Abstract
Introduction
There are limited studies exploring the effects of n-3 PUFA supplementation on pregnancy outcomes. The goal of this study was to review relevant studies in order to determine the effect of n-3 polyunsaturated fatty acid (n-3 PUFA) supplementation on pregnancy outcomes based on eligible randomized controlled trials (RCTs).
Material and methods
Qualified studies were searched by keywords in PubMed, the Cochrane library and Embase. Studies from other pertinent sources were also reviewed, and RCTs published before January 2021 were reviewed. For each study, we assessed and synthesized the outcomes by relative risk (RR) or weighted mean difference (WMD) combined with the 95% confidence interval (95% CI).
Results
We included 13 studies with 9069 patients. Compared with the control group, n-3 PUFA significantly decreased the incidence of preterm delivery (RR = 0.898, 95% CI: 0.819–0.984) and low birthweight (RR = 0.797, 95% CI: 0.655–0.970), and increased the birth weight (WMD = 99.340, 95% CI: 10.503–188.177) and birth length (WMD = 0.449, 95% CI: 0.236–0.663). There was no significant difference in pregnancy-induced hypertension, preeclampsia, intrauterine growth retardation (IUIG), early preterm delivery, anti-hypertensive therapy, gestational diabetes or head circumference at birth between the two groups.
Conclusions
The available evidence shows that n-3 PUFA is not beneficial in reducing the incidence of maternal pregnancy outcomes such as gestational diabetes mellitus and hypertension; but it is beneficial to neonatal health such as decreasing the incidence of preterm delivery and low birthweight and increasing birth weight and birth length.
Keywords: n-3 polyunsaturated fatty acid, pregnancy outcome, meta-analysis
Introduction
N-3 polyunsaturated fatty acids (N-3 PUFA) mainly include a-monolinolenic acid (ALA), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Different populations have different needs for n-3 PUFA. As an immunonutrient, n-3 PUFA plays a pharmacological role in the prevention and treatment of coronary heart disease, hypertension, arthritis, other inflammatory and autoimmune diseases, and tumors. The fetus receives nutrients from the mother through the placenta [1–4]. PUFAs are important components of biological cell membranes and transmitters of the neuroendocrine and immune systems. They regulate the fluidity of cellular membranes, affect the function of neurotransmitters, and regulate the production and activity of certain hormones. They have very important physiological functions in organisms. Fatty acids obtained from the mother are influenced by the composition of the maternal diet. The placenta also plays an important role in the transport of fatty acids from the mother to the fetus. Preeclampsia is often associated with fetal growth restriction and early placental development defects. The placenta is one of the main sources of lipid peroxidation, and n-3 PUFA plays a potential important regulatory role in lipid metabolism in the placenta and fetus. At the same time, n-3 PUFA, especially DHA, is an important component of the cellular membranes of the retina and the cerebral cortex, and plays a definite role in promoting the development of vision and the central nervous system [5–7]. However, according to the latest protocol from the European Atherosclerosis Society, currently marketed n-3 PUFA reduces neither cardiovascular morbidity nor mortality. Extensive research has been carried out on n-3 PUFA and has shown that it has a wide range of biological characteristics and functions, and plays an important role not only in regulating lipid metabolism but also in inflammation, and it is very important for brain development and function of the brain [8, 9]. Based on reviewing eligible randomized controlled trials (RCTs), the aim of this study was to explore the effect of n-3 PUFA supplementation on pregnancy outcomes.
Material and methods
Search strategy
The data of clinical indexes on the effect of n-3 PUFA supplementation on pregnancy outcomes based on eligible RCTs were obtained from the included studies. In the databases (Cochrane, PubMed, and Embase), all the relevant RCTs before January 2021 were reviewed. The references in eligible RCTs were also reviewed. The key words included fatty acids, omega-3, n-3 polyunsaturated fatty acid, PUFA, n-3 PUFA, a-linolenic acid, fish oil, EPA, DHA, ALA, pregnancy, random, randomized control study, randomized controlled trial, RCT. All the above key words were combined with “AND” or “OR”. Literature was retrieved by two investigators independently. However, when there was disagreement, a third investigator was involved to make a decision.
Following the PICOS (participants, intervention, comparison, outcome, study design) principle, the key search terms included (P, participants) pregnant women; (I, interventions) pregnant women in the treatment group treated with n-3 PUFA, pregnant women in the control group treated with standard care, normal diet, olive oil or placebo; (C/O, comparison/outcome) the comparison of pregnancy outcome; (S, study design) designed as a RCT. All analysis procedures followed the PRISMA principle and guideline.
Study selection criteria
The included studies had to meet all the following inclusion criteria: (1) the study was designed as an RCT; (2) the subjects were pregnant women; (3) patients were treated with n-3 PUFA; 4) articles were published in English or Chinese.
If a study met any of the following exclusion criteria, it was excluded: (1) duplicate articles or similar results; (2) obvious data errors; (3) cohort study, case control study, case series, theoretical research or reviews, guideline, reports, meta-analyses, or other forms of research or comments that were not designed as RCT; (4) data irrelevant to this study.
The studies were reviewed by two investigators independently to determine whether the included studies met the inclusion criteria and did not meet the exclusion criteria. A third investigator was asked to resolve any disagreement.
Data extraction and quality assessment
In all the included RCTs, two categories of data (the basic characteristics of articles and the main clinical indexes) were extracted. The basic characteristics of articles contained authors’ names, publication year, detailed interventions, sample size, age, and gestational age. The main clinical indexes contained preterm delivery (PD), preeclampsia, pregnancy-induced hypertension (PIH), low birthweight, intrauterine growth retardation (IUIG), early PD, anti-hypertensive therapy, gestational diabetes, and body length, body weight and head circumference at birth. The Jadad scoring checklist was used to appraise the quality of the included studies. We evaluated all the RCTs by the following five items: statement of randomization; appropriateness of generating a randomized sequence; use of double blinding; description of double blinding method; details of withdrawals and dropouts. Studies with a score < 3 indicated low quality and high bias risk, studies with a score > 3 indicated high quality. Data were extracted by two investigators independently.
Statistical analysis
All the data analysis was conducted using STATA v10.0 (TX, USA). The heterogeneity of the included RCTs was assessed by χ2 and I2 tests, and the fixed-effects or random-effects models were selected by the above results. When the included RCTs were of high heterogeneity (χ2 p ≤ 0.05 and I2 > 50%), we selected the random-effects model to analyze the indexes. When the included RCTs were of acceptable heterogeneity (χ2 p > 0.05 and I2 ≤ 50%), we selected the fixed-effects model to analyze the indexes. Mean ± standard deviation was used to describe continuous variables, which were then analyzed by the weighted mean difference (WMD). Percentage was used to describe categorical variables, which were analyzed by relative risk (RR). Body length, body weight and head circumference at birth were analyzed by WMD while other indexes were analyzed by RR. Multiple complementary methods (funnel plots, filled funnel plots, Begg’s and Mazumdar’s rank test, and Egger’s test) were used to assess the study quality and risk of bias. Sensitivity analysis was also performed to analyze the indexes.
Results
Overview of the included studies
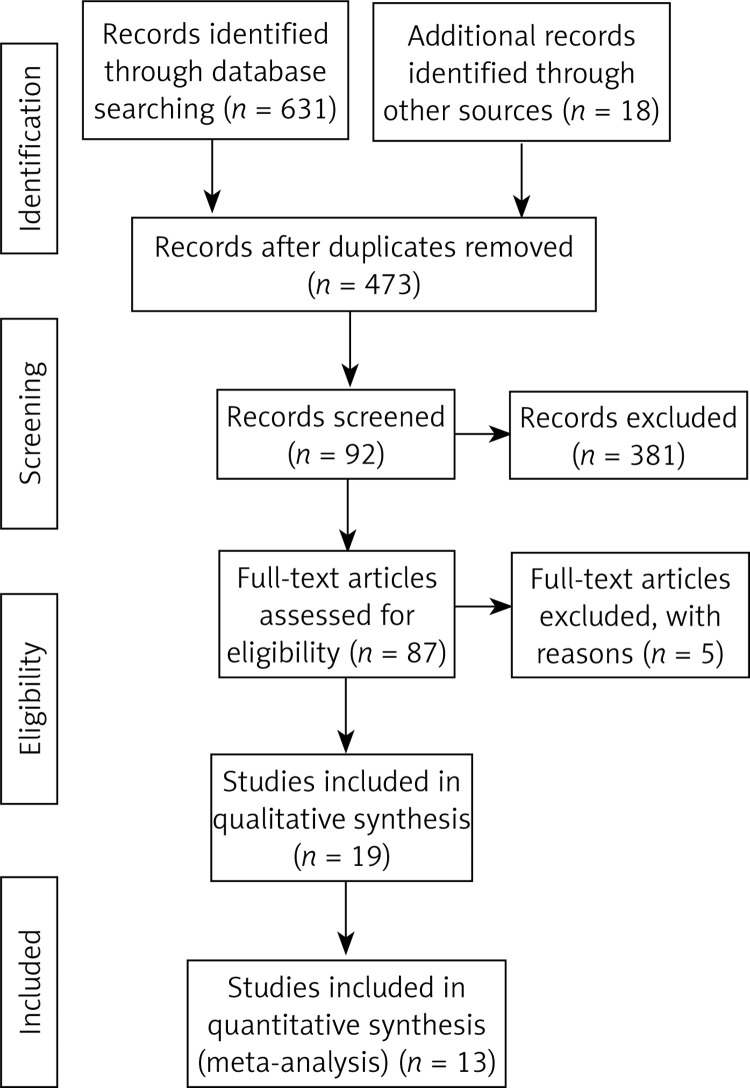
Finally, 649 articles were identified through search using initial keywords. After reviewing the titles and abstracts, we excluded 562 articles. The remaining 87 articles were evaluated by reading the full text, and then 74 articles were excluded using the selection criteria. The studies were excluded for the following reasons: no qualified studies (21), lack of clinical outcomes (27), review or theory research (8), and non-RCT studies (18). Finally, 13 studies [8, 10–22] with 9069 pregnant women that met the eligibility criteria were included in this meta-analysis. The article screening process is presented in Figure 1. The basic information of each study is summarized in Table I. The main Jadad score of the included studies was 4.46, and the main score was higher than 3, indicating that the 13 included RCTs were of high quality.
Figure 1.
Literature search and selection strategy
Table I.
Basic characteristics of included studies
| Study | Interventions | No. of patients | Age [years] | Gestational age [weeks] | Jadad score | ||||
|---|---|---|---|---|---|---|---|---|---|
| T | C | T | C | T | C | T | C | ||
| Sjrirhr F. Olsen 2000 a | Four capsules of either oil were given per day, fish oil 2.7 g (1.3 g eicosapentaenoic acid and 0–9 g docosahexaenoic acid) | Four capsules of olive oil were given per day | 110 | 122 | 29.3 | 30 | 131.8 days | 130.5 days | 5 |
| Sjrirhr F. Olsen 2000 b | 141 | 139 | 30 | 29 | 128.1 days | 131.2 days | 5 | ||
| Sjrirhr F. Olsen 2000 c | 184 | 202 | 30.3 | 28.9 | 129.6 days | 132.1 days | 5 | ||
| Sjrirhr F. Olsen 2000 d | 289 | 290 | 30.2 | 30.7 | 141.5 days | 141.5 days | 5 | ||
| Sjrirhr F. Olsen 2000 e | Nine capsules of either oil were given, fish oil 6.1 g (2.9 g eicosapentaenoic acid and 2–1 g docosahexaenoic acid) per day | Nine capsules of olive oil per day | 44 | 35 | 32.1 | 32.9 | 231.9 days | 219.6 days | 5 |
| Sjrirhr F. Olsen 2000 f | 36 | 37 | 29.3 | 29.8 | 227.1 days | 231.2 days | 5 | ||
| Cornelius M. Smuts 2003 | Docosahexaenoic acid-enriched eggs (mean of 133 mg of docosahexaenoic acid per egg) | Ordinary eggs (mean of 33 mg of docosahex-aenoic acid per egg) | 142 | 149 | 21.7 | 21.6 | 26 | 26.1 | 5 |
| Shao J. Zhou 2012 | DHA-enriched fish oil (800 mg/day) | Vegetable oil capsules without DHA | 1197 | 1202 | 28.9 | 28.9 | 19 | 19 | 5 |
| H. J. Huisje 1994 | 3 g eicosapentaenoic acid daily | Placebo | 32 | 31 | – | – | – | – | 3 |
| J. L. Onwude 1995 | 2.7 g of MaxEpa daily (1.62 g of eicosapentaenoic acid and 1.08 g of docosahexaenoic acid) | Placebo | 113 | 119 | 18-39 | 16-40 | 24 | 24.4 | 3 |
| Jannie Dalby Salvig 1996 a | Fish oil (2–7 g/day n-3 fatty acids (Pikasol)) | Olive oil | 266 | 136 | 29.4 | 29.7 | – | – | 4 |
| Jannie Dalby Salvig 1996 b | Fish oil (2–7 g/day n-3 fatty acids (Pikasol)) | No oil | 266 | 131 | 29.4 | 29.1 | – | – | 4 |
| Arminda D'Almeida 1992 a | Evening primrose oil (gamma linolenic acid) and fish oil (eicosapentaenoic + docahexaenoic acid) | Magnesium | 50 | 50 | – | – | – | – | 3 |
| Arminda D'Almeida 1992 b | Evening primrose oil (gamma linolenic acid) and fish oil (eicosapentaenoic + docahexaenoic acid) | Placebo | 50 | 50 | – | – | – | – | 3 |
| Spencer G. Kuper 2017 a | 1200 mg eicosapentaenoic acid (EPA, 20:5n-3) and 800 mg docosahexaenoic acid (DHA, 22:6n-3) for a total of 2000 mg of omega-3 long-chain polyunsaturated fatty acids daily | Placebo | 64 | 72 | 26 | 25.4 | 19.9 | 19.3 | 5 |
| Spencer G. Kuper 2017 b | 1201 mg eicosapentaenoic acid (EPA, 20:5n-3) and 800 mg docosahexaenoic acid (DHA, 22:6n-3) for a total of 2000 mg of omega-3 long-chain polyunsaturated fatty acids daily | placebo | 370 | 345 | 28.1 | 28 | 19.4 | 19.6 | 5 |
| Margaret Harper 2010 | Daily omega-3 supplement (1,200 mg eicosapentaenoic acid and 800 mg docosahexaenoic acid) | Placebo | 434 | 418 | 28 | 27 | 32 | 31 | 5 |
| Cornelius M. Smuts 2003 a | High-DHA hen eggs (135 mg DHA/egg) | Ordinary eggs (18 mg DHA/egg) | 18 | 19 | 19.9 | 24.8 | 26.1 | 26.7 | 4 |
| Cornelius M. Smuts 2003 b | High-DHA hen eggs (135 mg DHA/egg) | Low egg | 18 | 16 | 19.9 | 21.3 | 26.1 | 27 | 4 |
| Cornelius M. Smuts 2003 c | Ordinary eggs (18 mg DHA/egg) | Low egg | 19 | 16 | 24.8 | 21.3 | 26.7 | 27 | 4 |
| Susan E. Carlson 2013 | 600 mg/day of the n23 LCPUFA docosahexaenoic acid (DHA) | Placebo | 178 | 172 | 25.3 | 24.8 | – | – | 5 |
| Ellen L. Mozurkewich 2013 a | EPA-rich fish oil (1060 mg EPA plus 274 mg DHA) | Placebo | 39 | 41 | 29.9 | 30.4 | 15.9 | 16.2 | 5 |
| Ellen L. Mozurkewich 2013 b | DHA-rich fish oil (900 mg DHA plus 180 mg EPA) | Placebo | 38 | 41 | 30.6 | 30.4 | 17 | 16.2 | 5 |
| Bassel H. Al Wattar 2019 | High intake of nuts, extra virgin olive oil, fruits, vegetables, non-refined grains, and legumes; moderate to high consumption of fish; low to moderate intake of poultry and dairy products; low intake of red and processed meat; and avoidance of sugary drinks, fast food, and food rich in animal fat versus usual care | Standard care | 553 | 585 | 31.4 | 30.9 | – | – | 5 |
Pregnancy outcomes
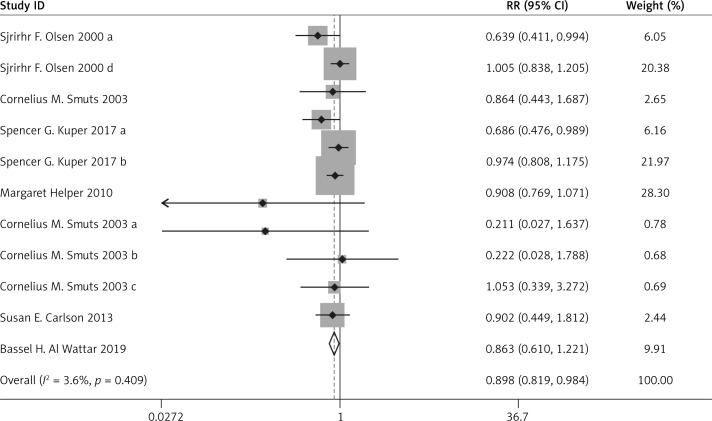
Compared with the control group, n-3 PUFA significantly decreased the incidence of PD (RR = 0.898, 95% CI: 0.819–0.984), and low birthweight (RR = 0.797, 95% CI: 0.655–0.970). Sensitivity analysis of low birthweight is presented in Figure 2.
Figure 2.
Sensitivity analysis of the included studies
Compared with the control group, n-3 PUFA significantly increased the body weight (WMD = 99.340, 95% CI: 10.503–188.177) and body length at birth (WMD = 0.449, 95% CI: 0.236–0.663).
There was no significant difference in the incidence of PIH (RR = 1.035, 95% CI: 0.891–1.202), preeclampsia (RR = 0.938, 95% CI: 0.741–1.187), IUIG (RR = 1.131, 95% CI: 0.957–1.337), early PD (RR = 0.603, 95% CI: 0.263–1.383), the rate of anti-hypertensive therapy (RR = 1.070, 95% CI: 0.518–2.210), gestational diabetes (RR = 0.869, 95% CI: 0.731–1.033), or head circumference at birth (WMD = 0.295, 95% CI: –0.005–0.595) between the two groups.
The above results are presented in Figures 3–6 and Table II.
Figure 3.
Forest plot for preterm delivery
Figure 6.
Forest plot for low birthweight
Table II.
Other results of meta-analysis
| Index | N (case/control) | ES (95%CI) | P * | I 2 | P # | P-value | |
|---|---|---|---|---|---|---|---|
| Begg’s | Egger’s | ||||||
| Intrauterine growth retardation IUIG | 685/689 | 1.131 (0.957, 1.337)a | 0.835 | 0.0% | 0.150 | 0.999 | – |
| Early PD | 394/403 | 0.603 (0.263, 1.383)a | 0.101 | 62.7% | 0.232 | 0.999 | – |
| Anti-hypertensive therapy | 550/574 | 1.070 (0.518, 2.210)a | 0.038 | 64.3% | 0.855 | 0.999 | 0.704 |
| Gestational diabetes | 2108/2137 | 0.869 (0.731, 1.033)a | 0.122 | 38.6% | 0.112 | 0.999 | 0.324 |
| Birth weight [g] | 2189/2207 | 99.340 (10.503, 188.177)b | < 0.001 | 96.0% | 0.028 | 0.350 | 0.374 |
| Birth length [cm] | 1572/1574 | 0.449 (0.236, 0.663)b | 0.823 | 0.0% | < 0.001 | 0.999 | 0.063 |
| Birth head circumference [cm] | 1572/1574 | 0.295 (–0.005, 0.595)b | 0.052 | 54.3% | 0.054 | 0.999 | 0.508 |
P-value of heterogeneity χ2.
P-value of pooled statistic.
RR (95% CI),
WMD (95% CI).
Figure 4.
Forest plot for preeclampsia
Figure 5.
Forest plot for pregnancy-induced hypertension
Quality and bias assessment
Multiple complementary methods (funnel plots, Begg’s and Mazumdar’s rank test, and Egger’s test) were used to assess the study quality and risk of bias. The funnel plot was based on the log RR funnel plot for low birthweight for all these studies (Figure 7), which showed a clear symmetry, indicating a low publication bias. There still was no significant bias risk of the included studies by Begg’s and Mazumdar’s rank test (Z = 0.99, p = 0.024) or Egger’s test (p = 0.042). The filled funnel plot was based on the log RR funnel plot for low birthweight for all these studies (Figure 8), which also showed a clear symmetry, indicating a low publication bias (Q = 26.828, p = 0.013).
Figure 7.
Funnel plot of the included studies
Figure 8.
Filled funnel plot of the included studies
Discussion
Compared with the control group, n-3 PUFA significantly decreased the incidence of PD and low birthweight while increasing birth weight and birth length. In addition, these significant efficacies were all associated with neonatal health status. Maternal deficiency of n-3PUFA during pregnancy can lead to fetal growth retardation in utero, which is closely related to low birth weight. Body weight, body length and head circumference are important indicators of infant physical development. Weight can reflect recent nutritional status, body length reflects the long-term nutritional status, and head circumference reflects the development of the brain and skull. If the growth of the physical development index is slow, it indicates that the nutrition and energy needed for growth and development may be insufficient. The incidence of eclampsia was reduced, and this difference was considered to be related to a reduced inflammatory response by n-3 PUFA. However, there was no significant difference in PIH, preeclampsia, IUIG, early PD, anti-hypertensive therapy, gestational diabetes, or head circumference at birth between the two groups. These results were all related to maternal pregnancy outcomes, so there still need to be more high-quality studies to confirm the efficacy of n-3 PUFA in maternal pregnancy outcomes.
N-3 PUFA contains a variety of polyunsaturated fatty acids starting from the methyl end and having the first unsaturated double bond between the third and fourth carbon atoms. It mainly includes α-linolenic acid, EPA and DHA. α-linolenic acid is mainly found in vegetable oils, such as flaxseed oil and chory oil. EPA and DHA, on the other hand, are found mainly in fish oils. DHA is the most abundant polyunsaturated fatty acid in retinal photoreceptors, which is necessary to maintain the normal function of rhodopsin, and also promotes fetal brain development. EPA can reduce cholesterols and triglycerides, reduce blood viscosity, and prevent atherosclerosis and other cardiovascular diseases. Since n-3 PUFA cannot be synthesized in the human body, it must be obtained from food, and pregnancy is a period when the demand for various trace elements increases significantly, so we should eat more fish, especially deep-sea fish, to obtain sufficient n-3 PUFA.
Considering the effect of n-3 PUFA on blood lipid and cardiovascular diseases, as well as its ability to reduce inflammation, patients with diabetes are also recommended to eat more fish to increase the intake of n-3 PUFA. The Chinese Medical Nutrition Guidelines for Diabetes Treatment recommends that n-3 PUFA in the vegetable oil in the diet should be increased. Daily intake of 3.5 g n-3 PUFA can significantly reduce the level of TG in diabetes patients. There is no evidence to recommend whether pregnant women should take supplements of n-3 PUFA or at which specific doses, and there is some debate about the effects of n-3 supplementation. Pregnancy induces changes in the cardiovascular system in order to meet the increased metabolic demands of the mother and fetus, but there are no recommendations about the prevention and management of lipid disorders [23]. Some meta-analyses [24, 25] found that statin therapy would not increase the incidence of birth defects, and an increasing number of pregnant women at high CVD risk are in need of statin therapy. One study [26] also suggested that vitamin D supplementation may be useful in preventing preeclampsia. In the present study, n-3 PUFA had no significant influence on the incidence of PIH, anti-hypertensive therapy and gestational diabetes compared with the control group. Finally, the meta-analysis showed no significant advantage of n-3 PUFA in reducing the incidence of gestational diabetes mellitus and hypertension.
However, the present meta-analysis has some limitations; for example, the number of included RCTs was limited. The exclusion and inclusion criteria were different in different RCTs. Moreover, the results of the study were influenced by multiple factors such as the active ingredient of n-3 PUFA preparation, dosage, duration of intervention and the supplementation of other nutrients in pregnant women’s daily life. Finally, our analysis was based on secondary data as the original data were not available, which precluded in-depth analyses.
In conclusion, this meta-analysis shows that n-3 PUFA is not beneficial in reducing the incidence of maternal pregnancy outcomes such as gestational diabetes mellitus and hypertension, but it is beneficial in neonatal health status.
Acknowledgments
This study was funded by Natural Science Foundation of Liaoning Province (No. 20180550571).
Conflict of interest
The authors declare no conflict of interest.
References
- 1.de Castro GS, Deminice R, Simões-Ambrosio LM, Calder PC, Jordão AA, Vannucchi H. Dietary docosahexaenoic acid and eicosapentaenoic acid influence liver triacylglycerol and insulin resistance in rats fed a high-fructose diet. Marine Drugs 2015; 13: 1864-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwingshackl L, Strasser B, Hoffmann G. Effects of monounsaturated fatty acids on glycaemic control in patients with abnormal glucose metabolism: a systematic review and meta-analysis. Ann Nutrition Metabol 2011; 58: 290-6. [DOI] [PubMed] [Google Scholar]
- 3.Yang ZH, Emma-Okon B, Remaley AT. Dietary marine-derived long-chain monounsaturated fatty acids and cardiovascular disease risk: a mini review. Lipids Health Dis 2016; 15: 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen NM, de Oliveira Andrade F, Jin L, et al. Maternal intake of high n-6 polyunsaturated fatty acid diet during pregnancy causes transgenerational increase in mammary cancer risk in mice. Breast Cancer Res. 2017; 19: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simopoulos AP. An increase in the omega-6/omega-3 fatty acid ratio increases the risk for obesity. Nutrients 2016; 8: 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Expert consensus document of the diagnosis and treatment of pregnancy with heart disease. Zhonghua Fu Chan Ke Za Zhi 2016; 51: 401-9. [DOI] [PubMed] [Google Scholar]
- 7.Jamilian M, Samimi M, Kolahdooz F, Khalaji F, Razavi M, Asemi Z. Omega-3 fatty acid supplementation affects pregnancy outcomes in gestational diabetes: a randomized, double-blind, placebo-controlled trial. J Maternal Fetal Neonatal Med 2016; 29: 669-75. [DOI] [PubMed] [Google Scholar]
- 8.Zhou SJ, Yelland L, McPhee AJ, Quinlivan J, Gibson RA, Makrides M. Fish-oil supplementation in pregnancy does not reduce the risk of gestational diabetes or preeclampsia. Am J Clin Nutrition 2012; 95: 1378-84. [DOI] [PubMed] [Google Scholar]
- 9.DeGiorgio CM, Taha AY. Omega-3 fatty acids (f-3 fatty acids) in epilepsy: animal models and human clinical trials. Expert Rev Neurother 2016; 16: 1141-5. [DOI] [PubMed] [Google Scholar]
- 10.Al Wattar BH, Dodds J, Placzek A, et al. Mediterranean-style diet in pregnant women with metabolic risk factors (ESTEEM): a pragmatic multicentre randomised trial. PLoS Med 2019; 16: e1002857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olsen SF, Secher NJ, Tabor A, Weber T, Walker JJ, Gluud C. Randomised clinical trials of fish oil supplementation in high risk pregnancies. Fish Oil Trials In Pregnancy (FOTIP) Team. BJOG 2000; 107: 382-95. [DOI] [PubMed] [Google Scholar]
- 12.Smuts CM, Huang M, Mundy D, Plasse T, Major S, Carlson SE. A randomized trial of docosahexaenoic acid supplementation during the third trimester of pregnancy. Obstet Gynecol 2003; 101: 469-79. [DOI] [PubMed] [Google Scholar]
- 13.Olsen SF, Secher NJ. A possible preventive effect of low-dose fish oil on early delivery and pre-eclampsia: indications from a 50-year-old controlled trial. Br J Nutr 1990; 64: 599-609. [DOI] [PubMed] [Google Scholar]
- 14.Bulstra-Ramakers MT, Huisjes HJ, Visser GH. The effects of 3g eicosapentaenoic acid daily on recurrence of intrauterine growth retardation and pregnancy induced hypertension. Br J Obstet Gynaecol 1995; 102: 123-6. [DOI] [PubMed] [Google Scholar]
- 15.Onwude JL, Lilford RJ, Hjartardottir H, Staines A, Tuffnell D. A randomised double blind placebo controlled trial of fish oil in high risk pregnancy. Br J Obstet Gynaecol 1995; 102: 95-100. [DOI] [PubMed] [Google Scholar]
- 16.Salvig JD, Olsen SF, Secher NJ. Effects of fish oil supplementation in late pregnancy on blood pressure: a randomised controlled trial. Br J Obstet Gynaecol 1996; 103: 529-33. [DOI] [PubMed] [Google Scholar]
- 17.D’Almeida A, Carter JP, Anatol A, Prost C. Effects of a combination of evening primrose oil (gamma linolenic acid) and fish oil (eicosapentaenoic + docahexaenoic acid) versus magnesium, and versus placebo in preventing pre-eclampsia. Women Health 1992; 19: 117-31. [DOI] [PubMed] [Google Scholar]
- 18.Kuper SG, Abramovici AR, Jauk VC, Harper LM, Biggio JR, Tita AT. The effect of omega-3 supplementation on pregnancy outcomes by smoking status. Am J Obstet Gynecol 2017; 217: 476.e1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harper M, Thom E, Klebanoff MA, et al. Omega-3 fatty acid supplementation to prevent recurrent preterm birth: a randomized controlled trial. Obstet Gynecol 2010; 115: 234-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smuts CM, Borod E, Peeples JM, Carlson SE. High-DHA eggs: feasibility as a means to enhance circulating DHA in mother and infant. Lipids 2003; 38: 407-14. [DOI] [PubMed] [Google Scholar]
- 21.Carlson SE, Colombo J, Gajewski BJ, et al. DHA supplementation and pregnancy outcomes. Am J Clin Nutrition 2013; 97: 808-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mozurkewich EL, Clinton CM, Chilimigras JL, et al. The Mothers, Omega-3, and Mental Health Study: a double-blind, randomized controlled trial. Am J Obstet Gynecol 2013; 208: 313.e1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banach M. Where are the recommendations on healthy lifestyle and cardiovascular disease prevention for pregnant women? J Am Heart Assoc 2020; 9: e016052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maierean SM, Mikhailidis DP, Toth PP, et al. The potential role of statins in preeclampsia and dyslipidemia during gestation: a narrative review. Exp Opin Investig Drugs 2018; 27: 427-35. [DOI] [PubMed] [Google Scholar]
- 25.Vahedian-Azimi A, Makvandi S, Banach M, Reiner Ž, Sahebkar A. Fetal toxicity associated with statins: a systematic review and meta-analysis. Atherosclerosis 2021; 327: 59-67. [DOI] [PubMed] [Google Scholar]
- 26.Fogacci S, Fogacci F, Banach M, et al. Vitamin D supplementation and incident preeclampsia: a systematic review and meta-analysis of randomized clinical trials. Clin Nutr 2020; 39: 1742-52. [DOI] [PubMed] [Google Scholar]