Abstract
A novel immunoassay was developed for specific detection of cyanobacterial cyclic peptide hepatotoxins which inhibit protein phosphatases. Immunoassay methods currently used for microcystin and nodularin detection and analysis do not provide information on the toxicity of microcystin and/or nodularin variants. Furthermore, protein phosphatase inhibition-based assays for these toxins are not specific and respond to other environmental protein phosphatase inhibitors, such as okadaic acid, calyculin A, and tautomycin. We addressed the problem of specificity in the analysis of protein phosphatase inhibitors by combining immunoassay-based detection of the toxins with a colorimetric protein phosphatase inhibition system in a single assay, designated the colorimetric immuno-protein phosphatase inhibition assay (CIPPIA). Polyclonal antibodies against microcystin-LR were used in conjunction with protein phosphatase inhibition, which enabled seven purified microcystin variants (microcystin-LR, -D-Asp3-RR, -LA, -LF, -LY, -LW, and -YR) and nodularin to be distinguished from okadaic acid, calyculin A, and tautomycin. A range of microcystin- and nodularin-containing laboratory strains and environmental samples of cyanobacteria were assayed by CIPPIA, and the results showed good correlation (R2 = 0.94, P < 0.00001) with the results of high-performance liquid chromatography with diode array detection for toxin analysis. The CIPPIA procedure combines ease of use and detection of low concentrations with toxicity assessment and specificity for analysis of microcystins and nodularins.
Cyanobacteria (blue-green algae) produce a wide range of secondary metabolites which are hazardous to humans, livestock, and wildlife (2). Among these are a group of potent hepatotoxins, the microcystins and nodularins. Several bloom-forming cyanobacterial genera are capable of producing these toxins; these genera include Microcystis, Anabaena, Planktothrix, and Nostoc, which can produce the cyclic heptapeptide microcystins, and Nodularia, which can produce the cyclic pentapeptide nodularins. The toxins have a number of common structural features, in particular, the unique β-C20 amino acid 3-amino-9-methoxy-2,6,8-trimethyl-10-phenyldeca-4,6-dienoic acid (3, 9).
At the molecular level, microcystins bind irreversibly to and inhibit several serine/threonine protein phosphatases, including protein phosphatase 1 (PP1) and protein phosphatase 2A (PP2A) (17). Reports of animal intoxication and human illness from around the globe (9) and, more recently, the deaths of more than 50 hemodialysis patients in Caruaru, Brazil, have been linked to the presence of microcystins in water (7, 11, 20). There is a need for increased awareness and enhanced ability to detect these toxins for protection of health and management of bodies of water which are prone to cyanobacterial bloom development (3). Several detection methods are currently in use; these methods include high-performance liquid chromatography (HPLC) (13), small-animal bioassays (5), and enzyme inhibition assays (1, 22). The ability of microcystins to inhibit certain protein phosphatases has led to the development of a number of straightforward assays for detection and quantification of these toxins. Protein phosphatase inhibition assays include the use of 32P in the form of [32P]glycogen phosphorylase (10, 12) and colorimetric protein phosphatase inhibition assays (1, 22). The colorimetric assays may utilize the ability of the catalytic subunit of PP1, as expressed in Escherichia coli (23), to dephosphorylate the chromogenic substrate p-nitrophenylphosphate. However, the protein phosphatase inhibition assays used for detection and analysis of cyanobacterial hepatotoxins also respond to a wide variety of noncyanobacterial toxins and metabolites, including okadaic acid, tautomycin, and calyculin A. The lack of specificity of the protein phosphatase inhibition assays for cyanobacterial hepatotoxins requires that additional confirmatory analytical methods be employed for specific analysis of these toxins. However, the protein phosphatase inhibition assay remains a useful screening assay based on both ease of use and the toxicological information which it provides (1, 3).
Immunoassays using polyclonal (4, 8) and monoclonal (19) antibodies in enzyme-linked immunosorbent assay (ELISA) formats are also used for detection of the cyclic peptide hepatotoxins. These assays show greater specificity than protein phosphatase inhibition assays but do not indicate the relative toxicities of microcystin and nodularin variants. Previous studies employing the ability of microcystin-LR antibodies to bind to microcystins and nodularin have revealed in vitro protection of PP1 activity (16) and PP2A activity (15, 19) from inhibition by these toxins and prevention of in vivo toxicity (19).
In this paper, we describe a novel colorimetric protein phosphatase inhibition assay, termed the colorimetric immuno-protein phosphatase inhibition assay (CIPPIA). This assay provides a rapid, easy-to-use detection method for microcystins and nodularins, which indicates the relative toxicity of the PP1 inhibitor, and the immunospecificity provided by antibodies raised against microcystin-LR. The assay was validated with eight purified cyanobacterial toxins which inhibit PP1 and with noncyanobacterial PP1 inhibitors. In addition, extracts from cyanobacterial laboratory strains and environmental samples were assayed by the CIPPIA, and the results were compared to the results of an analysis of the same extracts by HPLC with diode array detection (DAD).
MATERIALS AND METHODS
Sources of cyanobacterial toxins and other protein phosphatase inhibitors.
Microcystin variants (microcystin-LR, -D-Asp3-RR, -LA, -LF, -LY, -LW, and -YR) and nodularin were purified from lyophilized hepatotoxic laboratory strains of cyanobacteria (13, 14). Okadaic acid, tautomycin, and calyculin A were purchased from Calbiochem (Novabiochem, Nottingham, United Kingdom).
Preparation of antisera.
A microcystin-LR–keyhole limpet hemocyanin conjugate was prepared and polyclonal antiserum was raised in female Dutch rabbits as described elsewhere (18). The preimmune serum and polyclonal antiserum were partially purified by ammonium sulfate precipitation (8, 18). Microcystin-LR antiserum and preimmune serum were dialyzed separately against 0.1 M sodium phosphate-buffered saline (pH 7.4) for 1 h and then with fresh buffer for 24 h and stored at −20°C.
Neutralization of the inhibitory activity of microcystin-LR against PP1.
Solutions of purified microcystin-LR (0 to 1,000 μg liter−1) were prepared by using Milli-Q (Millipore) water. These standards were preincubated separately at 37°C with microcystin-LR antiserum and preimmune serum for 1 h in 96-well polystyrene flat-bottom microplates (Greiner Labortechnik Ltd., Stonehouse, United Kingdom). The recombinant catalytic subunit of PP1 from rabbit skeletal muscle, expressed in Escherichia coli (23), was diluted in buffer containing 50 mM Tris-HCl, 1 mM Na2EDTA, 2 mM MnCl2, 0.5 g of bovine serum albumin per liter, and 0.1% (vol/vol) β-mercaptoethanol, the pH was adjusted to 7.4, and 10 μl was added to each well. para-Nitrophenylphosphate (20 mM; Sigma Chemical Co., Poole, United Kingdom) was dissolved in assay buffer containing 50 mM Tris-HCl, 0.2 mM MnCl2, and 20 mM MgCl2 adjusted to pH 8.1. The assay was then performed as previously described for 21 min with a constant rate of para-nitrophenol (p-NP) production (22).
Development of the CIPPIA.
The ability of microcystin-LR antiserum to protect PP1 from the inhibitory action of microcystin-LR and related cyanobacterial toxins was used as the basis for the CIPPIA procedure. A typical assay was performed as follows. Ten-microliter portions of the microcystin-LR standard were pipetted into the wells of a microtiter plate. For each standard and unknown sample, six wells were loaded. Microcystin-LR antiserum was diluted 1/200 with assay buffer, and 10-μl portions were added to three of the six wells. Ten-microliter portions of null serum at a 1/200 dilution were added to the remaining three wells. The microcystin-serum mixtures were covered and incubated for 15 min at 37°C. After this, the plate was removed from the incubator, and the colorimetric protein phosphatase inhibition assay was performed (22).
The effect of the methanol concentration of the sample on the CIPPIA was investigated as this solvent is frequently used to extract microcystins from cyanobacterial bloom material (13, 14, 22). A microcystin-LR standard (40 μg liter−1) was prepared with increasing methanol concentrations (0 to 100% [vol/vol]). The preparations were then assayed by the CIPPIA.
Characterization of the CIPPIA using cyanobacterial toxins and other PP1 inhibitors.
The ability of anti-microcystin-LR antibodies to prevent inhibition of PP1 by microcystin-LR was compared with the abilities of other purified cyanobacterial toxins which are PP1 inhibitors and noncyanobacterial PP1 inhibitors (tautomycin, calyculin A, and okadaic acid). The CIPPIA was performed as described above.
Characterization of PP1 inhibitors with a PI.
A protective index (PI) was devised to quantify the degree of protection from the activity of each purified toxin or inhibitor which the preincubation step in the presence of microcystin-LR antiserum provided to PP1. This was done by comparing the PP1 activity in the presence of the inhibitor after incubation of the sample with either preimmune serum or microcystin-LR antiserum. The rates involved were constant for the time period used (21 min). The PI was calculated as follows: PI = (%ActAS − %ActNS)/% InhibNS, where %ActAS is the rate of p-NP production in the protein phosphatase inhibition assay for PP1 inhibitors after incubation with microcystin-LR antiserum, expressed as a percentage of the microcystin-free control value; %ActNS is the rate of p-NP production in the protein phosphatase inhibition assay for PP1 inhibitors after incubation with null (preimmune) serum, expressed as a percentage of the microcystin-free control value; and %InhibNS is the rate of p-NP production in the protein phosphatase inhibition assay for PP1 inhibitors after incubation with null serum subtracted from the rate of p-NP production by the microcystin-free control, expressed as a percentage of the microcystin-free control value.
For PP1 inhibition which is due solely to microcystins and nodularins, a theoretical PI value of 1.00 should be recorded. A theoretical PI value of 0 would be expected if the inhibition was due to compounds not bound by the microcystin-LR antiserum. In practice, PI values greater than 1.00 are possible if the activity of PP1 in the presence of antiserum is greater than the rate in the control wells. As PI values are calculated as a function of PP1 reaction rates, the PI value does not change with the degree of PP1 inhibition, although high PI values can be achieved if there is minimal inhibition of PP1.
Detection of microcystins and nodularins in extracts of cyanobacterial strains and blooms.
Laboratory strains of cyanobacteria were maintained and grown for experimental purposes in BG11 medium (21) as described previously (22). Cyanobacterial bloom material was obtained from freshwater lakes and reservoirs in England and South Africa and harvested by centrifugation. All material was lyophilized. Extracts were prepared from lyophilized material by using 70% (vol/vol) methanol and were centrifuged at 14,000 × g for 10 min in an Eppendorf 5415 centrifuge, and the resulting supernatants were evaluated by CIPPIA and HPLC with DAD (13).
RESULTS
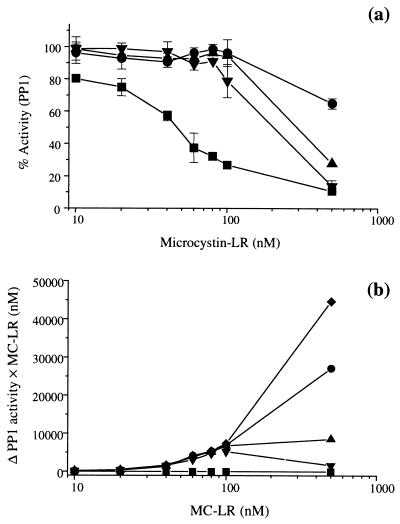
Preincubation of polyclonal microcystin-LR antiserum with purified microcystin-LR was found to completely neutralize the inhibitory effect of this toxin when PP1 was added up to a microcystin-LR concentration of 100 μg liter−1 (Fig. 1a). The ability of microcystin-LR antiserum to bind microcystin-LR and thereby protect PP1 from subsequent inhibition by the toxin was dependent upon the antiserum concentration. Preincubation of microcystin-LR with preimmune serum did not prevent inhibition of the PP1 enzyme. At a higher microcystin-LR concentration (500 μg liter−1), microcystin-LR antiserum at a 1/200 dilution was not able to completely prevent inhibition of PP1 activity by the toxin. However, the PP1 activities at this concentration of microcystin-LR were still higher if preparations were preincubated with a 1/200 dilution of microcystin-LR antiserum compared to toxin at equivalent concentrations preincubated with preimmune serum (Fig. 1a). The high microcystin-LR concentrations were beyond the linear detection range of the standard colorimetric protein phosphatase inhibition assay (1, 22). Calculating the differences in PP1 activity between assays that included microcystin-LR incubated in the presence of preimmune serum and assays that included microcystin-LR incubated in the presence of microcystin-LR antiserum and multiplying the values by the microcystin-LR equivalent concentration used to inhibit PP1 revealed that there is a dose-response relationship between complete theoretical protection of PP1 and complete theoretical inhibition of PP1 (Fig. 1b). At microcystin-LR concentrations greater than 100 nM, the calculated functions for PP1 and microcystin-LR showed values of 46,000, 28,000, 10,000, 4,000, and 0 for complete theoretical protection, 1/100, 1/200, and 1/500 dilutions of antiserum, and theoretical complete inhibition, respectively (Fig. 1b).
FIG. 1.
Neutralization of the inhibitory effect of microcystin-LR (MC-LR) on PP1 by preincubation of purified microcystin-LR with microcystin-LR antiserum. (a) Microcystin-LR was preincubated with microcystin-LR antiserum at 1/100 (●), 1/200 (▴), and 1/500 (▾) dilutions, and the results were compared to results obtained after preincubation of microcystin-LR with preimmune serum at a 1/100 dilution (■). Preparations were incubated for 1 h at 37°C before analysis by the colorimetric protein phosphatase inhibition assay. The vertical error bars indicate standard deviations (n = 3). (b) Mean delta PP1 activities (ΔPP1) were calculated (%ActAS − %ActNS) at each microcystin-LR concentration in the presence of antiserum at 1/100 (●), 1/200 (▴), and 1/500 (▾) dilutions and were multiplied by the microcystin-LR equivalent concentration, and the results were compared with the complete theoretical protection (⧫) and complete theoretical inhibition (■) data.
Once the CIPPIA had been optimized, the effect of sample methanol concentration on the ability of microcystin-LR antibodies to bind toxin and the ability of PP1 to dephosphorylate para-nitrophenylphosphate were studied (data not shown). The methanol concentration of the sample did not significantly affect either the activity of the enzyme or the ability of microcystin-LR antiserum to bind this toxin. Thus, samples dissolved in 100% (vol/vol) methanol can be incubated in the presence of microcystin-LR antiserum, resulting in a final methanol concentration of 50% (vol/vol), which does not interfere with the PP1 inhibition assay (data not shown) (PI ≥ 1.00; microcystin-LR concentration, 40 nM).
To characterize the specificity of the reduction in the percentage of inhibition of PP1 activity by microcystin-LR antiserum if preparations were preincubated in the presence of microcystin-LR, several related cyanobacterial toxins and noncyanobacterial PP1 inhibitors were studied (Table 1). For example, tautomycin inhibited PP1 activity, as did the other noncyanobacterial protein phosphatase inhibitors, regardless of whether preimmune serum, assay buffer, or microcystin-LR antiserum was used during preincubation with these inhibitors. The inability of the microcystin-LR antiserum to protect PP1 from inhibition by tautomycin, calyculin A, and okadaic acid was demonstrated by the low PI values obtained (0.02 to 0.18) (Table 1). The effect of the antiserum on inhibition of PP1 by these noncyanobacterial protein phosphatase inhibitors and toxin was negligible compared to the results of tests with the microcystin variants and nodularin. Of the microcystins, microcystin-D-Asp3-RR gave the lowest PI value (0.80), and the PI values for the other toxins were as high as 1.00 for microcystin-LY and nodularin when preparations were preincubated with microcystin-LR antiserum. Based on PI values, the purified microcystins and nodularin were thus clearly distinguishable from the noncyanobacterial PP1 inhibitors.
TABLE 1.
Characteristics of protein phosphatase inhibitors in the CIPPIA using anti-microcystin-LR antiserum (n = 3)a
| Protein phosphatase inhibitor or toxin | Detection limit (nM) | Linear concn range (nM) | IC50 (nM)b | PIc |
|---|---|---|---|---|
| Noncyanobacterial toxins | ||||
| Tautomycin | 50 | 50–800 | 326 | 0.18 |
| Calyculin A | 100 | 100–1600 | 652 | 0.07 |
| Okadaic acid | 300 | 300–10,000 | 2,000 | 0.02 |
| Cyanobacterial hepatotoxins | ||||
| Microcystin-LR | 10 | 20–100 | 47 | 0.90 |
| Microcystin-D-Asp3-RR | 40 | 40–500 | 300 | 0.80 |
| Microcystin-LA | 20 | 20–500 | 100 | 0.94 |
| Microcystin-LF | 40 | 40–80 | 80 | 0.84 |
| Microcystin-LY | 40 | 40–500 | 100 | 1.00 |
| Microcystin-LW | 10 | 20–500 | 160 | 0.82 |
| Microcystin-YR | 20 | 20–100 | 70 | 0.86 |
| Nodularin | 20 | 20–100 | 70 | 1.00 |
All data were obtained by using the same preparations of antiserum at 1/200 dilutions.
IC50, concentration of inhibitor or toxin resulting in 50% PP1 enzyme activity.
See Materials and Methods.
To further investigate specific detection of microcystins and nodularins by the CIPPIA, 70% (vol/vol) methanol extracts of laboratory strains and natural blooms of cyanobacteria were examined (Table 2). Of the 14 microcystin- and nodularin-producing laboratory strains studied, which represented four cyanobacterial genera, 12 (86%) resulted in PI values that were similar to or greater than the values obtained with purified microcystins and nodularin. Only two laboratory strains, DUN 901 (Nostoc sp.), and PCC 7804 (Nodularia sp.), produced PI values slightly lower than that achieved with purified microcystin-D-Asp3-RR. The three microcystin-containing natural bloom samples tested all produced PI values which were 1.00 or greater, indicating that microcystins were present and responsible for the inhibition of PP1. This was confirmed by HPLC with DAD (Table 2).
TABLE 2.
Determination of microcystin-LR equivalents in extracts of laboratory strains and environmental samples of cyanobacteria, as determined by CIPPIA (n = 3) and HPLC with DAD (n = 2)
| Strain or bloom | Cyanobacterial genus | Mean microcystin-LR equivalents (μg mg [dry wt]−1)
|
PIc | ||
|---|---|---|---|---|---|
| HPLC-DAD analysis | CIPPIA with preimmune seruma | CIPPIA with antiserumb | |||
| Strains | |||||
| NIES 102 | Microcystis | 0.24 | 0.27 | 0.03 | 0.90 |
| NIES 103 | Microcystis | 0.14 | 0.22 | NDd | 1.00 |
| NIES 298 | Microcystis | 2.26 | 3.79 | ND | 1.04 |
| PCC 7813 | Microcystis | 4.80 | 8.16 | 0.42 | 0.91 |
| PCC 7820 | Microcystis | 2.35 | 2.71 | 0.12 | 0.91 |
| RID I | Microcystis | 1.78 | 2.15 | 0.12 | 0.91 |
| CCAP1450/10 | Microcystis | 0.08 | 0.17 | ND | 1.10 |
| TH2 | Microcystis | 4.92 | 4.80 | 0.25 | 0.85 |
| NIES 595 | Planktothrix | 0.62 | 0.86 | ND | 0.97 |
| CCAP1459/22 | Planktothrix | 0.13 | 0.09 | ND | 1.00 |
| DUN 901 | Nostoc | 0.53 | 0.31 | 0.04 | 0.74 |
| T2 | Nodularia | 1.46 | 2.15 | 0.12 | 0.91 |
| PCC 7804 | Nodularia | 4.21 | 4.80 | 0.40 | 0.75 |
| Nod M1 | Nodularia | 0.53 | 0.71 | ND | 1.10 |
| Blooms | |||||
| Bray Lake (England) | Microcystis | 3.22 | 2.77 | ND | 1.04 |
| Farmoor Reservoir (England) | Microcystis | 0.49 | 0.71 | ND | 1.06 |
| Hartbeespoort Dam (South Africa) | Microcystis | 1.12 | 1.05 | ND | 1.00 |
CIPPIA analysis was performed by preincubating extract in the presence of preimmune (null) serum.
CIPPIA analysis was performed by preincubating extract in the presence of microcystin-LR antiserum.
See Materials and Methods. Extracts (50 mg ml−1) were prepared in 70% methanol. Extracts were preincubated separately in the presence of preimmune (null) serum or microcystin-LR antiserum after appropriate dilution for the assay.
ND, not detectable.
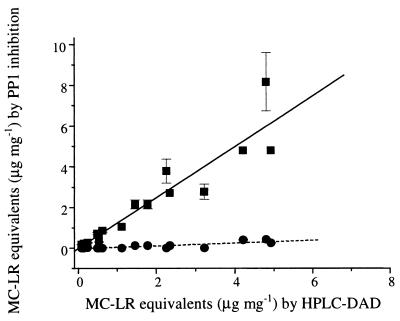
Linear regression analysis of the relationship between microcystin-LR equivalents, as determined by HPLC with DAD and by protein phosphatase inhibition solely in the presence of preimmune serum, revealed that the amounts of microcystin-LR equivalents were overestimated by the protein phosphatase inhibition assay (Fig. 2). However, the results of microcystin-LR equivalent determinations by the two methods were variable (Table 2). This was indicated by the gradient of the linear regression line (1.25), but the correlation coefficient revealed a good fit (R2 = 0.94, P < 0.00001). To improve this technique, preimmune serum was replaced with 70% methanol or assay buffer as the diluent for negative preincubation in the CIPPIA. Further analysis of microcystin-LR equivalents by HPLC with DAD and protein phosphatase inhibition in the presence of microcystin-LR antiserum (Fig. 2) revealed a large bias towards microcystin-LR equivalents when HPLC with DAD was used. Linear regression analysis revealed a gradient of 0.07 and an R2 value of 0.82 (P < 0.0001), indicating that preincubation of microcystin-LR antiserum prevented the microcystin and nodularin variants from inhibiting the PP1 enzyme.
FIG. 2.
Comparison of microcystin-LR (MC-LR) equivalents determined by HPLC with DAD and PP1 inhibition in extracts of laboratory strains and environmental samples of cyanobacteria. Extracts were analyzed by HPLC with DAD, and the results were compared to the results of PP1 analyses of the same extracts performed in the presence of preimmune (null) serum (■) and microcystin-LR antiserum (●), expressed in micrograms per milligram of cyanobacterial cells. The vertical error bars indicate standard deviations where their dimensions exceed those of the symbols. Regression lines are shown for HPLC-DAD analysis versus PP1 inhibition in the presence of preimmune serum (—) (y = 1.25x − 0.02; R2 = 0.94; P < 0.00001) and HPLC-DAD analysis versus PP1 inhibition in the presence of antiserum (–––) (y = 0.07x − 0.03; R2 = 0.82; P < 0.0001).
DISCUSSION
A developing awareness of the health risks posed by cyanobacterial hepatotoxins and the increasing anthropogenic eutrophication of potable and recreational waters (2) have increased the need for rapid, sensitive, and cost-effective methods for detection of these toxins. The colorimetric protein phosphatase inhibition assay has a number of features that are valuable for this purpose. It is more sensitive than classical methods, such as mouse (intraperitoneal) and other bioassays, and includes toxicity as a component for detection. Compared with HPLC methods, the need for large concentration steps is removed, and the unit cost of the colorimetric protein phosphatase inhibition assay and the level of expertise required to perform the assay are markedly lower (3). However, the protein phosphatase inhibition assay alone is not specific for cyanobacterial toxins. It responds to a wide range of additional protein phosphatase inhibitors (3). The use of antibodies in ELISA applications provides specificity, unlike the protein phosphatase inhibition assay. One drawback of current ELISA procedures, however, is that they do not provide information on the toxicity of the analytes being detected. The CIPPIA procedure aims to combine the advantages of the protein phosphatase inhibition and ELISA methods. Further improvements may involve the use of affinity-purified microcystin-LR antibodies, but as there are at present more than 65 known variants of microcystin (9a) and as keyhole limpet hemocyanin has not been shown to inhibit PP1, a wider variety of epitopes raised against microcystin-LR may improve this screening method. Furthermore, for routine screening the protective incubation step may be omitted unless positive inhibition of PP1 occurs. The positive sample should then be reassayed with the protective step included to ascertain the class of toxicant.
The use of antimicrocystin antibodies in a protein phosphatase inhibition assay has been shown to identify microcystin-LR and related compounds and to distinguish them from noncyanobacterial inhibitors of PP1 (16) and PP2A (15, 19). The CIPPIA described here combines determination of toxicity with determination of immunospecificity for microcystins and nodularin. The combination of these two detection methods should provide the validation which is required for both determination of the toxicity of an environmental sample and specific identification of the class of toxicant(s) detected (6). Using the assay described here, we distinguished several purified microcystin variants and nodularin from okadaic acid, calyculin A, and tautomycin of noncyanobacterial origin. Calculation of a simple PI allowed samples of cyanobacterial strains and environmental blooms to be quantitatively assessed for microcystin content. All eight purified cyanobacterial toxins were detected by the CIPPIA and had PI values of 0.80 to 1.00 (Table 1). When the applicability of CIPPIA analysis was investigated, 15 of the 17 hepatotoxin-containing cyanobacterial extracts produced PI values which were equal to or greater than those obtained with the purified cyanobacterial PPI inhibitors. The PI values of the remaining two samples were only marginally less than those of the toxin standards and significantly greater than the PI values for noncyanobacterial toxin protein phosphatase inhibitors (Table 2). Although the PI values were greater than 1.00 in some cases, this was thought to be due to the nature of the protein phosphatase inhibition assay and may also be accounted for by experimental error, such as pipetting of the enzyme. For actual quantitation of microcystins or nodularins, toxin concentrations can be estimated with reference to values for toxin standards determined by the CIPPIA performed with null serum, as indicated by comparison with HPLC-DAD analysis (Table 2 and Fig. 2). Furthermore, a comparison of the detection limits indicated that the CIPPIA is about 40 times more sensitive than HPLC with DAD; the detection limits, without sample concentration, were 10 and 400 μg liter−1, respectively. Recently, we have reduced the protein phosphatase inhibition detection limit to below the 1-μg liter−1 provisional guideline for drinking water (World Health Organization 1997) to permit specific toxicological testing for microcystins at around this concentration when the CIPPIA is used.
For rapid specific screening of cyanobacterial hepatotoxins, the combination of immunodetection and toxicity-based protein phosphatase inhibition in the CIPPIA provides a useful addition to the methods already available for detection of cyanobacterial hepatotoxins. The CIPPIA may also be used to screen for possible toxins and inhibitors from cyanobacteria and other sources which are not microcystins or nodularins, either by the assay described here or by using specific antibodies against other PP1 inhibitors. When used in conjunction with methods such as HPLC and assays for cyanobacterial neurotoxins, the CIPPIA is a useful first screening method for cyanobacterial cyclic peptide hepatotoxins in laboratory cultures and environmental samples.
ACKNOWLEDGMENTS
We thank E. Y. C. Lee for purified protein phosphatase, H. D. Black for valuable technical assistance, and K. A. Beattie for useful discussions. The Environment Agency (England and Wales) is thanked for the environmental samples from England, and Tamar Zohary is thanked for the environmental sample from South Africa.
This work was supported in part by the European Commission (contracts BIO4-CT96-0256 [BASIC] and ENV4-CT98-0802 [CYANOTOX]).
REFERENCES
- 1.An J, Carmichael W W. Use of a colorimetric protein phosphatase assay and enzyme linked immunoassay for the study of microcystins and nodularin. Toxicon. 1994;12:1495–1507. doi: 10.1016/0041-0101(94)90308-5. [DOI] [PubMed] [Google Scholar]
- 2.Bell S G, Codd G A. Cyanobacterial toxins and human health. Rev Med Microbiol. 1994;5:256–264. [Google Scholar]
- 3.Bell S G, Codd G A. Detection, analysis and risk assessment of cyanobacterial toxins. In: Hester R E, Harrison R M, editors. Agricultural chemicals and the environment. Issues in Environmental Science and Technology no. 5. Cambridge, United Kingdom: Royal Society of Chemistry; 1996. pp. 109–122. [Google Scholar]
- 4.Brooks W P, Codd G A. Immunoassay of hepatotoxic cultures and water blooms of cyanobacteria using Microcystis aeruginosa peptide toxin polyclonal antibodies. Environ Technol Lett. 1988;9:1343–1348. [Google Scholar]
- 5.Campbell D L, Lawton L A, Beattie K A, Codd G A. Comparative assessment of the specificity of the brine shrimp and Microtox assays to hepatotoxic (microcystin-LR containing) cyanobacteria. Environ Toxicol Water Qual. 1994;9:71–77. [Google Scholar]
- 6.Carmichael W W. The cyanotoxins. Adv Bot Res. 1997;27:211–260. [Google Scholar]
- 7.Carmichael W W, An J S, Azevedo S M F O, Lau S, Rinehart K L, Jochimsen E M, Holmes C E M, da Silva J B., Jr . Programa e Resumos, IV Simposio da Sociedade Brasileira de Toxinologia, 6–11 October, 1996. Recife, Brazil: Universidade federal de Pernambuco; 1996. Analysis for microcystins involved in an outbreak of liver failure and death of humans at a haemodialysis center in Caruaru, Pernambuco, Brazil; pp. 85–86. [Google Scholar]
- 8.Chu F S, Huang X, Wei R D, Carmichael W W. Production and characterization of antibodies against microcystins. Appl Environ Microbiol. 1989;55:1928–1933. doi: 10.1128/aem.55.8.1928-1933.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Codd G A. Cyanobacterial toxins: occurrence, properties and biological significance. Water Sci Technol. 1996;32:149–156. [Google Scholar]
- 9a.Codd, G. A., J. S. Metcalf, C. J. Ward, K. A. Beattie, S. G. Bell, K. Kaya, and K. G. Poon. J. Assoc. Off. Anal. Chem. Int., in press.
- 10.Holmes C F B. Liquid chromatography-linked protein phosphatase bioassay; a highly sensitive marine bioscreen for okadaic acid and related diarrhetic shellfish toxins. Toxicon. 1991;29:469–477. doi: 10.1016/0041-0101(91)90021-i. [DOI] [PubMed] [Google Scholar]
- 11.Jochimsen E M, Carmichael W W, An J S, Cardo D M, Cookson S T, Holmes C E M, Antunes M B D, deMelo D A, Lyra T M, Barreto V S T, Azevedo S M F O, Jarvis W R. Liver failure and death after exposure to microcystins at a hemodialysis center in Brazil. N Engl J Med. 1998;338:873–878. doi: 10.1056/NEJM199803263381304. [DOI] [PubMed] [Google Scholar]
- 12.Lambert T W, Boland M P, Holmes C F B, Hrudey S E. Quantitation of the microcystin hepatotoxins in water at environmentally relevant concentrations with the protein phosphatase bioassay. Environ Sci Technol. 1994;28:753–755. doi: 10.1021/es00053a032. [DOI] [PubMed] [Google Scholar]
- 13.Lawton L A, Edwards C, Codd G A. Extraction and high-performance liquid chromatographic method for the determination of microcystins in raw and treated waters. Analyst. 1994;119:1525–1530. doi: 10.1039/an9941901525. [DOI] [PubMed] [Google Scholar]
- 14.Lawton L A, Edwards C, Beattie K A, Pleasance S, Dear G J, Codd G A. Isolation and characterization of microcystins from laboratory cultures and environmental samples of Microcystis aeruginosa and from an associated animal toxicosis. Nat Toxins. 1995;3:50–57. doi: 10.1002/nt.2620030110. [DOI] [PubMed] [Google Scholar]
- 15.Lin J R, Chu F S. In vitro neutralization of the inhibitory effect of microcystin-LR to protein phosphatase 2A by antibody against the toxin. Toxicon. 1994;32:605–613. doi: 10.1016/0041-0101(94)90208-9. [DOI] [PubMed] [Google Scholar]
- 16.Liu B H, Yu F Y, Chu F S. Anti-idiotype and anti-anti-idiotype antibodies generated from polyclonal antibodies against microcystin-LR. J Agric Food Chem. 1996;44:4037–4042. [Google Scholar]
- 17.Mackintosh C, Beattie K A, Klumpp S, Cohen P, Codd G A. Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatase 1 and 2A from both mammals and higher plants. FEBS Lett. 1990;264:187–192. doi: 10.1016/0014-5793(90)80245-e. [DOI] [PubMed] [Google Scholar]
- 18.Metcalf J S, Bell S G, Codd G A. Production of novel polyclonal antibodies against the cyanobacterial toxin microcystin-LR and their application for the detection and quantification of microcystins and nodularin. Water Res. 2000;34:2761–2769. [Google Scholar]
- 19.Nagata S, Soutome H, Tsutsumi T, Hasegawa A, Sekijima M, Sugamata M, Harada K-I, Suganuma M, Ueno Y. Novel monoclonal antibodies against microcystin and their protective activity for hepatotoxicity. Nat Toxins. 1995;3:78–86. doi: 10.1002/nt.2620030204. [DOI] [PubMed] [Google Scholar]
- 20.Pouria S, de Andrade A, Barbosa J, Cavalcanti R L, Barreto V S T, Ward C J, Preiser W, Poon G K, Neild G H, Codd G A. Fatal microcystin intoxication in haemodialysis unit in Caruaru, Brazil. Lancet. 1998;352:21–26. doi: 10.1016/s0140-6736(97)12285-1. [DOI] [PubMed] [Google Scholar]
- 21.Stanier R Y, Kunisawa R, Mandel M, Cohen-Bazire G. Purification and properties of unicellular blue-green algae (order Chroococcales) Bacteriol Rev. 1971;35:171–205. doi: 10.1128/br.35.2.171-205.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ward C J, Beattie K A, Lee E Y C, Codd G A. Colorimetric protein phosphatase inhibition assay of laboratory strains and natural blooms of cyanobacteria: comparisons with high performance liquid chromatographic analysis for microcystins. FEMS Microbiol Lett. 1997;153:465–473. doi: 10.1016/s0378-1097(97)00290-5. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Z G, Bai G, Deans-Zirattu S, Browner M F, Lee E Y C. Expression of the catalytic subunit of phosphorylase phosphatase (protein phosphatase-1) in Escherichia coli. J Biol Chem. 1992;267:1484–1490. [PubMed] [Google Scholar]