Abstract
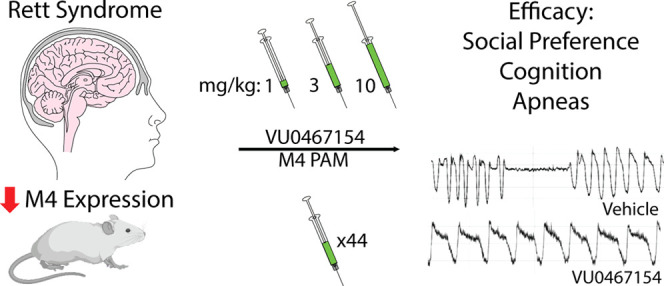
Hypofunction of cholinergic circuits and diminished cholinergic tone have been associated with the neurodevelopmental disorder Rett syndrome (RTT). Specifically, deletion of Mecp2 in cholinergic neurons evokes the same social and cognitive phenotypes in mice seen with global Mecp2 knockout, and decreased choline acetyltransferase activity and vesamicol binding have been reported in RTT autopsy samples. Further, we recently identified significant decreases in muscarinic acetylcholine receptor subtype 4 (M4) expression in both the motor cortex and cerebellum of RTT patient autopsies and established proof of concept that an acute dose of the positive allosteric modulator (PAM) VU0467154 (VU154) rescued phenotypes in Mecp2+/– mice. Here, we expand the assessment of M4 PAMs in RTT to address clinically relevant questions of tolerance, scope of benefit, dose response, chronic treatment, and mechanism. We show that VU154 has efficacy on anxiety, social preference, cognitive, and respiratory phenotypes in Mecp2+/– mice; however, the therapeutic range is narrow, with benefits seen at 3 mg/kg concentrations, but not 1 or 10 mg/kg. Further, sociability was diminished in VU154-treated Mecp2+/– mice, suggestive of a potential adverse effect. Compound efficacy on social, cognitive, and respiratory phenotypes was conserved with a 44-day treatment paradigm, with the caveat that breath rate was moderately decreased with chronic treatment in Mecp2+/+ and Mecp2+/– mice. VU154 effects on respiratory function correlated with an increase in Gsk3β inhibition in the brainstem. These results identify the core symptom domains where efficacy and adverse effects may present with M4 administration in RTT model mice and advocate for the continued evaluation as potential RTT therapeutics.
Keywords: Rett syndrome, brainstem, apneas, tolerance, M4 mAChR, Gsk3β
Introduction
Rett syndrome (RTT) is a neurodevelopmental disorder that is characterized by a myriad of symptoms that include developmental regression, loss of communicative ability, stereotyped hand movements, gross motor impairment, and apneas.1,2 The overwhelming majority of RTT cases are caused by loss-of-function mutations in the methyl CpG binding protein 2 (MECP2) gene,3 which encodes a methyl-reader protein that modifies chromatin structure to regulate transcription both locally and globally.4,5 There are currently only limited treatment options for individuals with RTT; however, recent discovery efforts have led to optimism that effective strategies may emerge in the near future.6,7
Historically, therapeutic development for RTT has followed the same path as other neurodevelopmental disorders, where targets are identified and optimized in mouse models. While not invaluable, given the high failure rate of promising compounds to translate into effective therapeutics,8 we recently adopted a reverse-translational approach to RTT target identification.9,10 Differential RNA-sequencing (seq) analysis of nine motor cortex and six cerebellar samples from RTT patient autopsies compared relative to matched controls identified disruption of muscarinic acetylcholine receptors (mAChRs) as a conserved aspect of the disease.9 Further, we demonstrated that selective targeting of the mAChR subtype 4 (M4) with the positive allosteric modulator VU0467154 (VU154) improved social and cognitive phenotypes in Mecp2+/– model mice. The finding of decreased mAChR levels aligns with well-established literature showing hypocholenergic tone in RTT patients and rodent models,11−13 as well as experiments demonstrating that methods of increasing cholinergic tone (dietary choline, donepezil, etc.) rescue social and cognitive phenotypes in mice.14−18
mAChRs are a class of G-protein coupled receptors (GPCRs) that are enriched in brain regions commonly associated with neurological disorders.19 The M4 receptor is located at both pre- and post-synaptic sites, where it negatively regulates adenyl cyclase activity via coupling to Gi/o signaling. M4 expression is enriched in forebrain structures and the striatum; however, broad expression is also observed in other brain regions relevant to RTT that include the hippocampus, cerebellar cortex, and brainstem.20−23 In support of its critical role in neurological function, M4 knockout mice show impaired social interactions, disruptions in sensory-motor gating, and hyperlocomotion,24 and pharmaceutical targeting of the M4 receptor rescues analogous symptom domains in mouse models of schizophrenia, Alzheimer’s disease, and Huntington’s disease.25−27
While encouraging, our proof-of-concept studies showing M4 PAM efficacy in Mecp2+/– mice did not address several questions central to its value as a therapeutic candidate for RTT.9 Specifically, the use of a single concentration and an acute dose sheds little light on the effective therapeutic range of the compound, the potential for the development of tolerance in RTT model mice, the adverse effect profile, or the full scope of phenotypes impacted by this approach. Since RTT is a lifelong condition with a broad spectrum of phenotypes, these represent salient questions to determining the value of M4 potentiation as a therapeutic strategy. Here, we perform dose–response and chronic administration experiments, coupled with comprehensive phenotypic profiling and molecular analysis to fill these critical knowledge gaps. Our data demonstrate that repeat administration of VU154 does not evoke a tolerance response, but that the range of effective concentrations is narrow. Repeat administration experiments showed efficacy in symptom domains involving social preference, spatial memory, and respiratory phenotypes while identifying hypolocomotion and diminished sociability as potential adverse effects in Mecp2+/– mice. Our data support the potential of M4 PAMs as RTT therapeutics but advocate for the continued development of compounds with a broader therapeutic range, as well as the careful monitoring for adverse effects.
Results and Discussion
Repeated M4 PAM Administration Does Not Impact M4 Expression
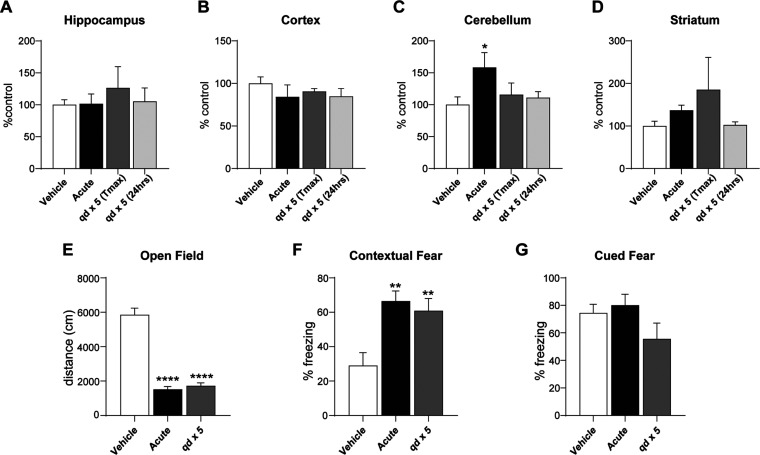
Several studies suggest that repeated potentiation does not desensitize the M4 receptor;27,28 however, it remains unknown whether additive changes in expression-based tolerance will present with sustained use.29,30 To determine whether M4-potentiation activates feedback mechanisms to regulate its own expression, we administered 10 mg/kg of VU154 (i.p) to wild-type mice either in an acute or 1 × 5 days paradigm. We then harvested the hippocampus, cortex, cerebellum, and striatum, isolated cDNA, and quantified M4 mRNA levels at Tmax (30 min),31 as well as after the compound had been cleared (24 h post-dose). As shown in Figure 1A–D, we observed no significant decrease in M4 expression with either treatment paradigm as would be predicted with a tolerance response, independent of the time point examined. A significant increase in M4 expression was observed in the cerebellum with a single dose of VU154; however, this finding was not sustained with repeat administration (Figure 1C). Before sacrifice (48 h), we also explored the functional consequences of repeat administration using two assays in which M4 PAMs consistently show efficacy: open field and fear conditioning. Consistent with previous data,28,32 acute treatment with VU154 significantly decreased spontaneous locomotion in the open field, and the results of daily administration of VU154 paralleled this finding (Figure 1E). Similar results were obtained in contextual fear (CF) conditioning, an assay of associative learning where time spent freezing in a previously aversive environment or in response to an associated-audio cue is a measure of cognition. In this assay, both acute and repeat administration of VU154 significantly increased freezing behavior, while having no impact on cued conditioning (Figure 1F,G). With the caveat that behavioral assays can alter gene expression in some contexts, these data confirm previous reports suggesting that daily administration of a single dose of VU154 does not evoke changes in M4 expression or functional tolerance responses.28
Figure 1.
Repeat administration of the M4 PAM VU0467154 (VU154) does not impact M4 expression or evoke tolerance to behavioral effects. N = 5 wild-type C57B6 female mice/treatment group. Comparisons are relative to acute vehicle treatment. (A–D) qRT-PCR. Test mice were administered vehicle or 10 mg/kg VU154, either once (acute) or once daily (qd) for 5 consecutive days, with tissue harvest at Tmax (30 min) or 24 h following the final dose. qRT-PCR of M4 mRNA shows no significant change in expression following M4 potentiation, with the exception of the cerebellum, where a transient increase was quantified following an acute dose. (E) Open Field. VU154’s ability to decrease spontaneous locomotion was not impacted by repeat dosing. (F, G) Fear conditioning. M4 potentiation with VU154 significantly increased contextual fear freezing behavior in both acute and repeat administration paradigms while having no impact on cued fear conditioning. One-way ANOVA with Tukey post hoc analysis. *p < 0.05, **p < 0.01, ****p < 0.0001. Data are expressed as mean ± SEM.
Dose Response of VU154 in Mecp2+/– Mice
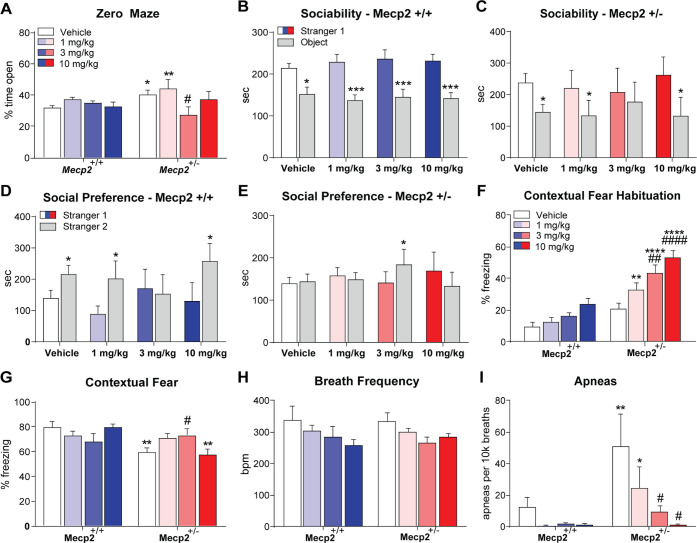
Our initial study examining VU154 efficacy in RTT model mice used a single 3 mg/kg concentration to show efficacy in social and cognitive phenotypes.9 To optimize dose in a model RTT with face and construct validity,7 we treated groups of Mecp2+/+ and Mecp2+/– mice (20w, N = 10–12/group) with either vehicle (10% tween 80) or a 1, 3, or 10 mg/kg dose of VU154. We then progressed mice through a phenotypic battery encompassing the major symptom domains of RTT, including elevated zero maze (anxiety), three-chamber social interaction (sociability, social preference), contextual fear conditioning (associative memory), and whole-body plethysmography (respiratory function). A minimum of a 5-day drug washout period was provided between assays.
In the elevated zero maze (EZM), vehicle-treated Mecp2+/– mice spent significantly more time in the open regions relative to Mecp2+/+ controls (Figure 2A). VU154 administration significantly normalized this phenotype a 3 mg/kg; however, no efficacy was observed at the 1 or 10 mg/kg doses, suggestive of a potentially narrow U-shaped range of effective concentrations.
Figure 2.
VU154 administration corrects multiple RTT phenotypes with a narrow therapeutic range. N = 10–12 mice/treatment/genotype; *denotes comparison to vehicle-treated Mecp2+/+ mice, # denotes comparison to vehicle-treated Mecp2+/– mice (A) Elevated Zero Maze. Relative to vehicle-treated Mecp2+/+ mice, vehicle-treated Mecp2+/– mice spent significantly more time in the open areas of the maze. Administration of 3 mg/kg VU154 reversed this phenotype; however, efficacy was not observed at 10 mg/kg. (B–E) Social Interaction Assay. (B, C) Sociability was not impacted by treatment or genotype, with the notable exception of 3 mg/kg VU154, which diminished sociability in Mecp2+/– mice. (D) Mecp2+/+ control mice showed a preference for the novel stranger (stranger 2) with the administration of vehicle, 1, and 10 mg/kg VU154; however, social preference was disrupted by the 3 mg/kg dose of VU154. (E) Mecp2+/– mice treated with vehicle, 1, or 10 mg/kg VU154 do not show a preference for the familiar (stranger 1) or novel stranger mouse. Administration of the 3 mg/kg dose of VU154 before the start of the assay significantly restored social preference in Mecp2+/– mice. (F, G) Contextual fear conditioning. When administered prior to the assay (day 1), VU154 significantly increased freezing behavior during the training phase. This finding did not impact efficacy on cognitive phenotypes on test day (day 2), where 3 mg/kg VU154 rescued impaired freezing behavior. (H, I) Whole-body plethysmography. No significant effect of genotype or treatment was observed on breath frequency. Conversely, vehicle-treated Mecp2+/– presented with a significant number of apneas, which was diminished to baseline in a dose-dependent manner with 3 and 10 mg/kg VU154 administration. Two-way ANOVA with Tukey post hoc. *p < 0.05, **p < 0.01, ****p < 0.0001. Data are expressed as mean ± SEM.
We next performed the three-chamber social interaction assay, which tests sociability (phase I) as a function of preference for a stranger 1 mouse over an empty cup, and social preference (phase II) by assessing the time spent with a novel mouse (stranger 2) relative to a familiar one (stranger 1). In phase I, Mecp2+/+ controls spent significantly more time with the mouse relative to the cup, and this was also true for Mecp2+/– mice treated with vehicle, 1, and 10 mg/kg VU154 (Figure 2B,C). Conversely, Mecp2+/– mice treated with 3 mg/kg did not show preference, indicative of diminished sociability (Figure 2C). As we previously reported, a 3 mg/kg dose of VU154 disrupted social preference (phase II) in Mecp2+/+ controls; however, this was not observed with the 1 or 10 mg/kg dose (Figure 2D). Similar to EZM, the 3 mg/kg dose of VU154 rescued social preference in Mecp2+/– mice, while the 1 and 10 mg/kg concentrations did not show efficacy (Figure 2E).
The contextual fear conditioning assay uses a freezing response following re-exposure to an aversive environment as an outcome measure for associative memory. Consistent with VU154’s well-characterized effects on spontaneous locomotion,28 we quantified significant increases in stagnant/freezing behavior during the preshock habituation phase of training day in Mecp2+/+ at 10 mg/kg, and in Mecp2+/– mice at 3 and 10 mg/kg (Figure 2F). Hypolocomotion following M4 potentiation is believed to be an exclusively motor phenotype linked to its role in the striatum33 and it does not appear to alter the pro-cognitive effects of M4 PAMs.28 Since the compound is not administered on test day in contextual fear conditioning, motor effects also do not impact the use of freezing in the assessment of fear memory. In this assay, no effect of dose was observed on freezing behavior in Mecp2+/+ control mice (Figure 2G). Consistent with previous reports, vehicle-treated Mecp2+/– mice showed reduced freezing behavior relative to controls, and this was significantly increased by 3 mg/kg VU154 administration (Figure 2G). As was true with EZM and SI, no efficacy was observed at the 1 and 10 mg/kg doses.
The final phenotypic assay we tested was whole-body plethysmography (WBP), which measures respiratory function in awake, freely moving mice. In the RTT field, WBP is used to quantify apneas, which represent a significant health concern for patients that is faithfully replicated in model mice. Following VU154 treatment, we observed a dose-dependent trend toward decreased breath frequency in both Mecp2+/+ and Mecp2+/– mice (Figure 2H). While this failed to reach statistical significance, a decrease in the number of breaths equates to a decrease in opportunities for apneas to present. To account for this variable, the number of quantified apneas was assessed per 10k breaths over the 30 min testing period. Following normalization, we quantified a substantial number of apneas in vehicle-treated Mecp2+/– mice, which showed a significant and dose-dependent reversal following VU154 administration (Figure 2I).
In summary, VU154 treatment rescues anxiety, social preference, associative memory, and respiratory phenotypes in Mecp2+/– mice. However, there appears to be a narrow bell curve response pattern where efficacy is observed at 3 mg/kg, but not 1 or 10 mg/kg.
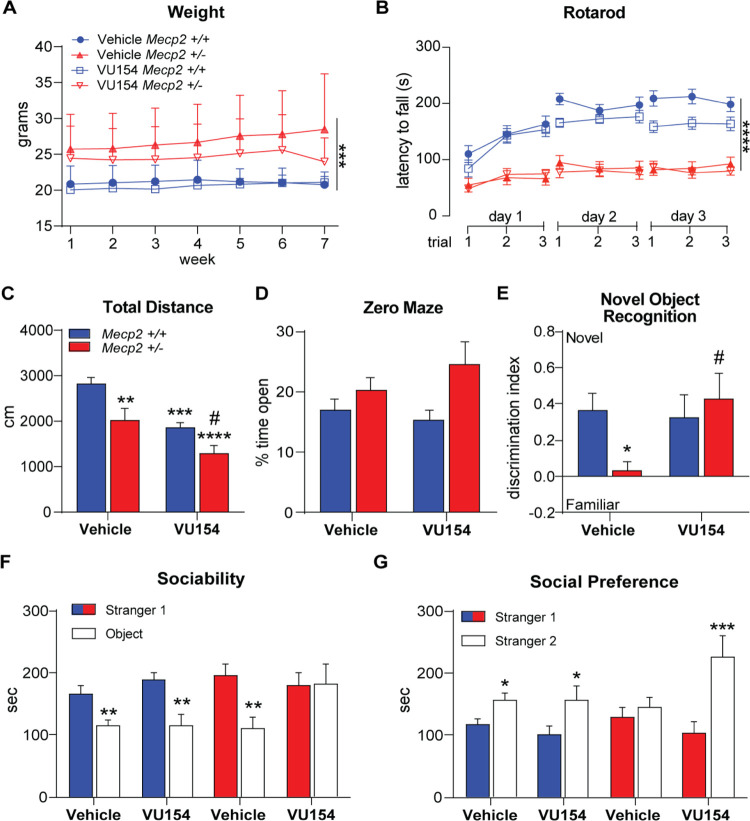
Chronic VU154 Treatment in Mecp2+/– Mice
With advancements in diagnosis and disease management, RTT patients often survive for many decades, and thus potential treatments must remain safe and effective over long periods of time. To assess VU154’s efficacy in a chronic treatment paradigm in RTT mice, 20-week-old Mecp2+/+ and Mecp2+/– mice were treated 1× daily (i.p., 3 mg/kg) for 44 days and progressed through a similar phenotypic battery as described above. Treatment began 21 days before phenotyping to determine whether additive effects of repeat administration would present, and relevant brain regions were harvested 48 h following the final assay and 30 min following the final dose of VU154. Phenotypic assays were selected to mirror and expand upon the motor (rotarod, open field), anxiety (open field, EZM), cognitive (novel object recognition, contextual fear conditioning), social (social interaction assay), and respiratory (WBP) symptom domains that were sensitive to M4 potentiation in dose–response experiments. Weights were taken weekly. As shown in Figure 3A, Mecp2+/– mice weighed significantly more than Mecp2+/+ mice throughout the experiment, and VU154 treatment did not affect the weight or general appearance (not shown).
Figure 3.
Chronic VU154 dosing reverses RTT-like neurological phenotypes in female Mecp2+/– mice. N = 14–16/genotype/treatment. 44-day chronic administration (3 mg/kg or vehicle, qd); * denotes comparison to vehicle-treated Mecp2+/+ mice, # denotes comparison to vehicle-treated Mecp2+/– mice. (A) Increased weight gain was observed in Mecp2+/– mice compared to Mecp2+/+ mice but was not significantly affected by repeat dosing of VU154. (B) Latency to fall from an accelerating rotarod over 3 days was decreased in Mecp2+/– mice but was not impacted by treatment. (C) Vehicle-treated Mecp2+/+ mice explored significantly more than vehicle-treated Mecp2+/– mice and chronic VU154 treatment significantly decreased spontaneous locomotion in both Mecp2+/+ and Mecp2+/– animals. (D) Time spent in the open area of the zero-maze assay was not impacted by treatment or genotype. (E) Discrimination for a novel object was significantly reduced in vehicle-treated Mecp2+/– mice relative to control Mecp2+/+ animals. Chronic VU154 administration restored discrimination for the novel object in Mecp2+/– mice. (F) Similar to acute studies, chronic VU154 treatment in Mecp2+/– mice diminished sociability in the social interaction assay. (G) Unlike Mecp2+/+ animals, preference for the novel stranger is not observed in vehicle-treated Mecp2+/– mice, but chronic VU154 treatment restored social preference. Data are expressed as mean ± SEM. Two-way ANOVA with Tukey post hoc analysis *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
To quantify motor phenotypes, we used both the accelerated rotarod and open field assays. Rotarod was conducted over three days, with three trials on each day. Throughout the test, Mecp2+/+ controls consistently performed better than Mecp2+/– mice, independent of treatment (Figure 3B). Additionally, Mecp2+/+ mice demonstrated motor learning and stayed on the rod longer with successive days while chronic vehicle- and VU154-treated Mecp2+/– mice did not. In the open field, spontaneous locomotion was significantly decreased in Mecp2+/– mice relative to Mecp2+/+ controls and was further reduced in both genotypes by repeated VU154 administration (Figure 3C).
Based on the results of our dose–response studies, we again performed EZM as a measure of anxiety. However, unlike what was observed in acute studies, we observed no change in the time spent by Mecp2+/– mice in the open areas of the EZM. This finding also stands in contrast to numerous published reports, where untreated or vehicle-treated Mecp2+/– mice routinely show decreased anxiety.10,34 While speculative, the changes in anxiety phenotypes observed here may be linked to the daily handling and injection associated with repeat dosing.
To assess cognitive phenotypes, we subjected mice to both the novel object recognition and contextual fear conditioning assays. Novel object recognition assesses spatial memory by exposing mice to two identical objects and then replacing one with a novel object and re-exposing test mice after a 1 h interval. In this assay, Mecp2+/+ mice showed recognition and preference for the novel object, while vehicle-treated Mecp2+/– mice failed to distinguish (Figure 3E). Repeated VU154 administration in Mecp2+/– mice rescued this phenotype, indicative of significantly improved spatial memory. Contextual fear conditioning was performed as described above; however, both chronic vehicle- and VU154-treated control Mecp2+/+ mice showed a marked reduction in freezing relative to acute studies (30 vs 80%) under identical assay conditions, rendering the results difficult to interpret (Figure S1).
In the social interaction assay, Mecp2+/+ mice demonstrated a preference for the stranger 1 mouse over the object independent of treatment, as did vehicle-treated Mecp2+/– mice (Figure 3F). Similar to acute studies using the 3 mg/kg dose, we again observed disruption of sociability in Mecp2+/– mice treated chronically with VU154. Also in agreement with acute studies, vehicle-treated Mecp2+/– mice showed no bias for the stranger 1 or stranger 2 mouse, and repeated VU154 administration rescued social preference phenotype (Figure 3G).
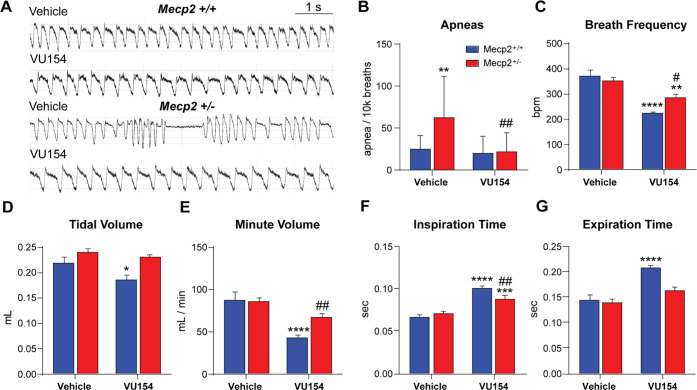
VU154 administration reduced apneas in Mecp2+/– mice in a dose-dependent manner in acute studies. Consistent with this finding, when we performed WBP on mice chronically treated with VU154, we again observed a significant reduction in apneas per 10k breaths in Mecp2+/– mice (Figure 4A,B). Paradoxically, we also quantified a significant reduction in breath frequency with VU154 treatment in both Mecp2+/+ control and Mecp2+/– RTT mice (Figure 4C). A significant reduction in individual breath volume (tidal volume) was also recorded in control mice (Figure 4D). When combined with decreased breath rate, this equated to a reduction in the total volume of air exchanged per minute (minute volume) in both genotypes (Figure 4E). When each breath was divided into inspiratory and expiratory phases, decreased breath rate in Mecp2+/+ controls was associated with significant increases in both inspiration and expiration time, while Mecp2+/– mice showed only significant increases in the inspiratory phase (Figure 4F,G). As distinct levels of the respiratory circuit control inspiration and expiration,35 these data point to a potential region-specific loss of M4 expression in Mecp2+/– mice and thereby, diminished response to VU154 in those that govern expiration time.
Figure 4.
M4 potentiation with VU154 significantly decreases apneas in Mecp2+/– mice. Whole-body plethysmography. N = 13–15/genotype/treatment; * denotes comparison to vehicle-treated Mecp2+/+ mice, # denotes comparison to vehicle-treated Mecp2+/– mice. (A) Sample plethysmography traces highlighting apneas in vehicle-treated Mecp2+/– mice. (B) Vehicle-treated Mecp2+/– mice had significantly more apneas relative to vehicle-treated Mecp2+/+ controls, and this was significantly reversed by chronic VU154 administration. (C) Average breaths per minute over 30 min was significantly decreased in both Mecp2+/+ and Mecp2+/– mice by chronic VU154 administration. (D, E) Reduced breath rate and tidal volume (in Mecp2+/+ mice) combined to result in significantly decreased minute volume in both genotypes (bpm × mL) following chronic VU154 administration. (F, G) The decrease in breaths-per-minute quantified with chronic VU154 treatment was associated with a significant increase in inspiration time in both genotypes, as well as an increase in expiratory time in Mecp2+/+ controls. Two-way ANOVA with Tukey post hoc analysis *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Data are expressed as mean ± SEM.
Chronic M4 Potentiation Does Not Impact mAChR Expression in Mecp2+/– Mice
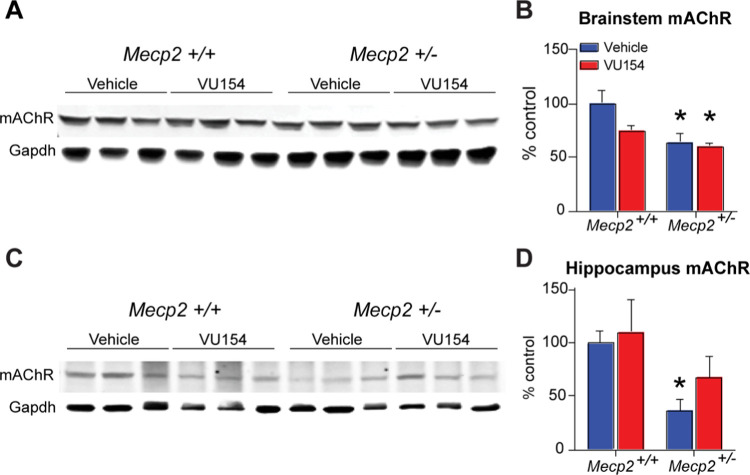
Our previous work has shown a decrease in mAChR expression in patient autopsy samples and in Mecp2+/– mice,9 and it is believed that VU154’s mechanism of action is to normalize cholinergic tone by potentiating a fewer number of receptors. However, an alternative hypothesis is that chronic treatment evokes compensatory mAChR expression changes in Mecp2+/– mice that normalize receptor number. To address this possibility, we isolated total protein from Mecp2+/+ and Mecp2+/– mice chronically treated with VU154 and ran Western blots for mAChR expression. As shown in Figure 5A–D, mAChR expression was significantly decreased in both the hippocampus and the brainstem of Mecp2+/– mice, brain regions associated with contextual fear conditioning and respiratory phenotypes, respectively. Further, VU154 treatment did not alter mAChR levels in either brain region when compared relative to vehicle-treated Mecp2+/– mice. These data suggest that VU154 is acting by potentiating M4 signaling and not by altering mAChR expression.
Figure 5.
Chronic VU154 administration does not alter mAChR expression. Western blot. N = 3–5/genotype/treatment. (A–D) Relative to Mecp2+/+ controls, mAChR expression is significantly decreased in the brainstem and hippocampus of vehicle-treated Mecp2+/– mice. Once daily administration of VU154 for 44 days does not significantly alter mAChR expression in either brain region. Two-way ANOVA with Tukey post hoc analysis *p < 0.05. Data are expressed as mean ± SEM.
VU154 Administration Increases S9 Gsk3β Phosphorylation
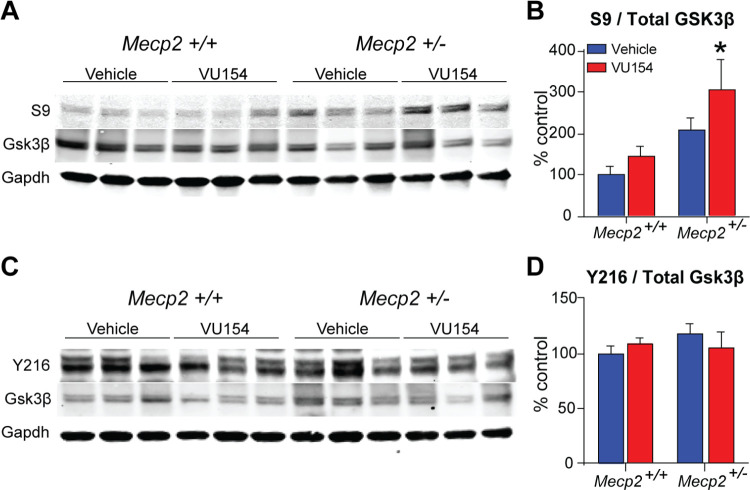
VU154’s efficacy on respiratory phenotypes aligns with data from targeting one of its well-studied signaling partners,36 glycogen synthase kinase 3β (Gsk3β), where pharmacological inhibition also rescues apneas in RTT model mice.37 To determine whether the two findings are linked, we harvested brainstem samples from mice treated chronically with VU154, isolated protein, and ran Western blots that probed for total Gsk3β, phospho-S9 (inhibitory site), and phospho-Y216 (activation site). As shown in Figure 6A–D, we observed a significant increase in the ratio of S9 to total Gsk3β, with no impact on the Y219 sites. These data point to a mechanism by which M4 potentiation improves apneas in Mecp2+/– mice via pathways that increase Gsk3β inhibition in the brainstem.
Figure 6.
S9 inhibitory site on Gsk3β is hyperphosphorylated in the brainstem of Mecp2+/– mice following chronic M4 PAM administration. Western Blot. N = 8–10/genotype/treatment. (A) Representative blot of the S9 inhibitory site and total Gsk3β. (B) The ratio of S9 to total Gsk3β phosphorylation is significantly increased in Mecp2+/– mice following chronic VU154 treatment. (C) Representative blot of the Y216 excitatory site and total Gsk3β. (D) Ratio of Y216 to total Gsk3β phosphorylation is not impacted by treatment or genotype. Two-way ANOVA with Tukey post hoc analysis *p < 0.05. Data are expressed as mean ± SEM.
Summary and Discussion
M4 PAMs represent an exciting class of potential treatments for the neurodevelopmental disorder Rett syndrome (RTT). The evidence supporting their utility is rooted in clinical data showing a decrease in M4 expression in the motor cortex and cerebellum of clinical RTT samples, as well as the efficacy of the M4 PAM VU154 on social and respiratory phenotypes in an acute study using Mecp2+/– mice.9 Here, we fill critical knowledge gaps regarding the potential for expression-induced tolerance, the scope of phenotypic benefits observed with M4 potentiation in Mecp2+/– mice in both acute and chronic dosing paradigms, and the potential for adverse effects associated with this strategy in RTT.
Proof-of-concept studies showing the efficacy of M4 PAMs in neurological disorders span indications that include schizophrenia, Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease.25−27 While the majority of these studies involved acute M4 PAM treatment, several examined the effects of repeat administration.27,28 Most notably, a comprehensive assessment of repeat administration in both wild-type mice and an MK-801-induced model of schizophrenia demonstrated that phenotypic efficacy was not impacted by daily M4 PAM administration.28 Our phenotypic data in wild-type mice parallels these findings and further suggests that repeat M4 PAM treatment does not impact M4 expression. As the majority of M4 PAM indications are lifelong conditions, these data provide optimism that chronic treatment is viable with this therapeutic mechanism.
In general, our dose–response and chronic VU154 treatment experiments yielded positive results in Mecp2+/– mice. We chose to employ a full battery of phenotypic assays to account for the breadth of symptom domains associated with RTT and the potential for M4 potentiation to impact them in both positive and negative directions. The capacity of VU154 to rescue preference for social novelty and reduce apneas were the most conserved findings across dose–response and chronic administration experiments. Efficacy was also observed in anxiety phenotypes in the elevated zero maze and associative memory in the contextual fear assay in acute dose–response studies, but these failed to translate to chronic administration experiments. It is unknown whether the loss in efficacy was linked to repeat administration over 44 days, or whether it was associated with the daily injections and handling. The dramatic decrease in contextual freezing response observed in control mice treated with either vehicle or VU154 argues for the latter point; however, further work will be required to determine whether this stressor was the sole factor.
Despite the positive phenotypic results, several negative findings were also observed. One cause for concern was the seemingly narrow U-shaped response of Mecp2+/– mice to VU154, where efficacy was limited to 3 mg/kg concentrations in most cases. This finding was not unique to our studies, as a similarly narrow U-shaped response was previously reported with VU154 in a pair-wise discrimination assay.28 Another potential concern was that the sociability phase of the social interaction assay was disrupted in Mecp2+/– mice by the 3 mg/kg dose of VU154 in both acute and chronic treatment paradigms. The potential clinical impact of reduced sociability in RTT is unknown, as are the mechanisms responsible for its presentation; however, it is clear that this represents a potential adverse effect that should be monitored throughout the development process.
Perhaps our most robust finding was that VU154 rescued apneas in Mecp2+/– mice in a manner that was both dose-dependent and conserved with chronic treatment. M4 expression is enriched in the frontal cortex and the striatum; however, it is also expressed in the cholinergic interneurons of the upper brainstem reticular core.22 The cholinergic reticular core is a signaling hub that integrates a complex network of circuits that mediate biological processes spanning cardiovascular control, consciousness, nociception, and motor function.38 Of relevance to this study are its descending pathways, whose damage results in loss of respiratory drive, among other phenotypes.39 With repeat dosing of 3 mg/kg VU154, we quantified a 50% decrease in apneas per 10k breaths. Apnea number had to be normalized to a defined number of breaths because we also observed a significant drop in breath rate in both Mecp2+/+ control and Mecp2+/– mice with chronic treatment. As the general health of all mice treated chronically with VU154 and vehicle was indistinguishable, it remains to be seen whether this statistically significant finding is also biologically significant. Further testing of structurally distinct M4 PAMs is required to determine whether the effects on apneas and respiratory rate are target-mediated, or if they can be uncoupled with chemical optimization, potentially via signaling bias. Given the diverse therapeutic requirements of their proposed indications, M4 PAM development would also merit employing a similar strategy to determine whether M4’s well-characterized effects on motor function can also be uncoupled from its robust efficacy on cognitive and social functions.
While approaches designed to fix or replace the mutant MECP2 gene are theoretically the optimal strategy to treat RTT, there remain significant hurdles in their therapeutic development. Further, it is unlikely that any single approach will cure every aspect of a disease as complex as RTT. As such, we contend that adjunct or symptomatic treatments will remain important tools in disease management for the foreseeable future. Our studies offer optimism that M4 potentiation is a viable approach to symptom management in RTT; however, they also highlight that continued development is required to improve the therapeutic range of M4-targeted compounds and to minimize the potential for adverse effects.
Methods
Study Design
The use of the Mecp2+/tm1.1 bird mouse model and selection of sample size was based on the standards established by the National Institute of Mental Health and RTT research community.7 Only female mice were used in this study to reflect the fact that RTT patients are overwhelmingly female. The mice were assigned to dosing groups at random and phenotyping or molecular quantitation was either performed by a blinded researcher or by automated software. Statistics were carried out using Prism 6.0 (GraphPad) and Excel (Microsoft), employing two-tailed unpaired or paired Student’s t-tests and one- or two-way analysis of variance (ANOVA), with Bonferroni’s or individual Student’s t-test post hoc analysis, as specified in each figure legend.
mRNA and Protein Analysis
Total RNA and cDNA were prepared from 20-week-old Mecp2+/– and 6-week-old WT C57B6 mice using standard trizol–chloroform, RNeasy DNase treatment, and Superscript VILO synthesis methodologies. Quantitative Real-Time (qRT)-PCR was performed on BioRad CFX96 instrumentation using a Thermo Fisher Assay on Demand primer-probe kit for CHRM4/M4 (Mm00432514_s1). G6pd was used as the internal control (Mm00656735_g1). qRT-PCR data were analyzed using the delta–delta Ct method.
Total protein was isolated from 200 mg of mouse tissue and Western blots were run as previously described.10 Primary antibodies were used at the following concentrations: Gsk3β Y216, (1:500, Fisher 44604G), Gsk3β (1:500, Abcam ab93926), Gsk3 β S9 (1:500, CST9323), Gapdh (1:5000, CST5174). The fluorescent secondary antibodies used were: Goat Anti-Mouse 680 (1:5000, LiCor #925-68070) and Goat Anti-Rabbit 800 (1:5000, LiCor #925-68071). Images were acquired, and fluorescence was quantified on a Li-Cor Odyssey Infrared Imaging System.
Compound Administration and Phenotyping
20-week-old Mecp2+/– and Mecp2+/+ females were used in the studies described herein, which represents an age where consistent phenotypes are observed in our colony. In all cases, either vehicle (10% Tween 80) or 1, 3, or 10 mg/kg of VU0467154 (VU154) was administered via intraperitoneal (i.p.) injection 30 min before the start of the phenotypic assay and/or tissue harvest. The order of phenotyping assays performed for dose–response experiments was elevated zero maze (EZM), social interaction, whole-body plethysmography (WBP), and contextual fear conditioning, with a minimum of 5 days washout between assays. For chronic dosing experiments, 3 mg/kg VU154 treatment began 21 days prior to phenotyping, and assays proceeded in the following order: rotarod, open field, EZM, novel object recognition, social interaction, contextual fear conditioning, and WBP.
Behavioral Assays
Open Field
To monitor spontaneous locomotion, test mice were injected with either VU154 or vehicle and placed in an activity monitoring chamber 30 min post-dose. Locomotor behavior in the open field was then monitored using Activity software to quantify beam breaks in the X, Y, and Z planes.
Accelerated Rotarod
Accelerated rotarod was used as a measure of motor function and motor learning. In this assay, mice were placed on a rotating rod that increased in speed from 4 to 40 rpm over 300 s. The duration of time the mouse was able to remain on the rod served as the outcome measure. VU154 was administered 30 min before the first trial, and the test was repeated three times per day for three days, with an hour spacing between trials. The difference in performance between day 1 and day 3 was used as a measure of motor learning.
Social Interaction and Preference Assay
Control and test mice were placed in a testing apparatus and allowed to habituate for 7 min. A novel mouse (stranger 1, 6-week-old C57B6, female) was restrained in a wire cage on one end of the apparatus and an empty cup was placed in the opposing end, with empty space in the center. The test mouse was then allowed to choose between the stranger 1 mouse and the empty cup for 7 min. The empty cup was then replaced with a novel stranger (stranger 2, 6-week-old C57B6, female) and social preference was quantified over 7 min using both AnyMaze and Noldus analysis software.
Novel Object Recognition Assay
Novel object recognition was conducted as described in.40 Briefly, test mice were placed inside a chamber with two identical objects (slide box or a beaker) and allowed to explore for 10 min. The animals were then returned to their home cage. After 1 h, test mice were returned to the chamber a final time for 5 min, and 1 of the 2 objects was replaced with a novel object. The familiar and novel objects were randomized in each test group. The test was video recorded and the seconds spent directly sniffing each object were scored by Noldus Software. The discrimination index was defined as (Timenovel – Timefamiliar)/(Timenovel + Timefamiliar).
Contextual Fear Conditioning
For contextual fear conditioning, VU154 was administered on day 1 of the assay and the percent of time spent freezing was assessed 24 h later. On training day, mice were placed into an operant chamber in the presence of a 10% vanilla odor cue with a shock grid (Med Associates Inc.). Following a 3 min habituation period, the mice were exposed to two 1 s, 0.7 mA foot shocks separated by 30 s. Test mice were placed back into the same shock chamber 24 h later with a 10% vanilla odor cue, and the percent of time spent freezing during a 3 min testing period was assessed by Med Associates software.
Whole-Body Plethysmography (WBP)
Mecp2+/– and Mecp2+/+ mice were placed in a WBP recording chamber (Buxco, 2-site system) with a continuous in-flow of air (1 liter/min). Following a habituation period (30 min), baseline recording was established for 30 min. Test mice were then injected with VU154 or vehicle, reacclimated (30 min), and respiratory measurements were made for an additional 30 min. FinePointe Research Suite (v2.3.1.9) was used for analysis, and apneas were defined as pauses spanning 2× the average expiratory time of the previous 2 min. Apneas were identified by the software and confirmed by random manual spot-checking of the larger data set. Only points of motion-free recording were analyzed. All filters were applied while the researchers were blinded to the genotype and treatment group.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acschemneuro.2c00113.
Chronic vehicle and VU154 administration decrease freezing responses in Mecp2+/+ control mice (Figure S1); repeat administration of both drug and vehicle resulted in a diminished fear response in controls, which was measured at 80% in acute studies was quantified as 30% in chronic studies; and the shift in control baseline freezing compromised the signal window relative to Mecp2+/– mutant mice and precluded the use of contextual fear as a reliable outcome measure for cognition in chronic administration studies (PDF)
Author Contributions
◆ J.C. and C.H. denote co-first authorship. J.C., C.H., B.A., M.S., C.H., and S.G., contributed substantially to the generation and analysis of the data. C.W.L. synthesized and provided VU154 to support the described experiments, C.M.N. helped in the planning, analysis, and interpretation of the data. R.G.G. contributed to all aspects of the paper, including data collection, analysis, interpretation, and drafting of the manuscript.
The authors thank P. Jeffrey Conn for his intellectual and strategic contributions to the project. R.G.G. received support from a Young Investigator Award from Brain and Behavior Research Foundation, K01MH112983, and R01NS112171. The authors acknowledge 3503 from IRSF (C.M.N.) and 3903 from IRSF (R.G.G., C.M.N.).
The authors declare the following competing financial interest(s): C.W.L. and C.M.N. receive research support from Acadia Pharmaceuticals and Boehringer Ingelheim, and C.W.L. also receives support from Ono Pharmaceutical. C.W.L. and C.M.N. are inventors on multiple patents for allosteric modulators of muscarinic acetylcholine receptors. All other authors declare no potential conflicts of interest.
Supplementary Material
References
- Percy A. K.; Lane J. B. Rett syndrome: clinical and molecular update. Curr. Opin. Pediatr. 2004, 16, 670–677. 10.1097/01.mop.0000143693.59408.ce. [DOI] [PubMed] [Google Scholar]
- Percy A. K. Rett syndrome. Curr. Opin. Neurol. 1995, 8, 156–160. 10.1097/00019052-199504000-00013. [DOI] [PubMed] [Google Scholar]
- Amir R. E.; Van den Veyver I. B.; Wan M.; Tran C. Q.; Francke U.; Zoghbi H. Y. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999, 23, 185–188. 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- Lyst M. J.; Ekiert R.; Ebert D. H.; Merusi C.; Nowak J.; Selfridge J.; et al. Rett syndrome mutations abolish the interaction of MeCP2 with the NCoR/SMRT co-repressor. Nat Neurosci. 2013, 16, 898–902. 10.1038/nn.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah R. R.; Bird A. P. MeCP2 mutations: progress towards understanding and treating Rett syndrome. Genome Med. 2017, 9, 17 10.1186/s13073-017-0411-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaze D. G.; Neul J. L.; Kaufmann W. E.; Berry-Kravis E.; Condon S.; Stoms G.; et al. Double-blind, randomized, placebo-controlled study of trofinetide in pediatric Rett syndrome. Neurology 2019, 92, e1912–e1925. 10.1212/WNL.0000000000007316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz D. M.; Berger-Sweeney J. E.; Eubanks J. H.; Justice M. J.; Neul J. L.; Pozzo-Miller L.; et al. Preclinical research in Rett syndrome: setting the foundation for translational success. Dis. Models Mech. 2012, 5, 733–745. 10.1242/dmm.011007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamo N. J.; Birknow M. R.; Sullivan D.; Kondo M. A.; Horiuchi Y.; Sakurai T.; et al. Valley of death: A proposal to build a ″translational bridge″ for the next generation. Neurosci Res. 2017, 115, 1–4. 10.1016/j.neures.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogliotti R. G.; Fisher N. M.; Stansley B. J.; Jones C. K.; Lindsley C. W.; Conn P. J.; Niswender C. M. Total RNA Sequencing of Rett Syndrome Autopsy Samples Identifies the M4 Muscarinic Receptor as a Novel Therapeutic Target. J. Pharmacol. Exp. Ther. 2018, 365, 291–300. 10.1124/jpet.117.246991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogliotti R. G.; Senter R. K.; Fisher N. M.; Adams J.; Zamorano R.; Walker A. G.; et al. mGlu7 potentiation rescues cognitive, social, and respiratory phenotypes in a mouse model of Rett syndrome. Sci Transl Med. 2017, 9, aai7459 10.1126/scitranslmed.aai7459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston M. V.; Hohmann C.; Blue M. E. Neurobiology of Rett syndrome. Neuropediatrics 1995, 26, 119–122. 10.1055/s-2007-979740. [DOI] [PubMed] [Google Scholar]
- Murasawa H.; Kobayashi H.; Imai J.; Nagase T.; Soumiya H.; Fukumitsu H. Substantial acetylcholine reduction in multiple brain regions of Mecp2-deficient female rats and associated behavioral abnormalities. PLoS One 2021, 16, e0258830 10.1371/journal.pone.0258830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenk G. L.; Mobley S. L. Choline acetyltransferase activity and vesamicol binding in Rett syndrome and in rats with nucleus basalis lesions. Neuroscience 1996, 73, 79–84. 10.1016/0306-4522(96)00019-X. [DOI] [PubMed] [Google Scholar]
- Ballinger E. C.; Schaaf C. P.; Patel A. J.; de Maio A.; Tao H.; Talmage D. A.; et al. Mecp2 Deletion from Cholinergic Neurons Selectively Impairs Recognition Memory and Disrupts Cholinergic Modulation of the Perirhinal Cortex. eNeuro 2019, 6, ENEURO.0134. 10.1523/ENEURO.0134-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nag N.; Berger-Sweeney J. E. Postnatal dietary choline supplementation alters behavior in a mouse model of Rett syndrome. Neurobiol. Dis. 2007, 26, 473–480. 10.1016/j.nbd.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Zhang S.; Johnson C. M.; Cui N.; Xing H.; Zhong W.; Wu Y.; Jiang C. An optogenetic mouse model of rett syndrome targeting on catecholaminergic neurons. J. Neurosci. Res. 2016, 94, 896–906. 10.1002/jnr.23760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Cao S. X.; Sun P.; He H. Y.; Yang C. H.; Chen X. J.; et al. Loss of MeCP2 in cholinergic neurons causes part of RTT-like phenotypes via alpha7 receptor in hippocampus. Cell Res. 2016, 26, 728–742. 10.1038/cr.2016.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Zhu Y.; Cao S. X.; Sun P.; Yang J. M.; Xia Y. F.; et al. MeCP2 in cholinergic interneurons of nucleus accumbens regulates fear learning. Elife 2020, 9, e55342 10.7554/eLife.55342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn P. J.; Jones C. K.; Lindsley C. W. Subtype-selective allosteric modulators of muscarinic receptors for the treatment of CNS disorders. Trends Pharmacol. Sci. 2009, 30, 148–155. 10.1016/j.tips.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey A. I. Immunological localization of m1-m5 muscarinic acetylcholine receptors in peripheral tissues and brain. Life Sci. 1993, 52, 441–448. 10.1016/0024-3205(93)90300-R. [DOI] [PubMed] [Google Scholar]
- Levey A. I.; Kitt C. A.; Simonds W. F.; Price D. L.; Brann M. R. Identification and localization of muscarinic acetylcholine receptor proteins in brain with subtype-specific antibodies. J Neurosci. 1991, 11, 3218–3226. 10.1523/JNEUROSCI.11-10-03218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugaya K.; Clamp C.; Bryan D.; McKinney M. mRNA for the m4 muscarinic receptor subtype is expressed in adult rat brain cholinergic neurons. Mol. Brain Res. 1997, 50, 305–313. 10.1016/S0169-328X(97)00199-X. [DOI] [PubMed] [Google Scholar]
- Vilaró M. T.; Mengod G.; Palacios G.; Palacios J. M. Receptor distribution in the human and animal hippocampus: focus on muscarinic acetylcholine receptors. Hippocampus 1993, 3, 149–156. 10.1002/hipo.1993.4500030718. [DOI] [PubMed] [Google Scholar]
- Koshimizu H.; Leiter L. M.; Miyakawa T. M4 muscarinic receptor knockout mice display abnormal social behavior and decreased prepulse inhibition. Mol. Brain 2012, 5, 10 10.1186/1756-6606-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder C. C.; Goldsmith P. J.; Jackson K.; Sanger H. E.; Evans D. A.; Mogg A. J.; Broad L. M. Current status of muscarinic M1 and M4 receptors as drug targets for neurodegenerative diseases. Neuropharmacology 2018, 136, 449–458. 10.1016/j.neuropharm.2018.01.028. [DOI] [PubMed] [Google Scholar]
- Foster D. J.; Choi D. L.; Conn P. J.; Rook J. M. Activation of M1 and M4 muscarinic receptors as potential treatments for Alzheimer’s disease and schizophrenia. Neuropsychiatr. Dis. Treat. 2014, 10, 183–191. 10.2147/NDT.S55104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancani T.; Foster D. J.; Moehle M. S.; Bichell T. J.; Bradley E.; Bridges T. M.; et al. Allosteric activation of M4 muscarinic receptors improve behavioral and physiological alterations in early symptomatic YAC128 mice. Proc. Natl. Acad. Sci. U.S.A. 2015, 112, 14078–14083. 10.1073/pnas.1512812112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould R. W.; Grannan M. D.; Gunter B. W.; Ball J.; Bubser M.; Bridges T. M.; et al. Cognitive enhancement and antipsychotic-like activity following repeated dosing with the selective M4 PAM VU0467154. Neuropharmacology 2018, 128, 492–502. 10.1016/j.neuropharm.2017.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzykowski A. Z.; Treistman S. N. The molecular basis of tolerance. Alcohol Res. Health 2008, 31, 298–309. [PMC free article] [PubMed] [Google Scholar]
- Williams J. T.; Christie M. J.; Manzoni O. Cellular and synaptic adaptations mediating opioid dependence. Physiol. Rev. 2001, 81, 299–343. 10.1152/physrev.2001.81.1.299. [DOI] [PubMed] [Google Scholar]
- Bubser M.; Bridges T. M.; Dencker D.; Gould R. W.; Grannan M.; Noetzel M. J.; et al. Selective activation of M4 muscarinic acetylcholine receptors reverses MK-801-induced behavioral impairments and enhances associative learning in rodents. ACS Chem. Neurosci. 2014, 5, 920–942. 10.1021/cn500128b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogliotti R. G.; Niswender C. M. A Coordinated Attack: Rett Syndrome Therapeutic Development. Trends Pharmacol. Sci. 2019, 40, 233–236. 10.1016/j.tips.2019.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ztaou S.; Maurice N.; Camon J.; Guiraudie-Capraz G.; Kerkerian-Le Goff L.; Beurrier C.; et al. Involvement of Striatal Cholinergic Interneurons and M1 and M4 Muscarinic Receptors in Motor Symptoms of Parkinson’s Disease. J. Neurosci. 2016, 36, 9161–9172. 10.1523/JNEUROSCI.0873-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abellán-Álvaro M.; Stork O.; Agustin-Pavon C.; Santos M. MeCP2 haplodeficiency and early-life stress interaction on anxiety-like behavior in adolescent female mice. J. Neurodev. Disord. 2021, 13, 59. 10.1186/s11689-021-09409-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. C.; Abdala A. P.; Borgmann A.; Rybak I. A.; Paton J. F. Brainstem respiratory networks: building blocks and microcircuits. Trends Neurosci. 2013, 36, 152–162. 10.1016/j.tins.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach K.; Loiacono R. E.; Felder C. C.; McKinzie D. L.; Mogg A.; Shaw D. B.; et al. Molecular mechanisms of action and in vivo validation of an M4 muscarinic acetylcholine receptor allosteric modulator with potential antipsychotic properties. Neuropsychopharmacology 2010, 35, 855–869. 10.1038/npp.2009.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorge-Torres O. C.; Szczesna K.; Roa L.; Casal C.; Gonzalez-Somermeyer L.; Soler M.; et al. Inhibition of Gsk3b Reduces Nfkb1 Signaling and Rescues Synaptic Activity to Improve the Rett Syndrome Phenotype in Mecp2-Knockout Mice. Cell Rep. 2018, 23, 1665–1677. 10.1016/j.celrep.2018.04.010. [DOI] [PubMed] [Google Scholar]
- Saladin K. S.; Gan C. A.; Cushman H. N.. Anatomy & Physiology: The Unity of Form and Function, 8th ed.; McGraw-Hill Education: New York, NY, 2018; Vol. 1, pp 518−519. [Google Scholar]
- Wang D. Reticular formation and spinal cord injury. Spinal Cord. 2009, 47, 204–212. 10.1038/sc.2008.105. [DOI] [PubMed] [Google Scholar]
- Leger M.; Quiedeville A.; Bouet V.; Haelewyn B.; Boulouard M.; Schumann-Bard P.; Freret T. Object recognition test in mice. Nat. Protoc. 2013, 8, 2531–2537. 10.1038/nprot.2013.155. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.