Abstract
During the past 30 years, 3D printing (3DP) technologies significantly influenced the manufacturing world, including innovation in biomedical devices. This special issue reviews recent advances in translating 3DP biomaterials and medical devices for metallic, ceramic, and polymeric devices, as well as bioprinting for organ and tissue engineering, along with regulatory issues in 3DP biomaterials. In our introductory article, besides introducing selected 3DP processes for biomaterials, current challenges and growth opportunities are also discussed. Finally, it highlights a few success stories for the 3D printed biomaterials for medical devices. We hope these articles will educate engineers, scientists, and clinicians about recent developments in translational 3DP technologies.
Introduction
Three dimensional printing (3DP), also known as additive manufacturing (AM), is an approach where functional parts can be printed directly from a computer-aided design (CAD) file without any part-specific tooling. Although the first commercial machine was available in the late 1980s, most industries have already accepted this disruptive manufacturing approach by now. And among them, biomedical device industries are probably at the forefront where the translation of various novel implant designs became a reality due to 3DP. The American Society for Testing and Materials (ASTM) has classified various 3DP technologies into seven broad categories based only on their part manufacturing approaches, summarized in Table I.1,2 These ASTM classified processes only focus on acellular materials; however, 3DP of cellular materials along with acellular materials is also becoming popular, commonly known as bioprinting. This Materials Research Society (MRS) Bulletin focuses on translating 3DP of biomaterials and biomedical devices. Before discussing some of the critical issues related to translation, the following section briefly discusses different 3DP processes relevant to biomaterials and biomedical devices.
Table I.
| 3DP Processes and Materials | Advantages and Disadvantages | Applications |
|---|---|---|
| Vat photopolymerization. Example—Stereolithography (SLA). Materials: Polymers, ceramics-polymer composites. |
A legacy process where high resolution parts can be made. Uses toxic monomers as feed stock material. | Mostly used with Polymers. Ceramic-loaded polymeric systems for dental implants and surgical tools. |
| Materials extrusion. Example—Fused deposition modeling (FDM). Materials: Polymer, Metal-polymer, and ceramics-polymer composites. |
The most popular 3DP process. Easy to use. Part resolution depends on nozzle diameter. | Extensively used for surgical models and bioprinting with cells. Also used for surgical tools. |
| Materials Jetting. Example—Poly jet. Materials:Thermoplastic and thermoset polymers. |
High-resolution multi-color parts can be made. Multi-material parts with variable stiffness can also be made. | Multi-material and multi-color parts for surgical models; surgical models and surgical tools. |
| Binder Jetting. Materials: Metals, ceramics, polymers, and glasses. |
A versatile process. Green strength for the parts is low. High-resolution parts can be made. Post-processing, such as sintering, is needed for metals and ceramics. | Porous scaffolds, surgical models, surgical tools. |
| Sheet Lamination. Materials: Paper, polymers, and composites. |
Paper-based parts are suitable for concept models with vibrant colors. | Color surgical models. |
| Powder bed fusion (PBF). Example—Selective laser sintering (SLS) and selective laser melting (SLM). Materials: Metals, ceramics, polymers, and glasses. |
They have been widely used for metal 3DP of biomedical devices: reliable and reproducible parts. High-resolution dense and porous parts can be made. | Metallic implants, surgical tools, scaffolds. Mostly metals and polymers. |
| Directed energy deposition (DED). Materials: Metals, ceramics, composites. |
An excellent process for new alloy design and multi-material part fabrication. Complex structures are challenging to process in a 3-axis DED system. | Coatings, multi-material structures, metallic implants. |
Materials extrusion is a standard 3DP technique for biomedical devices. This technology was first stated as fused deposition modeling (FDM). In FDM, a thermoplastic filament is pushed through a heated liquefier attached with a nozzle using counter-rotating rollers to extrude the build material along the x–y plane. The liquefier motion is controlled based on the tool-path of the part, and the extruded size and shape depend on the nozzle dimension. Multiple layers are built, one on top of another, to complete a part manufacturing. The layer thickness is controlled by either the liquefier or stage movement along the Z-dimension. In the indirect method of processing using 3DP, polymeric mold, negative of the device is made and then filled with the desired material for final part fabrication. Besides making polymer parts, metal or ceramic powder-filled thermoplastic polymers can also be used as a feedstock filament to make green metal or ceramic parts that will require further post-processing steps such as binder removal and sintering.1–3 Fugitive support structures are built using a different liquefier with another filament for complex shapes. Vat photopolymerization is the first commercial 3DP process, commonly known as “stereolithography (SLA).” A photopolymer resin is cured on a build plate using ultraviolet (UV) light to process parts using SLA. Since the printed part is fully immersed in the monomer liquid, the surface finish of processed SLA parts is better than most other 3DP processes. Generally, polymer parts are produced by SLA; however, ceramic parts can also be formed via SLA using ceramic-filled monomers as a stock material.1–3 Instead of point curing using a focused laser beam, area curing is used in digital light processing (DLP)-based stereolithography.
Apart from filament feed or curing monomers to make complex parts, powder bed-based processes are also standard for using 3DP where powders are either glued with a binder, (i.e., binder jetting) or joined using a heat source, (i.e., powder bed fusion [PBF]). For powder bed processes, metal, polymer, ceramics, or glass powders can be used. Typically, a roller spreads the powder from a powder bed onto a build plate to form a thin uniform layer. In the case of binder jetting, the water-soluble or organic binder is deposited based on the tool-path of the part file. The binder acts as a glue to bind the powders, and the surrounding loose powders act as support material. For PBF, the powder is heated using a laser or an electron beam for partial or complete densification during part fabrication. If the heat source is a laser and partial densification or sintering is achieved, then the process is called selective laser sintering or SLS, while the same for complete densification via melting is called selective laser melting or SLM. Because of this inherent support structure of unused powders in a powder bed-based process, complex parts with fine features can be made easily. Further heat treatment may be necessary for metal and ceramic parts to sinter for densification or stress relief for metal parts.1–4
For metal 3DP, directed energy deposition (DED) is another common approach where a high-energy heat source melts free-flowing powders at the focal point on the build plate. Different powders can be used simultaneously in DED.5,6 The part resolution of the DED is poor compared to the powder bed fusion process; however, DED-based hybrid machines are becoming popular to improve part resolution where additive and subtractive processes can be performed simultaneously.1,2 Material jetting is another process similar to the DED, where thermoplastic or thermoset polymers are jetted on a substrate. Polymeric parts with vibrant colors can be printed using this approach, used for 3D printed surgical models.2 Finally, sheet lamination is used for paper-based prototypes for multi-color surgical models.2 For ceramic 3DP, binder jetting, vat photopolymerization, FDM, DED, and SLS processes are commonly used.
Apart from these processes for acellular materials, 3DP is also used to manufacture parts with cellular and acellular materials, called bioprinting. Bioprinting is essentially a 3DP process where acellular materials are printed with living cells. Bioprinting uses a similar principle as any other 3DP operation by layerwise deposition of bioink. Typical bioink is developed with cell/tissue laden biopolymeric material specifically to print anatomical structures that can be cultured for organ/tissue engineering grafts.7,8 Bioink preparation through a combination of different cells and a medium such as collagen, gelatin are needed to provide necessary nutrition to the cells to survive the printing process. Several thermoresponsive polymers such as poly(N-isopropylacrylamide) (PNIPAM), pluronics, elastin polypeptides, and polycaprolactone (PCL) have found their applications in printing cell sheets for bioprinting operations. Growth factors are also added to the matrix to improve cell survivability.9,10 Three dimensional printing has also been used as an effective process toward drug manufacturing where multiple active drug or pharmaceutical agents be incorporated in a single dose, beneficial particularly to elderly patients.11,12 One needs to be careful about sequential release kinetics, post-processing limitations, and drug-drug interactions for such devices.
Current challenges in translating 3D printing for biomaterials and biomedical devices
Although 3DP technologies have been around for over three decades now, the translation of 3DP parts in different business sectors faces various challenges. Challenges that are important in biomedical devices may not be the same for aerospace or automobile sectors. Here we discuss specific challenges for biomaterials and biomedical devices. Perhaps the most critical point the 3DP community faced over the years is part quality with reproducibility. Can we produce the same quality part using 3DP compared to traditional manufacturing approaches? The last thing a biomedical device manufacturer needs is a 3D printed device failure inside the body. Extensive research and development of the past 25 years established that with good process monitoring and quality control, 3DP parts can be manufactured, offering similar part quality to conventionally processed metallic materials. Electron beam and laser-based PBF processes are now widely used worldwide to manufacture metallic implants approved by various regulatory bodies. Over 100,000 additively manufactured metallic devices were placed in the human body in the United States alone during 2021, a number that is expected to cross one million in the next 5 years. It is important to note that the US Food and Drug Administration (FDA) has only approved 3DP devices for about 10 years. Such explosive growth in 3DP of biomaterials and biomedical devices also puts a significant burden on the researchers to keep the innovation going to meet expectations. Although the 3DP approach is mainly used today to harness on-demand manufacturing of various traditional devices, new generation device designs are also evolving that can only be manufactured using 3DP. Innovations in various spinal implants are an excellent testament to understanding how 3DP will shape the future of biomedical devices.
Besides on-demand mass manufacturing of biomedical devices, patient-specific devices are also a niche for 3DP. Devices can be made for a specific disorder for an individual patient easily using 3DP. Although bioprinting has shown significant potential for patient-specific organ tissue engineering, its translation is slow. For metallic implants, 3DP is used regularly for patient-match implants, but applications are still in low volume than mass manufacturing of hip or knee implants. Three dimensional printing of ceramics still requires process optimization and finding solutions for challenging powder properties and post-processing issues for ceramic implants. In the recent past, one area growing is surgical models using polymeric materials. Surgical models are used for pre-surgical planning in complicated surgeries based on unique anatomical defects. Various 3DP processes have significantly impacted manufacturing polymeric surgical models with vibrant colors. Many prominent hospital radiology departments worldwide maintain 3D printers to print surgical models if needed. This trend is already making a difference in surgical outcomes for patients with complex health issues. However, the current challenge in this area is on handling big data. Computed tomography (CT) or magnetic resonance imaging (MRI) files are large, and in most cases, only a small segment is of interest to the physicians. Virtually how to extract that area of interest from a large CT or MRI file and adding relevant color to the final part for clarity are still challenging.
Apart from reliable machines, reproducibility in part quality, easy access to high-quality raw materials, and established guidelines for reusability of raw materials are all topics that need further understanding to sustain this exponential growth towards translating 3DP technologies to biomedical devices. Most manufacturers establish internal guidelines for quality control towards reproducibilities such as CT scans of all parts or selective testing of some parts per batch. Perhaps the time has come to establish global standards for these critical steps. Although most previous discussion is primarily suited for Class II and III devices, 3DP is quite popular for manufacturing Class I devices such as surgical tools. The development of low cost reliable 3DP machines will be essential to continue growing in that market segment. That trend will only continue with sustained market needs and cost–benefit analysis that favor on-demand manufacturing using 3DP.
Finally, the last critical challenge is the regulatory approval process. For any product to gain a broader acceptance worldwide, regulatory approval is helpful as it boosts customer confidence. For biomedical devices, it is impossible to gain market entry without necessary regulatory approval as the safety and efficacy of any device are of paramount concern to the patients, physicians, and device manufacturers. For a smooth transition of 3D printed parts in commercial products, it is essential to maintain the part quality and educate the regulators about the ins and outs of this new manufacturing paradigm. Early publications of anisotropic properties or low fatigue resistance of 3D printed parts erode confidence among the regulatory bodies for 3DP technologies. Extensive preclinical data and reliable equipment helped build the confidence among regulators worldwide to transition many 3D printed parts to commercial products. One article in this issue is focused on regulatory issues toward the translation of 3D printed parts to various biomedical devices.13
Translation of 3D printed metallic implants
Most commercial orthopedic, dental, fracture management, and cardiovascular devices are metallic materials.2 Due to the increased median age of our population and active lifestyle, a steadily increasing demand for biomaterials is seen. More than 5 out of every 100 persons above 50 years have knee implants in the United States alone. If dental implants and fracture fixation devices are considered, more than 7 out of every 100 Americans of all ages have some implant.5 Yet, considering different metallic materials used for biomedical devices, there are only three primary metallic materials—(1) stainless steel (SS) 316L that is primarily used in fracture management devices; (2) commercially pure titanium (CpTi) and Ti6Al4V that is used in dental, spinal and orthopedic implants; and (3) CoCr alloy for articulating surfaces. These alloys are borrowed from the aerospace industry because of their excellent corrosion and fatigue resistance. Since these alloys are not designed for biomedical devices, biocompatibility is poor. Typically, a variety of surface coatings are used to enhance the biocompatibility of metallic implants. Among them, perhaps the most popular one is a porous metal coating. Porosity in the metal surface reduces the stiffness and provides a path for biological fixation. However, manufacturing dense-porous metallic implants are challenging using conventional manufacturing approaches. During the past 15 years, extensive research has shown that powder bed- and directed energy deposition-based 3DP processes can be used to make complex metallic implants with both dense and porous structures.2 The main advantage of 3DP for metallic parts is the ease of manufacturability of complex parts in one simple operation. Today, many US FDA-approved commercial devices are available that are typically manufactured using powder bed fusion (PBF)-based 3DP.14 Figure 1a–b shows examples of 3D printed Ti6Al4V knee and spinal fusion implants.15,16 For porous structures, it is established that a pore size range of 300–600 microns is ideal for early-stage osseointegration. Apart from Ti alloys, 3DP has also been used in other metallic materials, such as Ta, Mg alloys, and nickel–titanium shape memory alloys.17 Porous Ta coating is used in commercially available load-bearing implants because Ta shows excellent biocompatibility. However, the melting point of Ta is > 3000°C, which makes processing Ta very difficult. Due to high laser absorption and low thermal conductivity, 3DP of Ta is relatively easy using laser-based PBF or DED processes. Both bulk and porous Ta structures have been processed using 3DP.18,19
Figure 1.
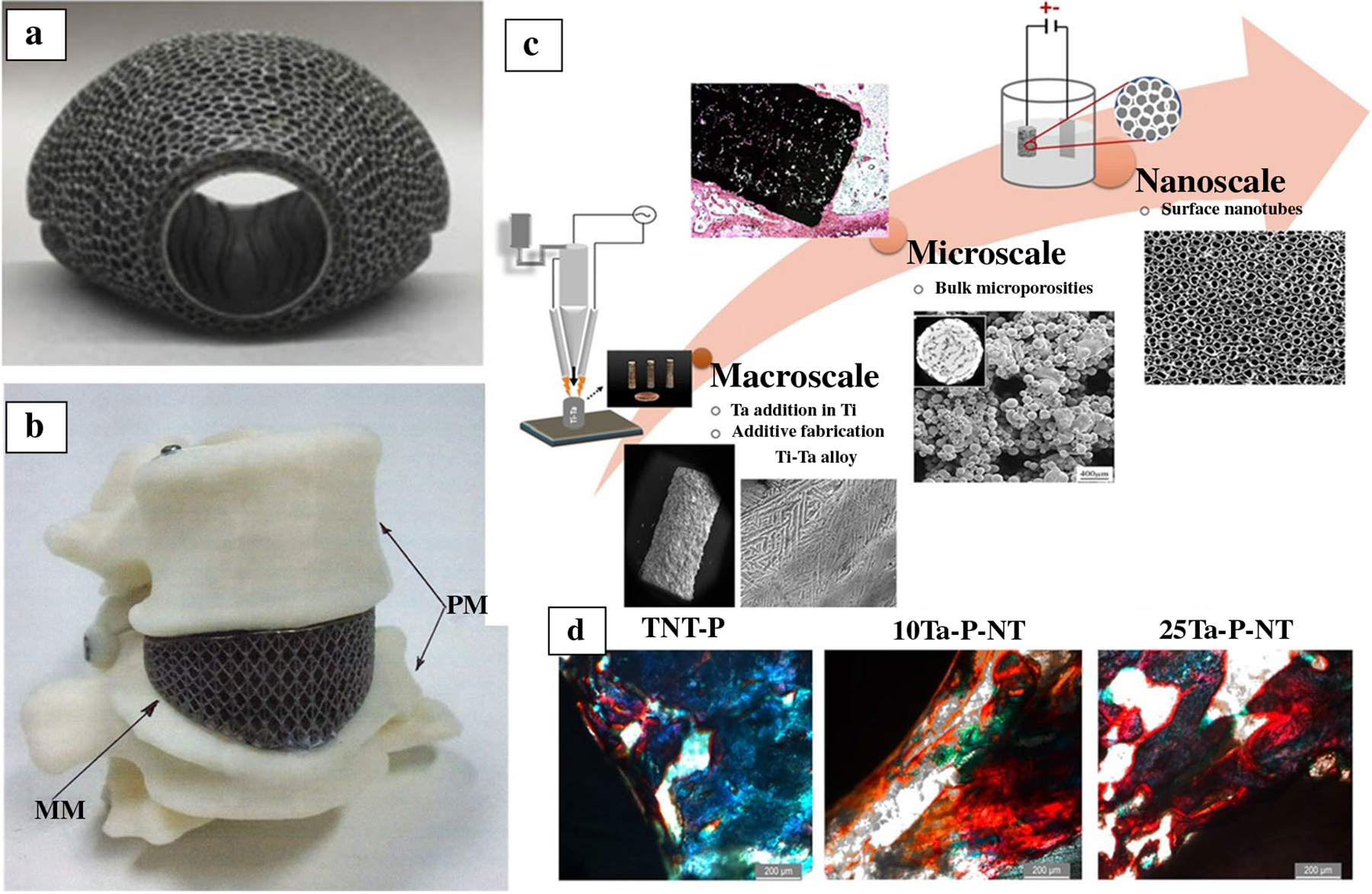
(a) Three dimensional printed titanium revision total knee arthoplasty augment for the proximal tibia.15 (b) Three dimensional printed patient-matched spinal cage of Ti6Al4V.16 (c) Three dimensional printing in alloy design. Ti–Ta alloys with inherent microporosity and nanoscale surface porosity via as grown titania nanotubes.20 (d) Histological evaluation on the 300-μm-thin sections of the Spurr embedded explant of rat femurs reveal early-stage osteoid formation in both the 10Ta-P-NT and 25Ta-P-NT at 5 weeks. Osteoid presence was marked via red color from Modified Masson Goldner’s stain. Non-uniform osteoid formation along the bone-implant interface was observed for the TNT-P (control). Scale bars measure 200 μm.20
Besides printing traditional alloys, 3DP can also design new alloys that offer unique properties. During metal 3DP, high cooling rates result in a non-equilibrium processing environment. Due to non-equilibrium processing, 3D printed parts show more refined grains and meta-stable phases in asprinted parts. Further heat treatments are necessary in many cases for 3D printed metallic parts. The question remains: Do we need the same alloying elements and their amounts for 3D printed compositions? Or the time has come to design new alloys for 3DP instead of printing the same legacy alloys. In a recent study, Ti–Ta alloys were processed via DED and showed that only 10%Ta in Ti is needed to achieve similar biocompatibility as 100% Ta.20 Figure 1c–d show 3DP Ti–Ta alloys having titania nanotubes on the surface and 3DP bulk microporosity. Histological evaluation reveals early-stage osteoid formation in both 3DP porous 10%Ta–90%Ti with TiO2 nanotubes (10Ta-P-NT) and 25%Ta–75%Ti with TiO2 nanotubes (25Ta-P-NT) at 5 weeks. Osteoid presence was marked via red color from Modified Masson Goldner’s stain. Non-uniform osteoid formation along the bone-implant interface was observed for the TNT-P (control).20 Since 3DP is inherently a shaping approach, the addition of alloy design makes it unique where new alloys can be placed in specific locations of a part while printing complex shapes to deliver the site-specific performance of the device. Similar innovations in 3D printed metallic implants are expected to continue in the coming decade.
Translation of 3D printed ceramic implants
Bone is a natural ceramic–polymer composite in which the inorganic part is a calcium phosphate ceramic. Synthetic ceramic compositions such as calcium phosphates are inherently brittle and only used for low load-bearing applications, bone void fillers as granules, or coatings.2 Generally, ceramics and glasses are bioinert (alumina and zirconia), bioactive (hydroxyapatite), or biosorbable (bioglass, tricalcium phosphate). Bioinert ceramics are typically used in dentistry and articulating surfaces of load-bearing implants such as femoral heads and liner, bioactive and bioresorbable ceramics are used in bone tissue engineering and drug delivery.21 Conventional ceramic processing involves injection molding, pressing, casting, and extrusion where green parts are binder removed and sintered at high temperatures. Green parts can be produced using 3DP, similar to conventional processing, followed by post-processing. Typically used are fused deposition of ceramics, robocasting or direct-write technique, binder jetting, and stereolithography. However, each process has its strengths and weaknesses. Apart from physical and mechanical properties, extensive research has been performed in vitro with different cells to measure the biocompatibility of various 3D printed ceramic structures.22–28 The laser-based directed energy deposition process has also been used for the 3DP of ceramics.29 For drug delivery applications, porous ceramic scaffolds are first made, followed by drug loading to avoid any degradation of drugs due to high-temperature processing. It has been shown that slight modifications in composition via dopant addition can significantly impact the biological performance of bioceramics in vivo.30,31 Three dimensional printed dense and porous ceramic structures are shown in Figure 2a–b. Figure 2c shows a histological micrograph for in vivo bone formation on Sr, Mg-doped TCP scaffolds after 4 and 8 weeks in a rat distal femur.30 The addition of dopants enhanced new bone formation through the interconnected porosities.
Figure 2.
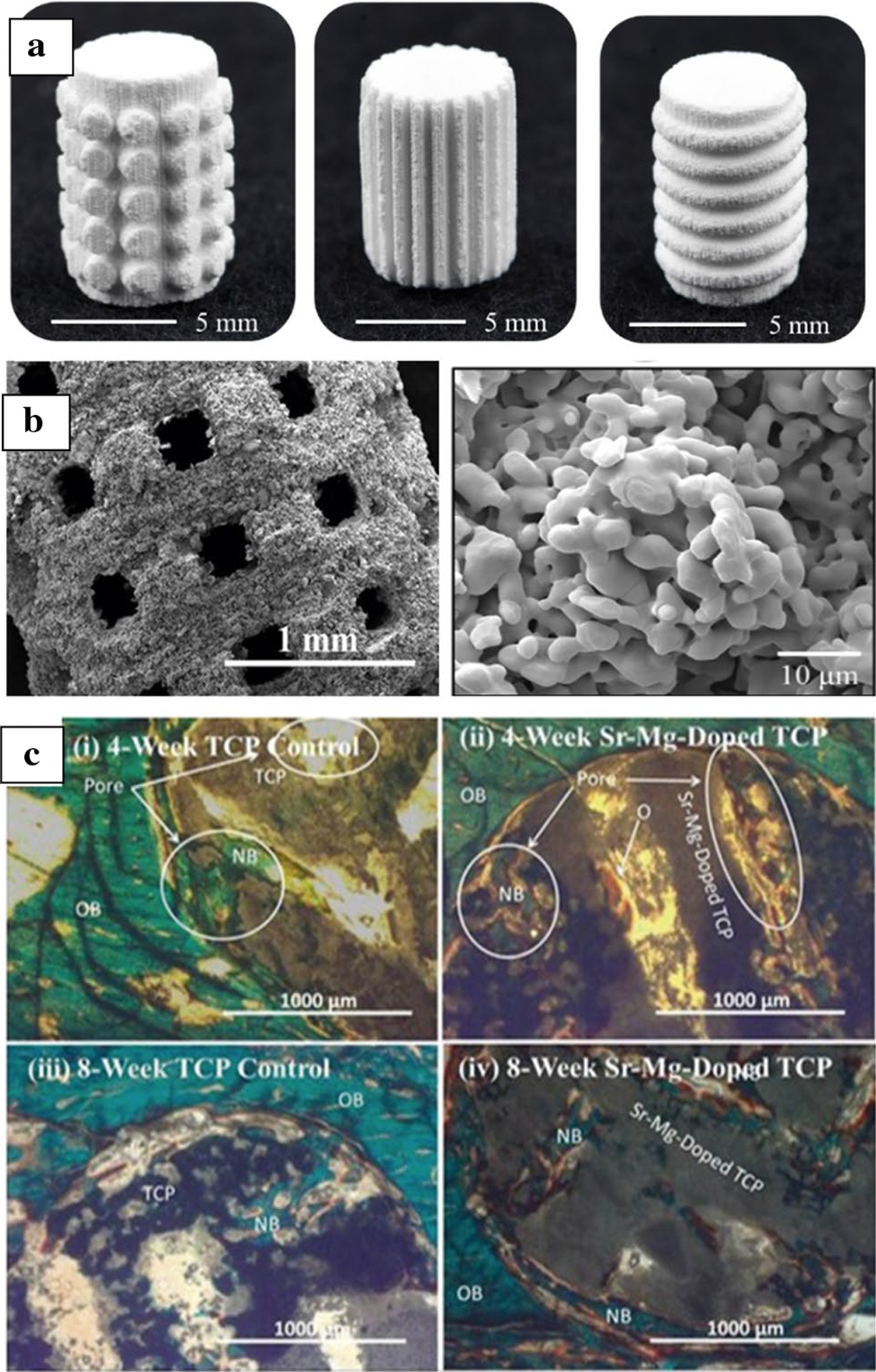
(a) Three dimensional printed tricalcium phosphate (TCP) ceramic scaffolds with different surface features, printed via binder jetting.32 (b) High magnification image of the microporous ceramic scaffolds and residual microporosity.32 (c) Histological micrograph for in vivo bone formation on Sr-, Mg-doped TCP scaffolds after 4 and 8 weeks in a rat distal femur model. Addition of dopants enhanced new bone formation through the interconnected porosities.30
Extensive research has been done on 3DP of ceramic-polymer composites.33–35 PCL, PLA, or polylactide glycolic acid (PLGA) are typically used with calcium phosphate ceramics.34 Ceramic polymer paste has also been extruded with calcium phosphate, PCL, and starch to make porous scaffolds for drug delivery applications.35 The 3DP techniques have also been used in bioinert ceramics. Due to their toughness and high mechanical strength, alumina and zirconia ceramics are used in dental and musculoskeletal bone regeneration.36–39 Stereolithography has been used with alumina ceramics and liquid phase zirconium by in situ precipitation.40 Strength increased due to metal infiltration compared to parts without infiltration. Alumina dental crown has also been studied for flexural strengths as a function of particle size and found that the strength is independent of particle size.41 Additionally, research has shown that bimodally distributed particle size can contribute to higher part density than uniform mono-sized alumina particles.42
Translation of 3D printed polymeric implants
Additive manufacturing may process non-biodegradable and biodegradable polymers for medical applications. Many advances in the clinical translation of 3DP-produced polymers have occurred in recent years. For example, Stratasys currently distributes three materials for use in dental applications that can be manufactured using its PolyJet printer: a flexible, transparent material called MED625FLX that can be used to manufacture indirect bonding trays and soft gingival masks, a rigid, transparent material called MED610 creates parts such as surgical guides orthopedic surgery or dental implantation procedures, and an opaque, rigid material called VeroGlaze MED620 for models and try-in (fit confirmation) devices.43 Gong et al. recently described the use of MED610 devices for naso-alveolar molding (NAM) devices for appropriate positioning of alveolar segments and soft tissues in infants with cleft lip and palate44 (Figure 3a). This device was part of an entirely digital workflow for patient care, including treatment planning and device manufacturing.
Figure 3.

Examples of polymer additive manufacturing for medical applications. (a) Manufacturing a series of NAM appliances by 3DP.44 (b) Confocal laser scanning micrograph of a three-by-one poly(tetrafluoroethylene) needle array in the planar orientation.51 (c) 5 mm-thick, 3D printed small tablet with a diameter of 15 mm, coaxial annulus tablet with an outer diameter of 15 mm and inner diameter of 10 mm, large tablet with a diameter of 20 mm, 4-circle pattern tablet with diameter of 20 mm and 4 circular holes of 4 mm in diameter, and honeycomb pattern tablet with a diameter of 20 mm and hexagonal holes with inner diameter of 4 mm.54 (d) Photos of printed scaffolds fabricated with laser frequency 12,000 Hz and printing speed 10 mm/s with infill density 20%, 30%, 40%, and 50%, respectively.57
In addition, clinical translation of 3DP-produced polyaryletherketone polymers has been accomplished in recent years. Polyetherketoneketone (PEKK), like other thermoplastic polymers in the polyaryletherketone family, exhibits unusual mechanical properties such as high mechanical strength, chemical resistance, biocompatibility, and radiolucency;45 these properties make it an ideal implant material. Oxford Performance Materials has commercialized a PEKK formulation called OXPEKK® for selective laser sintering of orthopedic and neurological implants; the selective laser sintering process involves using a CO2 laser to sinter thin layers of powder into a solid PEKK layer.45,46 The US FDA has approved these uses and granted a 501(k) clearance for manufacturing patient-specific implants in 2014.47 Oxford Performance Materials has manufactured 4000 cranial and spinal implants, including implants that replace up to 70% of the skull.48 Efforts are underway to translate additional high-performance polymers for clinical medicine applications.
The poly(tetrafluroethylene) (PTFE) is a fluoropolymer that exhibits unusual biological and chemical stability.49 Since it exhibits a glass transition temperature of 185°C, processing PTFE into medical devices remains a challenge; at this time, manufacturing PTFE parts involves sintering of PTFE powder.50 3M has developed a digital light processing (DLP)-based process that involves printing a PTFE solution that contains photopolymerizable binders. In this approach, the PTFE component does not experience polymerization during the printing process; the photopolymerizable binders were polymerized during the 3DP process and were removed after the printing process using a thermal processing step. This approach has been used to create medically relevant structures such as arrays containing twelve ~ 300 mm tall microneedles and arrays containing three hollow ~ 4.5 mm needles (Figure 3b); the functionality of these devices for drug delivery has been demonstrated using a model drug and human skin.51,52 These results show the promise of DLP-based 3DP for printing polymers with challenging physical properties.
Another exciting development of polymer 3DP research involves bio-based photoinitiators, such as using vitamin B2 as a photoinitiator and triethanolamine as a co-initiator. Nguyen et al. demonstrated that riboflavin (vitamin B2) and triethanolamine as a photoinitiator for a multiphoton absorption-based vat polymerization process called two-photon polymerization (2PP).53 Using a comet assay, which assesses double and single-strand DNA breaks, it showed that poly(ethylene glycol) diacrylate (PEGDA) polymerized with riboflavin and triethanolamine was much less genotoxic than PEGDA polymerized with Irgacure 2959 or Irgacure 369. Extracts from the riboflavin–triethanolamine polymerized material photopolymers exhibited viability similar to that of the glass control. A live/dead assay demonstrated the growth of bovine aortic endothelial cells on a scaffold that was made from this material. More recently, it has been demonstrated that the model drug ascorbic acid might be capsulated within the tablet, coaxial annulus, and honeycomb structures that were made from PEGDA with riboflavin and triethanolamine as photoinitiator and co-initiator, respectively, via a light-emitting diode based vat polymerization approach (Figure 3c).54 At least 80% of ascorbic acid is released from the honeycomb structure under 1 h of gastrointestinal conditions. In vitro and in vivo biocompatibility and mechanical properties of poly(glycerol sebacate) acrylate (PGSA) and poly(ε-caprolactone) diacrylate (PCLDA) resins were compared recently polymerized by different photoinitiators.55 Studies involving rabbit synovium fibroblast cells showed that PGSA with riboflavin and triethanolamine supported cell proliferation up to 10 days. A study with a murine model showed that this material was associated with a low immune response after 14 days post-implantation; the low levels of inflammation were associated with the presence of riboflavin, which enhanced the growth and proliferation of fibroblast cells in the mice. These studies indicate the promise of the combination of riboflavin and triethanolamine as a bio-based and biocompatible photoinitiator for polymer 3DP.
Efforts are also underway to develop more sustainable polymer 3DP based on soybeans. Recently, a polylactic acid filament containing 20–25% soy additive FilaSoy has been developed.56 The soy additive imparts antimicrobial activity and lowers brittleness to the filament. Laser-based vat polymerization of soybean oil epoxidized acrylate; Ciba Irgacure 819 was used as a photoinitiator57 (Figure 3d). The material exhibited shape memory activity, recovering its original shape at 37°C after being set in a temporary shape at −18°C. In addition, the material showed higher levels of multipotent human bone marrow mesenchymal stem cell adhesion and proliferation than PEGDA; the cell parameters were similar to those of polylactic acid and polycaprolactone. More recently, the use of a digital light processing lithography- and nonlinear laser lithography-based vat polymerization approach for 3DP with an acrylated epoxidized soybean oil-based resin has been demonstrated,58 and features below 1 mm were obtained. These studies indicate many opportunities for medical applications to move toward 3DP with sustainable materials over several length scales.
Translation of bioprinting-based products
The term bioprinting includes manufacturing processes in which living cells, growth factors, and other biologically active materials are utilized as building blocks during 3DP. Many applications have been proposed for bioprinted constructs, most notably artificial tissues and organs for implantation. At this time, the most successful translation of bioprinting technology has involved bioprinted constructs for drug development, toxicology screening, and cancer testing. In 2014, Organovo commercialized exVive3D™ Human Liver Tissue, intended for preclinical drug discovery testing.59 Three methods, droplet, inkjet, and continuous, have been used by Organovo to create tissues containing human cells.60 The construct exhibits stability for at least 42 days, synthesizes cholesterol, produces proteins (e.g., albumin and fibrinogen), and demonstrates inducible cytochrome P450 enzymatic activity. Potential future applications of this technology include the treatment of acute-on-chronic liver failure and pediatric inborn errors of metabolism.60
More recently, Poietis commercialized a skin construct that was made using a laser-assisted bioprinting-based approach.61 Printing layers of collagen created the dermal layer of the construct I using inkjet technology; primary human fibroblasts were printed onto the collagen layers using laser-assisted bioprinting technology. After 6 days, laser-assisted bioprinting was used to prepare an epidermal layer containing primary human keratinocytes using laser-assisted bioprinting technology. The laser-assisted bioprinting approach provided ~ 95% cell viability results for fibroblasts and keratinocytes. The scaffolds showed a dermal equivalent structure on Masson Trichrome staining; staining studies also showed stratification of the epidermal layer within the constructs.
In 2016, Aprecia Pharmaceuticals received FDA approval for its orodispersible tablet SPRITAM®, which treats epilepsy.62 The 3DP processing involves spreading powdered medication and applying liquid layer-by-layer to create a pill. The benefit of the bioprinting technology over conventional methods is that a high dose of up to 1 g of the drug levetiracetam can be loaded in a porous pill ~ one nickel in diameter and a stack of four nickels in height, which rapidly disintegrates with the consumption of a small amount of water. This technology may enhance the rate at which patients take difficult-to-swallow medications on time, thus minimizing medical issues associated with missed doses. Figure 4 shows the use of a three-dimensional printing process to manufacture SPRITAM® (levetiracetam) tablets.62 Another area of focus by the bioprinting community is printed meat. MeaTech Group recently demonstrated the production of 700 g of pure chicken fat biomass.63 If approved, MeaTech will produce additional meat at its pilot plant in 2022 and market it as a taste-enhancing ingredient.64
Figure 4.

Use of 3D printing process to manufacture SPRITAM® (levetiracetam) tablets.62 (a) Photo of dosage forms after printing and drying (contrast enhanced). (b) Photo of dosage forms after harvesting. (c) Side view schematic of Aprecia’s 3D printing process.
One of the limitations of conventional bioprinting technologies is that slow processing rates may limit the commercial viability of many bioprinting efforts.65,66 Recently bioprinting of materials containing L929 fibroblast-like cells and gelatin–norbornene bioink has been demonstrated at rapid rates 1000 mm s−1 using 2PP, which would allow a 300 μm × 300 μm × 300 μm cubic scaffold to be printed in under 10 min. The scaffolds were noted to support nearly 100% cell survival rates for laser power values under 100 mW. Another exciting area in bioprinting is the development of bioinks that contain biologically functional components. Recently broad-spectrum antimicrobial drug vancomycin was added within bioinks containing polycaprolactone, polyethylene oxide, and hydroxyapatite,67 where the drug showed rapid release from the scaffolds. This type of antimicrobial ink may be used to create scaffolds for bone tissue engineering. An immunomodulatory bioink, in which manganese silicate nanospheres incorporated within a hydrogel bioink, has also been demonstrated to repair vascularized tissue.68 The ink was shown to support the viability of endothelial cells and macrophages.
Concluding remarks
Nearly three and a half decades after the birth of commercial 3DP technologies in the United States, we focused this particular MRS Bulletin issue on how this disruptive technology platform is used to manufacture medical devices. Our focus is the translation of 3DP technology and what challenges remain. Six invited review articles discussed the areas of bioprinting, metallic implants, ceramic implants, cardiovascular devices, and regulatory issues in the translation of 3DP toward biomedical devices. And in all areas, the overall outlook is very positive, where significant growth is expected to happen in the coming decades. From the first FDA-approved device about 10 years back to over 100,000 devices per year today for human use in the United States alone, a phenomenal growth that is expected to continue to reach over four million in the next decade. Apart from the biomedical devices for human use, growth in anatomical models and bioprinting is also phenomenal, something that regulatory bodies like the FDA do not address explicitly. Although excitement is high with 3DP technologies, critical issues remain. Among the concerns, standardization of raw materials use, reproducibility in part quality from various 3D printers are deemed necessary to continue building consumer confidence in this technology platform.
In this issue, Kumar et al. discussed the translation of 3DP for orthopedic devices giving specific examples, unique advantages, and remaining challenges, including the areas of new alloy design.14 Raymond et al. discussed the translation of 3DP of ceramics in bone tissue engineering, drug delivery, and patient-matched personalized medicine along with current challenges.69 Chae et al. described the application of bioprinting with decellularized extracellular matrix-based bioinks in translational regenerative medicine, emphasizing musculoskeletal and cardiovascular tissue engineering.70 Mahajan et al. discussed the translation of bioprinted materials in skin, bone, cartilage, nerve, cardiac tissue, and vascular network repair, along with constructing organoids for toxicology testing as an alternative to animal studies.71 Zhou et al. described 3DP technology in scaffolds for facial reconstruction.72 And finally, Rafi et al. discussed the regulatory landscape for 3DP medical devices and offered guidance and various steps for regulatory approval of 3DP devices.13 We hope that information presented in this issue will be informative to scientists, engineers, clinicians, and the next generation of researchers to continue to push the frontiers of science and innovate medical devices using 3DP to solve critical human health issues in the coming days.
Acknowledgments
AB and SB would like to acknowledge financial support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Nos. R01 AR066361 (PI: Bose), R01 DE 029204 (PI: Bose), and R01 AR067306 (PI: Bandyopadhyay). The content is solely the authors’ responsibility and does not necessarily represent the official views of the National Institutes of Health. RN acknowledges financial support from the National Science Foundation under Grant Nos. 2029974 and 1762202.
Biographies

Amit Bandyopadhyay is a professor in the School of Mechanical and Materials Engineering at Washington State University. He has worked with 22 PhD and 30 MS students, is an inventor of 21 issued patents, and published more than 350 technical articles. Bandyopadhyay is a Fellow of the Society of Manufacturing Engineers, The American Ceramic Society, ASM International, American Institute for Medical and Biological Engineering, AAAS, National Academy of Inventors, and an elected member at the Washington State Academy of Sciences. His work has been cited more than 26,000 times, and the current h-index is 85 (Google Scholar). Bandyopadhyay can be reached by email at amitband@wsu.edu.

Susmita Bose is a professor in the School of Mechanical and Materials Engineering at Washington State University. She received the prestigious Presidential Early Career Award for Scientist and Engineers from the National Science Foundation. Bose, an inventor of 12 issued patents, has advised more than 45 students for their MS and PhD degrees, and published more than 300 articles. Her papers have been cited more than 24,000 times, and she has an h-index of 80 (Google scholar). She is a Fellow of the AAAS, National Academy of Inventors, the Materials Research Society, ASM International, American Institute for Medical and Biological Engineering, The American Ceramic Society, and the Royal Society of Chemistry. In 2017, she was elected to the Washington State Academy of Sciences. Bose can be reached by email at sbose@wsu.edu.

Roger Narayan is a professor in the Joint Department of Biomedical Engineering at the University of North Carolina and North Carolina State University. He is an author of more than 200 publications and several book chapters on the processing of biomedical materials. Narayan has edited several books, including the textbook Biomedical Materials, Second Edition, the handbook Materials for Medical Devices, and the Encyclopedia of Biomedical Engineering. He has been elected as Fellow of AAAS, The American Society of Mechanical Engineers, ASM International, the American Institute for Medical and Biological Engineering, and The American Ceramic Society. Narayan can be reached by email at rjnaraya@ncsu.edu.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Contributor Information
Amit Bandyopadhyay, W. M. Keck Biomedical Materials Research Lab, School of Mechanical and Materials Engineering, Washington State University, USA.
Susmita Bose, W. M. Keck Biomedical Materials Research Lab, School of Mechanical and Materials Engineering, Washington State University, USA.
Roger Narayan, Department of Biomedical Engineering, University of North Carolina and North Carolina State University, USA.
References
- 1.Bandyopadhyay A, Bose S, Additive Manufacturing, 2nd ed. (CRC Press, Boca Raton, FL, 2019) [Google Scholar]
- 2.Bose S, Ke D, Sahasrabudhe H, Bandyopadhyay A, Prog. Mater. Sci 93, 45 (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bandyopadhyay A, Mitra I, Bose S, Curr. Osteoporos. Rep 18, 505 (2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hossain MS, Gonzalez JA, Hernandez RM, Shuvo MAI, Mireles J, Choudhuri A, Lin Y, Wicker RB, Addit. Manuf 10, 58 (2016) [Google Scholar]
- 5.Bandyopadhyay A, Ghosh S, Boccaccini AR, Bose S, J. Mater. Res 36, 3713 (2021) [Google Scholar]
- 6.Bandyopadhyay A, Heer B, Mater. Sci. Eng. Rep 129, 1 (2018) [Google Scholar]
- 7.Liaskoni A, Wildman RD, Roberts CJ, Int. J. Pharm 597, 120330 (2021) [DOI] [PubMed] [Google Scholar]
- 8.Barthes J, Lagarrigue P, Riabov V, Lutzweiler G, Kirsch J, Muller C, Courtial E, Marquette C, Projetti F, Kzhyskowska J, Biomaterials 268, 120549 (2021) [DOI] [PubMed] [Google Scholar]
- 9.Gregor A, Filová E, Novák M, Kronek J, Chlup H, Buzgo M, Blahnová V, Lukášová V, Bartoš M, Nečas A, Hošek J, J. Biol. Eng 11, 1 (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun J, Tan H, Materials 6, 1285 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bloomquist CJ, Mecham MB, Paradzinsky MD, Janusziewicz R, Warner SB, Luft JC, Mecham SJ, Wang AZ, DeSimone JM, J. Control Release 278, 9 (2018) [DOI] [PubMed] [Google Scholar]
- 12.Liang K, Brambilla D, Leroux J, Adv. Mater 31, 1805680 (2019) [DOI] [PubMed] [Google Scholar]
- 13.Rafi K, Liu A, Di Prima M, Seifi M, MRS Bull 47(1) (2022) [Google Scholar]
- 14.Kumar M, Rappo S, Facchini L, Tomaselli M, MRS Bull 47(1) (2022) [Google Scholar]
- 15.Dion C, Yamomo G, Howard J, Teeter M, Willing R, Lanting B, J. Mech. Behav. Biomed. Mater 110, 103944 (2020) [DOI] [PubMed] [Google Scholar]
- 16.Murr LE, J. Mater. Res. Technol 9, 1087 (2020) [Google Scholar]
- 17.Xue W, Krishna BV, Bandyopadhyay A, Bose S, Acta Biomater 3, 1007 (2007) [DOI] [PubMed] [Google Scholar]
- 18.Balla VK, Banerjee S, Bose S, Bandyopadhyay A, Acta Biomater 6, 2329 (2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bandyopadhyay A, Mitra I, Shivaram A, Dasgupta N, Bose S, Addit. Manuf 28, 259 (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitra I, Bose S, Dernell WS, Dasgupta N, Eckstrand C, Herrick J, Yaszemski MJ, Goodman SB, Bandyopadhyay A, Mater. Today 45, 20 (2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bose S, Roy M, Bandyopadhyay A, Trends Biotechnol 30, 546 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trombetta R, Inzana JA, Schwarz EM, Kates SL, Awad HA, Ann. Biomed. Eng 45, 23 (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bose S, Sugiura S, Bandyopadhyay A, Scripta Mater 41, 1009 (1999) [Google Scholar]
- 24.Bergemann C, Cornelsen M, Quade A, Laube T, Schnabelrauch M, Rebl H, Weißmann V, Seitz H, Nebe B, Mater. Sci. Eng. C 59, 514 (2016) [DOI] [PubMed] [Google Scholar]
- 25.Bose S, Tarafder S, Bandyopadhyay A, Ann. Biomed. Eng 45, 261 (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sweet L, Kang Y, Czisch C, Witek L, Shi Y, Smay J, Plant GW, Yang Y, PLoS One 10, e0139820 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tarafder S, Balla VK, Davies NM, Bandyopadhyay A, Bose S, Tissue Eng J. Regen. Med 7, 631 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bose S, Vahabzadeh S, Bandyopadhyay A, Mater. Today 16, 496 (2013) [Google Scholar]
- 29.Balla VK, Bose S, Bandyopadhyay A, Int. J. Appl. Ceram. Technol 5, 234 (2008) [Google Scholar]
- 30.Tarafder S, Davies NM, Bandyopadhyay A, Bose S, Biomater. Sci 1, 1250 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bose S, Banerjee D, Robertson S, Vahabzadeh S, Ann. Biomed. Eng 46, 1241 (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vu AA, Burke DA, Bandyopadhyay A, Bose S, Addit. Manuf 39, 101870 (2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Placone JK, Engler AJ, Adv. Healthc. Mater 7, 1701161 (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nyberg E, Rindone A, Dorafshar A, Grayson WL, Tissue Eng. Part A 23, 503 (2017) [DOI] [PubMed] [Google Scholar]
- 35.Koski C, Onuike B, Bandyopadhyay A, Bose S, Addit. Manuf 24, 47 (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baino F, Minguella-Canela J, Korkusuz F, Korkusuz P, Kankılıç B, Montealegre MÁ, los Santos-López D, Antonia M, Vitale-Brovarone C, Int. J. Mol. Sci 20, 722 (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baino F, Montealegre MA, Minguella-Canela J, Vitale-Brovarone C, Coatings 9, 369 (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodriguez CA, Lara-Padilla H, Dean D, 3D Printing and Biofabrication (Springer, Cham, Switzerland, 2018), pp. 161–193 [Google Scholar]
- 39.Mussano F, Genova T, Serra FG, Carossa M, Munaron L, Carossa S, Int. J. Mol. Sci 19, 528 (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu W, Wu H, Zhou M, He R, Jiang Q, Wu Z, Cheng Y, Song X, Chen Y, Wu S, Ceram. Int 42, 17736 (2016) [Google Scholar]
- 41.Dehurtevent M, Robberecht L, Hornez J, Thuault A, Deveaux E, Béhin P, Dent. Mater 33, 477 (2017) [DOI] [PubMed] [Google Scholar]
- 42.Wu H, Cheng Y, Liu W, He R, Zhou M, Wu S, Song X, Chen Y, Ceram. Int 42, 17290 (2016) [Google Scholar]
- 43. https://www.stratasys.com/materials/search/biocompatible .
- 44.Gong X, Dang R, Xu T, Yu Q, Zheng J, J. Craniofac. Surg 31, 367 (2020) [DOI] [PubMed] [Google Scholar]
- 45.Roskies MG, Fang D, Abdallah M, Charbonneau AM, Cohen N, Jordan JO, Hier MP, Mlynarek A, Tamimi F, Tran SD, Laryngoscope 127, E392 (2017) [DOI] [PubMed] [Google Scholar]
- 46.Jose RR, Rodriguez MJ, Dixon TA, Omenetto F, Kaplan DL, ACS Biomater. Sci. Eng 2, 1662 (2016) [DOI] [PubMed] [Google Scholar]
- 47. https://oxfordpm.com/news-events/opm-press-releases?id=339780/opm-receives-fda-clearance-for-3d-printed-osteofab-patient-specific-facial-device .
- 48.Giordano G, Lamontagne ND, Plast. Eng 74, 16 (2018) [Google Scholar]
- 49.Teng H, Appl. Sci 2, 496 (2012) [Google Scholar]
- 50.Dhanumalayan E, Joshi GM, Adv. Compos. Hybrid Mater 1, 247 (2018) [Google Scholar]
- 51.Sachan R, Sachan A, Lu J, Erdmann D, Zhang JY, Narayan RJ, JOM (2021). 10.1007/s11837-021-04978-3 [DOI] [Google Scholar]
- 52.Sachan R, Nguyen AK, Lu J, Erdmann D, Zhang JY, Narayan RJ, MRS Commun (2021). 10.1557/s43579-021-00121-0 [DOI] [Google Scholar]
- 53.Nguyen AK, Gittard SD, Koroleva A, Schlie S, Gaidukeviciute A, Chichkov BN, Narayan RJ, Regen. Med 8, 725 (2013) [DOI] [PubMed] [Google Scholar]
- 54.Karakurt I, Aydoğdu A, Çıkrıkcı S, Orozco J, Lin L, Int. J. Pharm 584, 119428 (2020) [DOI] [PubMed] [Google Scholar]
- 55.Wang C, Chen J, Wang J, J. Biomed. Mater. Res. A 110, 204 (2022) [DOI] [PubMed] [Google Scholar]
- 56.Pakkanen J, Manfredi D, Minetola P, Iuliano L, Sustainable Design and Manufacturing (Springer, Cham, Switzerland, 2017), pp. 776–785. 10.1007/978-3-319-57078-5_73 [DOI] [Google Scholar]
- 57.Miao S, Zhu W, Castro NJ, Nowicki M, Zhou X, Cui H, Fisher JP, Zhang LG, Sci. Rep 6, 1 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Skliutas E, Lebedevaite M, Kasetaite S, Rekštytė S, Lileikis S, Ostrauskaite J, Malinauskas M, Sci. Rep 10, 1 (2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. https://ir.organovo.com/news-releases/news-release-details/organovo-announces-commercial-release-exvive3dtm-human-liver .
- 60.Tarassoli SP, Jessop ZM, Al-Sabah A, Gao N, Whitaker S, Doak S, Whitaker IS, J. Plast. Reconstr. Aesthet. Surg 71, 615 (2018) [DOI] [PubMed] [Google Scholar]
- 61. https://www.scintica.com/wp-content/uploads/2021/02/Poietis-Bioprinting-full-skin-tissues-with-NGB-R.pdf .
- 62.Fitzgerald S, Neurol. Today 15, 26 (2015) [Google Scholar]
- 63. https://www.prnewswire.com/news-releases/meatech-group-manufactures-over-half-a-kilogram-of-cultivated-fat-biomass-in-a-single-production-run-301376702.html .
- 64. https://3dprintingindustry.com/news/meatech-cultures-700-grams-of-chicken-fat-in-meat-printing-breakthrough-196303 .
- 65.Guillemot F, Guillotin B, Fontaine A, Ali M, Catros S, Kériquel V, Fricain J, Rémy M, Bareille R, Amédée-Vilamitjana J, MRS Bull 36(12), 1015 (2011) [Google Scholar]
- 66.Baldacchini T, Saksena J, Sklare SC, Vinson BT, Huang Y, Chrisey DB, Narayan RJ, MRS Bull 46(2), 174 (2021) [Google Scholar]
- 67.Zhang B, Nguyen AK, Narayan RJ, Huang J, J. Am. Ceram. Soc (2021). 10.1111/jace.18048 [DOI] [Google Scholar]
- 68.Wu J, Qin C, Ma J, Zhang H, Chang J, Mao L, Wu C, Appl. Mater. Today 23, 101015 (2021) [Google Scholar]
- 69.Raymond Y, Johansson L, Thorel E, Ginebra M-P, MRS Bull 47(1) (2022) [Google Scholar]
- 70.Chae S, Cho D-W, MRS Bull. 47(1) (2022) [Google Scholar]
- 71.Mahajan N, Yoo JJ, Atala A, MRS Bull 47(1) (2022) [Google Scholar]
- 72.Zhou Y, Grayson W, MRS Bull 47(1) (2022) [Google Scholar]


