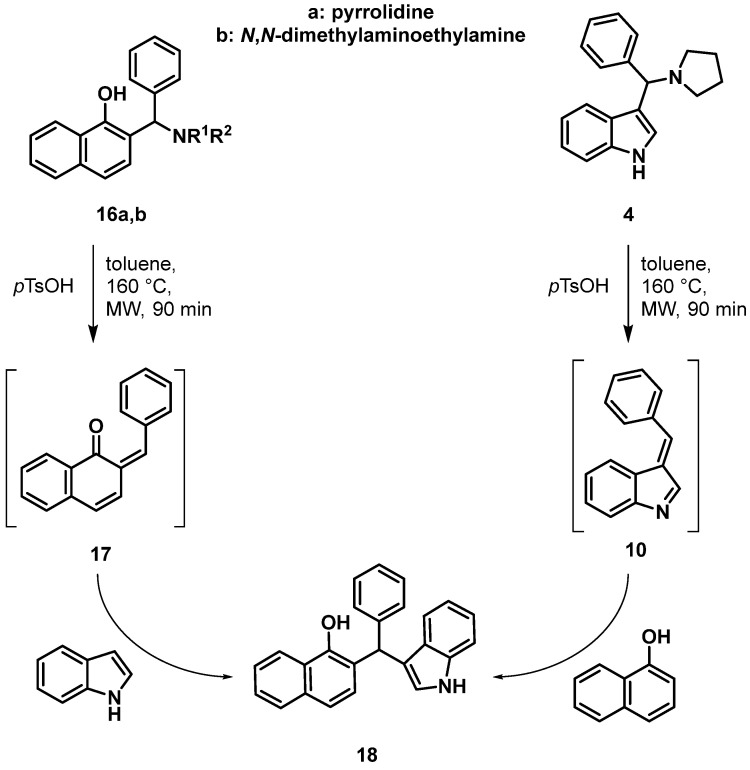
After successfully optimizing the reaction through route (iii), the synthesis of 7 was planned through the reaction of the KYNA Mannich base with indole (Scheme 1, route iv). KYNA Mannich derivatives synthesized previously are abundant [41]; however, compounds containing the crucial phenol structure were narrowed down only to a single compound (11, Scheme 5). Unfortunately, using this derivative in the reaction under conditions optimized previously did not result in the desired compound.
Scheme 5.
Synthesis of KYNA TRAM through aminoalkylated KYNA.
It is presumed that this may be due to the N,N-dimethylaminoethyl moiety being a bad leaving group. In order to fully support this hypothesis, a synthetic procedure was applied. Unfortunately, a Mannich base of KYNA containing a secondary amine function could not be synthesized, which is probably due to steric hindrance. Thus, considering the similarity of 1-naphthol to KYNA [41], the synthesis of Mannich bases 16a and 16b was carried out, as shown in Scheme 6.
Scheme 6.
Synthesis of aminoalkylated 1-naphthol derivatives 16a,b.
A comparison of the reaction of 1-naphthol with 6 and the reactions of 16a and 16b with indole (Scheme 7) allows us to arrive at two conclusions: (a) Mannich bases containing secondary amines (e.g., 11 and 16b) are less prone to undergo the transformation because of a bad leaving group character; (b) reactions through intermediate 10 are more preferable compared to reactions via possible ortho-quinone methide intermediates 12 and 17 derived either from 11 or from the Mannich bases of 1-naphthol (16a,b). This may be due to a possible hydrogen bridge between the hydroxy/oxo group in 16a,b and 11 in addition to the amine moiety, making the protonated form a more stable intermediate.
Scheme 7.
Comparison of the syntheses of 18. Conversions: starting from 16a 10%, 16b ~1 %, and 4 99%. Yield of 18 starting from 4: 70%.
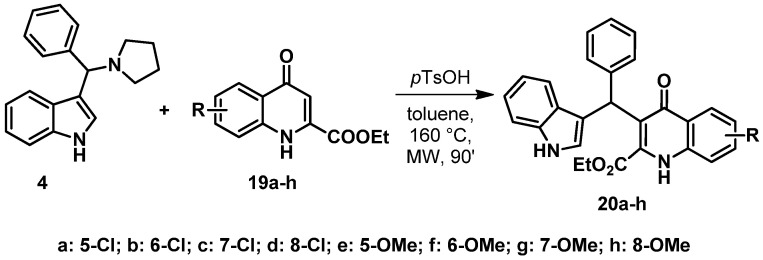
To further investigate the scope and limitations of the reaction, the synthesis of TRAMs containing different KYNA derivatives was planned. The reactions were carried out applying the optimized conditions (see Table 1, Entry #4) starting from KYNA derivatives 19a–h substituted at the B ring (Scheme 8). The reactions resulted in a diverse range of compounds (20a–h).
Scheme 8.
Synthesis of TRAMs 20a–h via aminoalkylated indole.
In the case of derivative 19e, the reaction applying microwaves as a heat source resulted in an exceptionally low conversion. To test whether a kinetic control takes place during the transformation, a longer reflux treatment was carried out. As the result with an almost full conversion was promising, reflux conditions were applied to the other derivatives as well, showing a general increase in conversions and supporting our hypothesis.
It is interesting to mention that the type of substituents on the B ring influenced the reactions to a lesser extent (e.g., Table 2, Entry #2, and #10) compared to the position of the substituents (e.g., Table 2, #10, and #16). Both chloro- and methoxy-KYNA derivatives, with substituents at C–5 and C–7, showed somewhat lower reactivity compared to the ethyl ester of KYNA (longer reaction times were needed). However, the same substituents in positions C–6 and C–8 caused a significant decrease in reactivity of the KYNA skeleton.
Table 2.
Comparison of the reactivity of substituted KYNA derivatives 19a–h.
|
Starting Material |
Temperature | Time (h) |
Conversion (%) a |
Yield (%) |
|---|---|---|---|---|---|
|
19a | 160 | 3 | 60 | 44 |
|
reflux | 8 | 99 | 91 | |
|
19b | 160 | 3 | 60 | 49 |
|
reflux | 8 | 90 | 83 | |
|
19c | 160 | 3 | 70 | 62 |
|
reflux | 8 | 85 | 76 | |
|
19d | 160 | 3 | 60 | 53 |
|
reflux | 8 | 50 | 38 | |
|
19e | 160 | 3 | 30 | 18 |
|
reflux | 8 | 90 | 81 | |
|
19f | 160 | 3 | 45 | 34 |
|
reflux | 8 | 70 | 58 | |
|
19g | 160 | 3 | 60 | 48 |
|
reflux | 5 | 99 | 95 | |
|
19h | 160 | 3 | 10 | 5 |
|
reflux | 18 | 20 | 10 |
a = determined from crude NMR spectra.
4. Conclusions
The synthesis of TRAM bioconjugates consisting of indole and the ethyl ester of kynurenic acid has been accomplished. The reactions take place through the cleavage of the C–N bond of indole Mannich base and subsequent C–C bond formation between the benzylidene intermediate and KYNA. To further investigate the scope and limitations of the reaction, KYNA derivatives bearing chloro and methoxy groups were also reacted yielding a wide variety of new TRAM KYNA derivatives with possible bioactivities. On the basis of acid and base catalysis, two possible catalytic pathways are hypothesized, both promoting the elimination of the amine moiety. To investigate the reactivity of ortho-quinone methides (oQm), an alternative reaction route via the Mannich bases of KYNA and its structural analogue 1-naphthol was also investigated. This showed surprisingly no conversion through the possible—and highly reactive—oQm intermediate. This could be contributed to the lack of elimination of the amine moiety, due to it having a bad leaving group character. Thus, upon comparison with the reactions applying the indole Mannich base, a prominent tilt toward the synthesis involving the benzylidene intermediate was observed.
Acknowledgments
The authors’ thanks are due to the Hungarian Research Foundation (OTKA No. K-138871) and the Ministry of Human Capacities, Hungary grant, TKP-2021-EGA-32, and to the Gedeon Richter Plc. Centenarial Foundation. The authors would also thank Rita Ambrus (Institute of Pharmaceutical Technology and Regulatory Affairs, University of Szeged) for the FTIR investigations.
Supplementary Materials
The 1H, 13C NMR spectra, FTIR spectra for all compounds and 2D NMR spectra for compound 7 as supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms23137152/s1.
Author Contributions
Conceptualization, I.S.; investigation, B.L. and P.S.; writing—original draft preparation, B.L. and P.S.; writing—review and editing, I.S. and B.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sundberg R.J. Indoles. Academic Press; London, UK: 1996. [Google Scholar]
- 2.Gribble G.W. Heterocyclic Chemistry II. Volume 26 Springer; Berlin, Germany: 2010. [Google Scholar]
- 3.Yang T., Lu H., Shu Y., Ou Y., Hong L., Au C.T., Qiu R. CF3SO2Na-Mediated, UV-Light-Induced Friedel–Crafts Alkylation of Indoles with Ketones/Aldehydes and Bioactivities of Products. Org. Lett. 2020;22:827–831. doi: 10.1021/acs.orglett.9b04272. [DOI] [PubMed] [Google Scholar]
- 4.Humphrey G.R., Kuethe J.T. Practical methodologies for the synthesis of indoles. Chem. Rev. 2006;106:2875–2911. doi: 10.1021/cr0505270. [DOI] [PubMed] [Google Scholar]
- 5.Cacchi S., Fabrizi G. Synthesis and Functionalization of Indoles Through Palladium-catalyzed Reactions. Chem. Rev. 2005;105:2873–2920. doi: 10.1021/cr040639b. [DOI] [PubMed] [Google Scholar]
- 6.Bartoli G., Bencivenni G., Dalpozzo R. Organocatalytic strategies for the asymmetric functionalization of indoles. Chem. Soc. Rev. 2010;39:4449–4465. doi: 10.1039/b923063g. [DOI] [PubMed] [Google Scholar]
- 7.Bandini M., Eichholzer A. Catalytic Functionalization of Indoles in a New Dimension. Angew. Chem. Int. Ed. 2009;48:9608–9644. doi: 10.1002/anie.200901843. [DOI] [PubMed] [Google Scholar]
- 8.Shiri M. Indoles in Multicomponent Processes (MCPs) Chem. Rev. 2012;112:3508–3549. doi: 10.1021/cr2003954. [DOI] [PubMed] [Google Scholar]
- 9.Szatmári I., Sas J., Fülöp F. Catalyst-free coupling of indole derivatives with 3,4-dihydroisoquinoline and related compounds. Tetrahedron Lett. 2013;54:5069–5071. doi: 10.1016/j.tetlet.2013.07.039. [DOI] [Google Scholar]
- 10.Takeda S., Yamamoto A., Okada T., Matsumura E., Nose E., Kogure K., Kojima S., Haga T. Identification of surrogate ligands for orphan G protein-coupled receptors. Life Sci. 2003;74:367–377. doi: 10.1016/j.lfs.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 11.Nikaido Y., Koyama Y., Yoshikawa Y., Furuya T., Takeda S. Mutation analysis and molecular modeling for the investigation of ligand-binding modes of GPR84. J. Biochem. 2015;157:311–320. doi: 10.1093/jb/mvu075. [DOI] [PubMed] [Google Scholar]
- 12.Fares F. The anti-carcinogenic effect of indole-3-carbinol and 3, 3′-diindolylmethane and their mechanism of action. Med. Chem. 2014;S1:1–8. doi: 10.4172/2161-0444.S1-002. [DOI] [Google Scholar]
- 13.Kiselev V.I., Drukh V.M., Muyzhnek E.L., Kuznetsov I.N., Pchelintseva O.I., Paltsev M.A. Preclinical antitumor activity of the diindolylmethane formulation in xenograft mouse model of prostate cancer. Exp. Oncol. 2014;36:90–93. [PubMed] [Google Scholar]
- 14.Pillaiyar T., Köse M., Sylvester K., Weighardt H., Thimm D., Borges G., Förster I., Kügelgen I., Müller C.E. Diindolylmethane Derivatives: Potent Agonists of the Immunostimulatory Orphan G Protein-Coupled Receptor GPR84. J. Med. Chem. 2017;60:3636–3655. doi: 10.1021/acs.jmedchem.6b01593. [DOI] [PubMed] [Google Scholar]
- 15.Safe S., Papineni S., Chintharlapalli S. Cancer chemotherapy with indole-3-carbinol, bis(3′-indolyl)methane and synthetic analogs. Cancer Lett. 2008;269:326–338. doi: 10.1016/j.canlet.2008.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kramer H.-J., Podobinska M., Bartsch A., Battmann A., Thoma W., Bernd A., Kummer W., Irlinger B., Steglich W., Mayser P. Malassezin, a novel agonist of the aryl hydrocarbon receptor from the yeast Malassezia furfur, induces apoptosis in primary human melanocytes. ChemBioChem. 2005;6:860–865. doi: 10.1002/cbic.200400247. [DOI] [PubMed] [Google Scholar]
- 17.Queiroz M.M.F., Queiroz E.F., Zeraik M.L., Ebrahimi S.N., Marcourt L., Cuendet M., Castro-Gamboa I., Hamburger M., Bolzani V.S., Wolfender J.-L. Chemical composition of the bark of Tetrapterys mucronata and identification of acetylcholinesterase inhibitory constituents. J. Nat. Prod. 2014;77:650–656. doi: 10.1021/np401003p. [DOI] [PubMed] [Google Scholar]
- 18.Liu X., Ma S., Toy P.H. Halogen Bond-Catalyzed Friedel-Crafts Reactions of Aldehydes and Ketones Using a Bidentate Halogen Bond Donor Catalyst: Synthesis of Symmetrical Bis-(indolyl)methanes. Org. Lett. 2019;21:9212–9216. doi: 10.1021/acs.orglett.9b03578. [DOI] [PubMed] [Google Scholar]
- 19.Pillaiyar T., Gorska E., Schnakenburg G., Müller C.E. General Synthesis of Unsymmetrical 3,3′-(Aza)diindolylmethane Derivatives. J. Org. Chem. 2018;83:9902–9913. doi: 10.1021/acs.joc.8b01349. [DOI] [PubMed] [Google Scholar]
- 20.Xiao J., Wen H., Wang L., Xu L.B., Hao Z.H., Shao C.-L., Wang C.-Y. Catalyst-free dehydrative SN1-type reaction of indolyl alcohols with diverse nucleophiles on water. Green Chem. 2016;18:1032–1037. doi: 10.1039/C5GC01838B. [DOI] [Google Scholar]
- 21.Huo C.D., Sun C.G., Wang C., Jia X.D., Chang W.J. Triphenylphosphine-m-sulfonate/Carbon Tetrabromide as an Efficient and Easily Recoverable Catalyst System for Friedel–Crafts Alkylation of Indoles with Carbonyl Compounds or Acetals. ACS Sustain. Chem. Eng. 2013;1:549–553. doi: 10.1021/sc400033t. [DOI] [Google Scholar]
- 22.Bayindir S., Saracoglu N. A facile one-pot method to synthesise 2-alkylated indole and 2,2′-bis(indolyl)methane derivatives using ketones as electrophiles and their anion sensing ability. RSC Adv. 2016;6:72959–72967. doi: 10.1039/C6RA16192H. [DOI] [Google Scholar]
- 23.Sas K., Robotka H., Toldi J., Vécsei L. Mitochondria, metabolic disturbances, oxidative stress and the kynurenine system, with focus on neurodegenerative disorders. J. Neurol. Sci. 2007;257:221–239. doi: 10.1016/j.jns.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 24.Gigler G., Szénási G., Simó A., Lévay G., Hársing L.G., Jr., Sas K., Vécsei L., Toldi J. Neuroprotective effect of L-kynurenine sulfate administered before focal cerebral ischemia in mice and global cerebral ischemia in gerbils. Eur. J. Pharmacol. 2007;564:116–122. doi: 10.1016/j.ejphar.2007.02.029. [DOI] [PubMed] [Google Scholar]
- 25.Luchowska E., Luchowski P., Sarnowska A., Wielosz M., Turski W.A., Urbańska E.M. Endogenous level of kynurenic acid and activities of kynurenine aminotransferases following transient global ischemia in the gerbil hippocampus. Pol. J. Pharmacol. 2003;55:443–447. [PubMed] [Google Scholar]
- 26.Harrison B.L., Baron B.M., Cousino D.M., McDonald I.A. 4-[(Carboxymethyl)oxy]- and 4-[(carboxymethyl)amino]-5,7-dichloroquinoline-2-carboxylic acid: New antagonists of the strychnine-insensitive glycine binding site on the N-methyl-D-aspartate receptor complex. J. Med. Chem. 1990;12:3130–3132. doi: 10.1021/jm00174a005. [DOI] [PubMed] [Google Scholar]
- 27.Edmont D., Rocher R., Plisson C., Chenault J. Synthesis and evaluation of quinoline carboxyguanidines as antidiabetic agents. Bioorg. Med. Chem. Lett. 2000;16:1831–1834. doi: 10.1016/S0960-894X(00)00354-1. [DOI] [PubMed] [Google Scholar]
- 28.Bonina F.P., Arenare L., Ippolito R., Boatto G., Battaglia G., Bruno V., de Caprariis P. Synthesis, pharmacokinetics and anticonvulsant activity of 7-chlorokynurenic acid prodrugs. Int. J. Pharm. 2000;202:79–88. doi: 10.1016/S0378-5173(00)00421-X. [DOI] [PubMed] [Google Scholar]
- 29.Manfredini S., Pavan B., Vertuani S., Scaglianti M., Compagnone D., Biondi C., Scatturin A., Tanganelli S., Ferraro L., Prasad P., et al. Design, synthesis and activity of ascorbic acid prodrugs of nipecotic, kynurenic and diclophenamic acids, liable to increase neurotropic activity. J. Med. Chem. 2002;45:559–562. doi: 10.1021/jm015556r. [DOI] [PubMed] [Google Scholar]
- 30.Manfredini S., Vertuani S., Pavan B., Vitali F., Scaglianti M., Bortolotti F., Biondi C., Scatturin A., Prasad P., Dalpiaz A. Design, synthesis and in vitro evaluation on HRPE cells of ascorbic and 6-bromoascorbic acid conjugates with neuroactive molecules. Bioorgan. Med. Chem. 2004;12:5453–5463. doi: 10.1016/j.bmc.2004.07.043. [DOI] [PubMed] [Google Scholar]
- 31.Stone T.W. Development and therapeutic potential of kynurenic acid and kynurenine derivatives for neuroprotection. Trends Pharmacol. Sci. 2000;21:149–154. doi: 10.1016/S0165-6147(00)01451-6. [DOI] [PubMed] [Google Scholar]
- 32.Stone T.W. Inhibitors of the kynurenine pathway. Eur. J. Med. Chem. 2000;35:179–186. doi: 10.1016/S0223-5234(00)00121-5. [DOI] [PubMed] [Google Scholar]
- 33.Nichols A.C., Yielding K.L. Anticonvulsant activity of 4-urea-5,7-dichlorokynurenic acid derivatives that are antagonists at the NMDA-associated glycine binding site. Mol. Chem. Neuropathol. 1998;35:1–12. doi: 10.1007/BF02815112. [DOI] [PubMed] [Google Scholar]
- 34.Füvesi J., Somlai C., Németh H., Varga H., Kis Z., Farkas T., Károly N., Dobszay M., Penke Z., Penke B., et al. Comparative study on the effects of kynurenic acid and glucosamine-kynurenic acid. Pharmacol. Biochem. Behav. 2004;77:95–102. doi: 10.1016/j.pbb.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 35.Zhang L., Sun F., Li Y., Sun X., Liu X., Huang Y., Zhang L.H., Ye X.S., Xiao J. Rapid synthesis of iminosugar derivatives for cell-based in situ screening: Discovery of “hit” compounds with anticancer activity. ChemMedChem. 2007;2:1594–1597. doi: 10.1002/cmdc.200700120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brik A., Lin Y.C., Elder J., Wong C.H. A quick diversity-oriented amide-forming reaction to optimise P-subsite residues of HIV protease inhibitors. Chem. Biol. 2002;9:891–896. doi: 10.1016/S1074-5521(02)00184-9. [DOI] [PubMed] [Google Scholar]
- 37.Tossi A., Benedetti F., Norbedo S., Skrbec D., Berti F., Romeo D. Small hydroxyethylene-based peptidomimetics inhibiting both HIV-1 and C. albicans aspartic proteases. Bioorg. Med. Chem. 2003;11:4719–4727. doi: 10.1016/j.bmc.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 38.Knyihár-Csillik E., Mihály A., Krisztin-Péva B., Robotka H., Szatmári I., Fülöp F., Toldi J., Csillik B., Vécsei L. The kynurenate analog SZR-72 prevents the nitroglycerol-induced increase of c-Fos immunoreactivity in the rat caudal trigeminal nucleus: Comparative studies of the effects of SZR-72 and kynurenic acid. Neurosci. Res. 2008;61:429–432. doi: 10.1016/j.neures.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 39.Fülöp F., Szatmári I., Vámos E., Zádori D., Toldi J., Vécsei L. Syntheses, transformations and pharmaceutical applications of kynurenic acid derivatives. Curr. Med. Chem. 2009;16:4828–4842. doi: 10.2174/092986709789909602. [DOI] [PubMed] [Google Scholar]
- 40.Barta P., Fülöp F., Szatmári I. Mannich base-connected syntheses mediated by ortho-quinone methides. Beilstein J. Org. Chem. 2018;14:560–575. doi: 10.3762/bjoc.14.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lőrinczi B., Csámpai A., Fülöp F., Szatmári I. Synthesis of New C-3 Substituted Kynurenic Acid Derivatives. Molecules. 2020;25:937. doi: 10.3390/molecules25040937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deb M.L., Das C., Deka B., Saikla P.J., Baruah P.K. Hydrogen-Bond-Catalyzed Arylation of 3-(Aminoalkyl)indoles via C–N Bond Cleavage with Thiourea under Microwave Irradiation: An Approach to 3-(α,α-Diarylmethyl)indoles. Synlett. 2016;27:2788–2794. doi: 10.1055/s-0036-1588887. [DOI] [Google Scholar]
- 43.Deb M.L., Pegu C.D., Deka B., Dutta P., Kotmale A.S., Baruah P.K. Brønsted-Acid-Mediated Divergent Reactions of Betti Bases with Indoles: An Approach to Chromeno[2,3-b]indoles through Intramolecular Dehydrogenative C2-Alkoxylation of Indole. Eur. J. Org. Chem. 2016;2016:3441–3448. doi: 10.1002/ejoc.201600546. [DOI] [Google Scholar]
- 44.Pegu C.D., Nasrin S.B., Deb M.L., Das D.J., Saikia K.K., Baruah P.K. CAN-Catalyzed microwave promoted reaction of indole with betti bases under solvent-free condition and evaluation of antibacterial activity of the products. Synth. Commun. 2017;47:2007–2014. doi: 10.1080/00397911.2017.1360912. [DOI] [Google Scholar]
- 45.Saidi M.R., Azizi N., Naimi-Jamal M.R. Lithium perchlorate assisted one-pot three-component aminoalkylation of electron-rich aromatic compounds. Tetrahedron Lett. 2001;42:8111–8113. doi: 10.1016/S0040-4039(01)01732-4. [DOI] [Google Scholar]
- 46.Zhu P., Yi Y., Liu Y., Peng Y. Asymmetric Addition of α-Diazomethylphosphonate to Alkylideneindolenine Catalyzed by a Trifunctional BINAP-Based Monophosphonium Salt. Org. Lett. 2022;8:1657–1661. doi: 10.1021/acs.orglett.2c00213. [DOI] [PubMed] [Google Scholar]
- 47.Aghaalikhani S., Behbahani F.K. Three-Component Synthesis of 3-Aminoalkylindoles using Iron(III) Phosphate. ChemistrySelect. 2016;1:5530–5532. doi: 10.1002/slct.201600901. [DOI] [Google Scholar]
- 48.Depa N., Erothu H. One-Pot Three-Component Synthesis of 3-Aminoalkyl Indoles Catalyzed by Molecular Iodine. ChemistrySelect. 2019;4:9722–9725. doi: 10.1002/slct.201902832. [DOI] [Google Scholar]
- 49.Cao J.-F., Chen Y.-L., Guan Z., He Y.-H. Catalyst-free, Solvent-promoted and Scalable Multicomponent Synthesis of 3-Aminoalkylated Indoles via a Mannich-type Reaction. Z. Nat. B. 2014;69:721–727. doi: 10.5560/znb.2014-3313. [DOI] [Google Scholar]
- 50.Rajesh C., Kholiya R., Pavan V.S., Rawat D.S. Catalyst-free, ethylene glycol promoted one-pot three component synthesis of 3-amino alkylated indoles via Mannich-type reaction. Tetrahedron Lett. 2014;55:2977–2981. doi: 10.1016/j.tetlet.2014.03.112. [DOI] [Google Scholar]
- 51.Kumar A., Gupta M.K., Kumar M. L-Proline catalysed multicomponent synthesis of 3-amino alkylated indoles via a Mannich-type reaction under solvent-free conditions. Green Chem. 2012;14:290–295. doi: 10.1039/C1GC16297G. [DOI] [Google Scholar]
- 52.Deb M.L., Baruah P.K. Deamination of Indole Mannich Bases: An Efficient Route to 3-Benzyl/Alkylindoles via a Metal-Free Transfer Hydrogenation Under Microwave Irradiation. Curr. Organocatal. 2016;3:84–89. doi: 10.2174/2213337202666150728205653. [DOI] [Google Scholar]
- 53.Kumar A., Gupta M.K., Kumar M., Saxena D. Micelle promoted multicomponent synthesis of 3-amino alkylated indoles via a Mannich-type reaction in water. RSC Adv. 2013;3:1673–1678. doi: 10.1039/C2RA22428C. [DOI] [Google Scholar]
- 54.Lőrinczi B., Csámpai A., Fülöp F., Szatmári I. Synthetic- and DFT modelling studies on regioselective modified Mannich reactions of hydroxy-KYNA derivatives. RSC Adv. 2021;11:543–554. doi: 10.1039/D0RA08325A. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.