Abstract
Objectives:
Opioid use disorder remains undertreated in the United States. One of the primary mechanisms for expanding access to treatment has been the use of buprenorphine. In this study, we compare prescribing trends of buprenorphine paid through Medicaid versus other payer sources.
Methods:
Combined data from California’s prescription drug monitoring program and California’s Department of Health Care Services was used to obtain statewide quarterly prescription rates for buprenorphine, indicated for the treatment of opioid use disorder, from 2012 to 2018.
Results:
From 2012 to 2018, the rate of individuals treated with buprenorphine in Medicaid increased by 657% (1.39 to 10.5 Medicaid beneficiaries per 10,000) with increases beginning in 2014 and continuing through 2018. Rate of individual prescribing among non-Medicaid sources increased by 93.7% (6.54 to 12.7 non-Medicaid individuals per 10,000) with most increases occurring prior to 2014.
Conclusions:
California Medicaid has made considerable gains in buprenorphine access, with access growing steadily even after expansions through the Affordable Care Act plateaued. In contrast, recent gains in buprenorphine access for individuals without Medicaid are uninspiring, indicating that initiatives to improve buprenorphine access to patients without Medicaid are urgently needed.
Keywords: Medicaid, Buprenorphine, Opioid Use Disorder, Access
Introduction:
Recent trends reveal substantial increases in opioid-related overdoses.1 Underscoring the urgent need for increased access to medications to treat opioid use disorder (OUD). Buprenorphine has key advantages over other medicines used to treat OUD: it is safer than methadone, can be prescribed in an office-based setting, and is more effective than naltrexone.2
The Affordable Care Act (ACA) has been associated with increased access to medications for OUD among Medicaid beneficiaries,3,4 and has been linked to decreases in opioid-related mortality,5 though recent evidence may cast some doubt on the importance of expanded insurance coverage on improved access.6 California’s Medicaid program covers low-income children, pregnant women, families, and as of 2014, and due to the ACA, expanded to include low-income adults. Following ACA expansion, California’s Medicaid program introduced multiple initiatives to promote buprenorphine access.7–9 However, ACA-related gains in Medicaid enrollment plateaued in 201610 and it remains unclear how access to OUD treatment has grown since for individuals with versus without Medicaid. In this study, we compare prescribing trends of buprenorphine for opioid use disorder, from 2012–2018, for Medicaid and non-Medicaid payer sources in California.
Methods:
Combined data from California’s prescription drug monitoring program (PDMP) and the Department of Health Care Services (DHCS) was used to obtain quarterly totals of individuals who received buprenorphine, indicated for the treatment of OUD, from 2012 to 2018. PDMP data includes all outpatient controlled substance prescriptions dispensed in the state and therefore all dispensed prescriptions for buprenorphine regardless of payer type. PDMP records contain encrypted patient identifiers that allowed us to track individuals over time. Individuals with buprenorphine for OUD prescriptions were identified and totals determined for each quarter in the study period. DHCS reports statewide quarterly totals of Medicaid beneficiaries with claims for buprenorphine for OUD. Totals are calculated from county-level submitted claims for buprenorphine.11 Buprenorphine NDC codes were compared from both datasets (see Table, Supplemental Digital Content 1) to ensure valid comparisons and the exclusion of formulations indicated for the treatment of pain.
We were able to estimate total individuals receiving these medications through non-Medicaid payer sources, except for Veterans’ Affairs, Department of Defense, and Indian Health Services, by taking the difference of the totals from the two data sources. Medicaid and non-Medicaid population totals were then used to determine quarterly rates of buprenorphine treatment. The non-Medicaid population was estimated by subtracting the Medicaid eligible population, (i.e., totals of all California residents eligible for enrollment obtained from DHCS)12 from the total population, estimated by interpolating annual counts obtained from the California Department of Finance.13
This study was approved by the University of California Davis Institutional Review Board and the California Committee for the Protection of Human Subjects.
Results:
Trends of buprenorphine prescription rates are shown for Medicaid and non-Medicaid populations (Figure 1). From 2012 to 2018, the rate of individuals treated with buprenorphine in Medicaid increased by 657% (1.39 to 10.5 Medicaid beneficiaries per 10,000) with 94.4% of this growth taking place after 2013. In contrast, buprenorphine prescribing rates for individuals without Medicaid increased only 93.7% (6.54 to 12.7 non-Medicaid individuals per 10,000) with 60.2% of this growth taking place prior to 2014. Medicaid prescribing of buprenorphine increased steadily through 2018 even though Medicaid enrollment plateaued in 2016 at just under 35% of the state population (Table 1). While only 4.8% of buprenorphine prescriptions written in California were paid through Medicaid in 2012, by 2016 this proportion had increased to 20.1%. From 2016 to 2018, this fraction further increased to 27.5% despite decreases in Medicaid coverage (34.5% to 33.0% of the total population).
Figure 1.
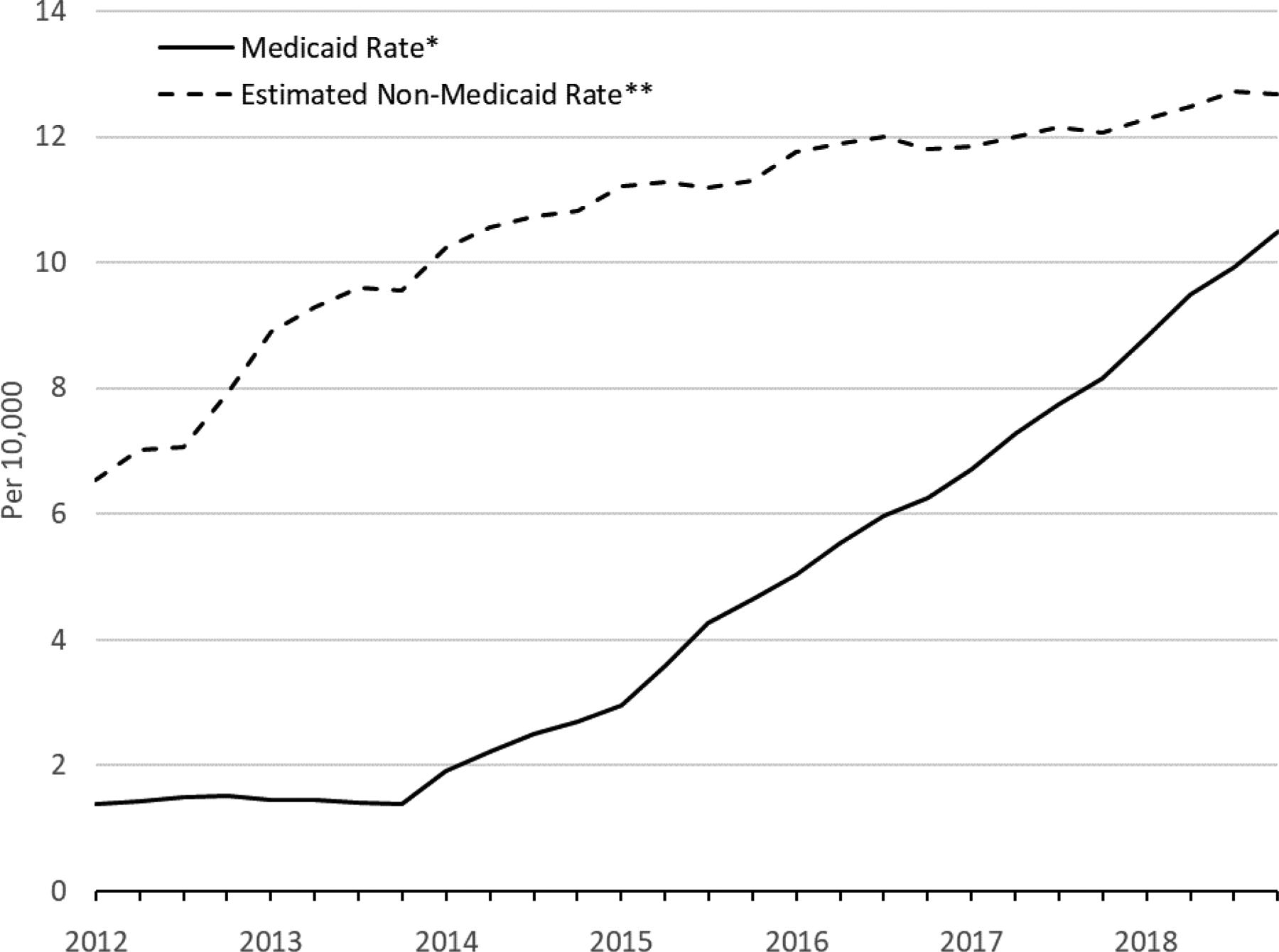
Quarterly Rates of Buprenorphine Treatment for Opioid Use Disorder by Payer Type per Total Medicaid and non-Medicaid Population: California, 2012—2018
*Per 10,000 California Residents Eligible for Medicaid
**Per 10,000 California Residents Ineligible for Medicaid
Table 1.
Annual Estimates of Medicaid Coverage and Buprenorphine Treatment for Opioid Use Disorder Payment Characteristics in California: 2012—2018
| Year | California Residents Eligible for Medicaid n (%*) | California Residents Treated with Buprenorphine | |
|---|---|---|---|
| Medicaid n (%**) | Non-Medicaid n (%**) | ||
| 2012 | 7,627,757 (20.0%) | 1,111 (4.8%) | 21,891 (95.2%) |
| 2013 | 8,385,417 (21.8%) | 1,195 (4.1%) | 28,230 (95.9%) |
| 2014 | 11,302,108 (29.2%) | 2,653 (8.3%) | 29,245 (91.7%) |
| 2015 | 12,848,284 (32.9%) | 4,982 (14.4%) | 29,646 (85.6%) |
| 2016 | 13,572,589 (34.5%) | 7,731 (20.1%) | 30,744 (79.9%) |
| 2017 | 13,403,761 (33.9%) | 10,019 (24.0%) | 31,646 (76.0%) |
| 2018 | 13,157,397 (33.0%) | 12,730 (27.5%) | 33,549 (72.5%) |
Among total population of California
Among all patients who filled prescriptions for Buprenorphine
Discussion:
The rate of Medicaid beneficiaries receiving buprenorphine increased steadily even after ACA-related gains plateaued. Results suggest that growth in buprenorphine prescribing cannot be explained solely by expanded Medicaid coverage. In 2015, Medicaid removed restrictions on buprenorphine indicated for OUD and clarified that primary care providers could be reimbursed for treating substance use disorders.7,8 California also launched the Treating Addiction in Primary Care Safety Net program in 2016 to encourage safety net clinics to increase the number of waivered physicians, reduce stigmatization, and increase compassion for OUD patients. This program was launched to augment federal funding provided through the Health Resources & Services Administration to 36 qualified health centers in California with the aims of increasing the numbers of waivered physicians and patient access to medication.9 These policy changes and service improvements are likely major contributors to the sustained increases in buprenorphine treatment rates observed among Medicaid beneficiaries after California’s Medicaid expansion plateaued.
Conversely, buprenorphine prescribing for patients without Medicaid showed relatively little growth post-2014. This may be partially explained by individuals who previously paid cash for buprenorphine enrolling in Medicaid. However, expanded Medicaid coverage would likely not be a factor in the commercially insured population, who remain a large proportion of individuals receiving buprenorphine,4 particularly as findings from a national study suggest a lack of improved access to buprenorphine. They reported that though OUD diagnosis rates increased from 2010 and 2014, treatment duration for commercially insured individuals decreased.14 Slow growth in the non-Medicaid population may also be due to already high base rates of prescribing which would lend to slower growth than if the base rates of prescribing were very low, as they were among Medicaid beneficiaries pre-ACA. Additionally, the burden of OUD may be comparatively higher among those newly eligible for Medicaid,3 so increases might be expected to occur faster and at a greater rate in this population than in the non-Medicaid one.
The use of different data sources for the comparison presents a limitation. The PDMP receives data submitted by pharmacies for the purpose of tracking controlled substance prescriptions while the Medicaid program receives claims to pay for prescriptions for Medicaid beneficiaries. To ensure a valid comparison, authors reviewed both data sources and verified that they contained the same set of national drug codes used to identify buprenorphine prescriptions indicated for the treatment of OUD. Additionally, the non-Medicaid estimate does not include individual prescriptions dispensed through Veterans’ Affairs, Department of Defense, or Indian Health Services. However, these sources make up a small fraction of total non-Medicaid payers so their exclusion has a minimal impact on results. California has low rates of opioid prescribing and opioid-related overdoses relative to the rest of the country.15 Results must therefore be understood in the context of California’s comparatively lower rates of opioid-related harms.
Conclusion:
Access to buprenorphine for OUD increased substantially for the Medicaid population and extended beyond the coverage expansion due to the ACA. The lack of growth in the prescribing of non-Medicaid covered buprenorphine, however, is sobering; especially given the overwhelming need for patient access to OUD treatment. Initiatives implemented by California’s Medicaid program appear to have contributed to the sustained growth of buprenorphine treatment of OUD in the state. These policies may provide commercial insurers with a framework to address the substantial barriers preventing access to OUD treatment among individuals ineligible for Medicaid.
Supplementary Material
Acknowledgements:
This work was supported by the Bureau of Justice Assistance, the Centers for Disease Control and Prevention, and the National Institutes of Health.
Financial Support:
This work was supported by Bureau of Justice Assistance grant 2015-PM-BX-K001, Centers for Disease Control and Prevention grant 1U17CE002747, and National Institutes of Health grant R01DA044282
Footnotes
Disclaimer: The findings and conclusions in this article are those of the author(s) and do not necessarily represent the views or opinions of the State of California, the Centers for Disease Control and Prevention, the Bureau of Justice Assistance, or the National Institutes of Health. This study was approved by the University of California Davis Institutional Review Board and the California Committee for the Protection of Human Subjects.
Conflicts of Interest: The authors have no conflicts of interest to disclose.
References:
- 1.Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and Opioid-Involved Overdose Deaths - United States, 2013–2017. MMWR Morb Mortal Wkly Rep. 2018;67(5152):1419–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strang J, Volkow ND, Degenhardt L, et al. Opioid use disorder. Nat Rev Dis Primers. 2020;6(1):3. [DOI] [PubMed] [Google Scholar]
- 3.Cher BAY, Morden NE, Meara E. Medicaid Expansion and Prescription Trends: Opioids, Addiction Therapies, and Other Drugs. Med Care. 2019;57(3):208–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saloner B, Levin J, Chang HY, Jones C, Alexander GC. Changes in Buprenorphine-Naloxone and Opioid Pain Reliever Prescriptions After the Affordable Care Act Medicaid Expansion. JAMA Netw Open. 2018;1(4):e181588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kravitz-Wirtz N, Davis CS, Ponicki WR, et al. Association of Medicaid Expansion With Opioid Overdose Mortality in the United States. JAMA Netw Open. 2020;3(1):e1919066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gertner AK, Robertson AG, Jones H, Powell BJ, Silberman P, Domino ME. The effect of Medicaid expansion on use of opioid agonist treatment and the role of provider capacity constraints. Health Serv Res. 2020;55(3):383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brooks S Professional Fees for Office Visits Associated with Alcohol and Substance Abuse Disorder Treatment Services. Managed Care Quality and Monitoring Division: Department of Health Care Services,. https://www.dhcs.ca.gov/formsandpubs/Documents/MMCDAPLsandPolicyLetters/APL2015/APL15-008.pdf. Published 2015. Accessed April, 2020.
- 8.Department of Health Care Services: Drug Use Review. Clinical Review: The Treatment of Opioid Addiction with Buprenorphine. https://files.medi-cal.ca.gov/pubsdoco/dur/Articles/dured_25096.pdf. Published 2016. Accessed April, 2020.
- 9.McGovern M Center for Care Innovations Treating Addiction in Primary Care: Evaluation. Center Behavioral Health Services and Implementation Research: Stanford University School of Medicine; 2018. [Google Scholar]
- 10.Baumgartner J, Collins S, Radley D, Hayes S. How the Affordable Care Act Has Narrowed Racial and Ethnic Disparities in Access to Health Care. The Commonwealth Fund. 2020. [Google Scholar]
- 11.Department of Health Care Services. Medication-Assisted Treatment in Medi-Cal for Opioid Use Disorders, by County. Department of Health Care Services. https://data.chhs.ca.gov/dataset/medication-assisted-treatment-in-medi-cal-for-opioid-use-disorders-quarterly-by-county. Published 2019. Accessed January, 2020.
- 12.Department of Health Care Services. Medi-Cal Certified Eligible Counts, by County and Dual Status, 2010 to the Most Recent Reportable Month. https://data.chhs.ca.gov/dataset/county-medi-cal-certified-eligible-counts-by-county-and-dual-status-2013-through-2017/resource/9ade93e4-0676-4117-adbe-a53378d7fe84. Published 2019. Accessed January, 2020.
- 13.California Department of Finance. E-2. California County Population Estimates and Components of Change by Year — July 1, 2010–2019. http://www.dof.ca.gov/Forecasting/Demographics/Estimates/E-2/index.html. Published 2019. Accessed January, 2020.
- 14.Morgan JR, Schackman BR, Leff JA, Linas BP, Walley AY. Injectable naltrexone, oral naltrexone, and buprenorphine utilization and discontinuation among individuals treated for opioid use disorder in a United States commercially insured population. J Subst Abuse Treat. 2018;85:90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Institute on Drug Abuse. Opioid Summaries by State. https://www.drugabuse.gov/drug-topics/opioids/opioid-summaries-by-state. Published 2020. Accessed July, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


