Abstract
Background
Communication about end of life (EoL) and EoL care is critically important for providing quality care as people approach death. Such communication is often complex and involves many people (patients, family members, carers, health professionals). How best to communicate with people in the period approaching death is not known, but is an important question for quality of care at EoL worldwide. This review fills a gap in the evidence on interpersonal communication (between people and health professionals) in the last year of life, focusing on interventions to improve interpersonal communication and patient, family member and carer outcomes.
Objectives
To assess the effects of interventions designed to improve verbal interpersonal communication about EoL care between health practitioners and people affected by EoL.
Search methods
We searched CENTRAL, MEDLINE, Embase, PsycINFO, and CINAHL from inception to July 2018, without language or date restrictions. We contacted authors of included studies and experts and searched reference lists to identify relevant papers. We searched grey literature sources, conference proceedings, and clinical trials registries in September 2019. Database searches were re‐run in June 2021 and potentially relevant studies listed as awaiting classification or ongoing.
Selection criteria
This review assessed the effects of interventions, evaluated in randomised and quasi‐randomised trials, intended to enhance interpersonal communication about EoL care between patients expected to die within 12 months, their family members and carers, and health practitioners involved in their care. Patients of any age from birth, in any setting or care context (e.g. acute catastrophic injury, chronic illness), and all health professionals involved in their care were eligible. All communication interventions were eligible, as long as they included interpersonal interaction(s) between patients and family members or carers and health professionals. Interventions could be simple or complex, with one or more communication aims (e.g. to inform, skill, engage, support). Effects were sought on outcomes for patients, family and carers, health professionals and health systems, including adverse (unintended) effects.
To ensure this review's focus was maintained on interpersonal communication in the last 12 months of life, we excluded studies that addressed specific decisions, shared or otherwise, and the tools involved in such decision‐making. We also excluded studies focused on advance care planning (ACP) reporting ACP uptake or completion as the primary outcome. Finally, we excluded studies of communication skills training for health professionals unless patient outcomes were reported as primary outcomes.
Data collection and analysis
Standard Cochrane methods were used, including dual review author study selection, data extraction and quality assessment of the included studies.
Main results
Eight trials were included. All assessed intervention effects compared with usual care. Certainty of the evidence was low or very low. All outcomes were downgraded for indirectness based on the review’s purpose, and many were downgraded for imprecision and/or inconsistency. Certainty was not commonly downgraded for methodological limitations.
A summary of the review's findings is as follows.
Knowledge and understanding (four studies, low‐certainty evidence; one study without usable data): interventions to improve communication (e.g. question prompt list, with or without patient and physician training) may have little or no effect on knowledge of illness and prognosis, or information needs and preferences, although studies were small and measures used varied across trials.
Evaluation of the communication (six studies measuring several constructs (communication quality, patient‐centredness, involvement preferences, doctor‐patient relationship, satisfaction with consultation), most low‐certainty evidence): across constructs there may be minimal or no effects of interventions to improve EoL communication, and there is uncertainty about effects of interventions such as a patient‐specific feedback sheet on quality of communication.
Discussions of EoL or EoL care (six studies measuring selected outcomes, low‐ or very low‐certainty evidence): a family conference intervention may increase duration of EoL discussions in an intensive care unit (ICU) setting, while use of a structured serious illness conversation guide may lead to earlier discussions of EoL and EoL care (each assessed by one study). We are uncertain about effects on occurrence of discussions and question asking in consultations, and there may be little or no effect on content of communication in consultations.
Adverse outcomes or unintended effects (limited evidence): there is insufficient evidence to determine whether there are adverse outcomes associated with communication interventions (e.g. question prompt list, family conference, structured discussions) for EoL and EoL care. Patient and/or carer anxiety was reported by three studies, but judged as confounded. No other unintended consequences, or worsening of desired outcomes, were reported.
Patient/carer quality of life (four studies, low‐certainty evidence; two without useable data): interventions to improve communication may have little or no effect on quality of life.
Health practitioner outcomes (three studies, low‐certainty evidence; two without usable data): interventions to improve communication may have little or no effect on health practitioner outcomes (satisfaction with communication during consultation; one study); effects on other outcomes (knowledge, preparedness to communicate) are unknown.
Health systems impacts: communication interventions (e.g. structured EoL conversations) may have little or no effect on carer or clinician ratings of quality of EoL care (satisfaction with care, symptom management, comfort assessment, quality of care) (three studies, low‐certainty evidence), or on patients' self‐rated care and illness, or numbers of care goals met (one study, low‐certainty evidence). Communication interventions (e.g. question prompt list alone or with nurse‐led communication skills training) may slightly increase mean consultation length (two studies), but other health service impacts (e.g. hospital admissions) are unclear.
Authors' conclusions
Findings of this review are inconclusive for practice. Future research might contribute meaningfully by seeking to fill gaps for populations not yet studied in trials; and to develop responsive outcome measures with which to better assess the effects of communication on the range of people involved in EoL communication episodes. Mixed methods and/or qualitative research may contribute usefully to better understand the complex interplay between different parties involved in communication, and to inform development of more effective interventions and appropriate outcome measures. Co‐design of such interventions and outcomes, involving the full range of people affected by EoL communication and care, should be a key underpinning principle for future research in this area.
Keywords: Humans, Anxiety, Communication, Physician-Patient Relations, Quality of Life, Randomized Controlled Trials as Topic, Terminal Care
Plain language summary
How can communication about the end of life and care in the last 12 months of life be improved?
Key messages
We did not find enough good quality evidence to be able to say which ways of communicating about end of life (EoL) are best for the people involved. One study of a family conference intervention found that communication interventions might increase the length of EoL discussions between families and health professionals in some situations, and one other found that an intervention which used a structured conversation guide might lead to earlier discussions between patients, carers, and health professionals about EoL and EoL care. We did not find any evidence of harmful or negative effects of communication interventions, and we are uncertain about effects on outcomes like knowledge or quality of EoL care.
Why is communication at the end of life important?
When people are in the last year of their life, it is important that they receive high‐quality care (refer to ACSQHC 2015 and 2015b references for more on care at end of life). Communication about EoL is a critical part of such care. It helps patients and their families and carers to understand what is happening, to know what to expect and what their options are, to ask questions and receive support, and to be involved in decisions and planning as much as they would like to be. Communication about EoL is not always done well and this can have negative effects. Understanding how to improve such communication between the different people involved in care at EoL (patients, family members, carers, health professionals) is important to help ensure that people receive the best possible care in the time leading up to death.
What did we want to find out?
We wanted to find out which ways of communicating with patients and carers might be best for improving people’s knowledge about the EoL (e.g. what to expect, treatment options).
What people thought about the communication (e.g. satisfaction, communication quality, how involved they were and wanted to be in consultations). Discussions about end of life (e.g. how often these happened, and when).
We also wanted to find out if communication interventions might increase unwanted or harmful effects, like fear or distress.
What did we do?
We searched for studies that looked at communication interventions compared with usual care (care that is provided routinely or as the standard way of treating people), or comparing one type of communication (e.g. providing information) with another (e.g. providing information together with support), in people of all ages from birth onwards and who were expected to die within 12 months. We summarised the results of the included studies and rated our confidence in the evidence based on factors such as study size, study methods, and the people studied by the trials.
To ensure this review's focus was maintained on interpersonal communication in the last 12 months of life, we excluded studies that addressed specific decisions, shared or otherwise, and the tools involved in such decision‐making. We also excluded studies focused on advance care planning (ACP) reporting ACP uptake or completion as the primary outcome. Finally, we excluded studies of communication skills training for health professionals unless patient outcomes were reported as primary outcomes.
What did we find?
We found eight studies that compared the effects of communication interventions for people at EoL with usual care. Interventions were varied and ranged from simple approaches like a list to help patients and carers ask questions in consultations, through to complex structured conversation interventions to engage patients and carers in discussions about EoL and the care they wished to receive.
We found that a family conference intervention may increase the length of EoL discussions in some situations, and a structured serious illness conversation guide might lead to earlier discussions between patients, carers and health professionals about EoL and EoL care.
We also found there may be little effect of communication interventions on knowledge, on what people thought about the communication (e.g. quality of communication, how involved in the discussion they would like to be) or on outcomes like numbers of questions asked by patients in consultations with their doctors. We did not find any evidence of harmful or negative effects of the interventions, but the studies were mostly small and not designed primarily to identify these.
There may also be little effect on the other outcomes we looked for, like quality of life, quality of EoL care, or numbers of care goals met. In other cases, we are unsure because there was little or no evidence available (e.g. health professional outcomes like knowledge or confidence to communicate, or health service use e.g. hospital admissions).
What are the limitations of the evidence?
We have very little confidence in the evidence: included studies only looked at communication for older adults in high‐income countries, whereas the review looked for evidence across the whole lifespan and irrespective of country and setting. Additionally, included studies often studied small numbers of people.
How up to date is this evidence?
The evidence is up to date to July 2018.
Summary of findings
Summary of findings 1. Summary of findings .
| Communication intervention compared with usual care for end of life care | |||
|
Patient or population: people approaching the end of life (within 12 months), their family members and/or carers Settings: any (residential care, hospital (inpatient and outpatient units) and community‐based clinics, palliative care services) Intervention: interventions to improve communication about EoL and/or EoL care Comparison: usual care | |||
| Outcomes | Intervention effects | Number of participants (studies) | Certainty of the evidence (GRADE) |
| Patient, family and/or carer outcomes | |||
| Knowledge and understanding Variable scales: information (amount and type) needs/preferences; discordance between patient and physician survival and curability estimates Timing: immediately to 1 month post‐consultation |
Overall, interventions to improve communication may have little or no effect on measures of knowledge of illness and prognosis, or information needs and preferences In 1 study (303 participants), discordant estimates of 2‐year survival between patients and doctors (intervention 59% versus usual care 62%) and curability (intervention 39% versus usual care 44%) were similar between groups (Epstein 2017). Another (79 participants) reported similar proportions of patients had their preferences for amount of information met or exceeded across intervention and usual care groups, but that type of information was met or exceeded more often in the intervention group (93% versus 80%) (Walczak 2017). 1 final study (170 participants) reported no differences in patients’ unmet information needs overall (Clayton 2007) |
552 (3 studies)c | ⊕⊕⊝⊝
lowa,b |
| Evaluation of the communication: different constructs (perceptions of communication quality; patient‐centredness of communication; involvement preferences; doctor‐patient relationship measures) Timing: immediately post‐consultation to 18 weeks post‐consultation |
Across constructs (patient‐centredness, involvement preferences, doctor‐patient relationship, satisfaction with consultation), there may be minimal or no effects of interventions to improve communication about EoL and EoL care (Agar 2017; Bernacki 2019; Clayton 2007; Epstein 2017; Walczak 2017), and uncertaintye about effects on quality of communication (Au 2012) |
6 studiesf | ⊕⊕⊝⊝ lowb,d |
| Discussions of EoL/EoL care: discussion timing and length EMR review post‐death Timing: at time of family conference (intervention) in ICU; post‐death |
The intervention may lead to longer and earlier discussions of EoL and EoL care, compared with usual care, but each result is based on a single study 1 study (108 participants) reported comparative data: median family conference intervention duration was 30 minutes (IQR 19 to 45 minutes) versus usual care (median 20 minutes, IQR 15 to 30 minutes) (Lautrette 2007) 1 study (376 participants) reported that the first documented Serious Illness Conversation happened earlier among intervention group participants (median 143 days prior to death (IQR 71 to 325) than usual care (71 days, IQR 33 to 166) (Bernacki 2019) |
484 (2 studies) | ⊕⊕⊝⊝ lowb,d |
| Discussions of EoL/EoL care: discussion occurrence EMR review post‐death; coding of consultations; self‐reported occurrence Timing: immediately, 1 or 2 weeks post‐consultation; after death |
Overall, we are uncertain about the effects of interventions to improve discussions about EoL care 2 studies indicated that the intervention increased the occurrence of EoL discussions, compared with usual care (RR 1.96, 95% CI 1.61 to 2.39; 2 trials, 537 participants); the others indicated little or no effect of the intervention on mean total numbers of patient questions in consultations (MD 1.58, 95% CI ‐1.82 to 4.98; 2 trials, 249 participants) |
786 (4 studies) | ⊕⊝⊝⊝
very lowb,g,h |
| Adverse (unintended) outcomes |
There is insufficient evidence to determine whether adverse (unintended) outcomes are associated with communication interventions. Patient and/or carer anxiety was reported (3 studies), but was judged as confounded, and no other unintended consequences, or worsening of desired outcomes, were reported | ‐ | ‐ |
| CI: confidence interval; EMR: electronic medical record; EoL: end of life; ICU: intensive care unit; IQR: interquartile range; MD: mean difference; RR: risk ratio | |||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of the effect. | |||
aDowngraded (‐1) due to inconsistency (different outcome measures and concepts assessed across studies). bDowngraded (‐1) for indirectness (all participants were older patients with advanced cancer or chronic obstructive pulmonary disease (COPD) or both, and results may not apply to other populations nearing EoL). c1 further study (Lautrette 2007, 108 participants) conducted in an ICU setting did not report useable data. dDowngraded (‐1) due to imprecision (results are from a single study and/or a small number of participants). eQuality of communication also downgraded (‐1) due to methodological limitations (sequence generation rated at unclear risk of bias). fMost of these outcomes under this broad construct were assessed by only 1 study; doctor‐patient relationship was reported by 3 studies (238 participants); and numbers of participants were consistently small across all outcome measures. gDowngraded (‐1) for inconsistency (2 of 4 studies indicated that the intervention had no effect, with residual variation despite similar populations and interventions). hDowngraded (‐1) for methodological limitations (the largest study rated as at unclear risk of bias on sequence generation).
Background
Description of the condition
Discussion about end of life (EoL) between health practitioners and affected people can be a confronting experience for all parties involved. According to the Australian Medical Association, "Death, dying and bereavement are all an integral part of life; however, reflecting on, and discussing death, can be profoundly confronting and difficult. Open and frank discussion of death and dying including EoL care options, approach to futile treatment, caring and bereavement should be encouraged within the profession and in the wider community" (AMA 2014). EoL and EoL care can be defined in many different ways. For this review, we have adopted the following definitions, developed as part of a national (Australian) consensus statement on end of life care.
End of life: "the period when a patient is living with, and impaired by, a fatal condition, even if the trajectory is ambiguous or unknown. This period may be years in the case of patients with chronic or malignant disease, or very brief in the case of patients who suffer acute and unexpected illnesses or events, such as sepsis, stroke or trauma" (ACSQHC 2015, page 33).
-
End of life care: "includes physical, spiritual and psychosocial assessment, and care and treatment delivered by health professionals and ancillary staff. It also includes support of families and carers, and care of the patient’s body after their death. People are 'approaching the end of life' when they are likely to die within the next 12 months. This includes people whose death is imminent (expected within a few hours or days) and those with:
advanced, progressive, incurable conditions;
general frailty and co‐existing conditions that mean that they are expected to die within 12 months;
existing conditions, if they are at risk of dying from a sudden acute crisis in their condition; and
life‐threatening acute conditions caused by sudden catastrophic events" (ACSQHC 2015, page 33).
Taken together, the above definitions show that the EoL period may be one of prognostic uncertainty and highly variable in duration. This review acknowledges this uncertainty and the difficulties associated with defining this period. When the selection criteria for this review were developed, the definition given by the above statement (i.e. people are approaching EoL when they are expected to die within 12 months) was the most recent available definition for Australian audiences and so was adopted as a working definition to define the scope of the review.
People involved in communication with health practitioners about EoL and EoL care may include the person at EoL and the family or carers of that person (Hjelmfors 2020; Wolfe 2020). Each of these people may have an important role in discussions about EoL care. For the purpose of this review, we needed to define these different people in a way that is not ambiguous, given the multiplicity of terms that are used in different health systems for all parties. Further, although the term 'patient' is not always suitable for someone who may often not be in a patient role, we needed to distinguish the person at EoL from that person's family member or carer. We therefore define affected people as follows.
Patient: identified as "the primary recipient of care" (ACSQHC 2015, page 34). In many health systems and countries, terms other than 'patient' are preferred. However, in this review we use this term to distinguish clearly between people who are approaching the end of their life, or dying (and to whom discussions about prognosis, treatment, and care relate directly), and people to whom these discussions relate indirectly (i.e. discussions about EoL and EoL care related to a family member or person in whose care they are involved).
Family: this review takes the broadest possible view of family members, considered to represent "those who are closest to the patient in knowledge, care and affection. This may include the biological family, the family of acquisition (related by marriage or contract), and the family and friends of choice" (ACSQHC 2015, page 33). It also includes First Nations definitions of family within the wider culture, such as those encompassed by the concept of kinship care (Palliative Care Australia 2016).
Carer: "a person who provides personal care, support and assistance to another individual who needs it because they have a disability, medical condition (including a terminal or chronic illness) or mental illness, or they are frail and aged. An individual is not a carer merely because they are a spouse, de facto partner, parent, child, other relative or guardian of an individual, or live with an individual who requires care" (ACSQHC 2015, page 32).
The focus of this review is interpersonal interactions occurring between patients, family, carers, and health practitioners at the EoL.
EoL discussions are often placed within the context of palliative care. The "WHO [World Health Organization] identified that, globally, palliative care needs are very high, with an estimated 20 million people needing end‐of‐life care each year" (AIHW 2014, page 2). This enormous demand exists across countries and healthcare systems (World Wide Palliative Care Alliance 2014), yet palliative care is only one of the contexts in which good communication about EoL care is essential.
Internationally, a large body of research has documented difficulties in EoL communication between healthcare professionals and people affected by EoL (i.e. patients, their families, and carers) (Anderson 2019; Clayton 2007a; Fawole 2012; Fujimori 2020; IoM 2014; NICE 2017; Walczak 2016). These difficulties include failure to communicate adequately with the person who is dying about his or her prognosis (Barnes 2006; Brighton 2016; Fawole 2012; Gott 2009; NICE 2017), or to provide understandable information on what the future holds, and decisions that the person and family members and carers may need to make (Alsakson 2012; Anselm 2005; Barnes 2012; Gutierrez 2012; NICE 2019; Selman 2007). It is also documented that patients receiving EoL care, or those closest to them, may not be given the opportunity to ask questions or to check their understanding of information that has been provided (Alsakson 2012; Clayton 2007a; Gutierrez 2012; Hjelmfors 2020). People often have misunderstandings about their prognosis and goals of treatment in the EoL period (Anderson 2019; Clayton 2007a; ; Gattellari 1999; Thode 2020; Weeks 1998). Misunderstandings may also arise from conflicting information given by multiple practitioners involved in the patient's care. Additionally, the patient and family members or carers may have their own questions about EoL care but may be unaware of how or whom they should approach to find answers to these questions (Alsakson 2012; Anselm 2005; Gutierrez 2012; NICE 2017).
Communication problems have significant potential to negatively impact the person who is dying and family members or carers. Communication problems may contribute to loss of trust in health practitioners (Clayton 2007a; NICE 2017), poorer quality of life and satisfaction, psychological harms, and avoidable distress (Chochinov 2000; Fawole 2012; Fujimori 2020; NICE 2017; Schofield 2003; Selman 2007; Wright 2008). These negative outcomes reflect poorly on the ability of existing healthcare systems to effectively deliver patient‐centred, responsive care during EoL (Anderson 2019; IoM 2014; NICE 2017; NICE 2019). In comparison, high‐quality communication about EoL is associated with improved quality of life and less aggressive approaches to treatment, as well as better outcomes for carers related to bereavement (Brighton 2016; Detering 2010; Heyland 2009; Wright 2008; Zhang 2009).
Communicating effectively about EoL is a difficult and complex task that is further complicated by uncertainty about the trajectory of the last stages of a person's life and the associated prognosis (Barnes 2012; Brighton 2016; Fawole 2012). Good communication remains the primary means of preparing the patient and affected people for the last months, weeks, and days of life (Anderson 2019; Fawole 2012). This review focuses on general communication between health practitioners and people affected by EoL ‐ not specifically on communication involving use of specific tools to achieve structured decision‐making (such as communication or discussion in which participants use a highly structured checklist to develop an advance care plan). Such specific, structured decision‐making approaches were therefore excluded. With focus on general communication, this review is able to evaluate the evidence on interventions intended to improve communication about EoL care among health professionals and patients and their family members and carers. Previous research has shown that discussion of prognosis and EoL is important to people who are dying and to their families (Brighton 2016; Fujimori 2020; NICE 2019; Steinhauser 2000; Walczak 2016; Wenrich 2001; Wolfe 2020). It is clear that for people to be able to articulate what they would like, and to participate in decisions about their care in the last stages of their life or the life of someone close to them, they must be adequately informed (Clayton 2007a; NICE 2019). A recent study seeking to develop quality indicators for EoL communication and decision‐making confirmed that these discussions are also important to health professionals and the systems in which EoL care is delivered (Sinuff 2015). The highest‐rated quality indicator overall was related directly to whether discussions about prognosis and the likelihood that the patient is approaching the end of life had actually been undertaken (Sinuff 2015). This review therefore seeks to evaluate how communication about EoL and EoL care might be better undertaken, and to assess the impact of verbal communication on the various people most directly involved.
Description of the intervention
Communication interventions can be broadly defined as "a purposeful, planned and formalised strategy associated with a diverse range of intentions or aims, including to inform, educate, communicate with, support, skill, change behaviour, engage and seek participation of people" (Hill 2011, page 30). This review follows this broad view and considers a communication intervention as a planned interaction provided by health practitioners to communicate with people about EoL and provision of EoL care. Although these interventions may take many forms and may reflect different purposes, to be eligible for this review interventions must have included direct interpersonal (verbal) communication between health practitioners and the patient and the patient's family members and/or carers. Specifically, these interventions could have taken the form of facilitating or improving EoL care discussions targeting a broad range of continuum of care, ranging from rapidly evolving situations to early preparatory stages of what may be a protracted period of terminal care. The review included any EoL communication intervention that involved a patient who was likely to die within 12 months (ACSQHC 2015, page 2; NICE 2017, page 7). Communication interventions must have been primarily interpersonal (verbal) in nature and preferably delivered in‐person, although if necessary they may have included the following channels for communication: in‐person, telephone, videoconferencing, remote video links, and internet‐enabled verbal discussions. Other non‐verbal forms of communication, such as written information, may also have been included as part of the intervention, and while data were collected on these approaches they were not the primary focus of the review.
The intervention may have focused on one or more of the following elements of EoL or EoL care: knowledge of what might happen around the disease and what a possible disease trajectory might be for the patient (prognosis); understanding of the possibilities for treatment, pain management, symptom management, and treatment or care to relieve suffering; preferences for care or treatment or both, including wishes regarding the location of living until dying; needs or concerns related to supportive, spiritual, cultural, or palliative care; needs or concerns related to the role of the family or carer, including support for family members/carers; needs or concerns associated with administrative paperwork, formal documentation, dying or the choice for assisted dying (for jurisdictions where relevant); and death. The intervention may have been tailored towards an individual or a small group, as long as the group includes patients and their family members or carers.
We considered the full range of EoL communication interventions identified as eligible for this review, and we anticipated that their dispersion and application across studies might vary. The needs and circumstances of the people involved were also expected be complex and highly varied. Accordingly, the elements of EoL and EoL care discussed were expected to be tailored to specific EoL contexts. EoL discussions are not limited to a specific healthcare setting, so it was important that this review was inclusive of EoL communication interventions applied irrespective of national, geographical, cultural, social, wealth, and healthcare access boundaries. Such diverse EoL experiences could be related to gender, ethnicity, race, religion, culture, refugee status, indigenous peoples, gender diversity, disability, socioeconomic status, education, poverty, and populations in low‐ and middle‐income countries (Welch 2010). For this reason, the review considered inequality and inequity issues as they relate to EoL communication interventions (Welch 2010).
To ensure this review's focus was maintained on interpersonal communication in the last 12 months of life, we excluded studies that addressed specific decisions, shared or otherwise, and the tools involved in such decision‐making. We also excluded studies focused on advance care planning (ACP) reporting ACP uptake or completion as the primary outcome. Finally, we excluded studies of communication skills training for health professionals unless patient outcomes were reported as primary outcomes.
How the intervention might work
Interventions to improve EoL verbal communication aim to provide more effective general communication between practitioners and the people directly affected by EoL and EoL care. Previous reviews have confirmed the highly complex and varied scope of EoL experiences, and support the need for the study intervention to be fully described and to include EoL context, details of what the intervention entails, and related primary patient outcomes (Fawole 2012; Walczak 2016).
We have described the content of the EoL communication intervention above. Practitioners could use a variety of modalities to deliver the intervention and to guide or influence the discussion about EoL or EoL care. Examples could include prompts for patients to promote or guide discussions about EoL care (Clayton 2007b; Fujimori 2020; Hjelmfors 2020; Sansoni 2014; Walczak 2017); web‐based collaboration tools to facilitate communication between practitioners and people affected by EoL (Voruganti 2017; Walczak 2016); nurse‐led discussions about EoL care (Sulmasy 2017); or EoL family meetings (Agar 2017; Bradford 2021; Walczak 2016). Outcomes chosen to measure effects of the interventions could reflect changes in the level of communication occurring (e.g. increasing the frequency or length or both of discussions between practitioners and patients and affected people), improved structure of the communication taking place (e.g. providing prompts to assist patients, family members, and carers to ensure that key questions are raised with practitioners, thereby improving knowledge and understanding about EoL care), or specific outcomes related to patient's/affected people's EoL care experiences and their experiences of the communication around EoL.
Why it is important to do this review
General EoL communication guidelines are already available. For example, in 2007, Medical Journal of Australia published a supplement titled 'Clinical practice guidelines for communicating prognosis and end of life issues with adults in the advance stages in a life limiting illness, and their caregivers' (Clayton 2007a). More recently published EoL guidelines related to paediatric patients and young people include the 'End of life planning series' (Together for Short Lives 2012), along with 'Difficult Conversations' (Together for Short Lives 2015). EoL care standards and quality markers and measures of EoL care related to communication are also available (ACSQHC 2015; NICE 2017). A more recent exploratory study conducted with paediatric practitioners confirmed that evidence‐based interdisciplinary interventions are needed to support general EoL discussions (Henderson 2017). A systematic review of communication quality improvement interventions for patients with advanced and serious illness completed in 2012 confirmed that better descriptions of communication interventions were needed for assessment of impact on the outcomes being researched (Fawole 2012). Although general guidelines on communication are available, they do not necessarily address or draw on rigorous research evidence related to the effectiveness of specific EoL communication interventions.
A systematic review and meta‐analysis undertaken by Oczkowski in 2016 examined communication tools for EoL decision‐making in ambulatory care settings (Oczkowski 2016). The Oczkowski review was focused on EoL decision‐making and advance care planning and concluded that use of structured communication tools should be the preferred approach to EoL decision‐making conversations (Oczkowski 2016). Another recent systematic review of studies of mixed designs (Thode 2020) assessed the role of communication tools such as decision aids for people considering life‐prolonging treatments. It similarly concluded that prompt lists and decision aids may be useful in communicating with patients about options for treatment, but was based on a small number of studies in a population that is not directly relevant to this current review. Two completed Cochrane Reviews ('Advance care planning for haemodialysis patients' (Lim 2016) and 'Advance care planning for adults with heart failure' (Nishikawa 2020) also have an indirect link with this review. The current review includes discussions on the topic of advance care planning, but only when these conversations are taking place in the last 12 months of life, and only when uptake of advance care planning (ACP) or advance directives (AD) is not the primary goal of the study.
Several other Cochrane Reviews, for example 'End‐of‐life care pathways for improving outcomes in caring for the dying' (Chan 2016); 'Hospital at home: home based end of life care' (Shepperd 2021); and 'Communication skills training for healthcare professionals working with people who have cancer' (Moore 2018), have addressed issues related to EoL, but they have not addressed the interventions to improve communication, with a distinct focus on patient outcomes, explored in this current review.
Studies of ACP or AD that did not meet these criteria were therefore deliberately excluded as they focused on the outcomes of the process (i.e. ACP completion) rather patient outcomes (this review's focus). Additionally, these strategies are commonly not closely related in time to the end of life, with many elderly people now asked to undertake ACP in preparation for death that may be years or even decades in the future. This variability in degree of temporal linkage to EoL, as well as heavy reliance on checklists and structured tools common with ACP, also led to the exclusion of these studies. As indicated above, studies focusing on specific decisions using structured tools (e.g. decision aids) were excluded in order to ensure the review maintained a focus on patient outcomes and how these were influenced by communication.
Communication skills training for health professionals was also excluded from this review, unless patient outcomes were reported as primary outcomes. This decision aligned with the reasoning above, as interventions to prepare professionals to communicate typically focus on evaluating the effectiveness of such strategies to improve clinician skills (how, and how well, clinicians communicate) ‐ a step influencing but preceding the communication encounter with patients, and typically reflected in a lesser focus on patient outcomes. One of the review's main underpinning principles was that interventions involved interpersonal interaction between health practitioner(s) and the patient, family, and/or carers in order that the focus on patients be maintained.
It is worth noting that had we included studies with a focus on structured decision‐making tools like those underpinning many approaches to ACP, or those on communication skills training, this review would have quickly become unfeasible and run to inclusion of potentially hundreds of trials ‐ as this represents a very substantial literature. Such a review however, would have a far more dispersed scope, and it would have been very difficult to untangle the effects of interpersonal communication for the people involved at EoL within this larger collective body of research.
Previous reviews of the literature have considered EoL communication interventions. Barnes 2012 undertook a critical review of the literature to explore patient‐professional communication about EoL issues in life‐limiting conditions. These review authors found limited evidence regarding successful interventions to improve discussions with patients about EoL care. Additionally, communication topics are often embedded in more specific EoL research. Walczak 2016 completed an important systematic review of evidence for EoL communication interventions. This review identified 45 studies through a search of the literature conducted in 2014, and review authors concluded that "Overall, greater use of validated measures, commonality of outcomes between studies and meta‐analyses allowing more concrete statements about the efficacy of end‐of‐life communication interventions are vital to the advancement of the field" (Walczak 2016, page 13). Bradford 2021 completed a systematic review regarding family meetings in paediatric palliative care, finding there was little guidance about how meetings should be organised or conducted, or when these should occur. Overall, the literature confirms that there is general agreement that EoL communication and interventions to improve such communication are important for providing quality care for patients and other people affected by EoL.
To inform how EoL communication can be improved in future practice, one must gain an understanding of the effectiveness of communication interventions in the EoL context, and the impact these interventions can have on measurable outcomes for patients, families, and carers. The findings of this review should prove important in this endeavour. Improved and more effective communication between health practitioners and people affected by EoL has the potential to help practitioners address gaps in care and to improve poor outcomes such as distress and lower quality of life associated with poor communication (Brighton 2016). This will provide a foundation where patients and others affected by EoL events can participate in shared decisions about treatment and care.
Objectives
To assess the effects of interventions designed to improve verbal interpersonal communication about end of life (EoL) care between health practitioners and people affected by EoL.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised and cluster‐randomised controlled trials (RCTs) and quasi‐RCTs evaluating the effects of interventions intended to enhance communication between health practitioners and patients and families or carers about end of life (EoL) care. We expected to find a limited number of RCTs on this topic and therefore planned to include quasi‐RCTs (defined as trials attempting, but not achieving, random allocation of participants).
Types of participants
We included the following participants.
Patients with a life‐limiting illness who were expected to die within 12 months (ACSQHC 2015).
Patients with cancer, end‐stage pulmonary disease, end‐stage cardiac failure, end‐stage renal failure, motor neuron disease, or other chronic conditions (e.g. dementia), as reported in the study.
Patients with a life‐threatening acute condition caused by sudden catastrophic events (ACSQHC 2015).
Vulnerable groups of patients with a life‐limiting illness, as reported in the study. For example, patients could be in a third world setting in which EoL is not explicitly defined. In such cases, researchers may use terms such as 'dying' and 'death', which can be used to identify the study as relevant.
Patients of any age from birth who met one of the criteria listed above.
We also included family or carers of a patient with a life‐limiting illness, as defined by the study. We defined family as "biological, family of acquisition (related by marriage or contract) and the family and friends of choice" (ACSQHC 2015, page 33). We defined a carer as "a person providing personal care, support and assistance for the patient with a life‐limiting illness" (ACSQHC 2015, page 32).
We did not exclude studies based on the setting of the communication or the person delivering the communication, although the communication must have involved a health practitioner. We defined health practitioners for inclusion in this review as follows.
Healthcare professionals may include doctors, nurses, midwives, allied health practitioners, social workers, and government healthcare workers.
The professional population could be identified as the healthcare team, the interdisciplinary team, the interprofessional team, or a group of healthcare providers, as reported in the study.
We included lay health workers, who are not health practitioners as such but who are educated/trained to deliver the intervention (e.g. may be applicable in resource‐poor/low‐ and middle‐income country settings or within a specific cultural context to promote cultural safety).
We included other community providers or volunteers, as reported in the study.
Types of interventions
We included any interventions provided to promote or improve interpersonal communication between health practitioners and people affected by EoL care versus usual care. We also included comparisons of one form of communication intervention versus another.
The communication may have focused on any aspect of EoL or EoL care, including the following.
Knowledge of what might happen around the disease and what a possible disease trajectory might be for the patient (prognosis).
Understanding of the possibilities for treatment, pain management, symptom management, and treatment or care to relieve suffering.
Preferences for care or treatment or both (e.g. resuscitation, feeding), including wishes regarding the location of living until dying.
Needs or concerns related to supportive, spiritual, cultural, or palliative care.
Needs or concerns related to the role of the family or carer, including support for family members/carers.
Needs or concerns associated with administrative paperwork, formal documentation, and dying or the choice for assisted dying (for jurisdictions where relevant).
The intervention must have involved interpersonal interaction between health practitioner(s) and the patient, family, and/or carers. We included videoconferencing, remote video links, or internet‐enabled discussions only if the parties involved could not be located physically together (e.g. in the case of patients living in rural, remote, or underserved areas).
The communication intervention might have included one or more of the following aims: to inform or educate, support, skill, engage, or seek the participation of patients and their families and carers in a communication episode with professionals around EoL care. Interventions could be simple or complex; we included interventions as long as the effects of the communication element of any complex intervention could be isolated by inclusion of an appropriate comparison group.
We excluded the following studies.
Studies focusing on specific decisions ‐ shared or otherwise. This review focused on general communication between health practitioners and patients and their family members and carers. Such communication may be viewed as a necessary and fundamental precursor to more specific decisions about treatment and other choices, which may often involve highly structured or specific communication tools (as described above).
Studies focusing on development or completion of an advance care planning (ACP) or advance directives (AD) for which uptake or completion is the primary outcome.
Studies assessing the effects of public education (e.g. on ACP), or of general individual education (e.g. about ACP, or about how to speak up).
Studies focusing on case conferencing for specific decision‐making needs, or case conferencing about choice of residence (e.g. discharging patient to a nursing home or to a palliative care service).
Studies focusing on communication skills training for health professionals (unless patient outcomes were reported as primary outcomes).
Studies involving health practitioner communication with a group of people, unless that group comprised the patient, family members, and/or carers.
Types of outcome measures
We collected data on a range of primary and secondary outcomes.
Primary outcomes
Patient, family, and/or carer (affected persons) outcomes
Knowledge and understanding about what might happen (prognosis), or what to do, or options.
Evaluation of the communication ‐ positive constructs (e.g. satisfaction, calmness or confidence about ability to manage the future).
Evaluation of the communication ‐ negative constructs (e.g. fear, anxiety, distress).
Discussions of EoL care/EoL (e.g. frequency, length, type, participants).
Adverse outcomes
-
Any adverse outcomes or harms identified in the included studies.
These might have included any negative effects on the primary outcomes listed above.
Secondary outcomes
Health practitioner knowledge and understanding of patient/family/carer knowledge, wishes, or preferences.
Health practitioner evaluation of his or her communication performance, the overall communication encounter, or self‐confidence or preparedness to communicate.
Patient/family member/carer quality of life.
Health systems impacts relevant to the impacts of communication
Costs of subsequent care.
Hospital admissions and re‐admissions (e.g. hospital bed days, intensive care unit (ICU) admissions).
Quality of EoL care (family/carer rated, practitioner rated).
Ratings of concordance with patient preferences for EoL care.
We did not exclude studies that were otherwise eligible based on the outcomes reported, except for the situation described above, in which the intervention focused on ACP/AD and the primary outcome sought was uptake or completion.
Main outcomes for the summary of findings tables
We reported the following outcomes.
Patient, family, and/or carer (affected persons) outcomes
Knowledge and understanding about what might happen (prognosis), what to do, or options.
Evaluation of the communication ‐ positive constructs (e.g. satisfaction, calmness or confidence about ability to manage the future, preparedness to plan for the future).
Evaluation of the communication ‐ negative constructs (e.g. fear, anxiety, distress).
Adverse events
These were reported as any negative changes in the above outcomes associated with the intervention.
We reported findings for each of the primary outcomes in the summary of findings tables.
If multiple outcomes were reported in a given outcome category, we collected information on all relevant outcomes. However, if the same outcome had been assessed by two or more outcome measures in the same trial, we planned for two review authors to:
select the primary outcome measure identified by the publication authors;
select the one specified in the sample size calculation when no primary outcome measure was identified; and
-
rank effect estimates (i.e. list them in order from largest to smallest) and select the median effect estimate if no sample size calculations were reported.
When an even number of outcome measures was reported, we planned to select the outcome measure whose effect estimate was ranked n/2, where n was the number of outcome measures.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases in July 2018, all from inception.
Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library (to 27 July 2018).
MEDLINE (OvidSP) (1946 to 27 July 2018).
Embase (OvidSP) (1947 to 27 July 2018).
PsycINFO (OvidSP) (1806 to 27 July 2018).
Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCOHost) (1937 to 27 July 2018).
Search strategies for all major databases are presented in Appendix 1 to Appendix 2.
There were no language or date restrictions.
We applied the Cochrane RCT Classifier to the database search results. The Classifier assigned a probability (from 0 to 100) of being a randomised trial to each citation retrieved. Those citations with a Classifier scores of nine or less were excluded from dual reviewer screening but were screened by a single reviewer (titles and abstracts) as part of a check of the accuracy of the Classifier and to ensure that no studies were misclassified and wrongly excluded from the search outputs.
Database searches were re‐run in June 2021. Studies potentially meeting the selection criteria are listed in studies Awaiting classification.
Searching other resources
We contacted experts in the field and authors of included studies for advice as to other relevant studies and searched reference lists of relevant studies. We searched (September 2019) relevant grey literature sources (ProQuest Dissertations & Theses, British Library Electronic Theses Online Service (EThOS)), conference proceedings (European Association for Palliative Care (EAPC), American Society of Clinical Oncology (ASCO), World Congress of Psycho‐oncology), and clinical trials registries (US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov and World Health Organization International Clinical Trials Registry Platform (WHO ICTRP)) to identify relevant trials.
Data collection and analysis
Selection of studies
Two review authors independently screened all titles and abstracts identified through searches to determine which met the inclusion criteria. We retrieved in full text any papers identified as potentially relevant by at least one review author. Two review authors independently screened full‐text articles for inclusion or exclusion and resolved discrepancies by discussion and by consultation with a third review author if necessary to reach consensus. We listed all potentially relevant papers excluded from the review at this stage as excluded studies and provided reasons for exclusion in the 'Characteristics of excluded studies' table. We also provided citation details and any available information about ongoing studies and collated and reported details of duplicate publications, so that each study (rather than each report) is the unit of interest in the review. We report the screening and selection process in an adapted PRISMA flow chart (Liberati 2009); see Figure 1.
1.
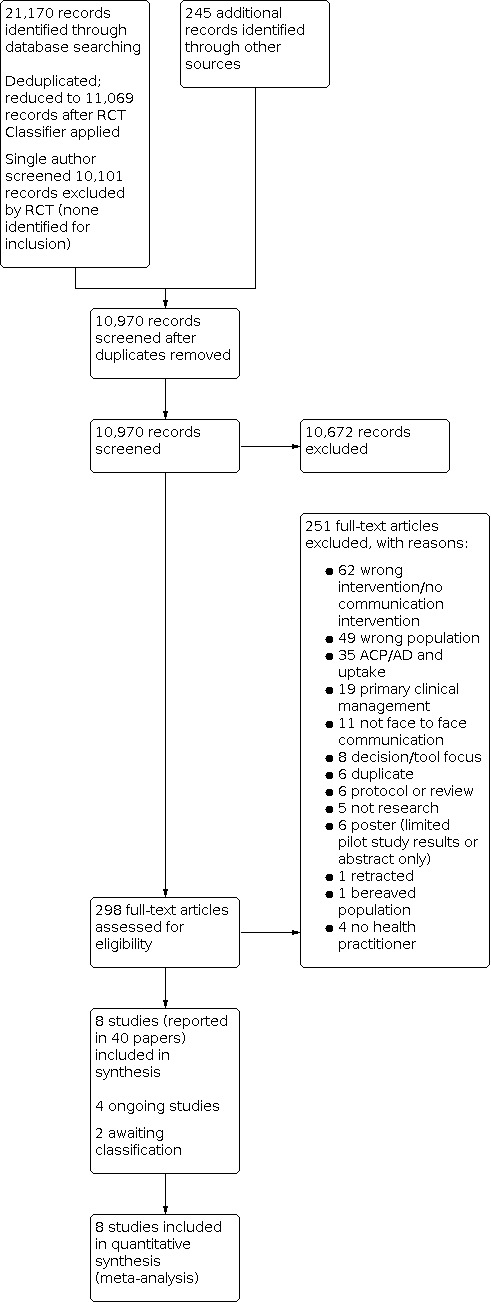
Study flow diagram.
Screening was performed by at least two review authors working independently, except those citations classified with a score of nine or less by the RCT Classifier, which were screened by a single review author. Citations from conference proceedings and trials registries were also screened by a single review author, who consulted with a second review author on any potentially relevant studies.
Data extraction and management
Two review authors extracted data from included studies. Ratings of risk of bias were made independently by two review authors, otherwise data were extracted by one review author and checked for accuracy by a second. We resolved any discrepancies by discussion until consensus was reached, or through consultation with a third review author when necessary. We developed and piloted a data extraction form using the Cochrane Consumers and Communication Group (CCCG) Data Extraction Template (available at cccrg.cochrane.org/author-resources)). Data extracted included the following: study details (aim of intervention, study design, description of the intervention and comparison group, outcomes, and data). One review author entered all extracted data into Review Manager 5 (Review Manager 2020), and a second review author working independently checked the data for accuracy against the data extraction sheets.
Assessment of risk of bias in included studies
We assessed and reported on the methodological risk of bias of included studies in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), as well as the guidelines of the Cochrane Consumers and Communication Group (Ryan 2013), which recommend explicit reporting of the following individual elements for RCTs: random sequence generation, allocation sequence concealment, blinding of participants and personnel, blinding of outcome assessment, completeness of outcome data, selective outcome reporting, and other sources of bias (baseline imbalances). We considered blinding separately for different outcomes when appropriate (e.g. blinding may have the potential to differently affect subjective versus objective outcome measures). We judged each item as being at high, low, or unclear risk of bias as set out in the criteria provided by Higgins 2011, and provided a quote from the study report or a justification for our judgement or both for each item in the risk of bias table.
We judged studies to be at highest risk of bias if they scored as at high or unclear risk of bias for either the sequence generation or the allocation concealment domain, based on growing empirical evidence that these factors are particularly important potential sources of bias (Higgins 2011).
In all cases, two review authors independently assessed the risk of bias of included studies and resolved disagreements by discussion to reach consensus. We contacted study authors for additional information about the included studies, or for clarification of study methods as required. We incorporated results of the risk of bias assessment into the review through standard tables and systematic narrative description and commentary about each of the elements, leading to an overall assessment of the risk of bias of included studies and a judgement about the internal validity of results of the review.
We planned to assess and report quasi‐RCTs as being at high risk of bias on the random sequence generation item of the risk of bias tool, but none were identified. For cluster‐RCTs, we also assessed and reported the risk of bias associated for an additional domain: selective recruitment of cluster participants.
Measures of treatment effect
For dichotomous outcomes, we analysed data based on the number of events and the number of people assessed in the intervention and comparison groups. We used these numbers to calculate the risk ratio (RR) and the 95% confidence interval (CI) where possible. For continuous measures, we analysed data based on the mean, the standard deviation (SD), and the number of people assessed for both intervention and comparison groups to calculate the mean difference (MD) and 95% CI. If the MD was reported without individual group data, we had planned to use this information to report the study results. If more than one study measured the same outcome using different tools, we calculated the standardised mean difference (SMD) and the 95% CI using the inverse variance method in Review Manager 5.
Unit of analysis issues
We checked cluster‐RCTs for unit of analysis errors. Levels of allocation and analysis were different in all four cluster‐RCTs, but all appropriately adjusted for clustering in their analyses.
If errors and sufficient information were available, we planned to re‐analyse the data using the appropriate unit of analysis, by taking account of the intracluster correlation (ICC). We planned to obtain estimates of the ICC by contacting authors of included studies, or impute them using estimates from external sources. Where it was not possible to obtain sufficient information to re‐analyse the data, we planned to report effect estimates, annotated with 'unit of analysis error'.
Dealing with missing data
We attempted to contact authors of all included studies to obtain missing data (participant, outcome, or summary data), or to clarify details of the trial's methods or conduct or both. All but two teams of authors were successfully contacted and provided additional information about their trial. We planned to analyse participant data based on an intention‐to‐treat basis, however we analysed the data as reported. We reported on the levels of loss to follow‐up and assessed this as a source of potential bias.
For missing outcome or summary data, we planned to impute missing data when possible, report any assumptions in the review, and investigate the effects of imputed data on pooled effect estimates through sensitivity analysis. We were unable to conduct these analyses due to the small number of studies contributing data for all outcomes.
Assessment of heterogeneity
When we considered studies similar enough (based on consideration of populations, interventions, or other factors) to allow pooling of data using meta‐analysis, we assessed the degree of heterogeneity by visually inspecting forest plots and by examining the Chi² test for heterogeneity. We quantified heterogeneity by using the I² statistic. We considered an I² value of 50% or more to represent substantial levels of heterogeneity, but we interpreted this value in the light of size and direction of effects and strength of the evidence for heterogeneity, based on the P value derived from the Chi² test (Higgins 2011).
We planned, when we detected substantial clinical, methodological, or statistical heterogeneity across included studies, not to report pooled results from meta‐analysis but instead to use a descriptive approach to data synthesis. However, where we judged studies to be similar enough clinically and methodologically to justify statistical pooling, and data were available, but heterogeneity was high, we reported the pooled result irrespective of high variability and accounted for this in our GRADE ratings of evidence certainty.
At protocol stage we planned to attempt to explore possible clinical or methodological reasons for variation across studies descriptively synthesised by grouping studies that were similar in terms of populations, intervention features, methodological features, or other factors to explore differences in intervention effects. However, numbers of studies contributing data to any one outcome were small and did not allow this type of analysis to go ahead.
Assessment of reporting biases
We assessed reporting bias qualitatively based on the characteristics of included studies (e.g. if only small studies that indicated positive findings were identified for inclusion), or where information that we obtained upon contacting experts and study authors suggested that there were relevant unpublished studies.
If we had identified sufficient studies (at least 10) for inclusion in the review, we planned to construct a funnel plot to investigate small‐study effects, which may indicate the presence of publication bias. In such instances, we planned to formally test for funnel plot asymmetry, after choosing the test based on advice provided in Higgins 2011, and bearing in mind when interpreting study results that there may be several reasons for funnel plot asymmetry.
Data synthesis
We decided whether to meta‐analyse data based on whether interventions in the included trials were similar enough in terms of participants, settings, comparisons, and outcome measures to ensure meaningful conclusions from a statistically pooled result. Owing to anticipated variability in populations and interventions, and possibly other factors, we used a random‐effects model for meta‐analysis.
Where we were unable to pool the data statistically using meta‐analysis, we prepared a descriptive synthesis of results. We presented data, organised by major outcome categories, and subcategories where applicable, in tables and in text. We had planned to explore the main comparisons of the review (intervention versus usual care; one intervention form versus another) within data categories but only the first comparison was assessed by included studies.
For each outcome/data category, we drew together results of meta‐analysis or descriptive synthesis or both to provide an overall synthesis of the effects of the intervention for each outcome category and subcategory.
Subgroup analysis and investigation of heterogeneity
We did not anticipate including enough studies with quantitative data to warrant subgroup analyses, but planned to attempt to explore potential effects of the following factors through systematic grouping of studies and synthesis when possible.
Type of EoL care: groupings might include palliative care, acute (emergency) care, and others. The rationale for considering effects separately in such (or similar) groupings is that communication needs, opportunities to communicate, and information and decisions needed are likely very different across such different types of EoL care.
Type or aim or both of intervention: groupings might include those to inform and educate, those to support communication, and those to promote communication or decision‐making skills. The rationale for separately considering these groupings is that interventions with different purposes have different underlying mechanisms of action.
Too few studies contributing data to any outcome were included in the review to enable the above analyses to proceed.
Sensitivity analysis
As anticipated, we did not include enough studies in any one pooled analysis to justify conducting sensitivity analyses. However, in future if we identify sufficient studies, we will consider removing those rated as having the highest risk of bias from the analysis and examining effects on the pooled effect estimates.
Ensuring relevance to decisions in health care
One of the co‐authors (Josephine Bothroyd (JB)) is a consumer representative for the Healthcare Consumers' Association of the Australian Capital Territory. She had input to the protocol at all stages.
We planned to consult more widely about the consumer perspective with consumer groups, industry, and/or government agencies. However, given the inconclusive findings of the review we did not perform these wider consultations at this stage. This may be an avenue to explore in future updates of the review.
A consumer provided feedback on the protocol and the review as part of standard CCCG editorial processes.
Summary of findings and assessment of the certainty of the evidence
We prepared a summary of findings table to present results for the main outcomes, based on the methods described in Chapter 11 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011). We presented the results of analysis for the major comparison of the review, for each of the major primary outcomes, including potential harms, as outlined in the Types of outcome measures section. We used the GRADE system to rank the certainty of evidence (GRADEpro GDT; Schünemann 2011). Two or more review authors independently assessed outcomes against the GRADE criteria, with discussion to reach consensus on final ratings of certainty.
Results
Description of studies
Results of the search
Database searches identified 21,170 records for screening. The RCT Classifier was used to assess these references, with 11,069 references remaining for screening with a 90% or higher likelihood of being true randomised controlled trials (RCTs). These references were screened, together with another 245 citations identified from searches of grey literature and other sources (e.g. author contact). After de‐duplicating references, two review authors independently screened 10,970 abstracts. Of these, 10,672 were excluded, with 298 papers screened in full text. In total 251 of these papers were excluded, four ongoing studies were identified, two are awaiting classification, and eight studies (reported in 40 papers) were included in the review; see Figure 1 for PRISMA chart.
One review author (Rebecca Ryan (RR)) screened the remaining 10,101 records excluded by the RCT Classifier as being of lower likelihood of being RCTs. No studies were identified for inclusion in the review from this secondary screening.
Included studies
Trial and participant features
Full details of the included studies are given in Characteristics of included studies tables.
We included eight trials, see Table a below. In four, participants were individually randomised to one of two arms, while in the remaining four, clusters of participants were randomised to one of two (three trials) or three (one trial) arms. All cluster trials appropriately adjusted for clustering in their analyses. See Additional Table 2 for participant numbers for each trial.
1. Participant numbers in trials.
| Participant numbers | Agar 2017 | Au 2012 | Bernacki 2019 | Clayton 2007 | Epstein 2017 | Lautrette 2007 | Reinhardt 2014 | Walczak 2017 |
| Eligible for inclusion | Nursing homes: 111 eligible Participants: 148 UC 171 intervention |
1292 1173 mailed introductory letter |
Clinicians: 133 recruited Patients: 9182 screened UC (8530 ineligible) 9395 screened intervention (8842 ineligible) |
196 | Physicians: 38 eligible Patients: 453 eligible |
132 | 214 | 363 |
| Excluded | Nursing homes: 91 (55 did not meet criteria; 36 declined) 14 UC 11 intervention |
30 screened out (did not meet criteria) 21 declined participation prior to screening |
Clinicians: 6 pilot clinicians ineligible Patients: 8987 UC 9211 intervention |
22 | Patients: 137 excluded (38 ineligible, 99 refused) | ‐ | ‐ | ‐ |
| Refused to take part | ‐ | 645 (did not wish to participate) 101 (did not keep appointment) |
Clinicians: 36 Patients: unclear |
18 | 99 | 6 | 104 | 253 |
| Randomised to intervention group(s) | 10 homes, 160 participants 156 received allocated intervention |
194 | Clinicians: 48 (20 clusters) Patients: 184 (20 clusters) |
92 | Physicians: 19 Patients: 139 Carers: 105 |
63 | 58 | 61 |
| Randomised to control (usual care) group | 10 homes, 134 participants 130 received allocation intervention |
182 | Clinicians: 43 (21 clusters) Patients: 195 (19 clusters) |
82 | Physicians: 19 Patients: 142 Carers: 99 |
63 | 52 | 49 |
| Excluded post‐randomisation (for each group; with reasons if relevant) | UC 66 Intervention 89 All excluded because did not die during the study period |
‐ | 96 (intervention) Death; lost to follow‐up; declined further surveys; completed 2 years 118 (UC) Death; lost to follow‐up; declined further surveys; completed 2 years Intervention: 184 (20 clusters) enrolled: 134 analysed (18 clusters); (50 total: no family/friend response, no baseline survey, withdrew) 34 (13 clusters) analysed: 74 died, 11 lost to follow‐up; 17 declined further surveys; 32 completed 2 years UC: 195 (19 clusters) enrolled: 144 analysed (17 clusters); (51 total: no family/friend response, no baseline survey, withdrew) 26 patients (13 clusters) analysed: 77 died. 12 lost to follow‐up, 21 declined further surveys; 34 completed 2 years |
‐ | ‐ | ‐ | ‐ | ‐ |
| Withdrawn (for each group; with reasons if relevant) | UC 4 Intervention 4 (died before intervention) |
Control 27 (14.5%) 3 patient‐clinician relationship changed 6 refuse to continue 9 not contactable 8 no target visit 1 too ill/deceased Intervention 43 (22.2%) 4 patient‐clinician relationship changed 15 refuse to continue 8 not contactable 10 no target visit 3 too ill/deceased 3 other |
Intervention 12 Control 8 |
None | Intervention 6 Control 5 |
Intervention 11 Control 7 |
‐ | ‐ |
| Lost to follow‐up (for each group; with reasons) | ‐ | ‐ | Intervention: 11, no reason given Control 12, no reason given |
Intervention: 2, 1 mistakenly seen by a junior physician who was not participating in the study, 1 due to mechanical failure of tape recorder Control: 2, 1 mistakenly seen by a junior physician who was not participating in the study 1 due to mechanical failure of tape recorder |
Intervention: 3 died Control: 1 died 1 lost to follow‐up |
Intervention: did not answer telephone n = 4; experiencing severe emotional distress n = 5; refused interview n = 2 Control: did not answer telephone n = 3; experiencing severe emotional distress n = 1; refused interview n = 2; patient still alive n = 1 |
Total numbers reported at each time point N = 96/110 completed 3‐month measures N = 90/110 completed 6‐month measures Plus an additional 3 where complete data were not available NB also for several outcomes data for slightly lower numbers in total are available i.e. table 3 – ranges from n = 65 to n = 81. Not clear what happened to these missing measures |
Intervention: 21 (34%) Unclear; data collection hampered by declining health, attrition high (higher in intervention group) but no systematic reasons for differential dropout identified. Reasons for loss not reported specifically Control: 9 (18%) Reasons for loss not reported specifically |
| Included in the analysis (for each group, for each outcome) | Intervention 67 Control 64 These numbers were analysed throughout, with losses for particular scales/assessments noted where applicable |
Intervention 194 Control 182 |
Intervention 38 patients (13 clusters) Control 26 patients (13 clusters) |
Intervention 90 Control 80 |
Intervention 19 physicians, 130 patients Control 19 physicians, 135 patients |
Intervention 52 (83%) Control 56 (89%) |
6‐month data (longest time point) Intervention 47 Control 40 |
Intervention 39 Control 40 |
| Assessment of attrition bias for RoB ratings | Assessed as low risk Large proportion of data (participants randomised) missing. However, these were comparable for the 2 study groups, and was due to participants not dying during the study period (outcomes assessed for this study were focused on those around death) Withdrawal rates for other reasons were low and comparable across groups |
Assessed as unclear risk Withdrawal 15% to 22% respectively control and intervention arms Reasons for withdrawal/dropout were reasonably comparable except that more (15 versus 6) refused to continue participation in the intervention group Authors report no differences in baseline characteristics regarding whether patients completed the study or were lost to follow‐up ITT analysis was used; effect of imputed data on results was examined in analysis models with authors reporting similar results where imputed and non‐imputed data were used in analysis |
Assessed as unclear risk Patient participation rates and numbers analysable were low but comparable between arms Authors note non‐participants and those not analysed were not significantly different from those who were analysed, and groups were still comparable (based on randomisation), although non‐participants were older, and less likely to have breast cancer than participants; and those patients with analysable data were more likely to be married and have higher incomes than those with non‐analysable data |
Assessed as low risk Low levels of loss to follow‐up 4/174; balanced across groups (n = 2 each), with comparable reasons |
Assessed as low risk "Fewer than 3% of follow‐up questionnaires were missing" (page 95) Data seem otherwise complete for outcomes reported in main paper and in supplement 3 |
Assessed as low risk Loss to follow‐up and withdrawals were acceptably low and comparable across study groups: 52/63 (83%) completed interviews at 90 days intervention group, 56/63 (89%) in control group Reasons for withdrawal/loss were similar across groups (not answering telephone, refused interview), although higher rates of severe emotional distress in intervention group (n = 5) than control group (n = 1) |
Assessed as unclear risk Missing data were reported 110 were randomly assigned and completed baseline interviews; 96 (87%) completed 3 month outcomes; 90 (82%) completed 6 month outcomes Losses were fairly comparable across groups and no major differences between those who completed the study and those who dropped out (on key demographic features) were noted by the authors However, numbers were lower for some outcomes (such as ratings of care management) where n = 65 (numbers fairly comparable between the 2 groups) Not clear what impact this may have had on the result, or what the reasons for this missing data were |
Assessed as high risk Attrition was high (31/110 (28%) lost to follow‐up), possibly largely explained by declining health of participants (patients) Higher in intervention group. No systematic reasons for differential attrition were identified by authors but 34% dropout in intervention group is substantial and may introduce bias Authors state that ITT analysis was used (according to group assignment) but dropout rates may be problematic |
| Additional notes | The study was underpowered for the primary outcome because fewer participants died during the study period than predicted Predicted sample size required recruitment of 272 participants (assuming 10% dropout rate) (17 per site) |
‐ | Sample size calculated as 200 evaluable patients per arm for required power, assuming 6% dropout | ‐ | ‐ | ‐ | ‐ | Trial is underpowered (sample size calculated at 140; 110 recruited; 79 completed) to detect differences between groups |
ITT: intention to treat; RoB: risk of bias; UC: usual care.
All studies were conducted in high‐income countries (four USA, three Australia, one France), and all in urban settings mostly associated with larger hospitals, clinics, or residential care facilities. All participants were older patients where the mean age was 60 years or more, despite inclusion criteria being wide (aged 18 years and older) for most studies, and even in the single study conducted in an intensive care unit (ICU) setting (Lautrette 2007). Most studies included minority ethnic groups to a small degree, and most explicitly excluded people not fluent in the majority language (English or French) and those with cognitive impairment.
Gender composition varied across trials: in the two dementia trials most patients were female (approximately 60% to 80%), whereas gender in chronic obstructive pulmonary disease (COPD) patients in Veteran's Affairs centres was almost exclusively male (96% or more). Other trials fell between these extremes. Only one trial reported surrogates' characteristics, with 70% or more of surrogate decision makers for ICU patients being female.
In two studies, patient participants had advanced dementia and the interventions targeted carers/ family members as surrogate decision makers, as did the study of patients in ICU where surrogate decision makers (usually family members) received the intervention. In remaining studies participants had diagnoses of advanced cancer and other advanced progressive diseases, and both the patient and carer or family member (and sometimes also professionals) were stated targets of the intervention.
Table a: Major trial and participant features
| Study |
Country, setting |
Diagnosis of person at end of life (EoL); selection criteria | Demographics | Intervention target |
|
Agar 2017 Cluster‐RCT 2 arms |
Australia Residential care facilities in major cities |
Advanced dementia; selection criteria identified people with average survival < 6 months Carers no information |
Age (years): intervention mean 84.7 (standard deviation (SD) 7.9), usual care (UC) 85.8 (SD 8.2) Gender: intervention 61% female, UC 58% female |
Residents with dementia Family member/ friend involved in decisions on patient's behalf |
|
Au 2012 Cluster‐RCT 2 arms |
USA Outpatient clinics, Veterans' Affairs centres |
COPD Clinician primarily responsible for COPD care (primary care and chest clinics) Excluded: cognitive dysfunction, language barriers or severe psychiatric disorders |
Age (years): patients mean 69.4 both arms Gender: patients: intervention 97.9% male, UC 96.2% male clinicians: intervention 50% male, UC 44% male |
Patients with COPD Clinicians Surrogates |
|
Bernacki 2019 Cluster‐RCT 3 arms (2 control; data from 2 arms available) |
USA Hospital clinics |
Advanced, incurable cancer; life expectancy < 12 months and identified surrogates (family member/ friend) Clinicians: oncology physicians, nurse practitioners, physician assistants caring for patients (advanced incurable cancer and life expectancy < 12 months) at least 1‐half day per week Excluded: cognitive impairment, unable to speak English or identify a surrogate. Clinicians participating in concurrent studies, or working with both intervention and control arms |
Age (years): patients: intervention 61.8, UC 62.1 Gender: patients: intervention 53.7% female, UC 52.8% female clinicians: intervention 62.5% female, UC 51.2% female |
Patients with cancer Clinicians Surrogates |
|
Clayton 2007 RCT 2 arms |
Australia Palliative care services |
Advanced, progressive, life‐limiting conditions Carer (spouse, partner, family member, friend) Clinicians: palliative care physicians who endorsed question prompt list (QPL) use |
Age (years): intervention: mean 65.5 (SD 12.6), UC 64.6 (SD 14.1) Gender: intervention 39% female, UC 40% female |
Patients Carer |
|
Epstein 2017 Cluster‐RCT 2 arms |
USA Community‐based cancer clinics, academic medical centres, community hospitals |
Advanced cancer; mean life expectancy 9 to 12 months Carers (family member, partner, friend, other involved in health care) Oncologists Excluded: inpatients, those in hospice, unable to understand spoken English or provide written informed consent, patient/carer without decisional capacity |
Age (years): patients: mean 64.4 carers: not reported physicians: mean 44 Gender: patients: 55% female carers: not reported physicians: 29% female |
Patients Carers Oncologists 73% of patients nominated a carer |
|
Lautrette 2007 RCT 2 arms |
France Intensive care (medical and surgical) units |
ICU (acute respiratory failure, coma, shock, acute renal failure, cardiac arrest; expected to die within days) Excluded: patients < 18 years, surrogates without sufficient French for telephone interview |
Age (years): patients: intervention median 68, UC 74 surrogates: intervention median 54, UC 54 Gender: patients: intervention 41% female, UC 48% female surrogates: intervention 77% female, UC 70% female |
Surrogate decision makers (primarily family members): 40% spouses, 48% children |
|
Reinhardt 2014 RCT 2 arms |
USA Large nursing facility in major city (New York) |
Advanced dementia Family members/surrogates (patient’s primary contact) |
Age (years): intervention: mean 59.6 (SD 12.3), UC mean 58.9 (SD 11.9) Gender: intervention: 78.7% female, UC 80% female |
Surrogates |
|
Walczak 2017 RCT 2 arms |
Australia Hospital‐affiliated cancer treatment centres |
Advanced, incurable cancer; oncologist‐assessed life expectancy 2 to 12 months Informal primary carers > 18 years participated if nominated by patient Excluded: non‐English speaking, those with cognitive impairment or significant psychological morbidity |
Age (years): intervention: mean 63.8, UC: mean 65.6 Gender: 34.5% female |
Patients, with or without primary informal carers |
Intervention and comparison features
All studies assessed the comparison intervention versus usual care; see Additional Table 3. The single three‐arm study (Bernacki 2019) included two control arms (one usual care, one control), with data available only for the usual care arm. Usual care showed some variability in levels of contact and support received, but most often participants received routine consultations or family conferences, with one (Reinhardt 2014) including social contacts via telephone in addition to usual care, in order to control for the effects of structured follow‐up calls in the intervention group.
2. Major intervention and comparator features.
| STUDY ID | Agar 2017 | Au 2012 | Bernacki 2019 | Clayton 2007 | Epstein 2017 |
Lautrette 2007* |
Reinhardt 2014 | Walczak 2017 |
| Intervention(s) aim and components | To improve EoL care Facilitated CC + patient‐centred palliative care training |
To improve communication about preferences for EoL care 1‐page patient‐specific feedback form based on patient’s self‐reported responses |
To evaluate the SICG SICG + training, including support for response documentation and patient/family materials |
To evaluate QPL effects on patient/carers’ EoL care topic discussions in consultations QPL for patients and caregivers |
To improve patient‐centred communication between physicians and patients/carers Complex patient‐centred communication training (2 components: physician, patient) |
To improve communication between family and ICU staff and support family decision‐making Proactive communication family conference plus bereavement information leaflet |
To provide information and support to surrogates of patients with advanced dementia Face‐to‐face structured conversation, telephone follow‐up |
To increase patients’ EoL care discussions and cue oncologists to endorse QPL and question asking Nurse‐led CSP (QPL, booster and verbal/written cueing of oncologists pre‐consultation) |
| Comparator (usual care) | Usual care (no additional education, training, support) | Usual care (no patient‐specific feedback) | Usual care | Usual care (routine consultation with PC physician) | Usual care (oncologist met with research assistant but received no training) | Usual care (routine family EoL conference); informs family of treatment limitations and that death is imminent; led by senior physician, with at least 1 family member | Usual care plus social contact by telephone (baseline and 2‐monthly to discuss whatever surrogate raised; each call mean 10 minutes) | Usual care (no contact with nurse, QPL, oncologists cueing to endorse QPL or question asking) |
| Provider (and training) and Recipient | Nurse‐led Trained as PCPC: organisation, facilitation and follow‐up of CC (family, multidisciplinary staff, external health professionals) Recipient: family members, in conference with healthcare professionals and residential home staff |
Clinicians No training described Recipient: patients, surrogates, clinicians |
Clinicians Training: 2.5 hour programme, small groups. Included demonstration and discussion of SICG, role play with feedback. Additional feedback after first SICG plus support (as needed) Recipient: patients, clinicians |
Physicians No training described Recipient: patients, carers |
Physicians, patient coaches Training: 3 days; included instructors in role of advanced cancer patient, role play, supporting materials Patient training: QPL with coaching to identify most important questions/concerns/priorities Recipient: patients, carers, physicians |
Physician‐led family conference Training: intervention meeting for investigators at each ICU site; copy of VALUE guideline. Member of study team visited each site to discuss guidelines and ensure differences between intervention and UC understood Recipient: family members |
PCT physician, social worker Training: structured meeting elements reviewed in training session Recipient: surrogates |
Trained senior nurses Training: 2 nurses; each receiving 40 hours’ training Recipient: patient/carer Oncologist |
| When and how much | Single session Timing: variable, median 48 minutes (IQR 30 to 60) |
Patients received 1‐page patient‐specific feedback via mail, to review with surrogate before consultation Patient‐specific feedback provided to clinician and patients on day of visit (for use during consultation) |
Intervention delivered over 1 or more consultations. Pre‐visit letter introducing SICG topics sent to patient (activate and prepare them for conversation) SICG used at consultation. Clinicians triggered by researchers to have SICG conversation (by email day before/ study materials on consultation day) |
Once Participants received QPL 20 to 30 minutes before PC physician consultation |
Physician training: 2 educational outreach sessions. 1st session 1 hour; 2nd booster session 45 minutes 1 month later Patients/carers: coaching session (approx. 35 to 40 minutes) 1 hour prior to consultation; follow‐up phone calls (up to 3 monthly intervals) |
Once, following 3 information meetings Proactive communication conference, conducted via guidelines. Planned in advance; included senior and junior physicians, nurses, psychologist, family and friends Family members given bereavement information leaflet, content explained orally |
Once, structured face‐to‐face meeting at care facility; mean duration 47 minutes (range 20 to 75 minutes) Social worker contacted surrogates at baseline and every 2 months via telephone |
Once, face‐to‐face session with nurse, 45 minutes, 1 week before follow‐up oncology consultation; private room. Carers attended where possible Follow‐up telephone (booster) 1 to 2 weeks after consultation following CSP delivery; 15 minutes Oncologists: verbally cued by nurses immediately before consultation following CSP; plus postcard |
| Tailoring | Yes; topics tailored to those important to the resident Discussion topics tailored to what was important to the resident. Could include care planning, current/future treatment decision‐making, information sharing, residents’ needs/preferences, ACP Agenda set with input from family members and staff involved in case |
Yes; patient‐specific feedback included patient‐specific highest‐ranked barrier and facilitator to EoL communication, with introductory sentence for clinician use to lower threshold to start conversations; patient’s 3 most important preferences for EoL experiences | Unclear; clinicians could split conversation across consultations but required to continue until all EMR module questions complete | Yes; QPL purpose to assist patients to identify questions of most importance and to raise these in consultation | Tailoring: oncologists’ training was individualised. Coaching tailored to patient/carer priorities and concerns for upcoming consultation |
Not stated explicitly; family members had opportunity to ask questions, discuss treatment options (both intervention and UC groups) | Yes; meetings and follow‐up phone calls aimed to cover issues surrogates wished to discuss | Yes; QPL explored with patients to identify priority questions and discuss skills for question asking |
| Content | Predefined clinical triggers for CC; shared agenda setting model (resident, family, multidisciplinary team); required attendance by resident and/or family/decision makers; facilitation by PCPC to ensure optimal participation by attendees; communication strategy to summarise CC actions and plan | Patient‐specific feedback form generated from patient questionnaire responses, selected automatically (computerised process) Selected responses included: whether their physician would know what care they would like, desire for communication about ACP, patient‐specific barriers and facilitators to communication about EoL care, preferences for CPR |
SICG guide for clinicians in values and goals conversations, 7 elements: illness understanding, decision‐making and information preferences; prognostic disclosure; patient goals and fears; views on acceptable function and trade‐offs; desires for family involvement Clinician documents discussion outcomes via structured EMR form (reminds clinicians of key discussion elements, enables documentation, able to be accessed by other clinicians). Family Communication Guide provided at consultation, suggesting approach for discussing illness/care preferences with family |
QPL: 16‐page A5 booklet containing 112 questions grouped into 9 topics encompassing issues that may be discussed with physician or another health professional | Physician and patient interventions focused on same 4 elements of patient‐centred communication: engaging patients in consultations, responding to emotions, informing patients about choices for treatment and prognosis, and framing information in a balanced way | Proactive family communication conference; information on diagnosis, prognosis, treatment and discussed appropriateness of treatment limitations with family. Intensivist leading conference sought to achieve values represented by VALUE mnemonic (Value and appreciate things family says, Acknowledge emotions, Listen, ask questions that allow Understanding of who the patient is as a person, and Elicit questions from family) Family bereavement information leaflet: 15 pages explaining EoL care, possible reactions after death of a family member, how to communicate with other family members, where to find help |
Structured meeting to provide information and support to surrogates, including about treatment decisions that may arise with worsening dementia severity. PCT available for further information/assistance with decision‐making; only 3 surrogates requested additional information Social worker follow‐up contacts provided support, presented opportunity for surrogates to raise concerns and designed to continue discussions about issues raised in face‐to‐face meeting |
Face‐to‐face session based on QPL, introduced by nurse. QPL systematically explored to identify questions (including prognosis, treatment options and decisions, palliative care, patient and family support, ACP and carer‐specific issues). Prognosis and EoL care issues highlighted, skills for question asking discussed Participants given DVD on ACP and documenting wishes for care relevant to New South Wales Participants prompted to identify 1 to 3 questions to ask at next consultation Follow‐up (booster) phone call; 15 minutes 1 to 2 weeks post‐consultation. Sought to reinforce face‐to‐face meeting content, prepare patients for future consultations using QPL Nurses verbally cued oncologists prior to consultation; oncologists received postcard with suggested endorsement phrasing |
ACP: advance care plan; CC: case conference; CSP: communication support programme; EMR: electronic medical record; EoL: end of life; ICU: intensive care unit; IQR: interquartile range; PC: palliative care; PCPC: palliative care planning co‐ordinator; PCT: primary care team; QPL: question prompt list; SICG: Serious Illness Conversation Guide; UC: usual care.
*Co‐intervention(s): ICUs were part of the FAMIREA study; providing 3 formal early information meetings for all families. First meeting 24 hours (general information on diagnosis, prognosis, treatments) plus information leaflet; second meeting 48 hours (answering questions, additional information check family understanding of situation); third meeting day 3 to 5 (treatments and prognosis explained, family questions answered).
If patient was expected to die (after 3 meetings) or shift to palliative care was indicated an EoL conference was held (i.e. intervention or routine conference). Co‐interventions involved extensive information provision; authors note this may have lessened differences between intervention and UC groups for some outcomes.
Interventions included both simple and complex approaches (single or multicomponent). For instance, in Clayton 2007 patients and carers received a question prompt list (QPL) as a written booklet just prior to a palliative care physician consultation; while in the trial by Epstein 2017 a patient QPL integrated within a coaching session and physician training (focusing on the same four elements of patient‐centred communication) were tailored to each group, and both participant groups received booster/follow‐up sessions or calls to reinforce the initial session. Almost all interventions were tailored to the participants, whether by allowing patients/family/carers to nominate or guide discussions towards topics of priority, by providing patient‐specific feedback to physicians, or enabling patients/family/carers to choose or prioritise questions for discussion in consultations with physicians.
All interventions were designed to be delivered as one‐off interventions, sometimes with the addition of a booster session or follow‐up by telephone. This depended in part on the purpose of the intervention, which varied across trials. Most interventions aimed explicitly to improve patient‐doctor communication, whether by targeting both parties’ communication or knowledge or both, upskilling patients/carers to be meaningfully involved in the consultation and to ask questions or identify priorities for discussion, or by providing a face‐to‐face forum for discussions about end of life care to happen between patients, family members/carers and doctors. In some cases (e.g. Bernacki 2019) the intervention could be delivered more than once over the course of the trial, reflecting the conversation‐based nature of the intervention.
Co‐interventions were delivered only in Lautrette 2007. Here, ICUs were participants of the FAMIREA study which provided a series of early information meetings for all families of ICU patients.
Outcomes and outcome measures
Outcomes for all primary outcome categories sought by this review were reported by the included studies, as were those for all but two secondary outcome categories (health practitioner knowledge and understanding of patient/family/carer knowledge, wishes, or preferences; health systems impacts hospital admissions and re‐admissions). However, outcomes reported within categories were often disparate, timing of assessment highly variable, and outcome measures rarely compatible with one another across studies. See Additional Table 4, Table 5; Table 6; Table 7; Table 8; Table 9; and Table 10 for details of outcomes reported within each review outcome category.
3. Outcomes: knowledge and understanding (primary).
Primary outcome: knowledge and understanding
| ||||
| Study ID | Information needs | Information preferences | Shared understanding | Timing (longest follow‐up) |
| Clayton 2007 | X Questionnaire; total score out of 11 tallied for items not discussed, items for which they did not receive enough information, or about which they received too much information |
X Questionnaire; amount of detail preferred, 1 item, Cassileth Information Styles Questionnaire 5‐point Likert scale |
‐ | 24 hours post‐consultation, 3 weeks post‐consultation |
| Epstein 2017 | ‐ | ‐ | X Discordance between prognosis ratings Researcher‐administered questionnaire/interview; 7‐point scale; ‘discordance’ defined as difference of ≥ 2 between category ratings |
Shortly after audio‐recorded consultation |
| Walczak 2017 | ‐ | X Preferences for amount and type of information Self‐administered, Cassileth Information Styles Questionnaire; scores subtracted from baseline preference scores; differences expressed dichotomously (preferences met or exceeded score > 0; unmet < 0) |
‐ | 1 month |
X: outcome assessed; ‐: outcome not reported.
The study by Lautrette 2007 reported outcomes related to knowledge (ratings of time allocated for information provision, information clarity, and information seeking by family). As these were assessed at 90 days after the death of the patient, following delivery of a one‐off family conference intervention, we judged that these outcomes were too far removed in time from the intervention to be meaningful. Data were not extracted for analysis from this study, but data are provided in Additional table 9, for transparency.
4. Outcomes: evaluation of the communication (primary).
Primary outcome: evaluation of the communication
| ||||||
| Study ID | Quality of communication | Satisfaction with consultation (communication) | Patient‐centred communication | Preferences for involvement | Goal‐consistent care | Patient‐physician relationship |
| Agar 2017 | ‐ | ‐ | X Person‐centred approach to care Care and Activities and Interpersonal Relationships and Interactions domain of Person‐Centred Environment and Care Assessment Tool; 18 items, each rated 0 (not at all) to 3 (all of the time); rated by observation, resident and family reports and documentation No data |
‐ | ‐ | ‐ |
|
Au 2012 |
X Quality of Communication questionnaire, (scored 0 to 100, higher better); 2 weeks |
‐ | ‐ | ‐ | ‐ | ‐ |
|
Bernacki 2019 |
X Quality of Communication scale, timing, scoring unclear No data |
‐ | ‐ | ‐ | X No goals met. Life Priorities Survey (patients), Family Perceptions Survey (surrogates). Baseline, 2‐monthly. Scored by matching patient final Life Priorities survey (within 3 months of death) to Family Perceptions; score 0 to 3 goals met |
X Therapeutic Alliance. Human Connection Scale. Baseline, 14, 24 weeks. Total score 7 to 28; higher better |
|
Clayton 2007 |
‐ | X Questionnaire, Roter and Korsch; 25‐item scale (25 to 125); 24 hours and 3 weeks post‐consultation; higher score better |
‐ | X Actual versus preferred involvement in consultation Questionnaire 24 hours post‐consultation 5‐item rating scale (ranging from doctor leads decisions to patient leads decisions) |
‐ | ‐ |
|
Epstein 2017 |
‐ | ‐ | X Composite patient‐centred (patient‐doctor) communication Composite of 4 communication measures; coded consultation; first visit after coaching session (intervention) or study entry (control) |
‐ | X Decision regret (family) Modified decision regret scale; 8 items, 2 months post‐mortem |
X Patient‐physician relationship Human Connection Scale, Health Care Communication Questionnaire, Perceived Efficacy in Patient‐Physician Interactions scale; 2 to 4 days after audio‐recorded consultation, then quarterly |
|
Walczak 2017 |
‐ | ‐ | ‐ | X Control preferences (doctor/patient +/‐ carer involvement in decisions) Self‐reported questionnaire; baseline and 1 month; Degner Control Preferences Scale; scores subtracted from baseline, differences dichotomised, preferences met/exceeded score > 0; unmet < 0 |
‐ | X Patient Communication Self‐Efficacy Self‐reported questionnaire; baseline and 1 month; Perceived Efficacy in Patient/Physician Interactions Scale |
X: outcome assessed; ‐: outcome not reported.
Satisfaction with the intervention was reported by both Clayton 2007 and Walczak 2017, but only for the intervention arm. This was therefore not extracted and reported in the review.
5. Outcomes: discussions of EoL/EoL care (primary).
|
Primary outcome: discussions of EoL/EoL care e.g. frequency, length, type, participants | |||||||
| Study ID | Discussion with clinicians | Discussion with surrogates | Documented discussion EoL care planning | Number of questions in consultation | Conversation duration | Communication content | Conversation timing |
| Agar 2017 | ‐ | ‐ | ‐ | ‐ | X Facilitated case conference intervention duration (non‐comparative) |
‐ | ‐ |
|
Au 2012 |
X (at last visit) Self‐reported questionnaire; 2 weeks after consultation |
X (since last visit) Self‐reported questionnaire; 2 weeks after consultation |
‐ |
‐ | ‐ | ‐ | ‐ |
|
Bernacki 2019 |
‐ | ‐ | X Conversation number/patient EMR review; after death |
‐ | X Physician report, post‐consultation (non‐comparative) |
X Conversation content/quality SIC domains/patient EMR review; after death; coded 0 to 4 on number of domains discussed and documented (≥ 1 SIC, discussion about: values/goals, prognosis/illness understanding, EoL care, life‐sustaining treatment preferences) |
X Timing of first documented SIC before death EMR review; after death |
|
Clayton 2007 |
‐ | ‐ | ‐ |
X Questions, concerns, items tallied across 9 QPL categories; post‐consultation; coded 112 questions QPL grouped in 9 categories; 85 issues covered by QPL |
‐ | ‐ | ‐ |
|
Lautrette 2007 |
‐ | ‐ | ‐ |
‐ | X At time of family conference with ICU staff |
‐ | ‐ |
|
Walczak 2017 |
‐ | ‐ | ‐ | X Number of questions, cues from patients, carers Coded audio‐recorded consultation; 1 week post‐CSP session; coding to identify number of direct questions, cues plus those on prognosis, EoL care, future care options, general issues |
‐ | ‐ | ‐ |
X: outcome assessed; ‐: outcome not reported.
CSP: communication support programme; EMR: electronic medical record; EoL: end of life; ICU: intensive care unit; QPL: question prompt list; SIC: serious illness conversation.
6. Outcomes: health practitioner outcomes.
| Secondary outcomes: health practitioner knowledge and understanding; evaluation of communication, communication encounter or preparedness to communicate | |||
| Study ID | Evaluation of communication | Knowledge and understanding | Preparedness to communicate |
| Agar 2017 | ‐ | Staff attitudes to, knowledge of providing palliative/EoL care PCPC assessed after training; other facility staff assessed after training by PCPC; Palliative Care for Advanced Dementia tool, 35 items (qPAD) No data |
Staff confidence in providing palliative/EoL care PCPC assessed after training; other facility staff assessed after training by PCPC; Palliative Care for Advanced Dementia tool, 35 items (qPAD) No data |
| Bernacki 2019 | ‐ | ‐ | Uptake and effectiveness of clinician training Use of conversation tool |
| Clayton 2007 | Physician satisfaction with consultation 24 hours and 3 weeks post‐consultation |
‐ | ‐ |
Bernacki 2019 also reported measures of clinician uptake and effectiveness of training to use their tool, and use of the tool. These were judged as measures related to implementation, rather than effects, of the intervention and data were therefore not analysed in this review.
EoL: end of life; PCPC: palliative care planning co‐ordinator.
7. Outcomes: health system impacts (quality of care, concordance with preferences).
| Secondary outcomes: health systems impacts (quality of EoL care, ratings of concordance with patient preferences for EoL care) | |||
| Study ID | Quality of EoL care (nurse rated) | Quality of EoL care (patient/family rated) | Ratings of concordance with patient preferences |
|
Agar 2017 |
X Nurse ratings of · CAD‐EOLD (higher scores = more comfort) · SM‐EOLD (higher scores = lower symptom frequency) Face‐to‐face/telephone interview; as soon as possible after patient death |
X Family ratings of · CAD‐EOLD last 7 days life (higher scores = more comfort) · SM‐EOLD last 90 days of life (higher scores = lower symptom frequency) · SWC‐EOLD last 90 days of life (higher scores = more satisfied) Face‐to‐face/telephone interview; 4 to 6 weeks after patient death |
‐ |
|
Bernacki 2019 |
‐ | ‐ | X PEACE scale, 2 subscales: · Peaceful Acceptance of Illness (acceptance of diagnosis, inner calm, feelings of being well‐loved); 5 questions, total score 5 to 20 · Struggle with Illness (feelings of upset, worry, anger, etc.), 7 questions, total score 7 to 28 Baseline, 2‐monthly |
|
Epstein 2017 |
‐ |
X Caregiver evaluation of quality of EoL care 2 months post‐mortem, 6 items |
‐ |
|
Reinhardt 2014 |
‐ | X Surrogates' ratings of · SM‐EOLD last 90 days of life (higher scores = lower symptom frequency) · SWC‐EOLD last 90 days of life (higher scores = more satisfied) · Satisfaction with care (higher scores = better care) Interview, questionnaire; baseline, 3 and 6 months |
‐ |
X: outcome assessed; ‐: outcome not reported.
CAD‐EOLD: Comfort Assessment In Dying with Dementia; EoL: end of life; SM‐EOLD: Symptom Management at the EoL in Dementia; SWC‐EOLD: Satisfaction with Care at EoL in Dementia.
8. Outcomes: health systems impacts (costs, service use).
| Secondary outcomes: health systems impacts (costs of care, hospital (re)admissions) | ||||||
| Study ID | Costs | Hospital (re)admissions | Consultation length | Treatments and hospice use | Timing (longest follow‐up) | Scale, scoring |
| Agar 2017 | X Training, CC and routine healthcare costs |
‐ | ‐ | ‐ | ‐ | Cost utility (benefit estimated as QALYs). QoL for economic analyses assessed by nurse‐rated EQ‐5D‐5L No data |
| Clayton 2007 | ‐ | ‐ | X | ‐ | ‐ | ‐ |
| Epstein 2017 | ‐ | ‐ | ‐ | X | ‐ | Treatments and hospice use in last month of life; medical records, composite score of 3 indicators of aggressive treatment in last 30 days of life: chemotherapy, potentially burdensome interventions, ED/hospital admission) and hospice utilization |
| Walczak 2017 | ‐ | ‐ | X | ‐ | ‐ | |
X: outcome assessed; ‐: outcome not reported.
Data from Epstein 2017 (composite of treatments and hospice use in last month of life) was judged as clinical, rather than primarily related to communication; data were not extracted on this outcome for the review.
CC: case conference; ED: emergency department; QoL: quality of life; QALY: quality‐adjusted life‐year.
9. Outcomes: patient/carer quality of life.
| Secondary outcomes: patient/family member/carer quality of life | |||
| Study ID | Patient quality of life | Timing (longest follow‐up) | Scale, scoring |
| Agar 2017 | X | 3‐monthly | Quality of life in Late‐stage Dementia (QUALID); 11‐item scale. Nurse‐rated No data |
| Bernacki 2019 | X | Unclear | SF‐12 V2 health survey (QoL and general physical health function) No data |
| Epstein 2017 | X | 3‐monthly to 3 years | Composite QoL score as average of 5 z‐scored subscales: McGillQoL scale single item, McGill Psychological Well‐Being subscale, McGill Existential Well‐Being subscale, FACT‐G Physical Functioning subscale, FACT‐G Social Functioning subscale Research‐administered questionnaire/interview |
| Walczak 2017 | X | 1 month | Health‐related QoL (FACT‐G) |
X: outcome assessed.
QoL: quality of life.
For instance, the primary outcome category of knowledge and understanding was reported by four of the eight included studies (Additional Table 4). Two studies reported 'information preferences' (Clayton 2007; Walczak 2017), assessed with similar tools and at comparable time points, but other outcomes within this category were each reported by only a single study (information needs, shared understanding, time for information, information clarity, additional information sought).
Similarly while six of eight studies reported outcomes within the category 'evaluation of the communication' (Additional Table 5), there was little comparability of measures across studies. For example, four studies reported measures of patient‐centred communication: one with no data available (Agar 2017), one reporting a composite of four measures (Epstein 2017), and the others reporting measures of patients' control preferences or desire for involvement in the consultation using different scales (Clayton 2007; Walczak 2017). Even in cases where outcome measures were comparable, data were often sparse or unavailable. As an example, quality of communication was assessed by two studies (Au 2012; Bernacki 2019), both using the quality of communication questionnaire but with data available only for one study (Au 2012).
Such differences in outcome measures across studies and within outcome categories prevented pooling of data statistically in some cases. Instead, where data could not be pooled, studies were grouped according to outcomes and synthesis was conducted descriptively.
No included studies reported outcomes relating to health practitioner knowledge and understanding of patient/family/carer knowledge, wishes, or preferences, or hospital admissions and re‐admissions (Additional Table 8). Epstein 2017 reported measures of treatments and hospice use in the last months of life, assessed via medical records and through calculation of a composite score of three indicators of aggressive treatment in last 30 days of life: chemotherapy, potentially burdensome interventions, emergency department (ED)/ hospital admission) and hospice utilisation. We judged these outcomes as clinical in focus and data were therefore not extracted for analysis in this review.
For several other outcomes, data were not reported, or were not yet available in a form that was usable for this review. This included health professionals' knowledge, attitudes and confidence, quality of life, costs and person‐centred approach to care (Agar 2017), and perception of the quality of communication and quality of life (Bernacki 2019). Additionally, outcomes with data for the intervention arm only, such as satisfaction with the intervention (Clayton 2007; Walczak 2017) were not analysed for this review.
We explored groupings of studies and the possibility of undertaking meta‐analysis to pool results where possible. It was not possible to conduct meta‐analysis, and we therefore conducted a descriptive synthesis of results without statistical pooling.
Excluded studies
Studies assessed in full text but excluded from the review are reported in the Characteristics of excluded studies table, with major reasons for exclusion. Studies were excluded most commonly for the following reasons: wrong intervention or no communication intervention (62 studies), population not at EoL according to the review's definition (49 studies), focus on advanced care planning/advanced directives and uptake (35 studies), focus of the study being primarily clinical management rather than communication (19 studies), wrong study design (19 studies), focus of the study on health provider communication skills training (19 studies), or the intervention was not delivered in face to face format (11 studies).
Risk of bias in included studies
Risk of bias, by tool domain, is reported below. Studies were at generally low risk of bias, particularly for selection bias. Blinding (performance bias) was the most obvious source of bias, but overall the included studies did not have major methodological limitations. See Figure 2.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Most (six of eight) studies used low risk methods (such as a computer‐generated random number sequence) to allocate participants to groups, with two studies providing inadequate details to judge the risk of bias (Au 2012; Reinhardt 2014). Allocation was adequately concealed in seven studies, using methods such as sealed, opaque envelopes to preserve the random number sequence until allocation, or an off‐site study statistician administering the sequence. One study (Reinhardt 2014) did not report enough details to make a clear judgement, and was rated as at unclear risk of bias.
Blinding
Blinding of participants and personnel was challenging in many of the included studies, due to the nature of the intervention. Five studies were rated as at unclear risk of performance bias, as it was not clear what effect any measures taken to blind participants or personnel, or the lack of blinding, might have been on outcomes assessed by these studies. Three studies (Lautrette 2007; Reinhardt 2014; Walczak 2017) were rated at high risk, stating that blinding was not possible and the lack of blinding may have affected delivery of the intervention to family members, and their interactions (Lautrette 2007); or that blinding was not possible and it was judged that self‐reported subjective outcomes may have been influenced by the lack of blinding (Reinhardt 2014; Walczak 2017).
Blinding of outcome assessment was judged as at low risk of bias for seven of the eight included studies. Only Walczak 2017 was rated as at high risk of bias as most outcomes were self‐reported and subjective (e.g. satisfaction, control preferences) and may have been influenced by knowledge of intervention assignment.
Incomplete outcome data
Participant numbers in trials from recruitment to follow‐up are given in Additional Table 2.
Four of eight studies were rated as at low risk of attrition bias, reporting low levels of losses (e.g. < 3% of questionnaire items missing reported by Epstein 2017) that were comparable or balanced across study groups (Agar 2017; Clayton 2007; Epstein 2017; Lautrette 2007). Three studies were rated as unclear risk of attrition bias: Au 2012 described withdrawals of 15% to 22% in control and intervention arms respectively, with mostly comparable reasons between groups. However, more people (15 versus 6) in the intervention arm refused to continue participation, and it is possible this may have introduced bias. Reinhardt 2014 reported 80% or more of participants completed both 3‐ and 6‐month follow‐ups. However, numbers for some outcomes were substantially lower, with some differences between study groups, and missing data may have influenced the results. Similarly, Bernacki 2019 reported patient participation rates that were low over the trial course, but comparable between study arms. However, there were some differences between those with analysable data and those without, and it is possible this may introduce bias.
One study (Walczak 2017) was rated at high risk of attrition bias, with 28% overall lost to follow‐up. This was possibly largely explained by declining health of participants. However, rates of loss were higher in the intervention group (34% intervention group versus 18% control) and no reasons for differential attrition were identified and may have introduced bias.
Selective reporting
Four of eight studies (Agar 2017; Au 2012; Bernacki 2019, Epstein 2017) had protocols available and all outcomes were reported as planned, or author contact confirmed that publication of data for outstanding outcomes is pending. The remaining studies typically reported all outcomes stated in the methods for the trial, but either we were not able to identify a protocol (Clayton 2007; Lautrette 2007; Reinhardt 2014), or outcomes stated in the protocol may be yet to be reported (Walczak 2017).
Other potential sources of bias
Most studies (six of eight) were at low risk of other sources of bias. This included all four cluster trials being assessed at low risk of selective recruitment of cluster participants.
Two studies were rated as at unclear risk (Au 2012; Clayton 2007): both reported some existing baseline differences between study arms, although the implications of these differences are uncertain.
Effects of interventions
See: Table 1
All included studies assessed the effects of interventions to improve interpersonal communication in comparison with usual care. None assessed the comparative effects of different interventions.
Where possible we pooled data statistically, but due to variability in outcome categories and measures reported across studies, much of the synthesis was descriptive, and is presented in tables and text.
We present below a synthesis of results, organised by outcome categories sought by the review, and outcome subcategories within the data where appropriate.
Interventions to improve interpersonal communication versus usual care
Knowledge and understanding
Four studies reported outcomes related to knowledge and understanding, or appraisals of information received, associated with EoL or EoL care.
Three were conducted in patients with advanced cancer or other life‐limiting conditions, with or without carers present. All included the use of a question prompt list (QPL) but the interventions ranged from simple (QPL alone; Clayton 2007) to complex (QPL with physician and patient training, or delivered alongside a nurse‐led communication skills programme; Epstein 2017; Walczak 2017). None of these studies reported substantial differences between intervention and usual care groups, despite measuring a range of outcomes related to information needs, information preferences, and understanding. Differences in outcome measures prevented statistical pooling of data.
Epstein 2017 reported shared understanding of prognosis, assessed as mismatch (discordance) between patient and physician estimates of 2‐year survival and curability of the patient’s cancer. Ratings were assessed on a seven‐point scale, with discordance defined as a difference of two or more points between category ratings performed by patients and clinicians. 2‐year survival discordance was similar across study groups (intervention 59% versus usual care 62%), as was discordance of curability estimates (intervention 39% versus usual care 44%).
Walczak 2017 assessed preferences for the amount and type of information received in consultations, dichotomising the data into ‘met/exceeded’ and ‘unmet’ by subtracting scores at 1‐month follow‐up from baseline scores (preferences met/exceeded giving a score > 0, unmet < 0). Preferences for amount of information were similar between arms (met/exceeded intervention 56.5% versus usual care 57.1%), while preferences for type of information were met or exceeded more often in the intervention group (92.6%) than in the group receiving usual care (80%).
Clayton 2007, reporting information needs and preferences at 3 weeks post‐consultation, reported few differences between intervention and usual care groups. There were no differences in patients’ unmet information needs overall, although these were slightly higher in the intervention group (27% versus usual care 12%). Similarly, there were no differences in total scores (out of 11) for patients’ perceptions of whether topics were discussed, whether they had unmet information needs, or there was too much information provided. Only one of 11 topic items showed a difference between groups, with 4% (intervention) compared with usual care (16%) reporting they had not discussed the topic (‘what is happening with my illness’) (P = 0.05) with the palliative care team.
In both the Walczak and Clayton studies it is noteworthy that there were few differences between groups in information needs and preferences following the use of interventions involving a QPL at minimum; and that in both studies a substantial proportion of participants in both arms (up to approximately 44%, ranging from 12% to 43.5%) responded that they had unmet information needs.
A final study (Lautrette 2007) was conducted in ICUs, with outcomes assessed by telephone interview with surrogates 90 days after the patient's death. This trial assessed the effects of a single, short (mean 10 minutes) family conference provided in addition to usual care and a substantial co‐intervention (a series of early family information sessions) in the time leading up to the patient’s death. We judged that outcomes assessed at a time point so removed from an intervention delivered once would likely be confounded by many factors and so do not report data for this study. Data extracted on surrogates’ ratings of information provided prior to death is provided for transparency in Additional Table 11.
10. Data extracted but not analysed.
| Study ID | Outcome category | Outcomes reported | Results | Assessment method and timing |
|
Lautrette 2007 |
Knowledge and understanding | Time for information | Sufficient time Intervention 51/56 (91%) Usual care 45/52 (87%) |
Surrogate telephone interview; 90 days after death of patient |
| Clarity of information | Information was clear Intervention 52/56 (93%) Usual care 45/52 (87%) |
Surrogate telephone interview; 90 days after death of patient |
||
| Additional information requested by family |
Additional information was requested Intervention 17/56 (30%) Usual care 24/52 (46%) |
Surrogate telephone interview; 90 days after death of patient |
||
| Bernacki 2019 | Anxiety (moderate to severe symptoms; GAD‐7) | 10.4% (intervention) versus 4.2% (usual care) | 24 weeks post‐baseline (approximately 12 weeks post‐intervention) | |
| Clayton 2007 | Anxiety (STAI) | 38.7 (intervention) versus 37.5 (usual care) | 3 weeks post‐intervention | |
| Lautrette 2007 | Anxiety (HADS, score > 8) | 44.6% (intervention) versus 67.3% (usual care) | 90 days after death of patient | |
Data at longest time point are reported unless otherwise indicated.
HADS: Hospital Anxiety and Depression Scales; STAI: State‐Trait Anxiety Inventory.
Overall, interventions to improve communication may have little or no effect on measures of knowledge of illness and prognosis, or information needs and preferences, although studies were small and measures used varied across trials. We assessed the certainty of evidence as low, downgrading (‐1) due to inconsistency (different outcome measures and concepts assessed across studies) and (‐1) for indirectness (all participants were older patients with advanced cancer, and results may not apply to other populations nearing EoL).
Evaluation of the communication (positive and/or negative constructs)
Six studies reported some measure of quality, patient‐centredness or other measure of the communication occurring between patients and/or carers and health professionals related to EoL or EoL care. All were assessed in patients with advanced life‐limiting conditions, including advanced cancer and COPD. Data were grouped by similar outcome constructs and are reported below.
Quality of communication
Quality of communication was measured by two studies (Au 2012; Bernacki 2019) using the Quality of Communication (QOC) questionnaire, but data were available for only one. Au 2012 reported a slightly higher mean total score 2 weeks post‐consultation in those receiving the patient‐specific feedback intervention (mean 34.0, 95% confidence interval (CI) 28.5 to 39.4), compared with usual care (mean 25.5, 95% CI 20.4 to 30.5). However, as 100 was the maximum possible score on this questionnaire, it is noteworthy that mean scores remained low in both arms, suggesting that the intervention may have limited effectiveness or there may have been implementation issues affecting its delivery or both.
We are uncertain about the effects of interventions on the quality of communication. We assessed the certainty of evidence as very low, downgrading (‐1) due to methodological limitations (sequence generation was at unclear risk of bias), (‐1) due to imprecision (results are from a single, small study) and (‐1) for indirectness (almost all participants were older males with COPD, and results may not apply to other population groups nearing EoL).
Patient‐centred communication
The trial by Epstein 2017 reported a composite measure of patient‐centred communication, developed from assessment of four communication domains (engaging, responding, informing, framing of decisions), the component domain scale scores transformed to z scores, and these averaged to give an overall measure. Overall mean scores across domains were slightly higher in the intervention group (mean 0.2, SD 0.8) compared with the group receiving usual care (mean 0, SD 0.7), assessed post‐consultation. This trial also reported that carers’ decisional regret was slightly lower with the intervention (mean 16.0, SD 6.6) compared with usual care (mean 18.1, SD 7.1), assessed 2 months after the patient’s death. The level of patient‐centred approach to care (using the Care and Activities and Interpersonal Relationships and Interactions domain of the Person‐Centred Environment and Care Assessment Tool, PCECAT) was assessed in one further study (Agar 2017) but data were not available.
Overall, interventions to improve communication may have little or no effect on measures of patient‐centred communication. We assessed the certainty of evidence as low, downgrading (‐1) for imprecision (results are based on a single small study) and (‐1) for indirectness (all participants were older patients with advanced cancer, and results may not apply to other populations nearing EoL).
Preferences for involvement
Two studies reported on achievement of preferred level of involvement in consultations (Clayton 2007) or decisions (Walczak 2017). Both assessed the effects of interventions which included a patient QPL, with the Walczak study including additional coaching. Clayton reported no difference between groups in numbers of patients achieving their preferred level of involvement (intervention 44% versus usual care 41%), or for numbers of patients being either more or less involved in the consultation than was their preference. Walczak reported similar findings, with small differences between groups on two measures of involvement assessed as the difference between follow‐up (1 month) and baseline scores. Fewer people in the intervention group had their preferences met or exceeded for the amount of doctor/patient involvement in decisions (mean intervention group 55.6% versus usual care 69.6%), while more in the intervention arm had preferences met or exceeded for the amount of doctor/patient/carer involvement in decisions (intervention mean 87.5% versus usual care 80.8%).
Overall, interventions to improve communication may have little or no effect on participants’ preferred level of involvement. Certainty was rated as low, and was downgraded (‐1) for imprecision (results are based on a small number of participants) and (‐1) for indirectness (all participants were older patients with advanced cancer, and results may not apply to other populations nearing EoL).
Doctor‐patient relationship
Three studies measured effects on the doctor‐patient relationship, all assessing complex interventions (Bernacki 2019; Epstein 2017; Walczak 2017), using a variety of scales including the Therapeutic Alliance and PEPPI (Patient Communication Self‐Efficacy) scales. Time points also differed: 14‐week data, rather than that at the longest time point (24 weeks) was selected for analysis from Bernacki in order to be most comparable to time points for the other two trials (Epstein 8 weeks; Walczak 4 weeks); and the data for Bernacki was recalculated based on reported 95% CIs, using group sample sizes of 38 and 26 for the intervention and control groups respectively.
Pooled analysis of the three studies indicated little or no effect of the intervention on doctor‐patient relationships, compared with usual care (standardised mean difference (SMD) 0.23, 95% CI ‐0.06 to 0.51; I2 = 17%; 3 trials, 238 participants; Analysis 1.1).
1.1. Analysis.

Comparison 1: Intervention versus usual care, Outcome 1: Doctor‐patient relationship
Interventions to improve communication may have little or no effect on measures of the doctor‐patient relationship. Evidence was rated as low certainty, downgraded (‐1) for imprecision (results are based on a small number of participants) and (‐1) for indirectness (all participants were older patients with advanced cancer, and results may not apply to other populations nearing EoL).
Satisfaction with the consultation
Satisfaction with the consultation was reported by Clayton 2007, with no difference between the QPL intervention group (mean 110.1) and usual care (mean 110.3) at 3 weeks post‐intervention. Both arms reported high mean satisfaction levels, the maximum score on this scale being 125.
Interventions to improve communication may therefore have little or no effect on consultation satisfaction. Evidence was rated as low certainty, downgraded (‐1) for imprecision (results are based on a single, small study) and (‐1) for indirectness (all participants were older patients with advanced cancer, and results may not apply to other populations nearing EoL).
Summary of effects on the broad outcome category ‘Evaluation of communication’
Overall, the results indicate that there may be minimal or no effects of interventions to improve communication about EoL and EoL care, compared with usual care, on outcome domains encompassed by the broader category of evaluation of the communication. Even where outcomes were reportedly statistically significantly different between groups, such as for quality of communication, mean ratings were low across both intervention and control groups, suggesting that the interventions assessed may not have profound effects on such outcomes. All outcomes were rated as low‐ or very low‐certainty evidence. Further research is likely to change these results.
Discussions of EoL/EoL care
Duration and timing of EoL discussions
Three studies (Agar 2017; Bernacki 2019; Lautrette 2007) reported this outcome, only one reporting comparative data. Lautrette 2007 reported that the median duration of the family conference intervention was 30 minutes (interquartile range (IQR) 19 to 45 minutes), compared with usual care (median 20 minutes, IQR 15 to 30 minutes). Certainty of the evidence was rated as low, being downgraded (‐1) for imprecision (results are based on a single small study) and (‐1) for indirectness (all participants were older patients in ICU, results may not apply to other populations approaching the EoL). The intervention may therefore increase the duration of discussion of EoL care in an ICU setting, but the significance of this for practice is not clear.
Bernacki 2019, assessing the timing of the first documented Serious Illness Conversation (SIC) prior to death, reported that conversations happened substantially earlier among those in the intervention group (median 143 days prior to death (IQR 71 to 325), compared with the usual care group (71 days, IQR 33 to 166) (P < 0.001). We rated the certainty of the evidence as low, downgrading (‐1) for imprecision (as results are based on a single small study) and (‐1) for indirectness (all participants were older patients with advanced cancer and results may not apply to other populations approaching the EoL). The intervention may therefore lead to earlier discussions of EoL and EoL care, when compared with usual care.
Occurrence of discussions of EoL care
Four studies reported data on measures of conversations occurring about EoL/EoL care. In Au 2012 and Bernacki 2019 these took the form of self‐reported or documented discussions about treatment preferences (and related issues in Bernacki), with interventions in both studies aiming to increase the quality and occurrence of EoL discussions. In comparison, Clayton 2007 and Walczak 2017 assessed question‐asking in consultations following the intervention (which included a QPL, with or without additional coaching), as measures of patient and carer engagement in consultations.
Outcome data from Au 2012 (number of self‐reported discussions of treatment preferences with their clinicians at their last visit) and Bernacki 2019 (numbers of patients with at least one Serious Illness Conversation (SIC) documented prior to death) were pooled statistically, indicating that the intervention increased the occurrence of such discussions, compared with usual care (risk ratio (RR) 1.96, 95% CI 1.61 to 2.39; I2 = 0%; 2 trials, 537 participants; Analysis 1.2).
1.2. Analysis.

Comparison 1: Intervention versus usual care, Outcome 2: Discussion of EoL care planning
Consistent with reported discussions with their clinicians, Au 2012 also reported that patients’ self‐reported discussions with surrogates since the last clinic visit was slightly higher amongst those in the intervention group (53.6%) than in those receiving usual care (45.2%). The intervention’s primary aim was to increase the rates (occurrences) of discussions between patients and their clinicians, with discussions between patients and surrogates forming a secondary aim. Although in this study the intervention did increase the numbers of patients reporting discussions with both their clinicians and their surrogates, it is worth noting that these remained quite low overall, particularly for clinician discussions, and suggests that the intervention has limited effectiveness. That rates of discussions were higher between patients and carers is perhaps unsurprising, but here too only around half of patients reported these discussions occurring, with little difference between study groups.
Bernacki 2019 also reported other measures reflecting the occurrence of SIC. The number of documented SIC per patient were higher in the intervention arm, overall and when considered per domain; see Table b below.
Clayton 2007 and Walczak 2017 assessed question asking in consultations, reflecting the use of a QPL as a component (or all) of the intervention under evaluation. Meta‐analysis indicated that there may be little or no effect of the intervention, compared with usual care, on mean total numbers of patient questions (MD 1.58, 95% CI ‐1.82 to 4.98; I2 = 81%; 2 trials, 249 participants; Analysis 1.3). Numbers of carer questions could not be pooled statistically as numbers of carers per group were not available for both studies. Findings were mixed, with approximately twice as many carer questions with the intervention, compared with usual care, reported by Clayton 2007 (intervention mean 4.4 (SD 3.49) versus usual care (2.1 (SD3.49)), and no difference between groups in Walczak 2017 (both groups mean 3.5 questions). Heterogeneity was high with no obvious reason identified: participants were all older, with advanced cancer or another life‐limiting condition; interventions both included a QPL, one (Walczak 2017, also including additional patient coaching), and numbers of questions representing relatively straightforward measures. These outcomes (data) however were obtained via coding of consultations and tallying of questions, and it is possible that this may not be a highly sensitive and/or reliable method for quantifying the effects of these interventions. The approach taken, together with other factors we cannot yet identify, may contribute to the variability in results.
1.3. Analysis.

Comparison 1: Intervention versus usual care, Outcome 3: Patient questions in consultation
We are uncertain about the effects of interventions to improve discussions about EoL care. Overall, certainty was rated as very low, downgraded (‐1) for inconsistency (two of four studies indicated that the intervention had no real effect, with residual variation despite similar populations and interventions), (‐1) for methodological limitations (the largest study rated as unclear risk of bias on sequence generation), and (‐1) for indirectness (participants were older patients with cancer or COPD and may not apply to other populations nearing EoL).
Content of communication
Bernacki 2019 reported numbers of SIC domains per patient, reflecting coverage of the four domains covered in these EoL conversations. This was reported as a measure of conversation quality by the trial; in this review we considered it a measure of conversation content, as we cannot determine from coverage alone how well the domains were covered. Intervention group participants had more documented SIC domains per patient, overall, and when SIC domains were considered individually. Clayton 2007 reported little difference between groups for number of QPL topics discussed in consultations. For the latter it is worth noting that, for a total score of 85 topics that could be covered, mean scores remained low in both trial arms (approximately 21 or fewer). See Table b.
Interventions to improve communication may have little or not effect of amount of content communicated in consultations. Certainty was rated as low, downgraded (‐1) for imprecision (results are based on a small number of participants) and (‐1) for indirectness (all participants were older patients with advanced cancer, and results may not apply to other populations nearing EoL).
Table b
| Study | Outcome | Intervention | Usual care |
| Bernacki 2019 | Number of documented SIC per patient (overall)* | Mean 3.1 (95% CI 2.5 to 3.6) | Mean 2.1 (95% CI 1.4 to 2.8) |
|
89% | 44% | |
|
91% | 48% | |
|
80% | 68% | |
|
63% | 32% | |
| Bernacki 2019 | Number of documented SIC domains per patient** | Mean 3.2 (95% CI 2.9 to 3.6) | Mean 1.9 (95% CI 1.6 to 2.3) |
| Clayton 2007 | Number of QPL topics discussed (out of 85 topics) | Mean 20.9 | Mean 17 |
*reported as statistically significant P = 0.02. **reported as statistically significant P < 0.001.
Adverse outcomes or unintended effects
Adverse outcomes are difficult to define for this review, and may more appropriately be considered as unintended effects of the intervention. These might include less understanding (confusion) about EoL information, options or decisions; worsened ratings for quality of care or communication or both at the end of life; or heightened fear, distress or anxiety in patients, family members and/or carers. Included studies did not report any outcomes that might be considered harms directly associated with the interventions: only anxiety (patient or carer or both) was reported as a potential unintended consequence. However, anxiety is very difficult to interpret in this population group: anxiety levels are likely to be high and there are many potentially confounding factors in play. Tools must be tailored to assess anxiety appropriately, and other influencing factors also need to be adequately taken into account at the time of such assessments (e.g. treatment with opiates), so that effects on such outcomes are interpreted in a meaningful way.
Three studies assessed and reported anxiety of patients and/or carers (Bernacki 2019; Clayton 2007; Lautrette 2007). However, tools used were typically generic (e.g. State‐Trait Anxiety Inventory (STAI)), and anxiety measured at weeks or months post‐intervention. We judged these outcomes to be seriously confounded and too far removed in time from the intervention delivery to allow meaningful interpretation in relation to the intervention’s effects. The data are reported in Additional Table 11, for transparency, but were not analysed further for the purposes of this review.
No other unintended consequences, or worsening of desired outcomes, was reported by the included studies.
Health practitioner outcomes (knowledge; preparedness; communication evaluation)
Three studies reported some measure related to health practitioners’ knowledge and understanding, preparedness to communicate or evaluation of the communication or of the communication encounter. Only Clayton 2007 reported useable data: physician ratings of satisfaction with communication during the consultation were comparable across intervention and usual care groups (e.g. mean ratings of ‘very satisfied’ 28% intervention versus 23% usual care; ratings of ‘not satisfied’ 12% in both groups).
Agar 2017 assessed staff knowledge, attitudes and confidence in providing palliative/EoL care after they had received training (using the Palliative Care for Advanced Dementia tool, qPAD), but useable data were not available. Bernacki 2019 assessed measures of clinician uptake and effectiveness of training to use their tool, and use of the tool. We judged these as measures related to implementation, rather than effects, of the intervention, and data were therefore not analysed in this review.
Interventions to improve communication may have little or no effect on health practitioner outcomes. We rated certainty as low, downgrading (‐1) for imprecision (results are based on single small study) and (‐1) for indirectness (all participants were older patients with advanced cancer).
Patient/carer quality of life
Four studies assessed quality of life (QoL), all using different scales, and two without useable data (Agar 2017; Bernacki 2019). Epstein 2017 reported no differences between intervention and usual care arms using a composite scale derived from several tools, noting that QoL was stable until 6 to 9 months prior to death, from which point it declined. Similarly, Walczak 2017 reported little difference between QoL ratings on the FACT‐G scale at 1 month post‐intervention (intervention mean 70.9 (SD 16.3) versus usual care mean 77.8 (SD 18.8)).
Interventions to improve communication may have little or no effect on patient/carer quality of life. Certainty was rated as low, downgraded (‐1) for imprecision (results are based on studies with small sample sizes) and (‐1) for indirectness (all participants were older patients with advanced cancer).
Health systems impacts relevant to the effects of communication
Quality of EoL care
Outcomes related to carer or clinician ratings of the quality of EoL care were reported in three studies (Agar 2017; Epstein 2017; Reinhardt 2014). In all of these studies, family member or surrogate ratings of care were reported, and one (Agar 2017) also reported ratings by nurses caring for patients at the EoL.
Agar 2017 and Reinhardt 2014 assessed quality of care perceptions in patients with advanced dementia, reporting against some of the same subscales of the End of Life in Dementia (EOLD) tool. Data corresponding to two subscales could be pooled: Satisfaction with Care at EoL in Dementia (SWC‐EOLD) and Symptom Management at the EoL in Dementia (SM‐EOLD) scales. Higher scores on both scales represented an improvement, with outcomes measured at 4 to 6 weeks (Agar 2017) and at 3 months (Reinhardt 2014) after the patient’s death. Data were analysed at 3 months, rather than the longest time point (6 months) for the Reinhardt study, so that timing of assessments would be as similar as possible to that of the Agar 2017 and Epstein 2017 studies.
Pooled analysis indicates that there may be little or no effect of the intervention, compared with usual care, on family members'/carers’ ratings of symptom management at the EoL (MD ‐1.98, 95% CI ‐4.38 to 0.43; I2 = 0%; 2 studies, 212 participants; Analysis 1.4) or their satisfaction with care (MD 0.44, 95% CI ‐0.99 to 1.87; I2 = 0%; 2 studies, 212 participants; Analysis 1.5).
1.4. Analysis.

Comparison 1: Intervention versus usual care, Outcome 4: Family‐rated symptom management (SM‐EOLD)
1.5. Analysis.

Comparison 1: Intervention versus usual care, Outcome 5: Family‐rated satisfaction with care at EoL (SWC‐EOLD)
Agar 2017 also reported data for the Comfort Assessment in Dying with Dementia (CAD‐EOLD) scale, with little or no difference between groups, a pattern reflected in nurse ratings of the CAD and SM subscales measured soon after the patient’s death (see Table c below).
Surrogate/carer ratings of care (satisfaction) and of the quality of care were assessed by two studies (Epstein 2017; Reinhardt 2014), again with little difference between groups when measured 2 to 3 months after the patient’s death.
Communication interventions may have little or no effect on the quality of EoL care. We rated the certainty as low, downgrading (‐1) for methodological limitations (as one study (Reinhardt 2014) was rated at unclear risk of bias for sequence generation and allocation concealment) and (‐1) for indirectness (as all participants were older patients with advanced cancer).
Table c
| Outcome, scale | Study | Intervention group mean (SD) | Usual care group mean (SD) |
| Comfort Assessment in Dying with Dementia (CAD‐EOLD), family rated (higher scores better) |
Agar 2017 | 34.7 (5.9) | 35.5 (5.9) |
| Comfort Assessment in Dying with Dementia (CAD‐EOLD), nurse rated (higher scores better) |
Agar 2017 | 32.1 (6.1) | 33.3 (5.7) |
| Symptom Management at the EoL in Dementia (SM‐EOLD), nurse rated (higher scores better) |
Agar 2017 | 22.4 (9.6) | 23.2 (8.3) |
| Surrogates’ satisfaction with care, 3 months | Reinhardt 2014 | 7.9 (1.4) | 7.8 (1.6) |
| Carers’ evaluation of quality of care, 2 months | Epstein 2017 | 49.6 (10) | 46.9 (9.7) |
Ratings of concordance between patient preferences for EoL care goals and care received
Concordance between goals of care and care provided at the EoL was reported by Bernacki 2019. This study also reported patients’ own ratings of their care and illness as they approached the end of life, reporting against two subscales of the Peace, Equanimity and Acceptance in the Cancer Experience (PEACE) questionnaire. This tool and its subscales assess different aspects of readiness to consider goals of care, for instance, identifying whether patients are accepting of their prognosis or whether they are still struggling to accept the approach of the end of their life. In the latter case, such struggles may need to be addressed before goals of care can be approached and discussed with the patient and their family members or carers.
This study reported little difference between intervention and usual care groups on patients’ own ratings of the two subscales: Peaceful Acceptance of Illness subscale (intervention mean 16.9 (95% CI 16.1 to 17.6) versus usual care mean 16.8 (95% CI 15.9 to 17.6)); and Struggle with Illness subscale (intervention mean 14.0 (95% CI 12.9 to 15.1) versus usual care mean 14.4 (95% CI 12.7 to 16.0)).
Concordance between goals of care and care provided at the EoL was calculated as the number of top‐3 rated goals of care identified by patients and assessed by family members as ‘met’ at the end of life (within 3 months of death). Scores could range from 0 to 3 goals met, and little or no difference between groups was reported (intervention 1.3 goals met (95% CI 1 to 1.6); usual care 1.5 (95% CI 0.9 to 2.2)).
Certainty of the evidence for both outcomes was rated as low, downgraded (‐1) for imprecision (results were based on a single small study) and (‐1) for indirectness (all participants were older adults with advanced cancer and results may not be applicable to other populations approaching the EoL).
Costs of care, hospital (re)admissions
Five studies reported health system impact outcomes but little data were presented, other than in Paladino 2020, which presented detailed healthcare utilization for the parent trial (Bernacki 2019). Agar 2017 measured costs associated with training and delivery of the intervention and usual care, and conducted a cost‐benefit utility analysis, but data were not available. Epstein 2017 reported a composite outcome assessing aspects of the aggressiveness of treatment in the last 3 months of life. However, this was judged as a primarily clinical outcome and data were not extracted for this review. Similarly, Paladino 2020b reported on measures of aggressiveness of treatment and chemotherapy receipt, and again we judged these as primarily clinical. This study also reported emergency department presentations, hospital and ICU admissions, hospice use and place of death (acute care setting), with no differences between intervention and usual care groups reported.
Both Clayton 2007 and Walczak 2017 reported consultation length, which may have implications for the costs of delivering care. In both, mean consultation length with the intervention was slightly longer (37.8 versus 30.5 minutes; 20.6 versus 20.4 respectively).
Discussion
Summary of main results
This review assessed the effects of interventions, evaluated in randomised trials, to improve or promote interpersonal communication about end of life (EoL) care between patients expected to die within 12 months, their family members and carers, and the health practitioners involved in their care. The review included all simple or complex interventions (to inform or educate, support, skill, engage, or seek participation) aiming to improve communication about EoL and EoL care. Effects were sought on a range of outcomes for health consumers, practitioners and systems, including unintended (adverse) outcomes.
Eight trials were included. All assessed the effects of interventions compared with usual care. Certainty of the evidence for all outcomes was low or very low. More specifically.
All outcomes were downgraded for indirectness based on the review’s purpose. Populations assessed by included trials were limited to older adults (60 years and older), conducted in urban settings in high‐income countries.
Outcomes were also often downgraded for imprecision or inconsistency or both. Methodological limitations were not a common reason for downgrading certainty.
A summary of the findings of the review is as follows.
Knowledge and understanding (four studies, low‐certainty evidence; one study without usable data): interventions to improve communication may have little or no effect on knowledge of illness and prognosis, or information needs and preferences, although studies were small and measures used varied across trials.
Evaluation of the communication (six studies measuring several constructs (communication quality, patient‐centredness, involvement preferences, doctor‐patient relationship, satisfaction with consultation), most low‐certainty evidence): across constructs there may be minimal or no effects of interventions to improve communication about EoL, and uncertainty about effects on quality of communication.
Discussions of EoL or EoL care (six studies measuring selected outcomes, low‐ or very low‐certainty evidence): interventions to improve communication may increase duration of EoL discussions in an intensive care unit (ICU) setting, and may lead to earlier discussions of EoL and EoL care (each assessed by one study). We are uncertain about effects on occurrence of discussions and question asking in consultations, and there may be little or no effect on content of communication in consultations.
Adverse outcomes or unintended effects (limited evidence): there is insufficient evidence to determine whether there are adverse outcomes associated with communication interventions for EoL and EoL care. Patient and/or carer anxiety was reported by three studies, but judged as confounded. No other unintended consequences, or worsening of desired outcomes, were reported.
Patient/carer quality of life (four studies, low‐certainty evidence; two studies without useable data): interventions to improve communication may have little or no effect on quality of life.
Health practitioner outcomes (three studies, low‐certainty evidence; two without usable data): interventions to improve communication may have little or no effect on health practitioner outcomes (satisfaction with communication during consultation; one study); effects on other outcomes (knowledge, preparedness to communicate) are unknown.
Health systems impacts: communication interventions may have little or no effect on carer or clinician ratings of quality of EoL care (satisfaction with care, symptom management, comfort assessment, quality of care) (three studies, low‐certainty evidence). Interventions to improve communication may have little or no effect on patients' self‐rated care and illness, or on numbers of care goals met (one study, low‐certainty evidence). Communication interventions may increase mean consultation length (two studies), but other health service impacts (e.g. hospital admissions) are unclear.
Overall completeness and applicability of evidence
The results of this review are inconclusive across the range of consumer, provider, and health system outcomes sought. This compares with the generally positive effects of interventions reported by most of the included trials. The scope of this review was, however, very broad and there are significant gaps in the evidence assembled from available trials. These gaps contributed to the generally low level of certainty we have in the results, and represent areas where future research might productively focus.
Included populations
Since EoL affects everyone, this review aimed to evaluate the effects of interventions to improve EoL communication for any and all people. Studies were therefore eligible for inclusion across the life span (neonatal to old age), in diverse settings (rural and remote, low‐ and middle‐income countries (LMIC), community, acute and chronic care), in ethnically diverse groups (e.g. culturally and ethnically diverse backgrounds), and reflecting the variability of the community (i.e. those of varying socioeconomic, health literacy, and educational status), including vulnerable or hard‐to‐reach groups (e.g. those experiencing homelessness). However, populations evaluated in trials were limited, all studying older adults in high‐income, urban settings. All outcomes were therefore downgraded for indirectness across the board, primarily because of the limited population group studied collectively, and represents a limitation – and gap for future research – in this literature.
This review defined the population of interest very broadly, but used a focused definition of EoL (a person expected to die within 12 months). Several studies were excluded on this basis (participants’ life expectancy was far longer e.g. 24 months). We adopted this criterion based on the ACSQHC 2015 consensus statement definition. However, this decision narrowed the review’s scope, and led to the exclusion of a number of studies that otherwise may have contributed data to the review. Future researchers may wish to consider this issue further if conducting studies or reviews in this area.
Related to this issue, we deliberately excluded advance care planning (ACP) from this review, as it may not be closely temporally linked to EoL. For example, older adults are increasingly encouraged to undertake ACP in preparation for future decisions. In some cases, this may be close to the end of the person’s life. In others, ACP is for an unspecified future occurrence that may be many years into the future. Due to this variability and the lack of immediacy of the decision‐making associated with ACP – as well as the heavy reliance on checklists and similar tools, and the strong focus on ACP uptake (rather than communication outcomes) – we excluded these studies. In comparison, voluntary assisted dying (VAD), was eligible for inclusion, although no relevant studies were identified. VAD focuses on decisions to end life within a short (6‐ to 12‐month) time frame, and so was consistent with the broader focus of this review. It is possible, for those trials excluded based on their ACP focus, that there may have been a small number of studies that included conversations about EoL within the final 12 months of life that were excluded from this review. We did not identify such situations during study screening for the review, but it is possible that these were not identified and this issue should be considered further in future updates to this review, or future related reviews.
Interventions evaluated
All communication purposes were eligible for inclusion. Most had a stated aim of improving some aspect of patient‐doctor communication, targeting patients and carers alone or together with practitioners. Almost all interventions were tailored to participants, whether by allowing patients/carers to nominate or guide discussions towards priority topics, by providing patient‐specific feedback to physicians, or enabling patients/carers to prioritise questions.
More specifically, interventions most often aimed to provide information to participants, or to support engagement in consultations or decision‐making. Less often were interventions focused on checking people’s knowledge and understanding; on eliciting preferences, views and goals of treatment and EoL care; or on determining people’s concerns about EoL care and communication, including those of the health practitioners involved in these communications. All of these represent areas worthy of further exploration.
Interventions included in the review ranged from the simple (e.g. question prompt list (QPL)) to the complex (e.g. QPL plus patient coaching plus health practitioner training). Almost all were delivered once, sometimes with an additional booster or brief follow‐up. Evaluating the effects of communication delivered over time is undoubtedly challenging. However, people’s priorities, preferences and understanding change over time as death nears, and communication that is responsive and tailored to people’s changing needs suggests that future studies may also fruitfully explore the delivery of communication interventions over time for people in the EoL period.
The review did not find any head‐to‐head comparisons between interventions. Future studies might consider such comparisons, along with stepwise addition of components to communication interventions, in order to systematically assess the relative effects and contributions to outcomes that might follow use of such strategies. Usual care was in all cases the control arm for the included trials, but the exact nature of this varied considerably. In some cases, usual care was standard clinical care, in others there was substantial information or support or both provided (or even a substantial co‐intervention delivered to both arms). This may have effectively narrowed any differences between intervention and usual care arms in at least some of the included trials. Future studies might consider this issue carefully and design studies accordingly.
Outcomes and outcome measures
This review sought information about the effects of interventions on a wide range of outcomes. Collectively, included studies reported outcomes across most broad categories of interest. This indicates researchers’ understanding of the complex effects of communication with different purposes related to EoL, and that different people are involved in such communication. Despite this, outcome measures (tools or timing or both) within categories were often highly varied. In general, few studies contributed data to any one outcome category or construct, so many were sparsely populated with data. As a result, findings were largely inconclusive. There were several outcomes where there were little data on which to base conclusions (e.g. those for which only a single small study contributed data); in other cases there were no data available or in usable form or both (e.g. costs). There were also a small number of specific gaps (health practitioner knowledge and understanding of patient/family/carer knowledge, wishes, or preferences; hospital admissions and re‐admissions). Despite our extensive searches for relevant evidence, it is possible that studies focused on cost‐effectiveness or health service use related to communication at the EoL were not identified for consideration for the review, and this may represent a limitation of the review.
Variability in outcome constructs and measures used in this research literature limits conclusions that can be made across studies, or pooling of data to identify whether effects of interventions exist, or both. Future studies might consider using ‐ or developing – validated, responsive tools to assess outcomes, to facilitate analysis and interpretation of findings by clinicians and by researchers.
Challenges of measuring the different constructs affected by EoL communication may also have contributed to the limited effects of the interventions across outcomes (Brighton 2016; Sansoni 2014). Such communication is complex (multidirectional, multifaceted, involving multiple people) and without a range of well‐established tools available, trialists may be left to rely on those used historically or to develop their own tools. Additionally, some outcome measures may not have been sensitive and/or specific enough to capture nuanced differences between intervention and control groups (e.g. coded qualitative data from audiotaped consultations converted to quantitative count data). In some cases, composite scores were reported, which can be difficult to interpret. In others, measures may have been a little blunt e.g. occurrence of discussions and coverage of topics may not reflect quality of the communication (how well was it done?), rather it more closely reflects content (were these topics covered?). Such challenges of measuring outcomes for EoL communication have been noted by other researchers (Brighton 2016; Sansoni 2014). Current results suggest that more sophisticated and nuanced ways of assessing communication at EoL may help to better understand the complex interactions between the people involved. It may be challenging to design studies to measure such outcomes, but continuing to conduct trials without appropriate and sensitive outcome measures will not optimally fill gaps in our knowledge or readily inform policy and practice decisions in this area.
Consideration should be given to the value of mixed‐methods or qualitative research or both, which may be better placed to inform decisions about outcomes that are meaningful to those involved in EoL consultations and discussions. Rather than rushing to replicate trials, research that explores a range of perspectives about communication at EoL may help to better understand the context in which communication is occurring, as well as barriers and enablers that may influence the complex interactions between the people involved in communication at the EoL.
Unintended (adverse) outcomes
Adverse outcomes are difficult to define amongst this population, and therefore difficult to measure and report. There may be many confounding factors present in this context of clinical care and communication. None of the included studies reported outcomes we judged as adverse (unintended effects) of the intervention, such as worsened understanding (confusion) about EoL, EoL care or options and decisions to be made; poorer ratings of quality of care or communication during the EoL period; or heightened fear, distress, anxiety or stress in patients, family members and carers, or in health practitioners involved in care.
Only patient and/or carer anxiety was reported as a potential unintended consequence of the intervention (3/8 studies), but effects were judged as confounded and there was no indication of other possible negative effects of communication interventions. This is a gap in the evidence, reflected by other studies and reviews (Brighton 2016; Sansoni 2014) and future studies should consider carefully the range, types and measurement of potential negative consequences.
Quality of the evidence
Overall, certainty of evidence was rated as low or very low for all outcomes. We rated down (‐1) for indirectness across all outcomes, as the populations assessed by the included trials were limited, as discussed above. We commonly also downgraded the evidence based on imprecision: several outcomes were measured only by single trials, and sample sizes were typically small. In a small number of cases, certainty was downgraded based on inconsistency (knowledge, discussion occurrence), as a result of variation in constructs and measures or persistent differences in findings despite similar population and intervention.
Methodological limitations (risk of bias) were not a major reason for downgrading across outcomes. Most trials were of good methodological quality, particularly on the key domains of sequence generation and allocation concealment, despite the challenges of conducting research in this area.
Potential biases in the review process
We used standard Cochrane methods to undertake this review, with few changes from protocol to review stage. The small changes made when conducting the review (changes to grey literature sources, decision to extract data at longest follow‐up) are unlikely to bias the results of the review.
Similarly, we searched extensively for relevant published and unpublished research, and conducted a range of supplementary search activities, including contacting authors of relevant trials. Despite this, it is possible that we have missed a relevant trial, or relevant publications arising from the included trials.
The review was established with detailed selection criteria that articulated several complicated distinctions in the topic area. However, making some of these distinctions operational was challenging. This included the intention to exclude studies in which ACP administration and completion were the main focus; clearly distinguishing between clinical care and communication across different clinical populations; and consistently identifying key features of the EoL patient population. These decisions were made through discussion to reach consensus amongst at least three review authors, and to ensure that decision rules were consistently applied. These screening decisions related to a large number of excluded studies (e.g. focus on ACP and uptake (30 studies excluded on this basis), population not at EoL according to the review's definition (30 studies), study focus primarily clinical management not communication (18 studies)). Others making these same selection decisions may reach different conclusions about the inclusion or exclusion of some of these studies. As a team we made every effort to apply decision rules consistently, and so do not believe that these decisions introduced bias.
Agreements and disagreements with other studies or reviews
This review’s findings ‐ which highlight uncertainty within current evidence ‐ differ substantially from those of most of the included trials, many of which individually concluded that communication interventions were effective, as assessed via a range of outcomes. Recent studies have, however, highlighted the challenges of delivering and evaluating such interventions in populations approaching EoL, and have called for rigorous evaluations of the effects of communication interventions for adult and paediatric patients and families (Ekberg 2019; Hjelmfors 2020; Wolfe 2020).
While many of the included studies reported statistically significant effects in favour of the intervention on key outcomes, the meaning of such results is in at least some cases unclear. For example, if quality of communication scores increase significantly with the intervention, but this effect is small and scores remain very much lower than the highest possible, it is very difficult to understand what this might mean for practice. Similarly, if knowledge increases significantly with an intervention relative to usual care, but 50% of people still have unmet information needs irrespective of their study group, this suggests the intervention’s effectiveness is limited.
We are not suggesting that an intervention could or should be 100% effective to be worthwhile and meaningful. However, we would urge trialists and systematic reviewers in this area to carefully consider the meaning of the findings arising from trials, particularly as they are undertaken in a vulnerable population group at a particularly stressful and distressing times of their lives. We also emphasise the importance of building carefully upon previous research to maximise the value and effectiveness of interventions for improving communication with people in the EoL period.
Such situations highlight the need for well‐established tools to measure communication interventions’ effects, and for researchers to consider the value of alternative methods for studying communication at the end of life – such as qualitative methods to unpack complexity and to understand barriers and enablers to good quality communication, or mixed methods approaches which might be harnessed to jointly understand complexity and to evaluate effects of interventions. Involvement of stakeholders in the co‐design and evaluation of interventions for communication at EoL also seems essential, and several of the included studies evaluated interventions which had been developed in just such a way. The importance of co‐design, coupled with meaningful stakeholder input to the development of interventions (and outcome measures) cannot be overstated – but the understanding of what this might involve in real terms has changed substantially over time (particularly the last decade) and is continuing to develop (Merner 2019; Merner 2021).
There are few recent systematic reviews published which have specifically focused on communication at the end of life. One new review was identified (Thode 2020), evaluating tools to support discussion of life‐prolonging treatments in hospital. This review identified a small number of studies of various designs, a summary of which suggested there may be mixed effects, including some positive effects on outcomes such as self‐efficacy. Other recent publications (Fujimori 2020; Van der Steen 2021) highlight that communication at EoL is a developing area and indicate a need for tailored approaches to communication. There is also a need to consider the complex interplay between the different people and their roles who may be involved in these discussions (Wolfe 2020), including shared decision‐making about goals for future care as diseases progress towards the end of life (e.g. for patients with dementia) (Van der Steen 2021).
Timing and timeliness of communication for EoL are factors with emerging importance in the literature, but only explored in this review to a small degree with the results of Bernacki 2019 indicating that the Serious Illness Conversation Guide (SICG) communication intervention led to much earlier discussions of EoL care. Early conversations about EoL may be better than later (Brighton 2016), although there is growing understanding that good EoL communication must take into account people’s different needs, preferences and priorities, and provide clear opportunities for preferences and concerns to be discussed, at different times (Hjelmfors 2020; NICE 2019; Thode 2020). Not all people will be ready to take part in such discussions at the same time point (Brighton 2016) and so the timing for offering information, and its staging, are critical (Anderson 2019). The length (duration) of consultations and meaning of these is also complex and not yet well understood. Sometimes longer consultations may indicate a more comprehensive discussion about EoL and care has taken place. At other times, longer meetings or consultations might indicate some level of disagreement or misunderstanding between those involved (Thompson 2009).
There must also be clear, ongoing opportunities for patients and carers to revisit and change decisions over time, tailored according to need (Anderson 2019; Brighton 2016; Ekberg 2019; Hjelmfors 2020; NICE 2019). For instance, patients and carers may have preferences for different types and amounts of information that depend at least in part on how close to the patient is to the EoL (Anderson 2019; Brighton 2016; Ekberg 2019). Cultural background and health literacy levels may also influence such preferences for information types and amounts (Anderson 2019; Hjelmfors 2020; Thode 2020). Opportunities for discussions about EoL therefore need to be provided, to account for people's varying preferences for information types and amounts (NICE 2019; Sansoni 2014). Considering such factors, likely among others, will help to determine how communication at the EoL might best take place, and whether or not it is able to meet the needs and priorities of all people involved in such discussions.
Authors' conclusions
Implications for practice.
There is currently no high‐certainty evidence to inform practice decisions about how healthcare practitioners can best communicate with patients, carers, and family members about end of life (EoL) and EoL care.
Implications for research.
There are several implications for research arising directly from the evidence assembled in this review.
Future research might usefully aim to fill identified gaps. This review highlights those particularly related to populations: research is needed in younger people (including neonates, children and young adults); people living in rural and remote areas, and in low‐ and middle‐income countries (LMIC); in people with diverse cultural, socioeconomic, educational and health literacy backgrounds; and including people from hard to reach or vulnerable groups.
Research is needed to establish valid outcome measures and tools that are responsive to the changes that might follow delivery of a communication intervention. Similarly, adverse or unintended effects need to be carefully considered and assessed.
Mixed methods or qualitative research or both may contribute usefully to this area, in order to better understand the complex interplay between different parties involved in communication. Such research may also help to identify barriers and enablers of good communication, and so inform development of more effective interventions as well as appropriate outcome measures. Outcomes should build on those reported to date in trials and in this review, and ensure that those important from a patient and family/carer, as well as health practitioner and system perspectives are adequately assessed and reported in relation to EoL communication.
Future trials might consider investigating comparisons in such a way that allow systematic evaluation of increasingly complex interventions, in order that the most effective approaches and combinations of strategies can be identified. Further investigation and evaluation of interventions which are responsive and tailored to people’s changing needs, and enable engagement in communication and shared decision‐making about EoL and EoL care would be valuable. Co‐design and evaluation of such interventions, involving a range of people affected by EoL communication and care, should be a key underpinning principle for future research in this area.
What's new
| Date | Event | Description |
|---|---|---|
| 22 July 2022 | Amended | Siegle 2018 removed from ongoing studies and added as a secondary ref to Krug 2021 (awaiting classification) following advice from Siegle 2018 author team. Number of ongoing studies reported in the results revised to reflect this change. |
History
Protocol first published: Issue 9, 2018 Review first published: Issue 7, 2022
Notes
This protocol is based on standard text and guidance provided by the Cochrane Consumers and Communication Group (CCCG 2016).
Acknowledgements
Michael Connolly was part supported by the Health Research Board (Ireland) and the HSC Public Health Agency (Grant number CBES‐2018‐001) through Evidence Synthesis Ireland/Cochrane Ireland.
We sincerely thank the authors of included trials for their help in identifying additional papers and for providing additional information about their studies (authors of the Agar 2017, Bernacki 2019, Clayton 2007, Epstein 2017, Reinhardt 2014, and Walczak 2017 trials).
We thank Lee‐Anne Pedersen for contributing to the title proposal for the review.
We thank Professor Declan Devane, Director, Evidence Synthesis Ireland and Cochrane Ireland, and Ray Kelly, Consumer, for providing peer review of this review. We thank Luisa M Fernandez Mauleffinch, Cochrane Copy Edit Support, for copy editing this review.
We also thank the editors and staff of the Cochrane Consumers and Communication Group, particularly our Contact Editor, Sophie Hill, for her input to the protocol and review, and Anne Parkhill, Information Specialist, for her work to develop and run the searches for this review.
Appendices
Appendix 1. MEDLINE search strategy
1. "Decision Support Techniques"/ 2. exp Decision Support Systems, Clinical/ 3. decision trees/ 4. (decision making or choice behavior).mp. and informed consent.sh. 5. "Truth Disclosure"/ 6. ((decision* or decid* or planning or choice* or plans or plan or discuss* or goal* or directive* or right*) adj3 (support* or aid* or tool* or instrument* or technolog* or technique* or system* or program* or algorithm* or process* or method* or intervention* or material* or conversation* or share or shared or sharing or inform* or making or behavior*)).ti,ab,kw. 7. (decision adj (board* or guide* or counseling)).tw. 8. ((risk communication or risk assessment or risk information) adj4 (tool* or method*)).tw. 9. decision‐making computer assisted/ 10. (computer* adj2 decision making).tw. 11. (communicati* or discuss* or ask* or understand*).ti,ab,kw. 12. (interactive adj (internet or online or graphic* or booklet*)).tw. 13. (interacti* adj4 tool*).tw. 14. ((interactiv* or evidence based) adj3 (risk information or risk communication or risk presentation or risk graphic*)).tw. 15. shared decision making.tw. 16. (informed adj (choice* or decision*)).tw. 17. adaptive conjoint analys#s.tw. 18. exp Decision Making/ 19. exp Communication/ 20. or/1‐19 21. exp Advance Directives/ 22. exp Advance Care Planning/ 23. advanced care plan*.ti,ab. 24. (advance* adj2 directive*).ti,ab. 25. living will*.ti,ab. 26. exp Terminal Care/ 27. "Terminally Ill"/ 28. Palliative Care/ 29. "Attitude to Death"/ 30. (end of life or (life adj limit*) or eol).ti,ab,kw. 31. (death or dies or die or dying or grief or bereav* or palliati*).ti,ab. 32. wills/ 33. right to die/ 34. patient self‐determination act/ 35. resuscitation orders/ 36. advance directive adherence/ 37. or/21‐36 38. "Caregivers"/ 39. "Interdisciplinary Communication"/ 40. exp Community Participation/ 41. Professional‐Patient Relations/ 42. "Physician‐Patient Relations"/ 43. "Professional‐Family Relations"/ 44. exp Family/ 45. ((patient$ or consumer$ or family or families or relative$ or parent$ or child$ or partner$ or women$ or carer$ or caregiver$ or advocate$ or surrogate* or subject*) adj5 (activat$ or involv$ or communicat* or initiat$ or engag$ or participat$ or contribut$ or collaborat$ or role or cooperat$ or assist$ or champion$ or advoc$ or help‐seek$ or document*)).tw. 46. exp legal guardians/ 47. health care agent*.tw. 48. power of attorney.tw. 49. proxy.tw. 50. or/38‐49 51. end of life.tw. 52. (death or die or dies or dying).tw. 53. or/51‐52 54. and/50,53 55. Patient Education as Topic/ 56. Patient Preference/ 57. or/54‐56 58. randomized controlled trial.pt. 59. controlled clinical trial.pt. 60. randomized.ab. 61. placebo.ab. 62. drug therapy.fs. 63. randomly.ab. 64. trial.ab. 65. groups.ab. 66. Practice Guidelines as Topic/ 67. Practice Guideline.pt. 68. or/58‐67 69. exp animals/ not humans.sh. 70. 68 not 69 71. and/20,37,57,70
For the search update conducted in July 2021 the following lines were added to the MEDLINE strategy:
72. 71 not (66 or 67) 73. 71 and (66 or 67) 2018 limit
Appendix 2. PsychINFO
1. exp decision making/
2. decision support systems/
3. truth/
4. preferences/ or preference measures/
5. (decision adj (board* or guide* or counseling)).tw.
6. ((risk communication or risk assessment or risk information) adj4 (tool* or method*)).tw.
7. (computer* adj2 decision making).tw.
8. (communicati* or discuss*).ti,ab.
9. (interactive adj (internet or online or graphic* or booklet*)).tw.
10. (interacti* adj4 tool*).tw.
11. ((interactiv* or evidence based) adj3 (risk information or risk communication or risk presentation or risk graphic*)).tw.
12. shared decision making.tw.
13. adaptive conjoint analys*.tw.
14. exp Communication/
15. or/1‐14
16. exp Advance Directives/
17. advanced care plan*.ti,ab.
18. (advance* adj2 directive*).ti,ab.
19. living will*.ti,ab.
20. "Terminally Ill"/
21. Palliative Care/
22. death attitudes/ or "death and dying"/ or death anxiety/
23. (end of life or (life adj limit*) or eol).ti,ab.
24. (death or dies or die or dying or grief or bereav* or palliati*).ti,ab.
25. euthanasia/
26. assisted suicide/
27. exp Terminally Ill Patients/
28. exp life sustaining treatment/
29. treatment refusal/ or treatment withholding/
30. or/15‐29
31. "Caregivers"/
32. interdisciplinary treatment approach/
33. client education/
34. exp Family/
35. health care agent*.tw.
36. power of attorney.tw.
37. proxy.tw.
38. end of life.tw.
39. (death or die or dies or dying).tw.
40. ((decision* or planning or plan or plans or discuss* or goal* or directive* or right*) adj3 (end of life or (death or die or dies or dying))).tw.
41. or/31‐40
42. random*.ti,ab,hw,id.
43. trial*.ti,ab,hw,id.
44. controlled stud*.ti,ab,hw,id.
45. placebo*.ti,ab,hw,id.
46. ((singl* or doubl* or trebl* or tripl*) and (blind* or mask*)).ti,ab,hw,id.
47. (cross over or crossover or factorial* or latin square).ti,ab,hw,id.
48. (assign* or allocat* or volunteer*).ti,ab,hw,id.
49. treatment effectiveness evaluation/
50. mental health program evaluation/
51. exp experimental design/
52. "2100".md.
53. or/42‐52
54. and/15,30,41,53
Appendix 3. CINAHL search strategy
| S44 | S31 AND S43 |
| S43 | S32 or S33 or S34 or S35 or S36 or S37 or S38 or S39 or S40 or S41 or S42 |
| S42 | TX allocat* random* |
| S41 | (MH "Quantitative Studies") |
| S40 | (MH "Placebos") |
| S39 | TX placebo* |
| S38 | TX random* allocat* |
| S37 | (MH "Random Assignment") |
| S36 | TX randomi* control* trial* |
| S35 | TX ( (singl* n1 blind*) or (singl* n1 mask*) ) |
| S34 | TX clinic* n1 trial* OR ( TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) ) OR ( TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) ) OR ( TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) ) |
| S33 | PT Clinical trial |
| S32 | (MH "Clinical Trials+") |
| S31 | S16 AND S25 AND S30 |
| S30 | S26 OR S27 OR S28 OR S29 |
| S29 | (MH "Guardianship, Legal+") OR (MH "Patient Education+") |
| S28 | ((patient* or consumer* or family or families or relative* or parent* or child* or partner* or women* or carer* or caregiver* or advocate* or surrogate* or subject*) N5 (activat* or involv* or communicat* or initiat* or engag* or participat* or contribut* or collaborat* or role or cooperat* or assist* or champion* or advoc* or help‐seek* or document*)) |
| S27 | (MH "Family+") OR (MH "Professional‐Family Relations") OR (MH "Patient‐Family Relations") OR (MH "Patient‐Family Conferences") |
| S26 | (MH "Caregivers") |
| S25 | S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 |
| S24 | TX advanced care plan* OR TX life limit* OR TX advance* N2 directive* OR TX living will* OR TX ( (end of life) or (eol) ) OR TX ( (death or dies or die or dying or grief or bereav* or palliati*) ) |
| S23 | (MH "Resuscitation Orders") |
| S22 | (MH "Right to Die") OR (MH "Treatment Refusal") |
| S21 | (MH "Attitude to Death+") |
| S20 | (MH "Palliative Care") OR (MH "Hospice and Palliative Nursing") |
| S19 | (MH "Terminal Care+") OR (MH "Terminally Ill Patients+") OR (MH "Nursing Care Plans, Computerized") |
| S18 | terminal care |
| S17 | (MH "Advance Directives+") OR (MH "Advance Care Planning") |
| S16 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 |
| S15 | (MH "Decision Making+") |
| S14 | adaptive conjoint analys* |
| S13 | (informed N (choice* or decision*)) |
| S12 | shared decision making |
| S11 | ((interactiv* or evidence based) N3 (risk information or risk communication or risk presentation or risk graphic*)) |
| S10 | (interacti* N4 tool*) |
| S9 | (interactive N (internet or online or graphic* or booklet*)) |
| S8 | communicati* OR discuss* |
| S7 | computer* N2 decision making |
| S6 | ((risk communication or risk assessment or risk information) N3 (tool* or method*)) |
| S5 | (decision N3 (board* or guide* or counseling)) |
| S4 | ((decision* or decid* or planning or choice* or plans or plan or discuss* or goal* or directive* or right*) N3 (support* or aid* or tool* or instrument* or technolog* or technique* or system* or program* or algorithm* or process* or method* or intervention* or material* or conversation* or share or shared or sharing or inform* or making or behavior*)) |
| S3 | (MH "Truth Disclosure") OR (MH "Self Disclosure") |
| S2 | MW choice |
| S1 | (MH "Decision Making, Patient+") OR (MH "Decision Making, Computer Assisted+") OR (MH "Decision Making, Clinical") OR (MH "Decision Making, Family") OR (MH "Decision Making, Ethical") |
Appendix 4. EMBASE search strategy
1. exp decision support system/
2. exp "decision tree"/
3. (truth adj3 disclosure).ti,ab.
4. ((decision* or decid* or planning or choice* or plans or plan or discuss* or goal* or directive* or right*) adj3 (support* or aid* or tool* or instrument* or technolog* or technique* or system* or program* or algorithm* or process* or method* or intervention* or material* or conversation* or share or shared or sharing or inform* or making or behavior*)).ti,ab,kw.
5. (decision adj (board* or guide* or counseling)).tw.
6. ((risk communication or risk assessment or risk information) adj4 (tool* or method*)).tw.
7. (computer* adj2 decision making).tw.
8. (communicati* or discuss* or ask* or understand*).ti,ab,kw.
9. (interactive adj (internet or online or graphic* or booklet*)).tw.
10. (interacti* adj4 tool*).tw.
11. ((interactiv* or evidence based) adj3 (risk information or risk communication or risk presentation or risk graphic*)).tw.
12. shared decision making.tw.
13. (informed adj (choice* or decision*)).tw.
14. adaptive conjoint analys#s.tw.
15. exp Decision Making/
16. or/1‐15
17. living will/
18. exp terminal care/
19. exp terminally ill patient/
20. exp palliative therapy/
21. attitude to death/
22. right to die/
23. patient self‐determination act/
24. resuscitation/
25. advanced care plan*.ti,ab,kw.
26. (advance* adj2 directive*).ti,ab,kw.
27. living will*.ti,ab,kw.
28. (end of life or (life adj limit*) or eol).ti,ab,kw.
29. (death or dies or die or dying or grief or bereav* or palliati*).ti,ab,kw.
30. or/17‐29
31. caregiver/
32. interdisciplinary communication/
33. exp interpersonal communication/
34. community participation/
35. professional‐patient relationship/
36. doctor patient relation/
37. exp family/
38. legal guardian/
39. ((patient$ or consumer$ or family or families or relative$ or parent$ or child$ or partner$ or women$ or carer$ or caregiver$ or advocate$ or surrogate* or subject*) adj5 (activat$ or involv$ or communicat* or initiat$ or engag$ or participat$ or contribut$ or collaborat$ or role or cooperat$ or assist$ or champion$ or advoc$ or help‐seek$ or document*)).ti,ab,kw.
40. health care agent*.tw.
41. power of attorney.tw.
42. proxy.tw.
43. patient education/
44. exp patient preference/
45. or/31‐44
46. randomized controlled trial/
47. controlled clinical trial/
48. single blind procedure/ or double blind procedure/
49. crossover procedure/
50. random*.tw.
51. placebo*.tw.
52. ((singl* or doubl*) adj (blind* or mask*)).tw.
53. (crossover or cross over or factorial* or latin square).tw.
54. (assign* or allocat* or volunteer*).tw.
55. or/46‐54
56. and/16,30,45,55
Appendix 5. CENTRAL search strategy
#1 MeSH descriptor: [Decision Support Techniques] this term only
#2 MeSH descriptor: [Decision Support Systems, Clinical] explode all trees
#3 MeSH descriptor: [Decision Trees] explode all trees
#4 MeSH descriptor: [Truth Disclosure] explode all trees
#5 MeSH descriptor: [Decision Making] explode all trees
#6 MeSH descriptor: [Communication] explode all trees
#7 ((decision making or choice behavior) and informed consent):ti,ab
#8 ((decision* or decid* or planning or choice* or plans or plan or discuss* or goal* or directive* or right*) near/2 (support* or aid* or tool* or instrument* or technolog* or technique* or system* or program* or algorithm* or process* or method* or intervention* or material* or conversation* or share or shared or sharing or inform* or making or behavior*)):ti,ab
#9 (decision and (board* or guide* or counseling))
#10 ((risk communication or risk assessment or risk information) and (tool* or method*)):ti,ab
#11 MeSH descriptor: [Decision Making, Computer‐Assisted] this term only
#12 (computer* and decision making):ti,ab
#13 (communicati* or discuss* or ask* or understand*):ti,ab
#14 (interactive near/3 (internet or online or graphic* or booklet*)):ti,ab
#15 (interacti* near/3 tool*)
#16 ((interactiv* or evidence based) near/3 (risk information or risk communication or risk presentation or risk graphic*))
#17 (shared decision making):ti,ab
#18 (informed next (choice* or decision*)):ti,ab
#19 (adaptive conjoint analys*):ti,ab
#20 {or #1‐#19}
#21 MeSH descriptor: [Advance Directives] explode all trees
#22 MeSH descriptor: [Advance Care Planning] explode all trees
#23 MeSH descriptor: [Terminal Care] this term only
#24 MeSH descriptor: [Terminally Ill] this term only
#25 MeSH descriptor: [Palliative Care] explode all trees
#26 MeSH descriptor: [Attitude to Death] this term only
#27 MeSH descriptor: [Wills] explode all trees
#28 MeSH descriptor: [Right to Die] this term only
#29 MeSH descriptor: [Resuscitation Orders] explode all trees
#30 MeSH descriptor: [Advance Directive Adherence] this term only
#31 (advanced care plan*):ti,ab
#32 (end of life or (life adj limit*) or eol):ti,ab
#33 (death or dies or die or dying or grief or bereav* or palliati*):ti,ab
#34 (living will*):ti,ab
#35 (advance* near/2 directive*):ti,ab
#36 {or #21‐#35}
#37 MeSH descriptor: [Caregivers] this term only
#38 MeSH descriptor: [Interdisciplinary Communication] explode all trees
#39 MeSH descriptor: [Community Participation] explode all trees
#40 MeSH descriptor: [Professional‐Patient Relations] this term only
#41 MeSH descriptor: [Physician‐Patient Relations] this term only
#42 MeSH descriptor: [Professional‐Family Relations] this term only
#43 MeSH descriptor: [Family] explode all trees
#44 MeSH descriptor: [Legal Guardians] this term only
#45 MeSH descriptor: [Patient Education as Topic] this term only
#46 MeSH descriptor: [Patient Preference] this term only
#47 ((patient* or consumer* or family or families or relative* or parent* or child* or partner* or women* or carer* or caregiver* or advocate* or surrogate* or subject*) near/5 (activat* or involv* or communicat* or initiat* or engag* or participat* or contribut* or collaborat* or role or cooperat* or assist* or champion* or advoc* or help‐seek* or document*)):ti,ab
#48 (health care agent*):ti,ab
#49 (power of attorney):ti,ab
#50 (proxy):ti,ab
#51 {or #37‐#50}
#52 {and #20, #36, #51} in Trials
Data and analyses
Comparison 1. Intervention versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Doctor‐patient relationship | 3 | 238 | Std. Mean Difference (IV, Random, 95% CI) | 0.23 [‐0.06, 0.51] |
| 1.2 Discussion of EoL care planning | 2 | 537 | Risk Ratio (M‐H, Random, 95% CI) | 1.96 [1.61, 2.39] |
| 1.3 Patient questions in consultation | 2 | 249 | Mean Difference (IV, Random, 95% CI) | 1.58 [‐1.82, 4.98] |
| 1.4 Family‐rated symptom management (SM‐EOLD) | 2 | 212 | Mean Difference (IV, Random, 95% CI) | ‐1.98 [‐4.38, 0.43] |
| 1.5 Family‐rated satisfaction with care at EoL (SWC‐EOLD) | 2 | 212 | Mean Difference (IV, Random, 95% CI) | 0.44 [‐0.99, 1.87] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Agar 2017.
| Study characteristics | ||
| Methods |
Aim: to assess the effects of facilitated case conferencing (FCC) versus usual care in improving EoL care for people with advanced dementia living in nursing homes Study design: cluster‐RCT; 2 arms (FCC intervention; usual care) Unit of randomisation: nursing home (stratified by organisational affiliation) Consumer involvement: none explicitly stated Funding source: Australian Department of Health. Authors declared they have no conflicts of interest |
|
| Participants |
Participants: people with advanced dementia and their carers Setting and geographic location: residential nursing homes in Sydney and Brisbane (Australia) Methods of recruitment: Sites: identified from Australian government list (websites) and approached in alphabetical order to participate (to minimise selection bias) 20 sites in major cities Family members provided consent for resident participation in trial Selection criteria for participation in study: Inclusion:
Diagnosis of person approaching EoL: as above for diagnostic markers; defined so as to identify people with FAST stage 7 and functional dependency, which in turn identifies people with an average survival of less than 6 months Target of intervention: Resident (person with dementia) Family member or friend who knows the person well (prior to dementia diagnosis) involved in making decisions on the patient’s behalf Protocol stated: visits the resident at least once/fortnight, knew resident prior to dementia diagnosis, willing to be involved in decisions about care, English proficiency at a level to allow completion of outcome assessments Age: patients: intervention 84.7 (SD 7.9), UC 85.8 (SD 8.2) Gender: intervention 61% female, UC 58% female Ethnicity/culture/language: born in Australia intervention 70%, UC 52% (significantly different) Other PROGRESS aspects: Focus was on advanced dementia/nursing homes with at least 50% patients with dementia, larger sized residential homes. Urban populations No exclusions mentioned re: literacy level, comorbidity, etc. but results may not be applicable to smaller/non‐specialist nursing home settings and populations Numbers of participants: see Additional Table 1 |
|
| Interventions |
Intervention: facilitated case conferencing (FCC) and patient‐centred palliative care training Aim of intervention: to improve care at the EoL (primary) Considerations included train‐the‐trainer, evidence‐based organisational culture change and expected advanced dementia trajectory Comparison: usual care No education, training or support provided over usual practice. No restriction on service‐provider training/education where this was usual practice Further details of UC not reported. Authors note however that the difference between intervention and UC sites may have been narrowed by the use of means other than CC to share decisions with residents/family members (e.g. 1:1 conversations). These may have influenced EoL care and satisfaction in the UC group (i.e. narrowed the gap between intervention and UC) Delivered by: nurse, trained as Palliative Care Planning Co‐ordinator (PCPC), worked 2 days/week or equivalent to:
Co‐intervention(s): not reported (other than palliative care training) Setting: residential nursing home; no further details Materials, procedures, content: Nurse, trained as Palliative Care Planning Co‐ordinator (PCPC), worked 2 days/week or equivalent to:
Content (key features): predefined specific clinical triggers for CC; shared agenda setting model (resident, family, multidisciplinary team) could identify areas for discussion; required attendance by resident and/or family/decision‐makers; facilitate by PCPC to ensure optimal participation by attendees; followed by communication strategy to summarise actions and plan from CC. In‐person meeting When and how much: author response indicates further data on these factors being analysed. Also reported median duration was 48 minutes (IQR 30 to 60). Sessions were always conducted at a single session (but some residents had more than 1 over time) Tailoring: discussion topics in FCC meeting tailored to what was important to the resident. Could include care planning, current and future treatment decision‐making, information sharing, meeting residents’ needs or preferences, ACP Author response indicated that topics were identified/put on the agenda for the CC meeting via the PCPC seeking "advice on what to include on the agenda from the resident’s family, GP and nursing staff beforehand (including people who were unable to attend). There was also usually a trigger for the CCs and any related issues were by default on the agenda (e.g. return from hospital, declining health, family concern)" Modified during study: no. Fidelity monitoring was modified to account for variations in level of detail of reporting across nursing homes Fidelity assessed: fidelity was assessed (‘dose at resident level’) for per protocol analyses at resident level. Not assessed as planned as many units did not collect information to make the assessment. Reverted to a simpler measure of intervention dose: resident received a CC or not Dose at nursing home level assessed extent to which PCPCs could: work 2 days/week; supported by managers; fulfilled expectations of training; diffused their role among other staff Theoretical base: factors considered when developing the intervention, and purpose, seem reasonable and logical. No further specific theoretical constructs |
|
| Outcomes |
Primary outcomes None reported Primary outcomes ‐ adverse events None reported Secondary outcomes Family‐rated EoL care: 3 subscales [Quality of EoL care, family‐rated]
Method: face‐to‐face or telephone interview with research team Timing: 4 to 6 weeks after death Scale and scoring: End of Life in Dementia Scales
Resident CAD‐EOLD [Quality of EoL care, nurse‐rated] Method: nurse‐rated. Face‐to‐face or telephone interview with research team Timing: as soon as possible after death of patient Scale and scoring: CAD‐EOLD; higher scores = better (more comfort) Resident SM‐EOLD [Quality of EoL care, nurse‐rated] Method: nurse‐rated. Face‐to‐face or telephone interview with research team Timing: as soon as possible after death of patient Scale and scoring: SM‐EOLD; higher scores = better (lower symptom frequency) Quality of life [Quality of life] Method: nurse‐rated Timing: 3‐monthly Scale and scoring: Quality of life in Late‐stage Dementia (QUALID); 11‐item scale Staff attitudes to, knowledge of and confidence in providing palliative/EoL care [Health practitioner evaluation of preparedness to communicate] Method and timing: assessed for PCPCs before and after training (by research staff) Assessed for other facility staff before and after training (by PCPC) Scale and scoring: Palliative Care for Advanced Dementia tool, 35 items (qPAD) Costs [Costs of subsequent care] Method: training, CC and routine healthcare costs to be considered Cost utility (benefit estimated as QALYs). QoL for economic analyses to be assessed by nurse‐rated EQ‐5D‐5L Person‐centred approach to care [Ratings of concordance with patient preferences for EoL care] Method: rated by observation, resident and family reports and documentation Timing: unclear Scale and scoring: Care and Activities and Interpersonal Relationships and Interactions domain of the Person‐Centred Environment and Care Assessment Tool (PCECAT). 18 items, each rated 0 (not at all) to 3 (all of the time) |
|
| Notes | Protocol prospectively registered 2012, updated 2017 (Improving dementia end of life care at local aged care facilities; www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12612001164886) Cluster‐RCT; ICC (0.050 used to calculate required sample size (not met because of unexpectedly low mortality rate amongst sample). Analyses described adjusted for ICC values (reported). Individual results presented. Seems likely that results were appropriately adjusted. ICCs scores reported for different scales and were reported as variable (some lower, some higher than predicted 0.05 level) (e.g. see page 6) This trial reported data related to care received in the last month of life. We did not extract these data as we judged them clinical, rather than fitting with the focus of this review on communication Data were not available/analysable for the following outcomes: quality of life (QUALID), staff attitudes to and knowledge of providing palliative/EoL care (qPAD), person‐centred care (PCECAT), or costs. Duration of EoL discussions was available only for the intervention arm (non‐comparative) and was therefore not included in the review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated allocation sequence; block randomisation Allocation (unit of randomisation) by nursing home (stratified by 2 factors: organisational affiliation (part of organisation or not); dementia‐specific unit or not) |
| Allocation concealment (selection bias) | Low risk | No details in trial report Protocol states that statisticians responsible for allocating sites were blinded to allocation (page 21) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Authors state that staff, family members and residents were blinded to the aim of the study (but those in the intervention sites may have noticed changes in practice) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Research staff were blinded to study aim; only collected data from sites in 1 arm to minimise the chance they would identify differences in practice between intervention and usual care sites |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Large proportion of data (participants randomised) missing. However, these were comparable for the 2 study groups, and was due to participants not dying during the study period (outcomes assessed for this study were focused on those around death) Withdrawal rates for other reasons were low and comparable across groups |
| Selective reporting (reporting bias) | Low risk | All primary outcomes set out in protocol are reported QUALID scores (nurse‐assessed) were planned for assessment 2‐weekly; in trial report this was 3‐monthly Other outcomes related to patient‐centred care, attitudes, knowledge, etc. were not reported but stated in protocol. These are unpublished and data were provided by the trial authors (not used in the review) |
| Other bias | Low risk | Baseline differences between groups: higher staff knowledge levels intervention group at baseline (16 (4) versus 14 (3)) in usual care group Less frequent visitors in intervention group (19% had daily visitors versus 34% usual care group) Unclear whether these represent important sources of bias Authors took measures to avoid contamination between sites Selective recruitment of cluster participants: authors approached nursing homes on Australian registry/list in alphabetical order to minimise selection bias |
Au 2012.
| Study characteristics | ||
| Methods |
Aim: to assess whether an intervention using patient‐specific feedback about preferences for EoL care would improve the occurrence and quality of communication between patients with COPD and their clinicians Study design: cluster‐RCT; 2 arms (intervention; usual care) Unit of randomisation: clinician (patients clustered per clinician) Consumer involvement: none explicitly stated Funding source: disclosures regarding funding support from industry are recorded. Authors state that the research was conducted independently of the research sponsor |
|
| Participants |
Participants: clinicians and patients with COPD Clinicians were physicians and non‐physicians from primary care and chest clinics Setting and geographic location: USA. University of Washington provided institutional review board approval for the protocol Methods of recruitment: Outpatient clinic at 2 veteran affairs facilities (1 university‐affiliated tertiary referral medical centre; 1 primarily non‐teaching outpatient facility). Participants were approved for participation by a clinician Other details of recruitment were not reported "All participants provided informed consent" (page 727) (not otherwise described) Selection criteria for participation in study: Inclusion:
Exclusion:
Diagnosis of person approaching EoL: COPD as defined by the GOLD (Global Initiative for Chronic Obstructive Lung Disease) criteria 14 Target of intervention: Clinicians and patients Surrogates were also included as a recipient of the intervention (patient‐specific feedback form mailed to patients prior to clinical consultation in order to review and share with surrogates; self‐reported discussions with surrogates reported as outcomes; no further details of who surrogates were, their characteristics or their relationship to the patient) Age: patients: mean 69.4 years both study arms. Not recorded for clinicians Gender: patients: intervention 97.9% male, UC 96.2% male Clinicians: intervention 50% male, UC 44% male Ethnicity/culture/language: Patient ‐ White 85.3% (intervention); 87.0% (UC) Not recorded for clinicians Other PROGRESS aspects: Focus on patients with COPD in a veterans’ care facility; predominantly male, predominantly White. Those with cognitive, psychiatric or language barriers were not included Numbers of participants: see Additional Table 1 |
|
| Interventions |
Intervention: 1 page patient‐specific feedback form based on patient’s self‐reported responses Aim of intervention: to improve the occurrence and quality of communication about preferences for EoL care between patients and their clinicians Comparison: usual care Neither clinician nor patients received patient‐specific feedback forms Further details not reported Delivered by: clinicians. No specific training is mentioned Setting: outpatient clinic Materials, procedures, content: Participants completed the following measures:
1‐page patient‐specific feedback form was generated automatically via computerised process. This selected the patient’s responses, including perspectives on whether their physician would know what type of care they would like, their desire for communication about ACP, patient‐specific barriers and facilitators to communication about EoL care, preferences for CPR and mechanical ventilation, severity of airflow limitation When and how much: intervention group were mailed their 1 page patient‐specific feedback form to the patient to review with surrogate prior to consultation with clinician On day of scheduled clinic visit the 1 page patient‐specific feedback form provided to clinicians and patients without endorsements to use during clinic visit Tailoring: patient‐specific feedback from provided Patient‐specific highest‐ranked barrier and facilitator to EoL communication, with introductory sentence that clinicians could use to lower the threshold to initiate conversations; patient’s 3 most important preferences for EoL experiences Modified during study: no Co‐intervention(s): not reported Fidelity assessed: not reported Theoretical base: social cognitive theory, with intervention designed to increase self‐efficacy of clinician and patient for discussing EoL |
|
| Outcomes |
Primary outcomes Quality of communication [Evaluation of the communication] Method: questionnaire; completed with research assistants’ help Timing: prior to (baseline) and after clinic visit (2 weeks) Scale and scoring: Quality of Communication (QOC) questionnaire, (0 to 100, higher score is better) Results for the 7 subscales of this tool also reported Reported discussion of treatment preferences with clinician at last visit [Discussions of EoL/ EoL care] Method: questionnaire (self‐reported rates; not clear how this was assessed exactly) Timing: 2 weeks after clinic visit Scale and scoring: unclear Discussion with surrogate since last clinic visit [Discussions of EoL/EoL care] Method: questionnaire (self‐reported rates; not clear how this was assessed exactly) Timing: 2 weeks after clinic visit Scale and scoring: unclear Primary outcomes ‐ adverse events None reported Secondary outcomes None reported |
|
| Notes | Protocol available from trial registry; approved by review board of University of Washington Cluster‐RCT; unit of allocation clinician, unit of analysis patient. All analyses adjusted for clustering (i.e. "All models accounted for the clustering of patients within clinician" page 729). Seems likely that results were appropriately adjusted The Preferences for Dying and Death Questionnaire, St George Respiratory Questionnaire, preferences for communication about EoL care and patient‐specific barriers and facilitators to this communication, and preferences for life‐sustaining treatments were given to patients and used to develop the 1‐page patient‐specific information sheet that formed the basis of the intervention Reported outcomes of 'Discussion of treatment preferences with clinician (ever)', 'Discussion with surrogate (ever)' were not reported by this review as 'ever' discussions could not be clearly linked to the effects of the intervention |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stratified random sampling. No further details provided |
| Allocation concealment (selection bias) | Low risk | Investigators and staff administering outcome measures were blinded to treatment assignment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not clear whether participants were blinded to allocation; unit of randomisation was clinician so this may be likely. Effect on self‐reported outcomes is not clear (patients) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Authors state that investigators and staff administering outcome measures were blinded to treatment assignment. Study staff members contacting patients (for survey 2 weeks post‐intervention) were blinded to treatment group assignment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawal 15% to 22% respectively control and intervention arms Reasons for withdrawal/dropout were reasonably comparable except that more (15 versus 6) refused to continue participation in the intervention group Authors report no differences in baseline characteristics regarding whether patients completed the study or were lost to follow‐up ITT analysis was used; effect of imputed data on results was examined in analysis models with authors reporting similar results where imputed and non‐imputed data were used in analysis |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes were reported as per protocol |
| Other bias | Unclear risk | Participants were mostly comparable at baseline although a few differences were noted between groups: greater proportion of control group reported at‐risk drinking; greater proportion intervention group reported hypertension Selective recruitment of cluster participants: details of randomisation and allocation are unclear |
Bernacki 2019.
| Study characteristics | ||
| Methods |
Aim: to assess the feasibility and acceptability of the Serious Illness Care Program Study design: cluster‐RCT; 3 arms original trial (intervention, usual care, secondary control group*). Data from 2 arms available (intervention, UC) Unit of randomisation: clinician (stratified by disease centre or satellite facility) Consumer involvement: project was informed by an advisory group which included clinicians from a range of specialties as well as patients. Feedback sought from DFCI Patient and Family Advisory Council on materials for use in the study through a series of meetings. The Council includes patients, family members, executive leaders and providers working in partnership to improve overall quality of care, policies and hospital programmes Focus groups (patient, family, clinician) were also held to inform choices of terminology, wording and format of materials Funding source: Branta Foundation; Charina Endowment Fund; Margaret T Morris Foundation; Richard A Canot Fund; Partners Healthcare; John A Hartford. Primary author supported by Health Resources Services Administration Grant (KO1HP2046); 2 authors declared conflicts of interest related to writing and editorial work; other authors declared no conflicts of interest |
|
| Participants |
Participants: Patients: people aged > 18 years with advanced incurable cancer and life expectancy < 12 months and their identified surrogates (friend or family member identified by the patient; over 18 years, able to speak English and to provide informed consent) Clinicians: oncology physicians, nurse practitioners (NP’s), physician assistants (PA’s) caring for patients with advanced incurable cancer and life expectancy < 12 months Setting and geographic location: Boston, USA. Hospital: Dana‐Faber Cancer Institute and 2 affiliated satellite clinics (Dana‐Faber Milford regional Medical Center and Dana‐Faber South Shore hospital) Methods of recruitment: Recruitment at meetings in the clinic or by email or in person Clinicians seeing patients at least 1‐half day per week were eligible. Enrolled clinicians identified patients through review of patient lists and answering the surprise question; patients for whom clinicians answered no were eligible Selection criteria for participation in study: Inclusion:
Exclusion:
Diagnosis of person approaching EoL: advanced incurable cancer and life expectancy < 12 months Target of intervention: multicomponent structured communication intervention used with patient and surrogate (communication quality improvement intervention) Age: patients 61.8 years (range 58.2 to 66) intervention; 62.1 years (58.2 to 66) UC Gender: patients: 53.7% female intervention; 52.8% female UC Clinicians: 62.5% female intervention; 51.2% female UC Ethnicity/culture/language: 93.2% White intervention; 92.7% White UC Other PROGRESS aspects: people not speaking English were excluded. Participants were majority White and predominantly college or professional school educated (80% or higher both groups) Numbers of participants: see Additional Table 1 |
|
| Interventions |
Intervention: Serious Illness Conversation Guide (SICG), a communication quality improvement intervention Aim of intervention:
Comparison: usual care Delivered by: clinicians (oncology physicians, NP’s, AP’s), who were triggered by research staff to have the SICG conversation (either by email day before scheduled visit or via packet of study materials on day of consultation) Social worker conducted bereavement telephone interview 2.5 hour training programme for intervention, small groups (6 to 10). Included didactic session on evidence base for ACP; demonstration and discussion of SICG use; individual practice using role‐plays with personalised feedback Aim of training: to teach clinicians how to orient patients to the conversation, ask permission to discuss future care desires, reassuring patients about continued treatment, starting support for patient/family, reiterating that no decisions have to be made during the initial discussion. Then summarise and confirm the conversation’s content; provide patients with Family Communication Guide All intervention clinicians received Clinician Reference Guide summarising the main training information, with guidance on challenging scenarios Tutorial completed on how to document conversations in EMR Feedback provided (individual) to clinicians after first SICG conversation. Additional support or coaching available to clinicians if desired Setting: at clinic on Dana‐Faber sites Materials, procedures, content: Development of the Serious Illness Conversation Guide (SICG) followed the following process:
Pre‐visit letter was sent (mailed) to patients to activate and prepare them for the conversation. The letter introduced SICG topics The Serious Illness Conversation Guide was used at the clinical visit. This contains 7 elements: illness understanding, decision‐making and information preferences; prognostic disclosure; patient goals and fears; views on acceptable function and trade‐offs; desires for family involvement Outcome documented by clinicians via structured format in EMR (reminds clinicians of key elements of discussion, eases burden of documentation, allows other clinicians easy access to the information in a consistent and structured way) Family guide provided at the time of consultation (suggesting an approach for discussing illness and care preferences with family) When and how much: Pre‐visit letter sent to patient to activate and prepare them for the conversation (introduces SICG topics) SICG used at consultation, and Family Guide provided to patients/carers (suggesting an approach for discussing illness and care preferences with family) Documentation of conversation via EMR Participants could receive the intervention more than once over the trial course. Data from authors indicate that of participants in the intervention group 3/76 (4%) had no intervention, 12/76 (16%) received the intervention once and 61/76 (80%) twice or more, compared with 18/85 (21%), 29/85 (34%) and 38/85 (45%) for 0, 1 or 2+ times the intervention was delivered in the usual care group Tailoring: unclear. Clinicians instructed that they were able to split the conversation across consultations but to continue to conduct the conversation until all questions in the EMR module were completed. (Time to fit in the conversation was identified as a significant issue discussed in training) Modified during study: modified after pilot study Co‐intervention(s): not reported Fidelity assessed: intervention clinicians readily used the intervention, and attended training and rated it as effective Theoretical base: not reported |
|
| Outcomes |
Primary outcomes Enhanced goal‐consistent care (number of goals met) [Evaluation of the communication] Method: Life Priorities survey for patients, Family Perceptions survey for identified surrogates Timing: baseline and every 2 months Scale and scoring: scoring system 0, 1, 2, 3 corresponding to top 3 goals met at EoL. Scored by matching patient final Life Priorities survey (within 3 months of death) to that of Family Perceptions: "scored each of the patient’s 3 highest ranking goals as concordant if the caregiver indicated the goal had been achieved to a large extent, resulting in a score of 0, 1, 2, or 3 goals met" (page 753) Therapeutic alliance [Evaluation of the communication] Method: Human Connection Scale Timing: baseline, 14 and 24 weeks Scale and scoring: 7/16 of the original scale items used; total score range 7 (lower) to 28 (higher therapeutic alliance) i.e. therapeutic alliance = patients’ sense of mutual understanding, caring, trust with their physicians (higher = better) Perception of quality of communication [Evaluation of the communication] Method: Quality of Communication scale Timing: unclear Scale and scoring: unclear Conversation numbers (per patient) [Discussions of EoL care/EoL] Method: EMR review Timing: post‐death Scale and scoring: not applicable Conversation content/quality (domains) [Discussions of EoL care/EoL] Method: EMR review Timing: post‐death Scale and scoring: thematic coding by multidisciplinary team. Documented SIC domains per patient (scored 0 to 4 according to number of domains discussed and documented); also reported in subdomains: patients with at least 1 serious illness conversation documented prior to death; patients with documented discussion about values/goals; about prognosis/illness understanding; about EoL care planning; about life‐sustaining treatment preferences Timing of first documented SIC before death (median days, IQR) [Discussions of EoL care/EoL] Method: EMR review Timing: post‐death Scale and scoring: not applicable Primary outcomes ‐ adverse events None reported Secondary outcomes Peacefulness at EoL [Quality of EoL care] Method: PEACE scale. 2 subscales ‘Peaceful Acceptance of Illness’ and ‘Struggle with Illness’ Timing: baseline and every 2 months Scale and scoring: struggle with illness (feelings of upset, worry, anger, etc.), 7 questions total score 7 to 28 Peaceful acceptance (acceptance of diagnosis, inner calm, feelings of being well‐loved); 5 questions, total score 5 to 20 Quality of life and general physical health function [Quality of life] Method: SF‐12 V2 health survey Timing: unclear Scale and scoring: unclear |
|
| Notes | Trial registered (NCT01786811) *Trial established as 3‐arm trial: intervention, usual care, and secondary control group. Main comparison is between the intervention and UC arms; secondary control arm is based on non‐participating physicians’ patients. Author response indicates that data have been collected but not yet available (public) for this secondary control arm All patients: assessments at baseline and every 2 months Intervention group: patients surveyed 1 week after SICG conversation to assess perception of the conversation and its acceptability Control group: parallel survey every 2 months around same time that intervention patients would have SIC conversation. These patients are asked about the number and content of ACP or EoL discussions with clinicians and family Cluster‐RCT; unit of randomisation: clinician, unit of analysis: patient "All comparisons across study arms accounted for clustering of patients within clinician teams" (page 753). Used Generalised Estimating Equations with Wald, t or Chi2 tests for analysis (depending on outcome). Seems likely that analyses were appropriately adjusted This trial also reported patient anxiety and depression, and survival. These were judged as clinical outcomes for the purpose of this review and data were not extracted and reported as results Uptake and effectiveness of clinician training, clinician use of the conversation tool, and conversation duration were reported by the trial and reported in this review as measures of intervention delivery (reported for the intervention group only; therefore not reported as comparative results). Duration was reported for the intervention arm only and therefore not collected for analysis for this review Data were not yet available for perceptions of quality of communication, or quality of life The study by Paladino 2020 on patient and clinician experiences of the Serious Illness Conversation Guide is included as a secondary reference for this trial. As data were reported for the intervention arm only these data were not extracted for inclusion in this review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Clinician clusters were stratified by disease centre or satellite facility and randomised within strata. Half randomised to UC (n = 21) and half to intervention (n = 20) Author contact confirmed use of computer‐generated random number sequence |
| Allocation concealment (selection bias) | Low risk | Author response indicated clusters were identified prior to randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Clinicians were not blinded (author contact confirms that clinicians knew that they were being trained); patients were blinded to assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Social workers not involved in the study conducted telephone bereavement interviews and were blinded to the study arm |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Sample size calculated as 200 evaluable patients per arm for required power, assuming 6% dropout Patient participation rates and numbers analysable were low but comparable between arms. Authors note non‐participants and those not analysed were not significantly different from those who were analysed, and groups were still comparable (based on randomisation), although non‐participants were older, and less likely to have breast cancer than participants; and those patients with analysable data were more likely to be married and have higher incomes than those with non‐analysable data |
| Selective reporting (reporting bias) | Low risk | Outcomes described in the protocol were reported. Primary outcomes were reported Data are not available for all outcomes assessed but author contact confirms that these data are pending publication (for several secondary outcomes) |
| Other bias | Low risk | Selective recruitment of cluster participants: clusters were identified before randomisation, therefore this risk of bias seems low Baseline imbalances: authors state that randomisation was maintained despite low participation rates. There were no differences at baseline between clinician groups or patient groups at baseline |
Clayton 2007.
| Study characteristics | ||
| Methods |
Aim: to determine whether the provision of a question prompt list (QPL) influences advanced cancer patients’/caregivers’ questions and discussion of topics relevant to EoL care Study design: RCT; 2 arms (intervention, usual care) Consumer involvement: development of the QPL (Clayton 2003): QPL based on focus groups and interviews with 19 patients, 24 carers, 22 palliative care health professionals. Draft QPL was reviewed by another 21 health professionals and piloted in 23 patients before being finalised with a list of 112 questions Funding source: supported by NHMRC grants; authors declared they have no potential conflicts of interest |
|
| Participants |
Participants: patients with diagnosis of an advanced progressive life limiting illness, English speaking, older than 18 years of age, and able and well enough to read QPL and complete questionnaires Setting and geographic location: 9 Australian palliative care (PC) services in 2 states. Setting unclear but QPL was administered at time of consultation with physician. Almost all patients were recruited from outpatient clinics Methods of recruitment: recruitment at the clinic of 15 PC physicians from 9 PC services. Consecutive eligible patients from each participating physician were invited to participate Enrolment was within 3 consultations from initial contact with the PC physician. After obtaining written consent and baseline data, patients were randomly assigned to study groups Selection criteria for participation in study: Inclusion:
Exclusion:
Diagnosis of person approaching EoL: patients with diagnosis of an advanced progressive life limiting illness Target of intervention: carer accompanying patient to consultation (spouse, partner, family member or friend) Age: QPL(intervention) 65.5 years (SD 12.6); UC 64.6 years (SD 14.1) Gender: intervention 39% female; UC 40% female Ethnicity/culture/language: QPL: Australian 73%; other English speaking country 5%; non‐English speaking country 8%; unknown 14% UC: Australian 79%; other English speaking country 5%; non‐English speaking country 6%; unknown 10% Other PROGRESS aspects: non‐English speakers; those who were too unwell to attend outpatient appointments; those from non‐urban centres were all excluded from the trial Numbers of participants: see Additional Table 1 |
|
| Interventions |
Intervention: QPL for advanced cancer patients and their caregivers, referred for palliative care (PC) Aim of intervention: to determine whether provision of a QPL influences advanced cancer patients’/caregivers’ questions and discussion of topics relevant to EoL care during consultations with a PC physician Comparison: usual care (routine consultation with PC physician) Delivered by: physicians Setting: at clinic where consultation occurred in 9 outpatient PC clinics Materials, procedures, content: Participants received the QPL 20 to 30 minutes before their PC physician consultations The QPL is a 16‐page A5 booklet containing 112 questions grouped into 9 topics encompassing issues that may be discussed with a physician or another health professional When and how much: once. Timing in terms of number of previous consultations with PC physician was variable Tailoring: QPL purpose is to assist patients to identify questions of most importance and to raise these when in consultation with physician Modified during study: no modification reported Co‐intervention(s): not reported Fidelity assessed: not reported, unclear Theoretical base: QPL based on focus group, interviews in a range of stakeholders; prior piloting in PC population |
|
| Outcomes |
Primary outcomes Total number of questions during consultation [Discussions about EoL/EoL care] Method: coding of audiotaped consultations Timing: after consultation with PC physician Scale and scoring: the QPL is a 16‐page A5 booklet containing 112 questions grouped into 9 topics encompassing issues that may be discussed with a physician or another health professional. Patient questions, concerns and items were tallied and categorised according to QPL categories. All patients’ consultations with the PC physician were audiotaped, transcribed, and coded. Question = direct request for information. Concern = patient/carer statement inviting response from physician. Items: "Items discussed (85 issues covered by questions in the QPL, whether or not prompted by a patient/caregiver question/concern), plus patient and caregiver questions/concerns were coded and tallied for each of the nine topics" (page 716) Achievement of patient information preferences [Knowledge and understanding] Method: questionnaire, 1 item Timing: 24 hours after consultation and 3 weeks after consultation Scale and scoring: Cassileth Information Styles Questionnaire (measures amount of detail preferred; 5‐point Likert scale) Achievement of patient information needs [Knowledge and understanding] Method: questionnaire Timing: 24 hours after consultation and 3 weeks after consultation Scale and scoring: totals out of 11 tallied for items not discussed, items for which they did not receive enough information, or about which they received too much information Patient satisfaction with consultation [Evaluation of the communication] Method: questionnaire, Roter and Korsch Timing: 24 hours after consultation and 3 weeks after consultation Scale and scoring: 25‐item scale, scores range from 25 to 125, higher scores reflect greater satisfaction Actual versus preferred involvement in consultation [Evaluation of the communication] Method: questionnaire Timing: 24 hours after consultation Scale and scoring: 5‐item rating scale (ranging from doctor leads decisions to patient leads decisions) Primary outcomes ‐ adverse events Patient anxiety [Evaluation of the communication] Method: questionnaire, STAI Timing: 24 hours after consultation and 3 weeks after consultation Scale and scoring: STAI, scores range 20 to 80; higher scores more anxiety Secondary outcomes Physician satisfaction with consultation [Health practitioner evaluation of communication] Method: unclear Timing: 24 hours after consultation and 3 weeks after consultation Scale and scoring: unclear Consultation length [Health systems impact] |
|
| Notes | Outcomes related to participants' views of QPL reported, alongside physician ratings of the QPL, but as these were rated only for the intervention group the data are non‐comparative and so not reported in the review Outcomes were analysed at longest follow‐up (3 weeks post‐consultation) For 'total questions during consultation' only mean total questions were extracted for the review (concerns, items were not collected but were reported by the trial) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Individually randomised and stratified by physician – random permuted blocks of 10 constructed using random number table (by research assistant not involved in recruitment) |
| Allocation concealment (selection bias) | Low risk | Allocations were concealed using sequentially numbered opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Physicians were unblinded; authors note that prior research has indicated that QPL require professional endorsement in consultations to be effective Unclear whether or not patients and carers were blinded to intervention and/or what effect this may have had on the outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Authors indicate that consultations were audiotaped, transcribed and analysed by blinded coders "Two coders were trained and blinded to group allocation. One coder coded all transcripts and recoded a random 10% to determine intrarater reliability. The second coder coded a random 10% of transcripts to determine inter‐rater reliability" (page 716) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low levels of loss to follow‐up 4/174; balanced across groups (n = 2 each), with comparable reasons |
| Selective reporting (reporting bias) | Unclear risk | All outcomes described in methods were reported but no protocol is available |
| Other bias | Unclear risk | Baseline differences between groups: authors note that groups were comparable on most characteristics but that some differences were present (> 5% differences between groups) including educational level (tertiary versus non‐tertiary (higher tertiary intervention group)); professional versus non‐professional occupation (higher professionals intervention group); carer presence (intervention group higher); timing of consultation (more seen previously intervention group) |
Epstein 2017.
| Study characteristics | ||
| Methods |
Aim: to determine the effects of a combined intervention (involving oncologists, patients and carers) on patient‐centred communication and other outcomes including shared understanding, patient‐physician relationships, QoL and aggressive treatments in the last 30 days of life Study design: cluster‐RCT, multisite (VOICE study); 2 arms (intervention, usual care) Unit of randomisation: clinician Consumer involvement: QPL was based on previous study developing this for cancer patients in palliative care; refined on the basis of a focus group and based on semistructured interviews with "demographically diverse patients with advanced cancer" (Rodenbach 2017) Funding source: grants from National Cancer Institute, National Institutes of Health supported the research. Funders had no involvement in design, conduct, data analysis or interpretation, preparation of manuscript or approval or decisions about submission. Authors report no conflicts of interest |
|
| Participants |
Participants: patients with advanced cancer, and their carers. Oncologists Setting and geographic location: community‐based cancer clinics, academic medical centres, community hospitals. Western New York, Sacramento, California; USA Methods of recruitment: Physicians: medical oncologists caring for non‐haemotologic cancer patients, recruited at practice meetings at participating clinics Patients: research assistants reviewed, with clinic staff, clinic rosters for enrolled clinicians to identify potentially eligible patients Carers: identified by patients (family member, partner, friend or other involved in health care, preferably a person who attended physician appointments with the patient) Patients recruited, consented and enrolled based on allocation of their physicians to intervention or control group All participants provided informed consent (written) From supplementary file 1 (protocol): page 23 "Method of Subject Identification and Recruitment: All Phase 1 and Phase 2 patients will be identified by research assistants working closely with participating physicians and their clinic staff by reviewing the clinic roster in detail to ascertain that all potentially eligible patients are identified. Potentially eligible patients will receive a brochure that describes the study (see attached study brochure). Office staff will explain to the patient that a research assistant will be calling him/her in the next two weeks to find out if he/she might be interested in participating in the study. Patients who do NOT want to be contacted about the study will be asked to return an enclosed opt‐out card to the study office within 4 days of receiving the study brochure. A research assistant will only call patients who have not returned the opt‐out card within the stated time period" Selection criteria for participation in study: Inclusion:
Exclusion:
Diagnosis of person approaching EoL: either stage IV non‐haematologic or stage III cancer "and whose physician 'would not be surprised’ if the patient were to die within 12 months" (page 93) Target of intervention: carers (family member, partner, friend or other involved in health care) 73% of enrolled patients nominated a carer Age: Physicians: 44 years Patients: 64.4 years Carers: not reported Gender: Physicians: 29% female Patients: 55% female Carers: not reported Ethnicity/culture/language: Physicians: 45% White, 42% Asian, 13% other race Patients: 11.5% non‐White Carers: not reported Other PROGRESS aspects: Those unable to understand spoken English or to provide written informed consent were excluded; participants were 89% White: those from minority groups, non‐English speaking, lower health and general literacy groups may not be well represented Numbers of participants: see Additional Table 1 |
|
| Interventions |
Intervention: combined patient‐centred communication (training) intervention Aim of intervention: to improve patient‐centred communication between physicians and patients/carers, and related outcomes Interventions developed based on previous studies on training of physicians, development and use of QPLs for patients Comparison: control (oncologist meets with research assistant but receives no training) Delivered by: physicians and patient coaches Trainers (for physicians) and coaches (for patients/carers) received 3 days of on‐site training Physician training included Standardized Patient Instructors (SPIs) adopting role of patient with advanced cancer with life expectancy of 12 months or less Setting: intervention setting not described Training for clinicians occurred in their clinical office. Training for patients occurred at the centre where their oncologist practiced Materials, procedures, content: 2 components to the intervention:
Physician and patient interventions focused on the same 4 elements of patient‐centred communication: engaging patients in consultations, responding to emotions, informing patients about choices related to treatment and prognosis, and framing information in a balanced (unbiased) way When and how much: Physician training: 2 educational outreach sessions. 1st session 1 hour; 2nd booster session 45 minutes 1 month later Patients/carers: coaching session (approx. 35 to 40 minutes in duration) prior to oncology consultation; follow‐up phone calls (up to 3 at monthly intervals) Tailoring: coaching was tailored to patient/carer priorities and concerns i.e. coaches helped patients to identify their most pressing questions in order to help these to be raised and addressed in physician consultation Modified during study: not applicable Co‐intervention(s): none Fidelity assessed: assessed and reported as 94% or higher (assessed by review of audio recordings of intervention sessions). All intervention physicians completed both training sessions; all intervention patients received in‐person coaching Theoretical base: based on previous work to develop interventions targeting patients |
|
| Outcomes |
Primary outcomes Patient‐centred (patient‐doctor) communication (composite measure) [Evaluation of the communication] Method: composite of 4 communication measures (engaging, responding, informing, framing of decisions) Audiotaped physician consultation, coded by trained university students (audited continuously, blinded to study aims and assignments) Timing: first physician visit following coaching session (intervention group) or study entry (control) Scale and scoring: Active Patient Participation Coding (engagement); Verona VR‐CoDES (response to emotions); Prognostic and Treatment Choices (PTCC) Informing subscale; PTCC Balanced Framing subscale. Component scores for each scale transformed to z scores; 4 scores averaged to give overall composite measure (authors report better sensitivity and precision than component individual scales) Patient‐physician relationship [Evaluation of the communication] Method: patient‐physician relationship Timing: shortly after audio recorded consultation (2 to 4 days, then quarterly) supplement 2 page 12 Scale and scoring: Human Connection Scale (THC); Health Care Communication Questionnaire (HCCQ); Perceived Efficacy in Patient‐Physician Interactions (PEPPI) scale Decision regret (caregiver) [Evaluation of the communication] Method: Modified Decision Regret Scale Timing: 2 months post‐death Scale and scoring: 8 items Shared understanding of prognosis (discordance between ratings) [Knowledge and understanding] Method: research‐administered questionnaire/interview Timing: shortly after audio recorded consultation Scale and scoring: 7‐point scale; discordance defined as difference of 2 or more categories of difference (i.e. between category ratings) Primary outcomes ‐ adverse events None reported Secondary outcomes QoL composite score [Quality of life] Method: research‐administered questionnaire/interview Timing: 3‐montly from study entry to 3 years Scale and scoring: composite QoL score as average of 5 z‐scored subscales: McGillQoL scale single item, McGill Psychological Well‐Being subscale, McGill Existential Well‐Being subscale, FACT‐G Physical Functioning subscale, FACT‐G Social Functioning subscale Treatments and hospice use in last month of life [Hospital admissions etc.] Method: trained nurse and physician‐abstracted data from medical records Timing: last 30 days of life Scale and scoring: composite score of 3 indicators of aggressive treatment in last 30 days of life: "chemotherapy, potentially burdensome interventions, emergency department [ED]/hospital admission) and hospice utilization" (page 95) Caregiver evaluation of quality of EoL care [Quality of EoL care] Method: caregiver Evaluation of Quality of EoL Care Scale Timing: 2 months post‐death Scale and scoring: 6 items |
|
| Notes | Clinical trial registration number NCT01485627 Mean survival of studied population was 16 months (19 months intervention, 14 months control group) See supplement 3 page 5 for detailed outline of all intervention components and delivery Cluster‐RCT; unit of randomisation: clinician, unit of analysis: clinician‐patient dyad (communication), patient ICC for all outcomes (except aggressive care at EoL) was < 0.1 "This is a cluster‐randomized trial, where our primary communication outcomes (Aim 1a) are measured at the level of the physician‐patient dyad and our secondary outcomes (Aims 1b, 2 & 3) are measured at the level of the patient. Analyses are based on published guidelines for group (cluster) randomized controlled trials" (supplement 1, page 16) "The physician‐patient dyad will be the unit of analysis, as measured in a single audio‐recorded clinical encounter. Because patients are clustered within physicians, in the data analysis, we may add random effects for physicians to account for the within‐physician correlation of each dyad. If analysis of the Phase 1 data identifies plausible confounding by physician (communication style) or patient factors (demographic, clinical status), these factors will be eligible for inclusion in final analyses as described above" (supplement 1 page 17) "As described in the BMC Protocol, we will primarily rely on regression models for clustered data to account for the stratified 325 cluster randomised longitudinal study design" (supplement 2 page 21) Seems likely that analyses were appropriately adjusted Treatments and hospice use in last month of life, assessed as a composite score of indicators of aggressive treatment in last 30 days of life was judged as primarily clinical in focus and data were not extracted for analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number sequence used Randomised by physician (physicians as the unit of randomisation) and stratified by 2 sites and by oncologist subspeciality Within strata physicians were randomly assigned 1:1 to intervention or control Patients enrolled based on allocation of their physicians to intervention or control group |
| Allocation concealment (selection bias) | Low risk | All but study statistician blinded to random number sequence and group assignment "To preserve blinding, assignment to the treatment or control conditions is maintained by the study statisticians and project manager, and not explicitly revealed to research assistants, transcriptionists, or coders of the audio‐recorded office visits" (supplement 2, page 12) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible to blind intervention group physicians; unclear what effect this might have Potentially patients and carers were aware of their treatment assignment; again not clear what effect this might have on outcomes sought (all but health services use (medical records) may have been influenced by knowledge of group assignment) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Only study statistician was aware of random number sequence and assignment: blinding preserved amongst transcriptionists, coders, abstractors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Fewer than 3% of follow‐up questionnaires were missing" (page 95) Data seems otherwise complete for outcomes reported in main paper and in supplement 3 |
| Selective reporting (reporting bias) | Low risk | Changes in subscale measured for primary outcome acknowledged in trial report (supplement 2 analysis plan), also other changes to outcome measures acknowledged in this report Several outcomes described in the protocol were reported in related papers |
| Other bias | Low risk | Selective recruitment of cluster participants: clusters were patients of an oncologist (unit of randomisation), these were identified before randomisation, therefore this risk of bias seems low Baseline imbalances: there were no differences at baseline between physician or patient groups at baseline. Low risk |
Lautrette 2007.
| Study characteristics | ||
| Methods |
Aim: to evaluate a proactive EoL conference and brochure to determine effects of bereavement Study design: 2 arms (intervention, usual care) Consumer involvement: none described Funding source: supported by grants from Assistance Publique‐Hopitaux de Paris and the French Society for Critical Care Medicine; supported by grant from the National institute of Nursing Research The last author disclosed funding support from Pfizer; no other potential conflicts of interest were reported |
|
| Participants |
Participants: surrogate decision‐makers for patients in ICU and expected to die within days Setting and geographic location: 22 ICUs in France; multisite ICUs: 68% teaching hospitals. Both medical and surgical ICUs Methods of recruitment: in each ICU unit, local investigator agreed to include surrogate decision‐makers of 6 consecutive patients expected to die within a few days Surrogates were either those designated by the patient or the person ranked highest in decision‐making hierarchy according to French law (spouse > parents/children > others) Oral informed consent obtained from surrogates Selection criteria for participation in study: Inclusion: physician belief that patient would die within days. Aged 18 years or older Exclusion: patients younger than 18 years. Those with insufficient French for phone interview Diagnosis of person approaching EoL: variable: included acute respiratory failure, coma, shock, acute renal failure, cardiac arrest Target of intervention: surrogates (primarily family members), approximately 40% spouses, approximately 48% children of patients Age: Patients: intervention median age 68 years, control 74 years Surrogates: intervention median 54 years, control 54 years Gender: Patients: intervention 41% female, control 48% female Surrogates: intervention 77% female, control 70% female Ethnicity/culture/language:around 90% of patients and surrogates in both study groups were of French descent Other PROGRESS aspects:those with inadequate French to enable telephone interviews were excluded; this may have restricted participation of other ethnic/minority groups (with almost 90% of participating surrogates were of French descent) Numbers of participants: see Additional Table 1 |
|
| Interventions |
Intervention: proactive communication family conference Aim of intervention: to decrease the effects of bereavement in family members of patients dying in the ICU (by improving communication between family members and ICU staff and to support families with decisions) Comparison: routine family conference Delivered by: investigators at each ICU attended meeting about the intervention; given a copy of the VALUE guidelines and of supporting research articles 1 member of study team visited each site to discuss the guidelines and ensure the differences between the intervention and routine family conference were understood Setting: in quiet separate room with seating for all; beepers and mobile phones off Usual care was delivered in ICU room or another available room (not specially designated for purpose; may or may not be in a separate room) Materials, procedures, content: Proactive communication strategy conference: conference conducted according to specific guidelines, also provision of bereavement information leaflet Participants planned several hours in advance; participants included senior and junior physicians, nurses, a psychologist, other health professionals, unrestricted number of family and friends; social worker and spiritual representative invited if requested by the family Guidelines for the conference based on previous research: based on detailed conference procedure, provided information on diagnosis, prognosis, treatment and discussed appropriateness of treatment limitation with family members. Intensivist leading the conference sought to achieve the 5r values outlined by the VALUE mnemonic: Value and appreciate things family says, Acknowledge emotions, Listen, ask questions that allow Understanding of who the patient is as a person, and Elicit questions from family At end of family conference family member was given a bereavement information leaflet, with content explained orally. 15 pages, explained EoL care, possible reactions after the death of a family member, how to communicate with other family members, where to find help Used previously but modified for this study to focus on adult ICU patients and optimising EoL care Usual care: routine EoL conference: held to inform family that death is imminent and to describe treatment‐limitation decisions and consequences of these. Family members may share in decisions if wished, but these decisions are under the authority of physicians and are made collegially by ICU team Led by senior physician in charge of patient; nurses may or may not attend. Conference may or may not be held in separate room Previous studies show mean duration is 10 minutes Occurred when at least 1 family member in the ICU When and how much: once, following 3 information meetings provided to all families Tailoring: not stated explicitly but family members had the opportunity to ask questions, discuss treatment options with physician and others (in both intervention and UC groups) Modified during study: not stated Co‐intervention(s): participating ICUs were members of the FAMIREA study group; 3 formal early information meetings held for all families (prior to randomisation). First at 24 hours (general information on diagnosis, prognosis, treatments) plus information leaflet; second at 48 hours (answering questions, additional information check family understanding of situation); third at day 3 to 5 (treatments etc. explained and prognosis explained, questions by family answered) If patient expected to die (following these 3 meetings) or shift to palliative care is indicated an EoL conference is held (i.e. intervention or routine conference) Co‐interventions delivered to all participants (fairly extensive information provision). Authors note that this may have lessened differences between intervention and routine care groups for some outcomes assessed by the study Fidelity assessed: quality of intervention: investigator attended all 3 EoL intervention conferences to ensure consistency of the conference format (prior to delivery of the intervention) Authors note that differences on various outcomes in the conduct of the conferences indicates that the guidelines for the conferences were followed No assessment was made of how many read the bereavement brochure, or how well understood the material (content) was Theoretical base: prior studies developing the communication guideline are cited |
|
| Outcomes |
Primary outcomes Effectiveness of information provided [Evaluation of the communication] Method: surrogate interview via telephone (ratings of time allotted to provide information, clarity of information, and whether additional information was requested by family members) Timing: 90 days after death Scale and scoring: unclear Primary outcomes ‐ adverse events None reported Secondary outcomes Duration of family conference [Health system impacts] Method: unclear Timing: at time of family conference with ICU staff Scale and scoring: unclear |
|
| Notes | Trial number NCT00331877 Co‐interventions delivered to all participants (fairly extensive information provision), and routine conference meeting with UC group. Authors note that this may have lessened differences between intervention and routine care groups for some outcomes assessed by the study Authors also note that the patient‐doctor relationship in France is typically more paternalistic than elsewhere, and since the standard consultation relied on this interaction that the effects of the intervention in France may have been greater than might be found in other countries where models of shared decision‐making are more commonly practiced. However, authors also note that interactions in control group were similar to those reported in North America and Europe One further issue may be that the control conferences were at least as good as routine care reported in other studies (i.e. longer duration, information needs of families largely met) Trial also reported the following outcomes for surrogates at 90 days after death of the patient: PTSD symptoms (Impact of Event Score), depression and anxiety (Hospital Anxiety and Depression Scales). Trial reported several outcomes rated by physician observation of family members during family conferences: expression of emotions by family members, family belief that patient's symptoms were controlled, family‐reported conflict with ICU staff, or ICU staff reported conflict with family members. ICU and patient medical data were also reported. None of these outcomes were judged as relevant for this review, based on the review's focus on communication at the end of life and were not included in analyses |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The study biostatistics department generated a randomization list stratified on the ICUs, using permutation blocks of six" (page 3 supplementary appendix) |
| Allocation concealment (selection bias) | Low risk | "Sealed consecutively numbered envelopes containing the name of the assigned group were sent to each ICU, with bereavement information leaflets. The leaflets were not in the sealed envelopes, so that the blind design was not broken. In each ICU, surrogate decision‐makers who consented to the study were assigned a study number, and the investigator opened the envelope bearing that number to determine group assignment" (page 3 supplementary appendix) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind investigators/providers of the intervention, or participants (surrogates) Possible that investigators with strong positive feelings about the intervention may have influenced family member interactions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Interviewer assessing surrogate outcomes was blinded to group assignment Unclear whether researchers were blinded to assignment when recording ICU and patient characteristics although the effects of this on clinical and treatment outcomes seem likely to be negligible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up and withdrawals were acceptably low and comparable across study groups: 52/63 (83%) completed interviews at 90 days intervention group, 56/63 (89%) in control group Reasons for withdrawal/loss were similar across groups (not answering telephone, refused interview), although higher rates of severe emotional distress in intervention group (n = 5) than control group (n = 1) |
| Selective reporting (reporting bias) | Unclear risk | Outcomes stated in methods are reported completely, however no protocol available for the trial |
| Other bias | Low risk | Baseline imbalances: groups were comparable on key features at baseline |
Reinhardt 2014.
| Study characteristics | ||
| Methods |
Aim: to assess the effects of a face‐to‐face, structured conversation about EoL intervention, compared with social contact via telephone only Study design: RCT, 2 arms: intervention, usual care Consumer involvement: none reported Funding source: Alzheimer’s Association provided funding support. No declarations of interest are provided from authors |
|
| Participants |
Participants: family members of residents with advanced dementia of a large skilled nursing facility Setting and geographic location: residential nursing facility; New York, USA Methods of recruitment: few details reported; surrogates were chosen from primary contacts of patients Surrogates provided informed consent (for themselves), and surrogate informed consent for patient participation Selection criteria for participation in study: Inclusion:
Exclusion:
Diagnosis of person approaching EoL: advanced dementia Target of intervention: surrogates: primary family member or friend contact, including healthcare agent Age: intervention 59.6 years (SD 12.3), UC 58.9 (11.9) Gender: intervention 37/47 (78.7%) female, UC 32/40 (80%) female Ethnicity/culture/language: 107/110 English speaking (3 Spanish) Black non‐Hispanic (intervention 42.5%, control 40%); White non‐Hispanic (intervention 31.9%, control 30%), Hispanic (intervention 23.4%, control 23%), other (intervention 2.1%, control 7%) Other PROGRESS aspects: study occurred in urban centre, in relatively highly educated group of surrogates (over 50% educated to college (university) level). Findings may not be applicable to rural or more remote populations, lower‐income countries and settings, or to those with lower levels of education and/or health literacy Authors also note that the intervention could only be conducted in this facility because of the employment of full‐time physicians by the care home, including palliative medicine physicians, which is not typical of most nursing homes Numbers of participants: see Additional Table 1 |
|
| Interventions |
Intervention: face‐to‐face structured conversation about EoL care, with telephone follow‐up Aim of intervention: to provide information and support to surrogates of patients with advanced dementia, including about the pros and cons of treatment decisions that may arise when the patient’s dementia severity worsens Comparison: social contact by telephone (to control for additional attention and interaction between staff and surrogates in intervention group) plus routine care. Delivered by trained research assistant Delivered by: intervention delivered by 1 of the PCT physicians and the social worker The elements listed in the structured meeting description were reviewed in a training session with the clinicians delivering the intervention Setting: at the care facility, no further details reported. Follow‐up calls via telephone Materials, procedures, content/When and how much: Structured meeting was one‐off; mean duration 47 minutes (range 20 to 75 minutes) Meeting was one‐off; PCT was available for further information or assistance with decision making but only 3 surrogates requested additional information Social worker contacted surrogates every 2 months via telephone to provide support and assess surrogate’s level of emotional comfort. This was an opportunity for surrogates to have concerns addressed, and designed to continue discussions about any issues raised in the initial meetings Each call lasted mean 10 minutes UC: baseline and 2‐month intervals telephone calls. Discussed whatever the surrogate raised on the call Mean 11 minutes at baseline, 9 minutes for follow‐up calls Tailoring: meetings and follow‐up phone calls aimed to cover those issues that surrogates wished to discuss Modified during study: not applicable Co‐intervention(s): not reported Fidelity assessed: quality of intervention not assessed explicitly. No further measures of fidelity reported Theoretical base: no theoretical basis cited but prior research mentioned |
|
| Outcomes |
Primary outcomes None reported Primary outcomes ‐ adverse events None reported Secondary outcomes Surrogate ratings of patient's symptom management (EOLD SM) [Quality of EoL care] Method: interview, questionnaire Timing: baseline, 3 and 6 months Scale and scoring: Symptom Management at the End of Life in Dementia Scale. Frequency of 9 symptoms rated on 6 point scale (0 = never, 6 = daily); range 0 to 45. Higher score = better symptom control Surrogate care satisfaction (EOLD SWC) [Quality of EoL care] Method: interview, questionnaire Timing: baseline, 3 and 6 months Scale and scoring: Satisfaction with Care at EoL in Dementia Scale. Frequency of 14 items, rated on 4‐point scale (strongly agree to strongly disagree; possible range 0 to 42). Higher score = greater satisfaction Surrogates' satisfaction with care [Quality of EoL care] Method: interview, questionnaire Timing: baseline, 3 and 6 months Scale and scoring: single item, 0 to 10 rating (0 worst possible care to 10 best possible care) |
|
| Notes | Trial also reported surrogate depressive symptoms, satisfaction with life, and patient medical data (medical record review) at 3 and 6 months, which are not reported in this review as they were judged to be primarily clinical outcomes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants randomly assigned but no further details provided |
| Allocation concealment (selection bias) | Unclear risk | No details reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No measures described to blind providers of intervention (and unlikely to be possible). Effect on outcomes unclear No measures described to blind surrogates to intervention, although comparison group received some telephone contact. May introduce bias if reporting on satisfaction with care etc. if participants knew they were part of the intervention group |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Interviewers assessing outcomes via surrogate interviews were blinded to study group allocation. Medical records were sourced for patient outcomes; not clear whether assessors were blinded to group allocation of patient but data are objective so risk of bias seems low |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Missing data were reported 110 were randomly assigned and completed baseline interviews; 96 (87%) completed 3‐month outcomes; 90 (82%) completed 6‐month outcomes. Losses were fairly comparable across groups and no major differences between those who completed the study and those who dropped out (on key demographic features) were noted by the authors However, numbers were lower for some outcomes (such as ratings of care management) where n = 65 (numbers fairly comparable between the 2 groups). For some outcomes, such as care satisfaction (intervention n = 45 and control n = 36) missing data may affect the results and there were differences between the groups Not clear what the impact might be on the result, or reasons for these missing data |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified. Outcomes seem complete based on those sought in methods |
| Other bias | Low risk | Baseline imbalances: groups were comparable on key features at baseline |
Walczak 2017.
| Study characteristics | ||
| Methods |
Aim: to evaluate effects of nurse‐facilitated communication support programme for patients with advanced, incurable cancer to assist them in discussing prognosis and EoL care Study design: RCT, 2 arms; intervention, usual care Consumer involvement: none stated Funding source: NHMRC grant 571346. Authors declaration: no conflicts of interest |
|
| Participants |
Participants: oncology patients with prognosis of less than 12 months. Advanced, incurable cancer diagnoses of various types, with oncologist‐assessed life expectancy of 2 to 12 months. Informal carers (adult) also participated if nominated by the patient Setting and geographic location: 6 hospital‐affiliated cancer treatment centres; Sydney, Australia Methods of recruitment: oncologists identified consecutive eligible patients at consultations, obtained consent for researcher contact. Oncologists referred patients they expected to die within 12 months but judged likely to live at least 2 months Selection criteria for participation in study: Inclusion:
Exclusion:
Diagnosis of person approaching EoL: oncology patients with prognosis of less than 12 months Target of intervention: patients, with or without carer Age: mean 64.4 years (intervention 63.8; control 65.6) Gender: 34.5% female Ethnicity/culture/language: not reported Other PROGRESS aspects: non‐English speaking excluded; psychologically/cognitively impaired excluded Differences between groups on education (higher levels amongst intervention group); more men than women overall in sample; differences in treatments received (chemotherapy rates higher in intervention group); otherwise groups comparable on demographic details. Not clear about representation for other factors Numbers of participants: see Additional Table 1 |
|
| Interventions |
Intervention: communication support programme (CSP) Aim of intervention: overall, to increase patients’ ability and motivation to discuss prognosis and EoL care early in their final year of life (i.e. to assist patients/carers in finding information related to EoL, prognosis, future care, ACP). Both patients’ autonomous motivation and competence were targets of the intervention (i.e. increases in both); and oncologists cued to endorse QPL us and support question asking to address social support needs (‘relatedness’) Comparison:standard care: no contact with nurse, no QPL, oncologists not cued to endorse QPL use or question asking prior to consultations Delivered by: trained senior nurses (experienced in oncology care) 2 nurses; each receiving 40 hours’ training Setting: cancer treatment centres, private environments (education or consultation rooms) Materials, procedures, content: Face‐to‐face nurse meeting; 45 minutes Question Prompt Lists (QPL) introduced by nurse (designed for patients and caregivers with incurable cancer); systematically explored to identify relevant questions Questions included those about prognosis, treatment options and decisions, palliative care, lifestyle, patient and family support, ACP and carer‐specific issues. Prognosis and EoL care issues were highlighted and skills for asking questions discussed Participants also given a DVD on ACP and documenting wishes for care relevant to NSW Participants prompted to identify 1 to 3 questions to ask at next consultation Follow‐up (booster) phone call; 15 minutes 1 to 2 weeks after consultation occurring following the CSP meeting Sought to reinforce content of face‐to‐face meeting and help prepare patients for future consultations using the QPL. Nurses verbally cued oncologists immediately prior to the consultation following the CSP session. Oncologists also received a postcard with suggested endorsement phrasing When and how much: single face‐to‐face session, approximately 1 week before follow‐up oncology consultation. Carers attended where possible Follow‐up telephone booster session 1 to 2 weeks after the consultation following the CSP delivery session Tailoring: tailored as QPL was explored with patients to identify priority questions and to discuss skills for question asking Modified during study: no Co‐intervention(s): none reported Fidelity assessed: fidelity was assessed and shown to be high: assessed after each face‐to‐face and telephone session Key goals completed for almost all participants (see page 34) for CSP Key goals for booster sessions completed for all participants Theoretical base: informed by self‐determination theory of health‐related behaviour change Evidence for effects of QPL and nurse communication support each described; rationale for combining the 2 seems sound (described in more detail in Walczak 2014 study report) |
|
| Outcomes |
Primary outcomes Information preferences (preferences for amount and type of information) [Knowledge and understanding] Method: Cassileth Information Styles Questionnaire (CISQ); self‐reported questionnaire Timing: baseline; 1 month Scale and scoring: validated and reference provided. Scores subtracted from baseline preference scores; differences expressed dichotomously (preferences met or exceeded if score > 0; unmet is difference < 0) Questions, cues (numbers; from patients, carers) [Discussions about EoL/EoL care] Method: coding of audio‐recorded consultation post CSP Timing: approximately 1 week post‐CSP session (consultation) Scale and scoring: coding scheme developed by authors to identify overall numbers of direct questions and cues for discussion, as well as those relating to specific aspects of care (prognosis, EoL care, future care options, and general issues (latter not targeted by the CSP intervention)) Control preferences (amount of doctor/patient +/‐ carer involvement in decisions) [Evaluation of the communication] Method: Degner Control Preferences Scale (CPS); self‐reported questionnaire Timing: baseline; 1 month Scale and scoring: validated and reference provided. Scores subtracted from baseline preference scores; differences expressed dichotomously (preferences met or exceeded if score > 0; unmet is difference < 0) Patient communication self‐efficacy (PEPPI: Perceived Efficacy in Patient/ Physician Interactions Scale) [Evaluation of the communication] Method: self‐reported questionnaire Timing: baseline; 1 month Scale and scoring: not stated but validated and reference provided Primary outcomes ‐ adverse events None reported Secondary outcomes Health‐related quality of life [Quality of life] Method: health‐related QoL (FACT‐G). Self‐reported questionnaire Timing: baseline; 1 month Scale and scoring: validated and reference provided; other details not stated Consultation length [Health system impacts] |
|
| Notes | Satisfaction with the face‐to‐face session and with follow‐up call were also reported for the intervention group but as data were not comparative it was not included in the review Numbers of questions and cues were also reported; for analysis only numbers of questions were used |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated random number table was used to generate blocks of 1:1 balanced randomization codes for each referring oncologist" (page 32) |
| Allocation concealment (selection bias) | Low risk | Allocation sequence concealed in sequentially numbered, opaque envelopes; sequentially opened by blinded research manager for oncologist to determine randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Participants and oncologists could not be blinded" (page 32) Questionnaire measures are self‐reported by patients/carers: lack of blinding may affect these ratings, particularly for several of the subjective outcome ratings |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "Participants and oncologists could not be blinded" (page 32) Questionnaire measures are self‐reported by patients/carers: lack of blinding may affect these ratings, particularly for several of the subjective outcome ratings No information on whether those coding consultation recordings for analysis were blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition was high (31/110 (28%) lost to follow‐up), possibly largely explained by declining health of participants (patients) Higher in intervention group (34% intervention group, 18% control). No systematic reasons for differential attrition were identified by authors but dropout in intervention group is substantial and may introduce bias Authors state that ITT analysis was used (according to group assignment) but dropout rates may be problematic |
| Selective reporting (reporting bias) | Unclear risk | Checking against the protocol in Walczak 2014 there are a number of outcomes not reported (e.g. acceptance of disease, preferences for future interventions, etc,) but primary outcomes are reported. It is possible that other outcomes are reported elsewhere |
| Other bias | Low risk | Groups were similar at baseline (other than higher educational levels and rates of chemotherapy in intervention group) Differences between groups on education (higher levels amongst intervention group); more men than women overall in sample; differences in treatments received (chemotherapy rates higher in intervention group); otherwise groups comparable on demographic details. Not clear about representation for other factors Contamination is possible as patients were the unit of randomisation (rather than at the level of the oncologist). Not clear if this is likely however Trial is underpowered (sample size calculated at 140; 110 recruited; 79 completed) to detect differences between groups |
ACP: advance care planning; COPD: chronic obstructive pulmonary disease; CPR: cardiopulmonary resuscitation; EMR: electronic medical record; EoL: end of life; ICC: intracluster correlation; ICU: intensive care unit; IQR: interquartile range; ITT: intention to treat; QALY: quality‐adjusted life‐year; QoL: quality of life; RCT: randomised controlled trial; STAI: State‐Trait Anxiety Inventory; SD: standard deviation; UC: usual care.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aaronson 1999 | Wrong intervention |
| Aasmul 2018 | ACP/AD and uptake |
| Abernethy 2006 | Primarily clinical management |
| Abernethy 2013 | Primarily clinical management |
| Abrahm 2016 | No communication intervention |
| ACTRN12614000150640 | Not communication at EoL |
| Agar 2016a | Non‐RCT/quasi |
| Agren 2019 | Population not at EoL |
| Ahrens 2003 | Non‐RCT/quasi |
| Aikman 1999 | ACP/AD and uptake |
| Akard 2020 | Not communication with healthcare professional |
| Akard 2021 | Not communication with healthcare professional |
| Akard 2021b | No healthcare professional involved in communication |
| Akyar 2019 | Abstract only; non‐RCT/quasi |
| Alexander 2006 | Communication skills training |
| Alghanim 2019 | Population not at EoL |
| Allen 2008 | Decision/tool focus |
| Ambuel 2001 | Non‐RCT/quasi |
| An 2019 | No‐ RCT/quasi (non‐comparative data only) |
| An 2020 | Data not separated by study arms, no communication intervention evaluated |
| Anandan 2020 | Abstract only; not EoL communication |
| Ang 2018 | Non‐RCT/quasi |
| Anonymous 2012 | Not research (commentary on cohort study) |
| Aoun 2015 | Non‐RCT/quasi |
| Au 2006 | Focus on resource use over last 6 months of life (no communication intervention) |
| Azoulay 2001 | No communication intervention |
| Azoulay 2002 | Not communication with patients at EoL |
| Azoulay 2007 | Retracted |
| Azoulay 2018 | Not communication with patients at EoL |
| Back 2007 | Communication skills training |
| Baharvandi | Population not at EoL |
| Bahary 2016 | Wrong intervention |
| Bajwah 2012 | Protocol |
| Baker 2000 | Bereaved population (non‐EoL population) |
| Baker 2017 | ACP/AD and uptake |
| Baker 2017a | Duplicate |
| Barrio‐Cantalejo 2009 | ACP/AD and uptake |
| Bartlow 2005 | Not research |
| Bauman 2015 | Non‐RCT/quasi |
| Bernacki 2014 | Not a research study |
| Best 2019 | Population not at EoL |
| Bhatia 2015 | Non‐RCT/quasi |
| Bickell 2017 | Communication skills training |
| Bickell 2018 | Communication skills training |
| Bickell 2018b | Population not at EoL |
| Bickell 2020 | Population not at EoL |
| Bloch 2015 | ACP/AD and uptake |
| Bose‐Brill 2016 | Not face‐to‐face communication |
| Boyd 2016 | Non‐RCT, ACP focus |
| Braus 2016 | No communication intervention |
| Brown 1999 | Not EoL; minority of participants had life expectancy < 12 months |
| Brown 2001 | Population is not at EoL (cancer patients) |
| Buck 2013 | Not research |
| Butow 1994 | Population is not at EoL |
| Carson 2016 | Wrong population |
| Chang 2020 | Population not at EoL |
| Chen 2019 | ACP/AD |
| Chung Vincent 2016 | No communication intervention |
| Clarke‐Pounder 2015 | Wrong intervention |
| Clayton 2012 | Non‐RCT/quasi |
| Coats 2018 | No communication intervention |
| Connors 1995 | Population is not at EoL |
| Cornbleet 2002 | Wrong intervention |
| Curtis 1997 | Qualitative; non‐RCT |
| Curtis 2004 | No communication intervention |
| Curtis 2005 | Review |
| Curtis 2013 | Communication skills training |
| Curtis 2016 | Wrong intervention |
| Curtis 2018 | Population is not at EoL (life expectancy > 12 months) |
| Curtis 2018a | Population is not at EoL (life expectancy > 12 months) |
| Dangayach 2011 | ACP/AD and uptake |
| Delgado‐Guay 2016 | Primarily focused on evaluation of wishes using tool |
| De Padova 2008 | No communication intervention |
| Dimoska 2008 | Systematic review |
| Doorenbos 2016 | Focus on goals of care including ACP, not EoL |
| Dose 2015 | No communication intervention |
| EAPC 2016 | No communication intervention |
| El‐Jawahri 2010 | Not face‐to‐face communication |
| El‐Jawahri 2019 | Decision tool focus |
| Enzinger 2020 | Population not at EoL |
| Ernecoff 2017 | Population not at EoL |
| Fakhri 2016 | Wrong intervention |
| Fakhri 2016a | Not face‐to‐face communication |
| Fallowfield 2002 | Communication skills training |
| Fischer 2021 | ACP/AD |
| Flannery 2019 | Population not at EoL |
| Flannery 2022 | Not communication at EoL |
| Freytag 2018 | Baseline (pre‐intervention) data only, no communication intervention |
| Fujimori 2014 | Communication skills training |
| Fujimori 2017 | Communication skills training |
| Garrouste‐Orgeas 2016 | Wrong intervention |
| Gilligan 2017 | Non‐RCT/quasi |
| Goelz 2010 | Communication skills training |
| Goldstein 2019 | Communication skills training |
| Gramling 2016 | Cross‐sectional data only, no communication intervention evaluated |
| Graul 2019 | Not EoL communication intervention |
| Graul 2020 | Not EoL communication intervention |
| Greer 2018 | Population not at EoL |
| Hancock 2016 | Poster with limited results (pilot study only) |
| Hannon 2015 | Wrong intervention |
| Hanson 2019 | Primarily clinical |
| Henselmans 2020 | Decision/decision tool focus |
| Hinton 1998 | Non‐RCT/quasi |
| Houben 2019 | Primarily ACP focus |
| Hudson 2018 | Population not at EoL |
| Hudson 2021 | Population not at EoL |
| ISRCTN36040085 | Primarily clinical management of care |
| Janssen 2011a | No communication intervention |
| Janssen 2011b | No communication intervention |
| Johnson 2016 | ACP/AD and uptake |
| Johnson 2016a | ACP/AD and uptake |
| Jones 2004 | Wrong intervention |
| Jones 2011 | Wrong population |
| Kirchhoff 2012 | ACP/AD and uptake |
| Knauft 2005 | Qualitative; non‐RCT |
| Knaus 1995 | Not EoL |
| Kruse 2013 | Wrong intervention |
| Lakin 2017 | Not EoL, not RCT |
| Lee 2015 | No communication intervention |
| Lee Brittney 2017 | No communication intervention |
| Lincoln 2020 | Abstract; not comparative |
| Lincoln 2020b | Protocol; not time frame for EoL communication |
| Loh 2020 | No communication intervention evaluated |
| Long 2013 | Duplicate |
| Long 2014 | No communication intervention |
| Lyon 2009 | ACP/AD and uptake |
| Lyon 2009a | ACP/AD and uptake |
| Lyon 2013 | ACP/AD and uptake |
| Lyon 2013a | ACP/AD and uptake |
| Lyon 2014 | ACP/AD and uptake |
| Lyon 2017 | ACP/AD and uptake |
| Lyon 2020 | ACP/AD and uptake |
| Maciasz 2013 | Not face‐to‐face communication |
| Maciasz 2013a | No communication intervention |
| Mah 2020 | Not EoL communication intervention |
| Malhotra 2019 | Population is not at EoL |
| Marbella 1998 | Wrong intervention |
| Martin 2020 | Communication skills training |
| Martinsson 2016 | Communication skills training |
| Matthys 2021 | Population not at EoL |
| McFarlin 2011 | Duplicate |
| Mehnert 2017 | Primarily clinical management (non‐communication) |
| Meier 2004 | Wrong intervention |
| Menon 2016 | Decision/tool focus |
| Murray 2008 | A protocol |
| Murray 2010 | Communication skills training |
| NCT00325611 | Primarily clinical management of care |
| NCT00374010 | ACP/AD and uptake |
| NCT00580515 | Wrong population |
| NCT01160367 | Wrong population |
| NCT01245621 | Primarily focussed on management of care |
| NCT01289444 | ACP/AD and uptake |
| NCT01670461 | Wrong population |
| NCT01828775 | No communication intervention |
| NCT01914848 | ACP/AD and uptake |
| NCT01944813 | ACP/AD and uptake |
| NCT01990742 | Primarily clinical management of care |
| NCT02112461 | No communication intervention |
| NCT02261935 | Not a communication intervention |
| NCT02349412 | Primarily clinical management (non‐communication) |
| NCT02445937 | No communication intervention |
| NCT02463162 | ACP/AD and uptake |
| NCT02606149 | Primarily focused on treatment decisions |
| NCT02723799 | Population not at EoL |
| NCT02730858 | Primarily clinical management of care |
| NCT02917603 | Not face‐to‐face communication |
| NCT02944344 | Not face‐to‐face communication intervention (primarily online) |
| NCT03068013 | No communication intervention |
| NCT03099746 | Not primarily face‐to‐face (doctor‐patient/carer) communication |
| NCT03138564 | No communication intervention |
| NCT03387436 | ACP/AD and uptake |
| NCT03506087 | ACP/AD and uptake |
| NCT03548142 | Population does not fit EoL criteria |
| NCT03626402 | Population does not fit EoL criteria |
| Nipp 2020 | Population not at EoL |
| Nishioka 2019 | Communication skills training |
| Norton 2019 | Qualitative data only, not separated by study arms, no communication intervention |
| O'Donnell 2016 | Wrong population |
| O'Donnell 2018 | ACP/AD and uptake |
| Oliver 2001 | Population not at EoL |
| Oliver 2012 | Primarily clinical management (non‐communication) |
| Paladino 2014 | Communication skills training |
| Paladino 2015 | ACP/AD and uptake |
| Paladino 2016 | Wrong population |
| Parker 2017 | Primarily clinical management (non‐communication) |
| Perry 2005 | ACP/AD and uptake |
| Pintova 2020 | Population not at EoL |
| Pirl 2019 | Abstract; data not interventional (associations only) |
| Pollak 2019 | ACP/AD focus and uptake |
| Pollak 2020 | Communication skills training |
| Porensky 2011 | Wrong population |
| Ramos 2013 | No communication intervention |
| Reinhardt 2015 | Data not presented by RCT arm data only, no communication intervention evaluated |
| Reinhardt 2017 | Retrospective data only, no communication intervention |
| Reinke 2011 | Non‐RCT/quasi |
| Reuther 2014 | Population not at EoL |
| Rousseau 2016 | Wrong population |
| Ruiz 2016 | Non‐RCT/quasi |
| Russell 2016 | No communication intervention |
| Saeed 2018 | Baseline (pre‐intervention) data, no communication intervention evaluated |
| Sanchez 2018 | Commentary (on Curtis 2018 trial report) |
| Schneiderman 2003 | Primarily clinical management |
| Smith 2017 | Primarily clinical management |
| Smucker 1993 | ACP/AD and uptake |
| Song 2009 | Wrong population |
| Song 2016 | Wrong population |
| Song 2018 | Protocol |
| Starks 2016 | Wrong population |
| Steinhauser 2008 | No communication intervention |
| Street 2010 | Population not at EoL |
| Sudore 2010 | No communication intervention |
| Sulmasy 2017 | Focus on decision‐making features (e.g. decisional control preferences) not broad communication focus |
| Szmuilowicz 2010 | Communication skills training |
| Temel 2017 | Primarily clinical management (non‐communication) |
| Temel 2020 | Abstract; not EoL communication intervention |
| Tierney 2001 | ACP/AD and uptake |
| Toles 2018 | Non RCT/quasi‐RCT; not comparative intervention data |
| Trevino 2019 | Baseline data only for trial, no communication intervention evaluated |
| Tulsky 2011 | Communication skills training |
| Uitdehaag 2012 | No communication intervention |
| Vaccaro 2016 | ACP/AD and uptake |
| Van Laarhoven 2018 | Communication skills training |
| Verhofstede 2012 | Primarily clinical management (non‐communication) |
| Verreault 2018 | No communication intervention |
| Volandes 2009 | Decision/tool focus |
| Volandes 2013 | Decision/tool focus |
| Von Blanckenburg | Population not at EoL |
| von Heymann‐Horan 2019 | Not EoL communication intervention |
| Voruganti 2017 | Not face‐to‐face communication |
| Walker 2017 | Wrong population |
| Walker 2017a | Duplicate |
| Wallen 2012 | Primarily clinical management (non‐communication) |
| Waller 2016 | ACP/AD and uptake |
| Walshe 2016 | Wrong population |
| Walshe 2016a | No health practitioner (peer‐peer communication) |
| Wanta Barbara 1998 | Non‐RCT/quasi |
| Wendlandt 2019 | Not RCT/quasi‐RCT; assessment of associations not intervention |
| Wentlandt 2012 | No communication intervention |
| Whisenant 2017 | Primarily clinical management (non‐communication) |
| White 2018 | Duplicate |
| White 2018a | Wrong population |
| Wilkinson 2015 | Not face‐to‐face communication |
| Wilson 2013 | Not face‐to‐face communication |
| Wilson 2015 | Not face‐to‐face communication |
| Wittenberg‐Lyles 2013 | No communication intervention |
| Wolfe 2014 | No communication intervention |
| Yilmaz 2020 | Not evaluation of communication intervention |
| Yun 2011 | Wrong intervention |
| Zaider 2012 | Duplicate |
| Zaider 2020 | No communication intervention |
| Zimmerman 2014 | Palliative care (non‐communication) intervention |
ACP/AD: advance care planning/advance directive; EoL: end of life; RCT: randomised controlled trial.
Characteristics of ongoing studies [ordered by study ID]
Fujimori 2020.
| Study name | Japan Supportive, Palliative and Psychosocial Oncology Group as J‐SUPPORT 1904 study protocol |
| Methods | RCT |
| Participants | 20 oncologists, 200 patients with advanced pancreatic cancer |
| Interventions | Oncologists receive 2.5‐hour individual communication skills training, and patients and caregivers receive a half‐hour coaching intervention to facilitate prioritising and discussing questions and concerns Control: no training |
| Outcomes | Patient‐centred communication behaviours |
| Starting date | Trial status: this study is currently enrolling participants. Enrolment period ends 31 July 2020; estimated follow‐up date is 31 March 2023 |
| Contact information | |
| Notes | Trial registration number UMIN Clinical Trial Registry (UMIN000033612); pre‐results |
NCT03770481.
| Study name | Using a nurse‐led communication strategy for surrogates in the intensive care unit |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | clinicaltrials.gov/ct2/show/study/NCT03770481. Completion date 2021 |
NL5388.
| Study name | CHOICE: CHOosing treatment together In Cancer at the End of life |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | Completed |
| Contact information | |
| Notes | www.trialregister.nl/trial/5388 |
R000038002.
| Study name | A randomized controlled trial of integrated empathic communication support program to promote end of life discussion among rapidly progressive cancer patient, caregiver and physician |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000038002. Completion 2012 |
RCT: randomised controlled trial.
Differences between protocol and review
The protocol stated we would search a range of grey literature sources. This was modified at review stage. The following were not searched as they were deemed less relevant: World Wide Hospice Palliative Care Alliance, TROVE and the Networked Digital Library of Theses and Dissertations (NDLTD). The following search was added at review stage as these were thought most likely to identify relevant trials: World Health Organization International Clinical Trials Registry Platform (WHO ICTRP).
At protocol stage we did not specify at which time points data would be collected. At review stage we decided (for studies reporting more than one time point) to extract data at the longest follow‐up, except where noted for purposes of comparisons between studies, as this is most likely to be meaningful for consumers and for practice.
Contributions of authors
Rebecca Ryan: was involved in all stages including drafting of the final review.
Michael Connolly: was involved in all stages including drafting of the final review.
Natalie Bradford: assisted with screening studies, commented and had input to the final review.
Simon Henderson: assisted with screening studies, commented and had input to the final review.
Anthony Herbert: assisted with screening studies, commented and had input to the final review.
Lina Schonfeld: assisted with extraction and checks of data.
Jeanine Young: assisted with screening studies, commented and had input to the final review.
Josephine Bothroyd: commented and had input to the protocol.
Amanda Henderson: conceived the review and was involved in all stages including drafting of the final review.
Sources of support
Internal sources
No sources of support provided
External sources
-
Rebecca Ryan, Australia
Position is supported by the National Health and Medical Research Council (NHMRC) funding to Australian Cochrane entities.
Declarations of interest
Rebecca Ryan: completed this work as part of her role as Joint Co‐ordinating Editor with the Cochrane Consumers and Communication Group. Her position is funded by a Cochrane Infrastructure Grant provided by the National Health and Medical Research Council (NHMRC).
Michael Connolly: completed this work as part of his Evidence Synthesis Ireland Fellowship.
Natalie Bradford: none to declare.
Simon Henderson: none to declare.
Anthony Herbert: received funding from Australia Research Council (2018 to 2020) and Gilead Sciences (2016).
Lina Schonfeld: none to declare.
Jeanine Young: none to declare.
Josephine Bothroyd: none to declare.
Amanda Henderson: none to declare.
Edited (no change to conclusions)
References
References to studies included in this review
Agar 2017 {published and unpublished data}
- ACTRN12612001164886. Improving dementia end of life care at local aged care facilities [Cluster randomised controlled trial of facilitated case conferencing versus usual care for improving end of life outcomes in aged care residents with advanced dementia and their families]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12612001164886 (first received 2 November 2012). [ACTRN12612001164886]
- Agar M, Beattie E, Luckett T, Phillips J, Luscombe G, Goodall S, et al. Pragmatic cluster randomised controlled trial of facilitated family case conferencing compared with usual care for improving end of life care and outcomes in nursing home residents with advanced dementia and their families: the IDEAL study protocol. BMC Palliative Care 2015;14(1):63. [DOI: 10.1186/s12904-015-0061-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agar M, Chenoweth L, Mitchell G, Goodall S, Beattie E, Luscombe G, et al. Cluster randomised controlled trial of facilitated case conferencing for aged care residents with advanced dementia. Palliative Medicine 2016;30(6):NP72-NP73. [DOI: 10.1177/0269216316646056] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agar M, Luckett T, Luscombe G, Phillips J, Beattie E, Pond D, et al. Effects of facilitated family case conferencing for advanced dementia: a cluster randomised clinical trial. PLOS One 2017;12(8):e0181020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckett T, Chenoweth L, Phillips J, Brooks D, Cook J, Mitchell G, et al. A facilitated approach to family case conferencing for people with advanced dementia living in nursing homes: perceptions of palliative care planning coordinators and other health professionals in the IDEAL study. International Psychogeriatrics 2017;29:1713-22. [DOI] [PubMed] [Google Scholar]
- Luckett T, Luscombe G, Phillips J, Beattie E, Chenoweth L, Davidson PM, et al. Australian long-term care personnel’s knowledge and attitudes regarding palliative care for people with advanced dementia. Dementia 2021;20(2):427-43. [DOI: 10.1177/1471301219886768] [DOI] [PubMed] [Google Scholar]
Au 2012 {published data only}
- Au DH, Udris EM, Engelberg RA, Diehr PH, Bryson CL, Reinke LF, et al. A randomized trial to improve communication about end-of-life care among patients with COPD. Chest 2012;141(3):726-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00106080. Improving the quality of end-of-life communication for patients with chronic obstructive pulmonary disease (COPD). clinicaltrials.gov/ct2/show/record/NCT00106080 (first received 21 March 2005). [NCT00106080]
Bernacki 2019 {published and unpublished data}
- Bernacki R, Hutchings M, Vick J, Smith G, Paladino J, Lipsitz S, et al. Development of the Serious Illness Care Program: a randomised controlled trial of a palliative care communication intervention. BMJ Open 2015;5(10):e009032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernacki R, Paladino J, Lamas D, Hutchings M, Lakin J, Neville BA, et al. Delivering more, earlier, and better goals-of-care conversations to seriously ill oncology patients. Journal of Clinical Oncology 2015;33(29 Suppl (October 10, 2015)):39. [Google Scholar]
- Bernacki R, Paladino J, Neville BA, Hutchings M, Kavanagh J, Geerse OP, et al. Effect of the Serious Illness Care Program in outpatient oncology: a cluster randomized clinical trial. JAMA Internal Medicine 2019;179(6):751-9. [DOI: 10.1001/jamainternmed.2019.0077] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01786811. Serious Illness Communication Project. clinicaltrials.gov/ct2/show/NCT01786811 (first received 8 February 2013). [NCT01786811]
- Paladino J, Bernacki R, Neville BA, Kavanagh J, Miranda SP, Palmor M, et al. Evaluating an intervention to improve communication between oncology clinicians and patients with life-limiting cancer: a cluster randomized clinical trial of the Serious Illness Care Program. JAMA Oncology 2019;5(6):801-9. [DOI: 10.1001/jamaoncol.2019.0292] [DOI] [PubMed] [Google Scholar]
- Paladino J, Koritsanszky L, Neal BJ, Lakin JR, Kavanagh J, Lipsitz S, et al. Effect of the Serious Illness Care Program on health care utilization at the end of life for patients with cancer. Journal of Palliative Medicine 2020;23(10):1365-9. [DOI: 10.1089/jpm.2019.0437] [DOI] [PubMed] [Google Scholar]
- Paladino J, Koritsanszky L, Nisotel L, Neville BA, Miller K, Sanders J, et al. Patient and clinician experience of a serious illness conversation guide in oncology: a descriptive analysis. Cancer Medicine 2020;9(13):4550-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paladino J, Lakin J, Miranda S, Gass J, Bernacki R, Koritsanszsky L, et al. Can we improve the quality of documented end-of-life conversations using a structured, multicomponent intervention? Journal of Clinical Oncology 2019;34(26 Supplement 1):49. [Google Scholar]
Clayton 2007 {published and unpublished data}
- Clayton J, Butow P, Tattersall M, Chye R, Noel M, Davis JM, et al. Asking questions can help: development and preliminary evaluation of a question prompt list for palliative care patients. British Journal of Cancer 2003;89(89):2069-77. [DOI: 10.1038/sj.bjc.6601380] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton J. Enhancing Doctor-Patient Communication in Palliative Medicine [Doctoral dissertation]. Sydney: The University of Sydney, 2005. [Google Scholar]
- Clayton JM, Butow PN, Tattersall MH, Devine RJ, Simpson JM, Aggarwal G, et al. Randomized controlled trial of a prompt list to help advanced cancer patients and their caregivers to ask questions about prognosis and end-of-life care. Journal of Clinical Oncology 2007;25(6):715-23. [DOI] [PubMed] [Google Scholar]
- Clayton JM, Butow PN, Tattersall MHN. The needs of terminally ill cancer patients versus those of their caregivers for information regarding prognosis and end-of-life issues. Cancer 2005;103:1957-64. [DOI] [PubMed] [Google Scholar]
Epstein 2017 {published and unpublished data}
- Duberstein PR, Kravitz RL, Fenton JJ, Xing G, Tancredi DJ, Hoerger M, et al. Physician and patient characteristics associated with more intensive end-of-life care. Journal of Pain and Symptom Management 2019;58(2):208-215.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duberstein PR, Maciejewski PK, Epstein RM, Fenton JJ, Chapman B, Norton SA, et al. Effects of the values and options in cancer care communication intervention on personal caregiver experiences of cancer care and bereavement outcomes. Journal of Palliative Medicine 2019;22(11):1394-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein RM, Duberstein PR, Fenton JJ, Fiscella K, Hoerger M, Tancredi D J, et al. Effect of a patient-centered communication intervention on oncologist-patient communication, quality of life, and health care utilization in advanced cancer: the VOICE randomized clinical trial. JAMA Oncology 2017;3(1):92-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton JJ, Kravitz RL, Duberstein P, Tancredi DJ, Xing G, Epstein RM. A cluster randomized trial of a patient-centered communication intervention in advanced cancer: the Values and Options In Cancer Care (VOICE) study. Journal of Clinical Oncology 2016;34(26 Suppl (October 9, 2016)):2. [DOI: 10.1200/jco.2016.34.26_suppl.2] [NCT01485627] [DOI] [Google Scholar]
- Hoerger M, Epstein RM, Winters PC, Fiscella K, Duberstein PR, Gramling R, et al. Values and options in cancer care (VOICE): study design and rationale for a patient-centered communication and decision-making intervention for physicians, patients with advanced cancer, and their caregivers. BMC Cancer 2013;13:188. [DOI: 10.1186/1471-2407-13-188] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamen CS, Duberstein P, Prigerson HG, Mohile SG, Asare M, Janelsins MC, et al. Agreement about end-of-life (EOL) care among advanced cancer patients and their caregivers: associations with care received. Journal of Clinical Oncology 2017;35(15 Suppl (May 20, 2017)):10021. [DOI: 10.1200/JCO.2017.35.15_suppl.10021] [NCT01485627] [DOI] [Google Scholar]
- Rodenbach RA, Brandes K, Fiscella K, Kravitz RL, Butow PN, Walczak A, et al. Promoting end-of-life discussions in advanced cancer: effects of patient coaching and question prompt lists. Journal of Clinical Oncology 2017;35(8):842-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lautrette 2007 {published data only}
- Jones T. A proactive communication strategy reduced post-traumatic stress disorder symptoms in relatives of patients dying in the ICU. Evidence-Based Nursing 2007;10:85. [DOI] [PubMed] [Google Scholar]
- Lautrette A, Darmon M, Megarbane B, Joly LM, Chevret S, Adrie C, et al. A communication strategy and brochure for relatives of patients dying in the ICU. New England Journal of Medicine 2007;356(5):469-78. [DOI: 10.1056/NEJMoa063446] [DOI] [PubMed] [Google Scholar]
- NCT00331877. A communication strategy for families of patients dying in the ICU. clinicaltrials.gov/ct2/show/record/NCT00331877?term=NCT00331877&draw=2&rank=1 (first received 31 May 2006). [NCT00331877]
Reinhardt 2014 {published and unpublished data}
Walczak 2017 {published and unpublished data}
- ACTRN12610000724077. Conversations with your doctor: making the most of medical consultations for patients with advanced incurable cancer and their carers [Improving communication and quality of life (QOL) at the end of life: a randomised controlled trial of a multifocal communication intervention for patients with advanced incurable cancer, carers and doctors]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12610000724077 (first received 31 August 2010). [ACTRN12610000724077]
- Brandes K, Butow PN, Tattersall MH, Clayton JM, Davidson PM, Young J, et al. Advanced cancer patients’ and caregivers’ use of a question prompt list. Patient Education and Counseling 2014;97(1):30-7. [DOI] [PubMed] [Google Scholar]
- Walczak A, Butow P, Tattersall M, Clayton J, Davidson P, Young J, et al. Communication at the end-of-life: an RCT of a nurse-delivered intervention incorporating a QPL for advanced cancer patients and caregivers. Psycho-oncology 2013;22(Suppl 3):4-5. [Google Scholar]
- Walczak A, Butow P, Tattersall M, Clayton J, Davidson P, Young J, et al. Discussing life expectancy and end-of-life care: preliminary results from a RCT/pre-post trial of a communication training and support intervention for patients, carers and oncologists. Psycho-oncology 2011;20(Suppl 2):119-20. [Google Scholar]
- Walczak A, Butow PN, Clayton JM, Tattersall MH, Davidson PM, Young J, et al. Discussing prognosis and end-of-life care in the final year of life: a randomised controlled trial of a nurse-led communication support programme for patients and caregivers. BMJ Open 2014;4(6):e005745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walczak A, Butow PN, Tattersall MH, Davidson PM, Young J, Epstein RM, et al. Encouraging early discussion of life expectancy and end-of-life care: a randomised controlled trial of a nurse-led communication support program for patients and caregivers. International Journal of Nursing Studies 2017;67:31-40. [DOI] [PubMed] [Google Scholar]
- Walczak A, Henselmans I, Tattersall MHN, Clayton JM, Davidson PM, Young J, et al. A qualitative analysis of responses to a question prompt list and prognosis and end-of-life care discussion prompts delivered in a communication support program. Psycho-oncology 2015;24:287-93. [DOI] [PubMed] [Google Scholar]
- Walczak A, Mazer B, Butow PN, Tattersall MHN, Clayton JM, Davidson PM, et al. A question prompt list for patients with advanced cancer in the final year of life: development and cross-cultural evaluation. Palliative Medicine 2013;27(8):779-88. [DOI] [PubMed] [Google Scholar]
- Walczak A. Beginning a Discussion About the End: Enhancing Advanced Cancer Patients' Communication About Prognosis and End-of-life Care [Doctoral dissertation]. Sydney: The University of Sydney, 2016. [Google Scholar]
References to studies excluded from this review
Aaronson 1999 {published data only}
- Aaronson NK. Assessing quality of life in clinical practice in oncology. European Journal of Cancer 1999;35:222 (Abstract No 870). [Google Scholar]
Aasmul 2018 {published data only}
- Aasmul I, Husebo BS, Flo E. Description of an advance care planning intervention in nursing homes: outcomes of the process evaluation. BMC Geriatrics 2018;18:26. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Abernethy 2006 {published data only}
- Abernethy AP, Currow DC, Hunt R, Williams H, Roder-Allen G, Rowett D, et al. A pragmatic 2 x 2 x 2 factorial cluster randomized controlled trial of educational outreach visiting and case conferencing in palliative care-methodology of the Palliative Care Trial [ISRCTN 81117481]. Contemporary Clinical Trials 2006;27(1):83-100. [PMID: ] [DOI] [PubMed] [Google Scholar]
Abernethy 2013 {published data only}
- Abernethy AP, Currow DC, Shelby-James T, Rowett D, May F, Samsa GP, et al. Delivery strategies to optimize resource utilization and performance status for patients with advanced life-limiting illness: results from the ‘‘Palliative Care Trial’’ [ISRCTN 81117481]. Journal of Pain and Symptom Management 2013;45:488e505. [DOI] [PubMed] [Google Scholar]
Abrahm 2016 {published data only}
- Abrahm J, Blonquist T, Catalano P, Lobach D, Braun I, Halpenny B, et al. Point of care clinical decision support for cancer symptom management: results of a group randomized trial. Supportive Care in Cancer 2016;24(1 Supplement 1):S45. [DOI: ] [Google Scholar]
ACTRN12614000150640 {published data only}
- ACTRN12614000150640. Acceptance and valued-living in palliative care patients, caregivers and significant others. anzctr.org.au/ACTRN12614000150640.aspx (first received 7 February 2014). [ACTRN12614000150640]
Agar 2016a {published data only}
- Agar M, Chenoweth L, Mitchell G, Goodall S, Beattie E, Luscombe G, et al. Implementing facilitated case conferencing for aged care residents with advanced dementia - development of a palliative care planning coordinator role. Palliative Medicine 2016;30(6):NP228-NP229. [DOI: ] [Google Scholar]
Agren 2019 {published data only}
- Agren S, Eriksson A, Fredrikson M, Hollman-Frisman G, Orwelius L. The health promoting conversations intervention for families with a critically ill relative: a pilot study. Intensive & Critical Care Nursing 2019;50:103-10. [DOI] [PubMed] [Google Scholar]
Ahrens 2003 {published data only}
- Ahrens T, Yancey V, Kollef M. Improving family communications at the end of life: implications for length of stay in the intensive care unit and resource use. American Journal of Critical Care 2003;12:317-23; discussion 324. [PubMed] [Google Scholar]
Aikman 1999 {published data only}
- Aikman PJ, Thiel EC, Martin DK, Singer PA. Proxy, health, and personal care preferences: implications for end-of-life care. Cambridge Quarterly of Healthcare Ethics 1999;8(2):200-10. [DOI] [PubMed] [Google Scholar]
Akard 2020 {published data only}
- Akard T, Gerhardt C, Ridner S, Gilmer MJ. Parent-child communication outcomes of a RCT testing a legacy intervention for children with advanced cancer (GP703). Journal of Pain and Symptom Management 2020;60(1):247-8. [Google Scholar]
Akard 2021 {published data only}
- Akard TF, Dietrich MS, Friedman DL, Wray S, Gerhardt CA, Hendricks-Ferguson V, et al. Randomized clinical trial of a legacy intervention for quality of life in children with advanced cancer. Journal of Palliative Medicine 2021;24(5):680-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Akard 2021b {published data only}
- Akard TF, Dietrich MS, Friedman DL, Gerhardt CA, Given B, Hendricks-Ferguson V, et al. Improved parent–child communication following a RCT evaluating a legacy intervention for children with advanced cancer. Progress in Palliative Care 2021;29(3):130-9. [DOI: 10.1080/09699260.2020.1826778] [DOI] [PMC free article] [PubMed] [Google Scholar]
Akyar 2019 {published data only}
- Akyar I, Dionne-Odom JN, Bakitas MA. Using patients and their caregivers feedback to develop ENABLE CHF-PC: an early palliative care intervention for advanced heart failure. Journal of Palliative Care 2019;34(2):103-10. [DOI] [PubMed] [Google Scholar]
Alexander 2006 {published data only}
- Alexander SC, Keitz SA, Sloane R, Tulsky JA. A controlled trial of a short course to improve residents' communication with patients at the end of life. Academic Medicine 2006;81(11):1008-12. [DOI: 10.1097/01.ACM.0000242580.83851.ad] [DOI] [PubMed] [Google Scholar]
Alghanim 2019 {published data only}
- Alghanim F, Zakaria S, Furqan M, Prichett L, Tao X. Increasing communication and trust in the ICU: the critical care collaboration and communication (C4) project. American Journal of Respiratory and Critical Care Medicine 2019;199:A4152. [Google Scholar]
Allen 2008 {published data only}
- Allen RS, Allen JY, Hilgeman MM, DeCoster J. End-of-life decision-making, decisional conflict, and enhanced information: race effects. Journal of the American Geriatrics Society 2008;56(10):1904-9. [DOI: 10.1111/j.1532-5415.2008.01929.x] [DOI] [PubMed] [Google Scholar]
Ambuel 2001 {published data only}
- Ambuel B, Mazzone MF. Breaking bad news and discussing death. Primary Care; Clinics in Office Practice 2001;28(2):249-67. [DOI] [PubMed] [Google Scholar]
An 2019 {published data only}
- An A, Ladwig S, Epstein R, Prigerson H, Duberstein P. The power of human connection (FR441B). Journal of Pain and Symptom Management 2019;57(2):421. [Google Scholar]
An 2020 {published data only}
- An AW, Ladwig S, Epstein RM, Prigerson HG, Duberstein PR. The impact of the caregiver-oncologist relationship on caregiver experiences of end-of-life care and bereavement outcomes. Supportive Care in Cancer 2020;28:4219–25. [DOI: ] [DOI] [PubMed] [Google Scholar]
Anandan 2020 {published data only}
- Anandan A, Kwekkeboom K, Zelenski A, Campbell T. A randomized controlled trial of TrialTALK: a designed conversation for cancer treatment decision making (GP705). Journal of Pain and Symptom Management 2020;60(1):248-9. [Google Scholar]
Ang 2018 {published data only}
- Ang K, Hepgul N, Gao W, Higginson IJ. Strategies used in improving and assessing the level of reporting of implementation fidelity in randomised controlled trials of palliative care complex interventions: a systematic review. Palliative Medicine 2018;32:500-16. [DOI: ] [DOI] [PubMed] [Google Scholar]
Anonymous 2012 {published data only}
- Anonymous. Summaries for patients: end-of-life care discussions between patients with advanced cancer and doctors. Annals of Internal Medicine 2012;156(3):I34. [DOI: ] [DOI] [PubMed] [Google Scholar]
Aoun 2015 {published data only}
- Aoun S, Deas K, Toye C, Ewing G, Grande G, Stajduhar K. Supporting family caregivers to identify their own needs in end-of-life care: qualitative findings from a stepped wedge cluster trial. Palliative Medicine 2015;29:508-17. [DOI: ] [DOI] [PubMed] [Google Scholar]
Au 2006 {published data only}
- Au DH, Udris EM, Fihn SD, McDonell MB, Curtis JR. Differences in health care utilization at the end of life among patients with chronic obstructive pulmonary disease and patients with lung cancer. Archives of Internal Medicine 2006;166(3):326-31. [DOI] [PubMed] [Google Scholar]
Azoulay 2001 {published data only}
- Azoulay E, Pochard F, Chevret S, Lemaire F, Mokhatari M, Le Gall JR, et al. Meeting the needs of intensive care unit patient families: a multicenter study. American Journal of Respiratory and Critical Care Medicine 2001;163:135-9. [DOI] [PubMed] [Google Scholar]
Azoulay 2002 {published data only}
- Azoulay E, Pochard F, Chevret S, Jourdain M, Bornstain C, Wernet A, et al. Impact of a family information leaflet on effectiveness of information provided to family members of intensive care unit patients: a multicenter, prospective, randomized, controlled trial. American Journal of Respiratory and Critical Care Medicine 2002;165:438-42. [DOI] [PubMed] [Google Scholar]
Azoulay 2007 {published data only}
- Azoulay E, Lautrette A, Darmon M, Megarbane B, Joly LM, Schlemmer B. A proactive communication strategy for family members of patients dying in the ICU: a multicenter randomized controlled trial. In: American Thoracic Society International Conference; 2007 May 18-23; San Francisco (California). 2007:Abstract No A509.
Azoulay 2018 {published data only}
- Azoulay E, Forel JM, Vinatier I, Truillet R, Renault A, Valade S, et al. Questions to improve family-staff communication in the ICU: a randomized controlled trial. Intensive Care Medicine 2018;44(11):1879-87. [DOI] [PubMed] [Google Scholar]
Back 2007 {published data only}
- Back AL, Arnold RM, Baile WF, Fryer-Edwards K, Alexander SC, Barley GE, et al. Efficacy of communication skills training for giving bad news and discussing transitions to palliative care. Archives of Internal Medicine 2007;167(5):453-60. [DOI] [PubMed] [Google Scholar]
Baharvandi {published data only}
- Baharvandi B, Moghadam Kobra K, Rashidi Homayoon H. The effectiveness of compassion-focused therapy on ambiguity tolerance and death anxiety in the elderly. Aging Psychology 2020;6(1):13-26. [Google Scholar]
Bahary 2016 {published data only}
- Bahary N, Claxton R, Childers J, Kavalieratos D, King L, Lembersky BC, et al. A pilot trial of early specialty palliative care for patients with advanced pancreatic cancer: challenges encountered and lessons learned. Journal of Clinical Oncology 2016;34:110-10. [DOI: 10.1200/jco.2016.34.26_suppl.110] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bajwah 2012 {published data only}
- Bajwah S, Higginson IJ, Wells AU, Koffman J, Ross J R, Birring SS, et al. Developing and evaluating a hospital2home palliative care service for patients with advanced progressive idiopathic fibrotic interstitial lung disease: phase 0-II. Palliative Medicine 2012;26(4):545. [DOI: 10.1177/0269216312446391] [DOI] [Google Scholar]
Baker 2000 {published data only}
- Baker R, Wu AW, Teno JM, Kreling B, Damiano AM, Rubin HR, et al. Family satisfaction with end-of-life care in seriously ill hospitalized adults. Journal of the American Geriatrics Society 2000;48(5 Supplement):S61-9. [DOI] [PubMed] [Google Scholar]
Baker 2017 {published data only}
- Baker J, Friebert S, Briggs L, Lyon M. Family-centered advance care planning for teens with cancer (FACE-TC): a demonstration of key components of the face intervention/respecting choices interview for AYA cancer patients. Pediatric Blood and Cancer 2017;64 Supplement 1:S73. [DOI: ] [Google Scholar]
Baker 2017a {published data only}
- Baker J, Friebert S, Briggs L, Lyon M. Family-centered advance care planning for teens with cancer (FACE-TC): a demonstration of key components of the face intervention/respecting choices interview for AYA cancer patients. Pediatric Blood and Cancer (30th Annual Meeting of the American Society of Pediatric Hematology/Oncology (ASPHO); 2017 April 26-29; Montreal (Canada)) 2017;64 Supplement 1:S73. [DOI: 10.1002/pbc.26591] [DOI] [Google Scholar]
Barrio‐Cantalejo 2009 {published data only}
- Barrio-Cantalejo IM, Molina-Ruiz A, Simón-Lorda P, Cámara-Medina C, Toral López I, Mar Rodríguez del Aguila M, et al. Advance directives and proxies' predictions about patients' treatment preferences. Nursing Ethics 2009;16(1):93-109. [DOI: 10.1177/0969733008097995] [DOI] [PubMed] [Google Scholar]
Bartlow 2005 {published data only}
- Bartlow B. Discussing end-of-life care or what do we know? Nephrology News & Issues 2005;19(4):55-6, 66. [PubMed] [Google Scholar]
Bauman 2015 {published data only}
- Bauman JR, Schleicher S, Nipp RD, El-Jawahri A, Pirl WF, Greer JA, et al. Feasibility of a pilot study of an intervention to Enhance Communication during Hospice care with Oncology (ECHO). Journal of Clinical Oncology 2015;33(29 Suppl):51-51. [DOI: 10.1200/jco.2015.33.29_suppl.51] [DOI] [Google Scholar]
Bernacki 2014 {published data only}
- Bernacki R, Block S. The Serious Illness Communication Checklist (FR408-B). Journal of Pain and Symptom Management 2014;47:420. [DOI: ] [DOI] [PubMed] [Google Scholar]
Best 2019 {published data only}
- Best M, McArdle MB, Huang YJ, Clayton J, Butow P. How and how much is spirituality discussed in palliative care consultations for advanced cancer patients with and without a question prompt list? Patient Education and Counseling 2019;102(12):2208-13. [DOI] [PubMed] [Google Scholar]
Bhatia 2015 {published data only}
- Bhatia N, Tibballs J. Deficiencies and missed opportunities to formulate clinical guidelines in Australia for withholding or withdrawing life-sustaining treatment in severely disabled and impaired infants. Journal of Bioethical Inquiry 2015;12(3):449-59. [DOI: ] [DOI] [PubMed] [Google Scholar]
Bickell 2017 {published data only}
- Bickell NA, Adelson K, Gonsky J, Pintova S, Levy B, Lin JJ, et al. Goals of care discussions: do advanced cancer patients have them and what difference do they make? Journal of General Internal Medicine 2017;32 (2 Supplement 1):S201. [Google Scholar]
Bickell 2018 {published data only}
- Bickell NA, Adelson K, Gonsky J, Lin JJ, Pintova S, Franco R, et al. Does training oncologists to have goals of care discussions increase and improve the quality of GoC discussions with advanced cancer patients? Journal of General Internal Medicine 2018;33 (2 Supplement 1):170-1. [Google Scholar]
Bickell 2018b {published data only}
- Bickell NA, Adelson KB, Gonsky JP, Lin JJ, Franco R, Egorova N, et al . Does training oncologists to have goals of care discussions affect healthcare utilization among patients with advanced cancer? Journal of Clinical Oncology 2018;36(15 Supplement):6595-6595. [DOI: 10.1200/JCO.2018.36.15-suppl.6595] [DOI] [Google Scholar]
Bickell 2020 {published data only}
- Bickell NA, Back AL, Adelson K, Gonsky JP, Egorova N, Pintova S, et al. Effects of a communication intervention randomized controlled trial to enable goals-of-care discussions. JCO Oncology Practice 2020;16(9):E1015-E28. [DOI] [PubMed] [Google Scholar]
Bloch 2015 {published data only}
- Bloch N, Krantz AC, Iqbal A, Frydryk A. Group discussions about future care planning. Journal of General Internal Medicine 2015;2:S534. [Google Scholar]
Bose‐Brill 2016 {published data only}
- Bose-Brill S, Kretovics M, Ballenger T, Modan G, Lai A, Belanger L, et al. Testing of a tethered personal health record framework for early end-of-life discussions. American Journal of Managed Care 2016;22(7):e258-e263. [PubMed] [Google Scholar]
Boyd 2016 {unpublished data only}
- Boyd KJ. Early palliative care for people with advanced illnesses: research into practice. EThos 2016. [The University of Edinburgh, thesis by publication 2015]
Braus 2016 {published data only}
- Braus N, Campbell TC, Kwekkeboom KL, Ferguson S, Harvey C, Krupp AE, et al. Prospective study of a proactive palliative care rounding intervention in a medical ICU. Intensive Care Medicine 2016;42:54-62. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 1999 {published data only}
- Brown R, Butow PN, Boyer MJ, Tattersall MH. Promoting patient participation in the cancer consultation: evaluation of a prompt sheetand coaching in question-asking. British Journal of Cancer 1999;80:242-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2001 {published data only}
- Brown RF, Butow PN, Dunn SM, Tattersall MH. Promoting patient participation and shortening cancer consultations: a randomised trial. British Journal of Cancer 2001;85:1273-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Buck 2013 {published data only}
- Buck HG. Help patients and families choose end-of-life care "wisely". Nursing 2013;43(7):16-7. [DOI: ] [DOI] [PubMed] [Google Scholar]
Butow 1994 {published data only}
- Butow PN, Dunn SM, Tattersall MHN, Jones QJ. Patient participation in the cancer consultation: evaluation of a question prompt sheet. Annals of Oncology 1994;5:199-204. [DOI] [PubMed] [Google Scholar]
Carson 2016 {published data only}
- Carson SS, Cox CE, Wallenstein S, Hanson LC, Danis M, Tulsky JA, et al. Effect of palliative care-led meetings for families of patients with chronic critical illness: a randomized clinical trial [Erratum appears in JAMA 2017;317(20):2134; PMID: 28535211]. JAMA 2016;316:51-62. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chang 2020 {published data only}
- Chang WT, Wang ST, Hsu CH, Tsai LM, Chan SH, Chen HM. Effects of illness representation-focused patient education on illness representations and self-care in patients with heart failure: a randomized clinical trial. Journal of Clinical Nursing 2020;29(17-18):3461-72. [DOI] [PubMed] [Google Scholar]
Chen 2019 {published data only}
- Chen CH, Chen JS, Wen FH, Chang WC, Chou WC, Hsieh CH, et al. An individualized, interactive intervention promotes terminally ill cancer patients' prognostic awareness and reduces cardiopulmonary resuscitation received in the last month of life: secondary analysis of a randomized clinical trial. Journal of Pain and Symptom Management 2019;57(4):705-14. [DOI] [PubMed] [Google Scholar]
Chung Vincent 2016 {published data only}
- Chung VM, Koczywas M, Williams A, Hurria A, Borneman TR, Cooper R, et al. Integration of palliative care for patients with solid tumors on phase I clinical trials. Journal of Clinical Oncology 2016;34:138-138. [DOI: 10.1200/jco.2016.34.26_suppl.138] [DOI] [Google Scholar]
Clarke‐Pounder 2015 {published data only}
- Clarke-Pounder JP, Boss RD, Roter DL, Hutton N, Larson S, Donohue PK. Communication intervention in the neonatal intensive care unit: can it backfire? Journal of Palliative Medicine 2015;18:157-61. [DOI: 10.1089/jpm.2014.0037] [DOI] [PubMed] [Google Scholar]
Clayton 2012 {published data only}
- Clayton JM, Natalia C, Butow PN, Simpson JM, O'Brien AM, Devine R, et al. Physician endorsement alone may not enhance question-asking by advanced cancer patients during consultations about palliative care. Supportive Care in Cancer 2012;20(7):1457-64. [DOI: 10.1007/s00520-011-1229-2] [DOI] [PubMed] [Google Scholar]
Coats 2018 {published data only}
- Coats H, Downey L, Sharma RK, Curtis JR, Engelberg RA. Quality of communication and trust in patients with serious illness: an exploratory study of the relationships of race/ethnicity, socioeconomic status, and religiosity. Journal of Pain and Symptom Management 2018;56(4):530-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Connors 1995 {published data only}
- Connors AF, Dawson NV, Desbiens NA, Fulkerson WJ, Goldman L, Knaus WA, et al. The SUPPORT principal investigators: a controlled trial to improve care for the seriously ill hospitalised patients: the Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatment (SUPPORT). JAMA 1995;274(20):1591-8. [PubMed] [Google Scholar]
Cornbleet 2002 {published data only}
- Cornbleet MA, Campbell P, Murray S, Stevenson M, Bond S. Patient-held records in cancer and palliative care: a randomized, prospective trial. Palliative Medicine 2002;16(3):205-12. [DOI: 10.1191/0269216302pm541oa] [DOI] [PubMed] [Google Scholar]
Curtis 1997 {published data only}
- Curtis JR, Patrick DL. Barriers to communication about end-of-life care in AIDS patients. Journal of General Internal Medicine 1997;12(12):736-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Curtis 2004 {published data only}
- Curtis JR, Engelberg RA, Nielsen EL, Au DH, Patrick DL. Patient-physician communication about end-of-life care for patients with severe COPD. European Respiratory Journal 2004;24(2):200-5. [DOI] [PubMed] [Google Scholar]
Curtis 2005 {published data only}
- Curtis JR, Engelberg RA, Wenrich MD, Au DH. Communication about palliative care for patients with chronic obstructive pulmonary disease. Journal of Palliative Care 2005;21(3):157-64. [PubMed] [Google Scholar]
Curtis 2013 {published data only}
- Curtis JR, Back AL, Ford DW, Downey L, Shannon SE, Doorenboos AZ, et al. Effect of communication skills training for residents and nurse practitioners on quality of communication with patients with serious illness: a randomized trial. JAMA 2013;310(21):2271-81. [DOI: 10.1001/jama.2013.282081] [DOI] [PMC free article] [PubMed] [Google Scholar]
Curtis 2016 {published data only}
- Curtis JR, Treece PD, Nielsen EL, Gold J, Ciechanowski PS, Shannon SE, et al. Randomized trial of communication facilitators to reduce family distress and intensity of end-of-life care. American Journal of Respiratory and Critical Care Medicine 2016;193(2):154-62. [DOI: 10.1164/rccm.201505-0900OC] [DOI] [PMC free article] [PubMed] [Google Scholar]
Curtis 2018 {published data only}
- Curtis JR, Downey L, Back AL, Nielsen EL, Paul S, Lahdya AZ, et al. Effect of a patient and clinician communication-priming intervention on patient-reported goals-of-care discussions between patients with serious illness and clinicians: a randomized clinical trial. JAMA Internal Medicine 2018;178(7):930-40. [DOI: 10.1001/jamainternmed.2018.2317] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01933789. Improving Communication About Serious Illness (ICSI). clinicaltrials.gov/show/NCT01933789 (first received 2 September 2013). [NCT01933789]
Curtis 2018a {published data only}
- Curtis JR, Downey L, Back A, Nielsen E, Treece P, Engelberg R. A patient and clinician communication-priming intervention increases patient-reported goals-of-care discussions between patients with serious illness and clinicians: a randomized trial. Palliative Medicine 2018;32(1 Supplement 1):9. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dangayach 2011 {published data only}
- Dangayach N, Mittal K, Raval D, Sharma K, Peddagovindu B, Kurungati G, et al. Evaluation of a standardized protocol for enhancing patient understanding of advanced directives and end of life care: a single center randomized controlled trial. Neurocritical Care 2011;15(1 Supplement 1):S168. [DOI: 10.1007/s12028-011-9625-5] [DOI] [Google Scholar]
Delgado‐Guay 2016 {published data only}
- Delgado-Guay MO, Rodriguez-Nunez A, De la Cruz V, Frisbee-Hume S, Williams J, Wu J, et al. Advanced cancer patients’ reported wishes at the end of life: a randomized controlled trial. Supportive Care in Cancer 2016;24:4273–81. [DOI: 10.1007/s00520-016-3260-9] [DOI] [PubMed] [Google Scholar]
De Padova 2008 {published data only}
- De Padova S, Carla G, Maniglia R, Salamina P, Lorusso V. Communication near the end of life. Tumori 2008;94(4):621; author reply 621-2. [DOI] [PubMed] [Google Scholar]
Dimoska 2008 {published data only}
- Dimoska A, Tattersall MHN, Butow PN, Shepherd P, Kinnersley P. Can a “prompt list” empower cancer patients to ask relevant questions? Cancer 2008;113:225-37. [DOI] [PubMed] [Google Scholar]
Doorenbos 2016 {published data only}
- Doorenbos AZ, Levy WC, Curtis JR, Dougherty CM. An intervention to enhance goals-of-care communication between heart failure patients and heart failure providers. Journal of Pain and Symptom Management 2016;52(3):353-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dose 2015 {published data only}
- Dose A, Holland D, Vanderboom C, Ingram C, Wild E. Sharing the journey: technology-enhanced transitional palliative care for rural patients/caregivers. Journal of Pain and Symptom Management 2015;49(2):422. [Google Scholar]
EAPC 2016 {published data only}
- European Association for Palliative Care. Abstracts of the 9th World Research Congress of the European Association for Palliative Care (EAPC), 2016 June 9-11; Dublin (Ireland). Palliative Medicine 2016;30(6):Meeting Abstracts. [Google Scholar]
El‐Jawahri 2010 {published data only}
- El-Jawahri A, Podgurski LM, Eichler AF, Plotkin SR, Temel JS, Mitchell SL, et al. Use of video to facilitate end-of-life discussions with patients with cancer: a randomized controlled trial [Erratum appears in Journal of Clinical Oncology 2010;28(8):1438]. Journal of Clinical Oncology 2010;28:305-10. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
El‐Jawahri 2019 {published data only}
- El-Jawahri A, Vanbenschoten O, Fenech AL, Jankowski AL, Markovitz N, Traeger L, et al. Randomized trial of a hospice video decision aid for patients with advanced cancer and their caregivers. Journal of Clinical Oncology 2019;37 (31 Supplement):42. [DOI: 10.1200/JCO.2019.37.15_suppl.11513] [DOI] [Google Scholar]
Enzinger 2020 {published data only}
- Enzinger AC, Uno H, McCleary N, Frank E, Sanoff H, Van Loon K, et al. Effectiveness of a multimedia educational intervention to improve understanding of the risks and benefits of palliative chemotherapy in patients with advanced cancer: a randomized clinical trial. JAMA Oncology 2020;6(8):1265-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ernecoff 2017 {published data only}
- Ernecoff N, Zimmerman S, Mitchell S, Song MK, Lin FC, Wessell K, et al. Goals of care and the dying experience in advanced dementia: what might be learned by implementing goals of care. Journal of Pain and Symptom Management 2017;53(2):416. [Google Scholar]
Fakhri 2016 {published data only}
- Fakhri S, Engelburg R, Downey L, Curtis JR. Factors affecting patients' discussions about end-of-life care. Journal of Investigative Medicine 2016;64(1):146-7. [DOI: ] [Google Scholar]
Fakhri 2016a {published data only}
- Fakhri S, Engelberg RA, Downey L, Nielsen EL, Paul S, Lahdya AZ, et al. Factors affecting patients' preferences for and actual discussions about end-of-life care. Journal of Pain and Symptom Management 2016;52(3):386-94. [DOI: 10.1016/j.jpainsymman.2016.03.012] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fallowfield 2002 {published data only}
- Fallowfield L, Jenkins V, Farewell V, Saul J, Duffy A, Eves R. Efficacy of a Cancer Research UK communication skills training model for oncologists: a randomised controlled trial. Lancet 2002;359(9307):650-6. [DOI] [PubMed] [Google Scholar]
Fischer 2021 {published data only}
- Fischer SM, Tropeano L, Lahoff D, Owens B, Nielsen E, Retrum J, et al. Integrating palliative care social workers into subacute settings: feasibility of the assessing and listening to individual goals and needs intervention trial. Journal of Palliative Medicine 2021;24(6):830-7. [DOI] [PubMed] [Google Scholar]
Flannery 2019 {published data only}
- Flannery MA, Culakova E, Poh Loh K, Epstein RM, Kamen CS, Obrecht S. Improving person-centered communication of goals, proxy, and advance directives in older patients with advanced cancer: secondary analysis from a University of Rochester NCI Community Oncology Research Program (NCORP) cluster randomized controlled trial (CRCT). Journal of Clinical Oncology 2019;37(15 Supplement):11523. [DOI: 10.1200/JCO.2019.37.15_suppl.11523] [DOI] [Google Scholar]
Flannery 2022 {published data only}
- Flannery MA, Mohile S, Culakova E, Norton S, Kamen C, Dionne-Odom JN, et al. Completion of patient-reported outcome questionnaires among older adults with advanced cancer. Journal of Pain and Symptom Management 2022;63(2):301-10. [DOI: 10.1016/j.jpainsymman.2021.07.032] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Freytag 2018 {published data only}
- Freytag J, Street RL, Xing G, Duberstein PR, Fiscella K, Tacredi DJ, et al. The ecology of patient and caregiver participation in consultations involving advanced cancer. Psycho-oncology 2018;27(6):1642-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fujimori 2014 {published data only}
- Fujimori M, Shirai Y, Asai M, Kubota K, Katsumata N, Uchitomi Y. Effect of communication skills training program for oncologists based on patient preferences for communication when receiving bad news: a randomized controlled trial. Journal of Clinical Oncology 2014;32:2166-72. [DOI: 10.1200/JCO.2013.51.2756] [DOI] [PubMed] [Google Scholar]
Fujimori 2017 {published data only}
- Fujimori M. Advanced directives - are they useful? Journal of Thoracic Oncology 2017;12(11 Supplement 2):S1622-s1623. [Google Scholar]
Garrouste‐Orgeas 2016 {published data only}
- Garrouste-Orgeas M, Max A, Lerin T, Gregoire C, Ruckly S, Kloeckner M, et al. Impact of proactive nurse participation in ICU family conferences: a mixed-method study. Annals of Intensive Care 2016;6 Supplement 1:1-236. [DOI: ] [DOI] [PubMed] [Google Scholar]
Gilligan 2017 {published data only}
- Gilligan T, Coyle N, Frankel RM, Berry DL, Bohlke K, Epstein RM, et al. Patient-clinician communication: American Society of Clinical Oncology consensus guideline. Journal of Clinical Oncology 2017;35(31):3618-32. [DOI: ] [DOI] [PubMed] [Google Scholar]
Goelz 2010 {published data only}
- Goelz T, Wuensch A, Stubenrauch S, De Figueiredo M, Bertz H, Wirsching M, et al. Improving communication during the transition to palliative care in oncology: a concise and individualized communication skills training demonstrates content specific effects in a randomized controlled trial. Psycho-oncology 2010;19:S93-S4. [Google Scholar]
Goldstein 2019 {published data only}
- Goldstein NE, Mather H, McKendrick K, Gelfman LP, Hutchinson MD, Lampert R, et al. Improving communication in heart failure patient care. Journal of the American College of Cardiology 2019;74(13):1682-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gramling 2016 {published data only}
- Gramling R, Fiscella K, Xing G, Hoerger M, Duberstein P, Plumb S, et al. Determinants of patient-oncologist prognostic discordance in advanced cancer. JAMA Oncology 2016;2(11):1421–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Graul 2019 {published data only}
- Graul A, Haggerty A, Stickley C, Kumar P, Morales K, Bogner H, et al . Effect of patient education on palliative care knowledge and acceptability of outpatient palliative care service among gynecologic oncology patients. Journal of Clinical Oncology 2019;37(15 Supplement):11583. [DOI: 10.1200/JCO.2019.37.15_suppl.11583] [DOI] [Google Scholar]
Graul 2020 {published data only}
- Graul A, Haggerty A, Stickley C, Kumar P, Morales K, Bogner H, et al. Effect of patient education on palliative care knowledge and acceptability of outpatient palliative care services among gynecologic oncology patients: a randomized controlled trial. Gynecologic Oncology 2020;156(2):482-7. [DOI] [PubMed] [Google Scholar]
Greer 2018 {published data only}
- Greer JA, Jacobs JM, El-Jawahri A, Nipp RD, Gallagher ER, Pirl WF, et al. Role of patient coping strategies in understanding the effects of early palliative care on quality of life and mood. Journal of Clinical Oncology 2018;36(1):53-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hancock 2016 {published data only}
- Hancock H, Pituch K, Uzark K, Bhat P, Fifer C, Silveira M, et al. Impact of early palliative care intervention on maternal stress in mothers of infants prenatally diagnosed with single ventricle heart disease: a randomized clinical trial. Journal of the American College of Cardiology 2016;1:920. [Google Scholar]
Hannon 2015 {published data only}
- Hannon B, Swami N, Pope A, Zimmermann C. Strengthened relationships: exploring the effects of an early palliative care intervention on patient-caregiver dyads. Supportive Care in Cancer 2015;23(1 Supplement 1):S209-S210. [DOI: 10.1007/s00520-015-2712-y] [DOI] [Google Scholar]
Hanson 2019 {published data only}
- Hanson LC, Kistler CE, Lavin K, Gabriel SL, Ernecoff NC, Lin FC, et al. Triggered palliative care for late-stage dementia: a pilot randomized trial. Journal of Pain and Symptom Management 2019;57(1):10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Henselmans 2020 {published data only}
- Henselmans I, Laarhoven HWM, Maarschalkerweerd P, Haes HCJM, Dijkgraaf MGW, Sommeijer DW, et al . Effect of a skills training for oncologists and a patient communication aid on shared decision making about palliative systemic treatment: a randomized clinical trial. Oncologist 2020;25:e578–e588. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hinton 1998 {published data only}
- Hinton J. An assessment of open communication between people with terminal cancer, caring relatives, and others during home care. Journal of Palliative Care 1998;14(3):15-23. [PubMed] [Google Scholar]
Houben 2019 {published data only}
- Houben CHM, Spruit MA, Luyten H, Pennings H-J, den Boogaart VEM, Creemers JPHM, et al. Cluster-randomised trial of a nurse-led advance care planning session in patients with COPD and their loved ones. Thorax 2019;74:328-36. [DOI] [PubMed] [Google Scholar]
- Houben CHM, Spruit MA, Wouters EFM, Janssen DJA. A randomised controlled trial on the efficacy of advance care planning on the quality of end-of-life care and communication in patients with COPD: the research protocol. BMJ Open 2014;4:e004465. [DOI: 10.1136/bmjopen-2013-004465] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hudson 2018 {published data only}
- Hudson P, Thomas K, Girgis A, Mitchell G, Philip J, Parker D, et al. Benefits of family meetings for hospitalized palliative care patients and their family caregivers. Journal of Pain and Symptom Management 2018;56(6):e56-e7. [Google Scholar]
Hudson 2021 {published data only}
- Hudson P, Girgis A, Thomas K, Philip J, Currow DC, Mitchell G, et al. Do family meetings for hospitalised palliative care patients improve outcomes and reduce health care costs? A cluster randomised trial. Palliative Medicine 2021;35(1):188-99. [DOI: 10.1177/0269216320967282] [DOI] [PubMed] [Google Scholar]
ISRCTN36040085 {unpublished data only}ISRCTN36040085
- ISRCTN36040085. ImproveCare - The management of clinical uncertainty in hospital settings. www.isrctn.com/ISRCTN36040085 (first received 18 January 2017).
Janssen 2011a {published data only}
- Janssen DJ, Curtis JR, Au DH, Spruit MA, Downey L, Schols JM, et al. Patient-clinician communication about end-of-life care for Dutch and US patients with COPD. European Respiratory Journal 2011;38(2):268-76. [DOI] [PubMed] [Google Scholar]
Janssen 2011b {published data only}
- Janssen DJ, Curtis JR, Au DH, Spruit MA, Downey L, Schols JM, et al. Patient-clinician communication about end-of-life care for patients with COPD in the Netherlands and the US. American Journal of Respiratory and Critical Care Medicine 2011;183(1):Meeting Abstracts. [DOI] [PubMed] [Google Scholar]
Johnson 2016 {published data only}
- Johnson S, Clayton J, Butow PN, Silvester W, Detering K, Hall J, et al. Advance care planning in patients with incurable cancer: study protocol for a randomised controlled trial. BMJ Open 2016;6:e012387. [DOI: 10.1136/bmjopen-2016-012387] [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnson 2016a {published data only}
- Johnson S, Vaccaro L, Butow PN, Kiely BE, Silvester W, Detering K, et al. Advance care planning increases communication between cancer patients, their oncologists and their family: an RCT. Asia-Pacific Journal of Clinical Oncology 2016;12(Supplement 5):93. [ACTRN12613001288718] [Google Scholar]
Jones 2004 {published data only}
- Jones T. Ethics consultations reduced hospital, ICU, and ventilation days in patients who died before hospital discharge in the ICU. Evidence Based Nursing 2004;7:53-53. [DOI] [PubMed] [Google Scholar]
Jones 2011 {published data only}
- Jones L, Harrington J, Barlow CA, Tookman A, Drake R, Barnes K, et al. Advance care planning in advanced cancer: can it be achieved? An exploratory randomized patient preference trial of a care planning discussion. Palliative & Supportive Care 2011;9(1):3-13. [DOI: 10.1017/S1478951510000490] [DOI] [PubMed] [Google Scholar]
Kirchhoff 2012 {published data only}
- Kirchhoff KT, Hammes BJ, Kehl KA, Briggs LA, Brown RL. Effect of a disease-specific advance care planning intervention on end-of-life care. Journal of the American Geriatrics Society 2012;60(5):946-50. [DOI: 10.1111/j.1532-5415.2012.03917.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Knauft 2005 {published data only}
- Knauft E, Nielsen EL, Engelberg RA, Patrick DL, Curtis JR. Barriers and facilitators to end-of-life care communication for patients with COPD. Chest 2005;127(6):2188-96. [DOI] [PubMed] [Google Scholar]
Knaus 1995 {published data only}
- KnausWA, Connors AF, Dawson NV. The SUPPORT Principal Investigators. A controlled trial to improve care for seriously ill hospitalized patients: the study to understand prognoses and preferences for outcomes and risks of treatments (SUPPORT). JAMA 1995;274(20):1591-8. [PubMed] [Google Scholar]
Kruse 2013 {published data only}
- Kruse RL, Parker Oliver D, Wittenberg-Lyles E, Demiris G. Conducting the ACTIVE randomized trial in hospice care: keys to success. Clinical Trials 2013;10(1):160-9. [DOI: 10.1177/1740774512461858] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lakin 2017 {published data only}
- Lakin JR, Koritsansky LA, Cunningham R, Maloney FL, Neal BJ, Paladino J, et al. A systematic intervention to improve serious illness communication in primary care. Health Affairs 2017;36:1264-9. [DOI] [PubMed] [Google Scholar]
Lee 2015 {published data only}
- Lee BC, Kimmel AL, Lyon ME. Who will speak for me? Identifying decision-makers in family-centered palliative care research. Journal of Adolescent Health 2015;1:S52-S53. [DOI: ] [Google Scholar]
Lee Brittney 2017 {published data only}
- Lee Brittney C, Houston PE, Rana SR, Kimmel AL, D'Angelo LJ, Lyon ME. Who will speak for me? Disparities in palliative care research with ''unbefriended'' adolescents living with HIV/AIDS. Journal of Palliative Medicine 2017;20:1135-8. [DOI: ] [Google Scholar]
Lincoln 2020 {published data only}
- Lincoln TE, Shields A, Petty K, Campbell T, Bellon J, Buddadhumaruk P, et al. Physicians' and family members' perception of a family support intervention in intensive care units. American Journal of Respiratory and Critical Care Medicine 2020;201:A2811. [URL: www.atsjournals.org/doi/abs/10.1164/ajrccm-conference.2020.201.1_MeetingAbstracts.A2811] [Google Scholar]
Lincoln 2020b {published data only}
- Lincoln T, Shields AM, Buddadhumaruk P, Chang CCh, Pike F, Chen H, et al. Protocol for a randomised trial of an interprofessional team-delivered intervention to support surrogate decision-makers in ICUs. BMJ Open 2020;10(3):e033521. [DOI: 10.1136/bmjopen-2019-033521] [DOI] [PMC free article] [PubMed] [Google Scholar]
Loh 2020 {published data only}
- Loh KP, Xu H, Epstein RM, Mohile SG, Prigerson HG, Plumb S, et al. Associations of caregiver-oncologist discordance in prognostic understanding with caregiver-reported therapeutic alliance and anxiety. Journal of Pain and Symptom Management 2020;60(1):20-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Long 2013 {published data only}
- Long AC, Engelberg RA, Downey L, Ford DW, Back AL, Kross EK, et al. Race, income, and education: associations with patient and family ratings of end-of-life care communication provided by physicians-in-training. American Journal of Respiratory and Critical Care Medicine 2013;187:Meeting Abstracts. [DOI] [PMC free article] [PubMed] [Google Scholar]
Long 2014 {published data only}
- Long AC, Engelberg RA, Downey L, Kross EK, Reinke LF, Cecere Feemster L, et al. Race, income, and education: associations with patient and family ratings of end-of-life care and communication provided by physicians-in-training. Journal of Palliative Medicine 2014;17(4):435-47. [DOI: 10.1089/jpm.2013.0214] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lyon 2009 {published data only}
- Lyon ME, Garvie PA, McCarter R, Briggs L, He J, D'Angelo LJ. Who will speak for me? Improving end-of-life decision-making for adolescents with HIV and their families. Pediatrics 2009;123(2):e199-206. [DOI: 10.1542/peds.2008-2379] [DOI] [PubMed] [Google Scholar]
Lyon 2009a {published data only}
- Lyon ME, Garvie PA, Briggs L, He J, McCarter R, D'Angelo LJ. Development, feasibility, and acceptability of the Family/Adolescent-Centered (FACE) advance care planning intervention for adolescents with HIV. Journal of Palliative Medicine 2009;12:363-72. [DOI: 10.1089/jpm.2008.0261] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lyon 2013 {published data only}
- Lyon ME. Family centered advance care planning for teens. Journal of Palliative Medicine 2013;16(4):A7-A8. [DOI: ] [Google Scholar]
Lyon 2013a {published data only}
- Lyon ME, Jacobs S, Briggs L, Cheng YI, Wang J. Family-centered advance care planning for teens with cancer. JAMA Pediatrics 2013;167(5):460-7. [DOI: 10.1001/jamapediatrics.2013.943] [DOI] [PubMed] [Google Scholar]
Lyon 2014 {published data only}
- Lyon ME, Jacobs S, Briggs L, Cheng YI, Wang J. A longitudinal, randomized, controlled trial of advance care planning for teens with cancer: anxiety, depression, quality of life, advance directives, spirituality. Journal of Adolescent Health 2014;54(6):710-7. [DOI: 10.1016/j.jadohealth.2013.10.206] [DOI] [PubMed] [Google Scholar]
Lyon 2017 {published data only}
- Lyon ME, D'Angelo LJ, Dallas RH, Hinds PS, Garvie PA, Wilkins ML, et al. A randomized clinical trial of adolescents with HIV/AIDS: pediatric advance care planning. AIDS Care 2017;29(10):1287-96. [DOI: 10.1080/09540121.2017.1308463] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lyon 2020 {published data only}
- Lyon M, Friebert S, Thompkins J, Baker J, Needle J, Cheng Y, et al. Let's talk: quality of facilitator communication as a determinant of satisfaction with family centered pediatric advance care planning for teens with cancer (FACE-TC PACP). Pediatric Blood & Cancer 2020;67 Supplement 4:e28742. [DOI: 10.1002/pbc.28742] [DOI] [Google Scholar]
Maciasz 2013 {published data only}
- Maciasz RM, Arnold RM, Chu E, Park SY, White DB, Vater LB, et al. Does it matter what you call it? A randomized trial of language used to describe palliative care services. Supportive Care in Cancer 2013;21:3411-9. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Maciasz 2013a {published data only}
- Maciasz R, Arnold R, Chu E, Park SY, Borgenheimer L, Schenker Y. Does it matter what you call It? a randomized trial of language used to describe palliative care services (TH340-A). Journal of Pain and Symptom Management 2013;45(2):359-60. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mah 2020 {published data only}
- Mah K, Shapiro GK, Hales S, Rydall A, Malfitano C, An E, et al. The impact of attachment security on death preparation in advanced cancer: the role of couple communication. Psycho-oncology 2020;29(5):833-40. [DOI] [PubMed] [Google Scholar]
Malhotra 2019 {published data only}
- Malhotra C, Rajasekaran T, Kanesvaran R, Yee A, Bundoc FG, Singh R, et al . Pilot trial of a combined oncologist-patient-caregiver communication intervention in Singapore. JCO Oncology Practice 2020 ;16(2):e190-e200. [DOI] [PubMed] [Google Scholar]
Marbella 1998 {published data only}
- Marbella AM, Desbiens NA, Mueller-Rizner N, Layde PM. Surrogates' agreement with patients' resuscitation preferences: effect of age, relationship, and SUPPORT intervention. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatment. Journal of Critical Care 1998;13(3):140-5. [DOI] [PubMed] [Google Scholar]
Martin 2020 {published data only}
- Martin M. Breaking bad news: providing communication guidance to doctor of nursing practice (DNP) students. Dissertation Abstracts International Section A: Humanities and Social Sciences 2020;81(3-A):No pagination specified. [Google Scholar]
Martinsson 2016 {published data only}
- Martinsson L, Heedman PA, Eriksson M, Tavelin B, Axelsson B. Increasing the number of patients receiving information about transition to end-of-life care: the effect of a half-day physician and nurse training. BMJ Supportive & Palliative Care 2016;6:452-8. [DOI: ] [DOI] [PubMed] [Google Scholar]
Matthys 2021 {published data only}
- Matthys O, De Vleminck A, Dierickx S, Deliens L, Vangoethem V, Hudson P, et al. Psychosocial and educational interventions for people with advanced cancer and their informal caregivers (diadic): protocol for a phase iii randomized controlled trial. Palliative Care and Social Practice 2021;15:28. [Google Scholar]
McFarlin 2011 {published data only}
- McFarlin J, Zomorodi M, Laskowitz D, Galanos A. Communication, healthcare attitudes, and the family conference (chat). Neurocritical Care 2011;15(1 Supplement 1):S176. [DOI: 10.1007/s12028-011-9625-5] [DOI] [Google Scholar]
Mehnert 2017 {published data only}
- Mehnert A, Koranyi S, Scheffold K, Philipp R, Engelmann D, Quintero-Garzon L, et al. Frequency and course of psychological distress among patients with advanced cancer: preliminary efficacy of the German CALM trial. Psycho-oncology 2017;26(Supplement 3):64. [DOI: ] [Google Scholar]
Meier 2004 {published data only}
- Meier DE, Thar W, Jordan A, Goldhirsch SL, Siu A, Morrison RS. Integrating case management and palliative care. Journal of Palliative Medicine 2004;7(1):119-34. [DOI: 10.1089/109662104322737395] [DOI] [PubMed] [Google Scholar]
Menon 2016 {published data only}
- Menon S, McCullough LB, Beyth RJ, Ford ME, Espadas D, Braun UK. Use of a values inventory as a discussion aid about end-of-life care: a pilot randomized controlled trial. Palliative & Supportive Care 2016;14(4):330-40. [DOI: 10.1017/S1478951515001091] [DOI] [PubMed] [Google Scholar]
Murray 2008 {published data only}
- Murray MA, O'Connor A, Stacey D, Wilson KG. Efficacy of a training intervention on the quality of practitioners' decision support for patients deciding about place of care at the end of life: a randomized control trial: study protocol. BMC Palliative Care 2008;7:4. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Murray 2010 {published data only}
- Murray MA, Stacey D, Wilson KG, O'Connor AM. Skills training to support patients considering place of end-of-life care: a randomized control trial. Journal of Palliative Care 2010;26(2):112-21. [PubMed] [Google Scholar]
NCT00325611 {unpublished data only}
- NCT00325611. Multidisciplinary inpatient palliative care intervention [A multi-site replication of an inpatient palliative care program]. clinicaltrials.gov/ct2/show/NCT00325611 (first received 15 May 2006). [NCT00325611]
NCT00374010 {unpublished data only}
- NCT00374010. Improving patient-clinician communication about end-of-life care. clinicaltrials.gov/show/nct00374010 (first received 8 September 2006).
NCT00580515 {unpublished data only}
- NCT00580515. Trial of family focused grief therapy in palliative care and bereavement. clinicaltrials.gov/show/nct00580515 (first received 24 December 2007).
NCT01160367 {unpublished data only}
- NCT01160367. Trial of ascertaining individual preferences for loved one's role in end-of-life. clinicaltrials.gov/show/nct01160367 (first received 12 July 2010).
NCT01245621 {unpublished data only}
- NCT01245621. Study of palliative care intervention for advanced cancer patients and their caregivers - Educate Nurture Advise Before Life Ends (ENABLE III). clinicaltrials.gov/ct2/show/NCT01245621 (first received 22 November 2010). [NCT01245621]
NCT01289444 {published data only}
- NCT01289444. Longitudinal pediatric palliative care: quality of life & spiritual struggle (FACE). clinicaltrials.gov/ct2/show/NCT01289444 (first received 3 February 2011). [NCT01289444]
NCT01670461 {unpublished data only}
- NCT01670461. FAmily CEntered (FACE) advance care planning for teens with cancer. clinicaltrials.gov/show/nct01670461 (first received 22 August 2012).
NCT01828775 {unpublished data only}
- NCT01828775. Palliative care intervention in improving quality of life, psychological distress, and communication in patients with solid tumors receiving treatment. clinicaltrials.gov/show/nct01828775 (first received 11 April 2013).
NCT01914848 {unpublished data only}
- NCT01914848. Multiprofessional advance care planning and shared decision making for end of life care (MAPS). clinicaltrials.gov/show/nct01914848 (first received 2 August 2013).
NCT01944813 {unpublished data only}
- NCT01944813. Advance care planning: a way to improve end-of-life care life care. clinicaltrials.gov/show/nct01944813 (first received 18 September 2013).
NCT01990742 {unpublished data only}
- NCT01990742. Improving Palliative Care Through Teamwork (IMPACTT). clinicaltrials.gov/ct2/show/NCT01990742 (first received 21 November 2013). [NCT01990742]
NCT02112461 {unpublished data only}
- NCT02112461. Hospice and end-of-life symptom monitoring & support using an automated system designed for family caregivers (SCP). clinicaltrials.gov/show/nct02112461 (first received 14 April 2014).
NCT02261935 {unpublished data only}
- NCT02261935. Supporting family caregivers of palliative patients at home: the carer support needs assessment intervention (CSNAT). clinicaltrials.gov/ct2/show/NCT02261935 (first received 10 October 2014). [NCT02261935]
NCT02349412 {unpublished data only}
- NCT02349412. Early palliative care with standard care or standard care alone in improving quality of life of patients with incurable lung or non-colorectal gastrointestinal cancer and their family caregivers. clinicaltrials.gov/show/nct02349412 (first received 28 January 2015).
NCT02445937 {unpublished data only}
- NCT02445937. PARTNER II: improving patient and family centered care in advanced critical illness. clinicaltrials.gov/show/nct02445937 (first received 15 May 2015).
NCT02463162 {unpublished data only}
- NCT02463162. Trial of advance care planning (ACP) & goals of care designations (GCD) discussions. clinicaltrials.gov/show/nct02463162 (first received 4 June 2015).
NCT02606149 {unpublished data only}
- NCT02606149. Truthful information on chemotherapy and its impact on chemotherapy at the end of life (HIPPOCRATE). clinicaltrials.gov/ct2/show/NCT02606149 (first received 17 November 2015). [NCT02606149]
NCT02723799 {unpublished data only}
- NCT02723799. Hope Promotion Program: effectiveness in palliative patients (HPP). clinicaltrials.gov/ct2/show/NCT02723799 (first received 30 March 2016). [NCT02723799]
NCT02730858 {unpublished data only}
- NCT02730858. Palliative and oncology care model in breast cancer. clinicaltrials.gov/ct2/show/NCT02730858 (first received 7 April 2016).
NCT02917603 {unpublished data only}
- NCT02917603. Shared decision making to improve palliative care in the nursing home. clinicaltrials.gov/show/nct02917603 (first received 28 September 2016).
NCT02944344 {unpublished data only}
- NCT02944344. Four conversations RCT. clinicaltrials.gov/ct2/show/NCT02944344 (first received 25 October 2016).
NCT03068013 {unpublished data only}
- NCT03068013. Managing Cancer and Living Meaningfully (CALM) adapted to Italian cancer care setting (CALM-IT). clinicaltrials.gov/show/nct03068013 (first received 1 March 2017).
NCT03099746 {unpublished data only}
- NCT03099746. Decision support among surrogate decision makers of the chronically critically ill (INVOLVE) [A clinical trial of decision support for end of life care among surrogate decision makers of the chronically critically ill]. clinicaltrials.gov/ct2/show/NCT03099746 (first received 4 April 2017). [NCT03099746]
NCT03138564 {unpublished data only}
- NCT03138564. An effectiveness-implementation trial of SPIRIT in ESRD. clinicaltrials.gov/show/nct03138564 (first received 3 May 2017).
NCT03387436 {unpublished data only}
- NCT03387436. The "Hand-in-Hand Study": improvement of quality of life in palliative cancer patients through collaborative advance care planning (COLAP). clinicaltrials.gov/show/nct03387436 (first received 2 January 2018).
NCT03506087 {unpublished data only}
- NCT03506087. Advance care planning coaching for patients with chronic kidney disease (MY WAY). clinicaltrials.gov/show/nct03506087 (first received 23 April 2018).
NCT03548142 {unpublished data only}
- NCT03548142. Advanced dementia and end-of-life. clinicaltrials.gov/ct2/show/NCT03548142 (first received 7 June 2018).
NCT03626402 {unpublished data only}
- NCT03626402. End-of-life care for African Americans: an outpatient intervention. clinicaltrials.gov/ct2/show/record/NCT03626402 (first received 13 August 2018).
Nipp 2020 {published data only}
- Nipp RD, Temel B, Fuh CX, Kay P, Landay S, Lage D, et al. Pilot randomized trial of a transdisciplinary geriatric and palliative care intervention for older adults with cancer. Journal of the National Comprehensive Cancer Network 2020;18(5):591-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nishioka 2019 {published data only}
- Nishioka M, Okuyama T, Uchida M, Aiki S, Ito Y, Osaga S, et al. What is the appropriate communication style for family members confronting difficult surrogate decision-making in palliative care?: a randomized video vignette study in medical staff with working experiences of clinical oncology. Japanese Journal of Clinical Oncology 2019;49(1):48-56. [DOI] [PubMed] [Google Scholar]
Norton 2019 {published data only}
- Norton SA, Wittink MN, Duberstein PR, Prigerson HG, Stanek S, Epstein RM. Family caregiver descriptions of stopping chemotherapy and end-of-life transitions. Supportive Care in Cancer 2019;27(2):669-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Donnell 2016 {published data only}
- O'Donnell AE, Schaefer KG, Stevenson LW, Mehra MR, Desai AS. A randomized controlled trial of a social worker-aided palliative care intervention in high risk patients with heart failure (SWAP-HF). Journal of Cardiac Failure 2016;22(11):940. [Google Scholar]
O'Donnell 2018 {published data only}
- O'Donnell AE, Schaefer KG, Stevenson LW, DeVoe K, Walsh K, Mehra MR, et al. Social worker-aided palliative care intervention in high-risk patients with heart failure (SWAP-HF): a pilot randomized clinical trial. JAMA Cardiology 2018;3:516-9. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Oliver 2001 {published data only}
- Oliver JW, Kravitz RL, Kaplan SH, Meyers FJ. Individualized patient education and coaching to improve pain control among cancer outpatients. Journal of Clinical Oncology 2001;19:2206-12. [DOI] [PubMed] [Google Scholar]
Oliver 2012 {published data only}
- Oliver DP, Demiris G, Wittenberg-Lyles E. The use of video technology for caregiver involvement in interdisciplinary hospice teams: preliminary experiences from the active randomized clinical trial. Journal of Pain and Symptom Management 2012;43(2):331. [DOI: ] [Google Scholar]
Paladino 2014 {published data only}
- Paladino J, Bernacki R, Hutchings M, Billings JA, Block S. Facilitators and barriers to advance care planning in oncology. Journal of Clinical Oncology 2014;32(31 Supplement):84-84. [DOI: 10.1200/jco.2014.32.31_suppl.84] [DOI] [Google Scholar]
Paladino 2015 {published data only}
- Paladino J, Bernacki R, Billings J, Block S. Triggering advance care planning conversations in oncology and identifying barriers to these discussions. Journal of Pain and Symptom Management 2015;49(2):445-6. [Google Scholar]
Paladino 2016 {published data only}
- Paladino J, Lamas D, Lakin J, Epstein S, Bernacki R. Delivering more, better and earlier goals of care conversations to seriously ill oncology patients in the clinical setting. Journal of Pain and Symptom Management 2016;51(2):380-1. [Google Scholar]
Parker 2017 {published data only}
- Parker O, Demiris G, Washington K, Kruse RL, Petroski G. Hospice family caregiver involvement in care plan meetings: a mixed-methods randomized controlled trial. American Journal of Hospice & Palliative Care 2017;34:849-59. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Perry 2005 {published data only}
- Perry E, Swartz J, Brown S, Smith D, Kelly G, Swartz R. Peer mentoring: a culturally sensitive approach to end-of-life planning for long-term dialysis patients. American Journal of Kidney Diseases 2005;46(1):111-9. [DOI] [PubMed] [Google Scholar]
Pintova 2020 {published data only}
- Pintova S, Leibrandt R, Smith CB, Adelson KB, Gonsky J, Egorova N, et al. Conducting goals-of-care discussions takes less time than imagined. JCO Oncology Practice 2020;16(12):E1499-E506. [DOI] [PubMed] [Google Scholar]
Pirl 2019 {published data only}
- Pirl WF, Lerner J, Traeger L, Greer JA, El-Jawahri A, Temel JS. Oncologists' dispositional affect and likelihood of end-of-life discussions. Journal of Clinical Oncology 2019;34(26 Supplement 1):9. [Google Scholar]
Pollak 2019 {published data only}
- Pollak KI, Gao X, Beliveau J, Griffith B, Kennedy D, Casarett D. Pilot study to improve goals of care conversations among hospitalists. Journal of Pain and Symptom Management 2019;58(5):864-70. [DOI] [PubMed] [Google Scholar]
Pollak 2020 {published data only}
- Pollak KI, Gao XM, Arnold RM, Arnett K, Felton S, Fairclough DL, et al. Feasibility of using communication coaching to teach palliative care clinicians motivational interviewing. Journal of Pain and Symptom Management 2020;59(4):787-93. [DOI] [PubMed] [Google Scholar]
Porensky 2011 {published data only}
- Porensky E. Breaking bad news: effect of physician communication on analog patients' response. Dissertation Abstracts International: Section B: Sciences and Engineering 2011;71:4492. [Google Scholar]
Ramos 2013 {published data only}
- Ramos KJ, Engelberg RA, Downey L, Treece PD, Nielsen EL, Ganz FD, et al. Nurse ratings of the quality of dying in the ICU: associations with nurse reports of physician-nurse and physician-family communication. American Journal of Respiratory and Critical Care Medicine 2013;187:A4960. [Google Scholar]
Reinhardt 2015 {published data only}
- Reinhardt JP, Boerner K, Downes D. The positive association of end-of-life treatment discussions and care satisfaction in the nursing home. Journal of Social Work in End-of-Life & Palliative Care 2015;11:307-22. [DOI] [PubMed] [Google Scholar]
Reinhardt 2017 {published data only}
- Reinhardt JP, Downes D, Cimarolli V, Bomba P. End-of-life conversations and hospice placement: association with less aggressive care desired in the nursing home. Journal of Social Work in End-of-Life & Palliative Care 2017;13(1):61-81. [DOI: 10.1080/15524256.2017.1282919] [DOI] [PubMed] [Google Scholar]
Reinke 2011 {published data only}
- Reinke LF, Slatore CG, Uman J, Udris EM, Moss BR, Engelberg RA, et al. Patient-clinician communication about end-of-life care topics: is anyone talking to patients with chronic obstructive pulmonary disease? Journal of Palliative Medicine 2011;14(8):923-8. [DOI: 10.1089/jpm.2010.0509] [DOI] [PubMed] [Google Scholar]
Reuther 2014 {published data only}
- Reuther S, Holle D, Buscher I, Dortmann O, Muller R, Bartholomeyczik S, et al. Effect evaluation of two types of dementia-specific case conferences in German nursing homes (FallDem) using a stepped wedge design: study protocol for a randomized controlled trial. Trials 2014;15:319. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rousseau 2016 {published data only}
- Rousseau PC. Curtis RJ, Treece PD, Nielsen EL, et al. Randomized trial of communication facilitators to reduce family distress and intensity of end-of-life care. Am J Respir Crit Care Med 2015;193:154-162. Journal of Palliative Medicine 2016;19(4):470-1. [DOI: 10.1089/jpm.2016.0039] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ruiz 2016 {published data only}
- Ruiz G, Holder RM, Graham N, Groninger H, Walker KA. Case study of initial patients with left-ventricular assist devices (LVADS) enrolled into palliative tele health connecting hospital to home (PATCH2) program. Journal of Cardiac Failure 2016;22(8, Supplement):S135. [DOI: ] [Google Scholar]
Russell 2016 {published data only}
- Russell J. The role of health care provider goals, plans, and physician orders for life-sustaining treatment (POLST) in preparing for conversations about end-of-life care. Journal of Health Communication 2016;21(9):1023-30. [DOI: 10.1080/10810730.2016.1204380] [DOI] [PubMed] [Google Scholar]
Saeed 2018 {published data only}
- Saeed F, Hoerger M, Norton SA, Guancial E, Epstein RM, Duberstein PR. Preference for palliative care in cancer patients: are men and women alike? Journal of Pain and Symptom Management 2018;56(1):1-6.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sanchez 2018 {published data only}
- Sanchez R, Mateo KF. Survey-based priming intervention linked to improved communication with the seriously ill. Journal of Clinical Outcomes Management 2018;25(7):300-3. [Google Scholar]
Schneiderman 2003 {published data only}
- Schneiderman LJ, Gilmer T, Teetzel HD, Dugan DO, Blustein J, Cranford R, et al. Effect of ethics consultations on nonbeneficial life-sustaining treatments in the intensive care setting: a randomized controlled trial. JAMA 2003;290:1166-72. [DOI] [PubMed] [Google Scholar]
Smith 2017 {unpublished data only}
- Smith C. The Feasibility of Research in Cancer Patients Close to Death and the Impact on Evaluating a Complex Intervention. EThos 2017. [Imperial College London, PhD thesis]
Smucker 1993 {published data only}
- Smucker WD, Ditto PH, Moore KA, Druley JA, Danks JH, Townsend A. Elderly outpatients respond favorably to a physician-initiated advance directive discussion. Journal of the American Board of Family Practice 1993;6(5):473-82. [PubMed] [Google Scholar]
Song 2009 {published data only}
- Song MK, Ward SE, Happ MB, Piraino B, Donovan HS, Shields AM, et al. Randomized controlled trial of SPIRIT: an effective approach to preparing African-American dialysis patients and families for end of life. Research in Nursing & Health 2009;32(3):260-73. [DOI: 10.1002/nur.20320] [DOI] [PMC free article] [PubMed] [Google Scholar]
Song 2016 {published data only}
- Song MK, Ward SE, Lin FC, Hamilton JB, Hanson LC, Hladik GA, et al. Racial differences in outcomes of an advance care planning intervention for dialysis patients and their surrogates. Journal of Palliative Medicine 2016;19:134-42. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Song 2018 {published data only}
- Song MK, Unruh ML, Manatunga A, Plantinga LC, Lea J, Jhamb M, et al. SPIRIT trial: a phase III pragmatic trial of an advance care planning intervention in ESRD. Contemporary Clinical Trials 2018;64:188-94. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Starks 2016 {published data only}
- Starks H, Doorenbos A, Lindhorst T, Bourget E, Aisenberg E, Oman N, et al. The Family Communication Study: a randomized trial of prospective pediatric palliative care consultation, study methodology and perceptions of participation burden. Contemporary Clinical Trials 2016;49:15-20. [DOI: 10.1016/j.cct.2016.05.004] [DOI] [PMC free article] [PubMed] [Google Scholar]
Steinhauser 2008 {published data only}
- Steinhauser KE, Alexander SC, Byock IR, George LK, Olsen MK, Tulsky JA. Do preparation and life completion discussions improve functioning and quality of life in seriously ill patients? Pilot randomized control trial. Journal of Palliative Medicine 2008;11:1234-40. [DOI: ] [DOI] [PubMed] [Google Scholar]
Street 2010 {published data only}
- Street RL, Slee C, Kalauokalani DK, Dean DE, Tancredi DJ, Kravitz RL. Improving physician-patient communication about cancer pain with a tailored education-coaching intervention. Patient Education and Counseling 2010;80(1):42-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sudore 2010 {published data only}
- Sudore RL, Fried TR. Redefining the “planning” in advance care planning: preparing for end-of-life decision making. Annals of Internal Medicine 2010;153(4):256-61. [DOI: 10.7326/0003-4819-153-4-201008170-00008] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sulmasy 2017 {published data only}
- Sulmasy DP, Hughes MT, Yenokyan G, Kub J, Terry PB, Astrow AB, et al. The Trial of Ascertaining Individual Preferences for Loved Ones' Role in End-of-Life Decisions (TAILORED) study: a randomized controlled trial to improve surrogate decision making. Journal of Pain and Symptom Management 2017;54:455-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Szmuilowicz 2010 {published data only}
- Szmuilowicz E, el-Jawahri A, Chiappetta L, Kamdar M, Block S. Improving residents' end-of-life communication skills with a short retreat: a randomized controlled trial. Journal of Palliative Medicine 2010;13(4):439-52. [DOI: 10.1089/jpm.2009.0262] [DOI] [PubMed] [Google Scholar]
Temel 2017 {published data only}
- Temel JS, Greer JA, El-Jawahri A, Pirl WF, Park ER, Jackson VA, et al. Effects of early integrated palliative care in patients with lung and GI cancer: a randomized clinical trial. Journal of Clinical Oncology 2017;35(8):834-41. [DOI: 10.1200/JCO.2016.70.5046] [DOI] [PMC free article] [PubMed] [Google Scholar]
Temel 2020 {published data only}
- Temel JS, Moy B, El-Jawahri A, Jackson VA, Kamdar M, Jacobsen J, et al. Randomized trial of a collaborative palliative and oncology care intervention to improve communication about end-of-life care in patients with metastatic breast cancer. Journal of Clinical Oncology 2020;38(15 Supplement):1008-1008. [DOI: 10.1200/JCO.2020.38.15-suppl.1008] [DOI] [Google Scholar]
Tierney 2001 {published data only}
- Tierney WM, Dexter PR, Gramelspacher GP, Perkins AJ, Zhou XH, Wolinsky FD. The effect of discussions about advance directives on patients' satisfaction with primary care. Journal of General Internal Medicine 2001;16(1):32-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Toles 2018 {published data only}
- Toles M, Song MK, Lin FC, Hanson LC. Perceptions of family decision-makers of nursing home residents with advanced dementia regarding the quality of communication around end-of-life care. Journal of the American Medical Directors Association 2018;19(10):879-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Trevino 2019 {published data only}
- Trevino KM, Maciejewski PK, Johnson Shen M, Prigerson HG, Mohile S, Kamen C, et al. How much time is left? Associations between estimations of patient life-expectancy and quality of life in patients and caregivers. Supportive Care in Cancer 2019;27(7):2487–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tulsky 2011 {published data only}
- Tulsky JA, Arnold RM, Alexander SC, Olsen MK, Jeffreys AS, Rodriguez KL, et al. Communication between oncologists and patients with a computer-based training program: a randomized trial. Annals of Internal Medicine 2011;155(9):593-601. [DOI: 10.7326/0003-4819-155-9-201111010-00007] [DOI] [PMC free article] [PubMed] [Google Scholar]
Uitdehaag 2012 {published data only}
- Uitdehaag MJ, Velden LA, Boer MF, Spaander MC, Steyerberg EW, Kuipers EJ, et al. Recordings of consultations are beneficial in the transition from curative to palliative cancer care: a pilot-study in patients with oesophageal or head and neck cancer. European Journal of Oncology Nursing 2012;16(2):109-14. [DOI: 10.1016/j.ejon.2011.04.006] [DOI] [PubMed] [Google Scholar]
Vaccaro 2016 {published data only}
- Vaccaro LD, Johnson S, Kiely BE, Clayton J, Clarke S, Beale P, et al. Acceptability of tailored life-expectancy information in patients with advanced cancer participating in an Australian nurse-led ACP RCR. Asia Pacific Journal of Clinical Oncology 2016;12:108. [DOI: 10.13140/RG.2.2.21221.81126] [DOI] [Google Scholar]
Van Laarhoven 2018 {published data only}
- Van Laarhoven HWM, Henselmans I, Van Maarschalkerweerd P, De Haes H, Sommeijer DW, Ottevanger PB, et al. Training oncologists and preparing patients for shared decision making about palliative systemic treatment: results from the randomized controlled CHOICE study. Annals of Oncology 2018;29:viii548. [Google Scholar]
Verhofstede 2012 {published data only}
- Verhofstede R, Smets T, Cohen J, Noortgate N, Heide A, Deliens L. Study protocol for a cluster randomized trial to evaluate the influence of the Liverpool care pathway on end-of-life care in acute geriatric hospital wards in Flanders. Palliative Medicine 2012;26(4):548-9. [DOI: 10.1177/0269216312446391] [DOI] [Google Scholar]
Verreault 2018 {published data only}
- Verreault R, Arcand M, Misson L, Durand P, Kroger E, Aubin M, et al. Quasi-experimental evaluation of a multifaceted intervention to improve quality of end-of-life care and quality of dying for patients with advanced dementia in long-term care institutions. Palliative Medicine 2018;32:613-21. [DOI: 10.1177/0269216317719588] [DOI] [PubMed] [Google Scholar]
Volandes 2009 {published data only}
- Volandes AE, Mitchell SL, Gillick MR, Chang Y, Paasche-Orlow MK. Using video images to improve the accuracy of surrogate decision-making: a randomized controlled trial. Journal of the American Medical Directors Association 2009;10:575-80. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Volandes 2013 {published data only}
- Volandes AE, Paasche-Orlow MK, Mitchell SL, El-Jawahri A, Davis AD, Barry MJ, et al. Randomized controlled trial of a video decision support tool for cardiopulmonary resuscitation decision making in advanced cancer. Journal of Clinical Oncology 2013;31(3):380-6. [DOI: 10.1200/JCO.2012.43.9570] [DOI] [PMC free article] [PubMed] [Google Scholar]
Von Blanckenburg {published data only}
- Von Blanckenburg P, Leppin N, Nagelschmidt K, Seifart C, Rief W. Matters of life and death: an experimental study investigating psychological interventions to encourage the readiness for end-of-life conversations . Psychotherapy and Psychosomatics 2021;90(4):243-54. [DOI: 10.1159/000511199] [DOI] [PubMed] [Google Scholar]
von Heymann‐Horan 2019 {published data only}
- Heymann-Horan A, Bidstrup PE, Johansen C, Rottmann N, Andersen EAW, Sjøgren P, et al. Dyadic coping in specialized palliative care intervention for patients with advanced cancer and their caregivers: effects and mediation in a randomized controlled trial. Psycho-oncology 2019;28(2):264-70. [DOI] [PubMed] [Google Scholar]
Voruganti 2017 {published data only}
- Voruganti T, Grunfeld E, Jamieson T, Kurahashi AM, Lokuge B, Krzyzanowska MK, et al. My team of care study: a pilot randomized controlled trial of a web-based communication tool for collaborative care in patients with advanced cancer. Journal of Medical Internet Research 2017;19(7):e219. [DOI: 10.2196/jmir.7421] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walker 2017 {published data only}
- Walker KA, Graham N, Brennan D, Groninger H, Holder RM, Malotte K, et al. PATCH2 program: the creation of a virtual palliative care clinic. Journal of Palliative Medicine 2017;20(4):A20-A21. [DOI: ] [Google Scholar]
Walker 2017a {published data only}
- Walker KA, Graham N, Brennan D, Groninger H, Holder RM, Malotte K, et al. PATCH2 program: the creation of a virtual palliative care clinic. In: Journal of Palliative Medicine. Abstracts from the Center to Advance Palliative Care National Seminar Practical Tools for Making Change; 2016 Oct 26–29; Orlando (Florida). Vol. 20(4). 2017:A20-A21. [DOI: 10.1089/jpm.2017.0051] [DOI] [PubMed]
Wallen 2012 {published data only}
- Wallen GR, Baker K, Stolar M, Miller-Davis C, Ames N, Yates J, et al. Palliative care outcomes in surgical oncology patients with advanced malignancies: a mixed methods approach. Quality of Life Research 2012;21(3):405-15. [DOI: 10.1007/s11136-011-0065-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Waller 2016 {published data only}
- Waller A, Proietto A, Ackland S, Clark K, Sanson-Fisher R, Mackenzie L. How can we enhance patient-centered communication at the end of life? Proof-of-concept RCT. Asia-Pacific Journal of Clinical Oncology 2016;12(Supplement 6):26. [Google Scholar]
Walshe 2016 {published data only}
- Walshe C, Preston N, Payne S, Algorta GP, Hill M, Ockenden N. "To wait or not to wait": lessons from running a wait list controlled trial (ELSA) of a volunteer befriending service at the end of life within NHS, hospice and voluntary sectors. Palliative Medicine 2016;30(4):S48-S49. [DOI: ] [Google Scholar]
Walshe 2016a {published data only}
- Walshe C, Dodd S, Hill M, Ockenden N, Payne S, Perez Algorta G, et al. Working with non-clinical staff to deliver research. Lessons from running a wait-list controlled trial (ELSA) of a volunteer befriending service towards the end of life. Palliative Medicine. EAPC2016: Abstracts 2016;30(6):NP283-NP284. [DOI: 10.1177/0269216316646056] [DOI] [Google Scholar]
Wanta Barbara 1998 {published data only}
- Wanta Barbara A. The relationship between family communication about death and dying, in terms of Bowen's family systems theory, and physicians' knowledge of preference for cardiopulmonary resuscitation. Dissertation Abstracts International: Section B: Sciences and Engineering 1998;59:1043. [Google Scholar]
Wendlandt 2019 {published data only}
- Wendlandt B, Ceppe A, Choudhury S, Cox CE, Hanson LC, Danis M, et al. Modifiable elements of ICU supportive care and communication are associated with surrogates’ PTSD symptoms. Intensive Care Medicine 2019;45(5):619-26. [DOI: 10.1007/s00134-019-05550-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wentlandt 2012 {published data only}
- Wentlandt K, Burman D, Swami N, Hales S, Rydall A, Rodin G, et al. Preparation for the end of life in patients with advanced cancer and association with communication with professional caregivers. Psycho-oncology 2012;21(8):868-76. [DOI: 10.1002/pon.1995] [DOI] [PubMed] [Google Scholar]
Whisenant 2017 {published data only}
- Whisenant M, Donaldson G, Wilson A, Mooney K. Cancer caregiver perception of symptom severity change. Supportive Care in Cancer 2017;25(2 Supplement 1):S235. [DOI: ] [Google Scholar]
White 2018 {published data only}
- White DB, Angus DC, Shields A, Pidro C, Paner C, Buddadhumaruk P, et al. A stepped wedge randomized controlled trial of a pragmatic, nurse-led intervention to support surrogate decision makers in ICUs. American Journal of Respiratory and Critical Care Medicine 2018;197:A6186. [Google Scholar]
White 2018a {published data only}
- White DB, Angus DC, Shields AM, Buddadhumaruk P, Pidro C, Paner C, et al. A randomized trial of a family-support intervention in intensive care units. New England Journal of Medicine 2018;378:2365-75. [DOI: ] [DOI] [PubMed] [Google Scholar]
Wilkinson 2015 {published data only}
- Wilkinson AM, Johnson CE, Walker H, Colgan V, Arnet H, Rai T. Evaluating the Liverpool care pathway for care of the terminally ill in rural Australia. Supportive Care in Cancer 2015;23:3173-81. [DOI: ] [DOI] [PubMed] [Google Scholar]
Wilson 2013 {published data only}
- Wilson ME, Akhoundi A, Hinds R, Krupa A, Kashani K. Use of a video to improve patient and surrogate understanding of cardiopulmonary resuscitation and resuscitation preference options in the ICU: a randomized controlled trial. American Journal of Respiratory and Critical Care Medicine 2013;187:A4962. [DOI] [PubMed] [Google Scholar]
Wilson 2015 {published data only}
- Wilson ME, Krupa A, Hinds RF, Litell JM, Swetz KM, Akhoundi A, et al. A video to improve patient and surrogate understanding of cardiopulmonary resuscitation choices in the ICU: a randomized controlled trial. Critical Care Medicine 2015;43:621-9. [DOI: ] [DOI] [PubMed] [Google Scholar]
Wittenberg‐Lyles 2013 {published data only}
- Wittenberg-Lyles E, Goldsmith J, Oliver DP, Demiris G, Kruse RL, Van Stee S. Using medical words with family caregivers. Journal of Palliative Medicine 2013;16(9):1135-9. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wolfe 2014 {published data only}
- Wolfe J, Orellana L, Cook EF, Ullrich C, Kang T, Geyer JR, et al. Improving the care of children with advanced cancer by using an electronic patient-reported feedback intervention: results from the PediQUEST randomized controlled trial. Journal of Clinical Oncology 2014;32(11):1119-26. [DOI: 10.1200/JCO.2013.51.5981] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yilmaz 2020 {unpublished data only}
- Yilmaz S. An Examination of Terminally-ill Cancer Patients’ Relational Well-being and Peaceful Acceptance of the Illness [Doctoral dissertation]. Rochester (NY): University of Rochester, 2020. [Google Scholar]
Yun 2011 {published data only}
- Yun YH, Lee MK, Park S, Lee JL, Park J, Choi YS, et al. Use of a decision aid to help caregivers discuss terminal disease status with a family member with cancer: a randomized controlled trial. Journal of Clinical Oncology 2011;29(36):4811-9. [DOI: 10.1200/JCO.2011.35.3870] [DOI] [PubMed] [Google Scholar]
Zaider 2012 {published data only}
- Zaider T, Kissane D. Therapeutic pathways to improved family communication in palliative care. Asia-Pacific Journal of Clinical Oncology 2012;8:183-4. [DOI: 10.1111/ajco.12029] [DOI] [Google Scholar]
Zaider 2020 {published data only}
- Zaider TI, Kissane DW, Schofield E, Li Y, Masterson M. Cancer-related communication during sessions of family therapy at the end of life. Psycho-oncology 2020;29(2):373-80. [DOI: 10.1002/pon.5268] [DOI] [PubMed] [Google Scholar]
Zimmerman 2014 {published data only}
- Zimmermann C, Swami N, Krzyzanowska M, Hannon B, Leighly N, Oza A, et al. Early palliative care for patients with advanced cancer: a cluster-randomised controlled trial. Lancet 2014;383(9930):1721-30. [DOI: 10.1016/S0140-6736(13)62416-2] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Bouleuc 2021 {published data only}
- Bouleuc C, Savignoni A, Chevrier M, Renault-Tessier E, Burnod A, Chvetzoff G, et al. A question prompt list for advanced cancer patients promoting advance care planning: a French randomized trial. Journal of Pain and Symptom Management 2021;61(2):331-41. [DOI] [PubMed] [Google Scholar]
Krug 2021 {published data only}
- Krug K, Bossert J, Deis N, Krisam J, Villalobos M, Siegle A, et al. Effects of an interprofessional communication approach on support needs, quality of life, and mood of patients with advanced lung cancer: a randomized trial. Oncologist 2021;26(8):e1445-e1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle A, Villalobos M, Bossert J, Krug K, Hagelskamp L, Krisam J, et al. The Heidelberg Milestones Communication Approach (MCA) for patients with prognosis. Trials 2018;19(1):438. [DOI: 10.1186/s13063-018-2814-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Fujimori 2020 {published data only}
- Fujimori M, Sato A, Jinno S, Okusaka T, Yamaguchi T Ikeda M, et al. Integrated communication support program for oncologists, caregivers and patients with rapidly progressing advanced cancer to promote patient-centered communication: J-SUPPORT1904 study protocol for a randomised controlled trial. BMJ Open 2020;10:e036745. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT03770481 {unpublished data only}
- NCT03770481. Using a nurse-led communication strategy for surrogates in the intensive care unit. clinicaltrials.gov/ct2/show/NCT03770481 (first received 10 December 2018).
NL5388 {unpublished data only}
- NL5388. CHOICE: CHOosing treatment together In Cancer at the End of life. www.trialregister.nl/trial/5388 (first received 15 September 2015).
R000038002 {unpublished data only}
- R000038002. A randomized controlled trial of integrated empathic communication support program to promote end of life discussion among rapidly progressive cancer patient, caregiver and physician. center6.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000038002 (first received 2 August 2018).
Additional references
ACSQHC 2015
- Australian Commission on Safety and Quality in Health Care (ACSQHC). National consensus statement: essential elements for safe and high quality end-of-life-care; 2015. www.safetyandquality.gov.au/publications/national-consensus-statement-essential-elements-for-safe-high-quality-end-of-life-care/ (accessed prior to 24 July 2018).
ACSQHC 2015b
- Australian Commission on Safety and Quality in Health Care (ACQSHC). How should care be delivered at the end of life? A guide for patients and their families and carers; 2015. www.safetyandquality.gov.au/publications-and-resources/resource-library/how-should-care-be-delivered-end-life-information-patients-and-their-families-and-carers (accessed prior to 24 July 2018).
Agar 2017
- Agar M, Luckett T, Luscombe G, Phillips J, Beattie E, Pond D, et al. Effects of facilitated family case conferencing for advanced dementia: a cluster randomised clinical trial. PLoS One 2017;12(8):e0181020. [DOI: 10.1371/journal.pone.0181020] [DOI] [PMC free article] [PubMed] [Google Scholar]
AIHW 2014
- Australian Institute of Health and Welfare (AIHW). Palliative care services in Australia 2014. www.aihw.gov.au/getmedia/035598a4-5dfa-4469-98ab-e29abb7a4940/17634.pdf.aspx?inline=true (accessed prior to 24 July 2018).
Alsakson 2012
- Alsakson RA, Wyskiel R, Thornton I, Copley C, Shaffer D, Zyra M, et al. Nurse-perceived barriers to effective communication regarding prognosis and optimal end-of-life care for surgical ICU patients: a qualitative exploration. Journal of Palliative Medicine 2012;15(8):910-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
AMA 2014
- Australian Medical Association (AMA). AMA position statement on end of life care and advance care planning; 2014. ama.com.au/sites/default/files/documents/AMA_position_statement_on_end_of_life_care_and_advance_care_planning_2014.doc_1.pdf (accessed prior to 24 July 2018).
Anderson 2019
- Anderson RJ, Bloch S, Armstrong M, Stone PS, Low JTS. Communication between healthcare professionals and relatives of patients approaching the end-of-life: a systematic review of qualitative evidence. Palliative Medicine 2019;33(8):926-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anselm 2005
- Anselm AH, Palda V, Guest CB, McLean RF, Vachon MLS, Kelner M, et al. Barriers to communication regarding end-of-life care: perspectives of care providers. Journal of Critical Care 2005;20(3):214-33. [DOI] [PubMed] [Google Scholar]
Barnes 2006
- Barnes S, Gott M, Payne S, Seamark D, Parker C, Gariballa S, et al. Communication in heart failure: perspectives from older people and primary care professionals. Health and Social Care in the Community 2006;14(6):482-90. [DOI] [PubMed] [Google Scholar]
Barnes 2012
- Barnes S, Gott M, Chady B, Seamark D, Halpin D. Enhancing patient-professional communication about end-of-life issues in life-limiting conditions: a critical review of the literature. Journal of Pain and Symptom Management 2012;44(6):866-79. [DOI: 10.1016/j.jpainsymman.2011.11.009] [DOI] [PubMed] [Google Scholar]
Bradford 2021
- Bradford N, Rolfe M, Ekberg S, Mitchell G, Beane T, Ferranti K, et al. Family meetings in paediatric palliative care: an integrative review. BMJ Supportive & Palliative Care 2021;11(3):288-95. [DOI: ] [DOI] [PubMed] [Google Scholar]
Brighton 2016
- Brighton LJ, Bristowe K. Communication in palliative care: talking about the end of life, before the end of life. Postgraduate Medical Journal 2016;92:466-70. [DOI: 10.1136/postgradmedj-2015-133368] [DOI] [PubMed] [Google Scholar]
CCCG 2016
- Cochrane Consumers and Communication Group. Standard protocol text and additional guidance for review authors; 2016. cccrg.cochrane.org/author-resources (accessed prior to 24 July 2018).
Chan 2016
- Chan R, Webster J, Bowers A. End-of-life care pathways for improving outcomes in caring for the dying. Cochrane Database of Systematic Reviews 2016, Issue 2. Art. No: CD008006. [DOI: 10.1002/14651858.CD008006.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chochinov 2000
- Chochinov HM, Tataryn DJ, Wilson KG, Ennis M, Lander S. Prognostic awareness and the terminally ill. Psychosomatics 2000;41(6):500-4. [DOI] [PubMed] [Google Scholar]
Clayton 2007a
- Clayton J, Hancock K, Butow P, Tattersall M, Currow D. Clinical practice guidelines for communicating prognosis and end-of-life issues with adults in the advanced stages of a life-limiting illness, and their caregivers. Medical Journal of Australia 2007;186(12):S77-S108. [DOI] [PubMed] [Google Scholar]
Clayton 2007b
- Clayton JM, Butow PN, Tattersall MHN, Devine RJ, Simpson JM, Aggarwal G, et al. Randomised controlled trial of a prompt list to help advanced cancer patients and their caregivers to ask questions about prognosis and end of life care. Journal of Clinical Oncology 2007;25(6):715-23. [DOI: 10.1200/JCO.2006.06.7827] [DOI] [PubMed] [Google Scholar]
Detering 2010
- Detering KM, Hancock AD, Reade MC, Silvester W. The impact of advance care planning on end of life care in elderly patients: randomised controlled trial. BMJ 2010;340:c1345. [DOI: 10.1136/bmj.c1345] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ekberg 2019
- Ekberg S, Herbert A, Johns K, Tarrant G, Sansone H, Yates P, et al. Finding a way with words: Delphi study to develop a discussion prompt list for paediatric palliative care. Palliative Medicine 2019;34(3):291-9. [DOI: 10.1177/0269216319888988] [DOI] [PubMed] [Google Scholar]
Fawole 2012
- Fawole OA, Dy SM, Wilson RF, Lau BD, Martinez KA, Apostol CC, et al. A systematic review of communication quality improvement interventions for patients with advanced and serious illness. Journal of General Internal Medicine 2012;28(4):570-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gattellari 1999
- Gattellari M, Butow PN, Tattersall MH, Dunn SM, MacLeod CA. Misunderstanding in cancer patients: why shoot the messenger? Annals of Oncology 1999;10(1):39-46. [DOI] [PubMed] [Google Scholar]
Gott 2009
- Gott M, Gardiner C, Small N, Payne S, Seamark D, Barnes S, et al. Barriers to advance care planning in chronic obstructive pulmonary disease. Palliative Medicine 2009;23(7):642-8. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed prior to 30 July 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2021. Available at gradepro.org.
Gutierrez 2012
- Gutierrez KM. Experiences and needs of families regarding prognostic communication in an intensive care unit: supporting families at the end of life. Critical Care Nursing Quarterly 2012;35(3):299-313. [DOI] [PubMed] [Google Scholar]
Henderson 2017
- Henderson A, Young J, Herbert A, Bradford N, Pedersen LA. Preparing pediatric healthcare professionals for end-of-life care discussions: an exploratory study. Journal of Palliative Medicine 2017;20(6):662-6. [DOI: 10.1089/jpm.2016.0367] [DOI] [PubMed] [Google Scholar]
Heyland 2009
- Heyland DK, Allan DE, Rocker G, Dodek P, Pichora D, Gafni A, et al. Discussing prognosis with patients and their families near the end-of-life: impact on satisfaction with end-of-life care. Open Medicine 2009;3(2):e101-10. [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook.
Hill 2011
- Hill S, editor(s). The Knowledgeable Patient: Communication and Participation in Health. Chichester, UK: Wiley Blackwell, 2011. [au.wiley.com/WileyCDA/WileyTitle/productCd-1444337173.html] [Google Scholar]
Hjelmfors 2020
- Hjelmfors L, Wal MHL, Friedrichsen M, Milberg A, Mårtensson J, Sandgren A, et al. Optimizing of a question prompt list to improve communication about the heart failure trajectory in patients, families, and health care professionals. BMC Palliative Care 2020;19:161. [DOI: 10.1186/s12904-020-00665-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
IoM 2014
- Institute of Medicine. Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Washington, DC: National Academies Press, 2014. [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLOS Medicine 2009;62(10):e1-34. [DOI: 10.1016/j.jclinepi.2009.06.006] [DOI] [PubMed] [Google Scholar]
Lim 2016
- Lim CED, Ng RWC, Cheng NCL, Cigolini M, Kwok C, Brennan F. Advance care planning for haemodialysis patients. Cochrane Database of Systematic Reviews 2016, Issue 7. Art. No: CD010737. [DOI: 10.1002/14651858.CD010737.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Merner 2019
- Merner B, Hill S, Colombo C, Xafis V, Gaulden CM, Graham-Wisener L, et al . Consumers and health providers working in partnership for the promotion of person-centred health services: a co-produced qualitative evidence synthesis.. Cochrane Database of Systematic Reviews 2019, Issue 2. Art. No: CD013274. [DOI: 10.1002/14651858.CD013274] [DOI] [Google Scholar]
Merner 2021
- Merner B, Lowe D, Walsh L, Synnot A, Stratil J, Lewin S, et al. Stakeholder involvement in systematic reviews: lessons from Cochrane’s Public Health and Health Systems Network. American Journal of Public Health 2021;111(7):1210-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2018
- Moore PM, Rivera S, Bravo-Soto GA, Olivares C, Lawrie TA. Communication skills training for healthcare professionals working with people who have cancer. Cochrane Database of Systematic Reviews 2018, Issue 7. Art. No: CD003751. [DOI: 10.1002/14651858.CD003751.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2017
- National Institute for Health and Care Excellence (NICE). End of life care for adults; 2017. www.nice.org.uk/guidance/qs13/resources/end-of-life-care-for-adults-pdf-2098483631557 (accessed prior to 24 July 2018).
NICE 2019
- National Institute for Health and Care Excellence (NICE). End of life care for adults: service delivery; October 2019. Available at www.nice.org.uk/guidance/ng142. [PubMed]
Nishikawa 2020
- Nishikawa Y, Hiroyama N, Fukahori H, Ota E, Mizuno A, Miyashita M, et al. Advance care planning for adults with heart failure. Cochrane Database of Systematic Reviews 2020, Issue 2. Art. No: CD013022. [DOI: 10.1002/14651858.CD013022.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Oczkowski 2016
- Oczkowski S, Chung H, Hanvey L, Mbuagbaw L, You J. Communication tools for end-of-life decision-making in ambulatory care settings: a systematic review and meta-analysis. PLOS One 2016;11(4):e0150671. [DOI: 10.1371/journal.pone.0150671] [DOI] [PMC free article] [PubMed] [Google Scholar]
Palliative Care Australia 2016
- Palliative Care Australia. Position statement: Improving access to quality care at the end of life for Aboriginal and Torres Strait Islander Australians; 2016. Available at palliativecare.org.au/wp-content/uploads/2015/08/PCA-Palliative-care-and-Indigenous-Australians-position-statement-updated-16-8-11.pdf.
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. The Cochrane Collaboration, 2020.
Ryan 2013
- Ryan R, Hill S, Prictor M, McKenzie J, Cochrane Consumers and Communication Group. Study quality guide; 2013. cccrg.cochrane.org/authorresources (accessed November 2016).
Sansoni 2014
- Sansoni J, Grootemaat P, Duncan C, Samsa P, Eagar K. Systematic literature review on question prompt lists in health care: final report. Centre for Health Service Development, University of Wollongong; 2014 June. [www.safetyandquality.gov.au/sites/default/files/migrated/Question-Prompt-List-Literature-Review.pdf]
Schofield 2003
- Schofield PE, Butow PN, Thompson JF, Tattersall MH, Beeney LJ, Dunn SM. Psychological responses of patients receiving a diagnosis of cancer. Annals of Oncology 2003;14(1):48-56. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook.
Selman 2007
- Selman L, Harding R, Beynon T, Hodson F, Coady E, Hazeldine C, et al. Improving end-of-life care for patients with chronic heart failure: "Let's hope it'll get better, when I know in my heart of hearts it won't". Heart 2007;93(8):963-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shepperd 2021
- Shepperd S, Goncalves-Bradley DC, Straus SE, Wee B. Hospital at home: home-based end-of-life care. Cochrane Database of Systematic Reviews 2021, Issue 3. Art. No: CD009231. [DOI: 10.1002/14651858.CD009231.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sinuff 2015
- Sinuff T, Dodek P, You JJ, Barwich D, Tayler C, Downar J, et al. Improving end-of-life communication and decision making: the development of a conceptual framework and quality indicators. Journal of Pain and Symptom Management 2015;49(6):1070-9. [DOI] [PubMed] [Google Scholar]
Steinhauser 2000
- Steinhauser KE, Christakis NA, Clipp EC, McNeilly M, McIntyre L, Tulsky JA. Factors considered important at the end of life by patients, family, physicians, and other care providers. JAMA 2000;284(19):2476-82. [DOI] [PubMed] [Google Scholar]
Sulmasy 2017
- Sulmasy DP, Hughes MT, Yenokyan G, Kub J, Terry PB, Astrow AB, et al. The Trial of Ascertaining Individual Preferences for Loved Ones' Role in End-of-life Decisions (TAILORED) study: a randomized controlled trial to improve surrogate decision making. Journal of Pain and Symptom Management 2017;54(4):455-65. [DOI: 10.1016/j.jpainsymman.2017.07.004] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thode 2020
- Thodé M, Pasman HRW, Vliet LM, Damman OC, Ket JCF, Francke AL, et al. Feasibility and effectiveness of tools that support communication and decision making in life-prolonging treatments for patients in hospital: A systematic review. BMJ Supportive & Palliative Care 2020;0:1-8. [DOI: doi. org/ 10.1136/bmjspcare-2020-002284] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thompson 2009
- Thompson AP. Whanau/Family Meetings in the Paediatric Intensive Care Unit: Content, Process and Family Satisfaction [Masters thesis]. Auckland (New Zealand): Massey University, 2009. [Google Scholar]
Together for Short Lives 2012
- Together for Short Lives. End of life planning series; 2012. www.togetherforshortlives.org.uk (accessed prior to 24 July 2018).
Together for Short Lives 2015
- Together for Short Lives. Difficult conversations; 2015. www.togetherforshortlives.org.uk/assets/0001/0096/Difficult_Conversations_for_Young_Adults_-_Final_PDF.pdf (accessed prior to 24 July 2018).
Van der Steen 2021
- Van der Steen J, Heck S, Juffermans CCM, Garvelink MM, Achterberg WP, Clayton J, et al. Practitioners’ perceptions of acceptability of a question prompt list about palliative care for advance care planning with people living with dementia and their family caregivers: a mixed-methods evaluation study. BMJ Open 2021;11:e044591. [DOI: 10.1136/bmjopen-2020-044591] [DOI] [PMC free article] [PubMed] [Google Scholar]
Voruganti 2017
- Voruganti T, Grunfeld E, Makuwaza T, Bender JL. Web-based tools for text-based patient-provider communication in chronic conditions: scoping review. Journal of Medical Internet Research 2017;19(10):e366. [DOI: 10.2196/jmir.7987] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walczak 2016
- Walczak A, Butow PN, Bua S, Clayton JM. A systematic review of evidence for end-of-life communication interventions: who do they target, how are they structured and do they work? Patient Education and Counseling 2016;99(1):3-16. [DOI] [PubMed] [Google Scholar]
Walczak 2017
- Walczak A, Butow PN, Tattersall MHN, Davidson PM, Young J, Epstein RM, et al. Encouraging early discussion of life expectancy and end-of-life care: a randomised controlled trial of a nurse-led communication support program for patients and caregivers. International Journal of Nursing Studies 2017;67:31-40. [DOI: 10.1016/j.ijnurstu.2016.10.008] [DOI] [PubMed] [Google Scholar]
Weeks 1998
- Weeks JC, Cook EF, O'Day SJ, Peterson LM, Wenger N, Reding D, et al. Relationship between cancer patients’ predictions of prognosis and their treatment preferences. JAMA 1998;279(21):1709-14. [DOI] [PubMed] [Google Scholar]
Welch 2010
- Welch V, Tugwell P, Petticrew M, De Montigny J, Ueffing E, Kristjansson B, et al. How effects on health equity are assessed in systematic reviews of interventions. Cochrane Database of Systematic Reviews 2010, Issue 12. Art. No: MR000028. [DOI: 10.1002/14651858.MR000028.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wenrich 2001
- Wenrich MD, Curtis JR, Shannon SE, Carline JD, Ambrozy DM, Ramsey PG. Communicating with dying patients within the spectrum of medical care from terminal diagnosis to death. Archives of Internal Medicine 2001;161(6):868-74. [DOI] [PubMed] [Google Scholar]
Wolfe 2020
- Wolfe J, Bluebond-Langer M. Paediatric palliative care research has come of age. Palliative Medicine 2020;34(3):259-61. [DOI: 10.1177/0269216320905029] [DOI] [PubMed] [Google Scholar]
World Wide Palliative Care Alliance 2014
- World Wide Palliative Care Alliance. Global atlas on palliative care at the end of life; 2014. www.thewhpca.org/resources/global-atlas-on-end-of-life-care (accessed prior to 24 July 2018).
Wright 2008
- Wright AA, Zhang B, Ray A, Mack JW, Trice E, Balboni T, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA 2008;300(14):1655-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2009
- Zhang B, Wright AA, Huskamp HA, Nilsson ME, Maciejewski ML, Earle CC, et al. Health care costs in the last week of life: associations with end-of-life conversations. Archives of Internal Medicine 2009;169(5):480-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


