Abstract
Type 1 diabetes is associated with increased intestinal inflammation and decreased abundance of butyrate-producing bacteria. We investigated the effect of butyrate on inflammation, kidney parameters, HbA1c, serum metabolites and gastrointestinal symptoms in persons with type 1 diabetes, albuminuria and intestinal inflammation. We conducted a randomized placebo-controlled, double-blind, parallel clinical study involving 53 participants randomized to 3.6 g sodium butyrate daily or placebo for 12 weeks. The primary endpoint was the change in fecal calprotectin. Additional endpoints were the change in fecal short chain fatty acids, intestinal alkaline phosphatase activity and immunoglobulins, serum lipopolysaccharide, CRP, albuminuria, kidney function, HbA1c, metabolites and gastrointestinal symptoms. The mean age was 54 ± 13 years, and the median [Q1:Q3] urinary albumin excretion was 46 [14:121] mg/g. The median fecal calprotectin in the butyrate group was 48 [26:100] μg/g at baseline, and the change was −1.0 [−20:10] μg/g; the median in the placebo group was 61 [25:139] μg/g at baseline, and the change was −12 [−95:1] μg/g. The difference between the groups was not significant (p = 0.24); neither did we find an effect of butyrate compared to placebo on the other inflammatory markers, kidney parameters, HbA1c, metabolites nor gastrointestinal symptoms. Twelve weeks of butyrate supplementation did not reduce intestinal inflammation in persons with type 1 diabetes, albuminuria and intestinal inflammation.
Keywords: type 1 diabetes, intestinal inflammation, albuminuria, butyrate, intestinal alkaline phosphatase
1. Introduction
An interesting interplay between gut microbes and host has been discovered in general as well as in diabetes, and the search for the central link between the gastrointestinal milieu and the development of diabetic complications has begun [1]. Inflammation is a key player in the development of diabetic complications, and inflammation in the gut has probable consequences beyond the gastrointestinal tract. Interestingly, fecal calprotectin, a marker of neutrophil activity, is increased in persons with type 1 diabetes, indicating a possible subclinical inflammatory state [2]. This might be related to disturbance of the bacterial composition, with a shift to lower bacterial diversity and less butyrate-producing bacteria, in persons with type 1 diabetes [3]. The gut microbiota has been suggested as a possible modulator of type 1 diabetes onset, and increased intestinal permeability in combination with intestinal dysbiosis is evident around diabetes onset in children [4,5]. The cause of this bacterial imbalance in diabetes is unknown, and the systemic effects of subclinical gut inflammation in persons with type 1 diabetes are still to be investigated.
It has been demonstrated that the short chain fatty acid (SCFA) butyrate produced by colonic bacteria—mainly Firmicutes—by fermentation of fibers, exerts beneficial metabolic, anti-inflammatory and anti-carcinogenic effects in epithelial cells, immune cells and adipocytes [6,7,8,9]. The anti-inflammatory effect is essential for maintaining the intestinal mucosal defense that segregates commensal and pathogenic bacteria, e.g., by upregulation of intestinal alkaline phosphatase (IAP) gene expression [10,11]. The brush-border enzyme IAP plays an important role by suppressing inflammatory mediators, including microbial compounds (e.g., endotoxins and polyphosphates) and luminal ATP by dephosphorylation [12,13]; IAP also increases immunoglobulin (Ig) A, which is crucial for the mucosal host–microbiota interplay, demonstrated in a mouse model [2,14]. Decreased luminal IAP activity, which has been reported in type 1 diabetes [2], possibly increases gut permeability and translocation of toxic proinflammatory compounds, e.g., lipopolysaccharides (LPS), into the intestinal vasculature, which can induce a systemic inflammatory response and thereby increase the risk of kidney or other organ injury [15,16]. An impaired intestinal barrier and an altered bacterial composition are also present in chronic kidney disease [17]; the causation is probably bidirectional, as accumulating uremic toxins are responsible for integrity loss in the intestinal barrier [18]. In a rat study, induction of kidney disease was associated with loss of intestinal tight junctions and colonic mucin, simultaneous with an increase in circulating LPS; supplementation with butyrate improved the kidney function, strengthened the intestinal barrier and reduced the circulating LPS [19]. The kidney-protective effects of SCFA have also been demonstrated in animal models of diabetic kidney disease and acute kidney injury, via local and systemic anti-inflammatory actions [20,21]. In humans, butyrate supplement has shown beneficial effects in persons with various gastrointestinal conditions, e.g., irritable bowel syndrome and inflammatory bowel disease (IBD) [22,23]. These results indicate that early identification and proper management of gastrointestinal inflammation in persons with type 1 diabetes prevents the development of inflammation-mediated systemic disease. Butyrate’s effect on glucose metabolism has been tested in persons with type 2 diabetes, in whom it increased glucagon-like peptide 1 concentration, compared to a placebo [24]. Whether or not this effect is also present in type 1 diabetes is unknown. To our knowledge, butyrate’s effect on intestinal inflammation, IAP activity, kidney parameters, metabolites and HbA1c has never been tested in type 1 diabetes. We hypothesized that oral supplementation of butyrate for 12 weeks would have anti-inflammatory effects locally, resulting in decreased fecal calprotectin, but also systemically, thereby reducing albuminuria and HbA1c and improving kidney function in persons with type 1 diabetes, albuminuria and intestinal inflammation.
2. Materials and Methods
This dual-center, double-blinded, parallel randomized controlled trial included participants from the Steno Diabetes Center Copenhagen (SDCC), Denmark and the Folkhälsan Research Center Helsinki, Finland. The intervention period was from 23 August 2019 to 29 December 2020. The study was registered on 29 August 2019 at clinicaltrials.org (NCT04073927). All participants gave written informed consent. The study was approved by the Regional Ethics Committee in Denmark (26 March 2019, ID: H-18062027) and the Ethics Committee of the Helsinki and Uusimaa Hospital District in Finland and conducted in accordance with the Declaration of Helsinki. Adults with type 1 diabetes and a history of or present albuminuria (two out of three consecutive measurements of urinary albumin creatinine ratio (UACR) above 30 mg/g or 30 mg albumin in 24-h urine collections) were invited for screening. Inclusion required fecal calprotectin ≥ 50 µg/g, determined at home by PreventID CalDetect 50/200 (Preventis GmbH, Bensheim, Germany). Participants were given detailed oral and written test instructions. If the home test was without a visible control line, it was judged as invalid, and the participant was given a second test. If the second test also was without a visible control line, the participant was excluded (n = 1). The home test was only used for screening, and not for assessment of fecal calprotectin levels at baseline and end-of-study (EOS). Key exclusion criteria were the presence of IBD, symptoms of IBD (this was justified based on clinical assessment and, if the investigator was in doubt, after discussion with a gastroenterologist), celiac disease, estimated glomerular filtration rate (eGFR) < 15 mL/min/1.73 m2, dialysis or kidney transplantation, non-diabetic chronic kidney disease, systemic anti-inflammatory therapy, antibiotic therapy within 30 days, pregnancy or lactation. Participants were randomized in blocks of four by the Capital Region Pharmacy, Herlev, Denmark to receive capsules with granular sodium butyrate (1.8 g, corresponding to 6 capsules, twice daily) coated with sodium alginate to ensure delayed release, or matching placebo (microcrystalline cellulose) produced by Sensilab d.o.o., Ljubljana, Slovenia for 12 weeks. Participants, investigators and outcome assessors were all blinded to group assignment. Adherence was predefined as a returned capsule count by the investigator of maximum 20% at EOS. Antibiotic treatment initialized during the intervention period did not cause discontinuation but was registered for sensitivity analysis.
2.1. Study Procedures
The UACR was calculated as the geometric mean of three morning urine samples at baseline and EOS. Blood sampling and measurements of height, weight and blood pressure were obtained at baseline and EOS. Information on demographic data and medical history was collected by interview and from medical records. Fecal samples at baseline, week four, week eight and week twelve (EOS) were collected and immediately frozen at home to −18 degrees Celsius. For the Danish participants, samples were delivered to the site of investigation a maximum 48 h after collection, and transported in provided equipment to avoid thawing, before storing at −80 degrees Celsius. In Finland, participants submitted their fecal samples by mail, and samples were stored at −80 degrees Celsius after a median of 46 [25:64] hours after collection. Assisted questionnaires were completed at baseline and EOS, for evaluation of changes in gastrointestinal symptoms.
2.2. Endpoints
The primary endpoint was change in fecal calprotectin. Secondary endpoints included change in fecal IAP activity, SCFAs, UACR, kidney function and HbA1c. Tertiary endpoints were change in serum lipopolysaccharide (LPS), high sensitivity-C-reactive protein (hs-CRP), fecal immunoglobulins (Ig), 38 selected serum metabolites (Supplementary Table S1) and gastrointestinal symptoms.
2.3. Analyses
The eGFR was estimated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation. Commercial Enzyme-linked Immunosorbent Assay (ELISA) (Bühlmann, Schönenbuch, Switzerland) was used for precise determination of fecal calprotectin concentrations. Fecal SCFA (acetate, propionate, butyrate and valerate) concentrations were quantitated by gas chromatography-mass spectrometry. For the analyses of IAP and immunoglobulins, fecal samples (50 mg) were homogenized in 500 µL extraction buffer (0.1 mM ZnCl2, 1 mM MgCl2, 10 mM Tris–HCl, pH 8.0) using 0.1-mm glass beads (Precellys, Bertin Technologies, Montigny, France). After centrifugation (16,000× g, 10 min, +4 °C), supernatants were collected for the downstream analyses of fecal IAP activity levels, immunoglobulins (IgA, IgG and IgM) and protein concentrations as described earlier [2]. Serum LPS activities were determined with a Limulus Amebocyte Lysate (LAL) assay on 1:5 diluted samples in accordance with the manufacturer’s instructions (HyCult Biotech, Uden, The Netherlands). A panel of 38 metabolites, including bile acids, amino acids and other compounds, was quantified in serum using a targeted platform based on ultra-high-performance liquid chromatography coupled to mass spectrometry, as described previously [25]. The metabolite panel included compounds recognized as predictors of diabetes-related complications: among them, creatinine, gama-butyrobetaine, beta-hydroxybutyrate, N-methylnicotinamide, kynurenine, leucine, isoleucine, phenylalanine, azelaic acid, tryptophan, asymmetric dimethyl arginine, amino adipic acid, taurine, trimethylamine-N-oxide, glycine, glutamine, glutamic acid, tyrosine and indoxyl sulfate. Metabolites were excluded if the concentration (ng/mL) was below the quantification limit in more than 70% of the samples.
2.4. Statistical Analysis
The sample size calculation was based on 80% power to detect a treatment effect of >10% reduction in fecal calprotectin at a significance level of 0.05, which required a sample size of a minimum 42 participants. To allow for dropouts, we included 53 participants. Categorical variables are reported as numbers (%). Continuous variables are presented as mean ± standard deviation (SD) if normal distributed, and the non-normal distributed variables are presented as median [lower quartile (Q1):upper quartile (Q3)] and transformed with a natural logarithm before analysis. The effect of butyrate compared to placebo on the EOS level with baseline value included as covariate was tested with the analysis of covariance (ANCOVA). According to the prespecified analysis plan, the main analyses evaluated the effect of butyrate compared to placebo after 12 weeks of treatment. Due to different fecal collection methods in Denmark and Finland, we tested for site-by-treatment interaction. The proportions of participants with a specific gastrointestinal symptom at baseline who experienced improvement were calculated, and proportions of all participants who experienced worsening or onset of a symptom were calculated for interpretation. In supplementary analysis we: (1) restricted the analyses of calprotectin and UACR to participants with elevated levels (>50 µg/g with ELISA test and >30 mg/g, respectively) at baseline (n = 29 and n = 30, respectively); (2) excluded participants who received antibiotics during the treatment period from analysis of changes in inflammatory parameters (n = 2); (3) excluded participants with dosage change in renin-angiotensin inhibitor (RASi) treatment during the study for the analysis of change in UACR (n = 3); (4) included calprotectin measurements from all four timepoints in a constrained linear mixed model with random intercept. Results are presented for the “intention-to-treat population”, that consisted of all randomized subjects, but an analysis restricted to adherent participants was also performed for the primary and secondary endpoints. All analyses were performed using SAS statistical software version 7.1 (SAS Institute Inc, Cary, NC, USA). A two-sided p value < 0.05 was considered significant.
3. Results
3.1. Baseline
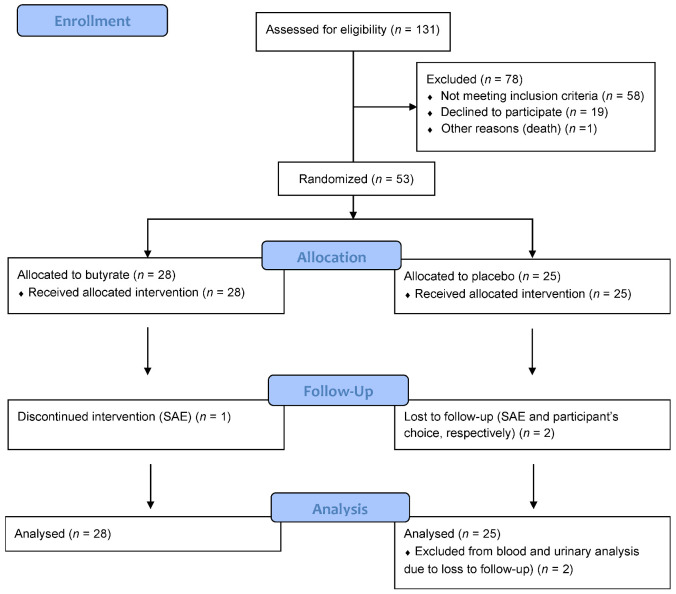
A total of 131 persons consented to participate. Twelve dropped out before the calprotectin home test or were excluded after performing two invalid tests. Of the 119 persons who performed the calprotectin test correct at home, 56 (47%) had <50 μg/g, 48 (40%) had 50–200 μg/g and 15 (13%) had >200 μg/g calprotectin. The two latter groups were invited for randomization, and all 53 persons were randomized (28 butyrate, 25 placebo). One participant terminated the intervention after a serious adverse event (SAE) (ischemic stroke), and seven other participants were non-adherent. Two participants dropped out after week eight, and fecal samples from week eight were used as EOS samples for these two persons in the main analysis of the fecal endpoints, whereas they were not included in analyses of the other endpoints. A flow diagram is presented in Figure 1. Baseline characteristics are displayed in Table 1. Among the 53 individuals, 23 (43%) were women, mean ± SD age was 54 ± 13 years and diabetes duration was 30 ± 15 years. At baseline, median [Q1:Q3] UACR was 46 [14:21] mg/g with 21 (40%), 24 (45%) and 8 (15%) participants having normo-, micro- and macroalbuminuria, respectively. Clinical characteristics at baseline were balanced between the butyrate and placebo group, apart from current smokers, who were all in the placebo group, and treatment with metformin and statin was more frequent in the butyrate group. We found no cases of subclinical celiac disease or IBD at baseline. Only 29 (55%) of the participants had elevated fecal calprotectin (≥50 μg/g) at baseline, despite a positive calprotectin test at home. When all four fecal calprotectin measurements during the study were taken into consideration, 42 (79%) participants had fecal calprotectin value ≥ 50 μg/g at minimum one timepoint during the study period.
Figure 1.
Flow diagram.
Table 1.
Baseline characteristics.
| Total | Sodium Butyrate | Placebo | |
|---|---|---|---|
| Number | 53 (100) | 28 (53) | 25 (47) |
| Site (Denmark) | 41 (77) | 22 (79) | 19 (76) |
| Sex (Female) | 23 (43) | 11 (39) | 12 (48) |
| Age, years | 54 ± 13 | 56 ± 11 | 52 ± 15 |
| Diabetes duration, years | 30 ± 15 | 29 ± 17 | 32 ± 14 |
| Current smoking | 7 (13) | 0 | 7 (28) |
| Body Mass Index, kg/m2 | 29 ± 5.8 | 30 ± 6.1 | 29 ± 5.7 |
| Systolic blood pressure, mm Hg | 135 ± 18 | 136 ± 15 | 134 ± 21 |
| Diastolic blood pressure, mm Hg | 78 ± 10 | 80 ± 10 | 77 ± 10 |
| Urinary albumin creatinine ratio, mg/g | 46 [14:121] | 39 [18:121] | 49 [14:121] |
| Normoalbuminuria | 21 (40) | 11 (39) | 10 (40) |
| Microalbuminuria | 24 (45) | 13 (46) | 12 (44) |
| Macroalbuminuria | 8 (15) | 4 (14) | 3 (12) |
| eGFR, mL/min/1.73 m2 | 84 ± 24 | 86 ± 26 | 8.1 ± 1.1 |
| HbA1c, % (mmol/mol) | 8.0 ± 1.0 | 8.0 ± 0.8 | 8.1 ± 1.1 |
| Fecal calprotectin ≥ 50 µg/g | 29 (55) | 14 (48) | 15 (52) |
| RASi treatment | 44 (83) | 23 (82) | 21 (84) |
| Statin treatment | 37 (70) | 21 (75) | 16 (64) |
| Metformin treatment | 10 (19) | 7 (25) | 3 (12) |
Data are shown as number (%), mean ± standard deviation, or median [Q1:Q3]. eGFR indicates estimated glomerular filtration rate; RASi, renin-angiotensin system inhibitor.
3.2. Changes in Fecal and Circulating Markers of Inflammation
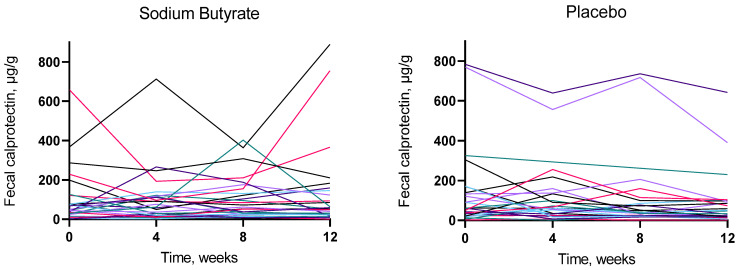
None of the tested fecal biomarkers (calprotectin, IAP, immunoglobulins, SCFAs) changed significantly after butyrate supplementation, compared to placebo (Table 2). From a baseline median [Q1:Q3] calprotectin level of 46 [26:100] μg/g, the median change in the butyrate group was −1.0 [−20:10] μg/g, and from a baseline median level of 61 [25:139] μg/g, the median change was −12 [−95:1] μg/g in the placebo group, with no difference between treatment groups (p = 0.24). Fecal LPS and serum hs-CRP did not change with butyrate supplementation compared to placebo (p = 0.13 and 0.093, respectively) (Table 2). Analyses restricted to participants with calprotectin measured by ELISA of minimum 50 µg/g at baseline (n = 29) showed a significant difference in the effect on calprotectin (p = 0.044) between the treatment groups, driven by a substantial decrease in the placebo group of median −44 [−151:−3] μg/g from a baseline level of 133 [62:304] μg/g, compared to a decrease in the butyrate group of −13 [−50:73] μg/g from a baseline level of 100 [72:229] μg/g. The other results were confirmatory. We found no site-by-treatment interaction (p ≥ 0.20) for any of the fecal markers. We checked that the protein normalized results (IAP and immunoglobulins) were not biased by a change in fecal protein concentrations. No change in fecal protein concentration was demonstrated (p for paired t-test = 0.59 and 0.43 in the butyrate and placebo group, respectively). No indication of a temporary effect on fecal parameters was demonstrated when we visualized the results from all four timepoints of the study (Figure 2).
Table 2.
Change in fecal, blood, urinary markers and HbA1c after 12 weeks intervention, compared by ANCOVA.
| Baseline | After Intervention | Change | ANCOVA p Value |
|
|---|---|---|---|---|
| Fecal calprotectin (µg/g) | ||||
|
48 [26:100] | 50 [19:135] | −1.0 [−20:10] | 0.24 |
|
61 [25:139] | 47 [19:95] | −12 [−95:1] | |
| Fecal IAP activity/Protein (U/g) | ||||
|
92 [49:609] | 83 [39:605] | −3.9 [−25:51] | 0.98 |
|
55 [38:133] | 53 [22:106] | −0.24 [−22:38] | |
| Fecal butyrate (µg/mg) | ||||
|
6.4 [2.8:12] | 6.4 [3.7:12] | 0.7 [−1.5:4.2] | 0.34 |
|
4.3 [2.1:7.6] | 4.4 [2.5:8.8] | 0.052 [−3.5:2.0] | |
| Fecal acetate (µg/mg) | ||||
|
21 ± 8.8 | 21 ± 12 | 0.080 ± 12 | 0.33 |
|
18 ± 9.6 | 17 ± 8.7 | −1.3 ± 10 | |
| Fecal propionate (µg/mg) | ||||
|
8.0 [4.2:12] | 6.8 [3.5:11] | −0.15 [−3.3:1.1] | 0.93 |
|
5.4 [3.6:7.9] | 5.5 [3.7:8.7] | −0.94 [−2.3:1.8] | |
| Fecal valerate (µg/mg) | ||||
|
2.0 ± 1.0 | 2.1 ± 1.6 | 0.13 ± 1.8 | 0.67 |
|
1.7 ± 1.0 | 1.8 ± 1.2 | 0.15 ± 1.3 | |
| Fecal immunoglobulin G/Protein (ng/g) | ||||
|
28 [16:110] | 40 [16:116] | −1.8 [−0.067:0.034] | 0.10 |
|
27 [14:67] | 17 [9.9:36] | −1.9 [−15:18] | |
| Fecal immunoglobulin A/Protein (µg/mg) | ||||
|
1.6 [0.50:7.14] | 2.3 [0.72:4.9] | 0.30 [−1.2:1.31] | 0.69 |
|
2.4 [1.1:7.8] | 1.7 [0.73:9.9] | 0.077 [−2.0:3.7] | |
| Fecal immunoglobulin M/Protein (ng/mg) | ||||
|
0.61 [0.32:2.0] | 1.1 [0.36:2.7] | 0.38 [−0.0011:1.5] | 0.20 |
|
1.0 [0.36:1.9] | 0.72 [0.46:1.8] | 0.18 [−1.4:0.70] | |
| Urinary albumin creatinine ratio (mg/g) | ||||
|
39 [18:121] | 37 [27:82] | 1.5 [−9.5:21] | 0.69 |
|
49 [14:121] | 37 [18:208] | −1.0 [−19:6.0] | |
| Estimated glomerular filtration rate (mL/min/1.73 m2) | ||||
|
86 ± 26 | 84 ± 25 | −2.0 ± 8.3 | 0.30 |
|
82 ± 22 | 83 ± 23 | 0.39 ± 7.1 | |
| Serum high sensitivity CRP (mg/L) | ||||
|
1.7 [0.91:3.1] | 1.7 [0.95:3.0] | −0.06 [−0.65:0.55] | 0.13 |
|
1.9 [1.0:4.0] | 2.0 [1.5:6.0] | −0.18 [−0.84:0.38] | |
| Serum lipopolysaccharide (EU/mL) | ||||
|
0.66 ± 0.18 | 0.66 ± 0.20 | −0.0025 ± 0.20 | 0.093 |
|
0.73 ± 0.11 | 0.80 ± 0.26 | 0.076 ± 0.26 | |
| HbA1c (% (mmol/mol)) | ||||
|
8.0 ± 0.8 | 8.0 ± 0.9 | 0.1 ± 0.4 | 0.32 |
|
8.1 ± 1.1 | 8.3 ± 1.3 | 0.2 ± 0.6 | |
Values are mean ± standard deviation, median [Q1:Q3]. Parameters with a non-normal distribution were log transformed before analysis. p value for the group-wise comparison of participants treated with sodium butyrate or placebo was calculated using baseline-corrected linear regression.
Figure 2.
Effects of 12 weeks of treatment with sodium butyrate or placebo on fecal calprotectin. Each colored line represents one participant.
3.3. Changes in Kidney Parameters, HbA1c and Metabolites
The UACR did not change with butyrate supplementation compared to placebo (Table 2). From a median baseline UACR level of 39 [18:121] mg/g, the median change was 1.5 [−9.5:21] mg/g after butyrate and −1.0 [−19:6.0] mg/g after placebo from a baseline of 49 [14:121] mg/g (p = 0.69 for difference between groups). For eGFR, the mean (SD) change was −2.0 (8.3) mL/min/1.73 m2 from a baseline of 86 (26) mL/min/1.73 m2 for butyrate and 0.39 (7.1) mL/min/1.73 m2 from a baseline of 82 (22) mL/min/1.73 m2 for placebo (p = 0.30, for difference between groups). Analyses restricted to the 30 participants with albuminuria at the baseline visit were consistent with the main analysis and did not show any significant effect on the UACR (p = 0.96, for difference between groups). There was no significant change in HbA1c, which was mean 0.1 (0.4)% (mmol/mol) in the butyrate group and 0.2 (0.6)% (mmol/mol) in the placebo group (p = 0.32 for difference between groups). Out of 38 metabolites, 8 were excluded due to non-detectable values for more than 70% of the samples. None of the 30 metabolites were changed after butyrate supplementation compared to placebo (Supplementary Table S2).
3.4. Changes in Gastrointestinal Symptoms
The proportions of participants who experienced changes in gastrointestinal symptoms in the two treatment groups are presented in Supplementary Table S3. No convincing effect of butyrate supplementation in any direction could be demonstrated: improvement, as well as worsening and onset of gastrointestinal symptoms during the study period, was reported in both the butyrate and in the placebo group. The numbers of participants who experienced changes in gastrointestinal symptoms were too small for statistical testing.
3.5. Supplementary Analysis
The results were consistent after the two participants that received antibiotics during the treatment period were excluded from the analyses of the inflammatory parameters; and after the three participants with change in RASi dosage during the study were excluded from the analyses of the UACR. The results of the analysis only including the 43 adherent participants (excluding 8 non-adherent participants and two dropouts) were confirmatory. We did not find a difference between the effect of butyrate and placebo on fecal calprotectin when including all four measurements in a mixed model (p = 0.31).
3.6. Safety
Eighteen adverse events were registered in the butyrate group, and 16 in the placebo group. Eight SAEs were reported. including: one death before randomization; one debut of multiple sclerosis; one ischemic stroke; one worsening of angina leading to elective percutaneous transluminal coronary angioplasty; one lower limb arterial thrombosis in the group receiving butyrate; one thrombosis and non-ST segment elevation myocardial infarction following a planned femoral bypass; and one hospitalization due to an infected diabetic leg ulcer in the placebo group.
4. Conclusions
In this study including persons with type 1 diabetes, intestinal inflammation and albuminuria, we could not demonstrate any effect on inflammatory markers, SCFAs, kidney parameters, HbA1c or selected metabolites after 12 weeks of butyrate supplementation. Furthermore, no convincing effect on gastrointestinal symptoms was found. Adherence was assessed by returned capsule count, and 43 of the 50 persons (86%) who completed the study met our pre-specified adherence criterion of a maximum 20% returned capsules, even though the daily dose counted as many as 12 capsules split in two doses. To our knowledge, this is the first study to evaluate the effect of butyrate supplementation on gut inflammation and diabetic nephropathy in type 1 diabetes.
Butyrate supplementation has beneficial effects on IBD, which shares genetic and immunological aspects with type 1 diabetes [26]. Both multifactorial autoimmune diseases are associated with increased risk of cardiovascular disease and premature mortality [27,28], and the prevalence of IBD is approximately 6-fold higher in adults with type 1 diabetes than in non-diabetic adults [29]. Interestingly, our finding of increased calprotectin level in 53% of the screened persons indicates a subclinical inflammatory state in this type 1 diabetes population, even when the possible overestimation by the calprotectin home test and the risk of selection bias is considered. Butyrate supplementation (of 4 g) as an add-on therapy showed significant reduction in fecal calprotectin after six months, compared to standard treatment in a population with ulcerative colitis and a median calprotectin level of 226 [167:309] μg/g [23]. Compared to our findings, one might reflect that butyrate is only beneficial in severe intestinal inflammation.
As butyrate is an energy source for colonocytes, the excretion rate is only 5–10% and is furthermore affected by transit time and diet, which makes the inter- and intraindividual variability substantial [30,31]. The implication of fecal SCFA measurements is disputed as the correlation with colonic availability is unknown. We included SCFAs as endpoints to examine whether changes in SCFA were correlated to a potential change in calprotectin level. We did not find a change in fecal butyrate in the group treated with butyrate. In a recent clinical trial by de Groot et al., butyrate supplementation reduced fecal butyrate levels in subjects with type 1 diabetes. The authors speculate that butyrate supplementation may have a counterregulatory effect on the microbial butyrate production [32]. Several drugs affect the gut microbiota and inflammatory markers and are therefore possible confounders. Non-steroidal inflammatory drugs have been reported to increase fecal calprotectin, but also proton pump inhibitors are associated with increased fecal calprotectin [33]. These over-the-counter-drugs are often used intermittently, and treatment onset or termination may bias the results in both directions. Additionally, metformin and statins might increase the number of butyrate-producing bacteria [34,35], and there was a higher frequency of treatment with these drugs in the group treated with butyrate. This imbalance would potentially bias the results towards an overestimation of the true effect of butyrate.
We were not able to demonstrate that butyrate supplementation changed the IAP activity (which preserve the gut blood-barrier) nor the concentration of circulating LPS. Neither did butyrate supplementation promote a beneficial shift in gut-related metabolites. Besides the proinflammatory effect of endotoxins passing a defect gut barrier, the inflammatory cascade can also be triggered by microbiota-derived metabolites [36]. An individual’s metabolic profile is associated with development of diabetic complications including chronic kidney disease [37], but metabolomics has not previously been evaluated in relation to butyrate supplementation in humans.
This study was not primarily designed to evaluate changes in gastrointestinal symptoms, as the presence of symptoms was not an inclusion criterion. However, one explanation for our findings could be that the gastrointestinal symptoms in our population were due to neuropathy. We are not aware of previous investigations of the effect of butyrate on gastrointestinal symptoms in diabetes. Neither has the effect of butyrate on kidney parameters been tested, though an association between intestinal dysbiosis and diabetic kidney disease has been described in humans [38].
This randomized placebo-controlled trial could not demonstrate that butyrate supplementation is the loophole to intestinal health in persons with intestinal inflammation with the applied dose. The link between dysbiosis and intestinal inflammation is evident [39], but the causal relationships and methods to interfere with the human intestinal environment are not understood. A fiber rich diet, shifting the microbiome towards butyrate-producing bacteria may be ideal, but profound lifestyle changes are hard to realize [40]. Facilitating butyrate production by increasing availability of fibers and butyrate-producing bacteria could potentially mimic a healthier diet. Supplementation with combinations of pre- and probiotics, synbiotics, has already shown beneficial effects in different populations [41,42]. The next step in the anti-inflammatory pathway, IAP, has also been investigated as a treatment target. Exogenous IAP administration has shown beneficial effects on calprotectin level in ulcerative colitis, kidney function in acute kidney injury and inflammatory markers in both populations, but the enzyme is challenging to administer [43,44,45].The question is if a shortcut to improve intestinal environment in susceptible persons is within reach or if the only way forward is to feed the beneficial strains.
The design of this dual-center, double-blinded randomized placebo-controlled trial is a major strength. Intervention targeting the colon is pharmacologically challenging, as protection against gastric pH and enzymic degradation is crucial for orally administered products. In this study, capsules with delayed release were used to ensure the delivery of butyrate to the terminal ileum and colon. The compliance of the study participants may have been an issue because of the capsule quantity, counting 12 capsules daily. The daily dose was split in two, to ease ingestion. This regimen required compliant participants, and the mean baseline HbA1c level of 8.0% (mmol/mol) suggests that the participants did not follow a strict medical regimen in general. On the other hand, the information about an inflammatory condition that was given to the participants during inclusion might explain why 86% of the participants who completed the study were adherent based on returned capsule count with a prespecified threshold of 80%. We aimed to study the effect of butyrate in a population with intestinal inflammation. To facilitate the screening proces, we used a calprotectin home test for screening. Of the screened persons, 53% had increased fecal calprotectin measured by this test, and were randomized, but only 55% had increased fecal calprotectin measured with ELISA test at randomization. This discrepancy can be explained by the time lag (1−3 weeks) between screening and randomization, intra-individual variability and/or low specificity of the home test. We accounted for the unintended inclusion of persons with normal calprotectin level by performing supplementary analyses restricted to participants with elevated fecal calprotectin level at randomization, though this sample size reduction may have resulted in insufficient power. Only one person was excluded, due to two invalid home calprotectin tests, limiting the influence of pre-analytical errors. Guided by previous human studies, we used a dose of butyrate of 3.6 g/day, but this might be an ineffective dose, as beneficial effects have been demonstrated with 10-times higher butyrate doses in studies with domestic animals [46].
In conclusion, in this randomized placebo-controlled trial of persons with type 1 diabetes, a butyrate supplement of 3.6 g/day was without adverse events, but also unable to change fecal and circulating inflammatory markers, SCFAs, kidney parameters, Hba1c, selected metabolites and gastrointestinal symptoms within 12 weeks. Even though butyrate plays a central role in the intestinal dysbiosis that is evident in both type 1 diabetes and diabetic kidney disease, a supplement of this bacterial product is not a shortcut to rebalance the gut microbiome in type 1 diabetes.
Acknowledgments
We thank the participants and laboratory technicians (Dorthe Riis, Tina Ragnholm Juhl, Jessie Armand Hermann, Tina Nielsen and Maja Lis Dybdahl Halkjær) from Steno Diabetes Center Copenhagen, Denmark; the participants, study nurses (Anna Sandelin, Mira Korolainen and Jaana Tuomikangas) and laboratory technicians (Hanna Olanne and Heli Krigsman) from Folkhälsan Research Center Helsinki, Finland; and Sirpa Rannikko from University of Oulu, Finland for technical assistance.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11133573/s1: Table S1: Metabolite panel; Table S2: Change in serum metabolites after 12 weeks intervention compared by ANCOVA; Table S3: Gastrointestinal symptoms before and after 12 weeks intervention.
Author Contributions
Conceptualization: N.H.T., M.F.-M., M.L. and P.R.; Methodology: N.H.T., M.F.-M., M.L. and P.R.; Formal analysis: N.H.T.; Investigation: N.H.T., H.S., E.B.S., A.D.Z. and I.M.M. Data Curation: N.H.T.; Writing—original draft preparation: N.H.T.; Writing—review and editing: All authors have read and edited; Visualization: N.H.T.; Supervision: T.W.H., M.F.-M., C.L.-Q., S.H., C.F., P.-H.G., M.L. and P.R.; Project administration: N.H.T. and M.L.; Funding acquisition: M.F.-M., P.-H.G., M.L. and P.R. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was approved by the Regional Ethics Committee in Denmark (26 March 2019, ID: H-18062027) and the Ethics Committee of the Helsinki and Uusimaa Hospital District in Finland and conducted in accordance with the Declaration of Helsinki.
Informed Consent Statement
All participants gave written informed consent.
Data Availability Statement
Data is available upon reasonable request to the corresponding author.
Conflicts of Interest
M.F.-M. reports having received research grants from Novo Nordisk and speaking fees from Boehringer Ingelheim, Novartis, Baxter and Sanofi. T.W.H. has shares in Novo Nordisk A/S. P.H.G. is an advisory board member of AbbVie, Astellas, Astra Zeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Janssen, Medscape, MSD, Mundipharma, Nestlé, Novartis, Novo Nordisk and Sanofi, and has received lecture honoraria from Astellas, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Elo Water, Genzyme, Medscape, MSD, Mundipharma, Novartis, Novo Nordisk, Sanofi and SCIARC. P.R. has received research support and personal fees from AstraZeneca and Novo Nordisk, and personal fees from Astellas, Boehringer Ingelheim, Eli Lilly, Gilead, Mundipharma, Sanofi, and Vifor; all fees are given to the Steno Diabetes Center Copenhagen. N.H.T., H.S., E.B.S., A.D.Z., I.M.M., C.L.Q., S.H., C.F. and M.L. have no conflicts of interest.
Funding Statement
The Novo Nordisk Foundation: PROTON: PeRsOnalizing Treatment of diabetic Nephropathy (grant number NNFOC0013659).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Winther S.A., Henriksen P., Vogt J.K., Hansen T.H., Ahonen L., Suvitaival T., Zobel E.H., Frimodt-Møller M., Hansen T.W., Hansen T., et al. Gut microbiota profile and selected plasma metabolites in type 1 diabetes without and with stratification by albuminuria. Diabetologia. 2020;63:2713–2724. doi: 10.1007/s00125-020-05260-y. [DOI] [PubMed] [Google Scholar]
- 2.Lassenius M.I., Fogarty C.L., Blaut M., Haimila K., Riittinen L., Paju A., Kirveskari J., Jaervelae J., Ahola A.J., Gordin D., et al. Intestinal alkaline phosphatase at the crossroad of intestinal health and disease—A putative role in type 1 diabetes. J. Intern. Med. 2017;281:586–600. doi: 10.1111/joim.12607. [DOI] [PubMed] [Google Scholar]
- 3.Mishra S., Wang S., Nagpal R., Miller B., Singh R., Taraphder S., Yadav H. Probiotics and Prebiotics for the Amelioration of Type 1 Diabetes: Present and Future Perspectives. Microorganisms. 2019;7:67. doi: 10.3390/microorganisms7030067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou H., Sun L., Zhang S., Zhao X., Gang X., Wang G. Evaluating the Causal Role of Gut Microbiota in Type 1 Diabetes and Its Possible Pathogenic Mechanisms. Front. Endocrinol. 2020;11:125. doi: 10.3389/fendo.2020.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harbison J.E., Roth-Schulze A.J., Giles L., Tran C.D., Ngui K.M., Penno M., Thomson R.L., Wentworth J.M., Colman P.G., Craig M.E., et al. Gut microbiome dysbiosis and increased intestinal permeability in children with islet autoimmunity and type 1 diabetes: A prospective cohort study. Pediatr. Diabetes. 2019;20:574–583. doi: 10.1111/pedi.12865. [DOI] [PubMed] [Google Scholar]
- 6.Vinolo M.A., Rodrigues H.G., Nachbar R.T., Curi R. Regulation of inflammation by short chain fatty acids. Nutrients. 2011;3:858–876. doi: 10.3390/nu3100858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vinolo M.A.R., Rodrigues H.G., Hatanaka E., Sato F.T., Sampaio S.C., Curi R. Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils. J. Nutr. Biochem. 2011;22:849–855. doi: 10.1016/j.jnutbio.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Tilg H., Moschen A.R. Microbiota and diabetes: An evolving relationship. Gut. 2014;63:1513–1521. doi: 10.1136/gutjnl-2014-306928. [DOI] [PubMed] [Google Scholar]
- 9.Liu H., Wang J., He T., Becker S., Zhang G., Li D., Ma X. Butyrate: A Double-Edged Sword for Health? Adv Nutr. 2018;9:21–29. doi: 10.1093/advances/nmx009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melo A.D.B., Silveira H., Bortoluzzi C., Lara L., Garbossa C., Preis G., Costa L., Rostagno M. Intestinal alkaline phosphatase and sodium butyrate may be beneficial in attenuating LPS-induced intestinal inflammation. Genet. Mol. Res. 2016;15:gmr15048875. doi: 10.4238/gmr15048875. [DOI] [PubMed] [Google Scholar]
- 11.Lallès J.-P. Intestinal alkaline phosphatase: Multiple biological roles in maintenance of intestinal homeostasis and modulation by diet. Nutr. Rev. 2010;68:323–332. doi: 10.1111/j.1753-4887.2010.00292.x. [DOI] [PubMed] [Google Scholar]
- 12.Lallès J.-P. Recent advances in intestinal alkaline phosphatase, inflammation, and nutrition. Nutr. Rev. 2019;77:710–724. doi: 10.1093/nutrit/nuz015. [DOI] [PubMed] [Google Scholar]
- 13.Ghosh S.S., Wang J., Yannie P.J., Ghosh S. Intestinal Barrier Dysfunction, LPS Translocation, and Disease Development. J. Endocr. Soc. 2020;4:bvz039. doi: 10.1210/jendso/bvz039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gutzeit C., Magri G., Cerutti A. Intestinal IgA production and its role in host-microbe interaction. Immunol. Rev. 2014;260:76–85. doi: 10.1111/imr.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bilski J., Mazur-Bialy A., Wojcik D., Zahradnik-Bilska J., Brzozowski B., Magierowski M., Mach T., Magierowska K., Brzozowski T. The Role of Intestinal Alkaline Phosphatase in Inflammatory Disorders of Gastrointestinal Tract. Mediat. Inflamm. 2017;2017:9074601. doi: 10.1155/2017/9074601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nighot M., Rawat M., Al-Sadi R., Castillo E., Nighot P., Ma T.Y. Lipopolysaccharide-Induced Increase in Intestinal Permeability Is Mediated by TAK-1 Activation of IKK and MLCK/MYLK Gene. Am. J. Pathol. 2019;189:797–812. doi: 10.1016/j.ajpath.2018.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramezani A., Raj D.S. The gut microbiome, kidney disease, and targeted interventions. J. Am. Soc. Nephrol. 2014;25:657–670. doi: 10.1681/ASN.2013080905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lehto M., Groop P.-H. The Gut-Kidney Axis: Putative Interconnections between Gastrointestinal and Renal Disorders. Front. Endocrinol. 2018;9:553. doi: 10.3389/fendo.2018.00553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalez A., Krieg R., Massey H.D., Carl D., Ghosh S., Gehr T.W.B., Ghosh S.S. Sodium butyrate ameliorates insulin resistance and renal failure in CKD rats by modulating intestinal permeability and mucin expression. Nephrol. Dial. Transplant. 2018;34:783–794. doi: 10.1093/ndt/gfy238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andrade-Oliveira V., Amano M., Correa-Costa M., Castoldi A., Felizardo R., De Almeida D.C., Bassi J., Vieira P., Hiyane M.I., Rodas A.C., et al. Gut Bacteria Products Prevent AKI Induced by Ischemia-Reperfusion. J. Am. Soc. Nephrol. 2015;26:1877–1888. doi: 10.1681/ASN.2014030288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang H., Zhang Z., Peng R., Zhang L., Liu H., Wang X., Tian Y., Sun Y. RNA-Seq analysis reveals critical transcriptome changes caused by sodium butyrate in DN mouse models. Biosci. Rep. 2021;41:bsr20203005. doi: 10.1042/BSR20203005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borycka-Kiciak K., Banasiewicz T., Rydzewska G. Butyric acid—A well-known molecule revisited. Gastroenterol. Rev. 2017;2:83–89. doi: 10.5114/pg.2017.68342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vernero M., De Blasio F., Ribaldone D., Bugianesi E., Pellicano R., Saracco G., Astegiano M., Caviglia G. The Usefulness of Microencapsulated Sodium Butyrate Add-on Therapy in Maintaining Remission in Patients with Ulcerative Colitis: A Prospective Observational Study. J. Clin. Med. 2020;9:3941. doi: 10.3390/jcm9123941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roshanravan N., Mahdavi R., Alizadeh E., Jafarabadi M.A., Hedayati M., Ghavami A., Alipour S., Alamdari N.M., Barati M., Ostadrahimi A. Effect of Butyrate and Inulin Supplementation on Glycemic Status, Lipid Profile and Glucagon-Like Peptide 1 Level in Patients with Type 2 Diabetes: A Randomized Double-Blind, Placebo-Controlled Trial. Horm. Metab. Res. 2017;49:886–891. doi: 10.1055/s-0043-119089. [DOI] [PubMed] [Google Scholar]
- 25.Ahonen L., Jäntti S., Suvitaival T., Theilade S., Risz C., Kostiainen R., Rossing P., Orešič M., Hyötyläinen T. Targeted Clinical Metabolite Profiling Platform for the Stratification of Diabetic Patients. Metabolites. 2019;9:184. doi: 10.3390/metabo9090184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gjymishka A., Coman R.M., Brusko T.M., Glover S.C. Influence of host immunoregulatory genes, ER stress and gut microbiota on the shared pathogenesis of inflammatory bowel disease and Type 1 diabetes. Immunotherapy. 2013;5:1357–1366. doi: 10.2217/imt.13.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lind M., Svensson A.-M., Kosiborod M., Gudbjörnsdottir S., Pivodic A., Wedel H., Dahlqvist S., Clements M., Rosengren A. glycemic control and excess mortality in type 1 diabetes. N. Engl. J. Med. 2014;371:1972–1982. doi: 10.1056/NEJMoa1408214. [DOI] [PubMed] [Google Scholar]
- 28.Gandhi S., Narulak N., Marshall J.K., Farkouh M. Are patients with inflammatory bowel disease at increased risk of coronary artery disease? Am. J. Med. 2012;125:956–962. doi: 10.1016/j.amjmed.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 29.Penny H.A., Leeds J.S., Kurien M., Averginos A., Hopper A.D., Hadjivassiliou M., Tesfaye S., Sanders D.S. The relationship between inflammatory bowel disease and type 1 diabetes mellitus: A study of relative prevalence in comparison with population controls. J. Gastrointest. Liver Dis. 2015;24:125–126. [PubMed] [Google Scholar]
- 30.Wong J.M.W., de Souza R., Kendall C.W.C., Emam A., Jenkins D.J.A. Colonic Health: Fermentation and Short Chain Fatty Acids. J. Clin. Gastroenterol. 2006;40:235–243. doi: 10.1097/00004836-200603000-00015. [DOI] [PubMed] [Google Scholar]
- 31.McOrist A.L., Miller R.B., Bird A.R., Keogh J., Noakes M., Topping D.L., Conlon M.A. Fecal butyrate levels vary widely among individuals but are usually increased by a diet high in resistant starch. J. Nutr. 2011;141:883–889. doi: 10.3945/jn.110.128504. [DOI] [PubMed] [Google Scholar]
- 32.De Groot P.F., Nikolic T., Imangaliyev S., Bekkering S., Duinkerken G., Keij F.M., Herrema H., Winkelmeijer M., Kroon J., Levin E., et al. Oral butyrate does not affect innate immunity and islet autoimmunity in individuals with longstanding type 1 diabetes: A randomised controlled trial. Diabetologia. 2020;63:597–610. doi: 10.1007/s00125-019-05073-8. [DOI] [PubMed] [Google Scholar]
- 33.Poullis A., Foster R., Mendall M.A. Proton pump inhibitors are associated with elevation of faecal calprotectin and may affect specificity. Eur. J. Gastroenterol. Hepatol. 2003;15:573–574. doi: 10.1097/00042737-200305000-00021. [DOI] [PubMed] [Google Scholar]
- 34.Wu H., Esteve E., Tremaroli V., Khan M.T., Caesar R., Mannerås-Holm L., Ståhlman M., Olsson L.M., Serino M., Planas-Fèlix M., et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat. Med. 2017;23:850–858. doi: 10.1038/nm.4345. [DOI] [PubMed] [Google Scholar]
- 35.Vieira-Silva S., Falony G., Belda E., Nielsen T., Aron-Wisnewsky J., Chakaroun R., Forslund S.K., Assmann K., Valles-Colomer M., Nguyen T.T.D., et al. Statin therapy is associated with lower prevalence of gut microbiota dysbiosis. Nature. 2020;581:310–315. doi: 10.1038/s41586-020-2269-x. [DOI] [PubMed] [Google Scholar]
- 36.Mosterd C., Kanbay M., Born B.V.D., van Raalte D., Rampanelli E. Intestinal microbiota and diabetic kidney diseases: The Role of microbiota and derived metabolites inmodulation of renal inflammation and disease progression. Best Pract. Res. Clin. Endocrinol. Metab. 2021;35:101484. doi: 10.1016/j.beem.2021.101484. [DOI] [PubMed] [Google Scholar]
- 37.Izundegui D.G., Nayor M. Metabolomics of Type 1 and Type 2 Diabetes: Insights into Risk Prediction and Mechanisms. Curr. Diabetes Rep. 2022;22:65–76. doi: 10.1007/s11892-022-01449-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zaky A., Glastras S.J., Wong M.Y.W., Pollock C.A., Saad S. The Role of the Gut Microbiome in Diabetes and Obesity-Related Kidney Disease. Int. J. Mol. Sci. 2021;22:9641. doi: 10.3390/ijms22179641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dai Z.F., Ma X.Y., Yang R.L., Wang H.C., Xu D.D., Yang J.N., Guo X.B., Meng S.S., Xu R., Li Y.X., et al. Intestinal flora alterations in patients with ulcerative colitis and their association with inflammation. Exp. Ther. Med. 2021;22:1322. doi: 10.3892/etm.2021.10757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cronin P., Joyce S.A., O’Toole P.W., O’Connor E.M. Dietary Fibre Modulates the Gut Microbiota. Nutrients. 2021;13:1655. doi: 10.3390/nu13051655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rossi M., Johnson D.W., Morrison M., Pascoe E.M., Coombes J.S., Forbes J.M., Szeto C.-C., McWhinney B.C., Ungerer J.P., Campbell K.L. Synbiotics Easing Renal Failure by Improving Gut Microbiology (SYNERGY): A Randomized Trial. Clin. J. Am. Soc. Nephrol. 2016;11:223–231. doi: 10.2215/CJN.05240515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng H.J., Guo J., Jia Q., Huang Y.S., Huang W.-J., Zhang W., Zhang F., Liu W.J., Wang Y. The effect of probiotic and synbiotic supplementation on biomarkers of inflammation and oxidative stress in diabetic patients: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2019;142:303–313. doi: 10.1016/j.phrs.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 43.Lukas M., Drastich P., Konecny M., Gionchetti P., Urban O., Cantoni F., Bortlik M., Duricova D., Bulitta M. Exogenous alkaline phosphatase for the treatment of patients with moderate to severe ulcerative colitis. Inflamm. Bowel Dis. 2010;16:1180–1186. doi: 10.1002/ibd.21161. [DOI] [PubMed] [Google Scholar]
- 44.Estaki M., DeCoffe D., Gibson D.L. Interplay between intestinal alkaline phosphatase, diet, gut microbes and immunity. World J. Gastroenterol. 2014;20:15650–15656. doi: 10.3748/wjg.v20.i42.15650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pickkers P., Heemskerk S., Schouten J., Laterre P.-F., Vincent J.-L., Beishuizen A., Jorens P.G., Spapen H., Bulitta M., Peters W.H., et al. Alkaline phosphatase for treatment of sepsis-induced acute kidney injury: A prospective randomized double-blind placebo-controlled trial. Crit. Care. 2012;16:R14. doi: 10.1186/cc11159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Banasiewicz T., Domagalska D., Borycka-Kiciak K., Rydzewska G. Determination of butyric acid dosage based on clinical and experimental studies—A literature review. Gastroenterol. Rev. 2020;15:119–125. doi: 10.5114/pg.2020.95556. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is available upon reasonable request to the corresponding author.