Abstract
Although Mycobacterium spp. often cause disease in domestic birds (chickens and companion birds), there are few data on avian tuberculosis in wild populations, especially in birds of prey. We describe here a case of a young adult female, free-living Eurasian griffon vulture (Gyps fulvus) that was found dead. Granulomas were grossly evident in the lungs at autopsy, and tuberculosis was suspected. Ziehl–Neelsen staining revealed large numbers of intracellular acid-fast–positive bacteria within granulomas. Examination on Löwenstein–Jensen medium was negative, but mycobacteria growth indicator tube medium results were positive. For the molecular detection of Mycobacterium spp., the primer set IS901F and IS901R was used. Positive results were observed on gel electrophoresis, indicating the presence of Mycobacterium avium subsp. avium DNA. Although tuberculosis is not considered to be a common cause of death in wild birds, it undoubtedly deserves special attention because vultures are generally considered to be a species resistant to a large number of pathogens. Determination of the cause of death of griffon vultures is important for future conservation measures for this sensitive wild species.
Keywords: bacteriology, Gyps fulvus, histopathology, Mycobacterium avium, PCR
Avian tuberculosis (ATB) is a contagious chronic disease affecting poultry, birds in zoologic gardens, and wild bird species.1,16,18 ATB is an infectious disease that typically affects the lungs, air sacs, and bone marrow in birds. It is caused by various mycobacteria, most often Mycobacterium avium subsp. avium, and less frequently M. genavense or M. bovis. 1 Among the different serovars of Mycobacterium that are isolated from a broad range of vertebrate hosts, the most widely distributed is the M. avium complex (MAC). 8
In wild birds, such as raptor species, mycobacteriosis is very rarely documented. 18 Most often TB is diagnosed in birds from zoologic gardens or pets.1,9,16 Only a few cases of TB in wild raptor species such as Buteo jamaicensis, B. lineatus, Bubo virginianus, Gyps rueppelli, and Harpia harpyja have been reported.15,16,18 MAC infections have been reported to be responsible for 1–30% of deaths in raptors.8,16
Antemortem diagnostic testing of ATB in live birds is difficult mainly because of the absence of pathognomonic clinical signs of mycobacteriosis. Clinical signs that can be observed are nonspecific and include depression, muscle atrophy, weakness, and decreased body weight.2,17 ATB is often suspected based on autopsy findings. The liver, spleen, bone marrow, and intestines are involved most frequently. Characteristic findings include granulomas with caseous necrotic centers in various organs. Given а thicker cell wall, growth, and pathogenicity, Mycobacterium spp. differ from other bacteria, therefore special methods for isolation and identification are required. Identification of the organism is difficult; acid-fast staining can be used but is not sensitive. Ziehl–Neelsen (ZN) stains of smears from infected tissue can be effective in identifying mycobacterium; however, other tests are necessary for characterization. Culture methods provide identification and antimicrobial susceptibility testing. Molecular methods allow for identification of specific agents. 17
The Eurasian griffon vulture (Gyps fulvus) is a large colonial species of the Mediterranean, alpine, steppe, and semi-desert areas given a sufficient supply of dead livestock and large wild mammals. The vulture is regularly present at official feeding stations and areas in which livestock carcasses are disposed. 7 Because diversity and population of vulture species are constantly decreasing globally, it is of great importance to investigate the cause of death of every individual. A better understanding of causes of death will help inform conservation and welfare practices. 19
We aimed to determine the cause of death of a Eurasian griffon vulture in the Special Nature Reserve “Uvac”, in the municipality of Nova Varos, Serbia. The carcass of a young, female, 6.4 kg, Eurasian griffon vulture with a 2.7-m wingspan was delivered by nature keepers to the Department of Veterinary Forensics Medicine, Faculty of Veterinary Medicine, University of Belgrade, for autopsy.
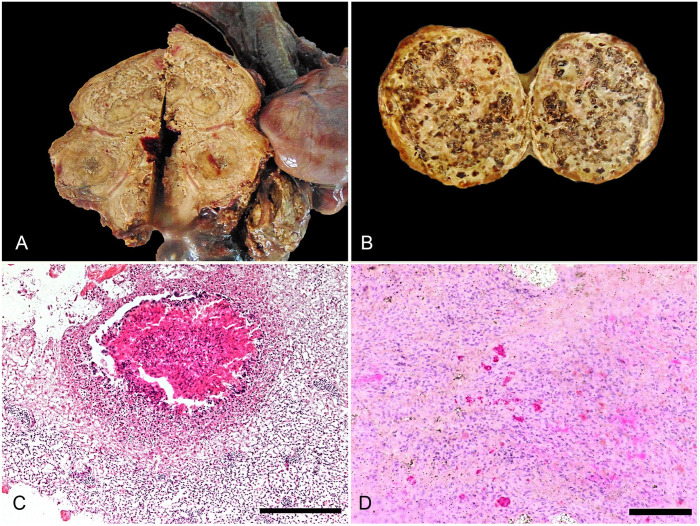
On gross examination, the griffon vulture was emaciated, with atrophied musculature and lack of subcutaneous fat tissue. Many ectoparasites (Dermanyssus sp.) were on the feathers. Also, maggots and fly eggs were in the left eye orbit. The talons were undamaged. The air sacs were not transparent and were thickened by adhesions; dark discoloration was noted on the inner sternal surface. Granulomas, in oval and circular shapes, involved 50–55% of the lung parenchyma. In the right lung lobe, there was a 10 × 5 cm, yellow-to-white, irregularly shaped nodule of caseous necrosis. The left lung had a large number of 1 × 1 cm, yellow, round granulomas with caseous, necrotic centers (Fig. 1A). The spleen was markedly enlarged (7-cm diameter), round, and had granulomas with a centrally located necrotic mass (Fig. 1B). The liver was diffusely enlarged with rounded edges and miliary white nodules in the parenchyma.
Figure 1.
Avian tuberculosis in a Eurasian griffon vulture. A. Pulmonary granulomas, with caseous, necrotic centers. B. Granulomas with a centrally located necrotic mass, in the spleen. C. A pulmonary granuloma surrounded by multinucleate giant cells and epithelioid cells. H&E. Bar = 200 μm. D. Acid-fast mycobacteria in a section of air sac. Ziehl–Neelsen stain. Bar = 50 μm.
Tissue samples collected at autopsy for histologic analysis included lungs, liver, spleen, kidneys, and brain. The samples were fixed in 10% neutral-buffered formalin, processed routinely, and stained with H&E; ZN staining was used for the detection of acid-fast bacilli in tissues.
Histologic examination revealed granulomas in the liver and lung composed of central necrosis and necrotic eosinophilic debris surrounded by multinucleate giant cells and epithelioid cells (Fig. 1C). The periphery of the granulomas had numerous lymphocytes, plasma cells, heterophils, and a fibrous capsule. ZN staining showed large numbers of intracellular acid-fast–positive bacteria (Fig. 1D).
Portions of lung and liver samples were stored at 4°C and used within 24 h for further testing. Isolation of M. tuberculosis from these samples was attempted on Löwenstein–Jensen (L-J) medium and mycobacteria growth indicator tube medium (BBL MGIT; Becton Dickinson). Inoculated tubes of L-J medium were incubated for up to 56 d and those on MGIT medium for 17 d at 37 ± 1°C. After incubation, the colonies were examined by adspection, and the results of bacterial growth on MGIT were confirmed using a UV transilluminator (365 nm) and microscope. After 56 d on L-J medium, bacterial growth was not observed. After 17 d, positive results were observed on the MGIT medium, and light-orange fluorescence was evident.
Final detection of the causative agent was performed by PCR testing. Lung and liver samples stored at 4°C for 24 h and bacterial culture material isolated on MGIT were used for extraction. In both cases, the extraction material was heated for 20 min on a thermoshaker at 95°C, and then frozen for 10 min at −20°C. After thawing, the Eppendorf centrifuge tubes were centrifuged for 3 min at 20,238 × g, and the supernatant was used for DNA extraction (BioExtract SuperBall extraction kit, Biosellal; KingFisher mL system, Thermo Fisher), according to the manufacturers’ protocols. After extraction, the DNA was stored at −20°C until use. DNA of a previously isolated and confirmed M. tuberculosis strain from a sample of domestic poultry was used as a positive PCR control, and nuclease-free water was used as a negative PCR control.
The primer set (Invitrogen Custom Primers; Thermo Fisher) IS901F (5′-AAGCCGAGGTGGTGTATGT-3′) and IS901R (5′-AGGGAAGATGGCGGTGAGCAT-3′) 13 was used to amplify insertion sequences (IS) of the mycobacterial DNA that characteristically are present as multiple copies. PCR product size was 257 bp. DNA amplification was carried out in a 50-μL reaction volume mixing 22.5 μL of PCR-grade water, 25 μL of master mix, 0.25 μL of each primer (concentration 50 μM), and 2 μL of DNA extracted from the test sample.
The PCR reaction was performed (thermocycler 2720; Applied Biosystems) using a protocol consisting of 3 min of initial denaturation at 94°C, followed by 50 cycles of denaturation (94°C, 15 s), primer annealing at 57.7°C for 30 s, extension (72°C, 30 s), and a final extension step at 72°C, which lasted 7 min. Separation of PCR products was performed by horizontal electrophoresis in 2% agarose gel with ethidium bromide (E-gel iBase power system, 48 V, 0.8 A, 50/60 Hz; Invitrogen) for 26 min, as recommended. A total of 20 μL of PCR products were applied directly to E-gel wells. The results were read by illuminating 2% agarose gel in a UV gel photography chamber. PCR detection was positive for M. tuberculosis (M. avium subsp. avium). On agarose gel, 257-bp fragments were observed.
Wild birds can be reservoirs of zoonotic pathogens, which is why the epidemiology of wildlife diseases is a focus of public health programs. MAC is associated frequently with human and animal diseases. 11 These agents can be isolated from birds and small terrestrial mammals and can cause organized or disseminated pathologic changes in various organs. 3 Because of its conservation status, the griffon vulture population in Serbia is constantly monitored, and thanks to conservation programs, the griffon vulture is now in the category of species of least concern on the IUCN list, with populations increasing each year. 4 Feeding on carcasses, these birds play an important role in ecosystems because they prevent the spread of many infectious diseases. The highly corrosive environment in the digestive tract of vultures destroys most pathogenic microorganisms. 5 Nevertheless, ATB can affect vultures and other raptor species,5,16 perhaps because of immunosuppression, immunodeficiency, exhaustion, or the existence of other infectious diseases and intoxications.
ATB is transmitted to susceptible birds by ingestion and inhalation of aerosolized organisms. 6 The route of transmission of Mycobacterium spp. depends on the species infected. Wild animals, such as deer and rabbits, are often infected through direct contact with feces of infected animals or the ingestion of contaminated vegetation in pastures or drylots. 14 These wild animals can be a risk factor for transmission to cattle on pasture. Although the vultures in Uvac fed on the carcasses of cows that had died while grazing free on pastures, all cattle were tested every year for TB. Therefore, even though the most common route of infection for susceptible birds is the alimentary tract, 6 in our case, the respiratory tract may have been the source of infection because the most significant pathologic changes were observed in the lungs, spleen, and liver, and no gross pathologic changes were seen in the alimentary tract. In addition, the bird was in poor nutritional condition, supporting chronic disease.16,18
Although we identified acid-fast organisms in lung and liver tissues of a griffon vulture with ZN stain, this was expected, given the presence of Mycobacterium spp. However, ZN stain can be used only as a screening method because it may also detect other acid-resistant bacteria (e.g., Nocardia spp.), which could lead to misidentification.12,16 On L-J medium, bacterial growth was not observed, unlike on MGIT medium, which is consistent with previous observations that L-J medium may fail to culture MAC 20 ; only 121 of 277 isolates of MAC were positive on L-J medium. The reason is probably contamination, which is more frequent when L-J medium is used, compared with other substrates (e.g., Bactec 12B, Middlebrook 7H11). The results in our case are also congruent with a report in which MAC was detected in 36 cases using MGIT and in only 13 cases using L-J medium. 10 The general sensitivity for detecting Mycobacterium clinical isolates was 86.5% for MGIT medium and 59.7% for L-J medium. 10 To establish the final diagnosis in our case, it was necessary to use end-point PCR, which proved to be an accurate, fast, and reliable method for the detection of M. avium.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: Our study was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia (contract 451-03-68/2022-14/200143).
ORCID iD: Vladimir Nesic  https://orcid.org/0000-0001-9608-5202
https://orcid.org/0000-0001-9608-5202
Contributor Information
Vladimir Nesic, Faculty of Veterinary Medicine, University of Belgrade, Belgrade, Serbia.
Darko Marinkovic, Faculty of Veterinary Medicine, University of Belgrade, Belgrade, Serbia.
Kazimir Matovic, Veterinary Specialised Institute, Kraljevo, Serbia.
Milos Radakovic, Institute for Nature Conservation of Serbia, Novi Beograd, Serbia.
Darko Davitkov, Faculty of Veterinary Medicine, University of Belgrade, Belgrade, Serbia.
Nikola Vaskovic, Veterinary Specialised Institute, Kraljevo, Serbia.
Dajana Davitkov, Faculty of Veterinary Medicine, University of Belgrade, Belgrade, Serbia.
References
- 1. Álvarez PP, et al. Avian tuberculosis in a Lady Amherst’s pheasant Chrysolophus amherstiae. Austral J Vet Sci 2017;49:213–215. [Google Scholar]
- 2. Baghal ML, et al. Comparison of PCR and designed ELISA methods to detect avian tuberculosis in suspected pigeons. Iran Vet J 2020;16:29–37. [Google Scholar]
- 3. Biet F, et al. Zoonotic aspects of Mycobacterium bovis and Mycobacterium avium-intracellulare complex (MAC). Vet Res 2005;36:411–436. [DOI] [PubMed] [Google Scholar]
- 4. BirdLife International. Gyps fulvus, 2021. Aug 11. The IUCN Red List of Threatened Species 2021: e.T22695219A157719127. https://www.iucnredlist.org/species/22695219/157719127
- 5. Cunha MV, et al. Exposure of threatened Accipitridae to Mycobacterium bovis, calls for active surveillance. Ecohealth 2017;14:310–317. [DOI] [PubMed] [Google Scholar]
- 6. Dhama K, et al. Tuberculosis in birds: insights into the Mycobacterium avium infections. Vet Med Int 2011;2011:712369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grubac B. [The Griffon Vulture]. Institute for Nature Conservation of Serbia, 2014. Serbian. [Google Scholar]
- 8. Hoenerhoff M, et al. Mycobacteriosis in an American bald eagle (Haliaeetus leucocephalus). Avian Dis 2004;48:437–441. [DOI] [PubMed] [Google Scholar]
- 9. Jones MP. Selected infectious diseases of birds of prey. J Exot Pet Med 2006;15:5–17. [Google Scholar]
- 10. Lu D, et al. Comparison of the automated mycobacteria growth indicator tube system (BACTEC 960/MGIT) with Löwenstein-Jensen medium for recovery of mycobacteria from clinical specimens. Am J Clin Pathol 2002;118:542–545. [DOI] [PubMed] [Google Scholar]
- 11. Marras TK, Daley CL. Epidemiology of human pulmonary infection with nontuberculous mycobacteria. Clin Chest Med 2002;23:553–567. [DOI] [PubMed] [Google Scholar]
- 12. Mathur S, et al. Cytologic diagnosis of pulmonary nocardiosis: a report of 3 cases. Acta Cytol 2005;49:567–570. [DOI] [PubMed] [Google Scholar]
- 13. Miller JM, et al. Polymerase chain reaction identification of Mycobacterium avium in formalin-fixed, paraffin-embedded animal tissues. J Vet Diagn Invest 1999;11:436–440. [DOI] [PubMed] [Google Scholar]
- 14. Raizman EA, et al. Mycobacterium avium subsp. paratuberculosis from free-ranging deer and rabbits surrounding Minnesota dairy herds. Can J Vet Res 2005;69:32–38. [PMC free article] [PubMed] [Google Scholar]
- 15. Sadar MJ, et al. Multifocal respiratory and vertebral mycobacteriosis in a red-tailed hawk (Buteo jamaicensis). J Zoo Wildl Med 2015;46:150–154. [DOI] [PubMed] [Google Scholar]
- 16. Skoric M, et al. Avian tuberculosis in a captured Ruppell’s griffon vulture (Gyps ruppellii): a case report. Vet Med (Praha) 2010;55:348–352. [Google Scholar]
- 17. Srivastava V, et al. Diagnostic approaches to avian tuberculosis. Worlds Poult Sci J 2017;73:857–871. [Google Scholar]
- 18. Tell LA, et al. Avian mycobacteriosis in free-living raptors in California: 6 cases (1997–2001). J Avian Med Surg 2004;18:30–40. [Google Scholar]
- 19. Vidal A, et al. Microbiological diagnosis and antimicrobial sensitivity profiles in diseased free-living raptors. Avian Pathol 2017;46:442–450. [DOI] [PubMed] [Google Scholar]
- 20. Wilson ML, et al. Comparison of recovery rates for mycobacteria from BACTEC 12B vials, Middlebrook 7H11-selective 7H11 biplates, and Lowenstein Jensen slants in a public health mycobacteriology laboratory. J Clin Microbiol 1995;33:2516–2518. [DOI] [PMC free article] [PubMed] [Google Scholar]