Abstract
Although systemic bacterial infection (SBI) is a common cause of sepsis and death in dogs, the neuropathology of canine SBI has been poorly characterized. Here we describe the neuropathologic features of SBI in a retrospective series of 28 dogs. The mean age of affected dogs was 5.5 y, and there was no sex or breed predisposition. Gross lesions in the brain were reported in 13 cases (46%) and consisted mainly of leptomeningeal hemorrhages in 10 of these cases (77%). Associated extraneural lesions included suppurative mitral valve endocarditis (12 cases; 43%) and pneumonia (10 cases; 36%). The main neurohistologic findings were neutrophilic (suppurative) and/or fibrinous meningoencephalitis with hemorrhage, vasculitis, thrombosis, and neuronal necrosis. Intralesional bacteria were observed in neutrophils or macrophages in 10 cases (77%). The putative primary site of infection was determined in 16 cases (57%) and consisted of pneumonia (6 cases; 38%), pyelonephritis (4 cases; 25%), and skin lesions (3 cases; 19%). Bacterial culture of fresh or frozen tissue samples yielded bacterial growth in 26 cases (93%), including Streptococcus canis (6 cases; 23%), Escherichia coli (4 cases; 15%), and Staphylococcus intermedius (3 cases; 12%).
Keywords: bacteremia, brain, dogs, neuropathology, sepsis
Septicemia, or systemic bacterial infection (SBI), is characterized by viable bacteria in the bloodstream (bacteremia) and is one of the most common causes of sepsis in humans and companion animals.2,9,20 Sepsis is the clinical syndrome associated with the systemic and often dysregulated inflammatory response to disseminated infection.3,9,10,15,18 Although significant advances have been made in the understanding of its pathophysiology and clinical management, sepsis continues to be a major medical emergency and one of the leading causes of multisystem organ dysfunction and death in the United States.10,20 Although the true incidence of sepsis remains poorly known in veterinary medicine, it is a common and life-threatening disorder of dogs and cats,3,8,14,22 with mortality rates reportedly of 20–50%.8,22
One of the main complications of SBI and sepsis is multisystem organ dysfunction with or without involvement of the central nervous system (CNS).2,15 In humans, bacterial infections of the CNS are less common but more clinically devastating and lethal than bacterial infection in other organs, even if caused by the same bacterial organism. 4 Bacteria can reach the CNS through the bloodstream as part of septicemia or following thromboembolism from extraneural lesions, such as valvular endocarditis. 4 Sepsis associated encephalopathy (SAE) affects nearly one-third of patients, leading to altered mental status (delirium and coma) and increased mortality.13,16 Patients who survive SAE often experience long-term and often disabling cognitive deficits, including decreased memory, learning, and visual abilities. 16
The neuropathology of SBI in dogs is poorly characterized. Here we describe the neuropathology, diagnostic features, associated extraneural lesions, and most common causes of SBI in a retrospective series of 28 dogs autopsied at the Athens Veterinary Diagnostic Laboratory (AVDL; University of Georgia, Athens, GA, UGA).
We searched the Web-based archives of the AVDL for autopsy cases of dogs with a confirmed diagnosis of sepsis and neurologic involvement between 2010 and 2020 using the search terms sepsis, septicemia, meningitis, encephalitis, meningoencephalitis, myelitis, meningomyelitis, meningoencephalomyelitis, and vasculitis. Retrieved cases in which systemic lesions of sepsis were reportedly associated with CNS inflammation underwent further evaluation. Archived histology slides were examined independently by both authors, and we included cases in which representative tissue sections of brain (telencephalon, brainstem, cerebellum) were available. The signalment, clinical signs, pathologic findings, and diagnosis were retrieved from the submission forms and autopsy reports.
We assessed neuropathologic changes according to the methods used in a previous study. 17 The distribution of inflammatory and degenerative neuropathologic changes was recorded according to their neuroanatomic localization as telencephalon, brainstem, or cerebellum. Perivascular inflammation was characterized as neutrophilic (suppurative), lymphoplasmacytic, or mixed (neutrophils, macrophages, lymphocytes, plasma cells). The location of the perivascular inflammation was recorded as leptomeningeal, neuroparenchymal (gray or white matter), choroid plexus, or ventricular (ependyma and/or lumen). Other examined findings included the presence or absence of vasculitis (with or without fibrinoid change), thrombosis, hemorrhage, neuronal necrosis (neurons with shrunken and hypereosinophilic cytoplasm and pyknotic nuclei), focal neuroparenchymal necrosis (clusters of foamy macrophages infiltrating the neuroparenchyma), gliosis, and neuroparenchymal spongiosis (interpreted as edema).The presence of intralesional bacteria was assessed using H&E-stained or Gram-stained tissue sections of brain.
We found 250 cases of suspect sepsis in dogs in the searched period and selected 28 confirmed cases of sepsis with CNS involvement (Suppl. Tables 1, 2). A pathology diagnosis was achieved in all cases based on histologic evidence of systemic inflammation and intralesional bacteria with confirmation by bacterial culture in 26 cases (93%). In all cases, neuropathologic changes were reported in the brain. Spinal cord had not been collected during autopsy. Affected dogs were 3-d to 16-y-old (x̄ = 5.5-y-old). Females and males were affected in 10 and 18 cases, respectively, with no evident breed predisposition. Clinical signs varied greatly, depending on the degree of systemic compromise and/or CNS lesions, and included depression, seizures, and recumbency (Suppl. Table 1). Affected dogs died in 17 cases (61%) and were euthanized in 11 cases (39%).
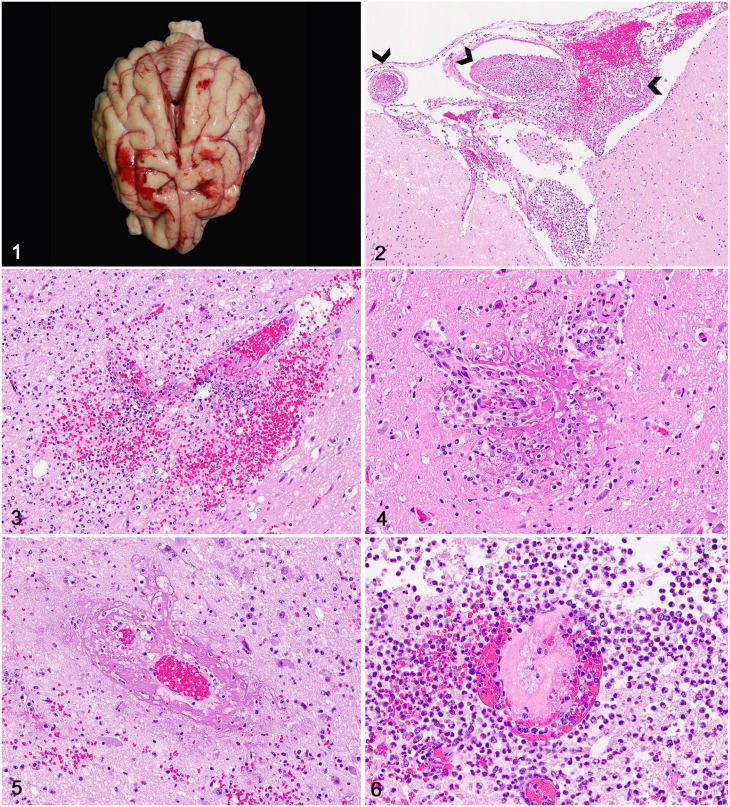
Gross lesions in the brain were reported in 13 cases (46%) and consisted mainly of random leptomeningeal hemorrhages (Fig. 1) in 10 cases (77%) and neuroparenchymal necrosis with hemorrhage, ventricular hemorrhage, and ventricular empyema in 1 case each (8%). The extraneural lesions associated most commonly in all 28 cases included suppurative mitral valve endocarditis (12 cases; 43%), pneumonia (10 cases; 36%), suppurative myocarditis (7 cases; 25%), splenic infarcts (6 cases; 21%), and acute pyelonephritis (4 cases; 14%).
Figures 1–6.
Neuropathology of sepsis in dogs. Figure 1. Extensive leptomeningeal hemorrhages in the frontal and parietal telencephalic lobes; case 25. Figure 2. Extensive perivascular accumulations of neutrophils, fibrin, and hemorrhage expand the telencephalic leptomeninges; case 4. Thrombi (arrowheads) are also present. H&E. Figure 3. Clusters of neutrophils, fibrin, and hemorrhage partially efface a blood vessel and expand the perivascular space in the telencephalic gray matter; case 4. H&E. Figure 4. Inflammatory cells infiltrate the walls of 2 small arterioles, which are surrounded by fibrin; case 18. H&E. Figure 5. A venular wall is expanded and effaced by fibrinoid change; case 8. H&E. Figure 6. A neuroparenchymal blood vessel is occluded by a fibrin thrombus and surrounded by inflammatory cells and hemorrhage; case 21. H&E.
The primary source of infection was putatively identified in 16 of the 28 cases (57%) and consisted mainly of pneumonia (6 cases; 38%), acute pyelonephritis (4 cases; 25%), and skin lesions (3 cases; 19%). The main causes of the septicemia and neuropathologic changes were confirmed by bacterial culture of fresh or frozen tissue samples in 26 cases (93%), and included Streptococcus canis (6 cases; 23%), Escherichia coli (4 cases; 15%), and Staphylococcus intermedius (3 cases; 12%).
Neurohistologic changes (Suppl. Table 3) occurred predominantly in the telencephalon (21 cases; 75%) and less often in the telencephalon and cerebellum (5 cases; 18%), telencephalon and brainstem (1 case; 4%), and cerebellum and brainstem (1 case; 4%). The overall intensity of the lesions was higher in the cerebral cortex, subcortical white matter, and basal nuclei, and lower in the brainstem and cerebellum. Briefly, lesions consisted of perivascular accumulations of neutrophils or a mixture of neutrophils and macrophages with fewer lymphocytes and plasma cells that were often associated with abundant fibrin exudation. Neuroinflammatory changes (Figs. 2, 3) occurred in the leptomeninges (24 cases; 86%) and throughout the gray matter (22 cases; 78%), white matter (18 cases; 64%), and less often in the choroid plexus (8 cases; 28%) or ependyma (3 cases; 11%).
Vascular changes included perivascular hemorrhage, which was observed in 20 cases (71%) and occurred predominantly in the leptomeninges in 16 cases (80%) followed by gray matter in 13 cases (65%) and white matter in 11 cases (55%). Vasculitis (14 cases; 50%) was characterized by arteriolar or venular wall infiltration by neutrophils and macrophages (Fig. 4) with vascular wall disruption as a result of fibrinoid change (Fig. 5). Thrombosis (6 cases; 21%) occurred mainly in leptomeningeal and superficial telencephalic cortical vessels (Fig. 6). Perivascular inflammation often extended into the immediately adjacent neuroparenchyma.
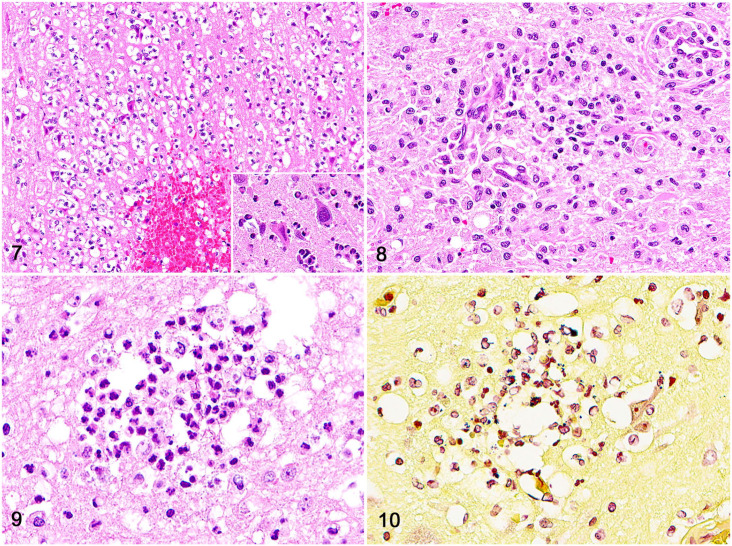
Neuroinflammatory and vascular changes were often accompanied by neuronal necrosis (Fig. 7) in 10 cases (36%), parenchymal necrosis with infiltration by neutrophils and foamy macrophages (Fig. 8) in 4 cases (14%), and variable degrees of gliosis in 11 cases (39%) and neuroparenchymal spongiosis in 7 cases (25%). In addition, dog 13 had an extensive area of hemorrhage that effaced the left lateral ventricle, and dog 16 had 2 abscesses in the left frontal and occipital telencephalic lobes. Intralesional bacteria (Figs. 9, 10) were observed within neutrophils or macrophages around blood vessels using H&E-stained tissue sections (8 cases; 28%) and Gram stain (10 cases; 36%). Intralesional bacteria were not observed in 18 cases.
Figures 7–10.
Neuropathology of sepsis in dogs. Figure 7. Necrotic neurons and scattered neutrophils in the telencephalic cortex; case 25. Inset: closer view of a necrotic neuron (left) compared to a normal neuron (right). H&E. Figure 8. An area of parenchymal necrosis in the telencephalic cortex characterized by clusters of foamy macrophages and fewer microglia. H&E. Figure 9. Rare intralesional bacteria within the cytoplasm of neutrophils. Figure 10. Intralesional gram-positive cocci within the cytoplasm of neutrophils. Gram stain.
Bacterial infection of the CNS in humans and small animals may occur as part of systemic bacteremia or when bacterial thromboemboli originating in other organs lodge in the encephalic microcirculation and spread into the adjacent neuroparenchyma.4,21 In humans, CNS complications are an important cause of death from bacterial valvular endocarditis, 4 which was reported in nearly 43% of our cases.
Multisystem organ dysfunction associated with sepsis occurs following tissue hypoperfusion caused by systemic and a dysregulated inflammatory response to infection. 6 The underlying mechanisms associated with the clinical signs and long-term cognitive decline during and post-sepsis in human patients have not been completely elucidated, but the neuropathologic changes in septic patients have been attributed to a combination of neuroinflammation, ischemia, and excitotoxicity. 13 Inflammation has been associated with endothelial cell injury, microglial activation, and oxidative injury within hours of the onset of experimentally induced sepsis in rats and nonhuman primates.1,11,12 Endothelial cell injury and impairment of the microvascular circulation leads to neuroparenchymal influx of neutrophils, as well as hemorrhagic and ischemic lesions in the brain.6,13 All of the cases in our study had considerable neutrophilic inflammation and hemorrhagic changes in the brain, which could account for the development of the reported clinical signs in the affected dogs. In addition, direct evidence of endothelial injury (vasculitis) and ischemia (neuronal or parenchymal necrosis) were observed, suggesting that mechanisms for the development of neuropathologic changes of sepsis in dogs may be similar to those in humans. The neuropathologic changes in our cases were consistent with other reports of acute CNS infections in dogs. 21
In humans, cerebral microinfarcts have been consistently found in septic patients, confirming the role of ischemia in the development of cognitive impairment in individuals who survive hospitalization for sepsis. 6 Cerebral microinfarcts are characterized by well-demarcated microscopic areas of neuroparenchymal necrosis (usually <3-mm diameter) that result from microvascular injury. 19 In our cases, neuroparenchymal necrosis consistent with microinfarcts were observed in only 4 cases, which suggests that these lesions may not be an important feature of sepsis in dogs, at least during acute neuroinflammation. Alternatively, the low number of cases with microinfarcts in our study may reflect the relatively low number of 3–5 brain tissue sections examined in each case. Further studies with dogs that survive sepsis may shed light on the role of microinfarcts in the post-hospitalization period.
Similar to the findings in our study, in which the most common sources of bacterial infection were pneumonia, pyelonephritis, and skin lesions, conditions that predispose dogs to sepsis include peritonitis, pancreatitis, pneumonia, pyometra, prostatitis, and cutaneous wound infections. 3 The mortality rate for dogs with sepsis is usually high despite the availability of antimicrobial therapy and surgery in most cases. 3 Severe clinical disease and bacteremia (confirmed via blood culture) have been associated with a greater chance of death than mild disease and negative culture results in septic dogs. 5 None of our cases had blood culture results available at the time of autopsy, but 17 dogs spontaneously died and 9 were euthanized because of the severity of the clinical signs, which highlights the progressive and lethal nature of sepsis in dogs.
Intralesional bacteria were observed in extraneural tissues in13 cases and in the brain in 10 cases. However, bacteria were challenging to visualize in the brain when compared to extraneural tissues. This finding is relevant because some of the vascular changes in these septic dogs may be similar to those reported in cases of steroid-responsive meningitis-arteritis, which can complicate the diagnosis when confirmatory testing is not available. 7
A final diagnosis was achieved based on the pathologic changes associated with bacterial culture using fresh or frozen tissues in 26 of our 28 cases. The most common extraneural lesions of sepsis in our cases included suppurative mitral valve endocarditis and pneumonia, which were observed grossly and likely prompted pathologists to submit tissues for bacterial culture after autopsy (mitral valve and/or lung were cultured in 12 cases). In addition, frozen brain tissue was submitted for bacterial culture in 9 cases. Similar to our findings, the most common bacteria isolated from septic dogs include Enterobacteriaceae (including E. coli, Klebsiella spp.), Streptococcus spp., and Staphylococcus spp. 3
Supplemental Material
Supplemental material, sj-pdf-1-vdi-10.1177_10406387221102899 for Neuropathologic changes associated with systemic bacterial infection in 28 dogs by Jessica A. Elbert and Daniel R. Rissi in Journal of Veterinary Diagnostic Investigation
Footnotes
Declaration of conflicting interests: The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors declared that they received no financial support for their research and/or authorship of this article.
ORCID iD: Daniel R. Rissi  https://orcid.org/0000-0003-4574-2836
https://orcid.org/0000-0003-4574-2836
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Jessica A. Elbert, Department of Pathology, College of Veterinary Medicine, University of Georgia, Athens, GA, USA
Daniel R. Rissi, Department of Pathology, College of Veterinary Medicine, University of Georgia, Athens, GA, USA Athens Veterinary Diagnostic Laboratory, College of Veterinary Medicine, University of Georgia, Athens, GA, USA.
References
- 1. Barichello T, et al. Oxidative variables in the rat brain after sepsis induced by cecal ligation and perforation. Crit Care Med 2006;34:886–889. [DOI] [PubMed] [Google Scholar]
- 2. Chaudhry N, Duggal AK. Sepsis associated encephalopathy. Adv Med 2014;2014:762320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. de Laforcade AM, et al. Hemostatic changes in dogs with naturally occurring sepsis. J Vet Intern Med 2003;17:674–679. [DOI] [PubMed] [Google Scholar]
- 4. Deckert M. Bacterial infections. In: Love S, et al., eds. Greenfield’s Neuropathology. Vol. 2. 9th ed. CRC Press, 2015:1192–1229. [Google Scholar]
- 5. Dow SW, et al. Bacterial culture of blood from critically ill dogs and cats: 100 cases (1985–1987). J Am Vet Med Assoc 1989;195:113–117. [PubMed] [Google Scholar]
- 6. Ehlenbach WJ, et al. Association between sepsis and microvascular brain injury. Crit Care Med 2019;47:1531–1538. [DOI] [PubMed] [Google Scholar]
- 7. Elbert JA, et al. Neuroinflammatory diseases of the central nervous system of dogs: a retrospective study of 207 cases (2008–2019). Can Vet J 2022;63:178–186. [PMC free article] [PubMed] [Google Scholar]
- 8. Greenfield CL, Walshaw R. Open peritoneal drainage for treatment of contaminated peritoneal cavity and septic peritonitis in dogs and cats: 24 cases (1980–1986). J Am Vet Med Assoc 1987;191:100–105. [PubMed] [Google Scholar]
- 9. Greiner M, et al. A retrospective study of the clinical presentation of 140 dogs and 39 cats with bacteraemia. J Small Anim Pract 2008;49:378–383. [DOI] [PubMed] [Google Scholar]
- 10. Gyawali B, et al. Sepsis: the evolution in definition, pathophysiology, and management. SAGE Open Med 2019;7:2050312119835043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Handa O, et al. Role of endothelial nitric oxide synthase-derived nitric oxide in activation and dysfunction of cerebrovascular endothelial cells during early onsets of sepsis. Am J Physiol Heart Circ Physiol 2008;295:H1712–H1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hannestad J, et al. Endotoxin-induced systemic inflammation activates microglia: [11C]PBR28 positron emission tomography in nonhuman primates. Neuroimage 2012;63:232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Heming N, et al. Neuroanatomy of sepsis-associated encephalopathy. Crit Care 2017;21:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hosgood G, Salisbury SK. Generalized peritonitis in dogs: 50 cases (1975–1986). J Am Vet Med Assoc 1988;193:1448–1450. [PubMed] [Google Scholar]
- 15. Kenney EM, et al. Association between outcome and organ system dysfunction in dogs with sepsis: 114 cases (2003–2007). J Am Vet Med Assoc 2010;236:83–87. [DOI] [PubMed] [Google Scholar]
- 16. Lamar CD, et al. Sepsis-associated encephalopathy: review of the neuropsychiatric manifestations and cognitive outcome. J Neuropsychiatry Clin Neurosci 2011;23:237–241. [DOI] [PubMed] [Google Scholar]
- 17. Rissi DR. A retrospective study of the neuropathology and diagnosis of naturally occurring feline infectious peritonitis. J Vet Diagn Invest 2018;30:392–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Seymour CW, et al. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (Sepsis-3). J Am Med Assoc 2016;315:762–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Smith EE, et al. Cerebral microinfarcts: the invisible lesions. Lancet Neurol 2012;11:272–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sonneville R, et al. Understanding brain dysfunction in sepsis. Ann Intensive Care 2013;3:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vandevelde M, et al. Veterinary Neuropathology: Essentials of Theory and Practice. Wiley-Blackwell, 2012:63–64. [Google Scholar]
- 22. Woolfson JM, Dulisch ML. Open abdominal drainage in the treatment of generalized peritonitis in 25 dogs and cats. Vet Surg 1986;15:27–32. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-vdi-10.1177_10406387221102899 for Neuropathologic changes associated with systemic bacterial infection in 28 dogs by Jessica A. Elbert and Daniel R. Rissi in Journal of Veterinary Diagnostic Investigation