Abstract
PURPOSE:
The aim of the HYLAN A study was to investigate if symptoms and/or signs of patients suffering from severe dry eye disease (DED) in Saudi Arabia can be improved by substituting individually optimized artificial tear therapy by high molecular weight hyaluronan (HMWHA) eye drops.
METHODS:
The HYLAN M study, a multicenter prospective randomized open-label study, was performed in 11 centers in eight countries. Patients suffering from severe DED were electronically randomized in two parallel arms. patients with symptoms of at least ocular surface disease index (OSDI) 33 and corneal fluorescein staining (CFS) of at least Oxford grade 3 were included . The patients in the control group continued with their individual optimized therapy as by the time of inclusion. The patients in the hylan A group replaced their individual lubricant eye drops by preservative-free eye drops containing 0.15% hylan A. The total OSDI scores as well as the OSDI subscores for pain and for visual disturbances of each patient at baseline, at 4 weeks, and at 8 weeks of treatment was used to analyse the improvement of symptoms. We focus and report the results obtained at the two study centers in Riyadh ,Saudi Arabia (King Khaled Eye Specialist Hospital and Riyadh Military Hospital).
RESULTS:
A total of 13 patients were included in the study. The majority of the study participants were middle aged (40-65 years). Overall, female patients accounted for 76.9% of all study participants. At the initiation of the study, both hylan A and control groups had relatively similar total OSDI scores together with pain and vision subscores. At 4-week follow-up, both groups demonstrated a noticeable decrease in all study variables. Nevertheless, the OSDI scores improved significantly in the group of patients treated with hylan A eye drops at 8 weeks, whereas the scores increased in the control group.
CONCLUSION:
Saudi Arabia has a very high prevalence of patients with severe dry eye disease. Ethnicity, climate, and a high incidence of diabetes mellitus may contribute to this situation. Lubricant eye drops frequently do not provide adequate relief from ocular pain and instable vision in severe chronic ocular surface disease. High molecular weight hyaluronan (HMWHA) eye drops provide superior relief of symptoms of patients suffering from severe DED. This includes ocular pain as well as unstable vision.
Keywords: Corneal nerves, diabetes, dry eye, hyaluronan, hylan A
INTRODUCTION
Discomfort and pain at the surface of the eye, with or without signs of corneoconjunctival tissue damage, are the dominant symptoms of dry eye disease (DED) and other conditions affecting the ocular surface. Studies in the USA, Spain, and France found a prevalence of 14% to 39%, whereas studies in Asia showed a prevalence of 20% to 52% for symptomatic DED.[1] For the population in Saudi Arabia, a DED prevalence of 32% to 93% has been reported.[2,3,4,5,6,7] A high percentage of dry eye patients in Saudi Arabia reported severe symptoms.[4,5,8] Moreover, among the patients with DED, there seems to be a very high percentage simultaneously suffering from diabetes mellitus (DM).[4] DED has a significant impact on work productivity, in particular on Saudi office workers.[9] Tear replacement with lubricating, hydrating eye drops which provide palliative relief from symptoms are the mainstay for long-term treatment of DED. This type of treatment is not targeting the underlying pathophysiology of the disease.[10] Particularly, severe DED associated with chronic inflammation requires a personalized treatment, prescribing the artificial tears which provide the best symptom relief for the individual patient.[11] Most lubricant eye drop formulations were developed in the USA, Europe, or Japan, and were never subject to a controlled clinical study of dry eye patients of Arab ethnicity and under the conditions of the climate in Saudi Arabia. For the first time, the HYLAN M study on patients with severe DED compared data from two study centers in Riyadh, Saudi Arabia, with those from nine study centers in Europe.[12] Tear substitutes containing 0.15% hylan A were compared with other lubricant eye drops which had been used by the patients as individual best treatment by the time of their inclusion into the study. The Asia Dry Eye Society recently proposed to use subjective severity of dry eye as a marker for therapeutic efficacy.[13] Various questionnaires are available for the assessment of dry eye symptoms.[14] In our study, we used the Ocular Surface Disease Index (OSDI) questionnaire.[15] According to Miller et al., an OSDI score up to 12 is considered as normal eye, a score from 13 to 22 as mild, from 23 to 32 as moderate, and 33 or more as severe dry eye.[16]
METHODS
Study design
The HYLAN M study, a multicenter prospective randomized open-label study, was performed in 11 centers in eight countries. The study adhered to the Declaration of Helsinki, was approved by the Ethics Committees of each center, and registered under the number CIV-16-06-015964 on the EUDAMED database of the European Commission. Patients suffering from severe DED were electronically randomized in two parallel arms. The patients in the control group continued with their individual optimized therapy as by the time of inclusion. The patients in the hylan A group replaced their individual lubricant eye drops by preservative-free eye drops containing 0.15% hylan A dissolved in isotonic saline solution buffered with 1.20 mmol/L phosphate (Comfort Shield® eye drops; i.com medical, Munich, Germany). Hylan A is a very high-molecular weight hyaluronic acid (HA).[17] The intrinsic viscosity of hylan A in our study was 2.9 m≥/kg. Further details of the study design have been published elsewhere.[12] Here, we report on the results obtained at the two study centers located in Riyadh, Saudi Arabia.
Participants
Inclusion criteria included all patients over the age of 18 years known to present with DED of any etiology managed with dry eye regimen that have not been altered for at least 2 months. The primary criteria for severe DED according to Baudouin et al., i.e., OSDI score 33 or more in combination with corneal fluorescein staining (CFS) Oxford Grade 3 or more, were used for the study.[18,19] The eyes with the higher CFS score were defined as study eyes. Patients were excluded if they participated in any other clinical trial, suffered from eye diseases other than dry eye, had ocular surgery <3 months prior to study inclusion, were using punctual plugs, or had masquerading conditions, as identified by Karpecki.[20]
Efficacy assessment
The OSDI questionnaire was used for the assessment of therapeutic efficacy.[15] The patients answered the OSDI questions by the time of inclusion (baseline), after 4 weeks, and after 8 weeks. The difference between OSDI scores at week 8 and at baseline was used as the endpoint. To further analyze the improvement of symptoms, OSDI subscores for questions related to ocular pain and discomfort, OSDIpain (OSDI questions 1–3), and OSDI questions related to stability of vision, OSDIvision(OSDI questions 4–9), were separately calculated according to the following formulas:

n = number of questions answered (3 and 6, at most, for the pain and vision subscore, respectively).
Data analysis
Descriptive statistics of the patients were presented using frequency and percentage distribution. The independent t-test was used to test for the mean difference between the groups at baseline, after 4 weeks, and after 8 weeks. No assumptions of the independent t-test were violated.
The repeated measures analysis of variance with sphericity assumed was then used to examine the trend of OSDI scores and to test for the difference in OSDI scores from baseline, to 4 weeks, and to 8 weeks. All assumptions of the repeated-measures ANOVA were also tested and none was violated.
The Mauchly’s test of sphericity was used to test for the assumption of sphericity. The results indicated that the assumption of sphericity was not violated in the OSDI score repeated measures, OSDIpain, and OSDIvision, χ2 (2) = 1.44, P = 0.487, χ2 (2) = 0.72, P = 0.698, and χ2 (2) = 2.70, P = 0.26, respectively.
RESULTS
The majority of the study participants were middle aged (40–65 years). Overall, female patients accounted for 76.9% of all study participants. The mean age ± standard deviation (SD) for the hylan A and control groups were 39.7% (±11.1) and 46.7% (±11.0), respectively. The sociodemographic data for all patients are summarized in Table 1.
Table 1.
Sociodemographic data of study participants
| Characteristics | Hylan A group (n=6), n (%) | Control group (n=7), n (%) |
|---|---|---|
| Age (years) | ||
| <40 | 2 (33.3) | 1 (14.3) |
| 40-65 | 4 (66.7) | 6 (85.7) |
| Gender | ||
| Female | 5 (83.3) | 5 (71.4) |
| Male | 1 (16.7) | 2 (28.6) |
Table 2 provides a complete summary of the OSDI scores as well as the OSDI subscores for pain and for visual disturbances of each patient at baseline, at 4 weeks, and at 8 weeks of treatment. At the initiation of the study, both hylan A and control groups had relatively similar total OSDI scores together with pain and vision subscores ranging between 40 and 60. At 4-week follow-up, both groups demonstrated a noticeable decrease in all study variables. Nevertheless, the OSDI scores improved significantly in the group of patients treated with hylan A eye drops at 8 weeks, whereas the scores increased in the control group reflecting the worsening of DED symptoms, as demonstrated in Figure 1.
Table 2.
Total Ocular Surface Disease Index scores as well as Ocular Surface Disease Index pain and Ocular Surface Disease Index vision subscores for hylan A and control groups
| Treatment | n | Mean (±SD) | ||
|---|---|---|---|---|
|
| ||||
| Baseline | Week 4 | Week 8 | ||
| Total OSDI score | ||||
| Hylan A group | 6 | 62.3 (18.6) | 36.9 (31.7) | 26.5 (20.3) |
| Control group | 7 | 63.0 (20.3) | 49.7 (15.7) | 65.9 (12.6) |
| OSDI pain | ||||
| Hylan A group | 6 | 59.7 (25.5) | 37.5 (33.6) | 30.6 (23.4) |
| Control group | 7 | 59.5 (29.8) | 50.0 (29.7) | 59.5 (16.3) |
| OSDI vision | ||||
| Hylan A group | 6 | 50.0 (19.0) | 22.2 (29.1) | 13.9 (13.4) |
| Control group | 7 | 41.1 (24.2) | 35.1 (15.6) | 46.4 (14.3) |
OSDI: Ocular Surface Disease Index, SD: Standard deviation
Figure 1.
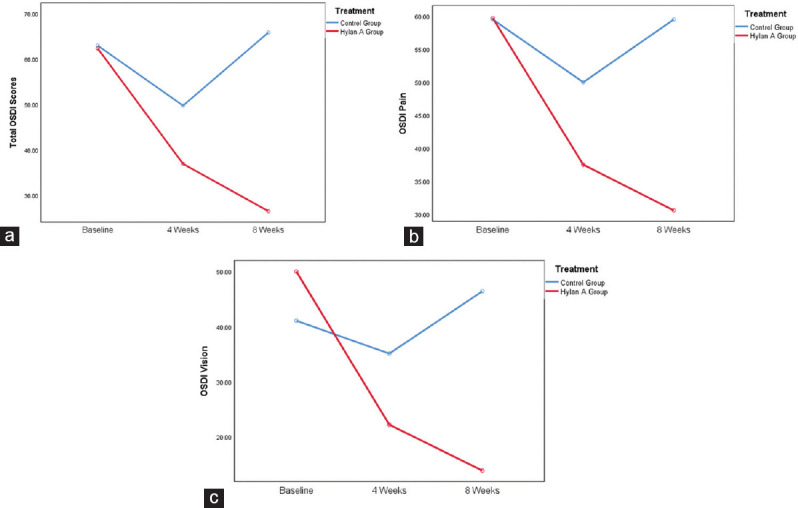
(a) Ocular Surface Disease Index score trend by time point, (b) Ocular Surface Disease Index pain trend by time point, (c) Ocular Surface Disease Index vision trend by time point
Table 3 presents the results of the repeated-measures ANOVA testing which showed that the total OSDI score was statistically significantly different between time points for the hylan A group and the control group (F [2, 10] = 5.123, P < 0.05) and (F [2, 12] = 4.72, P < 0.05), respectively. The results showed that while the total OSDI scores reduced for the hylan A group from baseline (mean = 62.3, SD = 18.4) to week 4 (mean = 36.9, SD = 31.7) and to week 8 (mean = 26.5, SD = 20.3), respectively, the total OSDI scores for the control group decreased from baseline (mean = 63.0, SD = 20.3) to week 4 (mean = 49.7, SD = 15.7) but increased significantly at week 8 (mean = 65.9, SD = 12.6).
Table 3.
Analysis of variance test results for total Ocular Surface Disease Index, Ocular Surface Disease Index pain, and Ocular Surface Disease Index vision for hylan A and control groups
| Treatment | Source | Type III sum of squares | df | Mean square | F | P |
|---|---|---|---|---|---|---|
| Hylan A group | OSDI score | 4089.8 | 2 | 2044.9 | 5.123 | 0.029 |
| Error (OSDI score) | 3991.9 | 10 | 399.2 | |||
| Control | OSDI score | 1033.9 | 2 | 517.0 | 4.72 | 0.031 |
| Error (OSDI score) | 1314.4 | 12 | 109.5 | |||
| Hylan A group | OSDI pain | 2785.5 | 2 | 1392.7 | 2.317 | 0.149 |
| Error (OSDI pain) | 6010.8 | 10 | 601.1 | |||
| Control | OSDI pain | 423.3 | 2 | 211.6 | 0.753 | 0.492 |
| Error (OSDI pain) | 3373.0 | 12 | 281.1 | |||
| Hylan A group | OSDI vision | 4290.1 | 2 | 2145.1 | 5.543 | 0.024 |
| Error (OSDI vision) | 3869.6 | 10 | 387.0 | |||
| Control | OSDI vision | 448.1 | 2 | 224.0 | 0.917 | 0.426 |
| Error (OSDI vision) | 2931.5 | 12 | 244.3 |
OSDI: Ocular Surface Disease Index
Regarding OSDI pain scores, the results showed that there was no statistically significant difference between the study time points for the hylan A group and the control group (F [2, 10] = 2.317, P = 0.149) and (F [2, 12] = 0.753, P ≤ 0.492), respectively. Results showed that OSDI pain mean reduced for the hylan A group from baseline (mean = 59.7, SD = 25.5) to week 4 (mean = 37.5, SD = 33.6) and to week 8 (mean = 30.6, SD = 23.4), respectively, while OSDI pain scores reduced for the control from baseline (mean = 59.5, SD = 29.8) to week 4 (mean = 50.0, SD = 29.7) and increased at week 8 (mean = 59.5, SD = 16.3). However, the results were not sufficient to conclude a significant reduction in pain at the different time points for both groups.
OSDI vision results demonstrated a statistically significant difference between time points for the hylan A group but not for the control group (F [2, 10] = 5.543, P < 0.05) and (F [2, 12] = 0.917, P ≤ 0.426), respectively. Post hoc analysis showed a statistically significant reduction in OSDI vision scores from (mean = 50, SD = 19) at baseline to (mean = 22.2, SD = 19.1) at 4 weeks to (mean = 13.9, SD = 13.3) at 8 weeks for the hylan A group but there was no significant reduction for the control group.
The independent t-test to compare both study groups’ total OSDI scores at baseline [Table 4] showed no statistically significant difference (t[11] = ‒0.06, P = 0.955); however, this score was statistically different after 8 weeks (t[11] = ‒4.27, P < 0.01]. Similarly, the OSDI pain scores for both groups were not different at baseline (t[11] = 0.01, P = 0.990), but they were statistically significantly different after 8 weeks of treatment (t[11] =‒2.63, P < 0.05). Likewise, the OSDI vision scores for hylan A and control groups were not significantly different at baseline (t[11] = 0.73, P = 0.481); yet, a significant difference was observed after 8 weeks of treatment (t[11] = ‒4.21, P < 0.01). The change of the OSDI scores and subscores from the baseline to the 8 weeks visit in the hylan A group as compared to the control group is visualized in Figure 2.
Table 4.
Student’s t-test results for hylan A and control groups at baseline and 8-week follow-up
| t | df | P | Mean difference | SE difference | 95% CI | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Lower | Upper | ||||||
| Baseline OSDI score | −0.06 | 11 | 0.955 | −0.63 | 10.87 | −24.57 | 23.30 |
| Week 8 OSDI score | −4.27 | 11 | 0.001 | −39.40 | 9.23 | −59.72 | −19.08 |
| Baseline OSDI pain | 0.01 | 11 | 0.990 | 0.20 | 15.55 | −34.02 | 34.42 |
| Week 8 OSDI pain | −2.63 | 11 | 0.023 | −28.97 | 11.02 | −53.23 | −4.71 |
| Baseline OSDI vision | 0.73 | 11 | 0.481 | 8.93 | 12.24 | −18.02 | 35.88 |
| Week 8 OSDI vision | −4.21 | 11 | 0.001 | −32.54 | 7.73 | −49.54 | −15.53 |
CI: Confidence interval, SE: Standard error, OSDI: Ocular Surface Disease Index
Figure 2.
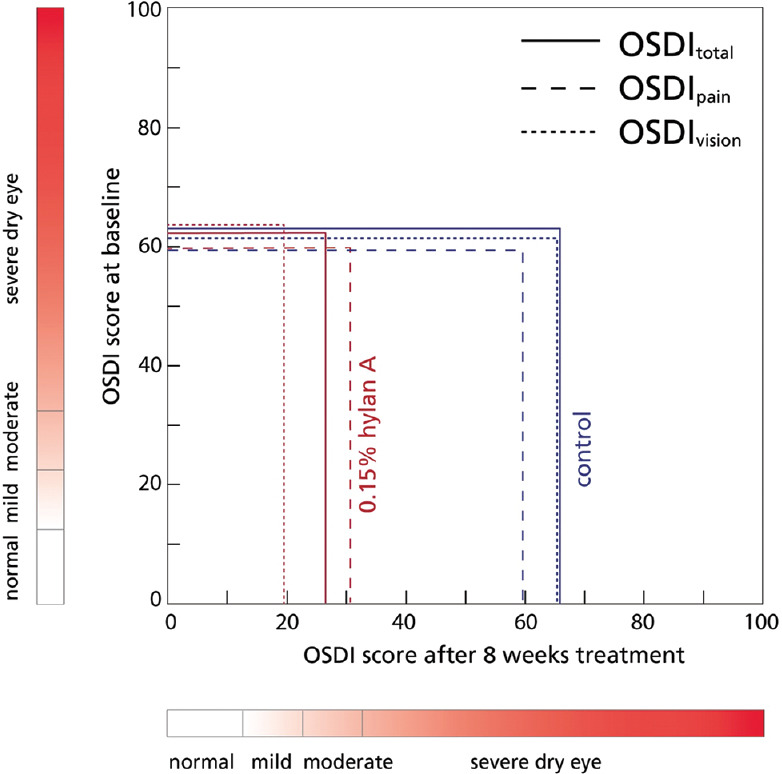
Change of the Ocular Surface Disease Index scores and subscores from baseline to 8 weeks in the hylan A group as compared to the control group
These results collectively demonstrate the efficacy of hylan A in reducing DED symptoms evidenced by a significant reduction in total OSDI scores. In addition, hylan A eye drops were likely to improve vision-related complaints, and to a lesser extent, pain-related symptoms in patients with DED.
DISCUSSION
Disturbed immunoregulation involving chronic inflammation is currently considered the characteristics of severe DED.[21] High-molecular weight HA is known to stabilize the epithelial barrier, suppress pain, and exercise immunosuppressive effects.[22,23,24] The Asia Dry Eye Society recently speculated that neuropathic components may determine the severity of dry eye symptoms.[13] Patients suffering from neuropathic pain have been reported to respond poorly to the treatment with lubricant eye drops.[25,26] Within the HYLAN M study, four of the participating centers had submitted images of the subbasal corneal nerve plexus taken by in vivo confocal microscopy at baseline and after 8 weeks of treatment with 0.15% hylan A eye drops. Significant nerve growth indicating the recovery of compromised corneal nerves under the treatment with 0.15% hylan A eye drops was found.[27] It is, therefore, hypothesized that the significant improvement of symptoms observed in the current study is at least partly due to the recovery of corneal nerves. Nerves provide essential support to the corneal epithelium.[28,29,30,31,32,33,34,35,36,37] 0.15% hylan A eye drops seem to contribute to regaining ocular surface homeostasis in eyes with chronic inflammation, although it needs to be emphasized that the observed improvement of symptoms has only been investigated in a small number of eyes.
Saudi Arabia is among the countries with the highest prevalence of DM.[38] DM is associated with progressive damage to corneal nerves and consequently epithelial cells, resulting in an increased risk for DED, corneal erosion, persistent epithelial defects, and eventually sight-threatening corneal ulcers.[39,40,41,42,43,44,45] About 50% of diabetic patients develop corneal neuropathy.[39,46,47,48,49] Corneal neuropathy is underrated due to the initial absence of ocular discomfort and pain.[49,50,51,52] The evidence that 0.15% hylan A eye drops support corneal nerve regeneration allows the assumption that these eye drops will offer a preventive therapy against the development and progression of diabetic keratopathy.[27] Other instances where 0.15% hylan A eye drops are likely to support the healing process of the ocular surface are ocular surgery, refractive surgery, corneal cross-linking, chemical burns, ocular trauma, keratoconus, or simply compromised corneal nerves due to aging.[53,54,55,56,57,58]
CONCLUSION
It has been shown that 0.15% hylan A eye drops provide superior relief of symptoms of patients suffering from severe DED. This includes ocular pain as well as unstable vision. 0.15% hylan A eye drops seem to simultaneously address the various and complex pathomechanisms of ocular surface disease, and in particular downregulate inflammation and provide trophic support to the corneal nerves. After any kind of ocular surgery, 0.15% hylan A eye drops may serve to ameliorate ocular discomfort and support the recovery of damaged nerves. 0.15% hylan A eye drops offer a therapeutic option for preventing and treating ocular surface disease, in particular conditions associated with corneal nerve damage like diabetic keratopathy.
Financial support and sponsorship
The HYLAN M study received unrestricted funding from i.com medical GmbH, Munich, Germany.
Conflicts of interest
Dr. Wolfgang G.K. Müller-Lierheim, the study director, is the CEO of the company i.com medical GmbH, Munich, Germany. The remaining authors declare no conflicts of interest.
Acknowledgment
We would like to greatly thank each of Dr. Ahmad Alsalem, Dr. Turki Alonaizan, Dr. Yaser Ben Thabet, Dr. Mohamed Alshafie, Ms. Eman Alghamdi, and Ms. Seena Elias from Prince Sultan Military Medical City, and Ms. Gharam AlZahrani and Ms. Sarah AlHarbi from KKESH, for their valuable contribution in conducting this study.
REFERENCES
- 1.Stapleton F, Alves M, Bunya VY, Jalbert I, Lekhanont K, Malet F, et al. TFOS DEWS II Epidemiology Report. Ocul Surf. 2017;15:334–65. doi: 10.1016/j.jtos.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Bukhari A, Ajlan R, Alsaggaf H. Prevalence of dry eye in the normal population in Jeddah, Saudi Arabia. Orbit. 2009;28:392–7. doi: 10.3109/01676830903074095. [DOI] [PubMed] [Google Scholar]
- 3.Alshamrani AA, Almousa AS, Almulhim AA, Alafaleq AA, Alosaimi MB, Alqahtani AM, et al. Prevalence and risk factors of dry eye symptoms in a Saudi Arabian population. Middle East Afr J Ophthalmol. 2017;24:67–73. doi: 10.4103/meajo.MEAJO_281_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alhamyani AH, Noor Kalakattawi RM, Noor Kalakattawi AM, Alhamyani AH, Alsuqati FA, Al-Shehri LA, et al. Prevalence of dry eye symptoms and its risk factors among patients of King Abdulaziz Specialist Hospital (Taif), Saudi Arabia. Saudi J Health Sci. 2017;6:140–4. [Google Scholar]
- 5.Alharbi1 AJ, Alanazi NA, Alhamad JR, Alabdulqader RA, Aljamea DA, Alabdulqader SA, et al. Prevalence of symptomatic dry eye and its risk factors among coastal population in Eastern Province of Saudi Arabia. EC Ophthalmol. 2019;10:503–9. [Google Scholar]
- 6.Yasir ZH, Chauhan D, Khandekar R, Souru C, Varghese S. Prevalence and determinants of dry eye disease among 40 years and older population of Riyadh (Except Capital), Saudi Arabia. Middle East Afr J Ophthalmol. 2019;26:27–32. doi: 10.4103/meajo.MEAJO_194_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alsweilem M, Alenzi MK, Almutairi SN, Alanazy TA. Prevalence of eye dryness among the general population of the Northern Region of Saudi Arabia. Int J Med Dev Ctries. 2019;10:841–8. [Google Scholar]
- 8.Almutairi AH, Alalawi BS, Badr GH, Alawaz RA, Albarry M, Elbadawy HM. Prevalence of dry eye syndrome in association with the use of contact lenses in Saudi Arabia. BMC Ophthalmol. 2021;21:147. doi: 10.1186/s12886-021-01912-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Binyousef FH, Alruwaili SA, Altammami AF, Alharbi AA, Alrakaf FA, Almazrou AA. Impact of dry eye disease on work productivity among Saudi workers in Saudi Arabia. Clin Ophthalmol. 2021;15:2675–81. doi: 10.2147/OPTH.S313158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones L, Downie LE, Korb D, Benitez-Del-Castillo JM, Dana R, Deng SX, et al. TFOS DEWS II management and therapy report. Ocul Surf. 2017;15:575–628. doi: 10.1016/j.jtos.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Aragona P, Giannaccare G, Mencucci R, Rubino P, Cantera E, Rolando M. Modern approach to the treatment of dry eye, a complex multifactorial disease: A P.I.C.A.S.S.O. board review. Br J Ophthalmol. 2021;105:446–53. doi: 10.1136/bjophthalmol-2019-315747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Setten GB, Baudouin C, Horwath-Winter J, Böhringer D, Stachs O, Toker E, et al. The HYLAN M study: Efficacy of 0.15% High molecular weight hyaluronan fluid in the treatment of severe dry eye disease in a multicenter randomized trial. J Clin Med. 2020;9:E3536. doi: 10.3390/jcm9113536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsubota K, Yokoi N, Watanabe H, Dogru M, Kojima T, Yamada M, et al. A new perspective on dry eye classification: Proposal by the asia dry eye society. Eye Contact Lens. 2020;46(Suppl 1):S2–13. doi: 10.1097/ICL.0000000000000643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolffsohn JS, Arita R, Chalmers R, Djalilian A, Dogru M, Dumbleton K, et al. TFOS DEWS II diagnostic methodology report. Ocul Surf. 2017;15:539–74. doi: 10.1016/j.jtos.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the ocular surface disease index. Arch Ophthalmol. 2000;118:615–21. doi: 10.1001/archopht.118.5.615. [DOI] [PubMed] [Google Scholar]
- 16.Miller KL, Walt JG, Mink DR, Satram-Hoang S, Wilson SE, Perry HD, et al. Minimal clinically important difference for the ocular surface disease index. Arch Ophthalmol. 2010;128:94–101. doi: 10.1001/archophthalmol.2009.356. [DOI] [PubMed] [Google Scholar]
- 17.Müller-Lierheim WG. Why chain length of hyaluronan in eye drops matters. Diagnostics (Basel) 2020;10:511. doi: 10.3390/diagnostics10080511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baudouin C, Aragona P, Van Setten G, Rolando M, Irkeç M, Benítez del Castillo J, et al. Diagnosing the severity of dry eye: A clear and practical algorithm. Br J Ophthalmol. 2014;98:1168–76. doi: 10.1136/bjophthalmol-2013-304619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 2003;22:640–50. doi: 10.1097/00003226-200310000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Karpecki PM. Why dry eye trials often fail: From disease variability to confounding underlying conditions, there are countless reasons why new dry eye drugs have come up short in FDA testing. Review of Optometry. 2013:50. [Google Scholar]
- 21.Baudouin C, Irkeç M, Messmer EM, Benítez-Del-Castillo JM, Bonini S, Figueiredo FC, et al. Clinical impact of inflammation in dry eye disease: Proceedings of the ODISSEY group meeting. Acta Ophthalmol. 2018;96:111–9. doi: 10.1111/aos.13436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansen IM, Ebbesen MF, Kaspersen L, Thomsen T, Bienk K, Cai Y, et al. Hyaluronic acid molecular weight-dependent modulation of mucin nanostructure for potential mucosal therapeutic applications. Mol Pharm. 2017;14:2359–67. doi: 10.1021/acs.molpharmaceut.7b00236. [DOI] [PubMed] [Google Scholar]
- 23.Ferrari LF, Khomula EV, Araldi D, Levine JD. CD44 signaling mediates high molecular weight hyaluronan-induced antihyperalgesia. J Neurosci. 2018;38:308–21. doi: 10.1523/JNEUROSCI.2695-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang D, Liang J, Noble PW. Hyaluronan as an immune regulator in human diseases. Physiol Rev. 2011;91:221–64. doi: 10.1152/physrev.00052.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galor A, Batawi H, Felix ER, Margolis TP, Sarantopoulos KD, Martin ER, et al. Incomplete response to artificial tears is associated with features of neuropathic ocular pain. Br J Ophthalmol. 2016;100:745–9. doi: 10.1136/bjophthalmol-2015-307094. [DOI] [PubMed] [Google Scholar]
- 26.Galor A, Moein HR, Lee C, Rodriguez A, Felix ER, Sarantopoulos KD, et al. Neuropathic pain and dry eye. Ocul Surf. 2018;16:31–44. doi: 10.1016/j.jtos.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Setten GB, Stachs O, Dupas B, Turhan SA, Seitz B, Reitsamer H, et al. High molecular weight hyaluronan promotes corneal nerve growth in severe dry eyes. J Clin Med. 2020;9:E3799. doi: 10.3390/jcm9123799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Müller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: Structure, contents and function. Exp Eye Res. 2003;76:521–42. doi: 10.1016/s0014-4835(03)00050-2. [DOI] [PubMed] [Google Scholar]
- 29.Garcia-Hirschfeld J, Lopez-Briones LG, Belmonte C. Neurotrophic influences on corneal epithelial cells. Exp Eye Res. 1994;59:597–605. doi: 10.1006/exer.1994.1145. [DOI] [PubMed] [Google Scholar]
- 30.Korsching S. The neurotrophic factor concept: A reexamination. J Neurosci. 1993;13:2739–48. doi: 10.1523/JNEUROSCI.13-07-02739.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shaheen BS, Bakir M, Jain S. Corneal nerves in health and disease. Surv Ophthalmol. 2014;59:263–85. doi: 10.1016/j.survophthal.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al-Aqaba MA, Dhillon VK, Mohammed I, Said DG, Dua HS. Corneal nerves in health and disease. Prog Retin Eye Res. 2019;73:100762. doi: 10.1016/j.preteyeres.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 33.Belmonte C, Acosta MC, Gallar J. Neural basis of sensation in intact and injured corneas. Exp Eye Res. 2004;78:513–25. doi: 10.1016/j.exer.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 34.Yang AY, Chow J, Liu J. Corneal innervation and sensation: The eye and beyond. Yale J Biol Med. 2018;91:13–21. [PMC free article] [PubMed] [Google Scholar]
- 35.Galor A, Felix ER, Feuer W, Levitt RC, Sarantopoulos CD. Corneal nerve pathway function in individuals with dry eye symptoms. Ophthalmology. 2021;128:619–21. doi: 10.1016/j.ophtha.2020.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dastjerdi MH, Dana R. Corneal nerve alterations in dry eye-associated ocular surface disease. Int Ophthalmol Clin. 2009;49:11–20. doi: 10.1097/IIO.0b013e31819242c9. [DOI] [PubMed] [Google Scholar]
- 37.Ueno H, Ferrari G, Hattori T, Saban DR, Katikireddy KR, Chauhan SK, et al. Dependence of corneal stem/progenitor cells on ocular surface innervation. Invest Ophthalmol Vis Sci. 2012;53:867–72. doi: 10.1167/iovs.11-8438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naeem Z. Burden of diabetes mellitus in Saudi Arabia. Int J Health Sci (Qassim) 2015;9:5–6. doi: 10.12816/0024690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ljubimov AV. Diabetic complications in the cornea. Vision Res. 2017;139:138–52. doi: 10.1016/j.visres.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Markoulli M, Flanagan J, Tummanapalli SS, Wu J, Willcox M. The impact of diabetes on corneal nerve morphology and ocular surface integrity. Ocul Surf. 2018;16:45–57. doi: 10.1016/j.jtos.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 41.Han SB, Yang HK, Hyon JY. Influence of diabetes mellitus on anterior segment of the eye. Clin Interv Aging. 2019;14:53–63. doi: 10.2147/CIA.S190713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Priyadarsini S, Whelchel A, Nicholas S, Sharif R, Riaz K, Karamichos D. Diabetic keratopathy: Insights and challenges. Surv Ophthalmol. 2020;65:513–29. doi: 10.1016/j.survophthal.2020.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Celiker H, Erekul G, Turhan SA, Kokar S, Yavuz DG, Gunduz OH, et al. Early detection of neuropathy in patients with type 2 diabetes with or without microalbuminuria in the absence of peripheral neuropathy and retinopathy. J Fr Ophtalmol. 2021;44:485–93. doi: 10.1016/j.jfo.2020.09.027. [DOI] [PubMed] [Google Scholar]
- 44.Issar T, Tummanapalli SS, Borire AA, Kwai NC, Poynten AM, Arnold R, et al. Impact of the metabolic syndrome on peripheral nerve structure and function in type 2 diabetes. Eur J Neurol. 2021;28:2074–82. doi: 10.1111/ene.14805. [DOI] [PubMed] [Google Scholar]
- 45.Tummanapalli SS, Issar T, Kwai N, Poynten A, Krishnan AV, Willcox M, et al. Association of corneal nerve loss with markers of axonal ion channel dysfunction in type 1 diabetes. Clin Neurophysiol. 2020;131:145–54. doi: 10.1016/j.clinph.2019.09.029. [DOI] [PubMed] [Google Scholar]
- 46.Schultz RO, Van Horn DL, Peters MA, Klewin KM, Schutten WH. Diabetic keratopathy. Trans Am Ophthalmol Soc. 1981;79:180–99. [PMC free article] [PubMed] [Google Scholar]
- 47.Manaviat MR, Rashidi M, Afkhami-Ardekani M, Shoja MR. Prevalence of dry eye syndrome and diabetic retinopathy in type 2 diabetic patients. BMC Ophthalmol. 2008;8:10. doi: 10.1186/1471-2415-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burda N, Mema V, Mahmudi ME, Selimi B, Zhugli S, Lenajni B, et al. Prevalence of dry eye syndrome at patients with diabetus melitus tip 2, one year retrospective study May 2011-June 2012. J Acute Dis. 2012;1:110–4. [Google Scholar]
- 49.Bikbova G, Oshitari T, Baba T, Bikbov M, Yamamoto S. Diabetic corneal neuropathy: Clinical perspectives. Clin Ophthalmol. 2018;12:981–7. doi: 10.2147/OPTH.S145266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kopf S, Groener JB, Kender Z, Fleming T, Bischoff S, Jende J, et al. Deep phenotyping neuropathy: An underestimated complication in patients with pre-diabetes and type 2 diabetes associated with albuminuria. Diabetes Res Clin Pract. 2018;146:191–201. doi: 10.1016/j.diabres.2018.10.020. [DOI] [PubMed] [Google Scholar]
- 51.Barsegian A, Lee J, Salifu MO, McFarlane SI. Corneal neuropathy: An underrated manifestation of diabetes mellitus. J Clin Endocrinol Diabetes. 2018;2:D–111. [Google Scholar]
- 52.Achtsidis V, Eleftheriadou I, Kozanidou E, Voumvourakis KI, Stamboulis E, Theodosiadis PG, et al. Dry eye syndrome in subjects with diabetes and association with neuropathy. Diabetes Care. 2014;37:e210–1. doi: 10.2337/dc14-0860. [DOI] [PubMed] [Google Scholar]
- 53.Ambrósio R, Jr, Tervo T, Wilson SE. LASIK-associated dry eye and neurotrophic epitheliopathy: Pathophysiology and strategies for prevention and treatment. J Refract Surg. 2008;24:396–407. doi: 10.3928/1081597X-20080401-14. [DOI] [PubMed] [Google Scholar]
- 54.Sarkar J, Milani B, Kim E, An S, Kwon J, Jain S. Corneal nerve healing after in situ laser nerve transection. PLoS One. 2019;14:e0218879. doi: 10.1371/journal.pone.0218879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Naderi K, Gormley J, O’Brart D. Cataract surgery and dry eye disease: A review. Eur J Ophthalmol. 2020;30:840–55. doi: 10.1177/1120672120929958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Niederer RL, Perumal D, Sherwin T, McGhee CN. Laser scanning in vivo confocal microscopy reveals reduced innervation and reduction in cell density in all layers of the keratoconic cornea. Invest Ophthalmol Vis Sci. 2008;49:2964–70. doi: 10.1167/iovs.07-0968. [DOI] [PubMed] [Google Scholar]
- 57.Peters MJ, Bakkers M, Merkies IS, Hoeijmakers JG, van Raak EP, Faber CG. Incidence and prevalence of small-fiber neuropathy: A survey in the Netherlands. Neurology. 2013;81:1356–60. doi: 10.1212/WNL.0b013e3182a8236e. [DOI] [PubMed] [Google Scholar]
- 58.Erie JC, McLaren JW, Hodge DO, Bourne WM. The effect of age on the corneal subbasal nerve plexus. Cornea. 2005;24:705–9. doi: 10.1097/01.ico.0000154387.51355.39. [DOI] [PubMed] [Google Scholar]


