Abstract
This study aimed to analyze the efficacy of exercise interventions on muscle strength, muscle mass, and physical performance in older adults with sarcopenia. Randomized controlled studies assessing exercise effects on sarcopenia were searched in Web of Science, PubMed, Cochrane Library, ProQuest, EBSCOhost, Scopus, EMBASE, and VIP and CNKI up to 31 March 2022. Data were expressed as weighted/standardized mean difference (MD/SMD) with 95% confidence intervals (CI). I2 index was employed for heterogeneity. The initial search identified 5379 studies, and 23 studies involving 1252 participants met the inclusion criteria for further analysis. Results revealed that exercise interventions can significantly improve grip strength (MD = 2.38, 95%CI = 1.33–3.43), knee extension strength (SMD = 0.50, 95%CI = 0.36–0.64), muscle mass of lower extremities (MD = 0.28, 95%CI = 0.01–0.56), walking speed (SMD = 0.88, 95%CI = 0.49–1.27), and functional mobility (MD = −1.77, 95%CI = −2.11–−1.42) among older adults with sarcopenia. No significant exercise effects were found on fat-free muscle mass, appendicular muscle mass, skeletal muscle mass, and muscle mass of the upper extremities. The results of subgroup analysis indicated that both resistance training and multicomponent exercise could significantly increase the muscle strength, while aerobic exercise did not. The findings suggest that exercise intervention can effectively improve muscle function and physical performance in older adults with sarcopenia, but has limited effects on the muscle mass of the upper extremities. In addition, it is highly recommended to apply group-based and supervised resistance training and multicomponent exercise in the prevention and treatment of sarcopenia among the older population.
Keywords: exercise, older adult, sarcopenia, muscle strength, muscle mass, muscle function, physical performance
1. Introduction
The remarkable increase in life expectancy and decrease in fertility rates result in an expanding ageing population worldwide. Because of ageing-related functional reduction, the wellbeing of older adults has been a focus for ageing-related research. A fact related to the advancing age is that older adults tend to have a sedentary lifestyle [1], which finally accelerates the loss of degradation of skeletal muscle mass and physical function.
Sarcopenia, as a critical component of frailty syndrome, refers to the progressive and generalized skeletal muscle disorder that involves the accelerated loss of muscle mass and function [2,3]. People with sarcopenia have been reported with declines in muscle strength and muscular functioning, which may ultimately lead to physical disability, reduced quality of life, and even death [4,5]. Although there are conflicting opinions about the diagnosis of sarcopenia, muscle strength, muscle mass, and physical functioning are the core diagnostic criteria for sarcopenia [6]. The accumulated evidence has indicated that sarcopenia is partially reversible, highlighting the importance of proper and early interventions for sarcopenia [7,8].
Among different interventions for sarcopenia, exercise has been well-evidenced to increase physical fitness, including but not limited to muscle mass, muscle strength/endurance, and cardiovascular capacities of older adults [7,9], highlighting its potential benefits to the prevention and treatment of sarcopenia. As a result of the literature review, most exercise interventions were conducted in healthy older adults or those at risk of sarcopenia [10,11,12]; while few in individuals with sarcopenia. Compared with those at the early stage of sarcopenia, people with sarcopenia would have a significantly lower physical function and thus show different responses to exercise interventions. The variations in participants would be one of the underlying reasons for the inconsistency in exercise efficacy for sarcopenia.
Even in individuals with sarcopenia, no consistent opinions have been reached regarding exercise efficacy. Lu and his colleagues found significantly improved walking speed after a 6-month exercise program in older adults with sarcopenia [13], while Zhu [14], Karina [15], and Iranzo [16] found limited improvements in walking speed, respectively. Inconsistent findings also exist in the related reviews. Vlietstra [17] found that exercise can significantly improve the muscle mass of older adults with sarcopenia, while Bao [18] did not found that. Therefore, this study systematically assessed the effects of exercise interventions on muscle strength, muscle mass, and physical performance in older adults with sarcopenia.
2. Materials and Methods
This study was conducted strictly following the reporting guidelines for systematic review and meta-analysis [19], and the study protocol was registered in the International Prospective Register of Systematic Reviews (CRD42021255735).
2.1. Search Strategy
We systematically searched the related randomized controlled trials (RCT) by implying exercise as the primary intervention among older adults with sarcopenia in the following databases, Web of Science, PubMed, Cochrane Library, ProQuest, EBSCOhost, Scopus, EMBASE, China National Knowledge Infrastructure (CNKI), and Chinese Science and Technology Periodical Database (VIP) from inception to 31 March 2022. The following MeSH terms were searched in English and Chinese: randomized controlled trial, sarcopenia, exercise, muscle function, physical performance, older adult, and the related terms used in their broadest sense. The detailed search strategies are described in Supplementary Material.
2.2. Eligibility Criteria
Inclusion criteria: (i) full-text published in English or Chinese; (ii) RCTs; (iii) taking exercise as the single intervention component in one group; (iv) participants were identified with sarcopenia; (v) participants aged 60 years and above; and (vi) primary outcomes include muscle function and physical performance indicators.
2.3. Study Selection and Data Collection
After removing the duplications, two researchers independently screened the study titles and abstracts. Then the full-text reviews were conducted with data extraction. Gray literature was explored by reviewing reference lists of selected primary studies and the related systematic reviews. Disagreements between the two researchers were resolved by consensus or a third researcher. The data included authors, publication year, sex, age, sample size, exercise interventions (types and intensity), and outcome measures. In cases of missing valuable data or information, the corresponding authors of these studies were contacted.
2.4. Risk of Bias Assessment
The methodological quality of the included studies was assessed using the Cochrane Collaboration risk of bias tool [20], which includes bias in selection (randomization and allocation), performance, detection, attrition, reporting, and other components. Two researchers performed the assessment independently, and the third researcher was consulted for resolution whenever discrepancies existed.
2.5. Statistical Analysis
The data analysis was performed using statistical software of the Cochrane Collaboration Review Manager (RevMan, version 5.3, Copenhagen, Denmark). Sample size, means, and standard deviations (SDs) were extracted for each RCT arm. The I2 statistic was used to describe the percentage of total variation across studies due to heterogeneity rather than chance [21]. A fixed-effect model was used to pool the results if the heterogeneity was not significant (I2 < 50%), and the random-effect model was applied when I2 > 50% [22]. Sensitivity analysis was carried out by removing studies one by one [23], and the subgroup analysis was performed for studies with high heterogeneity. Under the fix-model analysis, the weighted mean difference (MD) was used to assess the measure of effect, and the standardized mean difference (SMD) was applied if there were different outcome measures [24]. Finally, the STATA version 16 was used to assess the publication bias through the funnel plots and Egger test [25]. The statistical significance level was set at p < 0.05.
3. Results
3.1. Search Results
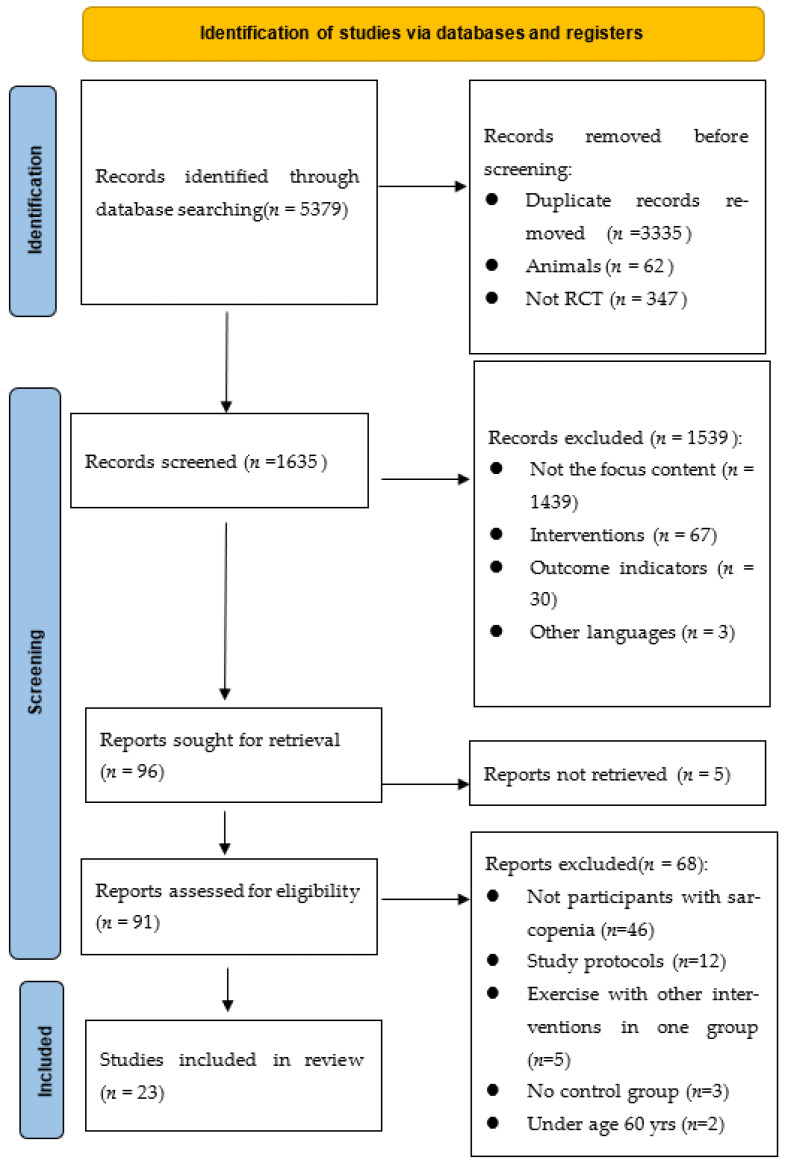
A total of 5379 studies were found in the initial search, from which 3335 duplications were removed, and 1948 records were removed for other reasons. Ninety-one studies were reviewed in full text, and 23 RCT studies involving 30 comparisons were included in this systematic review and meta-analysis (Figure 1).
Figure 1.
Flow diagram of the studies’ selection process.
3.2. Study Characteristics
The 23 RCT studies involving 1252 participants (665 received exercise interventions) were published between 2012 and 2022. Participants were aged from 60 to 101 years, with the mean age ranging between 63.2 ± 1.4 and 89.5 ± 4.4 years. Twelve studies included both men and women [14,16,26,27,28,29,30,31,32,33,34,35], while eight studies were conducted in women [15,36,37,38,39,40,41,42] and one study was conducted in older men [43]. Two studies did not specify the sex information [44,45]. The detailed information for data extraction can be found in Table 1.
Table 1.
Study characteristics.
| Author, Year, Country | Sample (Intervention/Control) | Age (Mean ± SD) |
Sex | Interventions | Dosage | Intensity | Control Group | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Zhu, L.Y. 2019, China [14] | 77(40/37) | 74.5 ± 7.1 | Male (24%) |
AT + RT | 40–50 min × 2 times/week × 12 weeks (16–20 h) | Monitored and adjusted by coach. | Maintain daily lifestyle | Hand grip strength, muscle mass of lower extremities, muscle mass of upper extremities, walking speed |
| Karina, S.S.V. 2016, Brazil [15] | 31(16/15) | 72 ± 4.6 | Female | RT | 60 min × 2 times/week × 10 weeks (20 h) | Intensity between moderate and somewhat severe monitored by modified Borg Scale | Telephone monitoring | Knee extension strength, walking speed |
| Iranzo, M.A. 2018, Spain [16] | 37(9,11/17) | 82.6 ± 9.1 | Male (25%) |
RT | 30 min × 3 times/week × 12 weeks (18 h) | Resistance:40–60% max isometric muscle strength | Maintain daily lifestyle | Hand grip strength, walking speed |
| Chen, H.T. 2017, China [26] | 60(15,15,15/15) | 68.9 ± 4.4 | Male (16%) |
RT/AT/AT + RT | 60 min × 2 times/week × 8 weeks (16 h) | Resistance: 60–70% of the maximum repetitions aerobic: Moderate | Maintain daily lifestyle | Hand grip strength, knee extension strength, skeletal muscle mass |
| Tsekoura, M. 2018, Greece [27] | 54(18,18/18) | 72.87 ± 7 | Male (13%) | AT + MT/AT + HT | AT + MT:40–50 min × 2 times/week × 12 weeks (16 h); AT + HT:30–35 min × 3 times/week × 12 weeks (18–21 h); | gradually increase: RPE from 6 to 20 | Maintain daily lifestyle | Hand grip strength, knee extension strength, fat-free mass, skeletal muscle mass, walking speed, functional mobility |
| Wang, L.Z. 2019, China [28] | 80(20,20,20/20) | 65.1 ± 3.4 | Male (53.8%) |
RT/AT/AT + RT | 20 min × 2 times/week × 8 weeks (5.4 h) | Moderate intensity: No obvious feeling of fatigue | Maintain daily lifestyle | Hand grip strength; knee extension strength |
| Zhu, G.F. 2019, China [29] | 65(33/32) | 66.32 ± 10.80 | Male (50.1%) |
Yi Jinjing exercise | 40 min × 7 times/week × 12 weeks (56 h) | No obvious feelings of fatigue | Health education | Hand grip strength |
| Li, Z. 2020, China [30] | 121(62/59) | 73.73 ± 5.69 | Male (37.1%) |
RT + OA | 20 min × 3 times/week × 12 weeks (18 h); 1 h × 3 times/week × 12 weeks (36 h) | RT:8 max rep.; OA: >800 steps in 10 min | Maintain daily lifestyle | Hand grip strength, appendicular muscle mass |
| Shao, W.H. 2020, China [31] | 71(41/30) | 69.3 ± 13.4 | Male (63.4%) |
RT | 30 min × 2 times/week × 24 weeks (24 h) | Moderate intensity | Health education | Hand grip strength, walking speed |
| Zhou, S.P. 2020, China [32] | 40(20/20) | 73.0 ± 8.5 | Male (42.5%) |
Ba duanjin exercise | 40 min × 5 times/week × 8 weeks (26.7 h) | Moderate intensity | Health education | Hand grip strength, functional mobility |
| Fang, L. 2020, China [33] | 36(18/18) | 82.8 ± 8.5 | Male (33.3%) |
Muscle-bone strengthening exercise | 30 min × 3 times/week × 24 weeks (36 h) | 60% maximum heart rate | Maintain daily lifestyle | Functional mobility |
| Wang, G.H. 2021, China [34] | 54(26/28) | 70.4 ± 5.1 | Male (33.3%) |
RT | 45 min × 3 times/week × 12 weeks (27 h) | Moderate: 50-70% 1RM | Maintain daily lifestyle | Hand grip strength, walking speed |
| Zhao, T. 2022, China [35] | 40(20/20) | 63.2 ± 1.4 | Male (40.0%) |
RT | 25 min × 14 times/week × 12 weeks (70 h) | 8RM (can be adjusted according to individual situation) | Health education | Hand grip strength, walking speed |
| Kim, H. 2012, Japan [36] | 78(39/39) | 79.0 ± 2.9 | Female | RT + Balance + Gait training | 50 min × 2 times/week × 12 weeks (20 h) | Moderate: RPE = 12–14 | Health education | Knee extension strength, muscle mass of lower extremities, appendicular muscle mass, walking speed |
| Kim, H. 2013, Japan [37] | 64(32/32) | 79.6 ± 4.2 | Female | RT + Balance + Gait training | 50 min × 2 times/week × 12 weeks (20 h) | Moderate: RPE = 12–14 | Health education | Hand grip strength, knee extension strength, Muscle mass of lower extremities, appendicular muscle mass, walking speed, functional mobility |
| Kim, H. 2016, Japan [38] | 69(35/34) | 81.4 ± 4.3 | Female | AT + RT and weight-bearing training | 60 min × 2 times/week × 12 weeks (24 h) | AT: start at 40 watts and gradually increase; RT: beginning with 1 set of 10 repetitions to 3 sets. | Health education | Hand grip strength, knee extension strength, muscle mass of lower extremities, muscle mass of upper extremities, appendicular muscle mass, walking speed |
| Liao, C.D. 2017, China [39] | 46(25/21) | 67.3 ± 5.2 | Female | RT | 35–40 min × 3 times/week × 12 weeks (21–24 h) | Moderate: RPE = 13 | No control | Hand grip strength, fat-free mass, functional mobility |
| Jung, W.S. 2019, Korea [40] | 26(13/13) | 75.0 ± 3.9 | Female | AT + RT | 25–55 min × 3 times/week × 12 weeks (15–60 h) | 60–80% heart rate reserve | Maintain daily lifestyle | Fat-free muscle mass |
| Lee, Y.H. 2021, China [41] | 27(15/12) | 70.13 ±4.41 | Female | RT | 40 min × 3 times/week × 12 weeks (24 h) | Moderate intensity: RPE = 12–14 | Allowed to exercise at home | Hand grip strength, functional mobility |
| Seo, M.Y. 2021, Korea [42] | 27(14/13) | 70.3 ± 5.38 | Female | RT | 50 min × 3 times/week × 16 weeks (32.5 h) | 0—extremely easy to 10—extremely hard | Maintain daily lifestyle | Hand grip strength, functional mobility, walking speed, appendicular muscle mass, fat-free mass |
| Zhu, Y.Q. 2019, China [43] | 79(24,28/27) | 89.5 ± 4. 4 | Male | Eight style TC/WBV | 20 min × 5 times/week × 8 weeks (13.3 h) | TC: Progressively increase; WBV: 5 groups/time, and 3 min/group | Maintain daily lifestyle | Hand grip strength, muscle mass of lower extremities, muscle mass of upper extremities |
| Liu, C. K. 2014, America [44] | 33(16/17) | 77.5 ± 4.2 | No clear | AT + RT + Balance + Flexibility training | 1–8 week:3 times/week; 9–24 week:2 times/week | Moderate | Not clear | Walking speed |
| Wei, N. 2016, China [45] | 40(20/20) | 75 ± 6 | No clear | WBV | 6 min × 3 times/week × 12 weeks (3.6 h) | 40 Hz/360 s per session; | Maintain daily lifestyle | Knee extension strength, walking speed, functional mobility |
AT, aerobic training; RT, resistance training; MT, multicomponent training; OA, outdoor activity; HT, home therapeutic exercise; TC, Tai Chi; WBV, whole-body vibration training; RPE, rating of perceived exertion.
Regarding the intervention content, different exercise types were used, including resistance training [15,26,28,31,34,35,39,41,42,43], aerobic exercise (e.g., Taichi, Qigong and Yi Jin Jing exercise) [26,28,29,32,33,34,43,45], and combined exercise [14,26,27,28,30,31,36,38,40,44]. The total duration of the exercise interventions ranged from 8 to 24 weeks, and most studies (n = 12) applied the 12-week duration [14,16,27,29,30,36,37,38,39,40,41,45]. Twice a week was the most adopted exercise frequency (n = 10) [14,15,26,27,28,31,36,37,38,44].
Variations exist in sample size among the included studies. Sample size ranged from less than 30 [15,40,41,42] to 80. Eleven studies included over 60 participants [14,26,28,29,30,31,36,37,38,43], and 10 studies comprise of 30–59 participants [16,27,30,32,33,34,35,39,44,45]. In addition, only half of the included studies reported detailed methods for sample size estimation [14,15,16,27,28,30,33,36,38,39,40,41].
3.3. Summary of Risk of Bias
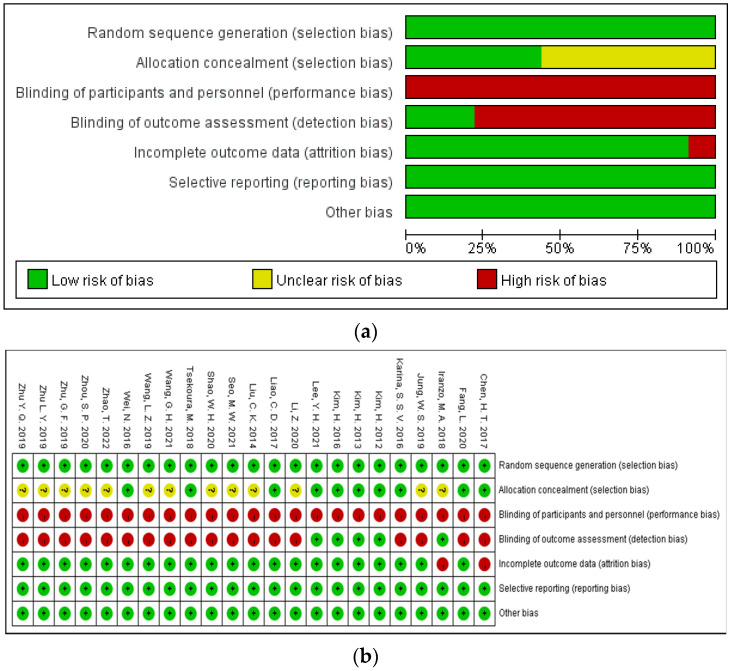
For the selection bias, all studies reported how random sequences were generated, and 10 of 23 trials reported the allocation concealment [15,26,27,33,36,37,38,39,41,45]. Twenty-two studies did not conceal the research purpose to the participants, and one study [40] did not mention whether informed consent was signed or not. Therefore, there is a high risk of performance bias in these studies and this is the common problem in exercise interventions since blinding participants from exercise groups is quite difficult. Only five studies blinded the outcome assessment to participants [16,36,37,38,41], and 18 studies had a high risk of detection bias. In the two studies [16,26], a large number of subjects were lost in the experimental process, but the specific reasons were not reported, so there was a high risk of attrition bias. In addition, all studies had low risk regarding reporting bias and other biases. The detailed information can be found in Figure 2.
Figure 2.
(a) The weighted plot for the assessment of the overall risk of bias; (b) summary of the bias for the trials included in this meta-analysis. Green indicates low risk of bias, yellow indicates unclear, and red indicates high risk of bias.
3.4. Effects of Exercise on Muscle Function and Physical Performance
Muscle function is the primary outcome of this study, and it includes muscle strength (i.e., grip strength and knee extension strength) and muscle mass (i.e., muscle mass of lower extremities, free fat mass, skeletal muscle mass, appendicular muscle mass, and muscle mass of upper extremities). The secondary outcome is physical performance, including walking speed and functional mobility as tested using the TUG.
3.4.1. Grip Strength
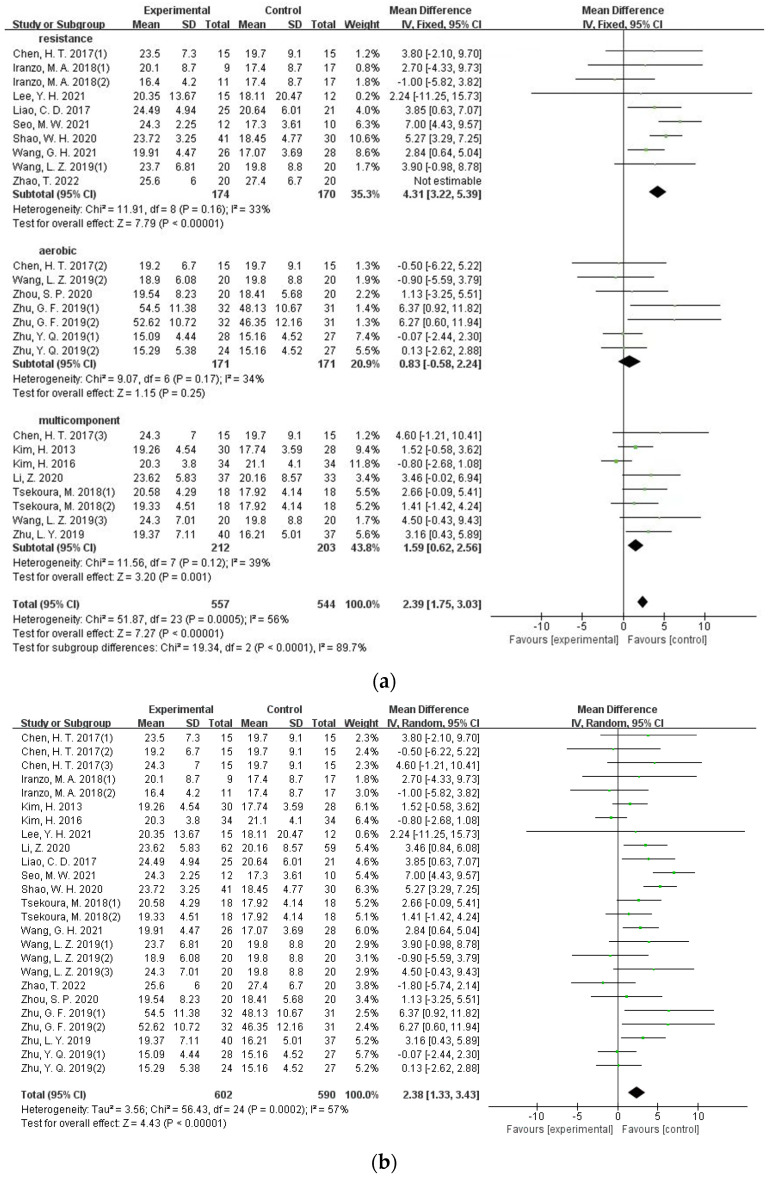
A total of 17 studies involving 954 participants had taken grip strength as one of the main outcomes. A relatively high level of heterogeneity was present among different studies (I2 = 57.0%), and the sensitivity analysis indicated that removing any one study cannot lower the heterogeneity. The high heterogeneity may be due to different types of exercise formats. Resistance training was used in nine studies, aerobic training was applied in five studies, and multicomponent training was adopted in seven. Subgroup analysis was performed (Figure 3a), and the results demonstrated that exercise format is the main source of heterogeneity (I2 = 56%, p < 0.001). The intra-group heterogeneity was low in each of the three exercise formats (RT: I2 = 33%, AT: I2 = 34%; MT: I2 = 39%). The intervention effect size with the fixed-effects model demonstrated that resistance training (MD = 4.31, 95%CI = 3.22–5.39, p < 0.001) and multi-component exercise (MD = 1.59, 95%CI = 0.62–2.56, p = 0.001) can significantly improve grip strength of the target group. Aerobic exercise, however, had a limited effect on the improvement of grip strength (MD = 0.83, 95%CI = −0.58–2.24, p = 0.25). At the same time, we also adopted a random effect model combining the effect size of exercise interventions on grip strength (Figure 3b). The result demonstrated that exercise interventions can significantly improve the grip strength of the target group (MD = 2.38, 95%CI = 1.33–3.43, p < 0.001).
Figure 3.
(a) Forest plot of the effectiveness of exercise interventions for grip strength improvement according to different exercise types; (b) forest plot of the effectiveness of exercise interventions for grip strength improvement in older adults with sarcopenia.
3.4.2. Knee Extension Strength
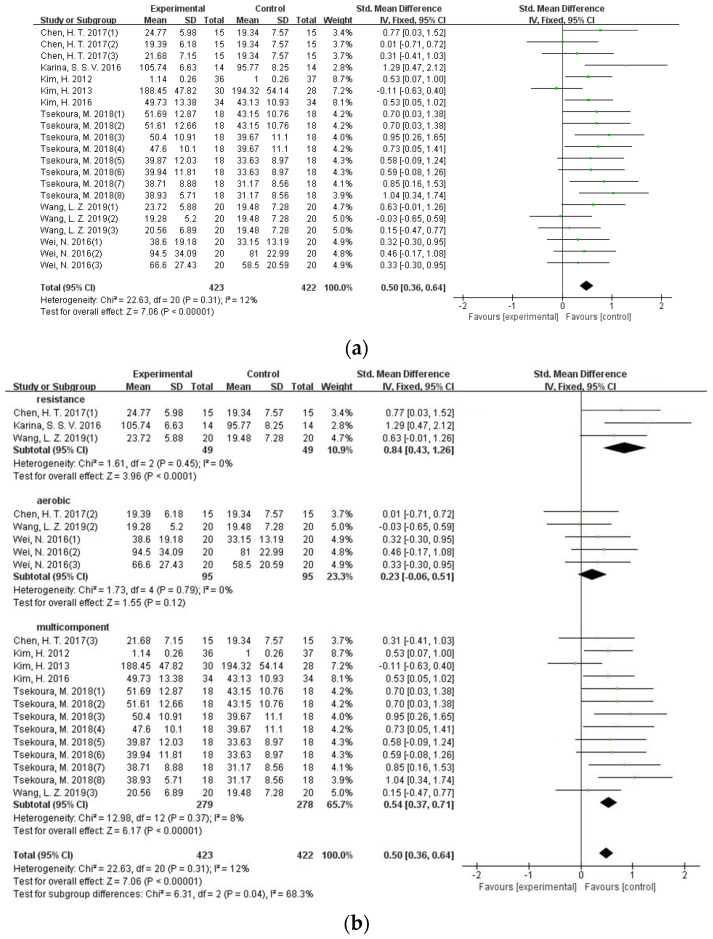
Eight studies involving 620 participants applied knee extension strength as the outcome. Since the measures of knee extension were different (isometric measures vs. isokinetic measures), the SMD was applied to calculate the effect size, and a low level of heterogeneity was found (I2 = 12%). Results of the meta-analysis indicated that exercise intervention can significantly improve muscle strength for knee extension (SMD = 0.50, 95%CI = 0.36–0.64, p < 0.001) (Figure 4a).
Figure 4.
(a) Forest plot of the effectiveness of exercise interventions for knee extension strength improvement in older adults with sarcopenia; (b) forest plot of the effectiveness of exercise interventions for knee extension strength improvement according to different exercise types.
In order to evaluate the effect of different exercise formats on knee extension strength in older adults with sarcopenia, a subgroup analysis was performed. Results showed that significant and positive effects of both resistance training (SMD = 0.84, 95%CI = 0.43–1.26, p < 0.001) and multi-component exercise (SMD = 0.54, 95%CI = 0.37–0.71, p < 0.001) on knee extension strength, while little is shown in the aerobic exercise on grip strength and is limited (SMD = 0.23, 95%CI = −0.06–0.51, p = 0.12) (Figure 4b).
3.4.3. Muscle Mass
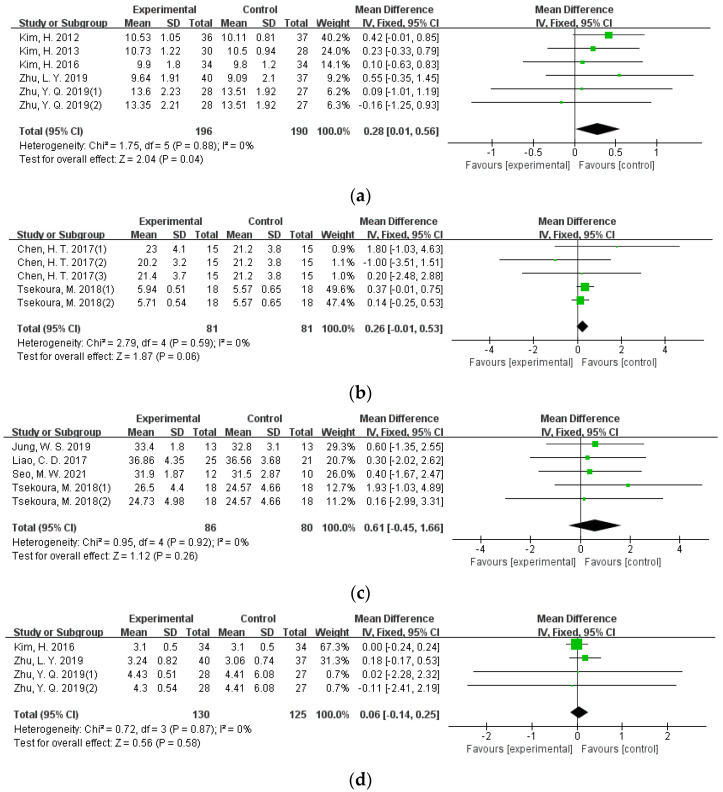
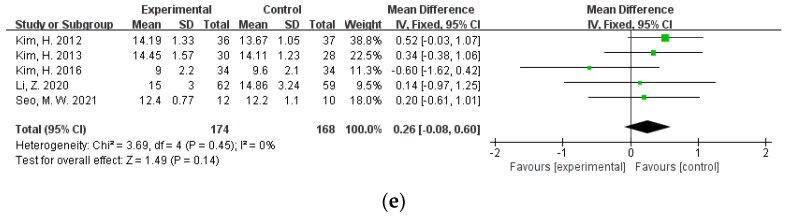
Eleven studies involving 648 participants set muscle mass as the main outcome, including muscle mass of lower extremities, free fat mass, skeletal muscle mass, appendicular muscle mass, and muscle mass of upper extremities. Results from sensitivity analysis indicated low heterogeneity among different studies (I2 = 0). Hence, the effect size was estimated using the fixed-effects model, and the results revealed significant exercise effects on the muscle mass of lower extremities (MD = 0.28, 95%CI = 0.01–0.56, p = 0.04) (Figure 5a), and limited effects were found in other muscle mass indicators of older adults with sarcopenia (Figure 5b–e).
Figure 5.
Forest plot of the effectiveness of exercise interventions for (a) muscle mass of lower extremities; (b) skeletal muscle mass; (c) free fat mass; (d) muscle mass of upper extremities; and (e) appendicular muscle mass improvement in older adults with sarcopenia.
3.4.4. Walking Speed
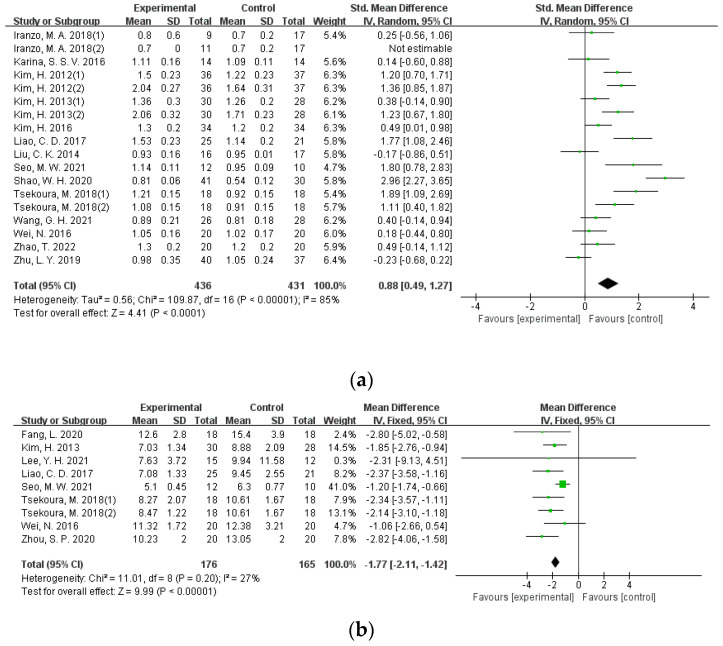
There were 14 studies involving 872 participants that applied walking speed as the outcome. The study heterogeneity is quite big (I2 = 85%). Subgroup analysis of three different exercise types showed high heterogeneity within each exercise format (I2 > 80%). This would be related to the fact that big variations exist in test procedures and requirements. Among these studies, different walking distances were used including 4 m [27], 5 m [38], 6 m [14], 10 m [15,16,45], and 400 m [44]. Some studies also assessed walking speed by calculating walking distance in 10 min [39]. Inconsistencies also exist in starting position (static vs. dynamic) and whether or not to emphasize the ending point. The effect size of exercise intervention under the random-effects model indicated that exercise could significantly improve the walking speed of older adults with sarcopenia (SMD = 0.88, 95%CI = 0.49–1.27, p < 0.001) (Figure 6a).
Figure 6.
Forest plot for physical performance. (a) Forest plot of the effectiveness of exercise interventions for walking speed improvement in older adults with sarcopenia; (b) forest plot of the effectiveness of exercise interventions for functional mobility improvement in older adults with sarcopenia.
3.4.5. Functional Mobility
Eight studies involving 364 participants assessed functional mobility using the time up ang go test (TUG). The heterogeneity among these studies was low (I2 = 27%), and sensitivity analysis showed that the exclusion of any single study had no significant effect on the total effect size. Results from the meta-analysis using the fixed-effect model showed that, in comparison with the placebo group, exercise intervention can improve functional mobility (MD = −1.77, 95%CI = −2.11–−1.42, p < 0.001) (Figure 6b).
3.5. Publication Bias
The funnel plot of the knee extension strength was asymmetrical (Supplementary Material). The Egger tests also revealed that there was a relatively higher level of publication bias in knee extension strength (t = 2.11, p = 0.048). The funnel plots of other indicators were symmetrical, and Egger tests also indicated a relatively low level of publication bias (p > 0.05).
4. Discussion
This meta-analysis of 23 RCT studies assessed the effects of exercise interventions on muscle function and physical performance in older adults with sarcopenia. Results indicated that exercise could effectively improve muscle strength, muscle mass of lower extremities, and physical performance. In addition, resistance and multicomponent exercises would be preferred for muscle strength improvement. Results from this study would enrich the evidence of exercise efficacy in reducing the risks of sarcopenia among older adults.
Muscle strength is an often applied indicator in various diagnostic criteria of sarcopenia [46]. The present results found that moderate resistance training, multicomponent exercises such as aerobic + resistance training [14,26,28,40], resistance + balance + gait training [36,37], and resistance training + outdoor activity [30], would have significant effects on muscle strength for the target population. However, moderate aerobic exercise has shown a limited effect on muscle strength improvement. It has been well documented that resistance training can generate increases in muscle strength [47], whereas aerobic exercise is known to improve cardiorespiratory fitness [48]. In addition, subjective methods such as the self-perceived exertion scale were often used in aerobic exercise to monitor exercise intensity, which would influence the expected quality of aerobic exercise. Compared to multicomponent exercise formats, the single aerobic exercise training may not be interesting and attractive enough; therefore, it would reduce participants’ compliance. Regardless of the exercise formats, group exercise with supervision and guidance instead of individual home-based exercise always show better motivation for exercise involvement and more efficacy in muscle strength improvements [27]. It is highly recommended that clinical practice enriches the content of exercise, stimulates exercise interest, and adopts group-based supervised exercise intervention to strengthen the muscle function of older adults with sarcopenia.
The present finding confirmed the positive training effects on muscle mass, especially in the lower body, while Bao and his colleagues found no exercise effect on appendicular skeletal muscle mass [18]. The controversial results would be mainly related to differences in population characteristics. Compared with younger adults, older adults would show a slower physical response to resistance training [49] and gain a lower increment in muscle mass [50,51,52,53]. The muscle mass growth rate depends on the synthesis and degradation rate of proteins [54]. Due to the reduced level of physical activity and the retardation of muscle cell metabolism, the metabolism and synthesis of proteins are degraded among older adults. In addition, the presence of various chronic illnesses, including various inflammatory, respiratory, and endocrinal diseases, would reduce the synthesis rate of proteins in older adults [55]. As discussed above, older adults with sarcopenia have relatively lower levels of physical function and have limited tolerance to high exercise intensity. In addition, it has been well evidenced that exercise intensity significantly affects muscle protein synthesis [56]. All these would finally affect the enhancement of muscle mass amplitude among older adults with sarcopenia.
The present study revealed significant training effects on the muscle mass of lower extremities, which is in line with the findings of the previous related meta-analysis [17]. However, the present study revealed limited effects on the muscle mass of the upper body. This can be explained by the movement features of exercise intervention that tend to emphasize lower body muscle movement. In a study conducted by Kim and his colleagues (2012), the exercise program consisted of repeated toe raises, heel raises, knee lifts, knee extensions, lateral leg raises and leg extensions, hip flexions by resistance bands, but only arms pulling down and biceps bending for the upper body [36]. In addition to the standing posture during exercise intervention, the muscle mass of the lower extremities would receive more exercise stimulations compared to that of the upper body. Further RCT studies are recommended to include more upper body movements to exert substantial training effects on upper body muscle mass.
Walking speed is a commonly used indicator of physical functioning and is highly correlated with falling risks, cognitive ability, and neuromuscular function [57,58,59,60]. Both the Asian Working Group for Sarcopenia Standards (AWGS) and the 2nd Edition of the European Working Group on Sarcopenia Standards (EWGSOP2) include walking speed as the diagnosis criteria of sarcopenia. The results of this study confirm that exercise can effectively increase the walking speed of the target group, which is consistent with previous studies [61,62,63]. However, a relatively higher level of heterogeneity existed among the included studies. This would be mainly related to the differences in walking speed test parameters, such as starting position, walking distance, and the set of the ending points. All these would influence the outcome accuracy [64]. Due to ageing-related functional deterioration, older adults with sarcopenia would require a longer time in signal identification, muscle recruitment, and movement implementation than their age-matched counterparts. Future RCT studies are suggested to apply the standardized walking speed measurement to increase study comparison and reduce study heterogeneity.
There are some limitations in the present study that may comprise the application of study results. First, the quality of the included RCTs was not high, and most studies showed risks of performance bias and detection bias because of the improper application of blinding methods. This would influence the validity of the study results. Second, most studies were conducted on female participants; whether gender differences exist in training effects is unknown. Finally, given the limited number of the included studies, the present findings should be interpreted cautiously when translating into practice.
5. Conclusions
Exercise can effectively improve muscle function and physical performance in older adults with sarcopenia, but has limited effects on the muscle mass of the upper extremities. In addition, it is highly recommended to apply group-based and supervised resistance training and multicomponent exercise to prevent sarcopenia among the older population.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijerph19138212/s1, Supplementary S1—Search strategy, Supplementary S2—Figure S1. Funnel plot of knee extension strength and Figure S2. Egger plot of knee extension strength.
Author Contributions
H.W. and W.Y.H. searched and screened the articles. H.W. performed the statistical analyses. Y.Z. provided additional suggestions and assisted in the interpretation of data. H.W. and Y.Z. reviewed the full-text articles and extracted the data. H.W. and Y.Z. drafted the manuscript. Y.Z. revised and finalized the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the National Natural Science Foundation of China, grant number 81801387.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.An H.Y., Chen W., Wang C.W., Yang H.F., Huang W.T., Fan S.Y. The Relationships between Physical Activity and Life Satisfaction and Happiness among Young, Middle-Aged, and Older Adults. Int. J. Environ. Res Public Health. 2020;17:4817. doi: 10.3390/ijerph17134817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jentoft A.J.C., Sayer A.A. Sarcopenia. Seminar. 2019;393:2636–2646. doi: 10.1016/S0140-6736(19)31138-9. [DOI] [PubMed] [Google Scholar]
- 3.Bhasin S., Travison T.G., Manini T.M., Patel S., Pencina K.M., Roger A., Jay M., Anne B., Douglas P., Cyrus C., et al. Sarcopenia definition: The position statements of the sarcopenia definition and outcomes consortium. JAGS. 2020;68:1410–1418. doi: 10.1111/jgs.16372. [DOI] [PubMed] [Google Scholar]
- 4.Nascimento C.M., Ingles M., Salvador-Pascual A., Cominetti M.R., Gomez-Cabrera M., Vina J. Sarcopenia, frailty and their prevention by exercise. Free Radic. Biol. Med. 2019;132:42–49. doi: 10.1016/j.freeradbiomed.2018.08.035. [DOI] [PubMed] [Google Scholar]
- 5.Walston J., Buta B., Xue Q.L. Frailty Screening and Interventions: Considerations for Clinical Practice. Clin. Geriatr. Med. 2018;34:25–38. doi: 10.1016/j.cger.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saeki C., Takano K., Oikawa T., Aoki Y., Kanai T., Takakura K., Nakano M., Torisu Y., Sasaki N., Abo M., et al. Comparative assessment of sarcopenia using the JSH, AWGS, and EWGSOP2 criteria and the relationship between sarcopenia, osteoporosis, and osteosarcopenia in patients with liver cirrhosis. BMC Musculoskelet. Disord. 2019;20:615. doi: 10.1186/s12891-019-2983-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruby Y., Moses W., Jason L., Lee J., Auytung T.W., Woo J. Incidence, reversibility, risk factors and the protective effect of high body mass index against sarcopenia in community-dwelling older chinese adults. Geriatr. Gerontol. Int. 2014;14:15–28. doi: 10.1111/ggi.12220. [DOI] [PubMed] [Google Scholar]
- 8.Morley J.E., Vellas B., van Kan A., Anker S.D., Bauer J.M., Bernabei R., Cesari M., Chumlea W.C., Doehner W., Evans J., et al. Frailty consensus: A call to action. J. Am. Med. Dir. Assoc. 2013;4:392–397. doi: 10.1016/j.jamda.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoo S.Z., No M.H., Heo J.W., Park D.H., Kang J.H., Kim S.H., Kwak H.B. Role of exercise in age-related sarcopenia. J. Exerc. Rehabil. 2018;14:551–558. doi: 10.12965/jer.1836268.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cruz-Jentoft A.J., Landi F., Schneider S.M., Zúñiga C., Arai H., Boirie Y., Chen L.K., Fielding R.A., Martin F.C., Michel J.P., et al. Prevalence of and interventions for sarcopenia in ageing adults: A systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS) Age Ageing. 2014;43:748–759. doi: 10.1093/ageing/afu115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi M., Kim H., Bae J. Does the combination of resistance training and a nutritional intervention have a synergic effect on muscle mass, strength, and physical function in older adults? A systematic review and meta-analysis. BMC Geriatr. 2021;12:639. doi: 10.1186/s12877-021-02491-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li P.S., Hsieh C.J., Tallutondok E.B., Peng H.J. The Dose-Response Efficacy of Physical Training on Frailty Status and Physical Performance in Community-Dwelling Elderly: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Healthcare. 2022;21:586. doi: 10.3390/healthcare10030586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu Y.X., Mathew N., Bee Y.K., Tan C.T.Y., Nynut M.S.Z., Feng L., Tan B., Chan G., Khoo S.A., Chan S.M., et al. Effects of multi-domain lifestyle interventions on sarcopenia measures and blood biomarkers: Secondary analysis of a randomized controlled trial of community-dwelling pre-frail and frail older adults. Aging. 2021;13:9330–9347. doi: 10.18632/aging.202705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu L.Y., Chan R., Kwok T., Cheng K.C., Ha A., Woo J. Effects of exercise and nutrition supplementation in community-dwelling older Chinese people with sarcopenia: A randomized controlled trial. Age Ageing. 2019;48:220–228. doi: 10.1093/ageing/afy179. [DOI] [PubMed] [Google Scholar]
- 15.Karina S.S.V., Dias J., Marilia C.A., Ana C.P., Bruno S.M., Rosangela C.D. Effects of a progressive resistance exercise program with high-speed component on the physical function of older women with sarcopenic obesity: A randomized controlled trial. Braz. J. Phys. Ther. 2016;20:432–440. doi: 10.1590/bjpt-rbf.2014.0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iranzo M.A., Bernat M.B., Chulia M.A.T., Parisi S.B. Effects of resistance training of peripheral muscles versus respiratory muscles in institutionalized older adults with sarcopenia: A randomized controlled trial. J. Aging Phys. Act. 2018;26:637–646. doi: 10.1123/japa.2017-0268. [DOI] [PubMed] [Google Scholar]
- 17.Vlietstra L., Hendrickx W., Waters D.L. Exercise interventions in healthy older adults with sarcopenia: A systematic review and meta-analysis. Australas J. Ageing. 2018;37:169–183. doi: 10.1111/ajag.12521. [DOI] [PubMed] [Google Scholar]
- 18.Bao W.X., Sun Y., Zhang T.F., Zou L.L., Wu X.H., Wang D., Chen Z.B. Exercise Programs for Muscle Mass, Muscle Strength and Physical Performance in Older Adults with Sarcopenia: A Systematic Review and Meta-Analysis. Aging Dis. 2020;11:863–873. doi: 10.14336/AD.2019.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shanseer L., Tetzlaff Z.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins J., Green S. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; London, UK: 2011. Version 5.1.0. [Google Scholar]
- 21.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 22.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;27:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tobias A. Assessing the influence of a single study in the meta-anyalysis estimate. Stata Tech. Bull. 1999;8:47. [Google Scholar]
- 24.Andrade C. Mean difference, standardized mean difference (smd), and their use in meta- analysis: As simple as it gets. J. Clin. Psychiatry. 2020;81:20f13681. doi: 10.4088/JCP.20f13681. [DOI] [PubMed] [Google Scholar]
- 25.Egger M., Smith G.D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen H.T., Chung Y.C., Chen Y.J., Ho S.Y., Wu H.J. Effects of different types of exercise on body composition, muscle strength, and igf-1 in the elderly with sarcopenic obesity. J. Am. Geriatr. Soc. 2017;65:827–832. doi: 10.1111/jgs.14722. [DOI] [PubMed] [Google Scholar]
- 27.Tsekoura M., Evdokia B., Elias T., Dimitriadis Z., Matzaroglou C., Tyllianakis M., Panagiotopoulos E., Gliatis J. The effects of group and home-based exercise programs in elderly with sarcopenia: A randomized controlled trial. J. Clin. Med. 2018;7:480. doi: 10.3390/jcm7120480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang L.Z., Guo Y.B., Luo K.H. Effects of home exercise training on obese sarcopenia in the elderly. Chin. J. Rehabil. Theory Pract. 2019;25:90–96. doi: 10.3969/j.issn.1006-9771.2019.01.012. [DOI] [Google Scholar]
- 29.Zhu G.F., Luo K.T., Shen Z.F., Gao F., Fu Y.X., Shen Q.H. Effect of Yijinjing on muscle strength of senile patients with sarcopenia. Chin. J. Gen. Pract. 2019;17:1388–1391. doi: 10.16766/j.cnki.issn.1674-4152.000951. [DOI] [Google Scholar]
- 30.Li Z., Cui M., Yu K., Zhang X.W., Li C.W., Nie X.D., Wang F. Effects of nutrition supplementation and physical exercise on muscle mass, muscle strength and fat mass among sarcopenic elderly: A randomized controlled trial. Appl. Physiol. Nutr. Metab. 2021;46:494–500. doi: 10.1139/apnm-2020-0643. [DOI] [PubMed] [Google Scholar]
- 31.Shao W.H., Gao L.X., Wang S.X., Lv C.X., Yao L.X. Effects of vitamin D combined with resistance training on skeletal muscle mass, activities of daily living and serological indices in elderly patients with sarcopenia. Chin. J. Mult. Organ. Dis. Elderly. 2020;19:656–660. doi: 10.11915/j.issn.1671-5403.2020.09.153. [DOI] [Google Scholar]
- 32.Zhou S.P., Zou Y., Sun X.F., Xu Q.L. Research on Baduanjin for Preventing Falls in Elderly Patients with Sarcopenia. Sep. Sci. Technol. 2020;41:27–30. [Google Scholar]
- 33.Fang L., Li Z.R., Tao X.C., Luo J. Effects of Yi Jin Jing on the risk of falling of sarcopenia and disequilibrium elderly. Chin. J. Rehabil. Med. 2020;35:319–323. [Google Scholar]
- 34.Wang G.H., Cai W.W., Shen X.J., Li C.Y., Xu Y.D. Effects of resistance training using elastic band for 12 weeks on muscle strength of elderly patients with sarcopenia in community. Chin. J. Clin. Healthc. 2021;24:90–94. doi: 10.16766/j.cnki.issn. [DOI] [Google Scholar]
- 35.Zhao T., Gao H.L., Guo N.Z., Chen Z.M., Wu X.Y., He D.L., Wei L.Q., Shi X.C., Lv W.Y. Effect of health education and whey protein combined with resistance exercise on the treatment of sarcopenia in middle-aged and elderly. Int. J. Clin. Exp. Med. 2022;21:90–94. doi: 10.3969/j.issn. [DOI] [Google Scholar]
- 36.Kim H.K., Suzuki T., Saito K., Yoshida H., Kobayashi H., Kato H., Katayama M. Effects of exercise and amino acid supplementation on body composition and physical function in community-dwelling elderly japanese sarcopenic women: A randomized controlled trial. J. Am. Geriatr. Soc. 2012;60:458–465. doi: 10.1111/j.1532-5415.2011.03776.x. [DOI] [PubMed] [Google Scholar]
- 37.Kim H., Suzuki T., Saito K., Yoshida H., Kojima N., Kim M., Sudo M., Yamashiro Y., Tokimitsu I. Effects of exercise and tea catechins on muscle mass, strength and walking ability in community-dwelling elderly Japanese sarcopenic women: A randomized controlled trial. Geriatr. Gerontol. Int. 2013;13:458–465. doi: 10.1111/j.1447-0594.2012.00923.x. [DOI] [PubMed] [Google Scholar]
- 38.Kim H.Y., Kim M., Kojima N., Fujino K., Hosoi E., Kobayashi H., Somekawa S., Niki Y., Yamashiro Y., Yoshida H. Exercise and nutritional supplementation on community-dwelling elderly japanese women with sarcopenic obesity: A randomized controlled trial. J. Am. Med. Dir. Assoc. 2016;17:1011–1019. doi: 10.1016/j.jamda.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 39.Liao C.D., Tsauo J.Y., Lin L.F., Huang S.W., Ku J.W., Chou L.C., Liou T.H. Effects of elastic resistance exercise on body composition and physical capacity in older women with sarcopenic obesity: A consort-compliant prospective randomized controlled trial. Medicine. 2017;96:e7115. doi: 10.1097/MD.0000000000007115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jung W.S., Kim Y.Y., Park H.Y. Circuit Training improvements in korean women with sarcopenia. Percept. Mot. Skills. 2019;126:828–842. doi: 10.1177/0031512519860637. [DOI] [PubMed] [Google Scholar]
- 41.Lee Y.H., Lee P.H., Lin L.F., Liao C.D., Liou T.H., Huang S.W. Effects of progressive elastic band resistance exercise for aged osteosarcopenic adiposity women. Exp. Gerontol. 2021;147:111272. doi: 10.1016/j.exger.2021.111272. [DOI] [PubMed] [Google Scholar]
- 42.Seo M.Y., Jung S.W., Kim S.W., Lee J.M., Jung H.C., Song J.K. Effects of 16 Weeks of Resistance Training on Muscle Quality and Muscle Growth Factors in Older Adult Women with Sarcopenia: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health. 2021;18:6762. doi: 10.3390/ijerph18136762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu Y.Q., Peng N., Zhou M., Liu P.P., Qi X.L., Wang N., Wang G., Wu Z.P. Tai chi and whole-body vibrating therapy in sarcopenic men in advanced old age: A clinical randomized controlled trial. Eur. J. Ageing. 2019;16:828–842. doi: 10.1007/s10433-019-00498-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu C.K., Leng X., Hsu F.C., Kritchevsky S.B., Ding J., Earnest C.P., Ferrucci L., Goodpaster J., Guralnik J.M., Lenchik L., et al. The impact of sarcopenia on a physical activity intervention: The lifestyle Interventions and Independence for elders pilot study (life-p) J. Nutr. Health Aging. 2014;18:59–64. doi: 10.1007/s12603-013-0369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wei N., Ng S.S.M., Ng G.Y.F., Lee R.S.Y., Lau M.C.K., Pang M.Y.C. Whole-Body Vibration training improves muscle and physical performance in community dwelling with sarcopenia: A randomized controlled trial. Int. J. Phys. Ther. Rehabil. 2016;2:1–6. doi: 10.15344/2455-7498/2016/116. [DOI] [Google Scholar]
- 46.Newman A.B., Kupelian V., Visser M., Simonsick E.M., Goodpaster B.H., Kritchevsky S.B., Tylavsky F.A., Rubin S.M., Harris T.B. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J. Gerontol. 2006;61:72–77. doi: 10.1093/gerona/61.1.72. [DOI] [PubMed] [Google Scholar]
- 47.Evans J.W. Periodized Resistance Training for Enhancing Skeletal Muscle Hypertrophy and Strength: A Mini-Review. Front. Physiol. 2019;10:13. doi: 10.3389/fphys.2019.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brooks G.A. Bioenergetics of exercising humans. Compr. Physiol. 2012;2:537–562. doi: 10.1002/cphy.c110007. [DOI] [PubMed] [Google Scholar]
- 49.Antoniak A.E., Greig C.A. The effect of combined resistance exercise training and vitamin D3 supplementation on musculoskeletal health and function in older adults:a systematic review and Meta-analysis. BMJ Open. 2017;7:e014619. doi: 10.1136/bmjopen-2016-014619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheng Y.W. Master’s Thesis. Kunming Medical University; Kunming City, China: 2018. Research on the Intervention of Regular Fitness Exercise on Female’s Muscle Mass, Grip Strength and Pace; pp. 21–23. [Google Scholar]
- 51.Chiara M., Francesco P., Corinna S., Pugliarello R., Zancanaro C. Effects of whole-body vibration with or without localized radiofrequency on anthropometry, body composition, and motor performance in young nonobese women. J. Altern. Complement. Med. 2012;18:69–75. doi: 10.1089/acm.2010.0324. [DOI] [PubMed] [Google Scholar]
- 52.Chiara M., Francesco P., Grazia Z.M., Paolo M., Carlo Z. Ten-week whole-body vibration training improves body composition and muscle strength in obese women. Int. J. Medical Sci. 2013;10:307–311. doi: 10.7150/ijms.5161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Figueroa A., Gil R., Wong A., Hooshmand S., Park S.Y., Vicil F., Gonzalez M.A. Whole-body vibration training reduces arterial stiffness, blood pressure and sympathovagal balance in young overweight/obese women. Hypertens. Res. 2012;35:667–672. doi: 10.1038/hr.2012.15. [DOI] [PubMed] [Google Scholar]
- 54.Zhou Y.Z., Chen P.J., Zheng L.F., Xiao W.H. Pathogenesis and treatment strategy of disuse muscular atrophy. Chin. J. Rehabil. 2017;32:1307–1313. [Google Scholar]
- 55.Shen L.B., Shen X. Research progress on the impact of sports on the elder’s muscle power. J. Sports Sci. 2016;37:49–51. [Google Scholar]
- 56.Kirsten E.B., Christopher S., Gianni P., Steven K.B., Stuart M.P. Day-to-Day Changes in Muscle Protein Synthesis in Recovery From Resistance, Aerobic, and High-Intensity Interval Exercise in Older Men. J. Gerontol. 2015;70:1024–1029. doi: 10.1093/gerona/glu313. [DOI] [PubMed] [Google Scholar]
- 57.Alessandra M., Stefania B., Elisa M.M., Jack G., Giovanni Z., Luigi F., Stefano V. Combining gait speed and recall memory to predict survival in late life: Population-based study. J. Am. Geriatr. Soc. 2017;65:614–618. doi: 10.1111/jgs.14705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee J.H., Dong S.H., Jeong J.C., Yoon B. Effects of transcranial direct current stimulation over the dorsolateral prefrontal cortex (pfc) on cognitive-motor dual control skills. Percept. Mot. Skills. 2020;127:803–822. doi: 10.1177/0031512520935695. [DOI] [PubMed] [Google Scholar]
- 59.Zhang B.Q., Ren D.H., Ruan M.F., Li Q.Q., Hou Q.P. Resistance exercise and prevention and treatment of sarcopenia. Zhejiang Sport Sci. 2021;43:87–107. [Google Scholar]
- 60.Wu T.T., Zhao Y.N. Associations between functional fitness and walking speed in older adults. Geriatr. Nurs. 2021;42:540–543. doi: 10.1016/j.gerinurse.2020.10.003. [DOI] [PubMed] [Google Scholar]
- 61.Chu S.F., Liou T.H., Chen H.C., Huang S.W., Liao C.D. Relative Efficacy of Weight Management, Exercise, and Combined Treatment for Muscle Mass and Physical Sarcopenia Indices in Adults with Overweight or Obesity and Osteoarthritis: A Network Meta-Analysis of Randomized Controlled Trials. Nutrients. 2021;13:1992. doi: 10.3390/nu13061992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shen Y.J., Liu D., Li S.Y., He Y.Z., Tan F.C., Sun X.L., Li D.P., Xia X., Hao Q.K. Effects of Exercise on Patients Important Outcomes in Older People With Sarcopenia: An Umbrella Review of Meta-Analyses of Randomized Controlled Trials. Front. Med. 2022;9:811746. doi: 10.3389/fmed.2022.811746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wen P.T., Zhang R.L., Hu W.Q., Wang X., Wu J.M., Li H.J. Effect of Different Modes of Exercise on Sarcopenia in Older Adults: A Meta-analysis. Chin. J. Rehabil. Theory Pract. 2021;27:1291–1298. [Google Scholar]
- 64.Zhao Y.N., Wu T.T., Wei Y. Effects of starting position, distance and ending point in a walking speed test among older adults. Geriatr. Gerontol. Int. 2020;20:680–684. doi: 10.1111/ggi.13938. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.