Abstract
Wall-associated kinases (WAKs) are important receptor-like proteins that play major roles in plant defense against pathogens. Fusarium head blight (FHB), one of the most widespread and devastating crop diseases, reduces wheat yield and leads to quality deterioration. Although WAK gene families have been studied in many plants, systematic research on bread wheat (Triticum aestivum) and its role in FHB resistance, in particular, is lacking. In this study, we identified and characterized 320 genes of the TaWAK family in wheat distributed across all chromosomes except 4B and divided them into three phylogenetic groups. Duplication and synteny analyses provided valuable information on the evolutionary characteristics of the TaWAK genes. The gene expression pattern analysis suggested that TaWAK genes play diverse roles in plant biological processes and that at least 30 genes may be involved in the response to Fusarium infection in wheat spikes, with most of the genes contributing to pectin- and chitin-induced defense pathways. Furthermore, 45 TaWAK genes were identified within 17 hcmQTLs that are related to wheat FHB resistance. Our findings provide potential candidate genes for improving FHB resistance and insights into the future functional analysis of TaWAK genes in wheat.
Keywords: wheat, wall-associated kinase, TaWAK, gene family, gene expression, Fusarium head blight, resistance
1. Introduction
Plants have evolved to have two layers of innate immune systems to prevent or minimize the damage caused by pathogenic infection. This is also referred to as the “zig-zag” model, which includes pathogen-associated molecular pattern (PAMP)-triggered immunity (PTI), and effector-triggered immunity (ETI) [1]. PTI is moderated by numerous receptor-like kinases (RLKs) and receptor-like proteins that act as pattern recognition receptors and detect PAMPs as the first layer of inducible defense; this is also considered to confer broad-spectrum resistance to nonadapted potential pathogens [2]. Wall-associated kinases (WAKs) are a unique subfamily of RLKs that typically contain the extracellular WAK galacturonan-binding (GUB), extracellular epidermal growth factor (EGF), transmembrane (TM), and intracellular serine/threonine kinase domains [3]. The extracellular domain of WAKs is involved in cytoplasm–cell wall communication, whereas the intracellular kinase domain is concerned with the activation of cytoplasmic signaling cascades in plant defense reactions [4]. WAKs are the only type of receptors involved in cell-wall signaling in addition to their roles in the growth and development and in the tolerance to abiotic stresses. A growing number of studies have indicated that WAKs play key roles in plant defense against pathogens [5].
Data on plant WAKs and their roles in signal transduction and pathogen stress responses are derived mostly from studies on Arabidopsis thaliana [6]. AtWAK1 was the first WAK-encoding gene identified in plants and was shown to bind with high affinity to pathogen- and damage-induced pectin fragments or oligogalacturonides, which have long been considered elicitors of plant defense [7,8]. Overall, 22 WAK genes have been detected in the Arabidopsis genome [3], and several studies have demonstrated that these are functional genes associated with pathogen defense. For example, AtWAK1 confers enhanced resistance to the necrotrophic fungus Botrytis cinerea [9]; AtWAKL22 plays a role in resistance to Fusarium wilt disease [10]; and AtWAKL10 is responsible for resistance to Pseudomonas syringae [11]. Furthermore, WAKs play a critical role in pathogen responses in other plant species. In maize (Zea mays), ZmWAK-RLK1 (encoded by Htn1) confers resistance to northern corn leaf blight and ZmWAK qHSR1 enhances resistance to head smut [12,13]. In rice (Oryza sativa), OsWAK1, OsWAK14, OsWAK91, and OsWAK92 positively regulate quantitative resistance to the blast fungus Magnaporthe oryzae [4,14]. Additionally, Xa4 encodes a WAK that strengthens the cell wall and enhances the resistance to bacterial infection [15]. SlWAK1 in tomatoes (Lycopersicon esculentum) promotes apoplastic immunity against the bacterial pathogen Pseudomonas syringae [16]. These findings assert that the WAK gene family is imperative for plant defense against pathogens.
Bread wheat (Triticum aestivum) is an important cultivated grain crop worldwide, and its products fulfill the food requirements of approximately 35% of the global population. Demands for crop production increase as the world’s population increases; however, pathogen-related diseases pose serious problems in agricultural production yield and quality [17]. Recently, several WAKs from wheat have been shown to be involved in defense against pathogens. The WAK-like protein Stb6 confers resistance to Septoria tritici blotch disease caused by Zymoseptoria tritici [18]. TaWAK6 is involved in adult plant resistance to wheat leaf rust [19], and TaWAK-6D and TaWAK7D mediate resistance to Fusarium pseudograminearum and Rhizoctonia cerealis infections, respectively [20,21]. Among these crop pathogens, Fusarium head blight (FHB), mainly caused by Fusarium graminearum (Fg), is a widespread and devastating crop disease. It affects small grain cereals and causes huge losses in grain yield and substantial deterioration in grain quality because of kernel contamination with harmful mycotoxins such as deoxynivalenol (DON). Although many quantitative trait loci (QTLs) associated with FHB resistance have been reported, only a few genes have been identified to confer FHB resistance [22]. For instance, WAK2 isolated from the QFhb.mgb-2A region of durum wheat, and the TaWAK2A-800 region of bread wheat is responsible for FHB resistance [23,24]. WAKs are now being recognized as playing important regulatory roles in plant immunity; therefore, the identification of new WAK genes that are critical for FHB responses facilitates the genetic manipulation of these candidates for improving FHB resistance in wheat breeding programs.
Genome sequences can provide valuable information for genome-based investigations. To date, the WAK gene family has been comprehensively identified in various plant species such as Arabidopsis [3], tomato [25], cotton [26], rice [27], barley [28], and walnuts [29]. However, systematic investigations of WAKs are yet to be performed in the wheat genome. The high-quality version of the hexaploid wheat genome assembled and annotated by the International Wheat Genome Sequencing Consortium allows the identification and characterization of WAK family members in wheat [30]. In this study, we identified and characterized the wheat WAK gene family comprising 320 genes in the bread wheat genome. A detailed characterization of gene structures, evolutionary relationships, expansion, expression levels, and subcellular localization of the wheat WAK genes is presented. Furthermore, online data were used to analyze the transcript accumulation of each family member in response to FHB, and the expression levels of 20 genes were evaluated after Fg infection and pectin inoculation via quantitative reverse transcription PCR (qRT-PCR). This study aimed to provide a global viewpoint for the molecular characterization of the WAK gene family in wheat and uncover new potential genes involved in FHB infection in crops so as to provide a basis for further functional studies and FHB-resistant wheat breeding.
2. Results
2.1. Genome-Wide Identification and Phylogenetic Analysis of the WAK Gene Family in Wheat
To characterize the putative WAKs in wheat, we performed a genome-wide analysis of the whole wheat genome with BLASTP [30] and identified the members of the WAK family using the AtWAK/AtWAKL proteins from Arabidopsis [31] as query sequences. All candidates filtered using the Pfam and SMART websites with three domains (WAK_GUB domain, TM domain, and protein kinase domain) were predicted to encode the WAK proteins. Overall, our analysis resulted in the identification of 320 TaWAK genes in wheat (Table S1). To analyze the gene characteristics, the encoded protein molecular weight (MW) and isoelectric points (pI) were predicted (Table S1). Among the 320 TaWAKs, TraesCS3D02G494200 had the highest MW of 119.2 kDa, whereas TraesCS5D02G374300 had the lowest MW of 65.4 kDa; the pI values ranged from 5.09 to 9.24.
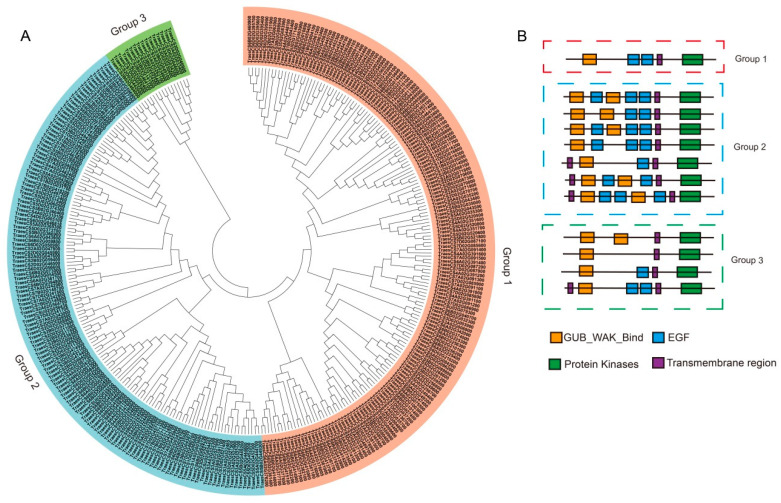
To explore the phylogenetic relationship among the WAKs in wheat, WAK protein sequences were used to construct a phylogenetic tree with the neighbor-joining (NJ) method. All members of the TaWAK family were classified into three groups (Figure 1A): Group 1 was the largest, with 166 TaWAKs; Group 2 was the second largest, with 138 TaWAKs; and Group 3 was the smallest, with 16 TaWAKs. The protein structures were further characterized, and the numbers of the WAK, EGF, and TM domains were determined (Figure 1B). The WAK domains were mostly located in the N-terminal of the proteins, whereas the kinase domains were mostly located in the C-terminal of the WAKs. Group 1 included one protein structure with a WAK-binding domain, two EGF domains, and a typical kinase domain. Group 2 comprised several structures, including varying positions and numbers of EGF structures. Finally, Group 3 included four types of structures with 0–2 EGF domains. All members were predicted to have at least one transmembrane region (Figure 1B).
Figure 1.
Phylogenetic and structural analysis of TaWAK proteins. (A) The phylogenetic tree of wall-associated kinase proteins from Arabidopsis thaliana and wheat constructed using the neighbor-joining method. (B) Protein structures of TaWAKs. The orange, blue, green, and purple boxes indicate the WAK_GUB domain, epidermal growth factor domain, protein kinase, and transmembrane domain, respectively.
2.2. Chromosomal Location and Duplication of TaWAK Genes in Wheat
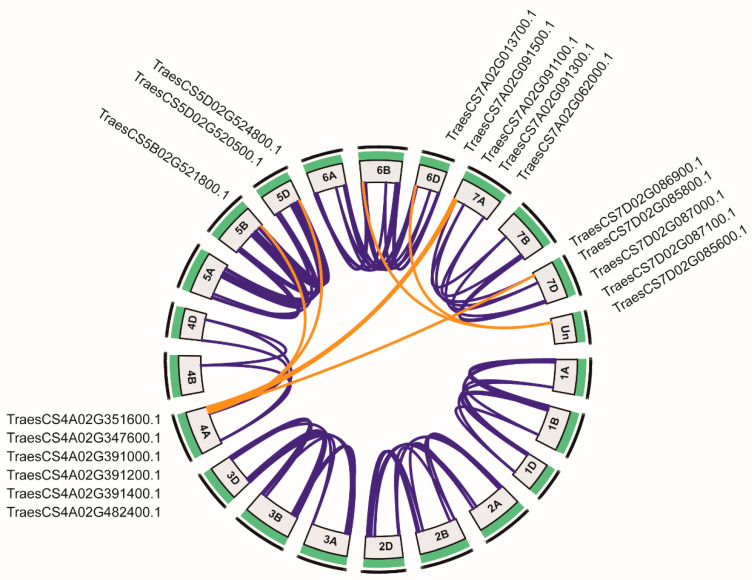
The chromosomal locations of TaWAKs were identified and mapped onto the corresponding wheat chromosomes to determine their genomic distribution (Figure 2). Only eight predicted TaWAK genes were marked on the scaffold; others were distributed on all 21 chromosomes except 4B, and some were clustered at the ends of chromosomes. Among them, 94, 110, and 108 TaWAKs were identified in the A, B, and D subgenomes, respectively. The numbers of TaWAKs in each chromosome varied from a minimum of 3 in chromosome 4D to a maximum of 36 in chromosome 6B. Chromosomes 5 and 6 were the most abundant in TaWAKs with 66 and 80 members, respectively. Among the numerous QTLs reported to improve FHB resistance in wheat, 77 high-confidence mQTLs (hcmQTLs) have been identified in wheat [32]. We further investigated the chromosomal locations of both TaWAKs and hcmQTLs to explore the potential role of TaWAKs in FHB resistance. In total, 45 TaWAKs were present in 17 hcmQTLs (Table 1), which provided valuable information that can aid in the discovery of new genes related to improving FHB resistance in wheat.
Figure 2.
Chromosomal location of the TaWAK genes. The distribution of TaWAKs on each wheat chromosome with a scale bar is displayed in megabase (Mb). The 45 TaWAKs in hcmQTLs are in red.
Table 1.
List of TaWAKs in FHB-related hcmQTLs in wheat.
| Gene Name | hcmQTL | Interval (Mb) | Chr. |
|---|---|---|---|
| TraesCS2A02G564700 | hcmQTL-13 | 763–771 | 2A |
| TraesCS2D02G046500 | hcmQTL-18 | 17–23 | 2D |
| TraesCS2D02G070600 | hcmQTL-19 | 26–36 | 2D |
| TraesCS2D02G070700 | hcmQTL-19 | 26–36 | 2D |
| TraesCS2D02G070800 | hcmQTL-19 | 26–36 | 2D |
| TraesCS2D02G508900 | hcmQTL-21 | 592–608 | 2D |
| TraesCS2D02G509200 | hcmQTL-21 | 592–608 | 2D |
| TraesCS2D02G509205 | hcmQTL-21 | 592–608 | 2D |
| TraesCS2D02G509500 | hcmQTL-21 | 592–608 | 2D |
| TraesCS2D02G511000 | hcmQTL-21 | 592–608 | 2D |
| TraesCS2D02G511300 | hcmQTL-21 | 592–608 | 2D |
| TraesCS3A02G033400 | hcmQTL-23 | 15–21 | 3A |
| TraesCS3A02G034300 | hcmQTL-23 | 15–21 | 3A |
| TraesCS3A02G034600 | hcmQTL-23 | 15–21 | 3A |
| TraesCS3A02G262100 | hcmQTL-28 | 466–485 | 3A |
| TraesCS4A02G310100 | hcmQTL-34 | 594–613 | 4A |
| TraesCS4A02G391000 | hcmQTL-35 | 660–676 | 4A |
| TraesCS4A02G391100 | hcmQTL-35 | 660–676 | 4A |
| TraesCS4A02G391200 | hcmQTL-35 | 660–676 | 4A |
| TraesCS4A02G391400 | hcmQTL-35 | 660–676 | 4A |
| TraesCS4A02G391600 | hcmQTL-35 | 660–676 | 4A |
| TraesCS4A02G448100 | hcmQTL-36 | 712–720 | 4A |
| TraesCS4A02G482400 | hcmQTL-37 | 728–743 | 4A |
| TraesCS5A02G249300 | hcmQTL-46 | 464–472 | 5A |
| TraesCS5A02G249400 | hcmQTL-46 | 464–472 | 5A |
| TraesCS5A02G249600 | hcmQTL-46 | 464–472 | 5A |
| TraesCS5A02G464700 | hcmQTL-47 | 644–662 | 5A |
| TraesCS5B02G259500 | hcmQTL-50 | 438–448 | 5B |
| TraesCS5B02G449200 | hcmQTL-53 | 610–623 | 5B |
| TraesCS5B02G449300 | hcmQTL-53 | 610–623 | 5B |
| TraesCS5D02G373400 | hcmQTL-55 | 444–454 | 5D |
| TraesCS5D02G373700 | hcmQTL-55 | 444–454 | 5D |
| TraesCS5D02G374300 | hcmQTL-55 | 444–454 | 5D |
| TraesCS5D02G374500 | hcmQTL-55 | 444–454 | 5D |
| TraesCS5D02G374600 | hcmQTL-55 | 444–454 | 5D |
| TraesCS5D02G374700 | hcmQTL-55 | 444–454 | 5D |
| TraesCS5D02G375200 | hcmQTL-55 | 444–454 | 5D |
| TraesCS5D02G375400 | hcmQTL-55 | 444–454 | 5D |
| TraesCS6A02G054500 | hcmQTL-58 | 26–35 | 6A |
| TraesCS6A02G061200 | hcmQTL-58 | 26–35 | 6A |
| TraesCS6B02G073000 | hcmQTL-61 | 47–65 | 6B |
| TraesCS6B02G394800 | hcmQTL-65 | 662–682 | 6B |
| TraesCS7A02G425700 | hcmQTL-68 | 611–629 | 7A |
| TraesCS7A02G425900 | hcmQTL-68 | 611–629 | 7A |
| TraesCS7B02G463200 | hcmQTL-74 | 709–728 | 7B |
Segmental and tandem duplications are important factors in gene family expansion. A total of 207 TaWAKs participated in duplication events (Figure 3), which indicated that the expansion of the TaWAK gene family in wheat could chiefly be attributed to whole-genome duplication or segmental duplication within genomes. A total of 176 pairs of TaWAK genes were identified to have undergone segmental duplication, with 21, 12, 27, 18, 38, 45, and 15 pairs located on chromosomes 1, 2, 3, 4, 5, 6, and 7, respectively (Figure 3). As per previous research [33], a tandem duplication event has been defined as the presence of two or more genes in a 200 kb-long segment. Fifty-four tandem duplication events were present on all chromosomes except 1B and 4B (Figure 2 and Figure 3). Six genes on chromosome 4A (TraesCS4A02G351600, TraesCS4A02G347600, TraesCS4A02G391000, TraesCS4A02G391200, TraesCS4A02G391400, and TraesCS4A02G482400) were paired with WAKs on the nonhomologous chromosomes 5B, 5D, 7A, and 7D and an unidentified chromosome. These results suggest the involvement of tandem and segmental duplication events in the expansion of the TaWAK gene family.
Figure 3.
Segmental duplication of TaWAK genes. Blue lines denote the segmental duplication of TaWAK gene pairs between homologous chromosomes, and the orange lines denote the segmental duplication of TaWAK gene pairs between nonhomologous chromosomes.
2.3. Synteny Analysis of WAKs in Wheat and Other Plants
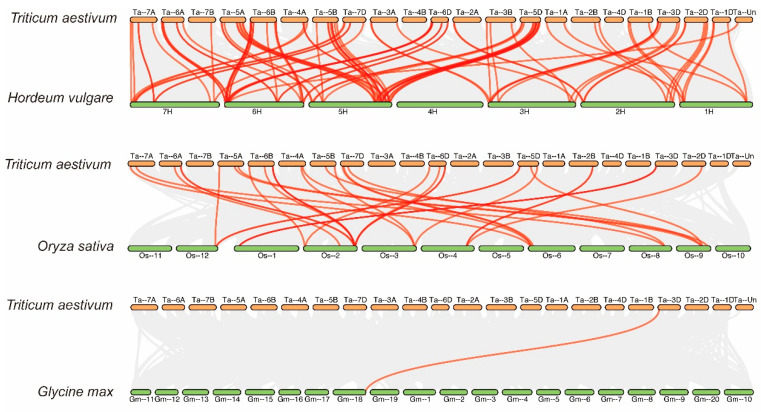
To further investigate the syntenic relationship of wheat TaWAK genes with other plants, such as barley (Hordeum vulgare), rice (Oryza sativa), and soybean (Glycine max), three comparative syntenic maps were constructed using the Multiple Collinearity Scan toolkit (Figure 4). A total of 101 and 34 orthologous gene pairs were identified between TaWAKs and other WAK genes in H. vulgare and O. sativa, respectively (Table S2). Some TaWAK family members were found to be associated with more than one syntenic gene pair between wheat and H. vulgare as well as between wheat and O. sativa; for instance, HORVU.MOREX.r3.2HG0194210 was associated with TraesCS2D02G442000 and TraesCS2B02G464000, and TraesCS6D02G361000 was associated with Os02g0811200 and Os02g0807200. However, in the dicotyledonous species G. max, only one orthologous gene pair was present between TaWAKs and WAK genes, which suggests the closer syntenic relationship of WAK genes in monocots than in dicots.
Figure 4.
Syntenic relationships of TaWAK genes among Hordeum vulgare, Oryza sativa, and Glycine max. The gray lines in the background represent the collinear blocks within Triticum aestivum and other plant genomes. The red lines highlight the syntenic WAK gene pairs.
2.4. Expression Profile Analysis of TaWAK Genes in Various Tissues
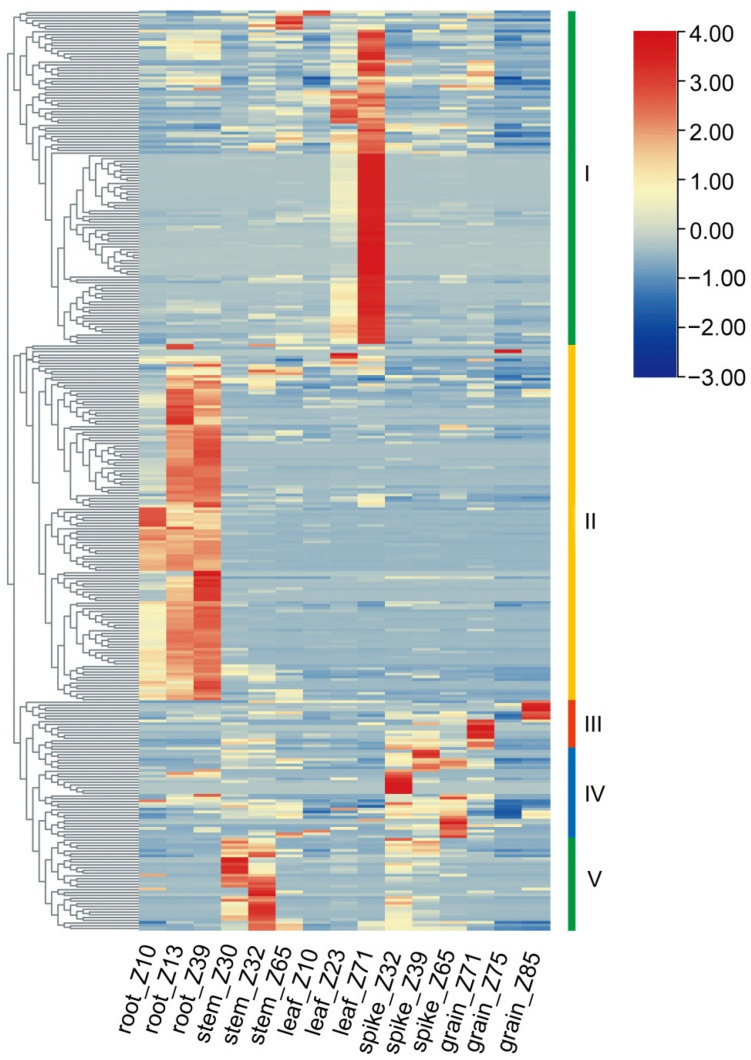
To understand the spatial and temporal expression patterns of TaWAK genes in wheat, transcriptomic data from different developmental stages of the root, stem, leaf, spike, and grain of the cultivar Chinese Spring were analyzed using the Zadoks scale from public data [34]. The expression levels of 307 TaWAKs in the five abovementioned tissues were determined and used to construct a heat map (Figure 5). The results revealed that different TaWAK genes exhibited differential expression profiles in different tissues and that they could be roughly clustered into five groups according to their specific expression. The expressions of 113 genes in Cluster 1 in the leaf, especially at the Z71 time, exceeded those in other tissues, whereas the expressions of 116 genes in Cluster 2 in the root exceeded those in the other tissues. The numbers of TaWAKs highly expressed in Cluster 3, Cluster 4, and Cluster 5 were 14, 20, and 44, respectively.
Figure 5.
Expression analyses of TaWAK genes in five different tissues at three different developmental stages. The TaWAK genes were divided into five clusters with different expression patterns.
2.5. Expression Profiles of TaWAK Genes in Response to Fusarium Graminearum
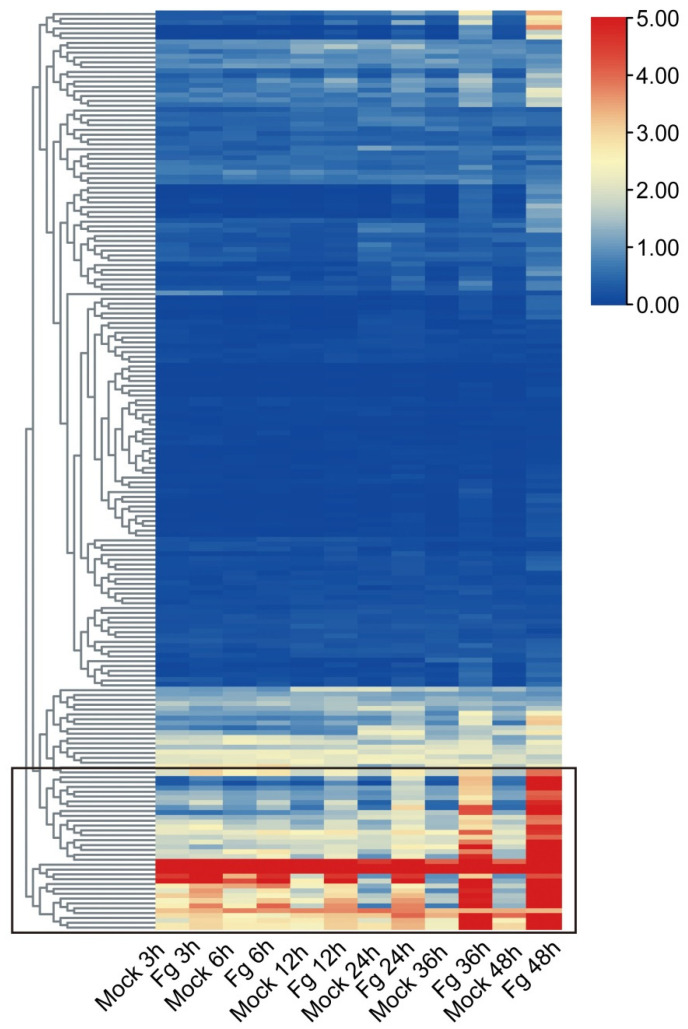
Previous studies have established the significant roles of plant WAK genes in the defense against pathogens [5]. To further investigate the putative role of TaWAKs in fungal disease resistance, the expression of TaWAKs was analyzed using the public RNA-seq data of the wheat spike after inoculation with Fg for 3, 6, 12, 24, 36, and 48 h from the WheatGene database. The heatmap showed that although most of the TaWAKs either did not change or changed slightly (<2 fold and FPKM < 1) at a series of times after the Fg treatment, 30 TaWAKs were differentially upregulated at least at one time point (Figure 6). For the upregulated TaWAKs, 16 were highly inoculated after 36 and 48 h of Fg treatment, whereas 14 were inoculated once after 3 h of Fg treatment. These results signify that the 30 Fg-responded TaWAKs are likely to be involved in wheat FHB resistance.
Figure 6.
Expression analysis of TaWAK genes in response to Fg infection. The red color indicates high expression and the blue color indicates low expression. Thirty TaWAKs that were upregulated after Fg treatment were clustered into a group.
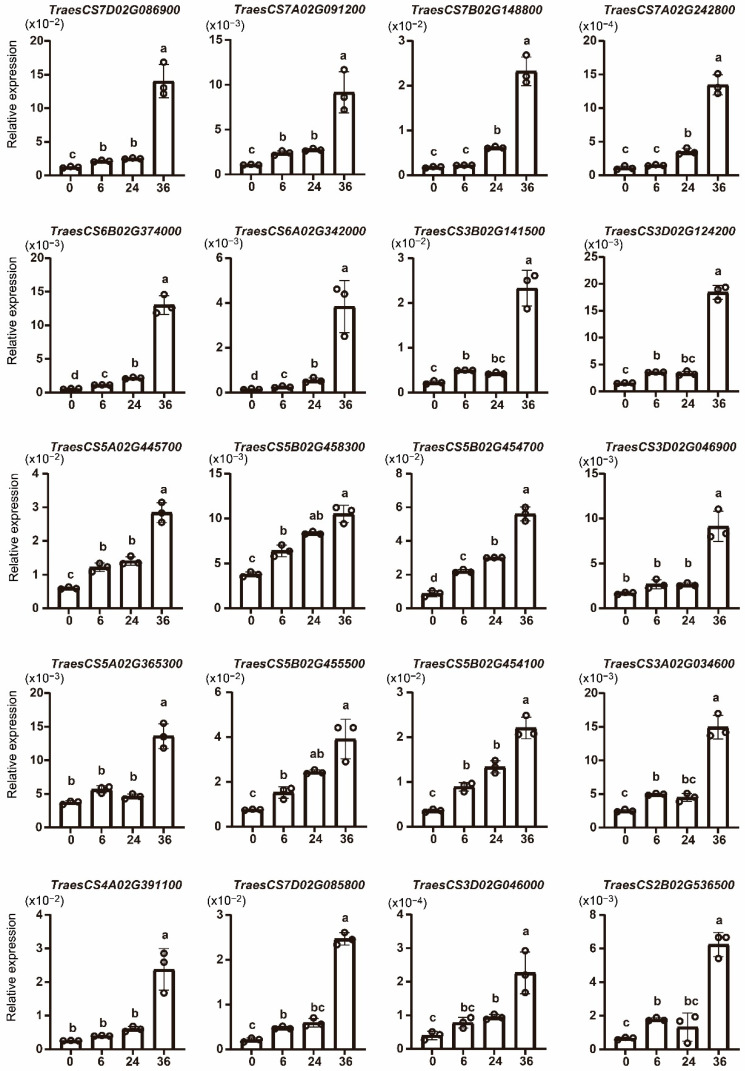
To verify the expression pattern of TaWAKs after Fg infection, 20 of the 30 upregulated TaWAKs were evaluated in the wheat spike after 6, 24, and 36 h of Fg infection using qRT-PCR. The findings showed that all 20 selected TaWAKs were significantly upregulated after 36 h of Fg treatment (Figure 7). For the 6 and 24 h Fg treatments, most of the TaWAKs were slightly but significantly incubated compared with the 0 h treatment. The TraesCS6A02G342000 gene demonstrated the highest relative expression at 36 h of Fg treatment compared with the rest of the tested genes. These results are in agreement with the RNA-seq data and indicate that these TaWAKs may be involved in the response to Fg infection.
Figure 7.
Quantitative real-time PCR analysis of selected TaWAK genes in response to 0, 6, 24, and 36 h of Fg infection to verify the RNA-seq data. The wheat tubulin gene was used as the internal control. The Student’s t-test was used to compare the significant differences. Error bars show the standard deviation. Data are mean ± SD (n = 3).
2.6. Pectin- and Chitin-Induced TaWAK Genes
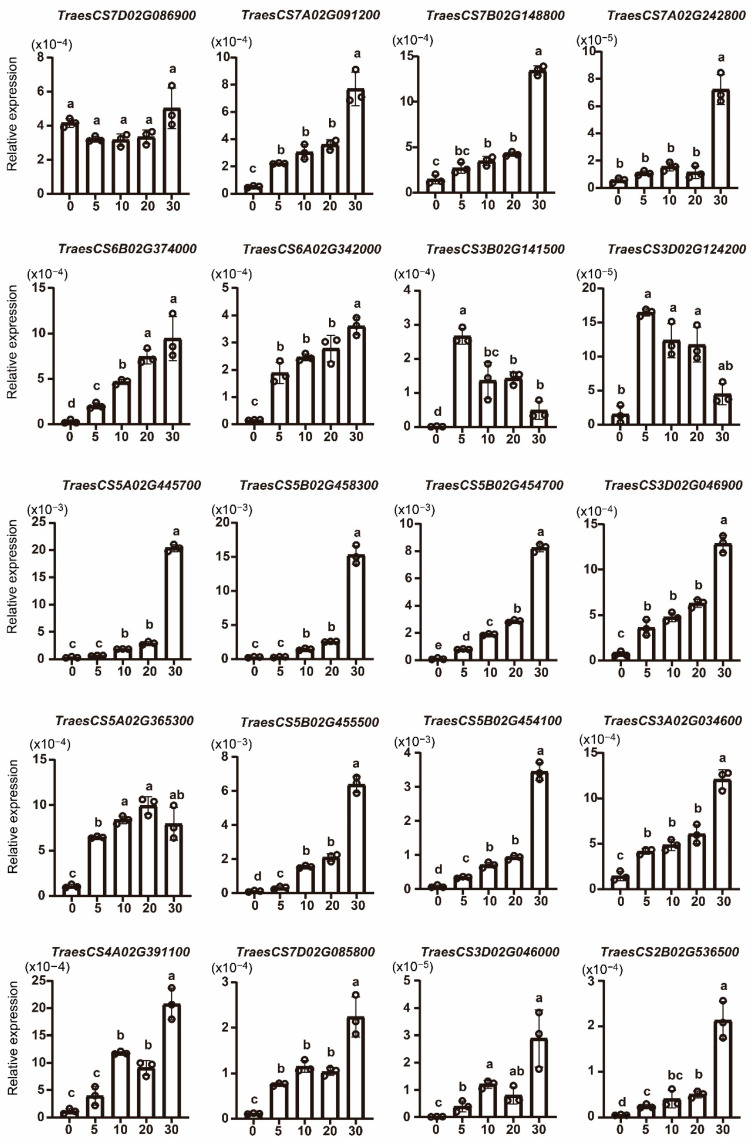
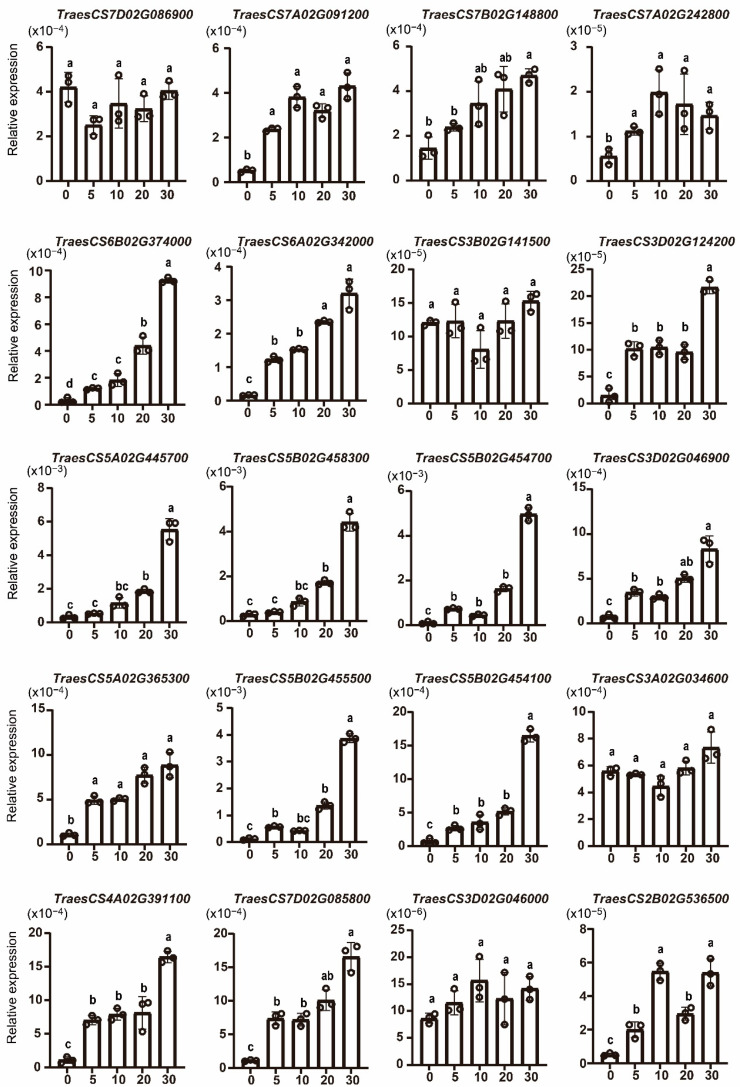
Pectin and chitin act as elicitors that trigger the plant’s defense response. To investigate the response of TaWAKs to exogenous pectin and chitin stimuli, we analyzed the transcriptional profiles of TaWAKs in wheat spikes treated with either pectin (100 µg/mL) or chitin (100 µg/mL), or mock solution for 0, 5, 10, 20, and 30 min. Most of the TaWAKs were elevated over time and reached their highest expression level after 30 min of exogenous pectin treatment. Only TraesCS3B02G141500 and TraesCS3D02G124200 were induced at 5 min and then decreased gradually (Figure 8). In the exogenous chitin treatment, 12 TaWAKs were elevated over time and reached their highest expression level after 30 min (Figure 9). TraesCS7D02G086900 was not induced by pectin or chitin treatment. TraesCS3B02G141500, TraesCS3A02G034600, and TraesCS3D02G046000 transcript levels were significantly elevated by exogenous pectin treatment but not by chitin treatment compared with the mock treatment. Collectively, these results suggest that the TaWAK family may contribute to pectin- and chitin-induced defense pathways in wheat.
Figure 8.
Expression analysis of TaWAK genes in wheat after treatment with exogenous pectin for 0, 5, 10, 20, and 30 min. The wheat tubulin gene was used as the internal control. The Student’s t-test was performed to compare the significant differences. Error bars show the standard deviation. Data are mean ± SD (n = 3).
Figure 9.
Expression analysis of TaWAK genes in wheat after treatment with exogenous chitin for 0, 5, 10, 20, and 30 min. The wheat tubulin gene was used as the internal control, and a Student’s t-test was used to compare the significant differences. Error bars show the standard deviation. Data are mean ± SD (n = 3).
2.7. Subcellular Localization of TaWAK Proteins
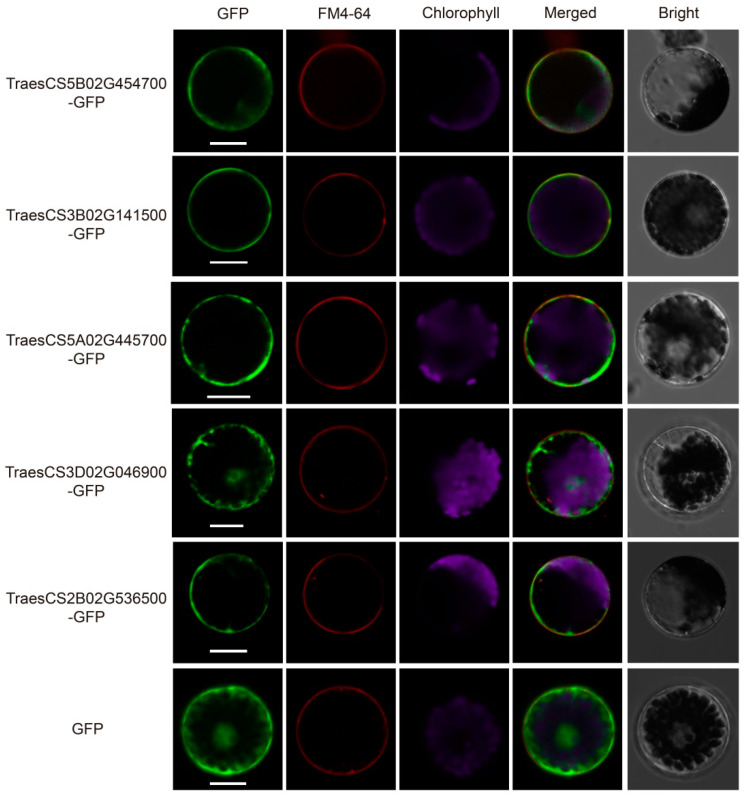
To investigate the subcellular localization of TaWAKs in wheat, Five TaWAK gene sequences from the abovementioned qRT-PCR analysis were fused with the green fluorescent protein (GFP) reporter gene in the presence of the maize ubiquitin promoter. The resulting fusion proteins and the control ubi:GFP were transiently expressed in the wheat protoplasts. The amphiphilic styryl dye FM4-64 was used as the plasma membrane marker owing to its ability to immediately stain the plasma membrane after application in plants [35]. Confocal microscopy revealed that the GFP fluorescence of four proteins, TraesCS5B02G454700, TraesCS3B02G141500, TraesCS5A02G445700, and TraesCS2B02G536500, was distributed in the plasma membrane of wheat protoplasts, just as FM4-64, whereas TraesCS3D02G046900 was expressed in all parts of the protoplasts, just as the GFP control (Figure 10). These results denote that TaWAKs have different subcellular localizations and varying functions in wheat.
Figure 10.
Subcellular localization of TaWAK proteins in wheat protoplasts. Plasmids labeled with GFP were separately transferred into the wheat protoplast, and the amphiphilic styryl dye FM4-64 was used as a plasma membrane marker. Scale bar = 20 μm.
3. Discussion
WAKs are important RLKs that typically comprise a putative extracellular domain, a hydrophobic TM region, and a cytoplasmic Ser/Thr kinase domain. It has been shown that WAKs function in signal transduction between the extracellular matrix and the cytoplasm and play pertinent roles in plant growth and development [26]. WAKs have recently gained attention as key factors in plant defense against pathogens [5]. Characterizing the WAK gene family will help identify many new candidate genes that may contribute to disease resistance. Presently, WAK gene families have been identified and characterized in diverse plant species, including 26 members in Arabidopsis [3], 125 in rice [27], 91 in barley [28], 27 in walnuts [29], 29 in tomato [25], 29 in potato [36], and 29 in cotton [26]. Although several wheat WAKs have been reported, their genome-wide characterization in the complicated wheat hexaploid genome is lacking.
In this study, 320 TaWAK genes containing the typical WAK_GUB, TM, and protein kinase domains were identified and characterized based on a high-quality version of the wheat genome assembled and annotated by IWGSC [30]. These genes were classified into three groups based on phylogenetic analysis. For the four typical domains, the protein structures were relatively conserved in each group, with only one similar structure for 166 TaWAK members in Group 1. The relative MW of the proteins was in the range of 65.4–119.2 kDa, and the range of the theoretical isoelectric point was 5.09–9.24, thus indicating a great functional diversity in TaWAK proteins. Chromosomal mapping of the TaWAK genes revealed that the 320 TaWAKs were almost uniformly distributed on three subgenomes, with 94, 110, and 108 genes in the A, B, and D subgenomes but unevenly distributed on each chromosome. The gene number on each chromosome varied from 0 in chromosome 4B to 36 in chromosome 6B. The number of WAK genes in wheat is quite high compared with rice and barley, and approximately >10 times that in dicotyledonous plants such as Arabidopsis and potato [3,27,28,36]. This variation could be attributed to the fact that wheat has a large genome with two whole-genome duplications, which implies more occurrences of WAK gene duplication events during domestication in monocotyledons than in dicotyledons. In fact, 297 of the 320 TaWAKs participated in duplication events. Further analysis revealed that 176 pairs of TaWAK genes were segmental duplications and that 54 tandem duplication events were concentrated on all chromosomes, except for chromosomes 1B and 4B. Moreover, WAK gene duplication events have been reported in other plant species such as cotton, rice, J. regia, and J. mandshurica. Thus, whole-genome and segmental duplications are among the common causes of WAK gene family expansion in different species [26,27,29]. The syntenic relationship revealed the presence of 101 and 34 orthologous gene pairs between wheat and H. vulgare and O. sativa, respectively, and only one orthologous gene pair between wheat and G. max. This finding suggests that TaWAK genes in wheat originated from a common ancestor during the evolutionary process in monocotyledonous plants but are distantly related to those in dicotyledonous species.
WAK genes exhibit selective and differential expression patterns in several plant species. For instance, GhWAKs have been observed to be expressed in a tissue-specific manner in cotton, with some highly expressed in young roots and some in a flower-specific manner [26]. TaWAKs have also been found to be expressed differently in different tissues and developmental stages, with five tissue-specific highly expressed pattern groups. For example, TraesCS7D02G086900, TraesCS7B02G148800, and TraesCS6B02G374000 genes were chiefly expressed in the leaf, root, and spike, respectively. The variations in the expression levels of TaWAKs indicate that these genes may be involved in the control of diverse biological processes related to the specific expression patterns shown in the corresponding tissues.
Several independent studies have established that WAK family proteins constitute a central pillar of plant cells that monitor and interact with their extracellular environments at both transcriptional and post-transcriptional levels. Moreover, they are important in the defense against pathogens and contribute to plant immunity [5,13,15,18,37,38,39]. Most of these immune-related WAK genes are highly induced upon infection by a pathogen. For example, GhWAK7A showed enhanced expression at 1 d after inoculation with V. dahliae [39], the expression of OsWAK25 was upregulated upon M. oryzae infection [6], and the TaWAK7D transcript level was increased after inoculation with R. cerealis [20]. FHB, mainly caused by Fg, is among the most widespread and devastating crop diseases and can exert damaging effects on wheat yield and quality. To identify new TaWAK genes essential for FHB resistance, the expression pattern of TaWAKs was analyzed. Although most of the TaWAKs did not change or changed slightly at a series of time points after Fg treatment, 30 TaWAKs were differentially upregulated. Twenty TaWAKs were further evaluated in the spike after 6, 24, and 36 h of Fg infection by qRT-PCR, indicating that they may be involved in the response to Fg infection.
For the genes that were not induced in this study, the possibility of their participation in FHB resistance cannot be eliminated. For example, TaWAK2A-800 reportedly participates positively in the resistance responses to FHB; however, it was significantly induced upon Fg inoculation in the FHB-resistant wheat cultivar, Sumai 3 [24] but not in this study. This difference may result from the expression analysis in different varieties or the conditions of Fg treatment. To date, numerous QTLs distributed over all 21 wheat chromosomes have been reported, and 77 high-confidence mQTLs (hcmQTLs) have been recently selected [32]. In this study, 45 TaWAKs in 17 hcmQTLs were identified, thereby providing valuable information for discovering new TaWAK genes related to improving FHB resistance in wheat.
Pectin and chitin are typical PAMPs on the fungal cell wall, which act as important elicitors in several plant–pathogen interactions [8,40]. According to previous research, pectin and chitin can trigger the expression of WAK genes. For example, TaWAK7D transcript levels were elevated after pectin and chitin treatments [20], TaWAK2A-800 was significantly induced by chitin treatment [24], and chitin triggered the expression of the rice OsWAK91/92/14 genes [14]. In this study, 19 of the 20 selected TaWAKs were elevated after exogenous pectin treatment, and 12 of the 20 TaWAKs were induced after exogenous chitin treatment, thus demonstrating that the TaWAK family may contribute to the pectin- and chitin-induced defense pathway in wheat. Many disease-resistant WAKs, including CsWAKL08 [41], OsWAK1 [4], ZmHtn1 [38], TaWAK-6D [21], and TaWAK7D, have been found to be localized in the plasma membrane [20]. We selected five TaWAKs to investigate their subcellular localization and observed that four were distributed in the plasma membrane, whereas the other one was expressed in both the plasma membrane and the nucleus. These findings suggest that the plasma membrane distribution of these TaWAKs may be responsible for their immune receptor roles, but the roles of these proteins in nuclear localization require further clarification.
4. Materials and Methods
4.1. Identification of the WAK Family in Wheat
The latest wheat reference genome (IWGSC) and hidden Markov model (HMM) profile (PF08488) of the WAK family were retrieved from the Ensembl Plants website (http://plants.ensembl.org/index.html accessed on 9 August 2021) and protein family database (Pfam) (http://pfam.sanger.ac.uk/ accessed on 9 August 2021) website. The AtWAK protein sequences of Arabidopsis thaliana obtained from the Arabidopsis Information Resource database (https://www.arabidopsis.org/ accessed on 9 August 2021) as previously described [3] were used as queries to identify putative WAK genes in bread wheat in a local database using BLASTp. The WAK HMM profile was employed for functional annotation filters using HMMER software (version 3.0). Subsequently, all candidate protein sequences were further verified for the existence of WAK_GUB, TM, EGF, and protein kinase domains using the Pfam (http://pfam.xfam.org/ accessed on 22 August 2021) and SMART (http://smart.embl-heidelberg.de/ accessed on 22 August 2021) websites. Finally, the proteins that coexisted with the WAK_GUB, TM, and protein kinase domains were considered TaWAKs. MW and pI were predicted using the ExPasy website (https://www.expasy.org/ accessed on 26 August 2021).
4.2. Phylogenetic Analysis
Multiple sequence alignment of the full-length amino acid sequences of TaWAKs was initially performed using MUSCLE (http://www.ebi.ac.uk/Tools/msa/muscle/ accessed on 6 September 2021). An unrooted NJ phylogenetic tree was constructed using MEGA 6.0 with the parameters as follows: pairwise deletion, p-distance model, and 1000 bootstrap replications [42].
4.3. Chromosomal Distribution, Gene Duplication, and Synteny Analysis
The chromosomal distribution of each TaWAK gene was obtained from the wheat genome database published in IWGSC [30]. The physical map of the TaWAKs on the chromosomes of wheat was visualized using TBtools software (version v.1.098696) [43]. Tandem and segmental duplications in the TaWAK family were investigated using a Multiple Collinearity Scan toolkit (MCScanX) [44]. The genome information of H. vulgare, O. sativa, and G. max was downloaded from the Ensemble website. The syntenic relationships of the WAK family between bread wheat and H. vulgare, O. sativa, and G. max were analyzed using MCScanX. Segmental and tandem duplication relationships were virtualized using the Advanced Cicros function of TBtools software (version v.1.098696).
4.4. Expression Profiling of TaWAK Genes
RNA-seq data from root, stem, leaf, spike, and grain, each with three developmental stages, were retrieved from the Wheat Expression Browser (http://www.wheat-expression.com accessed on 28 September 2021) [34]. RNA-seq data from the wheat spike after Fg inoculation for 3, 6, 12, 24, 36, and 48 h were retrieved from the WheatGene database (http://wheatgene.agrinome.org/gene-expression-analysis accessed on 28 September 2021). The heat map was generated using TBtools (version v.1.098696).
4.5. RNA Isolation and qRT-PCR
The seeds of the wheat genotype Fiedler were grown in plastic pots in growth chambers in Nanjing at a relative humidity of 50% and a 16 h photoperiod under 22 °C (light) and 18 °C (dark) conditions. The spike at the heading stage was infected by Fg (Fg1312) for 0, 6, 24, and 36 h. The inoculated spike was covered with plastic bags to maintain humidity and promote Fg infection. The spike at the heading stage was treated with pectin (100 µg/mL) or chitin (100 µg/mL) for 0, 5, 10, 20, and 30 min.
Total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). The quality and quantity of each RNA extract were determined with agarose gel electrophoresis and Nanodrop 2000 (Thermo Fisher Scientific, Carlsbad, CA, USA). Subsequently, the RNA was purified and reverse-transcribed into cDNA using the FastQuant RT Kit (Takara, RR047A, Dalian, China). qRT-PCR was performed using the Roche Thermal Cycler 96 with the TB Green® Premix Ex Taq™ II reagent (Takara, RR820B, Dalian, China). A total of 10 µL reaction solution, including 5 µL of TB Green Enzyme, 3.2 µL of water, and 0.6 µL of primer, was used for qRT-PCR. The PCR procedure was performed as described previously [45], which started at 95 °C for 30 s, followed by 45 cycles of 95 °C for 5 s, 60 °C for 20 s, and 72 °C for 10 s. Melting curve analysis included 95 °C for 10 s, 65 °C for 15 s, and heating to 95 °C at a rate of 0.1 °C/s with continuous readings of fluorescence for each amplification. The wheat tubulin gene [46] was used as the normalization reference gene for the gene expression analysis. Table S3 shows the gene-specific primers used for qRT-PCR. All reactions were run in triplicate, technical replicates for each sample, and three biological replicates were performed.
4.6. Subcellular Localization of TaWAKs
The coding region of TaWAKs lacking the stop codon was amplified using gene-specific primers (Table S3). The amplified fragment was digested with restriction enzyme Kpn I and subcloned in-frame at the 5′-terminus of the GFP coding region in the pCAMBIA1300 vector with the maize ubiquitin promoter. The Ubi:TaWAK-GFP fusion construct or the ubi:GFP control construct was individually introduced into wheat protoplasts. GFP signals were detected and photographed using a confocal laser scanning microscope after incubation at 21 °C for 16 h (Zeiss LSM 980, Oberkochen, Germany).
4.7. Statistical Analysis
All experiments were conducted at least three times with technical and biological replicates. Data were analyzed using Microsoft Excel. A two-tailed Student’s t-test was used to compare the significant differences. Values of p < 0.05 were considered statistically significant.
5. Conclusions
WAKs are important RLKs and play key roles in plant defense against pathogens. FHB is one of the most widespread and devastating crop diseases, causing wheat yield reduction and quality deterioration. We identified and characterized 320 TaWAK family members on all the chromosomes except 4B in bread wheat; these members could be categorized into three phylogenetic groups. Duplication and synteny analysis yielded valuable information on the evolutionary characteristics of TaWAK genes. Thirty TaWAKs were incubated after Fg treatment and most of them could contribute to pectin- and chitin-induced defense pathways. Additionally, 45 TaWAKs in 17 FHB resistance-related hcmQTLs were identified in this study. These results provide valuable information that can be used for discovering new TaWAK genes to improve FHB resistance in wheat.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ijms23137157/s1.
Author Contributions
Y.H. and G.L. designed the research, supervised the work, and wrote the manuscript. X.X. performed the majority of the experiments. Y.Z., Q.A., L.W. (Lirong Wang) and L.Y. extracted the RNA and performed the qRT-PCR analysis. X.Z., Q.T., L.W. (Lei Wu), P.J. and P.Z. provided the analytical tools and assisted in analyzing the data. X.Z. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data used or analyzed in this study are included in this published article and its supplementary materials.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the National Key Project for the Research and Development of China (2017YFE0126700), Jiangsu Seed Industry Revitalization Project (JBGS2021006; JBGS2021049), Jiangsu key R & D plan (Modern Agriculture) (BE2021375), China Agricultural Research System Program (CARS-03), Collaborative Innovation Center for Modern Crop Production co-sponsored by Province and Ministry, and a start-up grant from Nanjing Agricultural University (to G.L.).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jones J.D., Dangl J.L. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- 2.Zipfel C. Plant pattern-recognition receptors. Trends Immunol. 2014;35:345–351. doi: 10.1016/j.it.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Verica J.A., He Z.H. The cell wall-associated kinase (WAK) and WAK-like kinase gene family. Plant Physiol. 2002;129:455–459. doi: 10.1104/pp.011028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li H., Zhou S.Y., Zhao W.S., Su S.C., Peng Y.L. A novel wall-associated receptor-like protein kinase gene, OsWAK1, plays important roles in rice blast disease resistance. Plant Mol. Biol. 2009;69:337–346. doi: 10.1007/s11103-008-9430-5. [DOI] [PubMed] [Google Scholar]
- 5.Stephens C., Hammond-Kosack K.E., Kanyuka K. WAKsing plant immunity, waning diseases. J. Exp. Bot. 2022;73:22–37. doi: 10.1093/jxb/erab422. [DOI] [PubMed] [Google Scholar]
- 6.Harkenrider M., Sharma R., De Vleesschauwer D., Tsao L., Zhang X., Chern M., Canlas P., Zuo S., Ronald P.C. Overexpression of Rice Wall-Associated Kinase 25 (OsWAK25) Alters Resistance to Bacterial and Fungal Pathogens. PLoS ONE. 2016;11:e0147310. doi: 10.1371/journal.pone.0147310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He Z.H., Cheeseman I., He D., Kohorn B.D. A cluster of five cell wall-associated receptor kinase genes, Wak1–5, are expressed in specific organs of Arabidopsis. Plant Mol. Biol. 1999;39:1189–1196. doi: 10.1023/A:1006197318246. [DOI] [PubMed] [Google Scholar]
- 8.Decreux A., Messiaen J. Wall-associated kinase WAK1 interacts with cell wall pectins in a calcium-induced conformation. Plant Cell Physiol. 2005;46:268–278. doi: 10.1093/pcp/pci026. [DOI] [PubMed] [Google Scholar]
- 9.Brutus A., Sicilia F., Macone A., Cervone F., De Lorenzo G. A domain swap approach reveals a role of the plant wall-associated kinase 1 (WAK1) as a receptor of oligogalacturonides. Proc. Natl. Acad. Sci. USA. 2010;107:9452–9457. doi: 10.1073/pnas.1000675107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diener A.C., Ausubel F.M. Resistance to fusarium oxysporum 1, a dominant Arabidopsis disease-resistance gene, is not race specific. Genetics. 2005;171:305–321. doi: 10.1534/genetics.105.042218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bot P., Mun B.-G., Imran Q.M., Hussain A., Lee S.-U., Loake G., Yun B.-W. Differential expression of AtWAKL10 in response to nitric oxide suggests a putative role in biotic and abiotic stress responses. PeerJ. 2019;7:e7383. doi: 10.7717/peerj.7383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hurni S., Scheuermann D., Krattinger S.G., Kessel B., Wicker T., Herren G., Fitze M.N., Breen J., Presterl T., Ouzunova M., et al. The maize disease resistance gene Htn1 against northern corn leaf blight encodes a wall-associated receptor-like kinase. Proc. Natl. Acad. Sci. USA. 2015;112:8780–8785. doi: 10.1073/pnas.1502522112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zuo W., Chao Q., Zhang N., Ye J., Tan G., Li B., Xing Y., Zhang B., Liu H., Fengler K.A., et al. A maize wall-associated kinase confers quantitative resistance to head smut. Nat. Genet. 2015;47:151–157. doi: 10.1038/ng.3170. [DOI] [PubMed] [Google Scholar]
- 14.Delteil A., Gobbato E., Cayrol B., Estevan J., Michel-Romiti C., Dievart A., Kroj T., Morel J.B. Several wall-associated kinases participate positively and negatively in basal defense against rice blast fungus. BMC Plant Biol. 2016;16:1–10. doi: 10.1186/s12870-016-0711-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu K., Cao J., Zhang J., Xia F., Ke Y., Zhang H., Xie W., Liu H., Cui Y., Cao Y. Improvement of multiple agronomic traits by a disease resistance gene via cell wall reinforcement. Nat. Plants. 2017;3:1–9. doi: 10.1038/nplants.2017.9. [DOI] [PubMed] [Google Scholar]
- 16.Zhang N., Pombo M.A., Rosli H.G., Martin G.B. Tomato Wall-Associated Kinase SlWak1 Depends on Fls2/Fls3 to Promote Apoplastic Immune Responses to Pseudomonas syringae. Plant Physiol. 2020;183:1869–1882. doi: 10.1104/pp.20.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Savary S., Willocquet L., Pethybridge S.J., Esker P., McRoberts N., Nelson A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019;3:430–439. doi: 10.1038/s41559-018-0793-y. [DOI] [PubMed] [Google Scholar]
- 18.Saintenac C., Lee W.S., Cambon F., Rudd J.J., King R.C., Marande W., Powers S.J., Berges H., Phillips A.L., Uauy C., et al. Wheat receptor-kinase-like protein Stb6 controls gene-for-gene resistance to fungal pathogen Zymoseptoria tritici. Nat. Genet. 2018;50:368–374. doi: 10.1038/s41588-018-0051-x. [DOI] [PubMed] [Google Scholar]
- 19.Dmochowska-Boguta M., Kloc Y., Zielezinski A., Werecki P., Nadolska-Orczyk A., Karlowski W.M., Orczyk W. TaWAK6 encoding wall-associated kinase is involved in wheat resistance to leaf rust similar to adult plant resistance. PLoS ONE. 2020;15:e0227713. doi: 10.1371/journal.pone.0227713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qi H.J., Zhu X.L., Guo F.L., Lv L.J., Zhang Z.Y. The Wall-Associated Receptor-Like Kinase TaWAK7D Is Required for Defense Responses to Rhizoctonia cerealis in Wheat. Int. J. Mol. Sci. 2021;22:5629. doi: 10.3390/ijms22115629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qi H.J., Guo F.L., Lv L.J., Zhu X.L., Zhang L., Yu J.F., Wei X.N., Zhang Z.Y. The Wheat Wall-Associated Receptor-Like Kinase TaWAK-6D Mediates Broad Resistance to Two Fungal Pathogens Fusarium pseudograminearum and Rhizoctonia cerealis. Front. Plant Sci. 2021;12:2322. doi: 10.3389/fpls.2021.758196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu F., Zhou Y., Shen Y., Sun Z., Li L., Li T. Linking Multi-Omics to Wheat Resistance Types to Fusarium Head Blight to Reveal the Underlying Mechanisms. Int. J. Mol. Sci. 2022;23:2280. doi: 10.3390/ijms23042280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gadaleta A., Colasuonno P., Giove S.L., Blanco A., Giancaspro A. Map-based cloning of QFhb.mgb-2A identifies a WAK2 gene responsible for Fusarium Head Blight resistance in wheat. Sci. Rep. 2019;9:6929. doi: 10.1038/s41598-019-43334-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo F., Wu T., Xu G., Qi H., Zhu X., Zhang Z. TaWAK2A-800, a Wall-Associated Kinase, Participates Positively in Resistance to Fusarium Head Blight and Sharp Eyespot in Wheat. Int. J. Mol. Sci. 2021;22:11493. doi: 10.3390/ijms222111493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun Z., Song Y., Chen D., Zang Y., Zhang Q., Yi Y., Qu G. Genome-Wide Identification, Classification, Characterization, and Expression Analysis of the Wall-Associated Kinase Family during Fruit Development and under Wound Stress in Tomato (Solanum lycopersicum L.) Genes. 2020;11:1186. doi: 10.3390/genes11101186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dou L., Li Z., Shen Q., Shi H., Li H., Wang W., Zou C., Shang H., Li H., Xiao G. Genome-wide characterization of the WAK gene family and expression analysis under plant hormone treatment in cotton. BMC Genom. 2021;22:85. doi: 10.1186/s12864-021-07378-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Oliveira L.F.V., Christoff A.P., de Lima J.C., de Ross B.C.F., Sachetto-Martins G., Margis-Pinheiro M., Margis R. The Wall-associated Kinase gene family in rice genomes. Plant Sci. 2014;229:181–192. doi: 10.1016/j.plantsci.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 28.Tripathi R.K., Aguirre J.A., Singh J. Genome-wide analysis of wall associated kinase (WAK) gene family in barley. Genomics. 2021;113:523–530. doi: 10.1016/j.ygeno.2020.09.045. [DOI] [PubMed] [Google Scholar]
- 29.Li M., Ma J., Liu H., Ou M., Ye H., Zhao P. Identification and Characterization of Wall-Associated Kinase (WAK) and WAK-like (WAKL) Gene Family in Juglans regia and Its Wild Related Species Juglans mandshurica. Genes. 2022;13:134. doi: 10.3390/genes13010134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.IWGSC. Appels R., Eversole K., Feuillet C., Keller B., Rogers J., Stein N., Pozniak C.J., Choulet F., Distelfeld A., et al. Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science. 2018;361:661. doi: 10.1126/science.aar7191. [DOI] [PubMed] [Google Scholar]
- 31.Kanneganti V., Gupta A.K. Wall associated kinases from plants—An overview. Physiol. Mol. Biol. Plants. 2008;14:109–118. doi: 10.1007/s12298-008-0010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng T., Hua C., Li L., Sun Z.X., Yuan M.M., Bai G.H., Humphreys G., Li T. Integration of meta-QTL discovery with omics: Towards a molecular breeding platform for improving wheat resistance to Fusarium head blight. Crop J. 2021;9:739–749. doi: 10.1016/j.cj.2020.10.006. [DOI] [Google Scholar]
- 33.Chen Z., Shen Z., Zhao D., Xu L., Zhang L., Zou Q. Genome-Wide Analysis of LysM-Containing Gene Family in Wheat: Structural and Phylogenetic Analysis during Development and Defense. Genes. 2020;12:31. doi: 10.3390/genes12010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.IWGSC. Mayer K.F., Rogers J., Doležel J., Pozniak C., Eversole K., Feuillet C., Gill B., Friebe B., Lukaszewski A.J. A chromosome-based draft sequence of the hexaploid bread wheat (Triticum aestivum) genome. Science. 2014;345:1251788. doi: 10.1126/science.1251788. [DOI] [PubMed] [Google Scholar]
- 35.Rigal A., Doyle S.M., Robert S. Live cell imaging of FM4–64, a tool for tracing the endocytic pathways in Arabidopsis root cells. Methods Mol. Biol. 2015;1242:93–103. doi: 10.1007/978-1-4939-1902-4_9. [DOI] [PubMed] [Google Scholar]
- 36.Yu H., Zhang W., Kang Y., Fan Y., Yang X., Shi M., Zhang R., Wang Y., Qin S. Genome-wide identification and expression analysis of wall-associated kinase (WAK) gene family in potato (Solanum tuberosum L.) Plant Biotechnol. Rep. 2022;36:1–15. doi: 10.1007/s11816-021-00739-5. [DOI] [Google Scholar]
- 37.Shi G., Zhang Z., Friesen T.L., Raats D., Fahima T., Brueggeman R.S., Lu S., Trick H.N., Liu Z., Chao W. The hijacking of a receptor kinase–driven pathway by a wheat fungal pathogen leads to disease. Sci. Adv. 2016;2:e1600822. doi: 10.1126/sciadv.1600822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang P., Praz C., Li B., Singla J., Robert C.A., Kessel B., Scheuermann D., Lüthi L., Ouzunova M., Erb M. Fungal resistance mediated by maize wall-associated kinase Zm WAK-RLK 1 correlates with reduced benzoxazinoid content. N. Phytol. 2019;221:976–987. doi: 10.1111/nph.15419. [DOI] [PubMed] [Google Scholar]
- 39.Wang P., Zhou L., Jamieson P., Zhang L., Zhao Z., Babilonia K., Shao W., Wu L., Mustafa R., Amin I. The cotton wall-associated kinase GhWAK7A mediates responses to fungal wilt pathogens by complexing with the chitin sensory receptors. Plant Cell. 2020;32:3978–4001. doi: 10.1105/tpc.19.00950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kohorn B.D., Johansen S., Shishido A., Todorova T., Martinez R., Defeo E., Obregon P. Pectin activation of MAP kinase and gene expression is WAK2 dependent. Plant J. 2009;60:974–982. doi: 10.1111/j.1365-313X.2009.04016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Q., Hu A., Qi J., Dou W., Qin X., Zou X., Xu L., Chen S., He Y. CsWAKL08, a pathogen-induced wall-associated receptor-like kinase in sweet orange, confers resistance to citrus bacterial canker via ROS control and JA signaling. Hortic. Res. 2020;7:42. doi: 10.1038/s41438-020-0263-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Y.L., Li Z.K., Chen N.Z., Huang Y., Huang S.J. Phase separation of Arabidopsis EMB1579 controls transcription, mRNA splicing, and development. PLoS Biol. 2020;18:e3000782. doi: 10.1371/journal.pbio.3000782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y., Tang H., Debarry J.D., Tan X., Li J., Wang X., Lee T.H., Jin H., Marler B., Guo H., et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012;40:e49. doi: 10.1093/nar/gkr1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He Y., Ahmad D., Zhang X., Zhang Y., Wu L., Jiang P., Ma H. Genome-wide analysis of family-1 UDP glycosyltransferases (UGT) and identification of UGT genes for FHB resistance in wheat (Triticum aestivum L.) BMC Plant Biol. 2018;18:67. doi: 10.1186/s12870-018-1286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alexandre P., Jia J., Amal K., Chanemougasoundharam A., Steven S., Sarah B., Emma W., Fiona D. TaFROG encodes a Pooideae orphan protein that interacts with SnRK1 and enhances resistance to the mycotoxigenic fungus Fusarium graminearum. Plant Physiol. 2015;169:2895–2906. doi: 10.1104/pp.15.01056. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used or analyzed in this study are included in this published article and its supplementary materials.