This Special Issue has focused on dissecting the neuroprotective and neurodegenerative components of neurological and neuropsychiatric diseases, highlighting the latest advance in understanding the etiology, pathomechanism, biomarkers, imaging techniques, and novel therapeutic targets of neurodegenerative diseases (NDDs). The articles published in the Special Issue have featured Alzheimer’s disease (AD), Parkinsons’s disease (PD), multiple sclerosis (MS), dementia with Lewy bodies, and salicylate-induced tinnitus.
Broadly speaking, the disease onset and course may be roughly understood by imagining a tug-of-war between neuronal damage and recovery, which takes place as early as in prodromal phase. Then, who pulls the rope first? NDDs are multifactorial diseases in which genetic susceptibility, environmental factors, infections, nutrition, and/or lifestyle make a complex interaction to form an initial causative complex which later progresses to formation of secondary complex, eventually leading to the onset of diseases [1,2]. NDDs are characterized by impairments of both cognitive function and social interaction. Indeed, these alterations in social cognition and social functioning, are attributed to altered activity within cortical and subcortical brain structures [3], which store sensory, motor, and affective information, fundamental for self-awareness and decisional process [4], which is a crucial aspect in the symptomatology of various neurodegenerative disorders. Clinical features and changing functional patterns in NDDs include impairments in memory and emotional learning, poor planning, altered capacity to adapt behavior to the environment, impaired working memory, apathy, depression, disinhibition, and/or a dysexecutive cognitive profile, which correlate with a typical cognitive pattern due to frontal lobe dysfunction [4,5,6,7,8,9,10]. Thus, a certain interaction of etiological factors and a unique pathological progression together composite a team which triggers the initial pull of the rope.
Featuring environmental factors as pathogenic culprits, Sini and colleagues explored the potential etiological link between microorganisms and NDD pathogenesis in an environmental scale. Blue-green algae cyanobacteria produce cyanotoxins which are released during cell lysis in an algal bloom into the surrounding water. Acute exposure to cyanotoxins cause gastrointestinal symptoms, allergic reactions, headache, and neurological symptoms including muscle weakness and dizziness. The cyanobacteria neurotoxin β-N-methylamino-l-alanine (BMAA) is considered to play a role in development of NDDs including AD, PD, and amyotrophic lateral sclerosis [11].
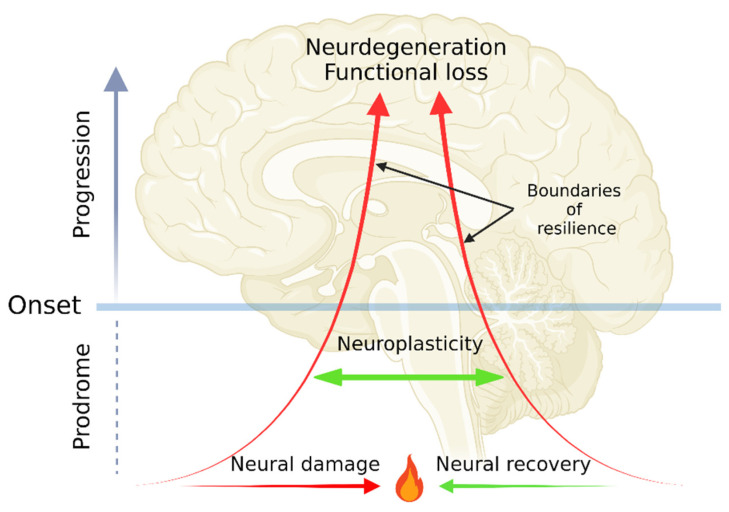
Diagnosis of a NDD is made through assembling a clinical picture interpreted by a doctor based on signs, symptoms, family history, and medical investigations including biomarkers, imaging tools, and medications [12]. The onset and disease course of NDDs may well be understood by envisioning a disease in analogy to a position vector: a position being the initial domain of symptoms; magnitude being severity in scales from molecular, tissue, neural correlate, to functional levels; and a direction being the domain of secondary symptoms. Neuroprotection, either through endogenous defense mechanisms or by exogenous supplements such as antioxidants may be able to intervene disease progression, slowing a free fall course with temporal remissions [13,14,15]. Following neural damage, direct repair mechanism neuroregeneration may help restore original or close to original brain functions in cellular and tissue levels [16]. Furthermore, overall neural activities can be maintained by the ability of the nervous system to recruit other components by reorganizing its structure, connections, and/or functions (that is neuroplasticity [17]). The capacity of neuroplasticity is bounded by resilience, which is the ability to be flexible and adaptive in response to harmful challenges [18]. The exacerbating disease course may well be exemplified by decreasing neural plasticity and weakening functional resilience. The endpoints of the plasticity, the resilience, and thus neural activities are neurodegeneration and eventually functional loss (Figure 1).
Figure 1.
The dynamics of neurodegenerative diseases (NDDs) from prodromal phase, onset, neurodegeneration, to functional loss. The pathogenesis of NDDs starts from a tug-of-war between neural damage and neural recovery in prodromal phase. The normal neural functions can be compensated by neuroplasticity, which is bounded by functional resilience. Decreasing neuroplasticity and resilience lead to neurodegeneration and functional loss. The figure was created with BioRender.com.
The initial neural damage can be repaired, and the function can be restored by the endogenous process of neuroprotection which refers to the salvage or recovery of the structure, function, neuronal cells, and/or network in the nervous system [19,20]. Microglial cells are responsible for inflammatory reaction in the nervous system. Czapski and Strosznajder discussed the roles of neuronal and microglial proteins including receptors, their involvement in the neural communication, and microglial-neuronal crosstalk in NDDs in search of neuroprotective and pharmacological targets [21]. The acute inflammatory response may proceed to neural recovery, but it may also lead to low-grade chronic inflammation and the state of immune tolerance. This is the allostatic state that maintains functional homeostasis at the cost of self-harm [22]. The healthy function in the network of excitatory glutamate and inhibitory γ-aminobutyric acid (GABA) neurotransmission is crucial to maintain neural homeostasis in the brain and the reciprocal excitatory-inhibitory balance has been observed to be compromised in neuroinflammation and AD [23]. Thus, the magnitude of organizational level is affected in AD through neuroinflammation.
Characterizing preclinical animal models simulating human diseases is an essential step for bench-to-bed translation research [24,25,26,27,28,29,30,31,32,33,34,35,36,37]. Mendes-Pinheiro and colleagues studied the behavioral domains of 6-hydroxydopamine (6-OHDA)-induced mouse model of PD. A progressive neurological disorder PD is widely considered to primarily affect movement of individuals. PD patients frequently experience psychological and behavioral symptoms named non-motor symptoms, which include sensory complaints, mental disorders, sleep disturbances, autonomic dysfunction, peripersonal space coding difficulties, motor disfunction and psychobehavioral symptoms such as apathy, agitation, hypersexuality, pathological gambling, psychoses, hallucinations, depression, and anxiety [2]. These symptoms can be present in the early stages of the disease, sometimes even before the appearance of classical motor symptoms, likely in relation to dopamine depletion in basal ganglia, suggesting how modulation of autonomic nervous system responses is fundamental for behavioral regulation. Evidence in healthy participants may suggest that these proprioceptive and motor mechanisms might be impaired in PD patients [38,39]. The authors investigated not only motor and coordination domain, but also the domains of positive and negative valences together with glial cell response. The mice showed despair-like behavior, decreased self-care, and less motivational behavior with proliferative and reactive microglia [40]. Thus, the authors successfully characterize a pharmacological animal model of PD, which also manifests the directional component of neural damage vector.
Biomarkers are measurable indicators to help evaluate risk, diagnosis, disease course, and therapeutic outcomes for a disease. MS is a chronic immunological neurodegenerative disease of which biomarkers certainly may be able to help identify various stages of MS and build personalized treatment plan. Biernacki and colleagues reviewed promising new biomarkers of blood and cerebrospinal fluid samples for MS, emphasizing need to develop biomarkers from blood samples and to establish biomarkers to complement current diagnostic strategies [41]. Furthermore, discovery of prodromal biomarkers is an urgent need not only for MS, but other NDDs in order to prevent the onset of diseases. Development of new diagnostic imaging techniques is under extensive research for NDDs. The accumulation of the tau protein is closely linked to neurodegeneration and thus cognitive impairment. The tau protein may serve as diagnostic and prognostic biomarkers. However, its precision calibration in vivo remained a great challenge. Ricci and colleagues reviewed recent advances in in vivo imaging by positron emission tomography (PET) using tau tracer 2-Deoxy-2-[18F]fluoroglucose (18F-FDG), describing the development of tau PET tracers and the distribution of tau tracers and pattern in the brain [42]. Accordingly, blood biomarkers and in vivo imaging techniques potentially serve as barometers of sustainability in the brain including neural plasticity and functional resilience.
Neuropeptides and neurohormones play an important role in cognitive, emotional, social, and arousal functions. Thus, neuropeptide fragments, receptor antagonists, and analogues are under extensive study in search of their beneficial use for neurological and psychiatric diseases [43,44,45,46]. Kisspeptin is a neuropeptide that plays a crucial role in the function of the hypothalamic–pituitary–gonadal axis. Simon and colleagues reported that A kisspeptin fragment Kisspeptin 10 (K-10) was observed to mitigate amyloid-β toxicity by direct biding. Simon and colleagues showed that low concentrations of KP-10 suppressed wild-type and E46K mutant α-syn-mediated toxicity in vitro and that KP-10 favorably binds to the active sites of wild-type and E46K 32 mutant α-syn in silico, concluding that KP-10 may be a potential therapeutic agent targeting the active sites of α-syn [47].
Drug repurposing is the fastest and the most economical drug developing strategy screening already approved drugs for other medical conditions in search of new indications [48]. Valproic acid (VA) is primarily indicated for epilepsy, bipolar disorder, and prophylaxis of migraine and seizures [49]. Song and colleagues investigated the neuroprotective effects of VA on an ototoxic drug salicylate-induced tinnitus model in vitro and in vivo, showing that salicylate-induced excitotoxicity and production of reactive oxygen species were attenuated by VA [50]. The study presented exogenous use of neuroprotective agent for temporary tinnitus and healing loss.
Accordingly, this Special Issue has successfully presented research articles covering a broad aspect of NDDs from environmental etiological factors, cellular and functional pathomechanism, preclinical models, clinical biomarkers, imaging technique, and endogenous neuroprotective peptides to exogenous neuroprotective medicine. Identifying combinations of multifactorial factors which increase susceptibility to an NDD including the initial causative and the subsequent causative complexes and detecting such risk factors in a prodromal phase would be of particular interest for prophylactic measures. Revealing the pathomechanism of NDD exacerbation, calibrating the levels of neuroplasticity, and measuring the strength of functional resilience would help plan personalized treatment to induce and secure remission. Monitoring the status of tryptophan-kynurenine metabolism which has close ties with neuroinflammation may shed some light on these approaches to neuropsychiatric symptoms [51,52,53,54,55,56,57,58]. Exploring endogenous and exogenous neuroprotective approaches would be able to complement current disease-modifying strategies and thus may be able to delay development of full-blown neurodegeneration and functional loss in NDDs.
Abbreviations
| AD | Alzheimer’s disease |
| BMAA | β-N-methylamino-l-alanine |
| 18F-FDG | 2-Deoxy-2-[18F]fluoroglucose |
| GABA | γ-aminobutyric acid |
| K-10 | Kisspeptin 10 |
| MS | multiple sclerosis |
| NDD | neurodegenerative disease |
| 6-OHDA | 6-hydroxydopamine |
| PD | Parkinson’s disease |
| PET | positron emission tomography |
| VA | Valproic acid |
Author Contributions
Conceptualization, M.T. and L.V.; writing—original draft preparation, writing—review and editing, M.T. and L.V.; supervision, L.V.; project administration, M.T. and L.V.; funding acquisition, L.V. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by National Scientific Research Fund OTKA138125, MTA-JSP-050609.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mackie J.L. The Cement of the Universe. A Study of Causation. Oxford University Press; Oxford, UK: 1976. [Google Scholar]
- 2.Tanaka M., Toldi J., Vécsei L. Exploring the Etiological Links behind Neurodegenerative Diseases: Inflammatory Cytokines and Bioactive Kynurenines. Int. J. Mol. Sci. 2020;21:2431. doi: 10.3390/ijms21072431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Battaglia S., Fabius J.H., Moravkova K., Fracasso A., Borgomaneri S. The Neurobiological Correlates of Gaze Perception in Healthy Individuals and Neurologic Patients. Biomedicines. 2022;10:627. doi: 10.3390/biomedicines10030627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sellitto M., Terenzi D., Starita F., di Pellegrino G., Battaglia S. The Cost of Imagined Actions in a Reward-Valuation Task. Brain Sci. 2022;12:582. doi: 10.3390/brainsci12050582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Battaglia S., Orsolini S., Borgomaneri S., Barbieri R., Diciotti S., di Pellegrino G. Characterizing cardiac autonomic dynamics of fear learning in humans. Psychophysiol. 2022 doi: 10.1111/psyp.14122. in press . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Battaglia S. Neurobiological advances of learned fear in humans. Adv. Clin. Exp. Med. 2022;31:217–221. doi: 10.17219/acem/146756. [DOI] [PubMed] [Google Scholar]
- 7.Battaglia S., Thayer J.F. Functional interplay between central and autonomic nervous systems in human fear conditioning. Trends Neurosci. 2022 doi: 10.1016/j.tins.2022.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Battaglia S., Harrison B.J., Fullana M.A. Does the human ventromedial prefrontal cortex support fear learning, fear extinction or both? A commentary on subregional contributions. Mol. Psychiatry. 2022;27:784–786. doi: 10.1038/s41380-021-01326-4. [DOI] [PubMed] [Google Scholar]
- 9.Garofalo S., Battaglia S., di Pellegrino G. Individual differences in working memory capacity and cue-guided behavior in humans. Sci. Rep. 2019;9:7327. doi: 10.1038/s41598-019-43860-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garofalo S., Timmermann C., Battaglia S., Maier M.E., di Pellegrino G. Mediofrontal Negativity Signals Unexpected Timing of Salient Outcomes. J. Cogn. Neurosci. 2017;29:718–727. doi: 10.1162/jocn_a_01074. [DOI] [PubMed] [Google Scholar]
- 11.Sini P., Dang T.B.C., Fais M., Galioto M., Padedda B.M., Lugliè A., Iaccarino C., Crosio C. Cyanobacteria, Cyanotoxins, and Neurodegenerative Diseases: Dangerous Liaisons. Int. J. Mol. Sci. 2021;22:8726. doi: 10.3390/ijms22168726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gómez-Río M., Caballero M.M., Górriz Sáez J.M., Mímz-nguez-Castellanos A. Diagnosis of Neurodegenerative Diseases: The Clinical Approach. Curr. Alzheimer Res. 2016;13:469–474. doi: 10.2174/1567205013666151116141603. [DOI] [PubMed] [Google Scholar]
- 13.Marmolejo-Martínez-Artesero S., Casas C., Romeo-Guitart D. Endogenous Mechanisms of Neuroprotection: To Boost or Not to Be. Cells. 2021;10:370. doi: 10.3390/cells10020370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teleanu R.I., Chircov C., Grumezescu A.M., Volceanov A., Teleanu D.M. Antioxidant Therapies for Neuroprotection—A Review. J. Clin. Med. 2019;8:1659. doi: 10.3390/jcm8101659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee K.H., Cha M., Lee B.H. Neuroprotective Effect of Antioxidants in the Brain. Int. J. Mol. Sci. 2020;21:7152. doi: 10.3390/ijms21197152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagappan P.G., Chen H., Wang D.Y. Neuroregeneration and plasticity: A review of the physiological mechanisms for achieving functional recovery postinjury. Mil. Med. Res. 2020;7:30. doi: 10.1186/s40779-020-00259-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dorszewska J., Kozubski W., Waleszczyk W., Zabel M., Ong K. Neuroplasticity in the Pathology of Neurodegenerative Diseases. Neural. Plast. 2020;202 doi: 10.1155/2020/4245821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ossenkoppele R., Lyoo C.H., Jester-Broms J., Sudre C.H., Cho H., Ryu Y.H., Choi J.Y., Smith R., Strandberg O., Palmqvist S., et al. Assessment of Demographic, Genetic, and Imaging Variables Associated With Brain Resilience and Cognitive Resilience to Pathological Tau in Patients With Alzheimer Disease. JAMA Neurol. 2020;77:632–642. doi: 10.1001/jamaneurol.2019.5154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitroshina E.V., Mishchenko T.A., Shirokova O.M., Astrakhanova T.A., Loginova M.M., Epifanova E.A., Babaev A.A., Tarabykin V.S., Vedunova M.V. Intracellular Neuroprotective Mechanisms in Neuron-Glial Networks Mediated by Glial Cell Line-Derived Neurotrophic Factor. Oxid. Med. Cell. Longev. 2019;2019:1036907. doi: 10.1155/2019/1036907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schirinzi T., Canevelli M., Suppa A., Bologna M., Marsili L. The continuum between neurodegeneration, brain plasticity, and movement: A critical appraisal. Rev. Neurosci. 2020;31:723–742. doi: 10.1515/revneuro-2020-0011. [DOI] [PubMed] [Google Scholar]
- 21.Czapski G.A., Strosznajder J.B. Glutamate and GABA in Microglia-Neuron Cross-Talk in Alzheimer’s Disease. Int. J. Mol. Sci. 2021;22:11677. doi: 10.3390/ijms222111677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanaka M., Vécsei L. Monitoring the kynurenine system: Concentrations, ratios or what else? Adv. Clin. Exp. Med. 2021;30:775–778. doi: 10.17219/acem/139572. [DOI] [PubMed] [Google Scholar]
- 23.Lopatina O.L., Malinovskaya N.A., Komleva Y.K., Gorina Y.V., Shuvaev A.N., Olovyannikova R.Y., Belozor O.S., Belova O.A., Higashida H., Salmina A.B. Excitation/inhibition imbalance and impaired neurogenesis in neurodevelopmental and neurodegenerative disorders. Rev. Neurosci. 2019;30:807–820. doi: 10.1515/revneuro-2019-0014. [DOI] [PubMed] [Google Scholar]
- 24.Hong F., He G., Zhang M., Yu B., Chai C. The Establishment of a Mouse Model of Recurrent Primary Dysmenorrhea. Int. J. Mol. Sci. 2022;23:6128. doi: 10.3390/ijms23116128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li T., Xu G., Yi J., Huang Y. Intraoperative Hypothermia Induces Vascular Dysfunction in the CA1 Region of Rat Hippocampus. Brain Sci. 2022;12:692. doi: 10.3390/brainsci12060692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martos D., Tuka B., Tanaka M., Vécsei L., Telegdy G. Memory Enhancement with Kynurenic Acid and Its Mechanisms in Neurotransmission. Biomedicines. 2022;10:849. doi: 10.3390/biomedicines10040849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin C.-C., Cheng P.-Y., Hsiao M., Liu Y.-P. Effects of RU486 in Treatment of Traumatic Stress-Induced Glucocorticoid Dysregulation and Fear-Related Abnormalities: Early versus Late Intervention. Int. J. Mol. Sci. 2022;23:5494. doi: 10.3390/ijms23105494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scalise S., Zannino C., Lucchino V., Lo Conte M., Scaramuzzino L., Cifelli P., D’Andrea T., Martinello K., Fucile S., Palma E., et al. Human iPSC Modeling of Genetic Febrile Seizure Reveals Aberrant Molecular and Physiological Features Underlying an Impaired Neuronal Activity. Biomedicines. 2022;10:1075. doi: 10.3390/biomedicines10051075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castillo-Mariqueo L., Giménez-Llort L. Impact of Behavioral Assessment and Re-Test as Functional Trainings That Modify Survival, Anxiety and Functional Profile (Physical Endurance and Motor Learning) of Old Male and Female 3xTg-AD Mice and NTg Mice with Normal Aging. Biomedicines. 2022;10:973. doi: 10.3390/biomedicines10050973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee E.C., Hong D.-Y., Lee D.-H., Park S.-W., Lee J.Y., Jeong J.H., Kim E.-Y., Chung H.-M., Hong K.-S., Park S.-P., et al. Inflammation and Rho-Associated Protein Kinase-Induced Brain Changes in Vascular Dementia. Biomedicines. 2022;10:446. doi: 10.3390/biomedicines10020446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smagin D.A., Kovalenko I.L., Galyamina A.G., Belozertseva I.V., Tamkovich N.V., Baranov K.O., Kudryavtseva N.N. Chronic Lithium Treatment Affects Anxious Behaviors and theExpression of Serotonergic Genes in Midbrain Raphe Nuclei of Defeated Male Mice. Biomedicines. 2021;9:1293. doi: 10.3390/biomedicines9101293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vila-Merkle H., González-Martínez A., Campos-Jiménez R., Martínez-Ricós J., Teruel-Martí V., Blasco-Serra A., Lloret A., Celada P., Cervera-Ferri A. The Oscillatory Profile Induced by the Anxiogenic Drug FG-7142 in the Amygdala–Hippocampal Network Is Reversed by Infralimbic Deep Brain Stimulation: Relevance for Mood Disorders. Biomedicines. 2021;9:783. doi: 10.3390/biomedicines9070783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Santana-Santana M., Bayascas J.-R., Giménez-Llort L. Fine-Tuning the PI3K/Akt Signaling Pathway Intensity by Sex and Genotype-Load: Sex-Dependent Homozygotic Threshold for Somatic Growth but Feminization of Anxious Phenotype in Middle-Aged PDK1 K465E Knock-In and Heterozygous Mice. Biomedicines. 2021;9:747. doi: 10.3390/biomedicines9070747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muntsant A., Giménez-Llort L. Genotype Load Modulates Amyloid Burden and Anxiety-Like Patterns in Male 3xTg-AD Survivors despite Similar Neuro-Immunoendocrine, Synaptic and Cognitive Impairments. Biomedicines. 2021;9:715. doi: 10.3390/biomedicines9070715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giménez-Llort L., Marin-Pardo D., Marazuela P., Hernández-Guillamón M. Survival Bias and Crosstalk between Chronological and Behavioral Age: Age- and Genotype-Sensitivity Tests Define Behavioral Signatures in Middle-Aged, Old, and Long-Lived Mice with Normal and AD-Associated Aging. Biomedicines. 2021;9:636. doi: 10.3390/biomedicines9060636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Correia B.S.B., Nani J.V., Waladares Ricardo R., Stanisic D., Costa T.B.B.C., Hayashi M.A.F., Tasic L. Effects of Psychostimulants and Antipsychotics on Serum Lipids in an Animal Model for Schizophrenia. Biomedicines. 2021;9:235. doi: 10.3390/biomedicines9030235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanaka M., Schally A.V., Telegdy G. Neurotransmission of the antidepressant-like effects of the growth hormone-releasing hormone antagonist MZ-4-71. Behav. Brain Res. 2012;228:388–391. doi: 10.1016/j.bbr.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 38.Ellena G., Battaglia S., Làdavas E. The spatial effect of fearful faces in the autonomic response. Exp. Brain Rres. 2020;238:2009–2018. doi: 10.1007/s00221-020-05829-4. [DOI] [PubMed] [Google Scholar]
- 39.Candini M., Battaglia S., Benassi M., di Pellegrino G., Frassinetti F. The physiological correlates of interpersonal space. Sci. Rep. 2021;11:2611. doi: 10.1038/s41598-021-82223-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mendes-Pinheiro B., Soares-Cunha C., Marote A., Loureiro-Campos E., Campos J., Barata-Antunes S., Monteiro-Fernandes D., Santos D., Duarte-Silva S., Pinto L., et al. Unilateral Intrastriatal 6-Hydroxydopamine Lesion in Mice: A Closer Look into Non-Motor Phenotype and Glial Response. Int. J. Mol. Sci. 2021;22:11530. doi: 10.3390/ijms222111530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Biernacki T., Kokas Z., Sandi D., Füvesi J., Fricska-Nagy Z., Faragó P., Kincses T.Z., Klivényi P., Bencsik K., Vécsei L. Emerging Biomarkers of Multiple Sclerosis in the Blood and the CSF: A Focus on Neurofilaments and Therapeutic Considerations. Int. J. Mol. Sci. 2022;23:3383. doi: 10.3390/ijms23063383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ricci M., Cimini A., Camedda R., Chiaravalloti A., Schillaci O. Tau Biomarkers in Dementia: Positron Emission Tomography Radiopharmaceuticals in Tauopathy Assessment and Future Perspective. Int. J. Mol. Sci. 2021;22:13002. doi: 10.3390/ijms222313002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Telegdy G., Tanaka M., Schally A.V. Effects of the growth hormone-releasing hormone (GH-RH) antagonist on brain functions in mice. Behav. Brain Res. 2011;224:155–158. doi: 10.1016/j.bbr.2011.05.036. [DOI] [PubMed] [Google Scholar]
- 44.Tanaka M., Telegdy G. Neurotransmissions of antidepressant-like effects of neuromedin U-23 in mice. Behav. Brain Res. 2014;259:196–199. doi: 10.1016/j.bbr.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 45.Tanaka M., Kádár K., Tóth G., Telegdy G. Antidepressant-like effects of urocortin 3 fragments. Brain Res. Bull. 2011;84:414–418. doi: 10.1016/j.brainresbull.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 46.Palotai M., Telegdy G., Tanaka M., Bagosi Z., Jászberényi M. Neuropeptide AF induces anxiety-like and antidepressant-like behavior in mice. Behav. Brain Res. 2014;274:264–269. doi: 10.1016/j.bbr.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 47.Simon C., Soga T., Ahemad N., Bhuvanendran S., Parhar I. Kisspeptin-10 Rescues Cholinergic Differentiated SHSY-5Y Cells from α-Synuclein-Induced Toxicity In Vitro. Int. J. Mol. Sci. 2022;23:5193. doi: 10.3390/ijms23095193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Durães F., Pinto M., Sousa E. Old Drugs as New Treatments for Neurodegenerative Diseases. Pharmaceuticals (Basel) 2018;11:44. doi: 10.3390/ph11020044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rahman M., Nguyen H. Valproic Acid. [(accessed on 1 June 2022)]; Available online: https://www.ncbi.nlm.nih.gov/books/NBK559112/
- 50.Song A., Cho G.-W., Vijayakumar K.A., Moon C., Ang M.J., Kim J., Park I., Jang C.H. Neuroprotective Effect of Valproic Acid on Salicylate-Induced Tinnitus. Int. J. Mol. Sci. 2022;23:23. doi: 10.3390/ijms23010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Balogh L., Tanaka M., Török N., Vécsei L., Taguchi S. Crosstalk between Existential Phenomenological Psychotherapy and Neurological Sciences in Mood and Anxiety Disorders. Biomedicines. 2021;9:340. doi: 10.3390/biomedicines9040340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tanaka M., Török N., Vécsei L. Are 5-HT1 receptor agonists effective anti-migraine drugs? Expert. Opin. Pharmacother. 2021;22:1221–1225. doi: 10.1080/14656566.2021.1910235. [DOI] [PubMed] [Google Scholar]
- 53.Gładka A., Zatoński T., Rymaszewska J. Association between the long-term exposure to air pollution and depression [published online as ahead of print on June 2, 2022] Adv. Clin. Exp. Med. 2022 June 2; doi: 10.17219/acem/149988. [DOI] [PubMed] [Google Scholar]
- 54.Tanaka M., Török N., Tóth F., Szabó Á., Vécsei L. Co-Players in Chronic Pain: Neuroinflammation and the Tryptophan-Kynurenine Metabolic Pathway. Biomedicines. 2021;9:897. doi: 10.3390/biomedicines9080897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen C. Recent advances in the study of the comorbidity of depressive and anxiety disorders. Adv. Clin. Exp. Med. 2022;31:355–358. doi: 10.17219/acem/147441. [DOI] [PubMed] [Google Scholar]
- 56.Spekker E., Tanaka M., Szabó Á., Vécsei L. Neurogenic Inflammation: The Participant in Migraine and Recent Advancements in Translational Research. Biomedicines. 2022;10:76. doi: 10.3390/biomedicines10010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carrera-González M.D.P., Cantón-Habas V., Rich-Ruiz M. Aging, depression and dementia: The inflammatory process. Adv. Clin. Exp. Med. 2022;31:469–473. doi: 10.17219/acem/149897. [DOI] [PubMed] [Google Scholar]
- 58.Tanaka M., Spekker E., Szabó Á., Polyák H., Vécsei L. Modelling the neurodevelopmental pathogenesis in neuropsychiatric disorders. Bioactive kynurenines and their analogues as neuroprotective agents-in celebration of 80th birthday of Professor Peter Riederer. J. Neural. Transm. (Vienna) 2022;129:627–642. doi: 10.1007/s00702-022-02513-5. [DOI] [PubMed] [Google Scholar]