To the editor.
Regarding the paper on clinical and treatment aspects of Familial Mediterranean fever (FMF) published by Manna & Rigante,1 we would like to add our experience on a unique case of a young FMF male who developed nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL) under treatment with canakinumab, and interleukin-1β (IL-1β) inhibitor.
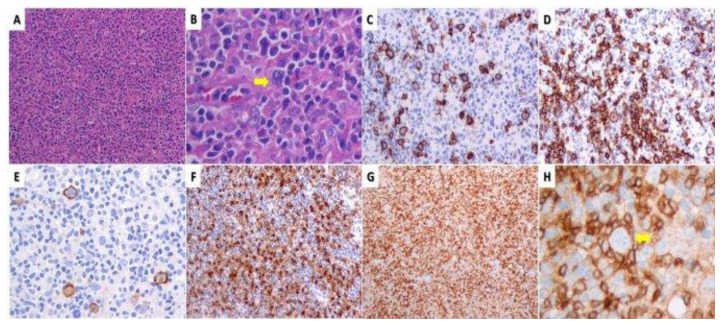
A 21-year-old male patient presented in December 2019 with asymptomatic right axillary and left inguinal lymphadenopathy over the preceding six months. Lymph node biopsy revealed NLPHL with a diffuse architectural pattern mimicking T-cell/histiocyte-rich large B-cell lymphoma (TCRBCL or DLBCL-like, Fan pattern E) (Figure 1).
Figure 1.
Nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL) with T-cell Rich B-cell lymphoma pattern (Pattern E): (A) and (B) Total effacement of normal lymph node architecture by a pleomorphic cell population consisting of abundant small lymphocytes, histiocytes and scattered large lymphocytes with Lymphocyte Predominant (LP) (arrow), Hodgkin cell, centroblast and immunoblast morphology (H&E). (C) In the largest area of the node scattered large B-cells are seen (CD20). (D) Rarely the large B-cells are surrounded by small reactive B-cells (NLPHL typical morphology) (CD20). (E) EMA positivity of large neoplastic B-cells. (F) Numerous histiocytes (PGM1). (G) Reactive background of small CD4 lymphocytes. (H) A PD1 positive T-rosette surrounding an LP cell (arrow).
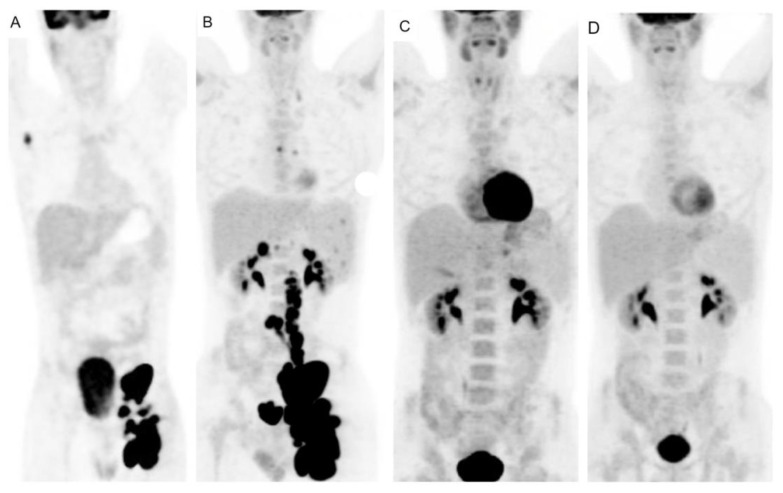
The clinical stage, confirmed by PET/CT, was IIIA with right axillary, mesenteric, and paraaortic lymphadenopathy and a 5.5 cm left iliac/inguinal mass (left inguinal SUVmax 35.8; Figure 2A). FMF was diagnosed at age 4 with homozygosity of pyrin gene mutation M694V. Since childhood, he experienced colchicine-refractory episodes of fever, abdominal and chest pain, and arthritis of the knees and ankles. He was placed on the anti-IL-1β canakinumab from age 13. No other symptoms such as aphthous stomatitis, pharyngotonsillitis, or lymphadenopathy were present, and the patient had not undergone tonsillectomy in childhood. Upon NLPHL diagnosis, canakinumab was discontinued due to the concern of adverse interaction with chemotherapy. ABVD chemotherapy was initiated. Interim PET/CT after ABVDx2 revealed progressive disease with new nodal lesions and spleen infiltration [SUVmax 33, Deauville 5-point scale score 5 (D5PSS 5)] (Figure 2B). Treatment was switched to R-CHOP. PET/CT after R-CHOPx3 revealed complete metabolic response (left inguinal SUVmax 2.2; D5PSS 3) (Figure 2C). End-of-treatment restaging after R-CHOPx6 in August 2020 confirmed complete remission (D5PSS 3, left iliac/inguinal SUVmax 1.8, dmax 2.8cm) (Figure 2D). The patient relapsed six months later with growing inguinal/iliac and abdominal lymphadenopathy. Salvage chemotherapy and autologous stem cell transplantation were scheduled, but he was admitted to another Center closer to his residency.
Figure 2.
Serial PET/CT evaluation during lymphoma course: (A) Baseline staging PET/CT: left submandibular, right axillary, paraortic, bilateral iliac and left inguinal lymphadenopathy (SUVmax of 35.8 at the left inguinal area). (B) Interim PET/CT after 2 cycles of ABVD: persistent disease at the initial sites, new left supraclavicular, subcarinal, hepatic portal, peripancreatic and right posterior diaphragmatic lymph node and spleen infiltration [SUVmax of 33, Deauville-5-point scale score (D5PSS) 5]. (C) Response assessment after 3 cycles of R-CHOP: slight 18FDG uptake in the left inguinal area (SUVmax of 2.2, D5PSS 3). (D) End-of-treatment PET/CT: complete remission (SUVmax of 1.8 at the left iliac/inguinal area, D5PSS 3).
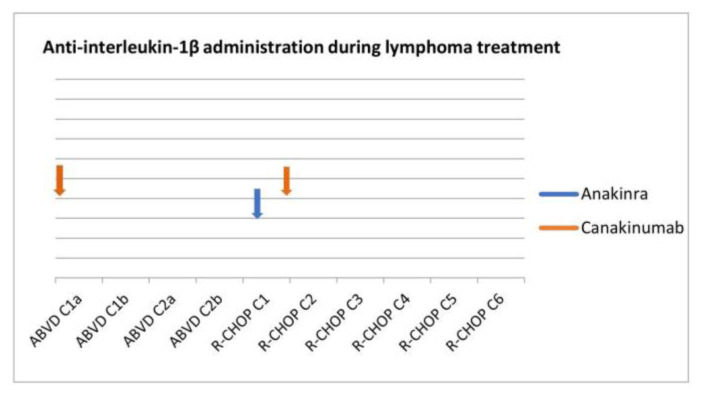
During ABVD and given the cessation of FMF-specific therapy, the patient had several minor FMF flares that required no intervention. However, following R-CHOP initiation, he suffered from 3 major episodes of fever and severe abdominal pain during a short period of ~ three weeks. The initial two events were treated with a single dose of betamethasone and anakinra, another anti-IL-1 (α and β) agent to which the patient had never been exposed. However, a single dose of canakinumab was eventually administered to control the severity of the third episode (Figure 3). After that, chemotherapy was completed uneventfully. Following rheumatology consultation, the patient occasionally received betamethasone for FMF after lymphoma presentation and remained on this until the last follow-up.
Figure 3.
The administration of anti-IL-β agents during lymphoma treatment.
The coexistence or overlap of periodic fever, aphthous stomatitis, pharyngitis, and cervical adenitis (PFAPA) with FMF has been described in the literature.2–4 However, our case is a typical, refractory to colchicine, FMF patient without any PFAPA-related symptoms or tonsillectomy in childhood. This diagnosis is also supported by the requirement of anti-IL-1 inhibitors to maintain full remission and the relapse of inflammatory attacks after the administration of R-CHOP, a therapeutic regimen containing corticosteroids, the mainstay treatment of PFAPA.
In contrast to cHL, very little data on the pathogenesis of NLPHL is available. In 2010 Celik et al. published a small study evaluating the frequency of MEFV gene mutations in patients with hematolymphoid neoplasms without FMF history. Gene carrier rate in patients with chronic lymphocytic leukemia, NHL, and HL was lower than in the general population; a higher frequency of MEFV gene mutations was observed in MM and acute lymphoblastic leukemia.5 Abnormal regulation of apoptosis and NF-κB pathway enhancement via pyrin were presumably implicated in the susceptibility to hematologic malignancy. However, data on the correlation between MEFV mutations and hematologic neoplasms remains limited, and the involvement of FMF in cancer remains speculative. Several studies have linked chronic inflammatory and autoimmune diseases with increased cancer incidence, including lymphomas. Still, a large cohort of 8,435 Israeli FMF patients included only 18 (5 males) to suffer from lymphomas (0.21% in total, 0.06% in males).6–8
The appearance of malignancy in patients treated with immunomodulators for various rheumatologic conditions has been widely discussed. Specifically, cancer incidence in rheumatoid arthritis patients under anti-IL-1 therapy with anakinra may not be different compared to the general population. However, lymphoma incidence in these patients is consistent with that reported in patients with rheumatoid arthritis and other autoimmune disorders, which may reflect that lymphomagenesis may be related to uncontrolled chronic inflammation and autoimmunity rather than biologic agents.9 Notably, FMF patients are characterized by chronic subclinical inflammation, even during attack-free periods, especially those carrying the homozygous M694V mutation.10 In addition, recent data on the long-term safety of canakinumab in pediatric FMF patients reveal an extremely low incidence of serious adverse events, including malignancy.11–12 However, most data come from retrospective studies in children with relatively short follow-up after initiating IL-1 inhibition. In our case, NLPHL appeared almost eight years after the first administration of canakinumab. Therefore, a causative relation between the canakinumab and the development of lymphoma, especially in adult patients under long-standing anti-IL-1 treatment, must be further elucidated.
In the context of preexisting FMF, only two cases of nodular sclerosing and mixed cellularity cHL have been reported worldwide,13–14 but no NLPHL case has been published so far. Our patient had advanced-stage NLPHL appearing under long-term anti-IL-1β therapy. Canakinumab was ceased before chemotherapy initiation, and managing FMF during concomitant (immuno)chemotherapy appeared to be fairly challenging. Soon after the initiation of R-CHOP, the presentation of severe, serial FMF episodes made IL-1 inhibitors anakinra and canakinumab inevitable. No early-onset side effects were observed with the concurrent administration of immunochemotherapy and anti-IL-1 agents, and hematologic toxicity did not differ from that typically expected, with only limited filgrastim use. There are published data supporting the successful prevention of the progression of prodrome to full-blown attacks by on-demand use of anakinra in selected patients.15 In difficult cases like the present one, on-demand anakinra, with its short half-life (4–6 hours), might be a beneficial and safer approach compared to the much longer half-life (23–26 days) canakinumab. Following remission, further administration of canakinumab was considered unsafe for the underlying malignancy, and the patient has been occasionally treated with betamethasone thereafter.
The novelty of this case report is dual: the description for the first time of NLPHL in FMF in a canakinumab-dependent case, pointing to the potential implication of autoinflammatory syndromes / biologic immunosuppressing agents in the pathogenesis of NLPHL. Secondly, this case highlights the query that IL-1 inhibitors and concurrent chemotherapy may be critical for successfully completing lymphoma treatment and preserving the quality of life.
Footnotes
Competing interests: The authors declare no conflict of Interest.
References
- 1.Manna R, Rigante D. Familial Mediterranean Fever: Assessing the Overall Clinical Impact and Formulating Treatment Plans. Mediterr J Hematol Infect Dis. 2019 May 1;11(1):e2019027. doi: 10.4084/mjhid.2019.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butbul Aviel Y, Harel L, Abu Rumi M, Brik R, Hezkelo N, Ohana O, Amarilyo G. Familial Mediterranean Fever Is Commonly Diagnosed in Children in Israel with Periodic Fever Aphthous Stomatitis, Pharyngitis, and Adenitis Syndrome. J Pediatr. 2019 Jan;204:270–274. doi: 10.1016/j.jpeds.2018.08.080. [DOI] [PubMed] [Google Scholar]
- 3.Gattorno M, Caorsi R, Meini A, Cattalini M, Federici S, Zulian F, Cortis E, Calcagno G, Tommasini A, Consolini R, Simonini G, Pelagatti MA, Baldi M, Ceccherini I, Plebani A, Frenkel J, Sormani MP, Martini A. Differentiating PFAPA syndrome from monogenic periodic fevers. Pediatrics. 2009 Oct;124(4):e721–8. doi: 10.1542/peds.2009-0088. [DOI] [PubMed] [Google Scholar]
- 4.Deneau M, Wallentine J, Guthery S, O’Gorman M, Bohnsack J, Fluchel M, Bezzant J, Pohl JF. Natural killer cell lymphoma in a pediatric patient with inflammatory bowel disease. Pediatrics. 2010 Oct;126(4):e977–81. doi: 10.1542/peds.2010-0486. [DOI] [PubMed] [Google Scholar]
- 5.Celik S, Erikci AA, Tunca Y, Sayan O, Terekeci HM, Umur EE, Torun D, Tangi F, Top C, Oktenli C. The rate of MEFV gene mutations in hematolymphoid neoplasms. Int J Immunogenet. 2010 Oct;37(5):387–91. doi: 10.1111/j.1744-313X.2010.00938.x. [DOI] [PubMed] [Google Scholar]
- 6.Theander E, Henriksson G, Ljungberg O, Mandl T, Manthorpe R, Jacobsson LT. Lymphoma and other malignancies in primary Sjögren’s syndrome: a cohort study on cancer incidence and lymphoma predictors. Ann Rheum Dis. 2006 Jun;65(6):796–803. doi: 10.1136/ard.2005.041186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ladouceur A, Clarke AE, Ramsey-Goldman R, Bernatsky S. Malignancies in systemic lupus erythematosus: an update. Curr Opin Rheumatol. 2019 Nov;31(6):678–681. doi: 10.1097/BOR.0000000000000648. [DOI] [PubMed] [Google Scholar]
- 8.Brenner R, Ben-Zvi I, Shinar Y, Liphshitz I, Silverman B, Peled N, Levy C, Ben-Chetrit E, Livneh A, Kivity S. Familial Mediterranean Fever and Incidence of Cancer: An Analysis of 8,534 Israeli Patients With 258,803 Person-Years. Arthritis Rheumatol. 2018 Jan;70(1):127–133. doi: 10.1002/art.40344. [DOI] [PubMed] [Google Scholar]
- 9.Rubbert-Roth A. Assessing the safety of biologic agents in patients with rheumatoid arthritis. Rheumatology (Oxford) 2012 Jul;51(Suppl 5):v38–47. doi: 10.1093/rheumatology/kes114. [DOI] [PubMed] [Google Scholar]
- 10.Kelesoglu FM, Aygun E, Okumus NK, Ersoy A, Karapınar E, Saglam N, Aydın NG, Senay BB, Gonultas S, Sarisik E, Can MZ, Atay S, Basbug D, Tiryaki FK, Ozer S, Durmus RB, Orem F, Atay T, Acar A, Yilmaz Y, Kaya S, Ciftkaya A, Sarac Z, Makar CC, Saracoglu B, Dogdu G, Omeroglu RE. Evaluation of subclinical inflammation in familial Mediterranean fever patients: relations with mutation types and attack status: a retrospective study. Clin Rheumatol. 2016 Nov;35(11):2757–2763. doi: 10.1007/s10067-016-3275-0. [DOI] [PubMed] [Google Scholar]
- 11.Gülez N, Makay B, Sözeri B. Long-term effectiveness and safety of canakinumab in pediatric familial Mediterranean fever patients. Mod Rheumatol. 2020 Jan;30(1):166–171. doi: 10.1080/14397595.2018.1559488. [DOI] [PubMed] [Google Scholar]
- 12.Sag E, Akal F, Atalay E, Akca UK, Demir S, Demirel D, Batu ED, Bilginer Y, Ozen S. Anti-IL1 treatment in colchicine-resistant paediatric FMF patients: real life data from the HELIOS registry. Rheumatology (Oxford) 2020 Nov 1;59(11):3324–3329. doi: 10.1093/rheumatology/keaa121. [DOI] [PubMed] [Google Scholar]
- 13.Langenberg-Ververgaert KPS, Laxer RM, Punnett AS, Dupuis LL, Finkelstein Y, Abla O. Chemotherapy-Colchicine Interaction in a Child with Familial Mediterranean Fever and Hodgkin Lymphoma. Mediterr J Hematol Infect Dis. 2018 Mar 1;10(1):e2018019. doi: 10.4084/mjhid.2018.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demir F, Bahadir A, Mungan S, Çobanoğlu Ü, Kalyoncu M. Systemic Amyloidosis in a Patient With Familial Mediterranean Fever and Hodgkin Lymphoma: A Case Report. J Pediatr Hematol Oncol. 2020 Apr;42(3):234–237. doi: 10.1097/MPH.0000000000001504. [DOI] [PubMed] [Google Scholar]
- 15.Babaoglu H, Varan O, Kucuk H, Atas N, Satis H, Salman R, Ozturk MA, Haznedaroglu S, Goker B, Tufan A. On demand use of anakinra for attacks of familial Mediterranean fever (FMF) Clin Rheumatol. 2019 Feb;38(2):577–581. doi: 10.1007/s10067-018-4230-z. [DOI] [PubMed] [Google Scholar]