Abstract
We investigated the platelet count (PLT), mean platelet volume (MPV), and plateletcrit (PCT) in dogs with type 1 diabetes mellitus (DM) compared to healthy controls, and their association with the major fraction of glycated hemoglobin (HbA1c). Blood samples from 33 clinically healthy dogs and 14 newly diagnosed diabetic dogs were included. CBCs were performed with the Advia 120; HbA1c was determined using a validated assay (Capillarys 2 flex-piercing; Sebia). Median [range] PLT and PCT were significantly higher (p = 0.040 and p = 0.010, respectively) in diabetic dogs (434 [176–987] × 109/L and 0.60 [0.26–1.22]%, respectively) compared to healthy dogs (297 [223–671] × 109/L and 0.35 [0.24–0.87]%, respectively]. Thrombocytosis was observed in 6 of 14 (43%) diabetic dogs. The median MPV was not significantly different (p = 0.114) between the diabetic (13.6 fL, 10.1–22.6 fL) and healthy dogs (11.9 fL, 8.6–19.1 fL). A significant, albeit weak, correlation was detected between HbA1c and PLT (rho = 0.298, p = 0.042) and PCT (rho = 0.340, p = 0.019), but no significant correlation was found with MPV (rho = 0.199, p = 0.180). Canine DM was associated with increased PLT and PCT, which was correlated with glycemic status. Our findings suggest dysregulated megakaryopoiesis in diabetic dogs, but this should be confirmed by large-scale studies, and the clinical implications should be investigated.
Keywords: diabetes mellitus, dogs, glycosylated hemoglobin, mean platelet volume, megakaryopoiesis, platelets, platelet mass, plateletcrit
Diabetes mellitus (DM) is the most common disease affecting the endocrine pancreas in dogs, with a reported prevalence of 0.32–1.33%.6,9 Canine DM is typically characterized by permanent hypoinsulinemia. Although canine DM and its comorbidities are generally well-studied, certain aspects such as platelets and hemostasis remain poorly investigated.
Platelets in human diabetic patients are characterized by a hyperreactive state, which is caused by dysregulation of various signaling pathways that affect their adhesion, activation, and aggregation. One of the suggested mechanisms for the dysregulated activity of platelets in DM is long-term hyperglycemia. 4 Among the various markers of platelet hyperreactivity, the mean platelet volume (MPV) is a simple and inexpensive index that is automatically generated by all common hematology analyzers. Platelets of increased volume are enzymatically and biochemically more active and, thus, MPV acts as an indirect measure of platelet activity. 7 Specifically, MPV was found to be increased in diabetic patients compared to healthy individuals,11,15 and was correlated significantly with poor glycemic control. 14 On the other hand, other indices such as platelet count (PLT), platelet mass (PM), or plateletcrit (PCT) have not been investigated to the same extent. PCT, as a measure of PM, is considered the most physiologically relevant platelet index given that it co-factors both platelet production and activity, and is therefore considered a more accurate reflection of in vivo platelet kinetics. 3 PM is normally kept constant by a tightly regulated and inversely related platelet size and count. 12 In a study in human diabetic patients, PM and PLT were significantly higher compared to healthy individuals. 18 To our knowledge, platelet indices have not been investigated in canine DM. Specifically, our current knowledge is limited to a retrospective study that reported canine DM as one of the causes of reactive thrombocytosis. 17
The major fraction of glycated hemoglobin (HbA1c) is a glycated derivative of hemoglobin A (HbA), which results from the irreversible, nonenzymatic, insulin-independent binding of glucose to the N-terminal valine residue of the β-globin chain. The glycosylation of Hb is a gradual and continuous process, and its magnitude depends on the erythrocyte lifespan and permeability to glucose, as well as on the blood glucose concentration during the lifespan of the circulating erythrocyte. In dogs, therefore, HbA1c levels reflect the average blood glucose concentration over a period of 2–3 mo. 13
Our objectives were: 1) to compare PLT, MPV, and PCT between dogs with type 1 DM and healthy controls, and 2) to correlate these platelet indices with the glycemic status, as reflected by HbA1c. We hypothesized that these platelet indices will be significantly different in diabetic dogs compared to healthy ones, and that the indices will be correlated positively with HbA1c.
We used aliquots of canine blood samples collected for routine health checks or diagnostic purposes in a teaching hospital in our study. Jugular venipuncture blood was collected into K3-EDTA tubes (Deltalab). CBCs were performed within 1 h of blood collection (Advia 120; Siemens). Blood films were prepared within 1 h, stained with Giemsa (Merck), and reviewed for the presence of platelet clumps. HbA1c was measured as a percentage of Hb within 4 h of blood collection using a validated capillary electrophoresis assay (Capillarys 2 flex-piercing; Sebia). 13
The control group comprised adult dogs with current vaccination status and antiparasitic prophylaxis, no history of illness or medication during the preceding month, unremarkable physical examination, and CBC results within RIs. Abnormal platelet indices were not considered an exclusion criterion. The diabetic group comprised dogs newly diagnosed with DM. The diagnosis was based on compatible anamnesis (polyuria, polydipsia, polyphagia, weight loss), persistent fasting hyperglycemia (>6.55 mmol/L), and glycosuria. Dogs with platelet clumps in the blood film were excluded from further analysis.
Sample size was calculated for a desired significance of 0.05 and power of 0.80. We assumed mean PLT, MPV, and PCT differences of 50 × 109/L, 1.0 fL, and 0.05%, respectively, between the groups, with pooled SDs of 41 × 109/L, 0.7 fL, and 0.045%, respectively. Data distribution was assessed using the Shapiro–Wilk test. Mean and median comparisons between the 2 groups were performed with the Welch 2-sample t-test or Wilcoxon rank sum test, respectively. The Spearman correlation coefficient was used for the correlation study. Statistical analysis was performed with a statistical language (R, https://www.r-project.org/).
According to our power analysis, 9–14 dogs were required in each group. Our diabetic population comprised 14 dogs (6 males, 8 females) with a mean ± SD age of 9.9 ± 2.8 y. The control group comprised 33 dogs (17 males, 16 females) with a mean age of 6.3 ± 3.8 y. Mean HbA1c was significantly higher (p < 0.001) in diabetic (5.4 ± 1.0%) compared to healthy (1.7 ± 0.5%) dogs. Mean Hb concentration was not significantly different (p = 0.196) between the diabetic (143 ± 36 g/L) and healthy dogs (156 ± 78 g/L).
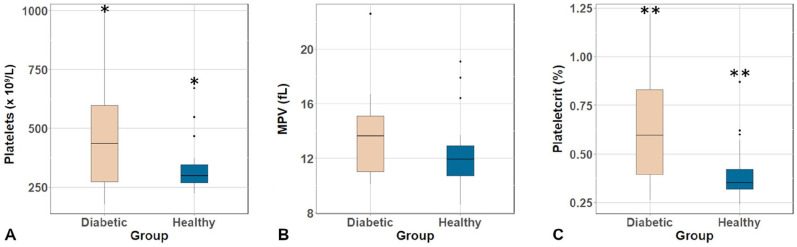
Median [range] PLT was significantly higher (p = 0.040) in diabetic dogs (434 [176–987] × 109/L) compared to healthy dogs (297 [223–671] × 109/L; Fig. 1A). Six of 14 (43%) diabetic dogs had thrombocytosis (defined as PLT > 500 × 109/L). Median MPV was not significantly different (p = 0.114) between the diabetic (13.6 fL, 10.1–22.6 fL) and healthy (11.9 fL, 8.6–19.1 fL; Fig. 1B) dogs. The median PCT was significantly higher (p = 0.010) in diabetic dogs (0.60 [0.26–1.22%]) compared to the control group (0.35 [0.24–0.87%]; Fig. 1C). A significant, weak, positive correlation was detected between HbA1c and both PLT (rho = 0.298, p = 0.042) and PCT (rho = 0.340, p = 0.019), but no significant correlation was found with MPV (rho = 0.199, p = 0.180).
Figure 1.
Boxplots of A. platelet count, B. mean platelet volume (MPV), and C. plateletcrit values of the 14 diabetic dogs and 33 control dogs. The boxes are the interquartile ranges; the central line is the median value. The whiskers are used to capture the remaining data. The dots are outliers. * p = 0.040. ** p = 0.010.
We found that diabetic dogs had significantly higher PLT and PCT compared to healthy dogs, and that these indices were correlated positively with HbA1c. On the other hand, no significant difference was detected in MPV between the 2 groups, and MPV was not correlated significantly with HbA1c.
PLT was significantly and substantially higher in diabetic dogs compared to healthy ones (mean difference of 138 × 109/L), with thrombocytosis detected in almost half of the diabetic dogs. Although our diabetic group was small, our results suggest that thrombocytosis is a common finding in dogs with DM, which concurs with the findings of a retrospective study. 17 DM should, therefore, be included in the differential diagnosis of reactive thrombocytosis in dogs. Interestingly, PLT was correlated significantly and positively, albeit weakly, with HbA1c. This suggests that platelet production may be influenced by the glycemic status. Alternatively, an interesting theory to explain this correlation would be an increased platelet half-life. Increased glycosylation has been associated with increased half-life of hormones and proteins,5,16 and we cannot exclude the possibility that the same principle applies to platelets. Additionally, we cannot rule out the possibility that an undefined factor influences both platelet production and glycemic status in dogs with type 1 DM. The significance of this finding is uncertain because the association between platelet count and glycemic control has hardly been investigated, even in diabetic humans.
PCT was significantly higher (>1.5 times) in dogs with DM compared to the control group and was correlated significantly, albeit weakly, with HbA1c. PCT is a measure of PM and is considered a better marker of in vivo platelet kinetics than PLT or MPV. 3 PM is kept constant under normal conditions by complex feedback mechanisms of megakaryopoiesis in the bone marrow, which regulate the platelet number and size in an inverse manner 2 ; therefore, PM is considered a marker of megakaryopoiesis. Importantly, PM has also been shown to be a reliable marker of megakaryopoiesis in different clinical settings in human medicine.8,10 The higher PCT in our study might suggest that canine DM could be characterized by dysregulated megakaryopoiesis, which may be related to the glycemic status, as discussed for PLT. However, we cannot rule out the possibility that another factor influences both megakaryopoiesis and glycemic status in dogs with type 1 DM. The clinical implication of this finding is uncertain and should be explored in a prospective cross-sectional study of larger scale, which could also employ bone marrow cytology and histology, as well as platelet viscoelastic and aggregation assays.
MPV is a readily available index of platelet activation. Specifically, larger platelets are more active hemostatically and enzymatically and have higher concentration of prothrombotic molecules (e.g., serotonin, platelet-derived growth factor) and greater aggregability in response to ADP. 1 MPV was not significantly different between the diabetic and healthy dogs in our study, and MPV was not correlated significantly with HbA1c. This is in contrast to most studies in human medicine,11,15 which report significantly higher MPVs in diabetic patients compared to healthy individuals. However, the human studies primarily evaluated patients with type 2 DM. Interestingly, in a study that included patients with type 1 DM, MPV was not significantly different between these patients and healthy individuals. 18 The discrepancies in MPV between type 1 and 2 DM could possibly be explained by the different underlying pathophysiology. Although type 1 DM is characterized by permanent hypoinsulinemia, type 2 DM is characterized by insulin resistance associated with metabolic abnormalities (e.g., obesity, dyslipidemia, hypertension, low-grade inflammation) that have been associated with platelet activation and increased platelet volume. 4 Increased MPV has been associated with a prothrombotic state characterized by increased expression of adhesion molecules (e.g., R-selectin, glycoprotein IIb/IIIa) and thromboxane A2. 7 The lack of platelet hyperreactivity (as reflected by MPV) in diabetic dogs supports the rarity of thrombotic events in these patients.
A limitation of our study is the relatively low number of diabetic patients that were included, precluding the comparison of platelet indices between diabetic dogs with different levels of HbA1c.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Theodora K. Tsouloufi  https://orcid.org/0000-0002-4032-0338
https://orcid.org/0000-0002-4032-0338
Ioannis L. Oikonomidis  https://orcid.org/0000-0002-1591-9891
https://orcid.org/0000-0002-1591-9891
References
- 1. Bath PM, Butterworth RJ. Platelet size: measurement, physiology and vascular disease. Blood Coagul Fibrinolysis 1996;7:157–161. [PubMed] [Google Scholar]
- 2. Bessman JD, et al. Mean platelet volume. The inverse relation of platelet size and count in normal subjects, and an artifact of other particles. Am J Clin Pathol 1981;76:289–293. [DOI] [PubMed] [Google Scholar]
- 3. Butkiewicz AM, et al. Platelet count, mean platelet volume and thrombocytopoietic indices in healthy women and men. Thromb Res 2006;118:199–204. [DOI] [PubMed] [Google Scholar]
- 4. Ferreiro JL, et al. Platelet abnormalities in diabetes mellitus. Diab Vasc Dis Res 2010;7:251–259. [DOI] [PubMed] [Google Scholar]
- 5. Flintegaard TV, et al. N-glycosylation increases the circulatory half-life of human growth hormone. Endocrinology 2010;151:5326–5336. [DOI] [PubMed] [Google Scholar]
- 6. Fracassi F, et al. Breed distribution of canine diabetes mellitus in Italy. Vet Res Commun 2004;28(Suppl 1):339–342. [DOI] [PubMed] [Google Scholar]
- 7. Gasparyan AY, et al. Mean platelet volume: a link between thrombosis and inflammation? Curr Pharm Des 2011;17:47–58. [DOI] [PubMed] [Google Scholar]
- 8. Gerday E, et al. Testing platelet mass versus platelet count to guide platelet transfusions in the neonatal intensive care unit. Transfusion 2009;49:2034–2039. [DOI] [PubMed] [Google Scholar]
- 9. Guptill L, et al. Time trends and risk factors for diabetes mellitus in dogs: analysis of veterinary medical data base records (1970–1999). Vet J 2003;165:240–247. [DOI] [PubMed] [Google Scholar]
- 10. Kim MJ, et al. Comparison of platelet parameters in thrombocytopenic patients associated with acute myeloid leukemia and primary immune thrombocytopenia. Blood Coagul Fibrinolysis 2014;25:221–225. [DOI] [PubMed] [Google Scholar]
- 11. Kodiatte TA, et al. Mean platelet volume in type 2 diabetes mellitus. J Lab Physicians 2012;4:5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kuter DJ. Milestones in understanding platelet production: a historical overview. Br J Haematol 2014;165:248–258. [DOI] [PubMed] [Google Scholar]
- 13. Oikonomidis IL, et al. Validation, reference intervals and overlap performance of a new commercially available automated capillary electrophoresis assay for the determination of the major fraction of glycated haemoglobin (HbA1c) in dogs. Vet J 2018;234:48–54. [DOI] [PubMed] [Google Scholar]
- 14. Park B-J, et al. The relationship of platelet count, mean platelet volume with metabolic syndrome according to the criteria of the American Association of Clinical Endocrinologists: a focus on gender differences. Platelets 2012;23:45–50. [DOI] [PubMed] [Google Scholar]
- 15. Shilpi K, Potekar RM. A study of platelet indices in type 2 diabetes mellitus patients. Indian J Hematol Blood Transfus 2018;34:115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sinclair AM, Elliott S. Glycoengineering: the effect of glycosylation on the properties of therapeutic proteins. J Pharm Sci 2005;94:1626–1635. [DOI] [PubMed] [Google Scholar]
- 17. Woolcock AD, et al. Thrombocytosis in 715 dogs (2011–2015). J Vet Intern Med 2017;31:1691–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zaccardi F, et al. Platelet indices and glucose control in type 1 and type 2 diabetes mellitus: a case-control study. Nutr Metab Cardiovasc Dis 2017;27:902–909. [DOI] [PubMed] [Google Scholar]