Abstract
Background
PRKAG2 cardiac syndrome is a rare autosomal dominant genetic disorder caused by a PRKAG2 gene variant. There are several major adverse cardiac presentations, including hypertrophic cardiomyopathy (HCM) and life‐threatening arrhythmia. Two cases with pathogenic variants in the PRKAG2 gene are reported here who presents different cardiac phenotypes.
Methods
Exome sequencing and variant analysis of PRKAG2 were performed to obtain genetic data, and clinical characteristics were determined.
Results
The first proband was a 9‐month‐old female infant (Case 1), and was identified with severe DCM and resistant heart failure. The second proband was a 10‐year‐old female infant (Case 2), and presented with HCM and ventricular preexcitation. Exome sequencing identified a de novo c.425C > T (p.T142I) heterozygous variant in the PRKAG2 gene for Case 1, and a c.869A > T (p.K290I) for Case 2. The mutated sites in the protein were labeled and identified as p.K290 in the CBS domain and p.T142 in the non‐CBS domain. Differences in the molecular functions of CBS and non‐CBS domains have not been resolved, and variants might lead to the different cardiomyopathy phenotypes. Single‐cell RNA analysis demonstrated similar expression levels of PRKAG2 in cardiomyocytes and conductive tissues. These results suggest that the arrhythmia induced by the PRKAG2 variant was the primary change, and not secondary to cardiomyopathy.
Conclusion
In summary, this is the first case report to describe a DCM phenotype with early onset in patients possessing a PRKAG2 c.425C > T (p.T142I) pathogenic variant. Our results aid in understanding the molecular function of non‐CBS variants in terms of the disordered sequence of transcripts. Moreover, we used scRNA‐seq to show that electrically conductive cells express a higher level of PRKAG2 than do cardiomyocytes. Therefore, variants in PRKAG2 are expected to also alter the biological function of the conduction system.
Keywords: AMPK signaling, case report, genomic sequence, PRKAG2 cardiac syndrome, scRNA‐seq
Differences in the molecular functions of CBS and non‐CBS domains have not been resolved, and mutations might lead to the different cardiomyopathy phenotypes. Single cell RNA analysis demonstrated similar expression levels of PRKAG2 in cardiomyocytes and conductive tissues. These results suggest that the arrhythmia induced by the PRKAG2 mutation was the primary change, and not secondary to cardiomyopathy.
1. INTRODUCTION
PRKAG2 cardiac syndrome (PCS) is a rare autosomal dominant genetic disorder caused by a PRKAG2 gene variant (Banankhah et al., 2018; Bayrak et al., 2006). In 2001, Gollob et al. confirmed that PRKAG2 variants have important implications for elucidating the pathogenesis of ventricular preexcitation (Gollob, Green, et al., 2001; Gollob, Seger, et al., 2001). The syndrome is caused by variants in the gene encoding for 5′adenosine monophosphate‐activated protein kinase (AMPK), specifically its γ2 regulatory subunit (PRKAG2) (Cao et al., 2017). AMPK disease is characterized by progressive conduction disease and cardiac hypertrophy, and hypertrophy cardiomyopathy is the most common manifestation in PRKAG2 syndrome. It includes extracardiac manifestations such as a skeletal myopathy, consistent with systemic glycogen storage disease. The incidence of PCS is low, and since the clinical manifestations are diverse, the misdiagnosis rate is relatively high (Banankhah et al., 2018). PRKAG2 syndrome is a progressive cardiomyopathy characterized by high rates of atrial fibrillation, conduction disease, advanced heart failure, and life‐threatening arrhythmias (Beyzaei et al., 2021; Coban‐Akdemir et al., 2020; Jääskeläinen et al., 2019; Maron & Maron, 2020; Pena et al., 2021; Spentzou et al., 2020; van der Steld et al., 2017). Dominant variants of PRKAG2 seldom occur in sporadic cases but appear to run in families. Variants recognized thus far include G100S, K290I, R302Q, V336L, S356P, R384T, H401D, K485E, N488I, E506D, R531Q, and R531G. These variants invariably cluster within the Bateman domain (defined as the region containing cystathionine beta‐synthase [CBS] domains) of the PRKAG2 gene, while only G100S has been reported as a non‐CBS variant (Zhang et al., 2013). The R302Q variant is the most common among the clinical isolates.
In vivo and in vitro models of PRKAG2 variants have successfully demonstrated that PRKAG2‐related cardiomyopathy is a distinct type of glycogen storage disease, involving primarily the heart. Consistent with a causative role of PRKAG2, heterozygous mice carrying a mutant PRKAG2 allele develop pathological cardiac changes resembling the human genetic disorder. Lopez‐Sainz et al. reported the largest cohort of cardiac glycogenosis with PRKAG2 variant (Lopez‐Sainz et al., 2020). They identified that the classical features of preexcitation and severe left ventricular hypertrophy were not uniformly presented. And a high rate of sudden cardiac death had been observed among such patients. However, the roles of PRKAG2 variants in regulating AMPK activity are controversial. Sidhu et al. (2005), and Scott et al. (2004) showed that the p.R302Q and p.R531G variants reduce AMPK activity, and Arad et al. (2003), and Banerjee et al. (2010) both showed that T400N induces early activation of myocardial AMPK. Therefore, different variants in PRKAG2 are related to opposite changes in AMPK activity, leading to diverse cardiac manifestations.
We report two cases with pathogenic variants of the PRKAG2 gene. The p.T142I variant, located in the non‐CBS domain, was association with dilated cardiomyopathy (DCM), while p.K290I, located in the CBS domain, predicted HCM. We also used single‐cell RNA (scRNA)‐seq analysis to assess the expression of PRKAG2 in different cardiac cell types. This is the first case report of DCM resulting from a PRKAG2 pathogenic variant. We provide a literature summary and protein structure analysis to help explain the diverse clinical presentations of PCS.
2. METHODS
2.1. Ethics compliance
The study was approved by the Ethics Committee of the West China Second Hospital of Sichuan University (approval number 2014–034). The patients' parents provided written informed consent to performing exome sequencing and to publication of this report.
2.2. DNA extraction and exome sequencing analysis
Peripheral blood samples were obtained from the patients in an EDTA anticoagulant blood sample tube stored at 4°C for less than 6 h. DNA was extracted using the Blood Genome Column Medium Extraction Kit (Tiangen Biotech, Beijing, China) in accordance with the manufacturer's instructions. Protein‐coding exome enrichment was performed using the xGen Exome Research Panel v.1.0, comprising protein‐coding regions (>23,000 genes) of the human genome. Exome sequencing was performed using the NovaSeq 6000 platform (Illumina, San Diego, CA, USA), and the raw data were processed using FastP to remove adapters and filter out low‐quality reads. Paired‐end reads were aligned to the ENSEMBL GRCh38/hg38 reference genome using the Burrows–Wheeler Aligner. Variant annotation was performed in accordance with database‐sourced minor allele frequencies (MAFs) and practical guidelines on pathogenicity issued by the American College of Medical Genetics. The annotation of MAFs was performed based on the 1000 Genomes, dbSNP, ESP, ExAC, Provean, Sift, Polypen2_hdiv, Polypen2_hvar, and Chigene in‐house MAF databases using R software (R Foundation for Statistical Computing, Vienna, Austria). The sequencing data have been deposited in the GSA database (http://ngdc.cncb.ac.cn/gsub/) (HRA001920).
2.3. Single‐cell RNA‐sequencing analysis
The public scRNA‐seq dataset for the heart was used to evaluate expression of PRKAG2 in all cell types, with emphasis on the conduction system and cardiomyocytes (CMs). All scRNA‐seq raw data have been deposited into the National Center for Biotechnology Information (NCBI)/GEO database under accession number GEO: GSE132658 (Goodyer et al., 2019). Embryonic day 16.5 (E16.5) wild‐type CD1 mouse hearts were harvested, and three regions were microdissected based on anatomical landmarks: Zone I—the sinoatrial node (SAN) region (superior vena cava/right atrial junction), Zone II—the atrioventricular node (AVN)/His region (crux of the heart), and Zone III—the bundle branch (BB)/Purkinje fiber (PF) region (luminal side of the ventricles). Droplet platform data were de‐multiplexed and mapped to the mouse genome MM10 using CellRanger from 10× Genomics with default parameters. Cell filter, data normalization, and unsupervised analysis were carried out in Seurat version 2 per their recommended steps. CMs isolated from Zone I were treated as atrial CMs and those isolated from Zone III were treated as ventricular CMs. Cells in the conduction system of Zone I were separated into compact SAN (cSAN, Hcn4 high /Gja5 neg /Gjc1 + ) cells and transitional cells (TC, Hcn4 low/neg /Gja5 + /Gjc1 + ) types; while cells from Zone II were separated into His bundle (His, Hcn4 + /TBX3 + /Kcne1 hi /Gjc1 + /Gja5 + /Etv1 + /Scn5a + /Cacna2d2 + ), Atrial Transitional Zone (ATZ, Myh6hi/Gjc1+/Gja5hi/Cacna2d2+), Transitional AV Ring (TAVR, Kcne1 + /Gjc1 + /Gja1 + /Scan5a + ), Nodal Atrioventricular Ring (NAVR, TBX3 + /Kcne1 + /Gjc1 + /Cacna2d2 + ), compact AVN (cAVN, Hcn4 hi /TBX3 hi /Kcne1 hi /Gjc1 + /Cacna2d2 + ), and Ventricular Transitional Zone (VTZ, Myl2 hi /Myh7 hi /Kcne1 hi /Gjc1 + /Gja + ) types; and cells from Zone II were separated into Transitional PF (TP, Gja1 hi /Gjc1 + /Scn5a + /Etv1 hi /Sema3a + /Nkx2.5 + ) and Standard Immature PF (PF, Hcn4 + /Gja5 hi /Gjc1 + /Scn5a + /Etv1 hi /Sema3a + /Nkx2.5 + ) types.
3. RESULTS
3.1. Clinical presentation and outcomes
3.1.1. Case 1
The proband was a 9‐month‐old female infant who was admitted to hospital due to an enlarged heart and heart failure for 1 month (Figure 1a). This patient exhibited shortness of breath and reduction of milk intake. Cyanosis was also occasionally observed, and the child suffered respiratory infection 1 month before onset of cardiac symptoms. Physical examination revealed increased heart rate (134 times per minute), elevated breathing rate (45 times per minute), and lower blood pressure (78/39 mm Hg). In addition, a pale face, dull heart sound, irregular rhythm, and second‐degree systolic bruits in the fourth intercostal space were observed. Results of physical examination of the respiratory and nervous systems were negative. Echocardiography revealed heart failure (EF = 21%, FS = 10%), and left ventricle enlargement (LV = 49 mm), left atrium enlargement (LA = 21 mm), normal right atrium and ventricle (RV = 9 mm, RA = 20 mm), moderate thickness of the endocardium at the posterior wall of the LV (3 mm), and moderate tricuspid and mitral valve regurgitation (Figure 1b). Routine ECG showed sinus tachycardia, abnormality of the left atrium, left ventricular hypertrophy, right ventricular hypertrophy, and changes in the ST‐T segment (II, III, aVF, V4‐V5). Holter ECG demonstrated recurrent ventricular premature beats (167 times/24 h) (Figure 1c). Cardiac MRI revealed an enlargement of the entire heart, but mainly in the left ventricle, in which there was significantly reduced systolic function (EF = 14%). Mitral regurgitation and pericardial effusion were also found.
FIGURE 1.
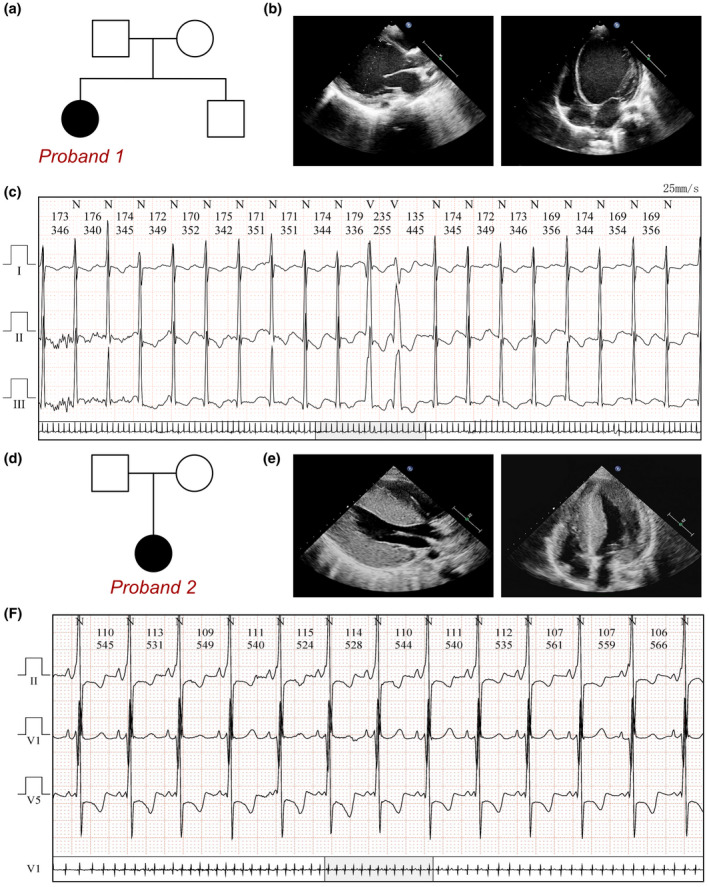
PRKAG2 variants in the two families. (a) Family pedigree revealing no carrier of PRKAG2 c.425C > T (p.T142I). The current proband (Case 1) exhibited severe DCM with a de novo heterozygous variant of PRKAG2 c.425C > T. (b) Echocardiography of the current proband, showing severe dilation of left ventricle. (c) ECG of the proband (c.425C > T), showing frequent ventricular pre‐mature beats. (d) Family pedigree revealing no carrier of PRKAG2 c.869A > T (p.K290I). The current proband (Case 2) exhibited severe HCM with de novo heterozygous variant of PRKAG2 c.869A > T. (e) Echocardiography in the current proband, showing a severe hypertrophy of left ventricle. (f) ECG of the proband (c.869A > T), showing ventricular preexcitation
Blood gas analysis indicated reduced BE‐Ecf (base excess in the extracellular fluid) of −4.6 (normal range [NR] −3.0–3.8 mmol/L) and increased lactase concentration (5.10, NR 0.7–3.0 mmol/L). Significantly increased levels of cTnI (0.254 μg/L, NR <0.034 μg/L) and NT‐BNP (19,200.00 pg/ml, NR <300 pg/ml) indicated myocardial damage. Creatine kinase (CK) and myoglobin (Mb) were elevated (148 U/L [NR 30–120 U/L] and 153.00 μg/L [(NR < 61.5 μg/L], respectively). In addition, abnormal thyroid function was assessed by TSH 4.72 mIU/L (NR 1.70–9.10 mIU/L), FT3 8.55 pmol/L (NR 4.3–10.6 pmol/L), FT4 24.54 pmol/L (NR 14.00–23.00 pmol/L), TGAb 78.0 U/ml (NR < 60 U/ml), and TPOAb 242.1 U/ml (NR < 60 U/ml). The results of routine blood tests and tests for rheumatic factor, autoimmune antibodies, antistreptolysin O, and CCP‐IgG were negative at admission.
DCM or myocarditis with heart failure was suspected. Methylprednisolone (10 mg/kg per day for 7 days), immunoglobulin (500 mg/kg per day for 4 days), and digoxin (6 μg/kg per day) were prescribed in addition to other treatments. Recovery from heart failure occurred and the patient was discharged. During follow‐up, frequent ventricular pre‐mature beats were detected, whereupon the patient received oral administration of prednisone (5 mg/day) for 1 year. Cardiac MRI revealed no evidence of fibrosis, at which point the diagnosis of myocarditis was questioned. Therefore, exome sequencing was performed and a pathogenic PRKAG2 variant at c.425C > T was identified. No other cardiomyopathy‐related pathogenic or uncertain variants had been identified, especially for other DCM‐related genes. Long‐term digoxin and captopril were then prescribed. During 4 years of follow‐up, her left ventricle remained large (41–50 mm), and EF remained at about 35–45%.
3.1.2. Case 2
This proband was a 10‐year‐old female infant, who was admitted to hospital suffering from fatigue, chest tightness, and fainting (Figure 1d). She experienced significant chest pain and palpitations shortly before syncope, with a decrease in tolerance of daily activities. A pale face, dull heart sound, and third‐degree systolic murmur in the fourth intercostal space were observed. Results of physical examination of the respiratory and nervous systems were negative. Echocardiography showed that the size of each ventricle was normal, and asymmetric hypertrophy was identified in the interventricular septum (IVS, 26 mm) and left ventricular posterior wall (LVPW 13 mm, IVS/LVPW = 0.5). Left ventricular outflow tract anterior blood flow was accelerated (PG = 30 mm Hg), indicating a narrow outflow tract due to hypertrophy, while the heart retained normal function (EF = 55%), which led to a diagnosis of hypertrophic obstructive cardiomyopathy (Figure 1e). Her cardiac MRI presented enlargement of the heart and increased thickness of the IVS and free wall of the left ventricle. The ventricular septum was obvious, with maximum thickness at the end of diastole of about 44 mm, and LVEF was normal at 66.4%. Moderate obstruction of the left ventricle was recorded. The cardiac MRI calculated stroke volume was 104.4 ml for the left ventricle and 70.8 ml for the right ventricle. In addition, Holter ECG examination revealed significant ventricular preexcitation and symptoms of Wolff–Parkinson–White (WPW) syndrome with a delta wave and shortened PR interval (Figure 1f).
These myocardial injuries resulted in significantly increased levels of cTnI (3.413 μg/L, NR < 0.034 μg/L) and NT‐BNP (4704.00 pg/ml, NR < 300 pg/ml), while CK and Mb were normal at 103 U/L (NR 30–120 U/L) and 32.00 μg/L (NR < 61.5 μg/L). In addition, thyroid function and biochemical tests were normal. The results of routine blood tests and tests for autoimmune antibodies, rheumatic factor, antistreptolysin O, and CCP‐IgG were negative at admission. All the tests for viral pathogens were negative. As there were no symptom of heart failure or cardiac enlargement, and ECG did not present any transduction blocks and escaping arrhythmias, myocarditis had been excluded.
Exome sequencing analysis demonstrated a heterozygous pathogenic variant of the PRKAG2 gene at c.869A > T. No other cardiomyopathy‐related pathogenic or uncertain variants had been identified. Finally, PRKAG2 syndrome was diagnosed from manifestations of HCM and ventricular preexcitation, upon which oral trimetazidine and coenzyme Q10 were prescribed. The patient then underwent radiofrequency ablation to terminate left ventricular outflow obstruction. During follow‐up after the ablation procedure, she still suffered chest pain and shortness of breath during daily activity. She subsequently developed recurrent respiratory tract infections and died of infection with irreversible heart failure approximately half a month after the ablation procedure.
3.2. Molecular results
Based on clinical manifestations and laboratory analyses, genetic disorders were strongly suspected. Exome sequencing was performed using the Illumina NovaSeq 6000 platform, which identified de novo heterozygous variants in the PRKAG2 gene at c.425C > T (p.T142I) for Case 1 and c.869A > T (p.K290I) for Case 2. These variants were absent from the patients' mothers and fathers. According to the American College of Medical Genetics, these variants have uncertain pathogenicity for both variant sites. The c.425C > T variant has not been reported in any publication and c.869A > T has never been reported in any population, and so this is the first report of these two variants (Figure 1e). MutationTaster, a tool to predict the effects of DNA variants, revealed that this variant is considered disease‐causing due to amino acid sequence changes, changes in protein structure, and splice‐site changes (probability = 0.995 for c.425C > T and 0.999 for c.869A > T). The PolyPhen‐2 tool predicted damage to protein structure and function for p.T142I (score: 0.998) and p.K290I (score: 1.000). The SIFT score demonstrated protein functional damage with rating of 0.001 for p.T142I and 0.000 for p.K290I. These results are summarized in Figure 2d.
FIGURE 2.
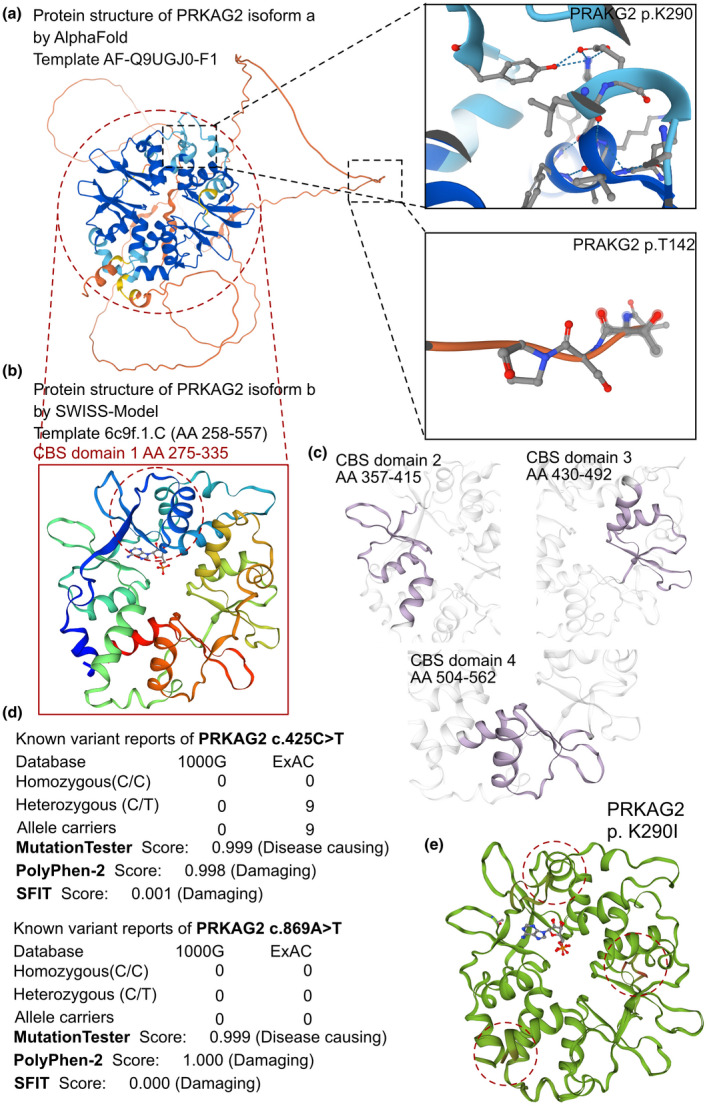
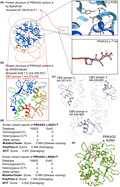
The effects of PRKAG2 c.425C > T and c.869A > T variants on the molecular structure of the protein. (a) the AlphaFold protein structure database was used to predict the PRKAG2 wild‐type protein crystal structure. Four important CBS domains were revealed and were clearly involved in AMPK signal transduction. (b) Protein structure of PRKAG2 isoform b (transcript‐b exon5‐exon16). This region contains four CBS domains with two tandem pairs. (c) Individual crystal structures of CBS domains not involved in the variant reported in this study. (d) The prevalence of PRKAG2 variants of c.425C > T and c.869A > T. (e) SWISS‐MODEL prediction of the wild‐type and p.K290I‐variant protein crystal structures. Changes of structure are indicated in the transporter region
AlphaFold2 was used to predict the protein structure of PRKAG2 using the AF‐Q9UGJ0‐F1 template with the entire AA sequence of isoform a. The mutated sequence site of the protein was labeled (Figure 2a), which revealed that p.K290 is located in the CBS domain and p.T142 in the non‐CBS domain. All potential templates were searched, but only parts of the protein had been analyzed previously, so the AlphaFold‐predicted structure was the only model that could be used. Although the predicted protein included the entire amino acid sequence, only CBS domains were solved with high confidence in the crystal structure (pLDDT > 70). Furthermore, PRKAG2 could be spliced into isoform b, which only contained the four CBS domains. The SWISS‐MODEL tool was then used to predict the crystal structures of isoform b (AA 258–557) (Figure 2b). Stable structures of the four CBS domains were solved (the AA for the four CBS domains were 275–335, 357–415, 430–492, and 504–562, respectively; Figure 2c). The CBS domain is most likely involved in regulating signaling of AMPK, an energy‐sensor protein kinase that plays a key role in regulating cellular energy metabolism. A comparison model was established to demonstrate changes in protein structure based on the p.K290I variant, which showed several alternate sites in the crystal structure (Figure 2e).
We then reviewed all reported variants in PRKAG2 (Table 1, Figure 3a), almost all of which were located in the CBS domains (isoform b). Reported clinical manifestations included HCM, ventricular preexcitation, bundle branch blocks, atrioventricular blocks (second and third degree), and sudden cardiac death. Although all patients received pacemakers or ICD implantation, unexpected deaths still occurred. Even though some patients exhibited bradycardia at the fetal stage, and a PRKAG2 variant was identified after birth, most were subjected to exome sequencing only after syncope or cardiac arrest. However, only two studies (14 cases) reported a non‐CBS domain variant, p.G100S, and that cohort also demonstrated HCM, ventricular preexcitation and AVBs. We report a pair of cases with one variant located in the CBS domain accompanied by the classic PRKAG2 syndrome manifestations of HCM, ventricular preexcitation and sudden cardiac death, and another variant identified in the non‐CBS domain that was associated with an unusual DCM phenotype and frequent premature ventricular beats. These results require further evaluation of the function of the non‐CBS domain, which is characterized by a disordered structure.
TABLE 1.
Summarization of reported PRKAG2 variants patients
| Reference | Variant site | Onset age/gender | Primary symptom | Cardiac manifestations | ECG | Echo | Clinical outcome |
|---|---|---|---|---|---|---|---|
| Austin et al. (2017) | p.G100S | 2.5 m/M | Generalized muscle weakness | HCM | sPR | Mild hypertrophy of the IVS | Alive |
| Zhang et al. (2013) | p.G100S (n = 13) | 16–38y/7 M&6F | Symptomatic arrhythmia | HCM |
Bradycardia CRBBB WPW II° or III°AVB |
Thickness of IVS |
4 died (30.7%) 4 PM 2 died after PM 1 died after treatment |
| van der Steld et al. (2017) | p.K290I | 27y/M | Syncope since infancy |
Arrhythmia LVH |
Sinus bradycardia RBBB |
LVH |
Alive Pacemaker |
| Banankhah et al. (2018) | p.R302Q | 23y/F | Persistent angina | HCM |
WPW RBBB |
LVH (22 mm) LVEF, 70%–75% |
Alive |
| Back Sternick et al. (2016) | p.R302Q | 52y/M | III°AVB |
Arrhythmia HCM |
III°AVB | LVH | Unknown |
| Roberts et al. (2010) | p.R302Q | 23y/M | Syncope |
HCM WPW |
sPR | LV hypertrophy and outflow obstruction | Cardiac transplantation |
| Charron et al. (2007) | p.R302Q | 22–66y/4F |
Dizzy Cardiopalmus |
Arrhythmia HCM |
sPR, RBBB, | Atrial hypertrophy | Alive |
| Spentzou et al. (2020) | p.Af304G | 13y/M | Ventricular fibrillation Cardiac arrest |
Arrhythmia LVH |
WPW SVT AVB AF |
LVH | Unknown |
| Yang et al. (2017) | p.V336L | 17–32y/1 M&1F |
Dizziness ECG anomaly |
HCM |
WPW V5‐V6 inverted T waves |
IVS (12 mm) LVH RVH EF, 46% |
1 Alive and 1 died |
| Laforêt et al. (2006) | p.S356P | 38y/M | Syncope |
Arrhythmia HCM |
SR (35/min) III°AVB LBBB |
IVST 18 mm LVEF = 60% |
Alive |
| Akman et al. (2007) | p.R384T | 10w/F | Fetal bradycardia |
Bradycardia HCM |
sPR |
IVST 15.2 mm LVEF = 81% |
Died |
| Liu et al. (2013) | p.K485E (n = 19) | 15–46y/15 M&4F |
Chest pain Palpitation Syncope |
HCM Arrhythmia |
Abnormal repolarization RBBB Bradycardia LVH WPW AVB |
LVH Thickness of IVS |
Unknown |
| Kelly et al. (2009) | p.E506D | 6 m/M |
WPW HCM |
HCM | sPR | Obliteration of the left ventricular cavity during systole | Cardiac transplantation |
| Gorla et al. (2018) | p.R531Q | 28wk of gestation/− | Echo\ECG anomaly | HCM | sPR | Severe biventricular hypertrophy and asymmetric septal hypertrophy | Died at 1 m |
| Burwinkel et al. (2005) | p.R531Q | 28 wk of gestation/− |
Bradycardia in fetus ARDS Multiserous cavity effusion |
Bradycardia HCM |
sPR (0.06 s) Enlarged QRS complexes Depressed ST segments |
Atrial hypertrophy Biventricular hypertrophy |
Died |
| Gollob, Seger et al. (2001) | p.R531G | 2y/M | Syncope |
Arrhythmia LVH |
AF WPW AVB |
LVH (11 mm) | Unknown |
| Murphy et al. (2005) | P.N488I (n = 40) | 3–45y (19)/− |
Asymptomatic (35%) Syncope (12.5%) Myalgia (18%) Stroke (3%) SCD (2.5%) |
arrhythmia may HCM |
WPW (58%) AF/SVT (30%) |
LVH (70%) 13–45 mm |
PM (30%) |
|
p.R302Q (n = 5) |
15–38y (26)/− | Syncope (100%) |
arrhythmia may HCM |
WPW (100%) AF/SVT (75%) |
LVH 15–16 mm |
PM (100%) | |
| Arad et al. (2002) |
p.R302Q (n = 43) |
39 ± 16y/− |
Arrhythmia SCD (n = 2) |
Arrhythmia LVH |
WPW (65%) SR (12%) AVB (37%) |
LVH (17.1 ± 7.6 mm) | PM (47%) |
| p.T400N | 42/− |
WPW Bradycardia |
Arrhythmia LVH |
WPW SR |
LVH (22 mm) | PM | |
|
p.N488I (n = 26) |
32 ± 13y/− |
WPW (23%) Bradycardia (31%) SCD (n = 2) |
Arrhythmia LVH |
WPW SR |
LVH (19.3 ± 9.3 mm) | PM (15%) | |
| Sri et al. (2019) |
p.N488I (n = 4) |
5–33y/3 M&1F |
Decreased activity tolerance Chest pain Echo\ECG anomaly |
HCM |
WPW sPR IRBBB |
LVH Thickness of IVS |
Alive |
|
p.E506D (n = 3) |
8–44y/2 M&1F |
Dizzy Palpitation Dyspnea |
HCM |
WPW sPR IRBBB |
LVH Thickness of IVS |
Alive | |
| Hu et al. (2020) |
p.R302Q (n = 8) |
15–40y/6 M&2F |
Chest pain Palpitation Syncope |
Arrhythmia HCM |
III°AVB Premature beat CLBBB WPW Bradycardia |
Thickness of IVS | PM |
| p.L341S | 15y/M | Negative |
Arrhythmia HCM |
II°AVB |
Thickness of IVS EF 42% |
PM | |
|
p.H401D (n = 2) |
14–26y/1 M&1F |
Palpitation Syncope |
Arrhythmia HCM |
2:1 heart block Premature beat WPW AF |
Thickness of IVS EF 40% |
Unknown | |
| p.K485E | 15y/M | Chest pain, chest distress, palpitation |
Arrhythmia HCM |
II°AVB WPW Bradycardia Premature beat |
Thickness of IVS (32 mm) EF 42% |
PM |
Abbreviations: AF, atrial fibrillation; AH, atrial hypertrophy; AVB, atrioventricular block; BH, biventricular hypertrophy; CMR, cardiac magnetic resonance; Echo, echocardiography; EF, ejection fraction; HCM, hypertrophic cardiomyopathy; IVS, interventricular septum; IVST, interventricular septum thickness; LAHB, Left anterior branch block; LBBB, left bundle branch block; LV, left ventricular; LVEF, left ventricular ejection fraction; LVH, left ventricular hypertrophy; LVPW, left ventricular posterior wall; LVWT, left ventricular wall thickness; MLVWT, maximal left ventricular wall thickness; PM, pacemaker implantation; RBBB, right bundle branch block; SCD, sudden cardiac death; sPR, short PR; SR, sinu rhythm; SVT, supraventricular tachycardia; WPW, Wolff–Parkinson–White syndrome.
FIGURE 3.
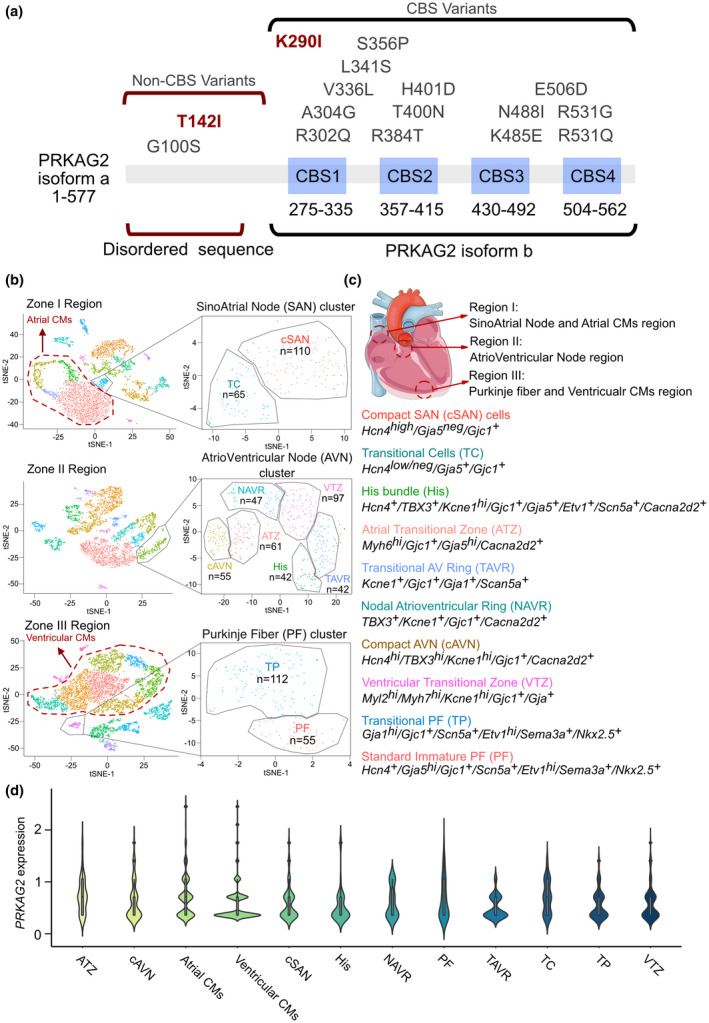
Using single‐cell RNA‐seq data (GSE132658), PRKAG2 was identified as being expressed both in CMs and conduction system cells. (a) Summary of current reports on individuals with PRKAG2 variants. (b) Cluster cell numbers and t‐distributed stochastic neighbor embedding (tSNE) plot for zone I, II, and III cells. (c) The isolated markers of conduction system‐related cells and the location of zone I, II, and III cells in the heart. (d) ViolinPlots of PRKAG2 expression in specific cell types in the heart
3.3. ScRNA‐seq analysis of PRKAG2 expression in the heart
PRKAG2 is involved in energy metabolism in heart tissue, which would affect glycogen storage. Moreover, the PRKAG2 variants affected both CMs and the conduction system, leading to hypertrophy or dilation cardiomyopathies and several types of conduction disorders, including pre‐exciting, bundle branch blocks, or AVBs. Therefore, it is important to understand expression of PRKAG2 among all kinds of cell types in the heart. Using the GSE132658 dataset, we isolated atrial CMs, ventricular CMs, and 10 kinds of conductive cells from SAN, AVN, and PF clusters (Figure 4b,c), and PRKAG2 expression was then measured in the 12 cell types. Similar expression levels of PRKAG2 were observed in CMs and the conduction system. This result illustrated the mechanisms of PRKAG2 variants, which affected both CM hemostasis and conduction system function.
4. DISCUSSION AND CONCLUSION
This study demonstrated two clinical cases having PRKAG2 genetic variants, c.524c > T in one and c.869A > T in the other. Both of them suffered severe adverse cardiac clinical manifestations, and onset of cardiac symptoms at a very young age. However, these two cases were characterized by totally different clinical manifestations, as Case 1 presented DCM with frequent ventricular pre‐mature beats, and Case 2 presented severe HCM with ventricular preexcitation, similar to other reported cases. We attempted to explore potential mechanisms affecting these cardiac phenotypes.
After reviewing all the published cases of PRKAG2, a case series of 175 patients was assembled and is summarized in Table 1. Among them, 172 cases presented with cardiomyopathy, and 174 with arrhythmias. All 172 cases were diagnosed as HCM or LVH according to echocardiography or cardiac MRI. Furthermore, 137 of the 174 patients with arrhythmias exhibited ventricular preexcitation. Such patients present a poor prognosis with a high rate of disorder in the conductive system at a young age, leading to ventricular preexcitation and high degree AVB, resistant heart failure, and severe hypertrophy of the left ventricle that induces obstruction of the outflow tract (Coban‐Akdemir et al., 2020; Jääskeläinen et al., 2019; Mo et al., 2019; Spentzou et al., 2020; Zhan et al., 2018). Moreover, syncope and sudden cardiac death have been observed in PRKAG2‐mutant patients, which constitute the dominate causes of lethality for PCS.
PRKAG2 encodes the AMPK γ2 subunit, regulating the function and activity of AMPK signaling in energy homeostasis. This gene has been associated with several transcripts and protein isoforms (Xie et al., 2016). Generally, there are two major transcripts: PRKAG2‐a, a full‐length transcript from 16 exons, encoding isoform a (569 AAs), and PRKAG2‐b, a truncated transcript from 12 exons, encoding isoform b (311 AAs). Both isoforms contain four identical consecutive CBS domains, a characteristic structure of the AMPK γ2 subunit, and two pairs of CBS domains located in tandem to form Bateman domains. The disruption of CBS domains would cause the impair enzymatic activity of AMPK. The impairment of AMPK would alter the expression of PGC‐1α, results in upregulation of gluconeogenesis by damaging mitochondrial biogenesis. So that, the variants of PRKAG2 in CBS domains would enhance the activity of AMPK which lead to mitochondrial oxidative metabolism, impaired glycolysis, increased glycogen synthase. Thus, the glycogen storage and related hypertrophic cardiomyocytes could be observed. The damaged mitochondrial function was associated with hyper‐activation of CaMKII by accumulation of reactive oxygen species (ROS) to generate abnormal calcium handling (Liu et al., 2022; Liu et al., 2021; Qi et al., 2022). So that, the variants of PRKAG2 were associated with cardiomyopathy and arrhythmia. A study from John et al., revealed that the four CBS domains in the γ2 subunit of AMPK form two allosteric binding sites for AMP and ATP. And variants within exons 5–16 could affect the activity of the tandem pairs of CBS domains and impair the energy sensor function of PRKAG2. Until now, more than 10 variants of PRKAG2 have been reported, including G100S, R302Q, V336L, S356P, R384T, H401D, K485E, N488I, E506D, R531Q, and R531G. Except for G100S, all the AA variants share common clinical presentations—ventricular preexcitation (or AVBs) and left ventricular hypertrophy (LVH)—and all could affect the activity of AMPK through its CBS domains (Hinson et al., 2016). Our report and analysis of Case 2 (K290I variant) also revealed a similar phenotype with ventricular preexcitation and LVH. And a sudden death occurred even after an ablation procedure to attenuate obstruction of the left ventricular outflow tract. Therefore, the CBS domains related to variants in PRKAG2 predict a stable cardiac presentation as LVH and arrhythmias, mainly for ventricular preexcitation. However, a well‐established molecular function of the non‐CBS domain, which is encoded by exons 1–4 and only exists in isoform a (full length transcript), is lacking. Only one disease‐causing variant, G100S (14 cases), has been reported in the non‐CBS domain (Austin et al., 2017; Zhang et al., 2013), revealing an important molecular function of the disordered non‐CBS sequence in maintaining normal functioning of PRKAG2. The study of Zhang et al. demonstrated that G100S in the non‐CBS domains has a similar but lesser effect than R302Q in the CBS domains (Zhang et al., 2013). This suggests that the mechanism by which G100S regulates AMPK activity via its non‐CBS domain may be consistent with that of R302Q in the CBS domains. The present study showed that non‐CBS variants lead to a decrease in AMPK activity and reduction of metabolic status and glycogen accumulation, which results in HCM. Furthermore, Scott et al. confirmed that the variant R302Q causes loss of enzymatic activity and revealed the mechanism to be reduced AMP binding (Scott et al., 2004). In vivo studies by Sidhu et al. showed that enzymatic activity of AMPK is significantly reduced, presumably owing to the variant disrupting the AMP binding site, and excessive cardiac glycogen is observed in the TG‐R302Q heart (Sidhu et al., 2005). Further in vivo studies by Davies et al. proposed that the R531G variant in the γ2 subunit leads to suppression of total cardiac AMPK activity secondary to increased glycogen accumulation in transgenic mice (Davies et al., 2006).
However, in our Case 1, the T142I variant is a non‐CBS variant that manifests as a rare DCM in PCS. This is the first case report to demonstrate a DCM patient related to a PRKAG2 variant. We used MutationTester, PolyPhen‐2, and SFIT to predict the impact of this variant on protein structure and function, showing that T142I is a diseasing‐causing and protein‐damaging variant. As there is no definitive crystal structure model for the full‐length isoform a, which contains the disordered sequence, we used AlphaFold with the AF‐Q9UGJ0‐F1 template to predict a structural model of the PRKAG2‐a protein. The T142 site is predicted to be located in a fold loop, and therefore may affect the formation of PRKAG2 transcripts. As the MutationTester tool predicted that the c.425C > T variant would affect its splicing site, T142 may induce RNA splicing to generate transcript‐b. More transcript‐b would in turn lead to more translation of tandem pairs of CBS domains and increased activity of AMPK. Activation of AMPK had been observed in two previous studies of the N488I variant early in the disease. Thus, we suspected T142I might induce a change opposite to that of other variants, leading to DCM. Due to the non‐CBS sequence being disordered, variants within this region might have variable effects on AMPK activity and lead to disparate reports in the literature.
Unlike HCM, individuals with PCS usually exhibit disorders in the heart's electrical conduction system, which require implantation of a pacemaker or ICD. So the question arises as to whether combined cardiac conduction disease is secondary to HCM or primary to a PRKAG2 variant. Taking advantage of scRNA‐seq, we assessed expression of specific genes in various cell types. Thus, we performed a re‐analysis of scRNA‐seq (GSE132658), and all conduction system‐related cells in the SAN cluster, AVN cluster and PF cluster were isolated, as well as atrial and ventricular CMs. After normalization, we found there was no significant difference of PRKAG2 expression between the conduction system cells and CMs. However, HCM is thought to be associated with disruption of sarcomeres or the cytoskeleton in CMs induced by variants in MYH7, TNNT2, MYBPC3 etc., which are mainly expressed in CMs. Therefore, the data from the scRNA‐seq analysis demonstrated a difference between PCS and typical HCM, and that conductive tissue diseases should be treated as a basis for PCS.
In summary, this is the first case report to underline a DCM phenotype in early‐onset PCS patients with a PRKAG2 c.425C > T (p.T142I) pathogenic variant. These results increase understanding of the molecular effects of non‐CBS domain variants, and suggest an explanation for controversial results concerning the role of the disordered sequence of transcript‐a (full‐length PRKAG2). Moreover, we used scRNA‐seq to show that conductive cells express a higher level of PRKAG2 than do CMs. Variants in PRKAG2 also alter the function of the conduction system. PCS‐related arrhythmia should therefore be recognized as a primary disorder, not secondary to hypertrophy.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
Xue Gong, Peiyu Yu and Ting Wu contributed equally to this work. He Huang and Tao Wang were the patient's physicians. Xue Gong and Yunru He reviewed the literature and contributed to manuscript drafting; Sha Lin performed the scRNA‐seq analysis. Yifei Li conceptualized and designed the study, coordinated and supervised data collection, and critically reviewed the manuscript for important intellectual content. Tao Wang, He Huang, and Yifei Li were responsible for the revision of the manuscript for important intellectual content; all authors issued final approval for the version to be submitted.
ETHICAL APPROVAL STATEMENT
This study was approved by the Ethics Committee of West China Second Hospital of Sichuan University (2014–034). And informed consent from the patient's parents prior to conducting the exome sequencing had been obtained, including the patient's clinical and imaging details in the manuscript for the purpose of publication.
ACKNOWLEDGMENT
This work was supported by grants from the National Natural Science Foundation of China (No. 81700360), Technology Project of Sichuan Province of China (2020YFS0102), Central Government Funds of Guiding Local Scientific and Technological Development for Sichuan Province (2021ZYD0105).
Gong, X. , Yu, P. , Wu, T. , He, Y. , Zhou, K. , Hua, Y. , Lin, S. , Wang, T. , Huang, H. , Li, Y. (2022). Controversial molecular functions of CBS versus non‐CBS domain variants of PRKAG2 in arrhythmia and cardiomyopathy: A case report and literature review. Molecular Genetics & Genomic Medicine, 10, e1962. 10.1002/mgg3.1962
Xue Gong, Peiyu Yu, and Ting Wu contributed equally to this article.
Contributor Information
Tao Wang, Email: 44871875@qq.com.
He Huang, Email: huanghe@wchscu.cn.
Yifei Li, Email: liyfwcsh@scu.edu.cn.
REFERENCES
- Akman, H. O. , Sampayo, J. N. , Ross, F. A. , Scott, J. W. , Wilson, G. , Benson, L. , Bruno, C. , Shanske, S. , Hardie, D. G. , & Dimauro, S. (2007, October). Fatal infantile cardiac glycogenosis with phosphorylase kinase deficiency and a mutation in the gamma2‐subunit of AMP‐activated protein kinase. Pediatric Research, 62(4), 499–504. 10.1203/PDR.0b013e3181462b86 [DOI] [PubMed] [Google Scholar]
- Arad, M. , Benson, D. W. , Perez‐Atayde, A. R. , McKenna, W. J. , Sparks, E. A. , Kanter, R. J. , McGarry, K. , Seidman, J. G. , & Seidman, C. E. (2002, February). Constitutively active AMP kinase mutations cause glycogen storage disease mimicking hypertrophic cardiomyopathy. Journal of Clinical Investigation, 109(3), 357–362. 10.1172/JCI14571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arad, M. , Moskowitz, I. P. , Patel, V. V. , Ahmad, F. , Perez‐Atayde, A. R. , Sawyer, D. B. , Walter, M. , Li, G. H. , Burgon, P. G. , Maguire, C. T. , Stapleton, D. , Schmitt, J. P. , Guo, X. X. , Pizard, A. , Kupershmidt, S. , Roden, D. M. , Berul, C. I. , Seidman, C. E. , & Seidman, J. G. (2003). Transgenic mice overexpressing mutant PRKAG2 define the cause of Wolff‐Parkinson‐white syndrome in glycogen storage cardiomyopathy. Circulation, 107(22), 2850–2856. 10.1161/01.Cir.0000075270.13497.2b [DOI] [PubMed] [Google Scholar]
- Austin, S. L. , Chiou, A. , Sun, B. , Case, L. E. , Govendrageloo, K. , Hansen, P. , & Kishnani, P. S. (2017). Alglucosidase alfa enzyme replacement therapy as a therapeutic approach for a patient presenting with a PRKAG2 mutation. Molecular Genetics and Metabolism, 120(1–2), 96–100. 10.1016/j.ymgme.2016.09.006 [DOI] [PubMed] [Google Scholar]
- Back Sternick, E. , de Almeida, A. S. , Ribeiro da Silva Camargos, E. , & Brasileiro Filho, G. (2016, December). Atrial pathology findings in a patient with PRKAG2 cardiomyopathy and persistent atrial fibrillation. Circulation: Arrhythmia and Electrophysiology, 9(12), e004455. 10.1161/CIRCEP.116.004455 [DOI] [PubMed] [Google Scholar]
- Banankhah, P. , Fishbein, G. A. , Dota, A. , & Ardehali, R. (2018). Cardiac manifestations of PRKAG2 mutation. BMC Medical Genetics, 19(1), 1. 10.1186/s12881-017-0512-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee, S. K. , McGaffin, K. R. , Huang, X. N. , & Ahmad, F. (2010). Activation of cardiac hypertrophic signaling pathways in a transgenic mouse with the human PRKAG2 Thr400Asn mutation. Biochimica et Biophysica Acta, 1802(2), 284–291. 10.1016/j.bbadis.2009.12.001 [DOI] [PubMed] [Google Scholar]
- Bayrak, F. , Komurcu‐Bayrak, E. , Mutlu, B. , Kahveci, G. , Basaran, Y. , & Erginel‐Unaltuna, N. (2006). Ventricular pre‐excitation and cardiac hypertrophy mimicking hypertrophic cardiomyopathy in a Turkish family with a novel PRKAG2 mutation. European Journal of Heart Failure, 8(7), 712–715. 10.1016/j.ejheart.2006.03.006 [DOI] [PubMed] [Google Scholar]
- Beyzaei, Z. , Ezgu, F. , Geramizadeh, B. , Alborzi, A. , & Shojazadeh, A. (2021). Novel PRKAG2 variant presenting as liver cirrhosis: Report of a family with 2 cases and review of literature. BMC Medical Genomics, 14(1), 33. 10.1186/s12920-021-00879-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burwinkel, B. , Scott, J. W. , Bührer, C. , van Landeghem, F. K. , Cox, G. F. , Wilson, C. J. , Grahame Hardie, D. , & Kilimann, M. W. (2005, June). Fatal congenital heart glycogenosis caused by a recurrent activating R531Q mutation in the gamma 2‐subunit of AMP‐activated protein kinase (PRKAG2), not by phosphorylase kinase deficiency. American Journal of Human Genetics, 76(6), 1034–1049. 10.1086/430840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, Y. , Bojjireddy, N. , Kim, M. , Li, T. , Zhai, P. , Nagarajan, N. , Sadoshima, J. , Palmiter, R. D. , & Tian, R. (2017). Activation of γ2‐AMPK suppresses ribosome biogenesis and protects against myocardial ischemia/reperfusion injury. Circulation Research, 121(10), 1182–1191. 10.1161/circresaha.117.311159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charron, P. , Genest, M. , Richard, P. , Komajda, M. , & Pochmalicki, G. (2007, August). A familial form of conduction defect related to a mutation in the PRKAG2 gene. Europace, 9(8), 597–600. 10.1093/europace/eum071 [DOI] [PubMed] [Google Scholar]
- Coban‐Akdemir, Z. H. , Charng, W. L. , Azamian, M. , Paine, I. S. , Punetha, J. , Grochowski, C. M. , Gambin, T. , Valdes, S. O. , Cannon, B. , Zapata, G. , Hernandez, P. P. , Jhangiani, S. , Doddapaneni, H. , Hu, J. , Boricha, F. , Muzny, D. M. , Boerwinkle, E. , Yang, Y. , Gibbs, R. A. , … Lalani, S. R. (2020). Wolff‐Parkinson‐white syndrome: De novo variants and evidence for mutational burden in genes associated with atrial fibrillation. American Journal of Medical Genetics. Part A, 182(6), 1387–1399. 10.1002/ajmg.a.61571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies, J. K. , Wells, D. J. , Liu, K. , Whitrow, H. R. , Daniel, T. D. , Grignani, R. , Lygate, C. A. , Schneider, J. E. , Noël, G. , Watkins, H. , & Carling, D. (2006). Characterization of the role of gamma2 R531G mutation in AMP‐activated protein kinase in cardiac hypertrophy and Wolff‐Parkinson‐white syndrome. American Journal of Physiology. Heart and Circulatory Physiology, 290(5), H1942–H1951. 10.1152/ajpheart.01020.2005 [DOI] [PubMed] [Google Scholar]
- Gollob, M. H. , Green, M. S. , Tang, A. S. , Gollob, T. , Karibe, A. , Ali Hassan, A. S. , Ahmad, F. , Lozado, R. , Shah, G. , Fananapazir, L. , Bachinski, L. L. , & Roberts, R. (2001). Identification of a gene responsible for familial Wolff‐Parkinson‐white syndrome. The New England Journal of Medicine, 344(24), 1823–1831. 10.1056/nejm200106143442403 [DOI] [PubMed] [Google Scholar]
- Gollob, M. H. , Seger, J. J. , Gollob, T. N. , Tapscott, T. , Gonzales, O. , Bachinski, L. , & Roberts, R. (2001). Novel PRKAG2 mutation responsible for the genetic syndrome of ventricular preexcitation and conduction system disease with childhood onset and absence of cardiac hypertrophy. Circulation, 104(25), 3030–3033. 10.1161/hc5001.102111 [DOI] [PubMed] [Google Scholar]
- Goodyer, W. R. , Beyersdorf, B. M. , Paik, D. T. , Tian, L. , Li, G. , Buikema, J. W. , Chirikian, O. , Choi, S. , Venkatraman, S. , Adams, E. L. , Tessier‐Lavigne, M. , Wu, J. C. , & Wu, S. M. (2019). Transcriptomic profiling of the developing cardiac conduction system at single‐cell resolution. Circulation Research, 125(4), 379–397. 10.1161/circresaha.118.314578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorla, S. R. , Raja, K. R. , Garg, A. , Barbouth, D. S. , & Rusconi, P. G. (2018, December). Infantile onset hypertrophic cardiomyopathy secondary to PRKAG2 gene mutation is associated with poor prognosis. Journal of Pediatric Genetics, 7(4), 180–184. 10.1055/s-0038-1657763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinson, J. T. , Chopra, A. , Lowe, A. , Sheng, C. C. , Gupta, R. M. , Kuppusamy, R. , O'Sullivan, J. , Rowe, G. , Wakimoto, H. , Gorham, J. , Burke, M. A. , Zhang, K. , Musunuru, K. , Gerszten, R. E. , Wu, S. M. , Chen, C. S. , Seidman, J. G. , & Seidman, C. E. (2016). Integrative analysis of PRKAG2 cardiomyopathy iPS and microtissue models identifies AMPK as a regulator of metabolism, survival, and fibrosis. Cell Reports, 17(12), 3292–3304. 10.1016/j.celrep.2016.11.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, D. , Hu, D. , Liu, L. , Barr, D. , Liu, Y. , Balderrabano‐Saucedo, N. , Wang, B. , Zhu, F. , Xue, Y. , Wu, S. , Song, B. , McManus, H. , Murphy, K. , Loes, K. , Adler, A. , Monserrat, L. , Antzelevitch, C. , Gollob, M. H. , Elliott, P. M. , & Barajas‐Martinez, H. (2020, April). Identification, clinical manifestation and structural mechanisms of mutations in AMPK associated cardiac glycogen storage disease. EBioMedicine, 54, 102723. 10.1016/j.ebiom.2020.102723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jääskeläinen, P. , Vangipurapu, J. , Raivo, J. , Kuulasmaa, T. , Heliö, T. , Aalto‐Setälä, K. , Kaartinen, M. , Ilveskoski, E. , Vanninen, S. , Hämäläinen, L. , Melin, J. , Kokkonen, J. , Nieminen, M. S. , FinHCM Study Group , Laakso, M. , & Kuusisto, J. (2019). Genetic basis and outcome in a nationwide study of Finnish patients with hypertrophic cardiomyopathy. ESC Heart Failure, 6(2), 436–445. 10.1002/ehf2.12420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly, B. P. , Russell, M. W. , Hennessy, J. R. , & Ensing, G. J. (2009, November). Severe hypertrophic cardiomyopathy in an infant with a novel PRKAG2 gene mutation: potential differences between infantile and adult onset presentation. Pediatric Cardiology, 30(8), 1176–1179. 10.1007/s00246-009-9521-3 [DOI] [PubMed] [Google Scholar]
- Laforêt, P. , Richard, P. , Said, M. A. , Romero, N. B. , Lacene, E. , Leroy, J. P. , Baussan, C. , Hogrel, J. Y. , Lavergne, T. , Wahbi, K. , Hainque, B. , & Duboc, D. (2006, March). A new mutation in PRKAG2 gene causing hypertrophic cardiomyopathy with conduction system disease and muscular glycogenosis. Neuromuscular Disorders, 16(3), 178–182. 10.1016/j.nmd.2005.12.004 [DOI] [PubMed] [Google Scholar]
- Liu, Y. , Bai, R. , Wang, L. , Zhang, C. , Zhao, R. , Wan, D. , Chen, X. , Caceres, G. , Barr, D. , Barajas‐Martinez, H. , Antzelevitch, C. , & Hu, D. (2013, May 31). Identification of a novel de novo mutation associated with PRKAG2 cardiac syndrome and early onset of heart failure. PLoS One, 8(5), e64603. 10.1371/journal.pone.0064603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, L. , Yue, P. , Zhang, Y. , Hua, Y. , Bi, W. , Yan, H. , Liao, H. , Li, J. , Zhou, K. , & Li, Y. (2022). Non‐cell‐autonomous manner of AAV administration to attenuate cardiomyocyte hypertrophy by targeting paracrine signaling on ECM to reduce viral dosage. Signal Transduction and Targeted Therapy, 7(1), 2. 10.1038/s41392-021-00715-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X. , Wang, S. , Guo, X. , Li, Y. , Ogurlu, R. , Lu, F. , Prondzynski, M. , de la Serna Buzon, S. , Ma, Q. , Zhang, D. , Wang, G. , Cotton, J. , Guo, Y. , Xiao, L. , Milan, D. J. , Xu, Y. , Schlame, M. , Bezzerides, V. J. , & Pu, W. T. (2021). Increased reactive oxygen species‐mediated ca(2+)/calmodulin‐dependent protein kinase II activation contributes to calcium handling abnormalities and impaired contraction in Barth syndrome. Circulation, 143(19), 1894–1911. 10.1161/circulationaha.120.048698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez‐Sainz, A. , Dominguez, F. , Lopes, L. R. , Ochoa, J. P. , Barriales‐Villa, R. , Climent, V. , Linschoten, M. , Tiron, C. , Chiriatti, C. , Marques, N. , Rasmussen, T. B. , Espinosa, M. Á. , Beinart, R. , Quarta, G. , Cesar, S. , Field, E. , Garcia‐Pinilla, J. M. , Bilinska, Z. , Muir, A. R. , … European Genetic Cardiomyopathies Initiative Investigators . (2020). Clinical features and natural history of PRKAG2 variant cardiac glycogenosis. Journal of the American College of Cardiology, 76(2), 186–197. 10.1016/j.jacc.2020.05.029 [DOI] [PubMed] [Google Scholar]
- Maron, B. J. , & Maron, M. S. (2020). PRKAG2 glycogen storage disease cardiomyopathy: Out of the darkness and into the light. Journal of the American College of Cardiology, 76(2), 198–200. 10.1016/j.jacc.2020.05.054 [DOI] [PubMed] [Google Scholar]
- Mo, X. , Zhang, H. , Zhou, Z. , Zhu, Z. , HuangFu, X. , Xu, T. , Wang, A. , Guo, Z. , & Zhang, Y. (2019). SNPs rs10224002 in PRKAG2 may disturb gene expression and consequently affect hypertension. Molecular Biology Reports, 46(2), 1617–1624. 10.1007/s11033-019-04610-3 [DOI] [PubMed] [Google Scholar]
- Murphy, R. T. , Mogensen, J. , McGarry, K. , Bahl, A. , Evans, A. , Osman, E. , Syrris, P. , Gorman, G. , Farrell, M. , Holton, J. L. , Hanna, M. G. , Hughes, S. , Elliott, P. M. , Macrae, C. A. , & McKenna, W. J. (2005, March 15). Adenosine monophosphate‐activated protein kinase disease mimicks hypertrophic cardiomyopathy and Wolff‐Parkinson‐White syndrome: natural history. Journal of the American College of Cardiology, 45(6), 922–930. 10.1016/j.jacc.2004.11.053 [DOI] [PubMed] [Google Scholar]
- Pena, J. L. B. , Santos, W. C. , Siqueira, M. H. A. , Sampaio, I. H. , Moura, I. C. G. , & Sternick, E. B. (2021). Glycogen storage cardiomyopathy (PRKAG2): Diagnostic findings of standard and advanced echocardiography techniques. European Heart Journal Cardiovascular Imaging, 22(7), 800–807. 10.1093/ehjci/jeaa176 [DOI] [PubMed] [Google Scholar]
- Qi, Y. , Ye, Y. , Wang, R. , Yu, S. , Zhang, Y. , Lv, J. , Jin, W. , Xia, S. , Jiang, W. , Li, Y. , & Zhang, D. (2022). Mitochondrial dysfunction by TFAM depletion disrupts self‐renewal and lineage differentiation of human PSCs by affecting cell proliferation and YAP response. Redox Biology, 50, 102248. 10.1016/j.redox.2022.102248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, J. D. , Veinot, J. P. , Rutberg, J. , & Gollob, M. H. (2010, September–October). Inherited cardiomyopathies mimicking arrhythmogenic right ventricular cardiomyopathy. Cardiovascular Pathology, 19(5), 316–320. 10.1016/j.carpath.2009.06.003 [DOI] [PubMed] [Google Scholar]
- Scott, J. W. , Hawley, S. A. , Green, K. A. , Anis, M. , Stewart, G. , Scullion, G. A. , Norman, D. G. , & Hardie, D. G. (2004). CBS domains form energy‐sensing modules whose binding of adenosine ligands is disrupted by disease mutations. The Journal of Clinical Investigation, 113(2), 274–284. 10.1172/jci19874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu, J. S. , Rajawat, Y. S. , Rami, T. G. , Gollob, M. H. , Wang, Z. , Yuan, R. , & Roberts, R. (2005). Transgenic mouse model of ventricular preexcitation and atrioventricular reentrant tachycardia induced by an AMP‐activated protein kinase loss‐of‐function mutation responsible for Wolff‐Parkinson‐white syndrome. Circulation, 111(1), 21–29. 10.1161/01.Cir.0000151291.32974.D5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spentzou, G. , McGowan, R. , Hares, D. , & McLeod, K. (2020). Novel PRKAG2 variant manifesting with a cardiac arrest in a child. Pediatric Cardiology, 41(4), 843–845. 10.1007/s00246-019-02245-6 [DOI] [PubMed] [Google Scholar]
- Sri, A. , Daubeney, P. , Prasad, S. , Baksi, J. , Kinali, M. , & Voges, I. (2019, March 26). A case series on cardiac and skeletal involvement in two families with PRKAG2 mutations. Case Reports in Pediatrics, 2019, 7640140. 10.1155/2019/7640140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Steld, L. P. , Campuzano, O. , Pérez‐Serra, A. , de Barros, M. , Zamorano, M. , Sousa Matos, S. , & Brugada, R. (2017). Wolff‐Parkinson‐white syndrome with ventricular hypertrophy in a Brazilian family. American Journal of Case Reports, 18, 766–776. 10.12659/ajcr.904613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, C. , Zhang, Y. P. , Song, L. , Luo, J. , Qi, W. , Hu, J. , Lu, D. , Yang, Z. , Zhang, J. , Xiao, J. , Zhou, B. , du, J. L. , Jing, N. , Liu, Y. , Wang, Y. , Li, B. L. , Song, B. L. , & Yan, Y. (2016). Genome editing with CRISPR/Cas9 in postnatal mice corrects PRKAG2 cardiac syndrome. Cell Research, 26(10), 1099–1111. 10.1038/cr.2016.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, K. Q. , Lu, C. X. , Zhang, Y. , Yang, Y. K. , Li, J. C. , Lan, T. , Meng, X. , Fan, P. , Tian, T. , Wang, L. P. , Liu, Y. X. , Zhang, X. , & Zhou, X. L. (2017, May 25). A novel PRKAG2 mutation in a Chinese family with cardiac hypertrophy and ventricular pre‐excitation. Scientific Reports, 7(1), 2407. 10.1038/s41598-017-02455-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan, Y. , Sun, X. , Li, B. , Cai, H. , Xu, C. , Liang, Q. , Lu, C. , Qian, R. , Chen, S. , Yin, L. , Sheng, W. , Huang, G. , Sun, A. , Ge, J. , & Sun, N. (2018). Establishment of a PRKAG2 cardiac syndrome disease model and mechanism study using human induced pluripotent stem cells. Journal of Molecular and Cellular Cardiology, 117, 49–61. 10.1016/j.yjmcc.2018.02.007 [DOI] [PubMed] [Google Scholar]
- Zhang, B. L. , Xu, R. L. , Zhang, J. , Zhao, X. X. , Wu, H. , Ma, L. P. , Hu, J. Q. , Zhang, J. L. , Ye, Z. , Zheng, X. , & Qin, Y. W. (2013). Identification and functional analysis of a novel PRKAG2 mutation responsible for Chinese PRKAG2 cardiac syndrome reveal an important role of non‐CBS domains in regulating the AMPK pathway. Journal of Cardiology, 62(4), 241–248. 10.1016/j.jjcc.2013.04.010 [DOI] [PubMed] [Google Scholar]


