Abstract
cis-9-Heptadecenoic acid (CHDA), an antifungal fatty acid produced by the biocontrol agent Pseudozyma flocculosa, was studied for its effects on growth and/or spore germination in fungi. Inhibition of growth and/or germination varied considerably and revealed CHDA sensitivity groups within tested fungi. Analysis of lipid composition in these fungi demonstrated that sensitivity was related primarily to a low intrinsic sterol content and that a high level of unsaturation of phospholipid fatty acids was not as involved as hypothesized previously. Our data indicate that CHDA does not act directly with membrane sterols, nor is it utilized or otherwise modified in fungi. A structural mechanism of CHDA, consistent with the other related antifungal fatty acids produced by P. flocculosa, is proposed in light of its activity and specificity. The probable molecular events implicated in the sensitivity of fungi to CHDA are (i) partitioning of CHDA into fungal membranes; (ii) a variable elevation in fluidity dependent on the buffering capability (sterol content) in fungi; and (iii) higher membrane disorder causing conformational changes in membrane proteins, increased membrane permeability and, eventually, cytoplasmic disintegration.
Pseudozyma flocculosa (Traquair, Shaw, et Jarvis) Boekhout et Traquair (= Sporothrix flocculosa Traquair, Shaw, et Jarvis) (8) is a yeast-like fungus with biocontrol properties against powdery mildew fungi (3, 14, 15, 20). Cytochemical observations revealed that it induces a rapid collapse of powdery mildew conidial chains and a cytoplasmic disintegration of the cells (16) through the production of unusual extracellular fatty acids with antifungal properties (1, 4, 11). These antifungal fatty acids cause the release of intracellular ions and proteins when in contact with sensitive fungi (16), suggesting that they disrupt properties and functions of the cytoplasmic membrane. Benyagoub et al. (5) hypothesized that this fungal sensitivity was related to a low sterol content and to a high degree of unsaturation of phospholipid fatty acids in fungal membranes, factors which increase membrane fluidity. Indeed, they showed that the antifungal fatty acids caused a dose-dependent elevation in fluidity in artificial membranes constructed from the total lipids of the sensitive fungus Cladosporium cucumerinum Ellis et Arth, whereas artificial membranes made with lipids of P. flocculosa demonstrated no changes in fluidity (5).
In general, elevated fluidity is known to cause disorder, i.e., a higher degree of mobility of phospholipid acyl chains in the membrane bilayer. This alteration in acyl chain packing can result in changes in membrane dynamics which would affect the activity of membrane-bound proteins (12). Since toxic fatty acids, in general, seem to interfere with multiple, apparently unrelated membrane enzymes (13), it has been proposed that the interaction between the fatty acids and cellular enzymes in sensitive fungi is indirect and nonspecific (19). However, to our knowledge, there are no documented cases which propose a specific mode of action of unusual fatty acids in living cells and explicitly discuss the differential response of cells to these compounds.
It has been suggested that free fatty acids alter membrane fluidity either by (i) partitioning into the lipid bilayer of cells (27) or by (ii) inclusion into fatty acyl chains of membrane phospholipids (13); toxic molecules containing double bonds, such as P. flocculosa antifungal fatty acids, may also act by causing changes in permeability towards low-molecular-weight substances (18) by (iii) binding to or altering membrane sterols (10, 26). In this study, these three hypotheses were tested using the free fatty acid cis-9-heptadecenoic acid (CHDA) produced by P. flocculosa to treat a range of fungi. To this end, our objectives were (i) to localize CHDA in the membranes of growing fungi exposed to a sublethal dose of this compound; (ii) to analyze its corresponding effect on fungal growth; (iii) to quantify cellular sterols and phospholipid fatty acid unsaturation for a number of fungi; and (iv) to determine a possible link between intrinsic membrane components in fungi and sensitivity to CHDA.
MATERIALS AND METHODS
Fungal material.
Botrytis cinerea Pers.:Fr., C. cucumerinum, Idriella bolleyi (Sprague) Arx (= Micodochium bolleyi [Sprague] de Hoog et Hermanides-Nijhof), Phytophthora infestans (Mont.) de Bary, Pseudozyma rugulosa (Traquair, Shaw, et Jarvis) Boekhout et Traquair (= Sporothrix rugulosa Traquair, Shaw, et Jarvis), and Pythium aphanidermatum (Edson) Fitzp. were maintained on potato dextrose agar. Sphaerotheca fuliginea (Schlechtend.:Fr.) Pollacci, a biotrophic fungus unable to grow on artificial media, was maintained on long English cucumber plants (Cucumis sativus L. cv. Corona).
Synthesis of CHDA.
CHDA was synthesized as previously described (1) and stored at −24°C in crystalline form.
Effects of CHDA on fungal growth and spore germination.
For assessment of fungal growth inhibition, two agar disks (15 mm) of the fungi under study were suspended in 100 ml of potato dextrose broth (PDB). CHDA dissolved in N,N-dimethylformamide (DMF) was added to the broth to give a final concentration of 0.15 mg/ml, a concentration shown previously to reduce growth of C. cucumerinum by approximately 50% on the basis of dry weight (1). An equivalent concentration of DMF (0.5%) was added to PDB to serve as a control. Growth was quantified by dry weight measurement following lyophilization after 3 days of culturing (25°C) on a rotary shaker (150 rpm).
To assess spore germination, fungal spores were suspended in 50 ml of PDB. CHDA was added as a DMF solution at a concentration of 0.15 mg/ml. DMF alone (0.5%) served as a control. Percent spore germination was determined after a 24-h incubation period at 25°C. One hundred spores were assayed for germination. Spores were considered to have germinated when the length of the germ tube equaled or exceeded that of the spore itself.
Extraction of lipids.
Total lipid extraction was carried out via a modified Bligh and Dyer method (6). Lyophilized fungal cells from the above-described experiment were suspended in chloroform-methanol-water (100:100:50 ml/g of dry weight) and homogenized with a Polytron (Kinematica; Brinkman Instruments, Ontario, Canada) for 1 min on ice. The homogenized solution was protected from light and extracted for 3 h on a rotary shaker (150 rpm) at 25°C. Solvents were separated from fungal biomass by filtration (Whatman paper no. 1), and the extraction procedure was repeated with chloroform-methanol-water (200:100:50 ml/g of dry weight) overnight. The combined extracts were diluted with chloroform and water (1:1 by volume), thus partitioning the water-soluble contaminants into the aqueous phase. Trace amounts of water were removed by dilution with benzene. The organic phase was evaporated on a rotary evaporator and taken to dryness under a stream of nitrogen. The resulting residue constituted total lipids.
Analysis of lipids.
Neutral and polar lipids were separated by acetone precipitation as described by Kates (21). Lipid fractions were separated by thin-layer chromatography on Silica Gel 60 (0.25 mm) using hexane-diethyl ether-acetic acid (78:20:4 by volume) for neutral lipids and chloroform-acetone-methanol-acetic acid-water (10:4:2:2:1 by volume) for polar lipids. Neutral lipid classes and individual polar lipids were visualized by iodine staining and were identified by comparing Rf values with authentic standards (Sigma, St. Louis, Mo.).
Fatty acid methyl esters (FAME) were prepared directly from phospholipids by transesterification using BF3-methanol (14%) for 60 min at 70°C. Individual FAME were quantified by a gas chromatograph (Model 5890 series II; Hewlett-Packard, Mississauga, Ontario, Canada) coupled to a flame-ionization detector (FID), using a 30-m DB-225 capillary column (J&W Scientific, Rancho Cordova, Calif.). Peaks were integrated with an HP integrator, model 3392A. The oven temperature program was as follows: 100°C, 20°C/min to 200°C, held 5 min, 10°C/min to 240°C, held 5 min. H2 injector and FID temperatures were 230 and 250°C, respectively. FAME were identified by comparing retention times with those of authentic standards (Chromatographic Specialties, Brockville, Ontario, Canada) and quantified by calibration curves of individual compounds. Methylheptadecanoate (17:0) was used as an internal standard. The degree of unsaturation (Δ/mol) was determined as previously described (31), where Δ/mol = [%18:1 + 2(% 18:2) + 3(% 18:3)]/100.
Sterols were obtained by alkaline hydrolysis of neutral lipids using 1 ml of KOH (33% wt/vol) in 10 ml of ethanol (95%) for 2 h at 90°C (5). The unsaponifiable fraction containing sterols was obtained by washing the hydrolysate with hexane. The hydrolysate was then brought to pH 1 to 2 with HCl (6 M), and the saponifiable fraction, containing the free fatty acids, was obtained by washing with hexane.
Total sterols were quantified by gas chromatography using a method proposed by Rangel et al. (28), with the following modifications. The oven temperature program was 100°C, held 2 min, 20°C/min to 200°C, held 1 min, 10°C/min to 300°C, held 5 min. He injector and detector temperatures were 290 and 300°C, respectively. Peaks were integrated with ChemStation software (Hewlett-Packard). Using this method, it was possible to separate C27, C28, and C29 sterols. C27 and C28 sterols were identified by analyzing mass spectrum data and comparing retention times with those for cholesterol and ergosterol, respectively. Sterol classes were quantified using calibration curves of cholesterol and ergosterol. 5, 24[28]-stigmastadien-3β-ol (C29) was used as an internal standard and was not present in any of the fungi. The sterol/phospholipid (S/P) molar ratio was determined using an average molecular weight of 725 for phospholipids (P), 386 for cholestane (C27) derivatives, and 396 for ergostane (C28) derivatives as follows: S/P molar ratio = (C27/386 + C28/396)/(P/725).
Evolution of phospholipid unsaturation.
I. bolleyi and P. aphanidermatum were subjected to further analyses of phospholipid unsaturation in the presence and absence of CHDA. Two agar disks (15 mm each) of fungal mycelia were suspended in 100 ml of PDB. CHDA was amended to the broth at a final concentration of 0.15 mg/ml in 0.5% DMF. DMF alone (0.5%) was added to the culture media to serve as a control. Growth was quantified daily over a 7-day culture period (25°C) on a rotary shaker (150 rpm) by dry-weight measurement following lyophilization. Growth curves were plotted, and phospholipid unsaturation of some samples was analyzed based on their growth stage (early and mid-logarithmic, early stationary, and stationary growth phases). Δ/mol values of fungi were calculated as described above.
Analysis of CHDA.
Following incubation of fungi with CHDA and extraction of lipids from both the fungal mass and the culture medium, free fatty acids and phospholipid fatty acids were analyzed for the presence of CHDA. FAME were prepared from both fatty acid fractions with BF3-methanol (14%) and were gas chromatographed as described above. CHDA methyl ester was identified by comparing the retention time with the synthetic standard and quantified by calibration curve.
Statistical analysis.
All experiments consisted of two replicates of each fungus and were repeated three times. Analysis of variance was performed, and Fisher's protected least significant difference (LSD) was used as a mean separation test.
RESULTS
General effects of CHDA on fungi.
CHDA exhibited activity against all tested fungi, although the degree of sensitivity varied considerably. For the concentration used in this study (0.15 mg/ml), growth inhibition was significantly higher for P. infestans and P. aphanidermatum than for the other fungi (Table 1). Mycelial growth was significantly more inhibited for B. cinerea and C. cucumerinum than for I. bolleyi. P. rugulosa had only minimal growth inhibition. Inhibition of conidial germination was nearly complete for S. fuliginea, elevated for B. cinerea and C. cucumerinum, and minimal for I. bolleyi and P. rugulosa (Table 1).
TABLE 1.
Inhibition and lipid analysis (percent dry weight) of selected fungi
| Fungus | % Inhibition of:
|
% Dry wt of d:
|
S/P ratiod | Δ/molde | |||
|---|---|---|---|---|---|---|---|
| Growthab | Germinationac | Total lipids | Sterols | Phospholipids | |||
| P. rugulosa | 8.0 a | 4.7 a | 38.7 d | 7.2 c | 9.6 cd | 1.4 c | 0.9 a |
| I. bolleyi | 32.3 b | 9.0 a | 36.1 d | 6.5 c | 9.1 cd | 1.3 c | 1.7 c |
| C. cucumerinum | 46.8 c | 60.9 b | 17.6 b | 2.1 b | 5.3 a | 0.7 b | 1.1 b |
| B. cinerea | 47.5 c | 58.0 b | 12.1 a | 3.2 b | 7.4 abc | 0.8 b | 1.6 c |
| S. fuliginea | ND | 92.5 c | 25.6 c | 3.4 b | 10.8 d | 0.6 b | 1.7 c |
| P. infestans | 64.9 d | ND | 25.4 c | 0.0 a | 8.7 bcd | 0.0 a | 0.9 a |
| P. aphanidermatum | 66.8 d | ND | 25.6 c | 0.0 a | 6.6 ab | 0.0 a | 0.8 a |
Within a column, means with same letter are not significantly different based on Fisher's protected LSD (P = 0.05). ND, not determined.
Inhibition expressed as a percentage of the reduction of mycelial dry weight of 3-day-old liquid cultures in the presence of CHDA (0.15 mg/ml) relative to the untreated control.
Inhibition expressed as a percentage of the reduction of conidial germination following a 24-h incubation period with CHDA (0.15 mg/ml) relative to the untreated control.
Within a column, means with same letter are not significantly different based on Fisher's protected LSD (P = 0.01).
Δ/mol = [% 18:1 + 2(% 18:2) + 3(% 18:3)]/100.
Total lipid composition.
Quantitative analysis of total lipids indicated that I. bolleyi and P. rugulosa had a significantly higher intrinsic lipid content than did the other five fungi (Table 1). P. infestans, P. aphanidermatum, and S. fuliginea had comparable lipid content, whereas C. cucumerinum had lower levels of total lipids. B. cinerea had the lowest lipid content of all fungi tested. I. bolleyi and P. rugulosa also had the highest proportion of total sterols, whereas B. cinerea, C. cucumerinum, and S. fuliginea had statistically similar content (Table 1). P. infestans and P. aphanidermatum contained no sterols. Thin-layer chromatography analysis revealed that phospholipids were the most abundant polar lipids in all fungi. Phospholipid contents varied twofold between the highest (S. fuliginea) and lowest (C. cucumerinum) values of tested fungi. The S/P molar ratio was significantly higher in I. bolleyi and P. rugulosa than in B. cinerea, C. cucumerinum and S. fuliginea. Because sterols were not detected in P. infestans and P. aphanidermatum, the S/P molar ratio was 0.0 (Table 1).
Phospholipid fatty acid composition.
For all tested fungal controls, palmitic acid (16:0) was the most prevalent saturated phospholipid fatty acid. The most quantitatively important unsaturated fatty acids were oleic acid (18:1) for P. infestans and P. rugulosa, linoleic acid (18:2) for B. cinerea, C. cucumerinum, I. bolleyi, and P. aphanidermatum, and linolenic acid (18:3) for S. fuliginea. Overall, B. cinerea, I. bolleyi, and S. fuliginea had a significantly higher degree of fatty acid unsaturation due to their high proportions of linoleic or linolenic acids. C. cucumerinum had an intermediately high Δ/mol value, whereas P. infestans, P. rugulosa, and P. aphanidermatum had lower values (Table 1). Among fungi treated with CHDA, the Δ/mol value remained unchanged for I. bolleyi and P. rugulosa but was significantly higher than that for the controls (P = 0.05) for B. cinerea (1.9 versus 1.6), C. cucumerinum (1.5 versus 1.1), P. infestans (1.2 versus 0.9), and P. aphanidermatum (0.9 versus 0.8). Only for S. fuliginea was the Δ/mol value significantly lower (1.3 versus 1.7) than for the controls (P = 0.05).
Evolution of phospholipid fatty acid unsaturation.
For I. bolleyi and P. aphanidermatum, the degree of fatty acid unsaturation evolved as a function of the growth stage (Table 2). For I. bolleyi, both mycelial controls and mycelia treated with CHDA entered different growth phases after equivalent culture periods. For I. bolleyi, there was no difference in the Δ/mol value between treatments at any growth phase. However, in the presence of CHDA, growth of P. aphanidermatum lagged behind that of the control. Thus, after 3 days of incubation, the P. aphanidermatum control had entered early stationary growth phase, whereas the CHDA-treated mycelia were in mid-logarithmic phase. At day 4, the CHDA-treated fungus was in early stationary phase when the control was well into its stationary growth phase. The Δ/mol value varied accordingly with the growth phase of P. aphanidermatum. Δ/mol was higher in mid-logarithmic phase than in early stationary growth phases at day 3. Also, the stationary growth phase was associated with a lower Δ/mol value than the early stationary phase at day 4.
TABLE 2.
Evolution of growth phase and phospholipid fatty acid unsaturation of selected fungi in the absence (control) and presence (treated) of a sublethal dose of CHDA
| Fungus | Growth period (days) | Treatment group | Growth phasea | Δ/molbc |
|---|---|---|---|---|
| I. bolleyi | 2 | Control | EL | 1.7 |
| Treated | EL | 1.7 | ||
| 3 | Control | ML | 1.6 | |
| Treated | ML | 1.7 | ||
| 4 | Control | ML | 1.6 | |
| Treated | ML | 1.7 | ||
| 6 | Control | ES | 1.6 | |
| Treated | ES | 1.6 | ||
| P. aphanidermatum | 2 | Control | EL | 0.9 |
| Treated | EL | 0.9 | ||
| 3 | Control | ES | 0.8 | |
| Treated | ML | 1.0∗∗ | ||
| 4 | Control | S | 0.8 | |
| Treated | ES | 0.9∗ | ||
| 7 | Control | S | 0.8 | |
| Treated | S | 0.9 |
EL, early logarithmic phase; ML, mid-logarithmic phase; ES, early stationary phase; S, stationary phase.
Δ/mol = [% 18:1 + 2(% 18:2) + 3(% 18:3)]/100.
Within control and treated fungi at the same growth period, ∗ = significantly different at P = 0.05; ∗∗ = significantly different at P = 0.01.
Analysis of CHDA.
CHDA was not detected in the culture medium or in the phospholipids of any fungus. However, CHDA was found in the free fatty acids in quantities consistent with the dose of application (P = 0.05) for all fungi.
DISCUSSION
P. flocculosa produces antifungal fatty acids known to affect the mycelial growth and conidial germination of fungi (2, 5, 17). Thus, understanding the mode and site of action of these antifungal fatty acids is of major importance in the development of P. flocculosa as a biocontrol agent. The sensitivity of fungi to these antifungal fatty acids was recently hypothesized to be linked to low sterol content and a high degree of phospholipid fatty acid unsaturation, factors which contribute to an elevated membrane fluidity (5). Here, we have tested a range of fungi differing in their specific lipid composition and their sensitivity to the antifungal fatty acids and have monitored CHDA, one of the antifungal fatty acids, in fungal membranes. This has allowed us to present new evidence on the relative sensitivities of fungi to this compound and provide insight into its specific mode of action.
I. bolleyi and P. rugulosa, the fungi most resistant to CHDA, had markedly higher sterol content than all the other fungi, while both P. infestans and P. aphanidermatum, the most sensitive fungi, lacked sterols altogether. These data support the concept (5) that a low proportion of sterols is linked to higher sensitivity to P. flocculosa antifungal fatty acids. These results were expected because sterols are known to buffer stress-induced modifications in membrane fluidity (7), thus protecting the fungus in the presence of potentially disruptive toxic fatty acids. This is of particular importance in the context of the use of P. flocculosa as a biocontrol agent for S. fuliginea, the pathogen that causes powdery mildew of cucumber. Indeed, the low sterol content in S. fuliginea relates well not only to its CHDA sensitivity but also to the effective biocontrol of cucumber powdery mildew by P. flocculosa.
The degree of phospholipid unsaturation did not correlate with the sensitivity of the fungi to CHDA. These results suggest that an elevated degree of phospholipid unsaturation is not as closely involved in sensitivity as was suggested previously (5). To address more deeply the involvement of phospholipid fatty acid unsaturation in CHDA-mediated events, untreated fungi were compared with those cultured in the presence of CHDA. For the most CHDA-resistant fungi, P. rugulosa and I. bolleyi, there was no change in the degree of unsaturation between treated and control mycelia, but sensitive fungi, with the exception of S. fuliginea, demonstrated a surprising elevation in the Δ/mol value. When a stress-induced elevation in membrane fluidity occurs, cells are expected to compensate by lowering their phospholipid unsaturation to maintain optimal membrane fluidity for growth (25). Results from I. bolleyi and P. aphanidermatum revealed that this elevation in the degree of phospholipid fatty acid unsaturation does not seem to be an adaptive response to the toxic effect of CHDA but rather a consequence of growth lag in sensitive fungi. Indeed, slower growth of sensitive fungi in the CHDA treatment led to sampling of these fungi in their most active phase (log phase), where a higher degree of fluidity is necessary. Other results (23, 30, 31) have demonstrated conclusively that cells need a higher degree of fluidity for such biological processes as germination and growth. This also explains why S. fuliginea spores treated with CHDA, which did not germinate, had a lower level of phospholipid fatty acid unsaturation than the germinated spores in the untreated controls.
Our results clearly indicate that sterols are not the targets of CHDA, since P. infestans and P. aphanidermatum, which do not contain or produce sterols, were very sensitive to CHDA. If sterols were the active sites of CHDA, these fungi would be unaffected by the treatment.
At the dose used in this study, CHDA was not retrieved from the media in which fungi had been cultured, indicating its uptake or insertion into fungal cells. When fungal lipid fractions were analyzed, CHDA was never present in the fatty acyl chains of phospholipids. This indicates that modification or utilization of CHDA in fungi is not involved in its toxicity. Moreover, CHDA was completely recovered from the free fatty acid fraction of fungi, indicating that CHDA is present in free form in fungal membranes. As described previously (22, 27), fatty acids, in general, are known to freely partition into membranes. Previous reports indicate that cis-unsaturated fatty acids induce disorder in neighboring acyl chains due to their bulkiness caused by the high motional freedom at a certain distance from the carboxyl group (24, 29). The cis double bond produces a fixed kink or bend in the fatty acid. Rotation of the molecule causes disorder in the neighboring membrane acyl chains, thus causing an elevation in membrane fluidity (9). Nonspecific changes in physical characteristics of the bilayer could therefore induce conformational changes in membrane proteins and thereby alter their normal function (12, 13). These results also explain the higher levels of activity of other P. flocculosa antifungal fatty acids (4) which possess additional methyl branch structures capable of disrupting membrane chain packing by occupying a larger cross-sectional area in the membrane bilayer (24).
Overall, our data and those of previous reports (5, 17) are best explained by a model in which the lipid bilayers of fungal membranes are the primary target of the action of CHDA and the other closely related fatty acids produced by P. flocculosa (Fig. 1). At the molecular level, current data suggest that a sublethal dose of CHDA would readily partition into fungal membranes. It is not modified or otherwise utilized but causes an elevation in membrane fluidity in its free form. Fungi with a high sterol content can buffer the stress-induced elevation in fluidity and can grow at their maximal rate (7). By contrast, fungi containing little or no sterol cannot deal as well with the presence of CHDA, and the ensuing loss of membrane integrity retards their growth rate. At higher doses of CHDA, the greater elevation in fluidity (higher disorganization) (5) would cause changes in membrane permeability. This would cause the release of intracellular electrolytes and of proteins (17) and, eventually, cytoplasmic disintegration (16) of mycelia and spores.
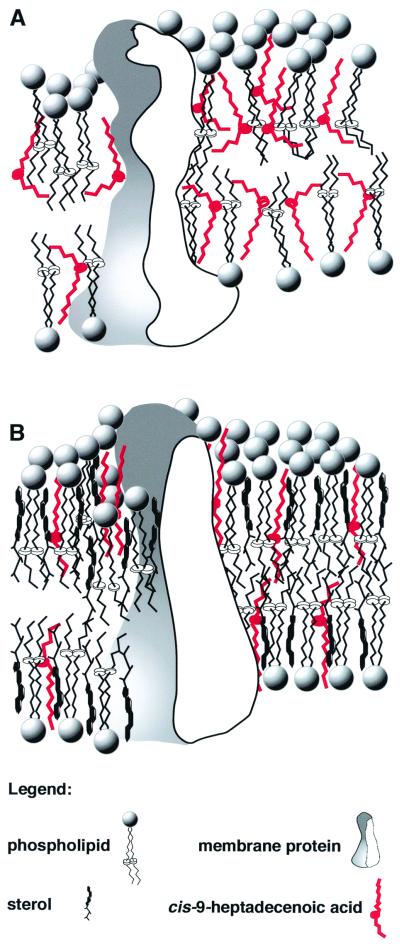
FIG. 1.
Proposed model of activity of CHDA, an antifungal compound produced by P. flocculosa. (A) Susceptible fungi. CHDA partitions into the hydrophobic region of fungal membranes, producing significant changes in the packing of lipid molecules by inducing disorder in neighboring acyl chains due to its high motional freedom. The resulting change in membrane dynamics would affect the activity of membrane-bound proteins. These effects result in alteration of membrane potentials which leads to its collapse, as reported for susceptible fungi exposed to P. flocculosa (17). (B) Resistant fungi. Sterols buffer stress-induced fluctuations in membrane fluidity by ordering fatty acyl chains. This would maintain more optimal activity of membrane-bound enzymes and proteins by minimizing the impact of the CHDA-induced disordering effect. Minimal membrane alterations are induced, and resistant fungi can overcome this stress, as observed with I. bolleyi (2). This model is consistent with the activity of the other related antifungal fatty acids produced by P. flocculosa, namely 6-methyl-9-heptadecenoic acid, whereby its methyl branch would cause an even greater disturbance in the lipid environment which would explain its greater activity in vitro (1, 5).
This study, in which living fungal cells were used as model membranes, is the first, to our knowledge, to propose a specific mode of action for unusual fatty acids and the molecular events involved in their toxicity. This is of great relevance in understanding the basis of the antagonistic effect of P. flocculosa on S. fuliginea, one of the targeted pathogens for use of the biocontrol agent. This study gives greater insight into the means by which P. flocculosa protects its habitat on the leaf surface and thus how it is able to exert its biocontrol activity against powdery mildew fungi, such as S. fuliginea, with which it shares its ecological niche.
ACKNOWLEDGMENTS
This work was supported by a grant from the Natural Sciences and Engineering Research Council of Canada and Plant Products Co. Ltd.
We thank R. Boulanger, S. Caron, and S. Couture for technical assistance, C. Labbé for graphic work, T. Carver for proofreading, and C. Willemot and M. Paquot for critical review of the manuscript and the model.
REFERENCES
- 1.Avis T J, Bélanger R R. Synthesis and biological characterization of (Z)-9-heptadecenoic and (Z)-6-methyl-9-heptadecenoic acids, fatty acids with antibiotic activity produced by Pseudozyma flocculosa. J Chem Ecol. 2000;26:987–1000. [Google Scholar]
- 2.Bélanger R R, Deacon J W. Interaction specificity of the biocontrol agent Sporothrix flocculosa: a video microscopy study. Phytopathology. 1996;86:1317–1323. [Google Scholar]
- 3.Bélanger R R, Labbé C, Jarvis W R. Commercial-scale control of rose powdery mildew with a fungal antagonist. Plant Dis. 1994;78:420–424. [Google Scholar]
- 4.Benyagoub M, Bel Rhlid R, Bélanger R R. Purification and characterization of new fatty acids with antibiotic activity produced by Sporothrix flocculosa. J Chem Ecol. 1996;22:405–413. doi: 10.1007/BF02033644. [DOI] [PubMed] [Google Scholar]
- 5.Benyagoub M, Willemot C, Bélanger R R. Influence of a subinhibitory dose of antifungal fatty acids from Sporothrix flocculosa on cellular lipid composition in fungi. Lipids. 1996;31:1077–1082. doi: 10.1007/BF02522465. [DOI] [PubMed] [Google Scholar]
- 6.Bligh E G, Dyer W J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 7.Bloch K. Sterol structure and membrane function. Crit Rev Biochem. 1983;14:47–92. doi: 10.3109/10409238309102790. [DOI] [PubMed] [Google Scholar]
- 8.Boekhout T. Pseudozyma Bandoni emend. Boekhout, a genus for yeast-like anamorphs of Ustilaginales. J Gen Appl Microbiol. 1995;41:359–366. [Google Scholar]
- 9.Burt J M, Massey K D, Minnich B N. Uncoupling of cardiac cells by fatty acids: structure-activity relationship. Am J Physiol. 1991;260:C439–C448. doi: 10.1152/ajpcell.1991.260.3.C439. [DOI] [PubMed] [Google Scholar]
- 10.Child J J, Défago G, Haskins R H. The effects of cholesterol and polyene antibiotics on the permeability of the protoplasmic membrane of Pythium PRL 2142. Can J Microbiol. 1969;15:599–603. doi: 10.1139/m69-102. [DOI] [PubMed] [Google Scholar]
- 11.Choudhury S R, Traquair J A, Jarvis W R. 4-Methyl-7, 11-heptadecadenal and 4-methyl-7, 11-heptadecadienoic acid: new antibiotics from Sporothrix flocculosa and Sporothrix rugulosa. J Nat Prod. 1994;57:700–704. doi: 10.1021/np50108a003. [DOI] [PubMed] [Google Scholar]
- 12.DeKruff B, DeGreef W J, Van Eyk R V W, Demel R A, Van Deen L L M. Influence of fatty acid and sterol composition on the lipid phase transition and activity of membrane-bound enzymes in Acholeplasma laidlawii. Biochim Biophys Acta. 1973;330:269–282. doi: 10.1016/0005-2736(73)90232-0. [DOI] [PubMed] [Google Scholar]
- 13.Garg A P, Müller J. Fungitoxicity of fatty acids against dermatophytes. Mycoses. 1993;36:51–63. doi: 10.1111/j.1439-0507.1993.tb00687.x. [DOI] [PubMed] [Google Scholar]
- 14.Hajlaoui M R, Bélanger R R. Comparative effects of temperature and humidity on the activity of three potential antagonists of rose powdery mildew. Neth J Plant Pathol. 1991;97:203–208. [Google Scholar]
- 15.Hajlaoui M R, Bélanger R R. Antagonism of the yeast-like phylloplane fungus Sporothrix flocculosa against Erysiphe graminis var. tritici. Biocontrol Sci Technol. 1993;3:427–434. [Google Scholar]
- 16.Hajlaoui M R, Benhamou N, Bélanger R R. Cytochemical study of the antagonistic activity of Sporothrix flocculosa on rose powdery mildew, Sphaerotheca pannosa var. rosae. Phytopathology. 1992;82:583–589. [Google Scholar]
- 17.Hajlaoui M R, Traquair J A, Jarvis W R, Bélanger R R. Antifungal activity of extracellular metabolites produced by Sporothrix flocculosa. Biocontrol Sci Technol. 1994;4:229–237. [Google Scholar]
- 18.Herbette L G, Favreau C, Segalman K, Napolitano C A, Watras J. Mechanisms of fatty acid effects on sarcoplasmic reticulum. II. Structural changes induced by oleic and palmitic acids. J Biol Chem. 1984;259:1325–1335. [PubMed] [Google Scholar]
- 19.Horsfal J G. Principles of fungicidal actions. Waltham, Mass: Chronica Botanica; 1956. pp. 89–112. [Google Scholar]
- 20.Jarvis W R, Shaw L A, Traquair J A. Factors affecting antagonism of cucumber powdery mildew by Stephanoascus flocculosus and S. rugulosus. Mycol Res. 1989;92:162–165. [Google Scholar]
- 21.Kates M. Techniques of lipidology: isolation, analysis, and identification of lipids. In: Burton T S, Van Knippenberg P H, editors. Laboratory techniques in biochemistry and molecular biology. Amsterdam, The Netherlands: Elsevier; 1986. pp. 186–278. [Google Scholar]
- 22.Klausner R D, Kleinfeld A M, Hoover R L, Karnovsky M J. Lipid domains in membranes: evidence derived from structural perturbations induced by free fatty acids and lifetime heterogeneity analysis. J Biol Chem. 1980;255:1286–1295. [PubMed] [Google Scholar]
- 23.Koch J L, Ratledge C. Changes in lipid composition and arachidonic acid turnover during the life cycle of the yeast Diplodascopsis uninucleata. J Gen Microbiol. 1993;139:459–464. doi: 10.1099/00221287-139-3-459. [DOI] [PubMed] [Google Scholar]
- 24.Macdonald P M, Sykes B D, McElhaney R N. Fatty acyl chain structure, orientational order, and the lipid phase transition in Acholeplasma laidlawii B membranes. A review of recent 19F nuclear magnetic resonance studies. Can J Biochem Cell Biol. 1984;62:1134–1150. doi: 10.1139/o84-147. [DOI] [PubMed] [Google Scholar]
- 25.Maresca B, Cossins A R. Fatty feedback and fluidity. Nature. 1993;365:606–607. doi: 10.1038/365606a0. [DOI] [PubMed] [Google Scholar]
- 26.Mukherjee M, Chakravarty A K, Sengupta S. Natural resistance of the mycelial culture of the mushroom, Panaeolus papillonaceus, towards growth inhibition by polyene antibiotics. Curr Microbiol. 1993;27:1–4. [Google Scholar]
- 27.Pjura W J, Kleinfeld A M, Karnovsky M J. Partition of fatty acids and fluorescent fatty acids into membranes. Biochemistry. 1984;23:2039–2043. doi: 10.1021/bi00304a024. [DOI] [PubMed] [Google Scholar]
- 28.Rangel H, Dagger F, Hernandez A, Liendo A, Urbina J A. Naturally azole-resistant Leishmania braziliensis promastigotes are rendered susceptible in the presence of terbinafine: comparative study with azole-susceptible Leishmania mexicana promastigotes. Antimicrob Agents Chemother. 1996;40:2785–2791. doi: 10.1128/aac.40.12.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rustenbeck I, Lenzen S. Regulation of transmembrane ion transport by reaction products of phospholipase A2. II. Effects of arachidonic acid and other fatty acids on mitochondrial Ca2+ transport. Biochim Biophys Acta. 1989;982:147–155. doi: 10.1016/0005-2736(89)90185-5. [DOI] [PubMed] [Google Scholar]
- 30.Watson K, Rose A H. Fatty-acyl composition of the lipids of Saccharomyces cerevisiae grown aerobically or anaerobically in media containing different fatty acids. J Gen Microbiol. 1980;117:225–233. [Google Scholar]
- 31.Weete J D, Sancholle M S, Montant C. Effects of triazoles on fungi. II. Lipid composition of Taphrina deformans. Biochim Biophys Acta. 1983;752:19–29. [Google Scholar]