Abstract
Osteoarthritis (OA) is a progressive degenerative joint disease which results in chronic degeneration of articular cartilage and sclerosis of bone. While tendons and ligaments may heal to a limited extent, articular cartilage has poor intrinsic regenerative potential, and critical-sized bone defects and pathological fractures cannot regenerate spontaneously. OA represents a significant burden of disease globally, affecting 240 million people in the world. The objective of tissue engineering is to recapitulate the natural healing cascade and developmental process by transplanting stromal and progenitor cells which can act directly or indirectly. As the ultimate goal of regenerative medicine is to avoid in vitro expansion of cells and its associated complications, the adipose-derived stromal cell (ASC) is an attractive progenitor cell for tissue engineering for treatment of OA. While clinical studies are still in their infancy, ASCs together with novel scaffold materials represent promising treatment options for patients suffering from OA. How ASCs exert their regenerative potential is a topic of debate, whereby it may be a result of direct differentiation of ASCs into the desired regenerating tissue, and/or through paracrine activity. With the advancement of material science, it is increasingly possible to enhance engraftment of ASCs through the use of biomaterials or to direct progenitor cell fate by activating biophysical signals through designed material microstructures. There are currently over 180 completed or ongoing registered early stage clinical trials involving ASCs, with 17 completed studies reviewed herein detailing the use of ASCs in OA. In order for ASC therapy to become an “off-the-shelf” option for treating OA, several strategies are currently being explored such as ASC cryopreservation and use of allogeneic ASCs. Newer approaches, such as exosome therapy, allow for the use of acellular ASC-derived therapies and are also currently the focus of ongoing investigations.
Keywords: Adipose derived stromal cells, Adipose derived stem cells, Tissue engineering, Exosomes, Scaffolds, Osteogenesis
1. Introduction
Osteoarthritis (OA) is a progressive degenerative joint disease which results in chronic degeneration of articular cartilage and sclerosis of bone. While tendons and ligaments may heal to a limited extent, articular cartilage has poor intrinsic regenerative potential, and critical-sized bone defects and pathological fractures cannot regenerate spontaneously. OA represents a significant burden of disease globally, affecting 240 million people in the world and accounts for 2.4% of years lived with disability worldwide, most notably among 9.6% of men and 18% of women over 60 years of age [1–3]. OA prevalence continues to increase globally as life expectancy and obesity rates rise [1]. OA can manifest as a spectrum of debilitation. While mild OA causes intermittent pain with minimal effect on daily living, severe cases are characterized by progressive irreversible structural deficits with progressive loss of function and an inability to work, often associated with psychological sequelae as well as increased mortality [1–4].
Tissue engineering (TE) can be defined as the implementation of a combination of cells, engineering materials, and biochemical factors to improve or divert biological processes [5]. A shared TE objective is to recapitulate the natural healing cascade and developmental process by transplanting stromal and progenitor cells or by endogenous manipulation of resident cells to augment their native regenerative potential [6]. Thus, by engineering and delivering tissues and/or cells with regenerative capacity, TE offers the potential to treat MSK disease, such as OA.
One must consider many issues in the regeneration of functional tissue in TE. A readily abundant source of cells, harvested with minimal donor site morbidity, and with the potential to express the phenotype of the desired tissue is required in addition to a biocompatible matrix to deliver and/or support cells in tissue regeneration [5]. In comparison to differentiated cells, the application of stem and progenitor cells is favorable because of their increased proliferation capacity, successful culture with a large number of passages, broader differentiation potential, and ability to promote vascularization [7]. In regenerative medicine, as we endeavor to harness the body’s own cells for treatment, innovation continues to advance stem cell-based therapies. Stem cells have been identified in many adult tissues [8–17], which contribute to both maintenance and regeneration. Postnatal (or “adult”) stem cells display tissue-specific differentiation patterns, proliferate in response to specific physiological cues, and are necessary for growth, homeostasis, and tissue regeneration [18].
The number and type of stem and stromal cells being investigated in human clinical trials for the treatment of OA continues to increase. Many approaches focus on mesenchymal stromal cells derived from bone marrow (BM-MSCs) and those derived from adipose tissue, known as adipose derived stromal cells (ASCs). As an overarching goal of regenerative medicine is to forgo in vitro expansion of cells and its associated complications, ASCs are an ideal cellular tissue engineering building block. Each mL of bone marrow contains 6,000 to 60,000 BM-MSCs, while over 200,000 ASCs can be isolated from 1 g of adipose tissue. Thus, when considering sources of progenitor/stromal cells, ASCs are more abundant and widely available source in comparison to BM-MSCs [5,19,20]. In this review, we will focus on the implementation of ASCs in MSK TE for the treatment of OA both in pre-clinical and clinical studies and the advantages and disadvantages of such treatment modalities, prior to describing future strategies (Fig. 1).
Fig. 1.
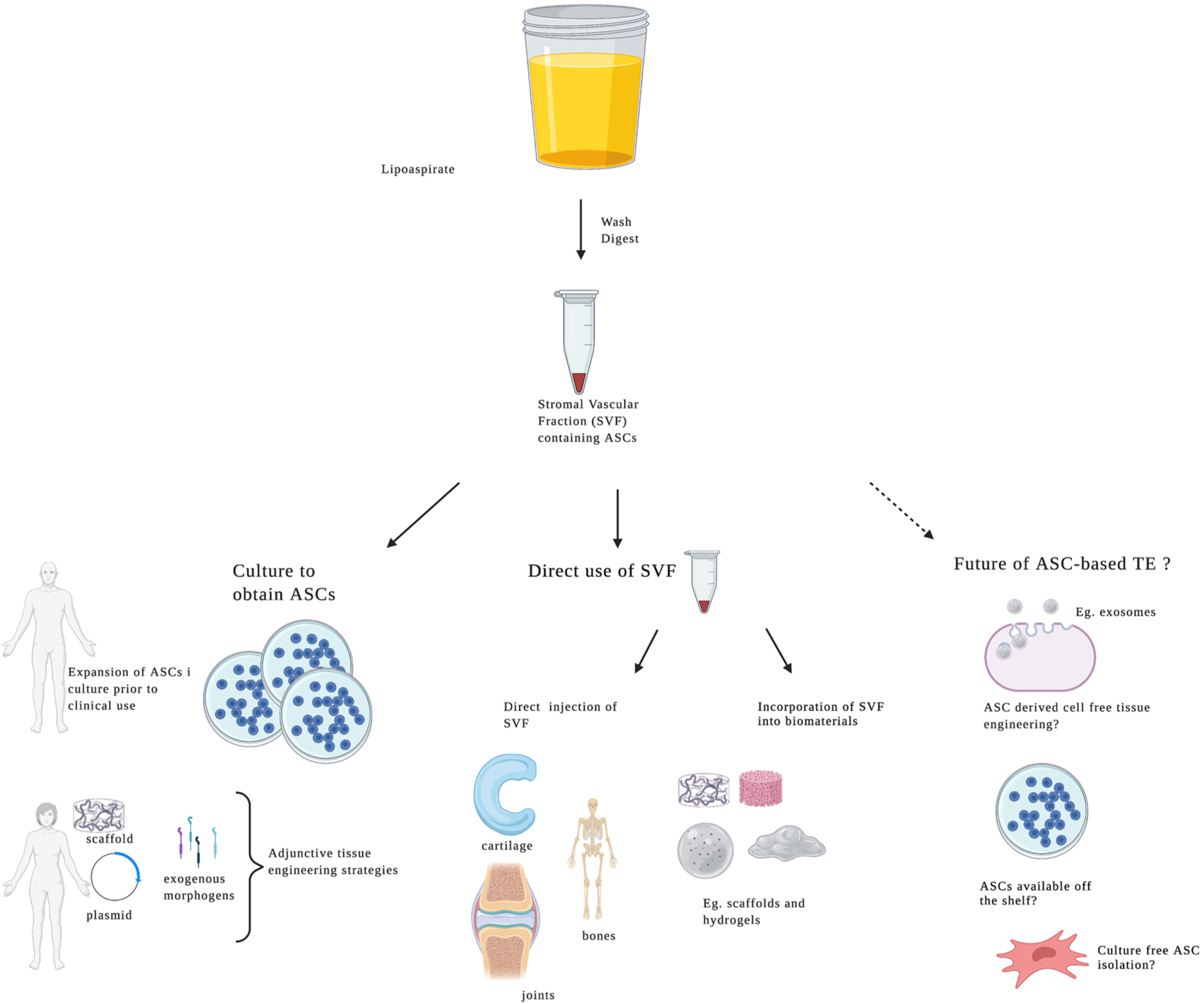
Implementation of ASCs in MSK Tissue Engineering
Lipoaspirate can be washed and digested to isolate the stromal vascular fraction (SVF), which contains ASCs. The SVF can be cultured to obtain ASCs, which can be augmented with plasmid DNA, or application of exogenous morphogens or incorporated into scaffolds to direct subsequent lineages, or it may be used directly in clinical applications to optimize regeneration of cartilage, ligaments and/or tendons, joints and bone. Figure created using BioRender.com.
1.1. Adipose tissue and characterization of ASCs
The main function of adipose tissue is to store free fatty acids in the form of triglycerides that are then released during starvation or physical activity [21]. Adipose tissue is the largest endocrine tissue of humans, regulating the body’s metabolism and immune system. There are two widely accepted sub-types of adipose tissue, white and brown adipose tissue. These differ considerably in function, metabolic activity, morphology, and distribution – white adipose tissue is the predominant fat in adults, whereas brown adipose tissue is the main adipose tissue of fetuses and newborns but declines with increasing age. In addition, lesser known discrete tissue-associated adipose depots have been described in the dermis, bone marrow, and mammary glands [22]. White adipose tissue is involved in lipid storage and is active in immune-endocrine responses, while brown adipose tissue is a key regulator of thermogenesis, whereby heat is generated by uncoupling of mitochondrial respiration as a result of sympathetic nervous system innervation.
White adipose tissue is composed of adipocytes, together with a mixture of endothelial, stromal, and immune cells and is where ASCs may be found. Zuk and colleagues first characterized ASCs and described their harvest from human subcutaneous adipose tissue in 2002 [10]. Since their characterization, these cells have been studied extensively by tissue engineers [10]. ASCs are described as a mesenchymal stromal cell (MSC) population that are harvested from the adipose tissue stromal vascular fraction (SVF) and share many regenerative properties as other MSCs. The International Society for Cellular Therapy reports that the term “ASC” only applies to the subset of progenitor cells that meet the following three minimal criteria: (i) plastic adherence, (ii) expression of CD34+/CD45−/CD31−/CD13+/CD73+/CD90+, and (iii) trilineage differentiation potential, as stated in a conjoined effort with the International Federation for Adipose Therapeutics (IFATS) in 2013 [23]. Contrastingly, the SVF is heterogenous containing fibroblasts, vascular smooth muscle cells, endothelial cells, lymphocytes, macrophages, and ASCs. ASCs, due to their capacity to differentiate into multiple cell lineages, are attractive building blocks to repair, maintain, or enhance various tissues.
Adipose tissue represents a viable alternative donor site to bone marrow due to its abundance, and reduced donor site morbidity. In addition, cells harvested from adipose tissue are exempt from ethical concerns pertaining to other stem cell sources, such as embryonic stem cells [24]. ASCs are harvested through liposuction and processed with washing, digestion, and centrifugation [24]. In the United States, over 250,000 liposuction procedures are performed annually, with a mean of 3 L of lipoaspirate discarded after each procedure [25]. Theoretically, by processing 3 L of discarded lipoaspirate, one could collect up to 6 billion ASCs from a single patient after a single passage [26].
1.2. Harvest of adipose tissue
Given the great interest in the regenerative capacity of ASCs, several studies have tried to discern the best method for fat harvest to ensure optimal preservation of these cells. In addition to traditionally used suction-assisted liposuction (SAL), more complex methods of liposuction have been developed to prioritize surgical removal of adipose tissue, mostly by increasing the ease and speed of liposuction, while optimizing patient results. These methods have not been developed to maximize cellular preservation for use in regenerative medicine. Ultrasound assisted liposuction (UAL), laser assisted liposuction (LAL), and mechanical assisted liposuction are examples of these new methods. Panetta et al. amongst others, reported that there was no difference in the biological properties and differentiation capacity of ASCs obtained from SAL versus UAL [27–30]. However, Chung and colleagues demonstrated that although ASCs derived from SAL and LAL successfully underwent osteogenic and adipogenic differentiation in pre-clinical studies, the cell yield, viability, proliferation, and frequency of ASCs in the stromal vascular fraction (SVF) were significantly less with LAL in vitro [31]. Furthermore, less efficient in vivo osteogenesis was also appreciated in ASCs derived from LAL relative to SAL in animal studies [31]. Thus, various liposuction methods can be used to harvest ASCs, however SAL and UAL yield higher quality ASCs than LAL for use in tissue engineering.
1.3. Isolation of ASCs
The protocol described by Zuk and colleagues continues to be the most widely used method of ASC isolation [10]. Here, lipoaspirate samples are extensively washed in equal volumes of phosphate-buffered saline and then digested at 37° Celsius for 30 min with 0.075% collagenase for tissue dissociation. When enzymatically digested, lipoaspirate yields a heterogenous mixture of endothelial cells, monocytes, lymphocytes, myeloid cells, pericytes, pre-adipocytes, smooth muscle cells, and mesenchymal cells [32]. SVF culture expansion then allows for selection of ASCs which are plastic adherent [32] (Fig. 2).
Fig. 2.
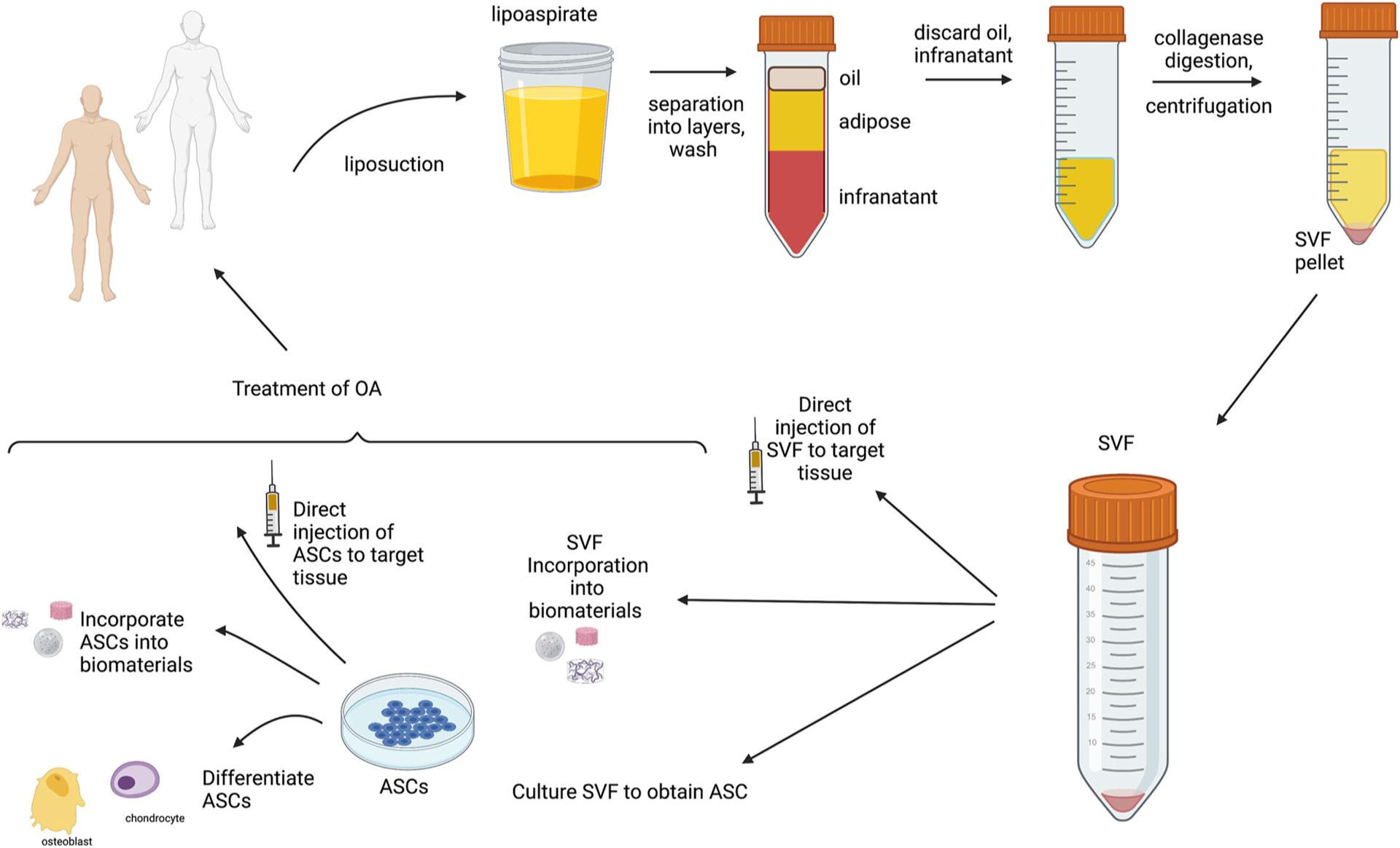
Harvest of ASCs for Tissue Engineering in OA
Adipose tissue is harvested by liposuction and the resultant lipoaspirate is washed and allowed to separate with removal of oil and infranatant layers (mixture of blood and tumescent solution). The adipose layer is then subjected to collagenase digestion and centrifugation to lead to the isolation of the SVF [148]. The SVF can be used in clinical applications in multiple methods: eg. by direct injection, incorporation into biomaterials or placed in culture for isolation of ASCs. ASCs can also be used in clinical applications in multiple methods: eg. by direct injection, incorporation into biomaterials or injection following differentiation into downstream progenitors. Figure created using BioRender.com.
Enzymatic digestion-based methods are mainly applicable in experimental purposes and are less favorable in clinical practice. The use of proteolytic enzymes (e.g. trypsin-EDTA solution, dispase, or collagenase) may reduce cell viability and alter surface antigen expression [33]. Digestion with animal-derived enzymes, such as collagenase, while efficient, is considered more than “minimally manipulated” by the US Food and Drug Administration as it can alter cellular characteristics [34]. Furthermore, preparation of cells using xenogeneic components may result in immune reactions when the resultant cells are used clinically [35–39]. Many groups are now turning to non-animal derived, manufactured enzymes to abate the risks of these xenogeneic components. However, manufactured enzymes are also flawed and several reports suggest that they also alter cellular phenotype [34,40], again resulting in more than minimal manipulation.
1.4. Optimizing the potential of ASC-based therapy: evidence from preclinical studies
The method by which ASCs exert their regenerative potential is a topic of ongoing debate. Several reports indicate that the improvement achieved with ASC application does not correlate directly with the levels of cellular engraftment and downstream differentiation, instead suggesting that paracrine activity could account for the beneficial effect of ASC-based therapy [41–48]. As Zuk and colleagues reported, rather than direct differentiation of ASCs into the desired regenerating tissue, it is entirely possible that the ASC “simply directs tissue formation from the sidelines” [49–51]. ASCs are known to secrete numerous factors and cytokines, including NGF, BDNF, VEGF, HGF, and multiple interleukins, thus leading to the “secretome” theory of ASCs [45,50–53]. In animal studies, ASCs have been proposed to improve tissue repair through both direct differentiation of injected cells and by the effect of their secretome (Fig. 3).
Fig. 3.
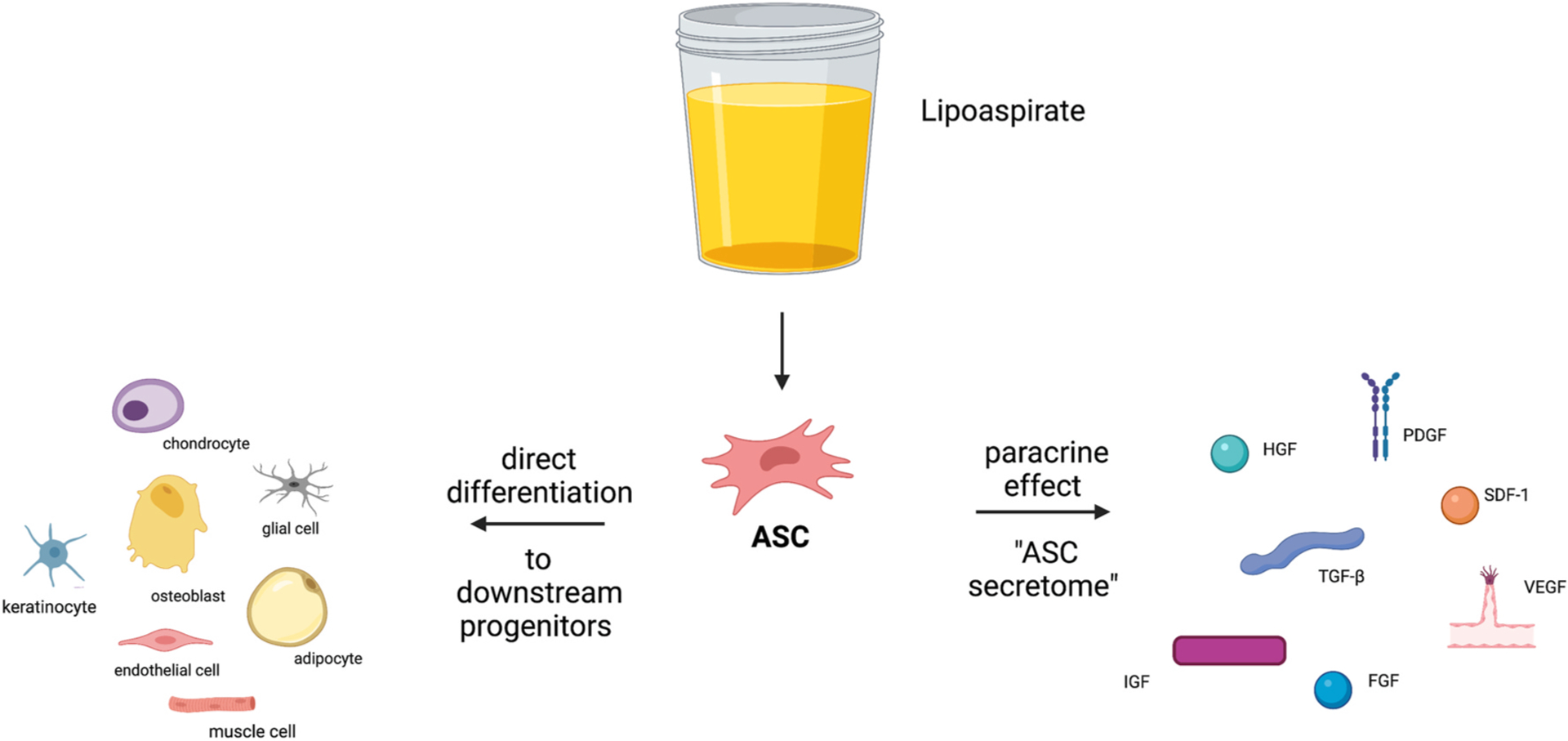
The role of ASCs in Tissue Engineering.
The method by which ASCs exert their regenerative potential is a topic of ongoing debate. Several reports indicate that the improvement achieved with ASC application does not correlate directly with the levels of cellular engraftment and downstream differentiation, instead suggesting that paracrine activity could account for the beneficial effect of ASC-based therapy [41–48]. Figure created using BioRender.com.
Specific subtypes of human ASCs have also been identified which demonstrate increased osteogenic potential. For example, surface expression of BMPR1b and CD90 have been shown to have an enhanced ability to form bone both in vitro and in vivo, while low expression of CD105 has been shown to promote osteogenesis in animal studies [54–56]. Given the need for isolation using fluorescence activated cell sorting, these methods require further innovation before promise can be seen in clinical studies.
ASCs have been implemented together with biomaterials to improve ASC engraftment, drive differentiation of ASCs to downstream progenitors, mimic the extracellular microenvironment, and offer mechanical support while coordinating the healing process [57]. ASCs can be incorporated into biomaterial scaffolds. Ideally, a scaffold should be biodegradable/absorbable, elicit minimal immunological and inflammatory response, and biocompatible. Gradual substitution of the scaffold by autologous tissue should also occur [47]. Two fundamental properties of a scaffold are porosity and stiffness. Stiffness provides adequate support for the engineering of bone and cartilage, whereas porosity results in vascular ingrowth. Thus, when considering scaffold design, the properties of porosity and stiffness need to be carefully considered. In addition, scaffolds can be osteo-inductive or osteo-conductive. Osteoconductive scaffolds act as inert support that guides bone extracellular matrix formation and cellular proliferation to regenerate bone. Thus, they can support ASCs in MSK TE but do not drive differentiation to specific downstream fates. Contrastingly, osteoinductive biomaterials can attract progenitor cells and drive downstream differentiation to osteoblasts, leading to de novo bone formation. In MSK TE, ASCs have been combined with scaffolds derived from combinations of both organic and inorganic sources such as decellularized matrices, inorganic ceramics (e.g. hydroxyapatite, glass ceramics, tricalcium phosphate) and synthetic biodegradable polymers such as polylactic acid (PLA) and polyglycolic acid (PGA) [47,58]. Calcium phosphate cements, for example, have intrinsic osteoinductive and osteoconductive features without the addition of osteogenic factors [47,59]. Using 3-dimensional (3D) printing, various biomaterials can be built in a layer-by-layer manner to result in an extracellular matrix scaffold with the goal of promoting regeneration of both functional bone and cartilage.
As ASCs are multipotent, an important strategy that has been pursued for TE is to determine how to drive specific ASC downstream lineage differentiation [58]. One such strategy is to increase osteogenic potential by the use of biomaterials and/or matrix proteins to recreate a niche micro-environment that can enhance differentiation of specific osteogenic cellular subpopulations. Natural polymers, such as fibrin, hyaluronic acid, and collagen are widely used in MSK TE. Fibrin, a biopolymer of the monomer fibrinogen involved in cell-matrix interactions, inflammation, blood coagulation, and wound healing, is an attractive biomaterial for this purpose. Fibrin use has repeatedly been shown to promote osteogenic differentiation, while also promoting vascularization [58,60,61]. It can also be loaded with osteoinductive agents to stimulate osteogenesis, as evidenced by functional bone regeneration when implanted in rat tibial defects [60]. Hyaluronic acid-based scaffolds support cell migration and differentiation and have been shown to be effective when combined with ASCs as an intra-articular injection in the prevention of OA disease progression and promotion of cartilage regeneration in sheep [46,62].
Engler et al. demonstrated that varied material physical properties could actively dominate biological behaviors of stem cells when they reported that varied matrix elasticity could guide specific lineage differentiation of MSCs (Fig. 4) [63]. This discovery that “pre-committing” stem cells to a specific lineage via appropriate material physical conditions established a solid scientific foundation for regenerative material design and application [63,64]. Simply put, soft matrices that mimic brain are neurogenic, stiffer matrices that mimic muscle are myogenic, and comparatively rigid matrices that mimic collagenous bone prove osteogenic [63]. Cartilage, especially articular cartilage, is constantly exposed to hydrostatic pressure. Ogawa and colleagues applied hydrostatic pressure (HP) to ASCs to mimic the native environment of chondrocytes and reported increased chondrogenic differentiation of ASCs in a three-dimensional collagen scaffolds following treatment with cyclic HP relative to control [65]. In addition, Safshekan et al. investigated the effects of both intermittent HP on chondrogenic differentiation of ASCs with or without induction medium. They respectively treated cell pellets with chemical induction or HP or combined chemical/mechanical stimuli for seven consecutive days and demonstrated that the chemical/mechanical group exhibited higher gene expression of chondrogenic mediators (e.g. Sox9, Collagen II) than the other two groups [66]. Thus, combined chemical and mechanical stimulation can optimize chondrogenic differentiation.
Fig. 4.
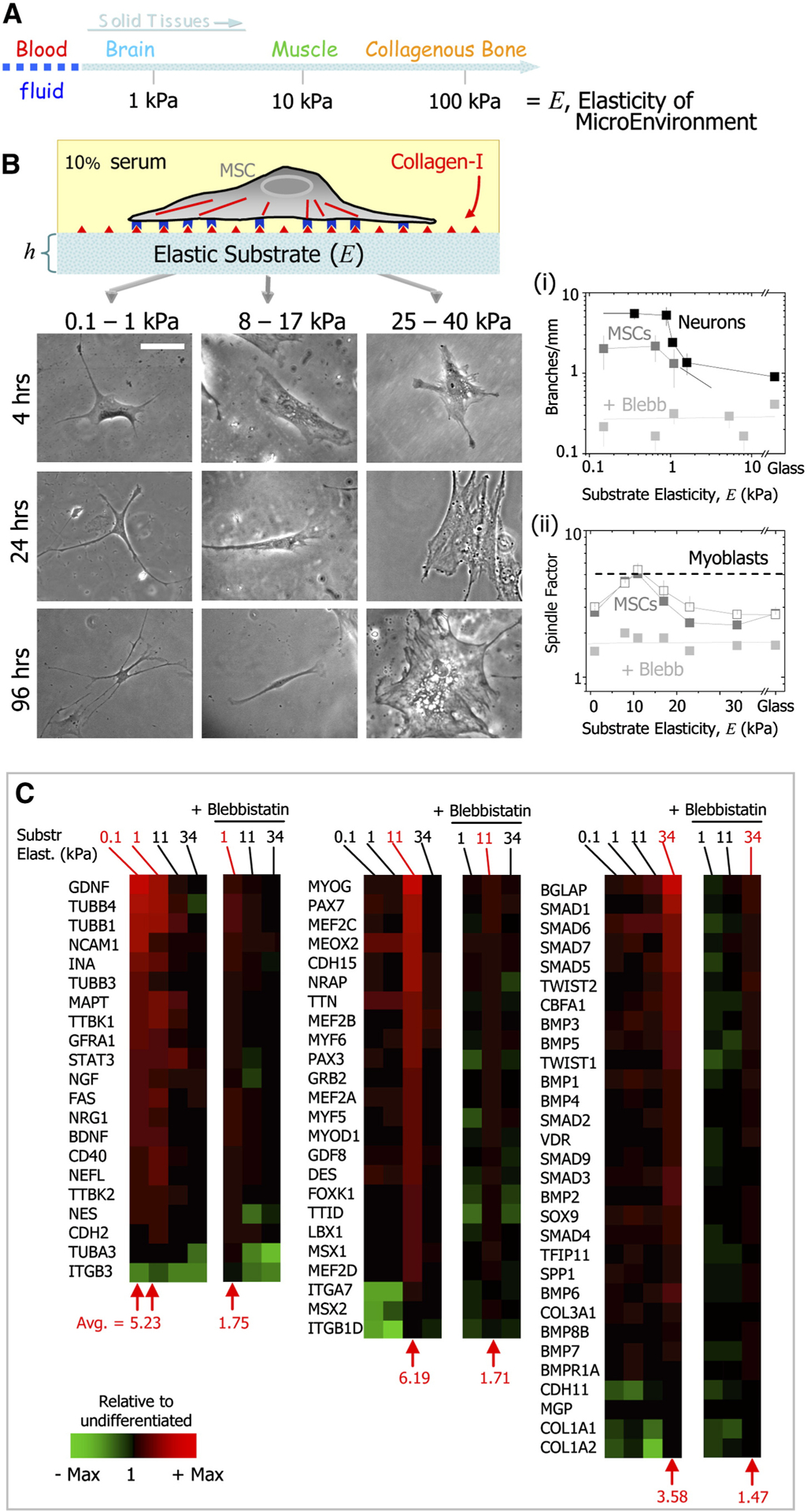
Matrix Properties Can Direct Stem Cell Differentiation.
(A) Solid tissues exhibit a range of stiffness, as measured by the elastic modulus, E. (B) The in vitro gel system allows for control of E through cross-linking, control of cell adhesion by covalent attachment of collagen-I, and control of thickness, h. Naive MSCs of a standard expression phenotype are initially small and round but develop increasingly branched, spindle, or polygonal shapes when grown on matrices respectively in the range typical of E-brain (0.1–1 kPa), E-muscle (8–17 kPa), or stiff crosslinked-collagen matrices (25–40 kPa). Scale bar is 20 mm. Figure from Engler et al. reproduced with permission [63].
Osteochondral TE is challenged by the difficulty in the simultaneous regeneration of hyaline cartilage and subchondral bone. Thus, strategies combining biomaterials that can conduct/induce bone and cartilage formation have been explored. For example, scaffolds combining hyaluronic acid, chitosan, and PLGA were developed that possess two different regions – one supporting hyaline chondrogenesis and another zone with bonded BMP-2 supporting osteogenesis. These osteochondral scaffolds were then seeded with ASCs, resulting in regeneration of rabbit osteochondral defects [67]. In addition to combining different scaffold polymers to induce cartilage and bone regeneration, strategies implementing differential concentrations of calcium have also been used for osteochondral TE. For example, Mellor and colleagues reported that human ASC seeded-stacked polylactic acid nanofibrous scaffolds containing either 0% or 20% tricalcium phosphate nanoparticles resulted in vitro site-specific osteogenesis (high calcium concentration) and chondrogenesis (basal calcium concentration) [68].
With the advancement of material science, it is increasingly possible that stem cell fate can be directed by activating biological and mechanical signals through specially designed material microstructures [69]. Designed biophysical signals include, but are not limited to structure micropatterning, material rigidity and elasticity, materials coated with extracellular matrix proteins, and mechanotransduction [70]. Surface micropatterning creates topography to guide cellular alignment, shape, and adhesion and can then be integrated with different matrices by 3D bioprinting, thus generating micropatterned 3D structures to guide stem cell based tissue regeneration [64]. For example, a difference in the scale of scaffold topography may improve tenogenic differentiation from ASCs for incorporation into tendon and tendon bone interfaces [64]. Zhou and colleagues demonstrated that micro-scale topography resulted in improved neo-tendon formation and stable tenogenic marker expression when compared to nanoscale topography [70]. Thus, smart scaffolds and matrices can promote downstream differentiation of ASCs for incorporation into MSK TE.
1.5. Clinical trials: ASCs in osteoarthritis
For the purpose of tissue regeneration, adipose tissue is often administered as unpurified SVF, which is obtained by enzymatic lipoaspirate digestion, centrifugation and removal of the adipocyte (floating) fraction (Fig. 2). As previously elaborated, this results in a heterogeneous cell suspension that contains ASCs together with hematopoietic cells, fibroblasts, and extracellular matrix components, the so-called SVF. The patient’s SVF may be combined with other materials, such as hyaluronic acid and/or platelet-rich plasma (PRP), which have been proposed as both a source of growth factors and a physical scaffolding to support the ASCs [71–73]. The resultant mixture is then introduced into the target joints via percutaneous intra-articular injection and is reported to support cartilage regeneration both through direct differentiation into chondrocytes, and through secretory/paracrine effects to support regenerative activity of tissue-resident cells [71–73]. The use of ASCs in clinical treatment of OA is still in its infancy and in the sections below, we will highlight the history of ASC use in clinical studies of OA and discuss additional ongoing studies.
The first case series of OA using ASCs was published in 2011. Here, Pak reported MRI-evident cartilage regeneration, as well as improvement in pain and functional metrics, in two patients with knee OA who were treated via intraarticular injection of autologous ASCs resuspended in hyaluronic acid, platelet-rich plasma (PRP), and calcium chloride [74]. Since then, additional studies have further investigated applications of ASCs in OA patients and reported similar results [75,76]. Early studies focused on establishing the safety and feasibility of ASC treatment for OA. In 2014, a dose-escalation study by Jo et al. found no treatment-related adverse events even at the highest dose of ASCs (1.0 × 108 cells) and observed regeneration of histologically “hyaline-like” joint cartilage within 6 months of treatment [77]. Similarly, in the context of knee OA, a 2016 study by Fodor and Paulseth [78] of 6 patients receiving SVF injection and a 2016 dose-escalation study by Pers et al. in 18 patients all reported no serious adverse events, with the longest study reporting patient follow-up up to 2 years [79]. Notably, in a 2020 study retrospectively examined outcomes for 34 knee OA patients treated with autologous ASCs and observed that pain scores tended to improve earlier than outcomes related to activities of daily living and sports/recreation. The authors also reported a significantly greater improvement in patients with more severe cartilage lesions, suggesting that ASC treatment may be most beneficial to patients with more severe disease [80].
There are currently over 180 completed or ongoing clinical trials of ASCs registered with the National Institutes of Health (NIH, www.clinicaltrials.gov - a database of privately and publicly funded clinical studies conducted around the world). Of the 180 clinical trials of ASCs, 45 target OA with 12 completed studies to date and 13 studies in the active enrollment phase as reported by the NIH [81]. Of the studies registered with the NIH, five are not yet recruiting and therefore do not have listed locations. Among the other studies that are recruiting, active, completed, or unknown, the most heavily represented countries are the United States (13 studies), China (8 studies), and South Korea (7 studies), France (3 studies), and Taiwan (3 studies). All other countries had less than three trials listed. The majority of studies focus on OA of the knee (n = 33), but additional studies focus on OA of the hip (n = 2), wrist (n = 2), spine (n = 1), or hand (n = 1). Some studies evaluate treatment of multiple joints: there are two studies of knee, hip, and shoulder, one study of knee, hip, ankle, shoulder, and wrist, and one study of spine, knee, and hip. Two studies do not identify a target joint for treatment. The majority of the published results from these studies detail the use of intra-articular injection in knee OA, mostly in the form of cells without the incorporation of additional biomaterials. Notably, most of these studies remain in early stages (phase I and II), and further investigation is required to fully characterize therapeutic effects, optimal treatment regimen, and potential long-term adverse sequelae of ASC treatment [81]. Early results have however been very favorable, with nearly all studies to date reporting significant clinical improvement in OA patients treated with autologous ASCs. Table 1 details the results of 17 completed studies – 12 completed studies which were identified using the NIH registry (www.clinicaltrials.gov) and 5 additional studies which were identified following a literature review of PubMed (search terms: osteoarthritis, ASC, clinical trials).
Multiple controlled studies have identified significant benefits of ASC treatment, either as a monotherapy or adjunct to other treatments/procedures, such as microfracture surgery, for OA patients [82,83]. A 2016 study by Kim et al. compared treatment with bone marrow stimulation (i.e., microfracture surgery) and ASC injection to treatment with microfracture alone in 62 patients with ankle OA [82,83]. They demonstrated that ASC treatment significantly improved outcomes, as measured by both symptom severity and arthroscopic assessment [82]. Similar results were found in a 2016 retrospective study by Kim and Koh [82], though a notable limitation is that patients in this study individually decided whether or not to receive ASC treatment. A 2019 study by Hong et al. studied 16 knee OA patients, each of whom were randomized to receive SVF injection in one knee and hyaluronic acid control injection in the contralateral knee [84]. SVF-treated knees had significantly improved functional, visual, and radiographic scores compared to HA-treated knees at 12 months [84]. Overall, while further randomized, controlled trials are needed with larger patient cohorts and longer-term follow-up, based on both clinical examination metrics and patient-reported outcome measures, all studies to date have suggested that intra-articular injection of ASC/SVF is a promising strategy for treating OA.
1.6. Challenges to clinical implementation
While early clinical studies have shown largely favorable results for ASCs’ ability to improve symptoms in patients with OA, key obstacles must be cleared prior to widespread clinical implementation. Perhaps the most significant issue is the ease of preparation of autologous ASCs. As described above, isolation of SVF typically requires enzymatic digestion of lipoaspirate with collagenase, which is considered “significant manipulation” of tissue and thus, requires FDA approval and equipment that meets rigorous FDA standards [85], which are beyond the means of many medical centers. A 2018 study by Roato et al. focused on circumventing this issue by using autologous concentrated adipose tissue, prepared by centrifuging lipoaspirate without enzymatic digestion [86]. While the authors did observe symptomatic improvement in patients treated with this “lightly manipulated” adipose tissue, they did not directly compare outcomes to patients treated with SVF isolated in the standard fashion. In addition, while no serious adverse events occurred, most patients reported limited knee mobility in the month following injection, which may be related to the large volume injected – 35 mL of processed adipose tissue – compared to the typical ~5 mL of SVF reported in other studies [86].
Emerging studies have also suggested that SVF may not be the ideal means to deliver ASCs in OA treatment. Yokota et al. retrospectively compared outcomes for patients treated with fresh SVF (prepared directly from lipoaspirate without intervening cell culture which is the means of ASC delivery typically used in clinical trials) versus cultured ASCs (ASCs selected from lipoaspirate by adherence to plastic, then expanded in cell culture - the form of ASCs used in most preclinical studies) [87]. Patients treated with cultured ASCs had accelerated resolution of symptoms and a more significant decrease in pain. Furthermore, patients treated with SVF had more frequent minor complications (including knee effusion), collectively suggesting that culture-expanded ASCs may be superior to SVF for treating OA. While this study was limited by a lack of blinding or randomization, these results are not surprising as SVF is a heterogeneous mix of cells including inflammatory cells and of which only 1–10% of cells are ASCs [88]. In light of these findings, non-enzymatic approaches have been proposed to optimize ASC isolation. Mechanical ASC isolation may be performed whereby adipose tissue is emulsified using syringes, a technique which results in a higher yield of multipotent CD34+/CD31−/CD45−/CD13+/CD73+/CD146− ASCs from lipoaspirate in comparison to collagenase digestion [89]. Another proposal relies on ASCs migrating out of the undigested solid adipose tissue in culture, thought to arise as ASCs are drawn out of the tissue to nutrient rich media [34]. This method yields ASCs with healthier morphology in comparison to collagenase treatment but cannot be used in same day harvest and administration, as it requires a minimum of 7 days ex vivo culture [34,90]. One such method, termed “ceiling culture”, allows adipose tissue fragments to adhere to the top inner surface of a culture flask, which is filled completely with medium allowing for culture and expansion of ASCs without tissue digestion [57]. This technique is flawed due to limited surface for attachment and migration of ASCs out of the adipose tissue. To overcome this limitation, Yang et al. implemented use of a highly hydrated fibrin matrix to securely support the adipose tissue and provide increased surface area for attachment and migration of ASCs [91].
ASC purification comes with further innate challenges. The additional processing steps required to purify ASCs increased the risk of contamination as well as substantially increasing expense and regulatory hurdles. As a result, very few medical centers have the required approved equipment to perform this cell expansion step. In comparison, SVF isolation typically takes only 60–90 min and can be performed in the operating room using automated devices, making it significantly more accessible and translatable [85]. Additionally, while ASCs are typically thought to inherently “home” to sites of tissue damage, there is some evidence that stem cells may lose this homing ability with increased passages in cell culture, potentially representing an additional limitation of extended ASC culture [92]. Furthermore, cell culture typically relies on serum additives (most commonly from xenogenic sources, e.g., fetal bovine serum) to provide a source of necessary growth factors and nutrients. The use of animal products however does lead to concerns for immunogenicity. While several alternatives (such as human PRP) are being actively explored and show promise in vitro studies, they remain to be validated for clinical applications [93].
In order for ASC therapy to be realized as a pre-packaged, “off the shelf” option for OA management, several strategies are currently being explored. First, the ability to cryopreserve ASCs for later use would significantly expand their potential use. While traditional cryopreservation conditions (typically, in media comprising 90% dimethyl sulfoxide and 10% fetal bovine serum) pose concerns for cell toxicity, inhibition of stem cell activity, and immunogenicity, recent studies have explored alternative methods using decreased dimethyl sulfoxide concentration and altered media composition that may improve the stemness/differentiation capacity and/or viability of ASCs following freezing and thawing [94,95].
An additional challenge is the fundamental limitation of relying on autologous ASCs. Stem cell properties are known to vary between different individuals; for instance, it has been well established that ASCs from older donors have decreased viability/proliferation and differentiation potential compared to those derived from younger individuals [96, 97] and that stem cell functions are impaired in ASCs from diabetic individuals [98,99]. Given the demographics of OA, which is most common in the elderly, it is likely that ASCs from OA patients in general may have reduced therapeutic chondrogenic regenerative potential than ASCs from healthy, younger individuals. As such, there is significant interest in developing therapies employing allogeneic ASCs such that ASCs could be derived from young, healthy donors, expanded, and then frozen, to be readily available for clinical use. There are obvious benefits to such an approach; for instance, donors could be pre-screened to optimize ASC viability, and large numbers of ASCs could be grown from a single healthy individual for use in multiple recipients. Allogeneic ASC-based therapeutics are currently in pharmaceutical production although no such products have been specifically developed for OA [100]. However, while ASCs are classically considered minimally immunogenic and in fact may exhibit immunosuppressive/anti-inflammatory properties [101–104], clinical studies have suggested that allogeneic ASC transplantation may result in an anti-donor immune response and lead to sensitization, particularly in patients requiring multiple rounds of treatment [105].
Finally, while early clinical data from patients treated with ASCs have been promising and have shown minimal short-term adverse effects, it is critical to thoroughly establish the long-term safety and efficacy of ASCs for the treatment of OA. To date, studies have been limited to 1–2 years of follow-up. Given the potential for a chronic inflammatory response over time (particularly with degradation of any animal-derived scaffolding materials [93]), as well as inevitable concerns regarding tumor tropism and tumorigenicity [85] (though no increased risk has been found in cancer-free patients receiving ASC transplantation [106]), future studies will need to robustly demonstrate the safety profile of ASCs at later timepoints. More rigorous studies (i.e., randomized, controlled, blinded, prospective clinical trials) with larger patient cohorts are also needed to fully establish the clinical benefits and guidelines for treatment with ASCs.
1.7. Future perspectives
In addition to ASCs showing promising results in early clinical studies in tissue regeneration by direct treatment, ex vivo genetic modification of ASCs could further enhance their intended therapeutic effect [107]. In gene therapy, viral and non-viral vectors are modified to encode a specific protein and consequently introduced into host cells, such as ASCs. The host cells are thus triggered to produce and release a therapeutic agent at concentrations reflective of physiological function, rather than supraphysiological levels as occurs following exogenous morphogen application [107]. In MSK TE, gene therapy using vectors is mostly applied to enhance bone regeneration by triggering host cells to produce bone morphogenetic proteins [107–119] or factors stimulating angiogenesis, e.g. VEGF [108,118]. Using virus-mediated gene transfer encoding bone morphogenetic protein (BMP)-2, BMP-7, BMP-4, or a combination thereof, ASCs have been modified to increase expression of osteogenic proteins in vitro [108–111] and enhanced osteogenesis in vivo in small [109,112–114] and large [116,120] animal models. For chondrogenic differentiation, manipulation of insulin like growth factor-1, transforming growth factor-β1, and BMP-2 expression in ASCs led to increased deposition of cartilage specific matrix, enhanced expression of proteoglycans, and increased collagen type II production in calf-derived MSCs [121,122].
While viral vectors are cost-effective and efficient, safety risks such as toxicity and high immunogenicity have been observed, and thus non-viral gene therapeutics have also been explored. Non-viral gene therapy implements plasmid DNA (pDNA), a circular double-stranded DNA found in bacterial cells, such as E. coli [123]. pDNA-transfected cells expressing BMP-2 have led to increased ALP activity, matrix mineralization and expression of osteogenic markers in vitro [117–119,123,124]) and enhanced osteogenesis and increased levels of osteocalcin and collagen I in vivo [123,124]. In contrast to viral vectors, pDNA has no mutagenic potential, triggers less immune response and delivers the therapeutic in a sustainable manner with no systemic side effects. pDNA approaches are limited however by poor transfection efficiency [108, 109,117–119,123–125]. Nonetheless, this form of gene therapy represents a promising approach to further enhance the physiological function of ASCs.
Finally, safety concerns regarding direct transplantation of ASCs still exist, and in order to circumvent these risks, research has focused on further exploration of paracrine signaling mechanisms of MSCs [51,117, 126]. An accumulating body of work has revealed that the positive effects of MSCs on tissue repair are not facilitated only by direct differentiation into parenchymal cells that repair and replace tissues, but rather by stimulation of recipient cells in recipient tissues via paracrine signaling [127,128]. Exosomes are biomolecular nanostructures, naturally secreted by MSCs and ASCs, and play key roles in paracrine signaling [129]. They are 40–150 nm in size and originate from multivesicular bodies. These nanostructures contain microRNA, messenger RNA, proteins, and lipids and have been demonstrated to directly modulate gene transcription and target cell signaling pathways (Fig. 5) [51,130–133].
Fig. 5.
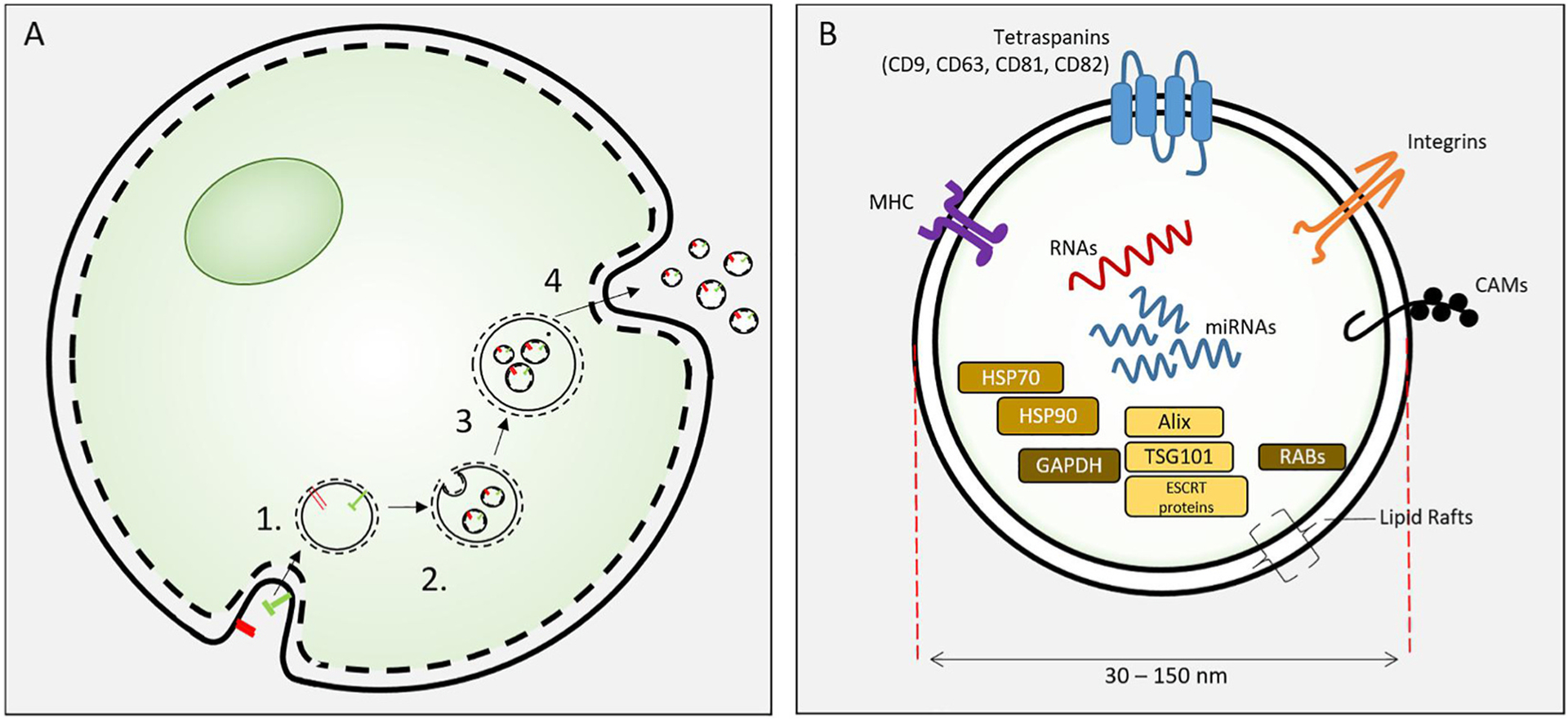
Exosome biosynthesis.
(A). Exosome biosynthesis. Exosomes are formed from endosomes [1] by an inward budding process to form intracellular vesicles [2]. These mature as multivesicular bodies [3] that fuse with the cell plasma membrane to release exosomes. (B) Exosomes are 30–150 nm extracellular vesicles containing specific proteins, RNAs and lipids. Proteins include HSP70, 90, GAPDH; proteins involved in synthesis (e.g. Alix, endosomal sorting complexes required for transport (ESCRT) proteins, TGS101), and membrane associated or transmembrane proteins (RABs, Annexins, CAMs, Integrins, Tetraspanins, MHC I and II) and other cytosolic proteins. Figure from Cooper et al. reproduced with permission [146].
Given the safety concerns regarding direct application of ASCs, exosomes represent a promising alternative for targeted drug delivery as they are non-teratogenic with a low immunogenic potential. The use of ASC-derived exosomes in tissue engineering is an opportunity to circumvent cell-based therapy, negating the risk of neoplastic transformation. MSC-derived exosomes have been shown to increase matrix mineralization and expression of osteogenic genes and proteins in vitro [133–138] and increased bone formation in animal models in vivo [134–140]. Increased osteogenic potential has been mostly ascribed to specific bone-related microRNA contained in exosomes, such as miR-218, miR-196a or miR-21 [141–144]. Many technical challenges still need to be overcome in the development of exosome-based therapeutics (145). The large-scale production of high quality exosomes is the most important factor in their therapeutic application [146]. Traditionally isolated using ultrafiltration, exosome isolation has proved challenging. Tangential flow filtration, rather than ultracentrifugation, has been proposed as the ideal method for industrial scale manufacturing of exosomes and has been implemented with good manufacturing practice in validated processes [145,147]. However, further investigations into standardization of exosomes and their effects are required before they can be implemented in clinical practice.
2. Conclusion
OA is a progressive degenerative joint disease which results in chronic degeneration of articular cartilage and sclerosis of bone. OA represents a significant burden of disease globally, affecting 240 million people in the world and accounts for 2.4% of years lived with disability worldwide. The high prevalence of OA has led to significant advancement and investment in the field of tissue engineering to augment healing and improve traditional surgical interventions. ASCs together with novel scaffold materials are under investigation as potential treatment options for patients suffering with OA. While progress has been made in pre-clinical and clinical studies to date, more rigorous studies are required to fully characterize the therapeutic effects, optimal treatment regimen, and potential long-term adverse effects of ASC treatment for OA. As the field develops greater understanding of material science, we can potentially drive ASC differentiation without the use of exogenous factors, instead opting for material cues to prime cellular differentiation. In order for ASC therapy to progress toward an “off-the-shelf” option for treating OA, several strategies are currently being explored such as ASC cryopreservation and the potential use of allogeneic ASCs. Newer approaches, such as exosome therapy, allow the use of acellular ASC-derived therapies and are currently the focus of ongoing investigation.
Supplementary Material
Acknowledgements
Figs. 1–3 were created using BioRender.com. We would like to thank Virginia Ford and Paulo Pereira for their dedication to excellent lab management and administration.
Funding
This work was supported by the Oak Foundation and the Hagey Laboratory for Pediatric Regenerative Medicine. R.T. was also supported by the Plastic Surgery Research Council Research Fellowship Grant and the Stanford University Transplant and Tissue Engineering Center of Excellence Research Grant. SDI was supported by the Stanford University Medical Scientist Training Program grant T32-GM007365. M.T.L. was supported by NIH Grant R01 DE026730 and R01 DE027323.
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.biomaterials.2022.121544.
References
- [1].Collaborators, G. B. o. D. S, Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013, Lancet 386 (2015) 743–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Nüesch E, Dieppe P, Reichenbach S, Williams S, Iff S, Jüni P, All cause and disease specific mortality in patients with knee or hip osteoarthritis: population based cohort study, Bmj 342 (2011) d1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cisternas MG, Murphy L, Sacks JJ, Solomon DH, Pasta DJ, Helmick CG, Alternative methods for defining osteoarthritis and the impact on estimating prevalence in a US population-based survey, Arthritis Care Res. 68 (2016) 574–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Felson DT, Clinical practice. Osteoarthritis of the knee, N. Engl. J. Med 354 (2006) 841–848. [DOI] [PubMed] [Google Scholar]
- [5].Tevlin R, Walmsley GG, Marecic O, Hu MS, Wan DC, Longaker MT, Stem and progenitor cells: advancing bone tissue engineering, Drug Deliv Transl Res 6 (2016) 159–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Loebel C, Burdick JA, Engineering stem and stromal cell therapies for musculoskeletal tissue repair, Cell Stem Cell 22 (2018) 325–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Si Z, Wang X, Sun C, et al. , Adipose-derived stem cells: sources, potency, and implications for regenerative therapies, Biomed. Pharmacother 114 (2019) 108765. [DOI] [PubMed] [Google Scholar]
- [8].Daniela F, Vescovi AL, Bottai D, The stem cells as a potential treatment for neurodegeneration, Methods Mol. Biol 399 (2007) 199–213. [DOI] [PubMed] [Google Scholar]
- [9].Chargé SB, Rudnicki MA, Cellular and molecular regulation of muscle regeneration, Physiol. Rev 84 (2004) 209–238. [DOI] [PubMed] [Google Scholar]
- [10].Zuk PA, Zhu M, Ashjian P, et al. , Human adipose tissue is a source of multipotent stem cells, Mol. Biol. Cell 13 (2002) 4279–4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chan CK, Seo EY, Chen JY, et al. , Identification and specification of the mouse skeletal stem cell, Cell 160 (2015) 285–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chan CKF, Gulati GS, Sinha R, et al. , Identification of the human skeletal stem cell, Cell 175 (2018) 43–56 e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Worthley DL, Churchill M, Compton JT, et al. , Gremlin 1 identifies a skeletal stem cell with bone, cartilage, and reticular stromal potential, Cell 160 (2015) 269–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Méndez-Ferrer S, Michurina TV, Ferraro F, et al. , Mesenchymal and haematopoietic stem cells form a unique bone marrow niche, Nature 466 (2010) 829–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Duchamp de Lageneste O, Julien A, Abou-Khalil R, et al. , Periosteum contains skeletal stem cells with high bone regenerative potential controlled by Periostin, Nat. Commun 9 (2018) 773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Tevlin R, Seo EY, Marecic O, et al. , Pharmacological rescue of diabetic skeletal stem cell niches, Sci. Transl. Med 9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tevlin R, McArdle A, Brett E, et al. , A novel method of human adipose-derived stem cell isolation with resultant increased cell yield, Plast. Reconstr. Surg 138 (2016) 983e–996e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cheung TH, Rando TA, Molecular regulation of stem cell quiescence, Nat. Rev. Mol. Cell Biol 14 (2013) 329–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Aust L, Devlin B, Foster SJ, et al. , Yield of human adipose-derived adult stem cells from liposuction aspirates, Cytotherapy 6 (2004) 7–14. [DOI] [PubMed] [Google Scholar]
- [20].Zhu Y, Liu T, Song K, Fan X, Ma X, Cui Z, Adipose-derived stem cell: a better stem cell than BMSC, Cell Biochem. Funct 26 (2008) 664–675. [DOI] [PubMed] [Google Scholar]
- [21].Frasca D, Blomberg BB, Adipose tissue, immune aging, and cellular senescence, Semin. Immunopathol 42 (2020) 573–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zwick RK, Guerrero-Juarez CF, Horsley V, Plikus MV, Anatomical, physiological, and functional diversity of adipose tissue, Cell Metabol. 27 (2018) 68–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Dominici M, Le Blanc K, Mueller I, et al. , Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement, Cytotherapy 8 (2006) 315–317. [DOI] [PubMed] [Google Scholar]
- [24].Brett E, Tevlin R, McArdle A, et al. , Human adipose-derived stromal cell isolation methods and use in osteogenic and adipogenic in vivo applications, Curr Protoc Stem Cell Biol 43 (2017) 2h.1.1–2h.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Surgery, A. S. o. P, in: A. S. o. P. Surgery (Ed.), Plastic Surgery Statistic Report, 2019. [Google Scholar]
- [26].Bateman ME, Strong AL, Gimble JM, Bunnell BA, Concise review: using fat to fight disease: a systematic review of nonhomologous adipose-derived stromal/stem cell therapies, Stem Cell. 36 (2018) 1311–1328. [DOI] [PubMed] [Google Scholar]
- [27].Panetta NJ, Gupta DM, Kwan MD, Wan DC, Commons GW, Longaker MT, Tissue harvest by means of suction-assisted or third-generation ultrasound-assisted lipoaspiration has no effect on osteogenic potential of human adipose-derived stromal cells, Plast. Reconstr. Surg 124 (2009) 65–73. [DOI] [PubMed] [Google Scholar]
- [28].Duscher D, Maan ZN, Luan A, et al. , Ultrasound-assisted liposuction provides a source for functional adipose-derived stromal cells, Cytotherapy 19 (2017) 1491–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Duscher D, Atashroo D, Maan ZN, et al. , Ultrasound-assisted liposuction does not compromise the regenerative potential of adipose-derived stem cells, Stem Cells Transl Med 5 (2016) 248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Duscher D, Luan A, Rennert RC, et al. , Suction assisted liposuction does not impair the regenerative potential of adipose derived stem cells, J. Transl. Med 14 (2016) 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chung MT, Zimmermann AS, Paik KJ, et al. , Isolation of human adipose-derived stromal cells using laser-assisted liposuction and their therapeutic potential in regenerative medicine, Stem Cells Transl Med 2 (2013) 808–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Gimble JM, Bunnell BA, Frazier T, et al. , Adipose-derived stromal/stem cells: a primer, Organogenesis 9 (2013) 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hendijani F, Explant culture: an advantageous method for isolation of mesenchymal stem cells from human tissues, Cell Prolif 50 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sherman LS, Condé-Green A, Kotamarti VS, Lee ES, Rameshwar P, Enzyme-free isolation of adipose-derived mesenchymal stem cells, Methods Mol. Biol 1842 (2018) 203–206. [DOI] [PubMed] [Google Scholar]
- [35].Chang H, Do BR, Che JH, et al. , Safety of adipose-derived stem cells and collagenase in fat tissue preparation, Aesthetic Plast. Surg 37 (2013) 802–808. [DOI] [PubMed] [Google Scholar]
- [36].Spees JL, Gregory CA, Singh H, et al. , Internalized antigens must be removed to prepare hypoimmunogenic mesenchymal stem cells for cell and gene therapy, Mol. Ther 9 (2004) 747–756. [DOI] [PubMed] [Google Scholar]
- [37].Horwitz EM, Gordon PL, Koo WK, et al. , Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: implications for cell therapy of bone, Proc. Natl. Acad. Sci. U. S. A 99 (2002) 8932–8937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Raposio E, Caruana G, Bonomini S, Libondi G, A novel and effective strategy for the isolation of adipose-derived stem cells: minimally manipulated adipose-derived stem cells for more rapid and safe stem cell therapy, Plast. Reconstr. Surg 133 (2014) 1406–1409. [DOI] [PubMed] [Google Scholar]
- [39].Guo J, Widgerow AD, Banyard D, et al. , Strategic sequences in fat graft survival, Ann. Plast. Surg 74 (2015) 376–382. [DOI] [PubMed] [Google Scholar]
- [40].Tsuji K, Ojima M, Otabe K, et al. , Effects of different cell-detaching methods on the viability and cell surface antigen expression of synovial mesenchymal stem cells, Cell Transplant. 26 (2017) 1089–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Gimble JM, Bunnell BA, Guilak F, Human adipose-derived cells: an update on the transition to clinical translation, Regen. Med 7 (2012) 225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Dubey NK, Mishra VK, Dubey R, Deng YH, Tsai FC, Deng WP, Revisiting the advances in isolation, characterization and secretome of adipose-derived stromal/stem cells, Int. J. Mol. Sci 19 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Frese L, Dijkman PE, Hoerstrup SP, Adipose tissue-derived stem cells in regenerative medicine, Transfus Med Hemother 43 (2016) 268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kapur SK, Katz AJ, Review of the adipose derived stem cell secretome, Biochimie 95 (2013) 2222–2228. [DOI] [PubMed] [Google Scholar]
- [45].Lombardi F, Palumbo P, Augello FR, Cifone MG, Cinque B, Giuliani M, Secretome of adipose tissue-derived stem cells (ASCs) as a novel trend in chronic non-healing wounds: an overview of experimental in vitro and in vivo studies and methodological variables, Int. J. Mol. Sci 20 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Lv X, He J, Zhang X, et al. , Comparative efficacy of autologous stromal vascular fraction and autologous adipose-derived mesenchymal stem cells combined with hyaluronic acid for the treatment of sheep osteoarthritis, Cell Transplant. 27 (2018) 1111–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Storti G, Scioli MG, Kim BS, Orlandi A, Cervelli V, Adipose-derived stem cells in bone tissue engineering: useful tools with new applications, Stem Cell. Int 2019 (2019) 3673857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Mancuso P, Raman S, Glynn A, Barry F, Murphy JM, Mesenchymal stem cell therapy for osteoarthritis: the critical role of the cell secretome, Front. Bioeng. Biotechnol 7 (2019) 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Zuk P The ASC: critical participants in paracrine-mediated tissue Health and function. In I. Open ed., Regenerative Medicine and Tissue Engineering, Vol. vol. 1. Peer-reviewed online chapter Intech Open; 2012. [Google Scholar]
- [50].Salgado AJ, Reis RL, Sousa NJ, Gimble JM, Adipose tissue derived stem cells secretome: soluble factors and their roles in regenerative medicine, Curr. Stem Cell Res. Ther 5 (2010) 103–110. [DOI] [PubMed] [Google Scholar]
- [51].Caplan AI, Dennis JE, Mesenchymal stem cells as trophic mediators, J. Cell. Biochem 98 (2006) 1076–1084. [DOI] [PubMed] [Google Scholar]
- [52].Giannasi C, Niada S, Magagnotti C, Ragni E, Andolfo A, Brini AT, Comparison of two ASC-derived therapeutics in an in vitro OA model: secretome versus extracellular vesicles, Stem Cell Res. Ther 11 (2020) 521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Ceccarelli S, Pontecorvi P, Anastasiadou E, Napoli C, Marchese C, Immunomodulatory effect of adipose-derived stem cells: the cutting edge of clinical application, Front. Cell Dev. Biol 8 (2020) 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].McArdle A, Chung MT, Paik KJ, et al. , Positive selection for bone morphogenetic protein receptor type-IB promotes differentiation and specification of human adipose-derived stromal cells toward an osteogenic lineage, Tissue Eng Part A 20 (2014) 3031–3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Chung MT, Liu C, Hyun JS, et al. , CD90 (Thy-1)-positive selection enhances osteogenic capacity of human adipose-derived stromal cells, Tissue Eng Part A 19 (2013) 989–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Levi B, Wan DC, Glotzbach JP, et al. , CD105 protein depletion enhances human adipose-derived stromal cell osteogenesis through reduction of transforming growth factor β1 (TGF-β1) signaling, J. Biol. Chem 286 (2011) 39497–39509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Sugihara H, Funatsumaru S, Yonemitsu N, Miyabara S, Toda S, Hikichi Y, A simple culture method of fat cells from mature fat tissue fragments, J. Lipid Res 30 (1989) 1987–1995. [PubMed] [Google Scholar]
- [58].Khan WS, Rayan F, Dhinsa BS, Marsh D, An osteoconductive, osteoinductive, and osteogenic tissue-engineered product for trauma and orthopaedic surgery: how far are we? Stem Cell. Int 2012 (2012) 236231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Yuan H, Li Y, de Bruijn JD, de Groot K, Zhang X, Tissue responses of calcium phosphate cement: a study in dogs, Biomaterials 21 (2000) 1283–1290. [DOI] [PubMed] [Google Scholar]
- [60].Gasparotto VP, Landim-Alvarenga FC, Oliveira AL, et al. , A new fibrin sealant as a three-dimensional scaffold candidate for mesenchymal stem cells, Stem Cell Res. Ther 5 (2014) 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Ibrahim A, Rodriguez-Florez N, Gardner OFW, et al. , Three-dimensional environment and vascularization induce osteogenic maturation of human adipose-derived stem cells comparable to that of bone-derived progenitors, Stem Cells Transl Med 9 (12) (2020) 1651–1666, 10.1002/sctm.19-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Feng C, Luo X, He N, et al. , Efficacy and persistence of allogeneic adipose-derived mesenchymal stem cells combined with hyaluronic acid in osteoarthritis after intra-articular injection in a sheep model, Tissue Eng Part A 24 (2018) 219–233. [DOI] [PubMed] [Google Scholar]
- [63].Engler AJ, Sen S, Sweeney HL, Discher DE, Matrix elasticity directs stem cell lineage specification, Cell 126 (2006) 677–689. [DOI] [PubMed] [Google Scholar]
- [64].Lin X, Shi Y, Cao Y, Liu W, Recent progress in stem cell differentiation directed by material and mechanical cues, Biomed. Mater 11 (2016), 014109. [DOI] [PubMed] [Google Scholar]
- [65].Ogawa R, Mizuno S, Murphy GF, Orgill DP, The effect of hydrostatic pressure on three-dimensional chondroinduction of human adipose-derived stem cells, Tissue Eng Part A 15 (2009) 2937–2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Safshekan F, Tafazzoli-Shadpour M, Shokrgozar MA, Haghighipour N, Mahdian R, Hemmati A, Intermittent hydrostatic pressure enhances growth factor-induced chondroinduction of human adipose-derived mesenchymal stem cells, Artif. Organs 36 (2012) 1065–1071. [DOI] [PubMed] [Google Scholar]
- [67].Zhang K, He S, Yan S, et al. , Regeneration of hyaline-like cartilage and subchondral bone simultaneously by poly(l-glutamic acid) based osteochondral scaffolds with induced autologous adipose derived stem cells, J. Mater. Chem. B 4 (2016) 2628–2645. [DOI] [PubMed] [Google Scholar]
- [68].Mellor LF, Mohiti-Asli M, Williams J, et al. , Extracellular calcium modulates chondrogenic and osteogenic differentiation of human adipose-derived stem cells: a novel approach for osteochondral tissue engineering using a single stem cell source, Tissue Eng Part A 21 (2015) 2323–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Li Y, Xiao Y, Liu C, The horizon of materiobiology: a perspective on material-guided cell behaviors and tissue engineering, Chem. Rev 117 (2017) 4376–4421. [DOI] [PubMed] [Google Scholar]
- [70].Zhou K, Feng B, Wang W, et al. , Nanoscaled and microscaled parallel topography promotes tenogenic differentiation of ASC and neotendon formation in vitro, Int J Nanomedicine 13 (2018) 3867–3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Comella K, Silbert R, Parlo M, Effects of the intradiscal implantation of stromal vascular fraction plus platelet rich plasma in patients with degenerative disc disease, J. Transl. Med 15 (2017) 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Fotouhi A, Maleki A, Dolati S, Aghebati-Maleki A, Aghebati-Maleki L, Platelet rich plasma, stromal vascular fraction and autologous conditioned serum in treatment of knee osteoarthritis, Biomed. Pharmacother 104 (2018) 652–660. [DOI] [PubMed] [Google Scholar]
- [73].Mehranfar S, Abdi Rad I, Mostafav E, Akbarzadeh A, The use of stromal vascular fraction (SVF), platelet-rich plasma (PRP) and stem cells in the treatment of osteoarthritis: an overview of clinical trials, Artif. Cell Nanomed. Biotechnol 47 (2019) 882–890. [DOI] [PubMed] [Google Scholar]
- [74].Pak J, Regeneration of human bones in hip osteonecrosis and human cartilage in knee osteoarthritis with autologous adipose-tissue-derived stem cells: a case series, J. Med. Case Rep 5 (2011) 296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Torres-Torrillas M, Rubio M, Damia E, et al. , Adipose-derived mesenchymal stem cells: a promising tool in the treatment of musculoskeletal diseases, Int. J. Mol. Sci 20 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Michalek J, Vrablikova A, Darinskas A, et al. , Stromal vascular fraction cell therapy for osteoarthritis in elderly: multicenter case-control study, J Clin Orthop Trauma 10 (2019) 76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Jo CH, Lee YG, Shin WH, et al. , Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial, Stem Cell. 32 (2014) 1254–1266. [DOI] [PubMed] [Google Scholar]
- [78].Fodor PB, Paulseth SG, Adipose derived stromal cell (ADSC) injections for pain management of osteoarthritis in the human knee joint, Aesthetic Surg. J 36 (2016) 229–236. [DOI] [PubMed] [Google Scholar]
- [79].Pers YM, Rackwitz L, Ferreira R, et al. , Adipose mesenchymal stromal cell-based therapy for severe osteoarthritis of the knee: a phase I dose-escalation trial, Stem Cells Transl Med 5 (2016) 847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Higuchi J, Yamagami R, Matsumoto T, et al. , Associations of clinical outcomes and MRI findings in intra-articular administration of autologous adipose-derived stem cells for knee osteoarthritis, Regen Ther 14 (2020) 332–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].N.I.o. Health, ClinicalTrials.gov, Available at: https://clinicaltrials.gov/ct2/results?cond=Osteoarthritis&term=adipose+derived+stem+cell&cntry=&state=&city=&dist=.
- [82].Kim YS, Koh YG, Injection of mesenchymal stem cells as a supplementary strategy of marrow stimulation improves cartilage regeneration after lateral sliding calcaneal osteotomy for varus ankle osteoarthritis: clinical and second-look arthroscopic results, Arthroscopy 32 (2016) 878–889. [DOI] [PubMed] [Google Scholar]
- [83].Kim YS, Lee M, Koh YG, Additional mesenchymal stem cell injection improves the outcomes of marrow stimulation combined with supramalleolar osteotomy in varus ankle osteoarthritis: short-term clinical results with second-look arthroscopic evaluation, J Exp Orthop 3 (2016) 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Hong Z, Chen J, Zhang S, et al. , Intra-articular injection of autologous adipose-derived stromal vascular fractions for knee osteoarthritis: a double-blind randomized self-controlled trial, Int. Orthop 43 (2019) 1123–1134. [DOI] [PubMed] [Google Scholar]
- [85].Şovrea AS, Boşca AB, Constantin AM, Dronca E, Ilea A, State of the art in human adipose stem cells and their role in therapy, Rom. J. Morphol. Embryol 60 (2019) 7–31. [PubMed] [Google Scholar]
- [86].Roato I, Belisario DC, Compagno M, et al. , Concentrated adipose tissue infusion for the treatment of knee osteoarthritis: clinical and histological observations, Int. Orthop 43 (2019) 15–23. [DOI] [PubMed] [Google Scholar]
- [87].Yokota N, Hattori M, Ohtsuru T, et al. , Comparative clinical outcomes after intra-articular injection with adipose-derived cultured stem cells or noncultured stromal vascular fraction for the treatment of knee osteoarthritis, Am. J. Sports Med 47 (2019) 2577–2583. [DOI] [PubMed] [Google Scholar]
- [88].Pak J, Lee JH, Pak N, et al. , Cartilage regeneration in humans with adipose tissue-derived stem cells and adipose stromal vascular fraction cells: updated status, Int. J. Mol. Sci 19 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Doornaert M, De Maere E, Colle J, et al. , Xenogen-free isolation and culture of human adipose mesenchymal stem cells, Stem Cell Res. 40 (2019) 101532. [DOI] [PubMed] [Google Scholar]
- [90].Miyazaki T, Kitagawa Y, Toriyama K, Kobori M, Torii S, Isolation of two human fibroblastic cell populations with multiple but distinct potential of mesenchymal differentiation by ceiling culture of mature fat cells from subcutaneous adipose tissue, Differentiation 73 (2005) 69–78. [DOI] [PubMed] [Google Scholar]
- [91].Yang YI, Kim HI, Choi MY, et al. , Ex vivo organ culture of adipose tissue for in situ mobilization of adipose-derived stem cells and defining the stem cell niche, J. Cell. Physiol 224 (2010) 807–816. [DOI] [PubMed] [Google Scholar]
- [92].Sohni A, Verfaillie CM, Mesenchymal stem cells migration homing and tracking, Stem Cell. Int 2013 (2013) 130763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Dai R, Wang Z, Samanipour R, Koo KI, Kim K, Adipose-derived stem cells for tissue engineering and regenerative medicine applications, Stem Cell. Int 2016 (2016) 6737345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].De Rosa A, De Francesco F, Tirino V, et al. , A new method for cryopreserving adipose-derived stem cells: an attractive and suitable large-scale and long-term cell banking technology, Tissue Eng. C Methods 15 (2009) 659–667. [DOI] [PubMed] [Google Scholar]
- [95].Miyamoto Y, Oishi K, Yukawa H, et al. , Cryopreservation of human adipose tissue-derived stem/progenitor cells using the silk protein sericin, Cell Transplant. 21 (2012) 617–622. [DOI] [PubMed] [Google Scholar]
- [96].Marędziak M, Marycz K, Tomaszewski KA, Kornicka K, Henry BM, The influence of aging on the regenerative potential of human adipose derived mesenchymal stem cells, Stem Cell. Int 2016 (2016) 2152435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Jin Y, Yang L, Zhang Y, et al. , Effects of age on biological and functional characterization of adipose-derived stem cells from patients with end-stage liver disease, Mol. Med. Rep 16 (2017) 3510–3518. [DOI] [PubMed] [Google Scholar]
- [98].Jumabay M, Moon JH, Yeerna H, Boström KI, Effect of diabetes mellitus on adipocyte-derived stem cells in rat, J. Cell. Physiol 230 (2015) 2821–2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Alicka M, Major P, Wysocki M, Marycz K, Adipose-derived mesenchymal stem cells isolated from patients with type 2 diabetes show reduced “stemness” through an altered secretome profile, impaired anti-oxidative protection, and mitochondrial dynamics deterioration, J. Clin. Med 8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Patrikoski M, Mannerström B, Miettinen S, Perspectives for clinical translation of adipose stromal/stem cells, Stem Cell. Int 2019 (2019) 5858247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Feisst V, Meidinger S, Locke MB, From bench to bedside: use of human adipose-derived stem cells, Stem Cells Cloning 8 (2015) 149–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].McIntosh KR, Lopez MJ, Borneman JN, Spencer ND, Anderson PA, Gimble JM, Immunogenicity of allogeneic adipose-derived stem cells in a rat spinal fusion model, Tissue Eng Part A 15 (2009) 2677–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Lin CS, Lin G, Lue TF, Allogeneic and xenogeneic transplantation of adipose-derived stem cells in immunocompetent recipients without immunosuppressants, Stem Cell. Dev 21 (2012) 2770–2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Griffin MD, Ritter T, Mahon BP, Immunological aspects of allogeneic mesenchymal stem cell therapies, Hum. Gene Ther 21 (2010) 1641–1655. [DOI] [PubMed] [Google Scholar]
- [105].Avivar-Valderas A, Martín-Martín C, Ramírez C, et al. , Dissecting allo-sensitization after local administration of human allogeneic adipose mesenchymal stem cells in perianal fistulas of crohn’s disease patients, Front. Immunol 10 (2019) 1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Minteer DM, Marra KG, Rubin JP, Adipose stem cells: biology, safety, regulation, and regenerative potential, Clin. Plast. Surg 42 (2015) 169–179. [DOI] [PubMed] [Google Scholar]
- [107].Raftery RM, Walsh DP, Castaño IM, et al. , Delivering nucleic-acid based nanomedicines on biomaterial scaffolds for orthopedic tissue repair: challenges, progress and future perspectives, Adv. Mater 28 (2016) 5447–5469. [DOI] [PubMed] [Google Scholar]
- [108].Evans C, Gene therapy for the regeneration of bone, Injury 42 (2011) 599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Wright V, Peng H, Usas A, et al. , BMP4-expressing muscle-derived stem cells differentiate into osteogenic lineage and improve bone healing in immunocompetent mice, Mol. Ther 6 (2002) 169–178. [DOI] [PubMed] [Google Scholar]
- [110].Wu CC, Wang F, Rong S, et al. , Enhancement of osteogenesis of rabbit bone marrow derived mesenchymal stem cells by transfection of human BMP-2 and EGFP recombinant adenovirus via Wnt signaling pathway, Exp. Ther. Med 16 (2018) 4030–4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Cao H, Sun ZB, Zhang L, et al. , Adenovirus-mediated bone morphogenetic protein-2 promotes osteogenic differentiation in human mesenchymal stem cells in vitro, Exp. Ther. Med 14 (2017) 377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Lieberman JR, Daluiski A, Stevenson S, et al. , The effect of regional gene therapy with bone morphogenetic protein-2-producing bone-marrow cells on the repair of segmental femoral defects in rats, J Bone Joint Surg Am 81 (1999) 905–917. [DOI] [PubMed] [Google Scholar]
- [113].Park S, Heo HA, Lee KB, Kim HG, Pyo SW, Improved bone regeneration with multiporous PLGA scaffold and BMP-2-transduced human adipose-derived stem cells by cell-permeable peptide, Implant Dent. 26 (2017) 4–11. [DOI] [PubMed] [Google Scholar]
- [114].Peterson B, Zhang J, Iglesias R, et al. , Healing of critically sized femoral defects, using genetically modified mesenchymal stem cells from human adipose tissue, Tissue Eng. 11 (2005) 120–129. [DOI] [PubMed] [Google Scholar]
- [115].Chang SC, Lin TM, Chung HY, et al. , Large-scale bicortical skull bone regeneration using ex vivo replication-defective adenoviral-mediated bone morphogenetic protein-2 gene-transferred bone marrow stromal cells and composite biomaterials, Neurosurgery 65 (2009) 75–81, discussion 81–73. [DOI] [PubMed] [Google Scholar]
- [116].Zhu L, Chuanchang D, Wei L, Yilin C, Jiasheng D, Enhanced healing of goat femur-defect using BMP7 gene-modified BMSCs and load-bearing tissue-engineered bone, J. Orthop. Res 28 (2010) 412–418. [DOI] [PubMed] [Google Scholar]
- [117].Feichtinger GA, Hacobian A, Hofmann AT, et al. , Constitutive and inducible co-expression systems for non-viral osteoinductive gene therapy, Eur. Cell. Mater 27 (2014) 166–184, discussion 184. [DOI] [PubMed] [Google Scholar]
- [118].Guo-ping W, Xiao-chuan H, Zhi-hui Y, Li G, Influence on the osteogenic activity of the human bone marrow mesenchymal stem cells transfected by liposome-mediated recombinant plasmid pIRES-hBMP2-hVEGF165 in vitro, Ann. Plast. Surg 65 (2010) 80–84. [DOI] [PubMed] [Google Scholar]
- [119].Loozen LD, Kruyt MC, Kragten AHM, et al. , BMP-2 gene delivery in cell-loaded and cell-free constructs for bone regeneration, PLoS One 14 (2019), e0220028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Dai KR, Xu XL, Tang TT, et al. , Repairing of goat tibial bone defects with BMP-2 gene-modified tissue-engineered bone, Calcif. Tissue Int 77 (2005) 55–61. [DOI] [PubMed] [Google Scholar]
- [121].Steinert AF, Palmer GD, Pilapil C, Nöth U, Evans CH, Ghivizzani SC, Enhanced in vitro chondrogenesis of primary mesenchymal stem cells by combined gene transfer, Tissue Eng Part A 15 (2009) 1127–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Palmer GD, Steinert A, Pascher A, et al. , Gene-induced chondrogenesis of primary mesenchymal stem cells in vitro, Mol. Ther 12 (2005) 219–228. [DOI] [PubMed] [Google Scholar]
- [123].Raftery RM, Mencía-Castaño I, Sperger S, et al. , Delivery of the improved BMP-2-Advanced plasmid DNA within a gene-activated scaffold accelerates mesenchymal stem cell osteogenesis and critical size defect repair, J. Contr. Release 283 (2018) 20–31. [DOI] [PubMed] [Google Scholar]
- [124].Wegman F, Bijenhof A, Schuijff L, Oner FC, Dhert WJ, Alblas J, Osteogenic differentiation as a result of BMP-2 plasmid DNA based gene therapy in vitro and in vivo, Eur. Cell. Mater 21 (2011) 230–242, discussion 242. [DOI] [PubMed] [Google Scholar]
- [125].Evans CH, Huard J, Gene therapy approaches to regenerating the musculoskeletal system, Nat. Rev. Rheumatol 11 (2015) 234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Katsha AM, Ohkouchi S, Xin H, et al. , Paracrine factors of multipotent stromal cells ameliorate lung injury in an elastase-induced emphysema model, Mol. Ther 19 (2011) 196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Liang X, Ding Y, Zhang Y, Tse HF, Lian Q, Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives, Cell Transplant. 23 (2014) 1045–1059. [DOI] [PubMed] [Google Scholar]
- [128].Baglio SR, Pegtel DM, Baldini N, Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy, Front. Physiol 3 (2012) 359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Hu GW, Li Q, Niu X, et al. , Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells attenuate limb ischemia by promoting angiogenesis in mice, Stem Cell Res. Ther 6 (2015) 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Yu B, Zhang X, Li X, Exosomes derived from mesenchymal stem cells, Int. J. Mol. Sci 15 (2014) 4142–4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Lai RC, Yeo RW, Lim SK, Mesenchymal stem cell exosomes, Semin. Cell Dev. Biol 40 (2015) 82–88. [DOI] [PubMed] [Google Scholar]
- [132].Kourembanas S, Exosomes: vehicles of intercellular signaling, biomarkers, and vectors of cell therapy, Annu. Rev. Physiol 77 (2015) 13–27. [DOI] [PubMed] [Google Scholar]
- [133].Raposo G, Stoorvogel W, Extracellular vesicles: exosomes, microvesicles, and friends, J. Cell Biol 200 (2013) 373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Narayanan R, Huang CC, Ravindran S, Hijacking the cellular mail: exosome mediated differentiation of mesenchymal stem cells, Stem Cell. Int 2016 (2016) 3808674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Li W, Liu Y, Zhang P, et al. , Tissue-Engineered bone immobilized with human adipose stem cells-derived exosomes promotes bone regeneration, ACS Appl. Mater. Interfaces 10 (2018) 5240–5254. [DOI] [PubMed] [Google Scholar]
- [136].Qi X, Zhang J, Yuan H, et al. , Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells repair critical-sized bone defects through enhanced angiogenesis and osteogenesis in osteoporotic rats, Int. J. Biol. Sci 12 (2016) 836–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Zhang J, Liu X, Li H, et al. , Exosomes/tricalcium phosphate combination scaffolds can enhance bone regeneration by activating the PI3K/Akt signaling pathway, Stem Cell Res. Ther 7 (2016) 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Martins M, Ribeiro D, Martins A, Reis RL, Neves NM, Extracellular vesicles derived from osteogenically induced human bone marrow mesenchymal stem cells can modulate lineage commitment, Stem Cell Rep. 6 (2016) 284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Liu S, Liu D, Chen C, et al. , MSC transplantation improves osteopenia via epigenetic regulation of notch signaling in lupus, Cell Metabol. 22 (2015) 606–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Furuta T, Miyaki S, Ishitobi H, et al. , Mesenchymal stem cell-derived exosomes promote fracture healing in a mouse model, Stem Cells Transl Med 5 (2016) 1620–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Xu JF, Yang GH, Pan XH, et al. , Altered microRNA expression profile in exosomes during osteogenic differentiation of human bone marrow-derived mesenchymal stem cells, PLoS One 9 (2014), e114627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Qin Y, Wang L, Gao Z, Chen G, Zhang C, Bone marrow stromal/stem cell-derived extracellular vesicles regulate osteoblast activity and differentiation in vitro and promote bone regeneration in vivo, Sci. Rep 6 (2016) 21961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Taipaleenmäki H, Bjerre Hokland L, Chen L, Kauppinen S, Kassem M, Mechanisms in endocrinology: micro-RNAs: targets for enhancing osteoblast differentiation and bone formation, Eur. J. Endocrinol 166 (2012) 359–371. [DOI] [PubMed] [Google Scholar]
- [144].Zhang WB, Zhong WJ, Wang L, A signal-amplification circuit between miR-218 and Wnt/β-catenin signal promotes human adipose tissue-derived stem cells osteogenic differentiation, Bone 58 (2014) 59–66. [DOI] [PubMed] [Google Scholar]
- [145].Lee JH, Ha DH, Go HK, et al. , Reproducible large-scale isolation of exosomes from adipose tissue-derived mesenchymal stem/stromal cells and their application in acute kidney injury, Int. J. Mol. Sci 21 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Cooper LF, Ravindran S, Huang CC, Kang M, A role for exosomes in craniofacial tissue engineering and regeneration, Front. Physiol 10 (2019) 1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Lai RC, Arslan F, Lee MM, et al. , Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury, Stem Cell Res. 4 (2010) 214–222. [DOI] [PubMed] [Google Scholar]
- [148].Zhu M, Heydarkhan-Hagvall S, Hedrick M, Benhaim P, Zuk P, Manual isolation of adipose-derived stem cells from human lipoaspirates, JoVE (2013), e50585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Qiao Z, Tang J, Yue B, et al. , Human adipose-derived mesenchymal progenitor cells plus microfracture and hyaluronic acid for cartilage repair: a Phase IIa trial, Regen. Med 15 (2020) 1193–1214. [DOI] [PubMed] [Google Scholar]
- [150].Garza J, Maria DS, Palomera T, Dumanian G, Dos-Anjos S, Use of Autologous Adipose- Derived Stromal Vascular Fraction to Treat Osteoarthritis of the Knee: A Feasibility and Safety Study, 2014. [Google Scholar]
- [151].Zhao X, Ruan J, Tang H, et al. , Multi-compositional MRI evaluation of repair cartilage in knee osteoarthritis with treatment of allogeneic human adipose-derived mesenchymal progenitor cells, Stem Cell Res. Ther 10 (2019) 308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Lee WS, Kim HJ, Kim KI, Kim GB, Jin W, Intra-articular injection of autologous adipose tissue-derived mesenchymal stem cells for the treatment of knee osteoarthritis: a phase IIb, randomized, placebo-controlled clinical trial, Stem Cells Transl Med 8 (2019) 504–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Koh YG, Kwon OR, Kim YS, Choi YJ, Tak DH, Adipose-derived mesenchymal stem cells with microfracture versus microfracture alone: 2-year follow-up of a prospective randomized trial, Arthroscopy 32 (2016) 97–109. [DOI] [PubMed] [Google Scholar]
- [154].Schiavone Panni A, Vasso M, Braile A, et al. , Preliminary results of autologous adipose-derived stem cells in early knee osteoarthritis: identification of a subpopulation with greater response, Int. Orthop 43 (2019) 7–13. [DOI] [PubMed] [Google Scholar]
- [155].Song Y, Du H, Dai C, et al. , Human adipose-derived mesenchymal stem cells for osteoarthritis: a pilot study with long-term follow-up and repeated injections, Regen. Med 13 (2018) 295–307. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


