Abstract
Zinc plays an important role in cardiomyocytes, where it exists in bound and histochemically reactive labile Zn2+ forms. Although Zn2+ concentration is under tight control through several Zn2+-transporters, its concentration and intracellular distribution may vary during normal cardiac function and pathological conditions, when the protein levels and efficacy of Zn2+ transporters can lead to zinc re-distribution among organelles in cardiomyocytes. Such dysregulation of cellular Zn2+ homeostasis leads to mitochondrial and ER stresses, and interrupts normal ER/mitochondria cross-talk and mitophagy, which subsequently, result in increased ROS production and dysregulated metabolic function. Besides cardiac structural and functional defects, insufficient Zn2+ supply was associated with heart development abnormalities, induction and progression of cardiovascular diseases, resulting in accelerated cardiac ageing. In the present review, we summarize the recently identified connections between cellular and mitochondrial Zn2+ homeostasis, ER stress and mitophagy in heart development, excitation–contraction coupling, heart failure and ischemia/reperfusion injury. Additionally, we discuss the role of Zn2+ in accelerated heart ageing and ageing-associated rise of mitochondrial ROS and cardiomyocyte dysfunction.
Keywords: zinc homeostasis, ER stress, mitophagy, cardiovascular disease, ageing
1. Introduction
Zinc is a redox inactive element, a crucial component of the antioxidant defence system, maintaining the cell redox balance, and is involved in a multitude of physiological functions [1]. In many cells and tissues, labile Zn2+ plays an important role as a signalling molecule, structural component or major regulator of macromolecules; therefore, even mild disturbance in Zn2+ homeostasis may impact human health, lead to neurodegenerative and neurodevelopmental disorders, diabetes and obesity, malfunction of reproductive system, cardiovascular diseases and other pathological conditions [2,3]. In particular, a high intracellular labile Zn2+ ([Zn2+]i) level is toxic for cardiomyocytes, where [Zn2+]i also takes part in excitation–contraction coupling and in excitation–transcription coupling [4]. Mammalian cells have different ways to regulate [Zn2+]i homeostasis. Zinc could be released from metalloenzymes or metalloproteins, several other intracellular storages and organelles, regulated through Zn2+-binding molecules and Zn2+ sensors, and transported by the two main Zn2+ transporter families Zrt-/Irt-like protein (ZIP) and zinc transporters (ZnT) [5].
It is known that the development of cardiac dysfunction is closely associated with increased mitochondrial reactive oxygen species (ROS) production, which could be caused by different pathological conditions (such as hyperglycaemia or hyperlipidaemia). In particular, Zn2+-associated signalling pathways are involved in the development of diabetic cardiomyopathy and other heart diseases [6,7]. In that regard, the close coordination of [Ca2+]i and [Zn2+]i homeostasis in mitochondrion balances the redox status and oxidative stress status of cardiomyocytes and provides a cardioprotective effect [8].
A number of studies suggest that both Zn2+ deficiency and excess zinc are harmful to cells, leading to impaired excitation–contraction cycling in cardiomyocytes, metabolic disorders and growth retardation [9,10]. Under normal physiological conditions, the cytosolic labile [Zn2+]i in cardiomyocytes is less than 1 nM, while it could increase ~2 fold under chronic hyperglycaemia conditions or even ~30-fold under acute oxidant exposure, although total cellular Zn2+ is about 200 µM [11,12]. Additionally, mitochondria were proved to be another crucial intracellular Zn2+-pool in cardiomyocytes, thus, further connecting mitochondria as a major cellular ROS producer, Zn2+-mediated alterations in mitochondria ultrastructure and their role in the pathogenesis of many cardiovascular diseases [13,14]. The role of Zn2+ homeostasis in diabetes mellitus and obesity-induced cardiac inflammation, remodelling, dysfunction and other cardiovascular diseases and complications was thoroughly covered in several recent excellent reviews [9,15,16]. Therefore, those topics will be excluded from our review. We wish to redirect interested readers to the suggested papers.
2. Zn2+ in Mitochondrial Homeostasis
Although mitochondria represent an important intracellular Zn2+ pool and both insufficient or excessive Zn2+ are deleterious to the mitochondrial structure and function, it is not known to date how Zn2+ is transported into and out of mitochondria. Surplus Zn2+ accumulation in mitochondria has diverse negative effects on the mitochondria, where it inhibits α-ketoglutarate dehydrogenase and complexes I and III of the electron transport chain (ETC), increases ROS production and causes loss of MMP (mitochondrial membrane potential) and activates mPTP (mitochondrial permeability transition pore), which leads to the release of pro-apoptotic factors and cell death [17]. Further, zinc overload causes abnormal morphological changes: mitochondria had shortened in length and increased in circularity, decreased in major axis and in aspect ratio [18]. Incubation of cardiomyocytes with a Zn2+ source (as Zn2+ pyrithione) increases the phosphorylation levels of several signalling proteins, such as Glycogen Synthase Kinase-3 Beta (GSK3β), Protein Kinase B (Akt) and nuclear factor-κ B (NFκB), and increases the level of endoplasmic reticulum (ER) stress proteins, such as Heat Shock Protein 70 Family Protein 5 (GRP78) and calregulin (Endoplasmic Reticulum Resident Protein 60) and ER-mitochondria contact sites protein promyelocytic leukemia protein (PML), thus, suggesting the involvement of Zn2+ in ER-mitochondria cross-talk and ER stress [19].
Zn2+ is involved in the regulation of mitochondrial dynamics (fission and fusion) and mitophagy, a quality control mechanism, specialised form of autophagy, targeted to degrade damaged and superfluous mitochondria to reinstate cellular homeostasis in response to various stresses. Mitochondrial fission is regulated by DRP1 (DNM1L or dynamin 1 like)/FIS1 (fission, mitochondrial 1) and fusion is regulated by MFN1, MFN2 (mitofusin 1 and 2)/OPA1 (optic atrophy protein 1) genes [20]. While there are several defined mitophagy mechanisms, in general, they could be divided into two main types: conventional PTEN-Induced Kinase 1/Parkin RBR E3 Ubiquitin Protein Ligase (PINK1/Parkin)-mediated and alternative Parkin-independent mitophagy [21,22]. After damaged parts of mitochondria are digested during mitophagy, healthy parts of mitochondria are fused back to the mitochondrial network and continue normal functioning [23]. Mitophagy and balanced fission/fusion are crucial for many cellular processes, normal organism development and functioning, and their dysregulation is associated with many developmental and neurodegenerative diseases, cancer, inflammageing and other pathologies [24,25]. As it was found, ROS promotes the release of lysosomal Zn2+ and increases mitochondrial Zn2+, which triggers mitochondrial division by promoting mitochondrial recruitment of Drp1 [26]. Further, a Drp1–ZIP1 interaction could stimulate Zn2+ entry into mitochondria, thus, reducing the MMP and promoting mitochondrial fission and mitophagy [27]. Furthermore, high mitochondrial Zn2+ might promote PINK/Parkin-mediated mitophagy by increasing BECN1 (Beclin1 or ATG6, Autophagy Related) expression in cardiomyocytes in response to hypoxia oxygenation (H/R) [28].
A recent study on the Caenorhabditis elegans model suggested that Znt9 (SLC-30A9, Solute Carrier Family 30 Member 9) is the mitochondrial Zn2+ exporter, the loss of which causes Zn2+ accumulation in mitochondria and dysregulation of the mitochondrial structure and functions, aggravating normal animal development and reducing lifespan. Moreover, SLC-25A25/SCaMC-2 was identified as an important regulator of mitochondrial Zn2+ import, the losing of which suppresses functional and structural defects caused by a loss of SLC-30A9. It was shown that mitochondria mostly import Zn2+ from the endoplasmic reticulum Zn2+ pool [29]. Similarly, the role of SLC-30A9 in Zn2+ export from mitochondria was also confirmed in humans, where loss of SLC-30A9 leads to excessive Zn2+ accumulation in mitochondria, severe mitochondrial swelling, increased ROS production and dysregulated metabolic function. Additionally, it was proposed that SLC-30A9 is crucial for sperm activation and organismal fertility, and in neurons, the SLC-30A9 mutation is responsible for Birk–Landau–Perez cerebrorenal syndrome—an autosomal recessive syndrome, characterized by nephropathy, muscle weakness, intellectual disability, camptocormia and oculomotor apraxia [30,31].
In summary, the dysregulation of cellular Zn2+ homeostasis leads to mitochondrial and ER stresses, interrupts normal ER/mitochondria cross-talk and mitophagy, and subsequently, results in increased ROS production and dysregulated metabolic function. Besides cardiac structural and functional defects, insufficient Zn2+ transport was associated with development abnormalities, such as Birk–Landau–Perez cerebrorenal syndrome. Therefore, further investigation of Zn2+-regulating treatments to prevent mitochondrial zinc overload and the associated changes in mitochondrial functions could provide vital mechanistic insights for understanding and treating these human diseases.
3. Zn2+ in Cardiac Function and Pathology
Impaired Zn2+ homeostasis is associated with developmental defects, including multiple types of cardiac abnormalities, a variety of cardiovascular disorders and diseases. Sufficient Zn2+ supply is crucial for normal heart electrical and mechanical functions and the operation of redox signalling pathways. However, Zn2+ levels beyond a narrow concentration range are toxic and damaging for cardiomyocytes [32]. Further, in this section, we discuss the role of Zn2+ in heart development, cardiac contraction and arrhythmia, heart failure, ischaemia/reperfusion injury and heart ageing.
3.1. Zn2+ in Heart Development
Heart development is a sophisticated process orchestrated by multiple transcription factor families, which regulate myocardial gene expression, vessel and muscle growth, and morphogenesis, which eventually result in the formation of a functionally competent ventricle wall. In the first step, the tubular heart is composed of an outer one-cell layer of myocardium and an inner one-cell layer of endocardium separated by cardiac jelly (or ECM—extracellular matrix). In the next step (trabeculation), cardiomyocytes protrude into the cardiac jelly and form rapidly growing structures—trabeculae. Trabeculae facilitate nutrient and oxygen exchange between the blood and the heart before coronary circulation is arranged. Accompanying the initiation of coronary circulation, trabeculae go through the compaction process, collapsing into the ventricle wall and becoming part of the compact myocardium. In a further stage, the ventricle wall has a thick compact myocardium composed of multi-layered spiral cardiomyocytes, with few trabeculae on the surface [33].
Zinc is an important microelement, involved in gene transcription, protein translation and maintaining the structural stability of organelles via synthesis and activity regulation of various metalloenzymes, transcription factors and RNA polymerase [34]. Recent research suggested that gestational zinc deficiency is a cause of congenital heart disease (CHD) in a mouse foetus [35]. CHD combines a wide range of birth defects, which are present from birth and affect the normal way the heart works. It was established that zinc deficiency induced SENP5 overexpression, which led to cardiac dysplasia. SUMO1/Sentrin Specific Peptidase 5 (SENP5) is responsible for the reversible posttranslational modification of proteins by the addition of small ubiquitin-like SUMO proteins, altering the conformation, localization and stability of the target protein, and is involved in regulation of the ribose biosynthesis, stem cell maintenance and differentiation, cell cycle, DNA damage repair, chromatin remodelling and gene transcription [36]. In-vitro experiments on cardiomyocytes differentiated from human-induced pluripotent stem cells under a zinc deficiency condition showed increased SENP5 expression, reducing conjugated SUMO during heart development and causing increased cell apoptosis and decreased viability, and further resulted in myocardial abnormality [35].
Recent research proposed the cellular zinc importer ZIP8 (Solute carrier family 39 member 8 or Slc39a8) as a novel regulator of ventricular myocardial development [37]. Left ventricular noncompaction (LVNC) is a form of CHD caused by arrested compaction, which usually affects both ventricles and is characterized by excessive trabeculation with deep intertrabecular recesses and a thin compact myocardium. Typical LVNC complications are systemic embolic events, ventricular arrhythmias and heart failure [38,39]. Homozygous Slc39a8-null mice embryos do not survive embryogenesis and exhibit an LVNC-similar cardiac phenotype. It was shown that SLC39A8 is expressed by endothelial cells in the developing mouse heart, where it is involved in ECM degradation through the MTF1 (metal regulatory transcription factor 1), which promotes the expression of several ECM-degrading A Disintegrin and Metalloproteinase with Thrombospondin Motifs 1 (ADAMTS) metalloproteinases. Therefore, SLC39A8 knockdown decreases cellular Zn uptake, with a subsequent reduction in MTF1 transcription activity, expression of Adamts metalloproteinases and impaired cardiac ECM degradation [37].
3.2. Zn2+ in Excitation–Contraction Coupling
Molecular and pharmacological studies have shown that labile Zn2+ can permeate membranes through both ligand-gated channels and voltage-activated Ca2+ channels, and also significantly affect the functionality of these channels. [Zn2+]i is widely recognized as a vital intracellular second messenger, known to regulate K+ ATP-channels (ATP-dependent K+-channels), M-type K+-channels, both L-type and T-type of Ca2+-channel currents and Cl−-conductance, thus, affecting the electrical activity of a single cardiomyocyte and further stimulating an induction of arrhythmia at the cellular level [40]. Additionally, an increase in [Zn2+]i levels is closely associated with increased production of ROS/reactive nitrogen species (RNS) and induction of thiol oxidation and hyper-phosphorylation of many cellular proteins and kinases, thus, affecting the contractile machinery of cardiomyocytes [32].
Recent research suggested that high [Zn2+]i could inhibit voltage-dependent K+-currents, thereby altering cardiac function by prolonging action potential. In particular, a high [Zn2+]i level significantly lowers the cellular ATP level, inhibits transient outward K+-currents, steady-state currents and inward-rectifier K+-currents, leading to a modulation of depolarization in resting membrane potential, prolongations in action potential repolarizing phases and induction of spontaneous action potentials [41].
Overall, the presented data suggested a crucial role for the high cardiomyocytes [Zn2+]i level in the induction of cardiac contraction and arrhythmia. Therefore, application of [Zn2+]i controlling drugs/compounds could be a good candidate for a novel therapeutic target in cardiac dysfunction. However, further studies to investigate the underlying physiology of [Zn2+]i action in cardiomyocytes are required.
3.3. Zn2+ in Heart Failure
Heart failure (HF) is a clinical syndrome of progressive functional degradation when cardiac output is inadequate to meet the needs of the organism. HF may be present from birth (congenital heart disease) or occur secondarily, because of various insults, such as atherosclerosis, myocardial infarction, hypertension, arrhythmia, toxic stimuli and others, affecting over 64 million people worldwide [42,43]. Mitochondrial dysfunction, including defects in mitophagy, redox signalling, biogenesis, oxidative phosphorylation, network organization, unfolded protein response, fission/fusion and substrate selection, are widely recognised as significant factors in HF progression [44]. Recent research shows that increased mitochondrial ROS is the main driver of both acute events (such as electrical instability, responsible for sudden cardiac death) and chronic HF-associated remodelling events (disordered expression and phosphorylation of proteins crucial for excitation–contraction and antioxidant defence system) [45].
Experimental and clinical evidence suggests a crucial role for the crosstalk between Zn2+ and Ca2+ transporters and channels in the control of cardiac contractility. In particular, the cardiac ryanodine receptor (RYR2), known to conduct Ca2+ in vivo and other divalent cations in vitro, has attracted much attention [7]. During the cardiac cycle, myocardial contraction is initiated by Ca2+ influx into the cell, where it binds to and activates the RyR2 receptor. Further, the opening of RyR2 channels releases Ca2+ from the sarcoplasmic reticulum (SR), which results in a transient rise in cytosolic Ca2+. Because of the combined action of RyR2 channel closure, extrusion of Ca2+ from the cell and uptake of Ca2+ back into the SR Ca2+ stores, the Ca2+ concentration is reduced and causes cardiac muscle relaxation. The current understanding suggests that HF RyR2 channels cannot remain closed during diastole and become “leaky”, leading to dysregulated Ca2+ homeostasis in cardiomyocyte and spontaneous Ca2+ spark frequency, resulting in irregular contractile activity and decreased systolic contraction. Additionally, there is some evidence for the involvement of unknown channels, facilitating an RyR2-independent mechanism of Ca2+ efflux [46].
Indeed, recent research suggested that pathophysiological concentrations of Zn2+ not only dysregulated RyR2-channel openings but also activated the transmembrane protein mitsugumin 23 (MG23) [47], a recently identified voltage-dependent non-selective cation-conducting channel abundant in the ER/SR, where it was proposed to participate in regulating Ca2+ dynamics alongside RyR2 [48].
Additionally, in HF, increased [Zn2+]i induces ER stress via increased total PKC, PKCα expression and PKCα-phosphorylation. Increased levels of [Zn2+]i were associated with significantly elevated levels of ER stress markers GRP78, CHOP/Growth Arrest and DNA Damage-Inducible Protein GADD153 (Gadd153) and calnexin. Further, HF was associated with increased expression of ZIP14 and ZNT8 and decreased levels of ZIP8 Zn2+ transporters [49]. Interestingly, that activated PKCα is known as an important mediator of induction of ventricular arrhythmias [50] and effector of oxidative tissue injury [51]. Similarly, experiments on a transverse aortic constriction (TAC) rat model, mimicking the development of cardiac hypertrophy-associated heart failure, demonstrated increased expression of ER stress markers (GRP78, CHOP/Gadd153 and calnexin) and apoptotic status markers (Glycogen Synthase Kinase-3 Beta (GSK3B), Bax-to-Bcl-2 ratio and BCL2-Binding Component 3 (BBC3)). Further, the ratios of phosphorylated to non-phosphorylated Akt, NFκB and PKCα proteins were significantly higher in the TAC group. Heart tissue from the TAC group had increased expression levels of ZIP7, ZIP14 and ZNT8 transporters, while the expression levels of ZIP8 and ZNT7 were decreased [52]. Recent research has identified the involvement of SLC39A2 (ZIP2) in the development of cardiac hypertrophy and heart failure [53]. Interestingly, ZIP2 knockdown enhances innate immune signalling pathways (NFκB, TOLL-like receptor and interferon regulatory factors (IRFs)), which are responsible for programmed cell death, inflammatory response and defence against microbes [54,55,56], while the expression of IκBα (Inhibitor of NFκBα) was reduced. Those data demonstrate the tight regulatory circuit between ZIP2-mediated zinc homeostasis and remodelling of innate immune signalling in cardiomyocyte hypertrophy [53].
Interestingly, zinc deficiency (caused by malnutrition, intestinal malabsorption or other reasons) is associated with the development and deterioration of HF via several pathobiological pathways (systemic inflammation, dysregulated apoptosis and oxidative stress) [57]. As it was shown on hearts of weaned piglets affected by subclinical zinc deficiency, the level of glutathione and the expression of ROS-detoxifying enzymes (glutathione, glutathione reductase, catalase and metallothionein 1A) was decreased, while the expression of pro-apoptotic genes (B-cell lymphoma 2–associated X protein (BAX) and caspase 9 (CASP9)) was increased and the expression of (FAS), etoposide-induced 2.4 (EI24) and cyclin-dependent kinase inhibitor 1A (CDKN1A) correlated positively to cardiac zinc level in piglets [12]. Recent clinical data clearly support such an association, suggesting to use zinc level as a possible biomarker of cardiovascular health and application of zinc supplementation on outcomes in patients with HF [58,59].
In conclusion, zinc deficiency is linked to the development and progression of HF syndromes, where [Zn2+]i (controlled via Zn2+ transporters) acts on the intersection between ER stress and PKCα activation pathways in HF induction. The number of available studies suggests the high therapeutic potential of zinc supplementation, which, however, requires further clinical confirmation, ideally, in randomized trials.
3.4. Zn2+ in Ischemia/Reperfusion Injury
Myocardial ischemia/reperfusion injury (I/R) is a very common cardiovascular disease with a high mortality rate, which is caused by an inadequate supply to the heart of blood and nutrients, because of a narrowing or closure of the coronary arteries or increased myocardial substrate demand. Pathogenesis of myocardial infarction is caused by intracellular Ca2+ overload, ATP depletion, increased migration of neutrophils to the ischemic tissue, acidosis, ROS overproduction and activation of inflammatory, ER stress, apoptosis and other pathways [60,61]. Recent research suggests protective effects from Zn2+ against I/R through stimulation of anti-oxidant defence, anti-inflammatory and other mechanisms, thus, reducing ischemic injury and facilitating recovery [62]. Further in this section, we cover recent research explaining the role of Zn2+ in ischemia/reperfusion injury and the related in-vitro model (hypoxia–reoxygenation injury).
3.4.1. Mitochondria as the Main Source of Chemically Reactive Species in I/R Injury
Chemically reactive species are a product of both normal cellular physiological processes and pathological conditions and are usually represented by oxygen and/or nitrogen (reactive oxygen and nitrogen species). In general, increased ROS and/or RNS has been associated with many cardiovascular diseases, including ischaemia/reperfusion injury. Imbalance between ROS/RNS generation and removal causes oxidative/nitrosative alterations of proteins, lipids, carbohydrates and nucleic acids, which result in derangements of cellular structures, transduction of hormonal stimuli, inflammatory and apoptosis signalling [63]. Because mitochondria occupy a significant volume of cardiomyocytes and utilize more than 90% of oxygen reaching the cardiac muscle, it is not surprising that a substantial part of ROS generation occurs in mitochondria. For this reason, mitochondria are the main targets of many signalling pathways involved in different cardioprotection strategies [64].
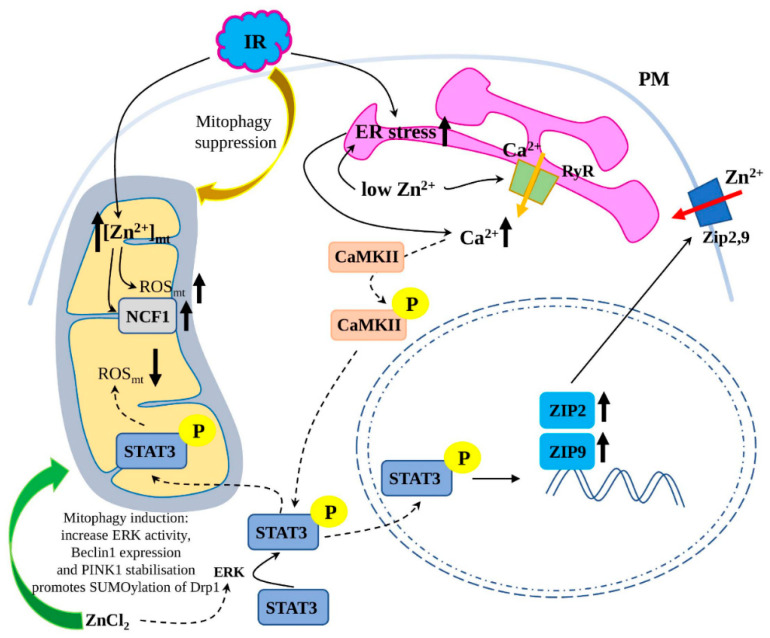
As it was shown on the HeLa cells, subjected to chemical ischaemia, stimulated by 30 min of oxygen and glucose deprivation, Zn2+ is released from intracellular stores and accumulated in mitochondria (Figure 1). Further, a rise in Zn2+ was followed by a rise in mitochondrial ROS production and congruent with an increase in the functional component of NADPH oxidase, p47phox (NCF1) [65], which produces superoxide anion [66]. Thus, a positive feedback loop during ischemic stress is suggested, where an excess of free Zn2+ and mitochondrial ROS closely connected via NADPH component p47phox [65].
Figure 1.
The role of Zn2+ in ischemia/reperfusion injury (I/R). I/R was shown to suppress mitophagy, increase mitochondrial reactive oxygen species (ROS) production and induce endoplasmic reticulum (ER) stress. I/R leads to increased Zn2+ accumulation in mitochondria, further increasing ROS production via (Neutrophil Cytosolic Factor 1) NCF1. I/R, similarly to Zn2+ deficiency, induces ER stress, which leads to ryanodine receptor (RyR) channel activation and increase in intracellular Ca2+ level and Ca2+-calmodulin-dependent protein kinase (CaMKII) phosphorylation, which subsequently acts on Signal Transducer and Activator of Transcription 3 (STAT3) to stimulate ZIP2 and 9 expression. Zn2+ treatment provides cardioprotective effect by stabilising PTEN Induced Kinase 1 (PINK1) and dynamin 1 like (Drp1) proteins, increasing ATG6, Autophagy Related (Beclin1) expression and ERK activity, thus stimulating mitophagy. Additionally, ERK phosphorylates STAT3 in a Zn2+-dependent way. The direction of Zn2+ transport is depicted with a red arrow, Ca2+—orange, increased/decreased levels of metabolites/gene expression—black; PM—plasma membrane.
Myocardial zinc homeostasis contributes to the cardioprotective effect from hypoxia/reoxygenation injury, ischemia/reperfusion injury and ischemia postconditioning. One of the Zn2+-mediated cardioprotective mechanisms relies on the activation of STAT3 (Figure 1), which is phosphorylated at Ser727 by Mitogen-Activated Protein Kinase 1 or ERK (MAPK1) and important for regulating cardioprotective proteins, mPTP opening, mitochondrial respiration and ETC activity [67,68]. As it was shown on mitochondria isolated from rat hearts subjected to reperfusion, ZnCl2 treatment increases the level of phospho-Ser727 STAT3 in mitochondria, which improves the mitochondrial oxidative phosphorylation and increases the mRNA level of the complex I subunit ND6, reduces mitochondrial ROS generation and preserves mitochondrial membrane potential (ΔΨm) [69].
Interestingly, another study demonstrated that ZIP2 expression was increased in an STAT3-dependent way at reperfusion in in-vivo mouse hearts in an attempt to compensate Zn2+ loss (Figure 1). Knockout of Zip2 genes (Zip2+/− and Zip2−/−) aggravates I/R injury, and STAT3 gene delivery failed to reduce I/R injury in Zip2−/− mice, while the delivery of both genes (Zip2 and STAT3) to wild-type mice reduced I/R injury [70].
3.4.2. Zn2+ Regulation of Mitophagy in I/R Injury
An increasing body of evidence shows a vital role for Zn2+ in the regulation of autophagy and cardiac homeostasis. Mitophagy, a specialised form of autophagy, selectively targets and degrades damaged or dysfunctional mitochondria, thus, preventing excessive production of ROS and playing a cardioprotective function in a wide range of cardiac diseases [71,72]. As it was shown on cardiac myoblasts (H9c2 cell line) subjected to hypoxia/reoxygenation, zinc treatment (in form of ZnCl2) induces autophagy and mitophagy by increasing ERK activity, Beclin1 expression and stabilising PINK1 (Figure 1). At the reoxygenation stage, those Zn2+-mediated effects prevented mitochondrial superoxide generation and dissipation of mitochondrial membrane potential (ΔΨm), thus, protecting the heart from H/R injury [28].
Similarly, recent research has shown that zinc regulates mitophagy and contributes to the cardioprotection by modulating the small ubiquitin-like modifier (SUMO) system, which is responsible for the involvement of modified proteins in different cellular processes (apoptosis, transcriptional regulation, protein stability and nuclear transport) [73]. Zn2+ treatment reverses the negative H/R effects (decreased SUMO1 modification level of proteins and increased myocardial apoptosis) and promotes SUMOylation of Drp1, the crucial regulator of mitophagy [74].
Myocardial reperfusion injury upregulates mitochondrial Zn2+ transporter ZIP7 and suppresses mitophagy [75]. As it was shown on mouse hearts and human heart samples with the cardiac-specific ZIP7 conditional knockout and subjected to ischemia/reperfusion in vivo, ZIP7 is upregulated at reperfusion, which leads to mitochondrial hyperpolarization and prevents PINK1 and Parkin accumulation in mitochondria, thus, suppressing mitophagy. Similarly, ZIP7 is markedly upregulated in cardiac mitochondria samples from patients with heart failure. On the contrary, ZIP7 knockout enhanced mitophagy upon reperfusion, reduced mitochondrial ROS generation and myocardial infarction size in a PINK1-dependent manner [75]. Therefore, timely normalisation of the mitochondrial Zn2+ level via downregulation or inactivation of the mitochondrial ZIP7 expression could be beneficial for patients with acute myocardial infarction and other cardiovascular diseases. Further, because impaired mitophagy is associated with many other diseases and disorders (such as Alzheimer’s and Parkinson’s diseases, premature ageing, atherosclerosis and others) [76,77], zinc-based modulation of the mitophagy rate with therapeutic or pharmacological approaches may have wide clinical applications.
3.4.3. Zn2+-Mediated Regulation of ER Stress in I/R Injury
ER is responsible for various cellular functions, including protein production and folding, regulation of Ca2+ levels, steroids and lipid synthesis and metabolism. However, different cellular stresses could interrupt normal protein synthesis, disturb proper protein folding or cause accumulation of non-properly folded proteins and activated so-called unfolded protein response (URP). Prolonged activation of the UPR disrupts normal ER functions and leads to an “ER stress” state, which is accompanied by the activation of various ER stress chaperones or proteins, such as calregulin, GRP78 and GRP94. Multiple studies have proved the close association of ER stress with I/R injury and other cardiovascular diseases (reviewed in [78,79]).
Interestingly, a recent report suggested that reperfusion initiates ER stress but not ischaemia. Analysis of GRP78 and GRP94 expression during ischaemia and reperfusion in combination with ER stress inhibitor tauroursodeoxycholic acid (TUDCA) and zinc chelator N,N,N′,N′-tetrakis-(2-pyridylmethyl) ethylenediamine (TREN) proved involvement of Zn2+ in ER stress inhibition-induced cardioprotection against I/R injury by modulation of the mPTP opening. Application of TUDCA increases intracellular free zinc, reduces infarct size, prevents ER and mitochondrial damages at reperfusion, thus, protecting the heart from reperfusion injury in a Zn2+-dependent way [80].
The treatment of cardiomyocytes with zinc pyrithione provides several beneficial effects after H/R; in particular, it normalises depleted intracellular zinc, enhances intracellular superoxide production, mitigates H/R-induced UPR and improves proteasomal function. Further, zinc pyrithione suppresses NADPH oxidase 2 (NOX2), which is responsible for ROS production and promotes Erb-B2 Receptor Tyrosine Kinase 2 (ErbB2) signalling, thus, improving cell survival after H/R [81]. The dysregulation of ErbB2 expression was associated with many cancer types, heart failure and ischemic heart disease; ErbB dimerization and activation of PI3K/Akt cascade suppresses cardiomyocyte cell death through caspase inactivation [82]. Thus, Zn2+ replenishment after myocardial H/R alleviates associated oxidative stress, UPR and improves cardiomyocytes survival.
Recent research has shown a close association between ER stress, Ca2+ signalling, STAT3 activation and Zn2+ homeostasis in I/R cardioprotection (Figure 1). Zinc deficiency triggers ER stress and enhances RyR2 expression in the ER, thus, mediating Ca2+ release and Ca2+-calmodulin-dependent protein kinase (CaMKII) phosphorylation. Subsequently, phosphorylated CaMKII activates STAT3, which promotes ZIP9 expression to normalise the imbalance in Zn2+ homeostasis [83]. CaMKII is a serine/threonine-specific protein kinase that is regulated by the Ca2+/calmodulin complex, mediating many of the second messenger effects of Ca2+, and is involved in the regulation of many cellular and developmental processes, including cardiac rhythm [84]. Further, researchers have confirmed that reperfusion can initiate ER stress and cause an increase in intracellular Ca2+ and CaMKII activation [85].
Interestingly, a ZIP13 zinc transporter was shown to modulate the Ca2+ signalling in myocardial I/R injury. In particular, heart-specific knockout of ZIP13 leads to CaMKII activation, mitochondrial swelling, increases the mitochondrial Ca2+, ROS production, decreases the mitochondrial respiration control rate and dissipation of mitochondrial membrane potential in a CaMKII-dependent manner, and finally, exacerbates myocardial infarction in mouse hearts subjected to I/R. On the contrary, ZIP13 overexpression suppresses I/R-induced CaMKII phosphorylation and reduces infarct size [86].
These data suggest the involvement of Zn2+ in the functioning of a new ER stress/CaMKII/STAT3 cardioprotective mechanism, activated in the heart to increase the resistance to I/R injury. Furthermore, the interplay of Zn2+ with ER stress/CaMKII/STAT3 axis provides new pharmacological and therapeutic targets for treating diseases associated with zinc deficiency.
4. Aged Heart
Ageing is the dominant risk factor for cardiovascular diseases, as it contributes to cardiac morbidity and mortality in the aged population. The pathogenesis of cardiac ageing is associated with several parameters: dysregulation in Ca2+ homeostasis and changes in other ions exchange across sarcolemma, action potential prolongation, increased fibrosis, rate of mitochondrial defects and oxidative stress pressure in cardiomyocytes. Mechanically, mitochondrial oxidative stress and lipid overload increase sarcoplasmic reticulum Ca2+-leak through RyR2 channels, cause structural remodelling and affect the electrical and mechanical activation of the left ventricle [87,88]. In general, it is known that increased systematic and cardiac ROS production and oxidative stress, alongside decreased antioxidant capacity, are the main triggers of the development of aged-related insufficiencies. A comparison of aged and adult rats showed that aged rats have depressed contraction and relaxation activities in aortic rings, increased heart rate and mean arterial pressure, significantly prolonged RR and QT intervals, decreased ejection-fraction and preload-recruitable stroke work, alongside clear insulin resistance and hypertrophy in aged rats with normal fasting blood glucose. Further, an investigation of aged rats with a microscope suggested irregularly clustered mitochondria and lysosomes around the myofilaments in cardiomyocytes, flattened and partial local splitting in elastic lamellas in the aorta and increased muscle fibre radius and amount of collagen fibres in the heart [89,90]. Zinc is crucial for many cellular processes, including memory formation, immune function, reproduction, and antioxidant capacity. However, both zinc overload and deficiency are associated with cellular dysfunction and disease. Zinc deficiency is associated with premature ageing and the development of age-related diseases. On the contrary, zinc supplementation was shown to decrease markers of oxidative stress and the production of pro-inflammatory cytokines in elderly patients. However, surplus zinc supplementation induces senescence of several cell types and reduces lifespan in Caenorhabditis elegans [91,92]. Further in this section, we focus on recent publications investigating the role of zinc in ageing-related functional and structural changes in the cardiovascular system.
It is known that some zinc transporters are upregulated by pro-inflammatory stimuli, such as IL-6 (interleukin 6), in an age-dependent manner. Ageing is responsible for a significant increase in serum IL-6 and tissue-specific increases in Zn2+ concentration of skeletal muscle and white adipose tissue. Additionally, the proper functioning of ZIP8 and ZIP14 is necessary to maintain trabecular and cortical bone density during ageing, and could influence the development of such diseases as sarcopenia and osteoporosis [93].
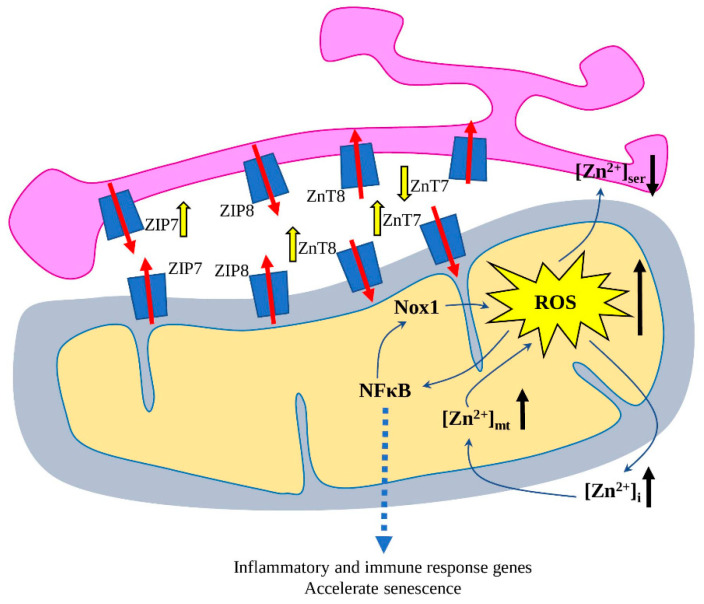
Aged cardiomyocytes had an increased [Zn2+]i level due to decreased levels of ZIP8 and ZnT7, and increased ZIP7 and ZnT8 transporters (Figure 2). Similarly, aged H9c2 cells demonstrated a significant increase in mitochondrial Zn2+ ([Zn2+]mt) with decreases in sarco/endoplasmic reticulum Zn2+ ([Zn2+]ser), where expression of mitochondrial Zn2+ transporters ZNT7 and ZNT8 was increased, and in the case of S/ER Zn2+ transporters, decreased expression of ZNT7 and increased ZIP7 [94].
Figure 2.
Redistribution of [Zn2+]i, [Zn2+]mt and [Zn2+]ser in the aged cardiomyocytes during the development of ageing-mediated cardiac dysfunction. The increased ROS production is one of the leading factors, leading to the redistribution of Zn2+ between cytosol, mitochondria and sarco/endoplasmic reticulum (SER) via modulated expression of Zn2+ transporters. ROS activates nuclear factor-κ B (NFκB), which translocates to the nucleus and activates different genes related to immune and inflammatory response. Further, NFκB increases NADPH Oxidase 1 (Nox1) expression, thus, further increasing ROS production and resulting in accelerated senescence. The ROS-mediated effects on the Zn2+ transporters are depicted with yellow arrows; the direction of Zn2+ transport—with red arrows, increased/decreased level of ROS and Zn2+—with black arrows.
Zinc overload increases the production of mitochondrial ROS, activates NFκB and contributes to the increase in NADPH Oxidase 1 (Nox1) expression and stimulates senescence vascular smooth muscle cells (VSMCs) (Figure 2) [95]. NFκB is a crucial transcription regulator, which is activated by various stimuli (such as cytokines, oxidant-free radicals, pathogens), translocates into the nucleus and stimulates the expression of a wide variety of inflammatory and immune-response-related genes [96,97]. Mechanically, Zn2+ acts via ZnT3 and ZnT10 transporters, which are known to reduce cytosolic zinc. Interestingly, other metals (copper, iron, cobalt and manganese) did not affect NOX1 expression, suggesting its zinc-specific upregulation. Further, Nox1 induces VSMCs’ senescence by both telomere-dependent and -independent pathways, which was also associated with increased DNA damage and reduced proliferation [95].
In total, the presented data demonstrated the crucial role of precise Zn2+ distribution among cytosol and organelles in heart ageing. Ageing-associated dysregulation of Zn2+ transporters leads to intracellular and mitochondrial Zn2+ overload, increased ROS production and accelerated senescence. However, application of direct mitochondria-targeting antioxidant treatment can be an effective therapeutic strategy to overcome the ageing-associated Zn2+-mediated rise of mitochondrial ROS and cardiomyocyte dysfunction. Further, target pharmacological inhibition/stimulation of the Zn2+ transporters, responsible for the Zn2+ redistribution among suborganelles in aged cardiomyocytes, could be another potential cardioprotective approach against ageing-associated cardiac dysfunction.
5. Conclusions
Zn2+ plays a vital role in normal cell physiology and in pathophysiological conditions, such as cardiovascular diseases, premature ageing and ageing-associated diseases, diabetes and insulin resistance. Zn2+ uptake, influx/efflux, distribution and storage in organelles are tightly controlled by many signalling pathways and associated with the homeostasis of the other two valent ions (primarily Ca2+). Both surplus zinc supply and deficiency have been associated with a number of cardiovascular diseases, cardiac developmental abnormalities and accelerated cardiac ageing. In this review, we documented the role of Zn2+ for mitochondrial function, heart development, electrical and mechanical functions, heart failure and ischemia/reperfusion injury. Because mitochondria are the primary source of ROS responsible for the induction and progression of many cardiovascular diseases and heart ageing, the application of pharmacological agents targeting mitochondrial Zn2+-transporters to normalise [Zn2+]mt and ROS levels could be an effective strategy for the prevention and/or therapy of cardiovascular dysfunction in humans.
Abbreviations
| [Zn2+]i | intracellular labile Zn2+ |
| [Zn2+]mt | mitochondrial Zn2+ |
| [Zn2+]ser | sarco/endoplasmic reticulum Zn2+ |
| ADAMTS | A Disintegrin And Metalloproteinase With Thrombospondin Motifs 1 |
| BAX | B-cell lymphoma 2–associated X protein |
| BBC3 | BCL2 Binding Component 3 |
| Beclin1 | ATG6, Autophagy Related |
| calregulin | Endoplasmic Reticulum Resident Protein 60 |
| CaMKII | Ca2+-calmodulin-dependent protein kinase |
| CASP | caspase 9 |
| CDKN1A | cyclin-dependent kinase inhibitor 1A |
| CHD | congenital heart disease |
| DRP1 | DNM1L or dynamin 1 like |
| ECM | extracellular matrix |
| EI24 | etoposide-induced 2.4 |
| ER | endoplasmic reticulum |
| ErbB2 | Erb-B2 Receptor Tyrosine Kinase 2 |
| ETC | electron transport chain |
| FAS | Fas cell-surface death receptor |
| GRP78 | Heat Shock Protein 70 Family Protein 5 |
| GSK3B | Glycogen Synthase Kinase-3 Beta |
| H/R | hypoxia-oxygenation |
| HF | Heart failure |
| I/R | ischemia/reperfusion injury |
| IκBα | NF-Kappa-B Inhibitor Alpha |
| LVNC | left ventricular noncompaction |
| MAPK1 | Mitogen-Activated Protein Kinase 1, or ERK |
| MG23 | transmembrane protein mitsugumin 23 |
| MMP | mitochondrial membrane potential |
| mPTP | mitochondrial permeability transition pore |
| MTF1 | metal regulatory transcription factor 1 |
| NOX2 | NADPH oxidase 2 |
| RNS | reactive nitrogen species |
| ROS | reactive oxygen species |
| SENP5 | SUMO1/Sentrin Specific Peptidase 5 |
| SR | sarcoplasmic reticulum |
| ZIP | Zrt-/Irt-like protein |
| ZnT | zinc transporters |
| Znt9 | SLC-30A9, Solute Carrier Family 30 Member 9 |
Author Contributions
S.A.D. and A.N.O. conceptualised the manuscript; S.A.D. wrote the manuscript text; N.K.S. and A.G.K. reviewed the text; E.E.B. and V.N.S. methodology; N.K.S., E.E.B. and A.G.K. formal analysis; V.N.S. and A.N.O. obtained funding and supervised. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Funding Statement
This work was supported by the Russian Science Foundation (22-15-00064).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hübner C., Haase H. Interactions of Zinc- and Redox-Signaling Pathways. Redox. Biol. 2021;41:101916. doi: 10.1016/j.redox.2021.101916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skalny A.V., Aschner M., Tinkov A.A. Zinc. Adv. Food Nutr. Res. 2021;96:251–310. doi: 10.1016/bs.afnr.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng Y., Chen H. Aberrance of Zinc Metalloenzymes-Induced Human Diseases and Its Potential Mechanisms. Nutrients. 2021;13:4456. doi: 10.3390/nu13124456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tuncay E., Turan B. Intracellular Zn(2+) Increase in Cardiomyocytes Induces Both Electrical and Mechanical Dysfunction in Heart via Endogenous Generation of Reactive Nitrogen Species. Biol. Trace Elem. Res. 2016;169:294–302. doi: 10.1007/s12011-015-0423-3. [DOI] [PubMed] [Google Scholar]
- 5.Kambe T., Taylor K.M., Fu D. Zinc Transporters and Their Functional Integration in Mammalian Cells. J. Biol. Chem. 2021;296:100320. doi: 10.1016/j.jbc.2021.100320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asghari K., Shargh Z., Fatehfar S., Chodari L., Sameei P. The Impact of Zinc on the Molecular Signaling Pathways in the Diabetes Disease. J. Trace Elem. Med. Biol. 2022;72:126985. doi: 10.1016/j.jtemb.2022.126985. [DOI] [PubMed] [Google Scholar]
- 7.Gaburjakova J., Gaburjakova M. The Cardiac Ryanodine Receptor Provides a Suitable Pathway for the Rapid Transport of Zinc (Zn2+) Cells. 2022;11:868. doi: 10.3390/cells11050868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Filippo E.S., Checcaglini F., Fanò-Illic G., Fulle S. H2O2/Ca2+/Zn2+ Complex Can Be Considered a “Collaborative Sensor” of the Mitochondrial Capacity? Antioxidants. 2022;11:342. doi: 10.3390/antiox11020342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang H., Cai L. Zinc Homeostasis Plays an Important Role in the Prevention of Obesity-Induced Cardiac Inflammation, Remodeling and Dysfunction. J. Trace Elem. Med. Biol. 2020;62:126615. doi: 10.1016/j.jtemb.2020.126615. [DOI] [PubMed] [Google Scholar]
- 10.Abregú F.M.G., Caniffi C., Arranz C.T., Tomat A.L. Impact of Zinc Deficiency During Prenatal and/or Postnatal Life on Cardiovascular and Metabolic Diseases: Experimental and Clinical Evidence. Adv. Nutr. 2022;13:nmac012. doi: 10.1093/advances/nmac012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thokala S., Bodiga V.L., Kudle M.R., Bodiga S. Comparative Response of Cardiomyocyte ZIPs and ZnTs to Extracellular Zinc and TPEN. Biol. Trace Elem. Res. 2019;192:297–307. doi: 10.1007/s12011-019-01671-0. [DOI] [PubMed] [Google Scholar]
- 12.Brugger D., Windisch W.M. Short-Term Subclinical Zinc Deficiency in Weaned Piglets Affects Cardiac Redox Metabolism and Zinc Concentration. J. Nutr. 2017;147:521–527. doi: 10.3945/jn.116.240804. [DOI] [PubMed] [Google Scholar]
- 13.Chabosseau P., Tuncay E., Meur G., Bellomo E.A., Hessels A., Hughes S., Johnson P.R.V., Bugliani M., Marchetti P., Turan B., et al. Mitochondrial and ER-Targeted ECALWY Probes Reveal High Levels of Free Zn2+ ACS Chem. Biol. 2014;9:2111–2120. doi: 10.1021/cb5004064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tuncay E., Bitirim V.C., Durak A., Carrat G.R.J., Taylor K.M., Rutter G.A., Turan B. Hyperglycemia-Induced Changes in ZIP7 and ZnT7 Expression Cause Zn2+ Release From the Sarco(Endo)Plasmic Reticulum and Mediate ER Stress in the Heart. Diabetes. 2017;66:1346–1358. doi: 10.2337/db16-1099. [DOI] [PubMed] [Google Scholar]
- 15.Tamura Y. The Role of Zinc Homeostasis in the Prevention of Diabetes Mellitus and Cardiovascular Diseases. JAT. 2021;28:1109–1122. doi: 10.5551/jat.RV17057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacKenzie S., Bergdahl A. Zinc Homeostasis in Diabetes Mellitus and Vascular Complications. Biomedicines. 2022;10:139. doi: 10.3390/biomedicines10010139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turan B., Tuncay E. The Role of Labile Zn2+ and Zn2+-Transporters in the Pathophysiology of Mitochondria Dysfunction in Cardiomyocytes. Mol. Cell. Biochem. 2021;476:971–989. doi: 10.1007/s11010-020-03964-8. [DOI] [PubMed] [Google Scholar]
- 18.Knies K.A., Li Y.V. Zinc Cytotoxicity Induces Mitochondrial Morphology Changes in Hela Cell Line. Int. J. Physiol. Pathophysiol. Pharmacol. 2021;13:43–51. [PMC free article] [PubMed] [Google Scholar]
- 19.Billur D., Tuncay E., Okatan E.N., Olgar Y., Durak A.T., Degirmenci S., Can B., Turan B. Interplay Between Cytosolic Free Zn2+ and Mitochondrion Morphological Changes in Rat Ventricular Cardiomyocytes. Biol. Trace Elem. Res. 2016;174:177–188. doi: 10.1007/s12011-016-0704-5. [DOI] [PubMed] [Google Scholar]
- 20.Forte M., Schirone L., Ameri P., Basso C., Catalucci D., Modica J., Chimenti C., Crotti L., Frati G., Rubattu S., et al. The Role of Mitochondrial Dynamics in Cardiovascular Diseases. Br. J. Pharmacol. 2021;178:2060–2076. doi: 10.1111/bph.15068. [DOI] [PubMed] [Google Scholar]
- 21.Onishi M., Okamoto K. Mitochondrial Clearance: Mechanisms and Roles in Cellular Fitness. FEBS Lett. 2021;595:1239–1263. doi: 10.1002/1873-3468.14060. [DOI] [PubMed] [Google Scholar]
- 22.Sobenin I.A., Mitrofanov K.Y., Zhelankin A.V., Sazonova M.A., Postnov A.Y., Revin V.V., Bobryshev Y.V., Orekhov A.N. Quantitative Assessment of Heteroplasmy of Mitochondrial Genome: Perspectives in Diagnostics and Methodological Pitfalls. BioMed Res. Int. 2014;2014:292017. doi: 10.1155/2014/292017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tilokani L., Nagashima S., Paupe V., Prudent J. Mitochondrial Dynamics: Overview of Molecular Mechanisms. Essays Biochem. 2018;62:341–360. doi: 10.1042/EBC20170104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Onishi M., Yamano K., Sato M., Matsuda N., Okamoto K. Molecular Mechanisms and Physiological Functions of Mitophagy. EMBO J. 2021;40:e104705. doi: 10.15252/embj.2020104705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chistiakov D.A., Sobenin I.A., Orekhov A.N., Bobryshev Y.V. Myeloid Dendritic Cells: Development, Functions, and Role in Atherosclerotic Inflammation. Immunobiology. 2015;220:833–844. doi: 10.1016/j.imbio.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 26.Abuarab N., Munsey T.S., Jiang L.-H., Li J., Sivaprasadarao A. High Glucose-Induced ROS Activates TRPM2 to Trigger Lysosomal Membrane Permeabilization and Zn2+-Mediated Mitochondrial Fission. Sci. Signal. 2017;10:eaal4161. doi: 10.1126/scisignal.aal4161. [DOI] [PubMed] [Google Scholar]
- 27.Cho H.M., Ryu J.R., Jo Y., Seo T.W., Choi Y.N., Kim J.H., Chung J.M., Cho B., Kang H.C., Yu S.-W., et al. Drp1-Zip1 Interaction Regulates Mitochondrial Quality Surveillance System. Mol. Cell. 2019;73:364–376. doi: 10.1016/j.molcel.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 28.Bian X., Teng T., Zhao H., Qin J., Qiao Z., Sun Y., Liun Z., Xu Z. Zinc Prevents Mitochondrial Superoxide Generation by Inducing Mitophagy in the Setting of Hypoxia/Reoxygenation in Cardiac Cells. Free Radic. Res. 2018;52:80–91. doi: 10.1080/10715762.2017.1414949. [DOI] [PubMed] [Google Scholar]
- 29.Ma T., Zhao L., Zhang J., Tang R., Wang X., Liu N., Zhang Q., Wang F., Li M., Shan Q., et al. A Pair of Transporters Controls Mitochondrial Zn2+ Levels to Maintain Mitochondrial Homeostasis. Protein Cell. 2022;13:180–202. doi: 10.1007/s13238-021-00881-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perez Y., Shorer Z., Liani-Leibson K., Chabosseau P., Kadir R., Volodarsky M., Halperin D., Barber-Zucker S., Shalev H., Schreiber R., et al. SLC30A9 Mutation Affecting Intracellular Zinc Homeostasis Causes a Novel Cerebro-Renal Syndrome. Brain. 2017;140:928–939. doi: 10.1093/brain/awx013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deng H., Qiao X., Xie T., Fu W., Li H., Zhao Y., Guo M., Feng Y., Chen L., Zhao Y., et al. SLC-30A9 Is Required for Zn2+ Homeostasis, Zn2+ Mobilization, and Mitochondrial Health. Proc. Natl. Acad. Sci. USA. 2021;118:e2023909118. doi: 10.1073/pnas.2023909118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turan B., Tuncay E. Impact of Labile Zinc on Heart Function: From Physiology to Pathophysiology. Int. J. Mol. Sci. 2017;18:2395. doi: 10.3390/ijms18112395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poelmann R.E., Gittenberger-de Groot A.C. Development and Evolution of the Metazoan Heart. Dev. Dyn. 2019;248:634–656. doi: 10.1002/dvdy.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin W., Li D. Zinc and Zinc Transporters: Novel Regulators of Ventricular Myocardial Development. Pediatr. Cardiol. 2018;39:1042–1051. doi: 10.1007/s00246-018-1859-y. [DOI] [PubMed] [Google Scholar]
- 35.Zhang X., Wang C., Zhao D., Chen X., Zhang C., Zheng J., Liu X. Zinc Deficiency Induces Abnormal Development of the Myocardium by Promoting SENP5 Overexpression. PLoS ONE. 2020;15:e0242606. doi: 10.1371/journal.pone.0242606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao X. SUMO-Mediated Regulation of Nuclear Functions and Signaling Processes. Mol. Cell. 2018;71:409–418. doi: 10.1016/j.molcel.2018.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin W., Li D., Cheng L., Li L., Liu F., Hand N.J., Epstein J.A., Rader D.J. Zinc Transporter Slc39a8 Is Essential for Cardiac Ventricular Compaction. J. Clin. Investig. 2018;128:826–833. doi: 10.1172/JCI96993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rohde S., Muslem R., Kaya E., Dalinghaus M., van Waning J.I., Majoor-Krakauer D., Towbin J., Caliskan K. State-of-the Art Review: Noncompaction Cardiomyopathy in Pediatric Patients. Heart Fail. Rev. 2022;27:15–28. doi: 10.1007/s10741-021-10089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soldatov V.O., Malorodova T.N., Balamutova T.I., Ksenofontov A.O., Dovgan A.P., Urozhevskaya Z.S. Endothelial Dysfunction: Comparative Evaluation of Ultrasound Dopplerography, Laser Dopplerflowmetry and Direct Monitoring of Arterial Pressure for Conducting Pharmacological Tests in Rats. Res. Results Pharmacol. 2018;4:73–80. doi: 10.3897/rrpharmacology.4.25529. [DOI] [Google Scholar]
- 40.Peralta F.A., Huidobro-Toro J.P. Zinc as Allosteric Ion Channel Modulator: Ionotropic Receptors as Metalloproteins. Int. J. Mol. Sci. 2016;17:1059. doi: 10.3390/ijms17071059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Degirmenci S., Olgar Y., Durak A., Tuncay E., Turan B. Cytosolic Increased Labile Zn2+ Contributes to Arrhythmogenic Action Potentials in Left Ventricular Cardiomyocytes through Protein Thiol Oxidation and Cellular ATP Depletion. J. Trace Elem. Med. Biol. 2018;48:202–212. doi: 10.1016/j.jtemb.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 42.Lippi G., Sanchis-Gomar F. Global Epidemiology and Future Trends of Heart Failure. AME Med. J. 2020;5:15. doi: 10.21037/amj.2020.03.03. [DOI] [Google Scholar]
- 43.Puchenkova O.A., Nadezhdin S.V., Soldatov V.O., Zhuchenko M.A., Korshunova D.S., Kubekina M.V., Korshunov E.N., Korokina L.V., Golubinskaya P.A., Kulikov A.L., et al. Study of antiatherosclerotic and endothelioprotective activity of peptide agonists of epor/cd131 heteroreceptor. Farm. Farmakol. 2020;8:100–111. doi: 10.19163/2307-9266-2020-8-2-100-111. [DOI] [Google Scholar]
- 44.Wu C., Zhang Z., Zhang W., Liu X. Mitochondrial Dysfunction and Mitochondrial Therapies in Heart Failure. Pharmacol. Res. 2022;175:106038. doi: 10.1016/j.phrs.2021.106038. [DOI] [PubMed] [Google Scholar]
- 45.Dey S., DeMazumder D., Sidor A., Foster D.B., O’Rourke B. Mitochondrial ROS Drive Sudden Cardiac Death and Chronic Proteome Remodeling in Heart Failure. Circ. Res. 2018;123:356–371. doi: 10.1161/CIRCRESAHA.118.312708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lemos F.O., Bultynck G., Parys J.B. A Comprehensive Overview of the Complex World of the Endo- and Sarcoplasmic Reticulum Ca2+-Leak Channels. Biochim. Biophys. Acta Mol. Cell. Res. 2021;1868:119020. doi: 10.1016/j.bbamcr.2021.119020. [DOI] [PubMed] [Google Scholar]
- 47.Reilly-O’Donnell B., Robertson G.B., Karumbi A., McIntyre C., Bal W., Nishi M., Takeshima H., Stewart A.J., Pitt S.J. Dysregulated Zn2+ Homeostasis Impairs Cardiac Type-2 Ryanodine Receptor and Mitsugumin 23 Functions, Leading to Sarcoplasmic Reticulum Ca2+ Leakage. J. Biol. Chem. 2017;292:13361–13373. doi: 10.1074/jbc.M117.781708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takeshima H., Venturi E., Sitsapesan R. New and Notable Ion-Channels in the Sarcoplasmic/Endoplasmic Reticulum: Do They Support the Process of Intracellular Ca2+ Release? J. Physiol. 2015;593:3241–3251. doi: 10.1113/jphysiol.2014.281881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Olgar Y., Durak A., Tuncay E., Bitirim C.V., Ozcinar E., Inan M.B., Tokcaer-Keskin Z., Akcali K.C., Akar A.R., Turan B. Increased Free Zn2+ Correlates Induction of Sarco(Endo)Plasmic Reticulum Stress via Altered Expression Levels of Zn2+ -Transporters in Heart Failure. J. Cell. Mol. Med. 2018;22:1944–1956. doi: 10.1111/jcmm.13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mathieu S., El Khoury N., Rivard K., Gélinas R., Goyette P., Paradis P., Nemer M., Fiset C. Reduction in Na(+) Current by Angiotensin II Is Mediated by PKCα in Mouse and Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Heart Rhythm. 2016;13:1346–1354. doi: 10.1016/j.hrthm.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 51.Guo W.-H., Wang X., Shang M.-S., Chen Z., Guo Q., Li L., Wang H.-Y., Yu R.-H., Ma C.-S. Crosstalk between PKC and MAPK Pathway Activation in Cardiac Fibroblasts in a Rat Model of Atrial Fibrillation. Biotechnol. Lett. 2020;42:1219–1227. doi: 10.1007/s10529-020-02843-y. [DOI] [PubMed] [Google Scholar]
- 52.Olgar Y., Ozdemir S., Turan B. Induction of Endoplasmic Reticulum Stress and Changes in Expression Levels of Zn2+-Transporters in Hypertrophic Rat Heart. Mol. Cell. Biochem. 2018;440:209–219. doi: 10.1007/s11010-017-3168-9. [DOI] [PubMed] [Google Scholar]
- 53.Fang Y., Wang S., Lv J., Zhao Z., Guo N., Wu G., Tong J., Wang Z. Slc39a2-Mediated Zinc Homeostasis Modulates Innate Immune Signaling in Phenylephrine-Induced Cardiomyocyte Hypertrophy. Front. Cardiovasc. Med. 2021;8:736911. doi: 10.3389/fcvm.2021.736911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.El-Zayat S.R., Sibaii H., Mannaa F.A. Toll-like Receptors Activation, Signaling, and Targeting: An Overview. Bull. Natl. Res. Cent. 2019;43:187. doi: 10.1186/s42269-019-0227-2. [DOI] [Google Scholar]
- 55.Heim V.J., Stafford C.A., Nachbur U. NOD Signaling and Cell Death. Front. Cell. Dev. Biol. 2019;7:208. doi: 10.3389/fcell.2019.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sobenin I.A., Salonen J.T., Zhelankin A.V., Melnichenko A.A., Kaikkonen J., Bobryshev Y.V., Orekhov A.N. Low Density Lipoprotein-Containing Circulating Immune Complexes: Role in Atherosclerosis and Diagnostic Value. BioMed Res. Int. 2014;2014:205697. doi: 10.1155/2014/205697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu X., Huang L., Zhao J., Wang Z., Yao W., Wu X., Huang J., Bian B. The Relationship between Serum Zinc Level and Heart Failure: A Meta-Analysis. Biomed. Res. Int. 2018;2018:2739014. doi: 10.1155/2018/2739014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rosenblum H., Wessler J.D., Gupta A., Maurer M.S., Bikdeli B. Zinc Deficiency and Heart Failure: A Systematic Review of the Current Literature. J. Card. Fail. 2020;26:180–189. doi: 10.1016/j.cardfail.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 59.Rosenblum H., Bikdeli B., Wessler J., Gupta A., Jacoby D.L. Zinc Deficiency as a Reversible Cause of Heart Failure. Tex. Heart Inst. J. 2020;47:152–154. doi: 10.14503/THIJ-17-6586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Heusch G. Myocardial Ischaemia–Reperfusion Injury and Cardioprotection in Perspective. Nat. Rev. Cardiol. 2020;17:773–789. doi: 10.1038/s41569-020-0403-y. [DOI] [PubMed] [Google Scholar]
- 61.Chistiakov D., Revin V., Sobenin I., Orekhov A., Bobryshev Y. Vascular Endothelium: Functioning in Norm, Changes in Atherosclerosis and Current Dietary Approaches to Improve Endothelial Function. Mini Rev. Med. Chem. 2015;15:338–350. doi: 10.2174/1389557515666150226114031. [DOI] [PubMed] [Google Scholar]
- 62.Akbari G. Role of Zinc Supplementation on Ischemia/Reperfusion Injury in Various Organs. Biol. Trace Elem. Res. 2020;196:1–9. doi: 10.1007/s12011-019-01892-3. [DOI] [PubMed] [Google Scholar]
- 63.Andreadou I., Schulz R., Papapetropoulos A., Turan B., Ytrehus K., Ferdinandy P., Daiber A., Di Lisa F. The Role of Mitochondrial Reactive Oxygen Species, NO and H2S in Ischaemia/Reperfusion Injury and Cardioprotection. J. Cell. Mol. Med. 2020;24:6510–6522. doi: 10.1111/jcmm.15279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boengler K., Lochnit G., Schulz R. Mitochondria “THE” Target of Myocardial Conditioning. Am. J. Physiol. Heart Circ. Physiol. 2018;315:H1215–H1231. doi: 10.1152/ajpheart.00124.2018. [DOI] [PubMed] [Google Scholar]
- 65.Slepchenko K.G., Lu Q., Li Y.V. Cross Talk between Increased Intracellular Zinc (Zn2+) and Accumulation of Reactive Oxygen Species in Chemical Ischemia. Am. J. Physiol. Cell Physiol. 2017;313:C448–C459. doi: 10.1152/ajpcell.00048.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao J., Ma J., Deng Y., Kelly J.A., Kim K., Bang S.-Y., Lee H.-S., Li Q.-Z., Wakeland E.K., Qiu R., et al. A Missense Variant in NCF1 Is Associated with Susceptibility to Multiple Autoimmune Diseases. Nat. Genet. 2017;49:433–437. doi: 10.1038/ng.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Meier J.A., Hyun M., Cantwell M., Raza A., Mertens C., Raje V., Sisler J., Tracy E., Torres-Odio S., Gispert S., et al. Stress-Induced Dynamic Regulation of Mitochondrial STAT3 and Its Association with Cyclophilin D Reduce Mitochondrial ROS Production. Sci. Signal. 2017;10:eaag2588. doi: 10.1126/scisignal.aag2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Banerjee K., Keasey M.P., Razskazovskiy V., Visavadiya N.P., Jia C., Hagg T. Reduced FAK-STAT3 Signaling Contributes to ER Stress-Induced Mitochondrial Dysfunction and Death in Endothelial Cells. Cell. Signal. 2017;36:154–162. doi: 10.1016/j.cellsig.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang G., Sheng M., Wang J., Teng T., Sun Y., Yang Q., Xu Z. Zinc Improves Mitochondrial Respiratory Function and Prevents Mitochondrial ROS Generation at Reperfusion by Phosphorylating STAT3 at Ser727. J. Mol. Cell. Cardiol. 2018;118:169–182. doi: 10.1016/j.yjmcc.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 70.Du L., Zhang H., Zhao H., Cheng X., Qin J., Teng T., Yang Q., Xu Z. The Critical Role of the Zinc Transporter Zip2 (SLC39A2) in Ischemia/Reperfusion Injury in Mouse Hearts. J. Mol. Cell. Cardiol. 2019;132:136–145. doi: 10.1016/j.yjmcc.2019.05.011. [DOI] [PubMed] [Google Scholar]
- 71.Zech A.T.L., Singh S.R., Schlossarek S., Carrier L. Autophagy in Cardiomyopathies. Biochim. Biophys. Acta Mol. Cell. Res. 2020;1867:118432. doi: 10.1016/j.bbamcr.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 72.Liuzzi J.P., Pazos R. Interplay Between Autophagy and Zinc. J. Trace Elem. Med. Biol. 2020;62:126636. doi: 10.1016/j.jtemb.2020.126636. [DOI] [PubMed] [Google Scholar]
- 73.Li X.-C., Zeng Y., Sun R.-R., Liu M., Chen S., Zhang P.-Y. SUMOylation in Cardiac Disorders—A Review. Eur. Rev. Med. Pharmacol. Sci. 2017;21:1583–1587. [PubMed] [Google Scholar]
- 74.Bian X., Xu J., Zhao H., Zheng Q., Xiao X., Ma X., Li Y., Du X., Liu X. Zinc-Induced SUMOylation of Dynamin-Related Protein 1 Protects the Heart against Ischemia-Reperfusion Injury. Oxidative Med. Cell. Longev. 2019;2019:1232146. doi: 10.1155/2019/1232146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang H., Yang N., He H., Chai J., Cheng X., Zhao H., Zhou D., Teng T., Kong X., Yang Q., et al. The Zinc Transporter ZIP7 (Slc39a7) Controls Myocardial Reperfusion Injury by Regulating Mitophagy. Basic Res. Cardiol. 2021;116:54. doi: 10.1007/s00395-021-00894-4. [DOI] [PubMed] [Google Scholar]
- 76.Dabravolski S.A., Nikiforov N.G., Zhuravlev A.D., Orekhov N.A., Grechko A.V., Orekhov A.N. Role of the MtDNA Mutations and Mitophagy in Inflammaging. Int. J. Mol. Sci. 2022;23:1323. doi: 10.3390/ijms23031323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Summerhill V.I., Grechko A.V., Yet S.-F., Sobenin I.A., Orekhov A.N. The Atherogenic Role of Circulating Modified Lipids in Atherosclerosis. Int. J. Mol. Sci. 2019;20:3561. doi: 10.3390/ijms20143561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang X., Xu L., Gillette T.G., Jiang X., Wang Z.V. The Unfolded Protein Response in Ischemic Heart Disease. J. Mol. Cell Cardiol. 2018;117:19–25. doi: 10.1016/j.yjmcc.2018.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang Y., Zhou Q., Gao A., Chen L., Li L. Endoplasmic Reticulum Stress and Focused Drug Discovery in Cardiovascular Disease. Clin. Chim Acta. 2020;504:125–137. doi: 10.1016/j.cca.2020.01.031. [DOI] [PubMed] [Google Scholar]
- 80.Wang G., Huang H., Zheng H., He Y., Zhang Y., Xu Z., Zhang L., Xi J. Zn2+ and MPTP Mediate Endoplasmic Reticulum Stress Inhibition-Induced Cardioprotection Against Myocardial Ischemia/Reperfusion Injury. Biol. Trace Elem. Res. 2016;174:189–197. doi: 10.1007/s12011-016-0707-2. [DOI] [PubMed] [Google Scholar]
- 81.Bodiga V.L., Vemuri P.K., Nimmagadda G., Bodiga S. Zinc-Dependent Changes in Oxidative and Endoplasmic Reticulum Stress during Cardiomyocyte Hypoxia/Reoxygenation. Biol. Chem. 2020;401:1257–1271. doi: 10.1515/hsz-2020-0167. [DOI] [PubMed] [Google Scholar]
- 82.Vermeulen Z., Segers V.F.M., De Keulenaer G.W. ErbB2 Signaling at the Crossing between Heart Failure and Cancer. Basic Res. Cardiol. 2016;111:60. doi: 10.1007/s00395-016-0576-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhao H., Liu D., Yan Q., Bian X., Yu J., Wang J., Cheng X., Xu Z. Endoplasmic Reticulum Stress/Ca2+-Calmodulin-Dependent Protein Kinase/Signal Transducer and Activator of Transcription 3 Pathway Plays a Role in the Regulation of Cellular Zinc Deficiency in Myocardial Ischemia/Reperfusion Injury. Front. Physiol. 2022;12:736920. doi: 10.3389/fphys.2021.736920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhao N., Li Q., Sui H., Zhang H. Role of Oxidation-Dependent CaMKII Activation in the Genesis of Abnormal Action Potentials in Atrial Cardiomyocytes: A Simulation Study. Biomed. Res. Int. 2020;2020:1597012. doi: 10.1155/2020/1597012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sheng M., Zhang G., Wang J., Yang Q., Zhao H., Cheng X., Xu Z. Remifentanil Induces Cardio Protection Against Ischemia/Reperfusion Injury by Inhibiting Endoplasmic Reticulum Stress Through the Maintenance of Zinc Homeostasis. Anesth. Analg. 2018;127:267–276. doi: 10.1213/ANE.0000000000003414. [DOI] [PubMed] [Google Scholar]
- 86.Wang J., Cheng X., Zhao H., Yang Q., Xu Z. Downregulation of the Zinc Transporter SLC39A13 (ZIP13) Is Responsible for the Activation of CaMKII at Reperfusion and Leads to Myocardial Ischemia/Reperfusion Injury in Mouse Hearts. J. Mol. Cell. Cardiol. 2021;152:69–79. doi: 10.1016/j.yjmcc.2020.12.002. [DOI] [PubMed] [Google Scholar]
- 87.Bou-Teen D., Kaludercic N., Weissman D., Turan B., Maack C., Di Lisa F., Ruiz-Meana M. Mitochondrial ROS and Mitochondria-Targeted Antioxidants in the Aged Heart. Free. Radic. Biol. Med. 2021;167:109–124. doi: 10.1016/j.freeradbiomed.2021.02.043. [DOI] [PubMed] [Google Scholar]
- 88.Ruiz-Meana M., Bou-Teen D., Ferdinandy P., Gyongyosi M., Pesce M., Perrino C., Schulz R., Sluijter J.P.G., Tocchetti C.G., Thum T., et al. Cardiomyocyte Ageing and Cardioprotection: Consensus Document from the ESC Working Groups Cell Biology of the Heart and Myocardial Function. Cardiovasc. Res. 2020;116:1835–1849. doi: 10.1093/cvr/cvaa132. [DOI] [PubMed] [Google Scholar]
- 89.Olgar Y., Degirmenci S., Durak A., Billur D., Can B., Kayki-Mutlu G., Arioglu-Inan E.E., Turan B. Aging Related Functional and Structural Changes in the Heart and Aorta: MitoTEMPO Improves Aged-Cardiovascular Performance. Exp. Gerontol. 2018;110:172–181. doi: 10.1016/j.exger.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 90.Olgar Y., Billur D., Tuncay E., Turan B. MitoTEMPO Provides an Antiarrhythmic Effect in Aged-Rats through Attenuation of Mitochondrial Reactive Oxygen Species. Exp. Gerontol. 2020;136:110961. doi: 10.1016/j.exger.2020.110961. [DOI] [PubMed] [Google Scholar]
- 91.Novakovic S., Molesworth L.W., Gourley T.E., Boag P.R., Davis G.M. Zinc Transporters Maintain Longevity by Influencing Insulin/IGF-1 Activity in Caenorhabditis Elegans. FEBS Lett. 2020;594:1424–1432. doi: 10.1002/1873-3468.13725. [DOI] [PubMed] [Google Scholar]
- 92.Wong C.P., Magnusson K.R., Sharpton T.J., Ho E. Effects of Zinc Status on Age-Related T Cell Dysfunction and Chronic Inflammation. Biometals. 2021;34:291–301. doi: 10.1007/s10534-020-00279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Aydemir T.B., Troche C., Kim J., Kim M.-H., Teran O.Y., Leeuwenburgh C., Cousins R.J. Aging Amplifies Multiple Phenotypic Defects in Mice with Zinc Transporter Zip14 (Slc39a14) Deletion. Exp. Gerontol. 2016;85:88–94. doi: 10.1016/j.exger.2016.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Olgar Y., Tuncay E., Turan B. Mitochondria-Targeting Antioxidant Provides Cardioprotection through Regulation of Cytosolic and Mitochondrial Zn2+ Levels with Re-Distribution of Zn2+-Transporters in Aged Rat Cardiomyocytes. Int. J. Mol. Sci. 2019;20:3783. doi: 10.3390/ijms20153783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Salazar G., Huang J., Feresin R.G., Zhao Y., Griendling K.K. Zinc Regulates Nox1 Expression through a NF-ΚB and Mitochondrial ROS Dependent Mechanism to Induce Senescence of Vascular Smooth Muscle Cells. Free. Radic. Biol. Med. 2017;108:225–235. doi: 10.1016/j.freeradbiomed.2017.03.032. [DOI] [PubMed] [Google Scholar]
- 96.Sun S.-C. The Non-Canonical NF-ΚB Pathway in Immunity and Inflammation. Nat. Rev. Immunol. 2017;17:545–558. doi: 10.1038/nri.2017.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Barnabei L., Laplantine E., Mbongo W., Rieux-Laucat F., Weil R. NF-ΚB: At the Borders of Autoimmunity and Inflammation. Front. Immunol. 2021;12:716469. doi: 10.3389/fimmu.2021.716469. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.