Dear Editor,
Long-term outcomes among coronavirus disease 2019 (COVID-19) survivors have been a cause for concern [1–3]. Similarly, patients surviving critical illness from other conditions have shown anxiety, depression and altered quality of life, contributing to post-intensive care syndrome (PICS). The specific contribution of COVID-19 beyond the non-specific contribution of critical illness, however, remains unknown. In this study, we matched and compared critically ill survivors admitted to the intensive care unit (ICU) for COVID-19 to critically ill patients admitted for pneumonia or acute respiratory distress syndrome unrelated to COVID-19. We explored hospital Anxiety and Depression Scale (HADS) and the Short Form (36) Health Survey (SF-36) scores 1 year after hospitalization.
We used two cohorts of critically ill patients: the French-COVID cohort (COVID-19 cohort, clinical trial NCT04262921) [4] and the FROG-ICU cohort (control cohort, clinical trial NCT01367093) [5]. We selected patients who survived 12 months post-hospitalization and subsequently had HADS and SF-36 scores assessed. 40 patients from each cohort were matched based on age, sex, comorbidities (diabetes, hypertension, chronic heart failure, previous stroke, obesity, chronic obstructive pulmonary disease, liver disease, smoking, asthma, and cancer), and treatments (renal replacement therapy, mechanical ventilation, and use of vasopressors/inotropes; Supplemental Table 1).
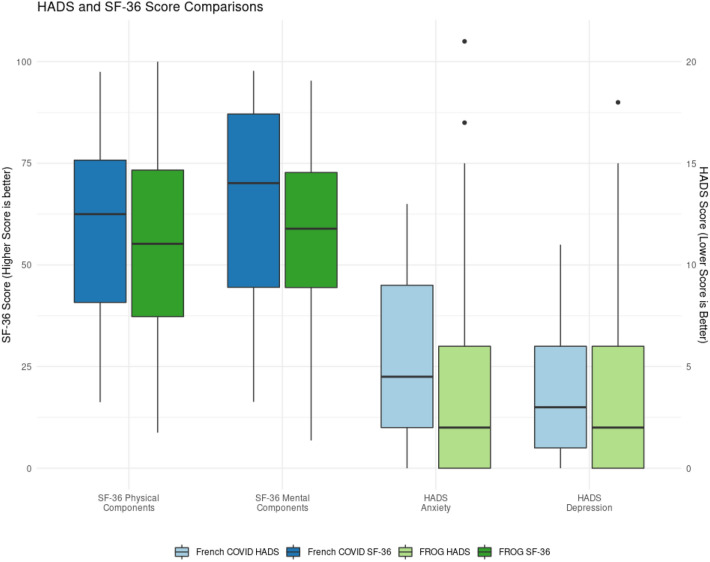
At 1 year, the COVID-19 vs control group median scores for HADS depression were 3 [1, 6] vs 2 [0, 6] (p = 0.807); for HADS anxiety were 4.5 [2, 9] vs 2 [0, 6] (p = 0.213); for the SF-36 physical component were 62.5 [40.8, 75.8] vs 55.2 [37.3, 73.3] (p = 0.264) and for the SF-36 mental component were 70.1 [44.5, 87.1] vs 58.9 [44.4, 72.8] (p = 0.08) (Fig. 1). SF-36 domains significantly higher in the COVID-19 vs controls were the emotional well-being (80 [65, 88] vs 64 [52, 72], p = 0.004) and the social functioning (75 [62.5, 100] vs 62.5 [50, 87.5], p = 0.047). Other domains were not significantly different between groups.
Fig. 1.
Domains of the Hospital Anxiety and Depression Scale (HADS)and Short Form 36 in 12 months (SF-36a), in the matched cohorts. The SF-36 Physical Components includes the physical function domain, bodily pain domain, general health domain, physical function domain. The SF-36 mental components includes the mental health domain, energy and fatigue domain, emotional wellbeing, and social function
This study has limits. The control cohort enrolled between 2011 and 2013, so changes in clinical practice over time may have occurred. It was carried out primarily in France and had a limited sample-size with substantial loss to follow up. In addition, the outcomes measured in this study are not exhaustive and other functional outcomes were not collected. Finally, patients were recruited primarily in the pre-vaccination pandemic phase and were infected with the alpha variant, so results may not be generalizable to other scenarios.
Long-term outcomes of patients with COVID-19 and critically ill patients have been concerning [1–3], however the interaction between COVID-19 and critical illness 1 year post-COVID-19 diagnosis has not yet been explored. In this case–control study, we identified no statistically significant difference in HADS and the physical and mental components of the SF-36 scores between groups. Of note, depression and anxiety scores were low and within normal range, although emotional well-being and social functioning domains were higher in COVID-19 survivors, suggesting better outcomes. This study provides reassuring preliminary data on the specific impact of COVID-19 on outcomes after critical illness. Future work should confirm these findings in larger cohorts and identify potential risk factors and drivers of poor long-term functional outcomes after critical illness to better understand strategies that could mitigate these outcomes.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank all participating centers, clinicians, nurses, research assistants of the French-COVID and the FROG-ICU studies. The members of the French-COVID and FROG-ICU cohort studies and investigators groups are here listed:
French-COVID study group: Laurent ABEL laurent.abel@inserm.fr; Inserm UMR 1163, Paris, France; Amal ABROUS amal.abrous@inserm.fr; Inserm sponsor, Paris, France; Claire ANDREJAK andrejak.claire@chu-amiens.fr; CHU Amiens, France; François ANGOULVANT francois.angoulvant@aphp.fr; Hôpital Necker, Paris, France; Delphine BACHELET delphine.bachelet@aphp.fr; Hôpital Bichat, Paris, France; Marie BARTOLI marie.bartoli@anrs.fr; ANRS, Paris, France; Sylvie BEHILILL sylvie.behillil@pasteur.fr; Pasteur Institute, Paris, France; Marine BELUZE marine.beluze@aphp.fr; F-CRIN Partners Platform, Paris, France; Krishna BHAVSAR krishna.bhavsar@aphp.fr; Hôpital Bichat, Paris, France; Lila BOUADMA lila.bouadma@aphp.fr; Hôpital Bichat, Paris, France; Minerva CERVANTES-GONZALEZ minerva.cervantes@inserm.fr; Hôpital Bichat, Paris, France; Anissa CHAIR anissa.chair@aphp.fr; Hôpital Bichat, Paris, France; Charlotte CHARPENTIER charlotte.charpentier@aphp.fr; Hôpital Bichat, Paris, France; Léo CHENARD leo.chenard@aphp.fr; Hôpital Bichat, Paris, France; Catherine CHIROUZE catherine.chirouze@univ-fcomte.fr; CHRU Jean Minjoz, Besançon, France; Sandrine COUFFIN-CADIERGUES sandrine.couffin-cadiergues@inserm.fr; Inserm sponsor, Paris, France; Camille COUFFIGNAL camille.couffignal@aphp.fr; Hôpital Bichat, Paris, France; Marie-Pierre DEBRAY marie-pierre.debray@aphp.fr; Hôpital Bichat, Paris, France; Dominique DEPLANQUE Dominique.DEPLANQUE@chru-lille.fr; Hôpital Calmette, Lille, France; Diane DESCAMPS diane.descamps@aphp.fr; Hôpital Bichat, Paris, France; Alpha DIALLO alpha.diallo@inserm.fr; ANRS, Paris, France; Fernanda DIAS DA SILVA fernanda.dias-da-silva@inserm.fr; Inserm sponsor, Paris, France; Céline DORIVAL celine.dorival@iplesp.upmc.fr; Inserm UMR 1136, Paris, France; Xavier DUVAL xavier.duval@aphp.fr; Hôpital Bichat, Paris, France; Philippine ELOY philippine.eloy@aphp.fr; Hôpital Bichat, Paris, France; Vincent ENOUF vincent.enouf@pasteur.fr; Pasteur Institute, Paris, France; Hélène ESPEROU helene.esperou@inserm.fr; Inserm sponsor, Paris, France; Marina ESPOSITO-FARESE marina.esposito-farese@aphp.fr; Hôpital Bichat, Paris, France; Manuel ETIENNE Manuel.Etienne@chu-rouen.fr; CHU Rouen, France; Aline-Marie FLORENCE aline-marie.florence@aphp.fr; Hôpital Bichat, Paris, France; Alexandre GAYMARD alexandre.gaymard@chu-lyon.fr; Inserm UMR 1111, Lyon, France; Jade GHOSN jade.ghosn@aphp.fr; Hôpital Bichat, Paris, France; Tristan GIGANTE T.GIGANTE@chru-nancy.fr; F-CRIN INI-CRCT, Nancy, France; Morgane GILG M.GILG@chru-nancy.fr F-CRIN INI-CRCT, Nancy, France; François GOEHRINGER f.goehringer@chru-nancy.fr; CHU Nancy, France; Jérémie GUEDJ jeremie.guedj@inserm.fr; Inserm UMR 1137, Paris, France; Ikram HOUAS ikram.houas@inserm.fr; Inserm sponsor, Paris, France; Isabelle HOFFMANN isabelle.hoffmann@aphp.fr; Hôpital Bichat, Paris, France; Jean-Sébastien HULOT jean-sebastien.hulot@aphp.fr; Hôpital Européen Georges Pompidou, Paris, France; Salma JAAFOURA salma.jaafoura@inserm.fr; Inserm sponsor, Paris, France; Ouifiya KAFIF ouifiya.kafif@aphp.fr; Hôpital Bichat, Paris, France; Antoine KHALIL antoine.khalil@aphp.fr; Hôpital Bichat, Paris, France; Nadhem LAFHEJ nadhem.lafhej@aphp.fr; Hôpital Bichat, Paris, France; Cédric LAOUÉNAN cedric.laouenan@aphp.fr; Hôpital Bichat, Paris, France; Samira LARIBI samira.laribi@aphp.fr; Hôpital Bichat, Paris, France; Minh LE minh.le@aphp.fr; Hôpital Bichat, Paris, France; Quentin LE HINGRAT quentin.lehingrat@aphp.fr Hôpital Bichat, Paris, France; Soizic LE MESTRE soizic.le mestre@anrs.fr ANRS-MIE, Paris, France; Sophie LETROU sophie.letrou@aphp.fr; Hôpital Bichat, Paris, France; Yves LEVY yves.levy@inserm.fr; Vaccine Research Insitute (VRI), Inserm UMR 955, Créteil, France; Bruno LINA bruno.lina@chu-lyon.fr Inserm UMR 1111, Lyon, France; Guillaume LINGAS guillaume.lingas@inserm.fr Inserm UMR 1137, Paris, France; Denis MALVY denis.malvy@chu-bordeaux.fr; CHU Bordeaux, France; France MENTRÉ france.mentre@inserm.fr; Hôpital Bichat, Paris, France; Hugo MOUQUET hugo.mouquet@pasteur.fr; Pasteur Institute, Paris, France; Nadège NEANT nadege.neant@inserm.fr; Inserm UMR 1137, Paris, France; Christelle PAUL christelle.paul@anrs.fr; ANRS-MIE, Paris, France; Aurélie PAPADOPOULOS aurelie.papadopoulos@inserm.fr; Inserm sponsor, Paris, France; Christelle PAUL christelle.paul@inserm.fr; ANRS-MIE, Paris, France; Ventzislava PETROV-SANCHEZ ventzislava.petrov-sanchez@anrs.fr; ANRS-MIE, Paris, France; Gilles PEYTAVIN gilles.peytavin@aphp.fr; Hôpital Bichat, Paris, France; Valentine PIQUARD valentine.piquard@aphp.fr; Hôpital Bichat, Paris, France; Olivier PICONE olivier.picone@aphp.fr; Hôpital Louis Mourier, Colombes, France; Manuel ROSA-CALATRAVA manuel.rosa-calatrava@univ-lyon1.fr Inserm UMR 1111, Lyon, France; Bénédicte ROSSIGNOL B.ROSSIGNOL@chru-nancy.fr; F-CRIN INI-CRCT, Nancy, France; Patrick ROSSIGNOL p.rossignol@chru-nancy.fr; CHU Nancy, France; Carine ROY carine.roy@aphp.fr Hôpital Bichat, Paris, France; Marion SCHNEIDER marion.schneider2@aphp.fr Hôpital Bichat, Paris, France; Richa SU richa.su@aphp.fr; Hôpital Bichat, Paris, France; Coralie TARDIVON coralie.tardivon@aphp.fr; Hôpital Bichat, Paris, France; Jean-François TIMSIT jean-francois.timsit@aphp.fr; Hôpital Bichat, Paris, France; Sarah TUBIANA sarah.tubiana@aphp.fr; Hôpital Bichat, Paris, France; Sylvie VAN DER WERF sylvie.van-der-werf@pasteur.fr; Pasteur Institute, Paris, France; Benoit VISSEAUX benoit.visseaux@aphp.fr; Hôpital Bichat, Paris, France; Aurélie WIEDEMANN aurelie.wiedemann@inserm.fr; Vaccine Research Insitute (VRI), Inserm UMR 955, Créteil, France.
FROG ICU study group: N Deye, C Fauvaux, A Mebazaa, C Damoisel, D Payen, E Gayat: Hopital Lariboisiere (Paris); E Azoulay, AS Moreau, L Jacob, O Marie, M Legrand: Hopital Saint Louis (Paris); M Wolf, R Sonneville, R Bronchard: Hopital Bichat (Paris); I Rennuit, C Paugam: Hopital Beaujon (Clichy); JP Mira, A Cariou, A Tesnieres: Hopital Cochin (Paris); N Dufour, N Anguel, L Guerin, J Duranteau, C Ract: Hopital Bicetre (Le Kremlin-Bicetre); M Leone, B Pastene: Chu De Marseille (Marseille); T Sharshar, A Fayssoyl: Hopital Raymond Poincare (Garches); J-L Baudel, B Guidet: Hopital Saint-Antoine; Q Lu, W Jie Gu, N Brechot, A Combes: Hopital De La Pitie—Salpetriere (Paris); S Jaber, A Pradel, Y Coisel, M Conseil: Chu St Eloi (Montpellier); A Veillard Baron, L Bodson: Hopital Ambroise Pare (Boulogne); Jy Lefrant, L Elotmani, A Ayral, S Lloret: Chu Caremeau (Nimes); S Pily-Flouri, Jb Pretalli: Hopital Jean Minjoz (Besançon); Pf Laterre, V Montiel, Mf Dujardin, C Berghe: Clinique Saint-Luc (Belgium).
Author contributions
ML designed and supervised the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. RTT, BD, NF, and JG contributed substantially to the study design, data analysis and interpretation, and the writing of the manuscript.
Funding
The French COVID cohort is funded by the REACTing (REsearch and ACtion emergING infectious diseases) consortium and by a grant of the French Ministry of Health (PHRC no. 20-0424). This study was supported by the National Institute Of General Medical Sciences of the National Institutes of Health under Award number T32GM008440. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Declarations
Conflicts of interest
All authors declare no conflict of interest.
Ethical approval
The French COVID cohort (COVID-19 cohort, clinical trial NCT04262921) awas approved by the institutional review board CPP-Ile-de-France VI (ID RCB: 2020-A00256-33). The FROG-ICU study (Control cohort, clinical trial NCT01367093) was approved by the institutional review board (board CPP-Ile-de-France IV, IRB n°00003835 and Commission d’éthique biomédicale hospitalo-facultaire de l’hôpital de Louvain, IRB n° B403201213352).
Footnotes
The members of the French-COVID and the FROG-ICU study groups are listed in Acknowledgements.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Matthieu Legrand, Email: Matthieu.Legrand@ucsf.edu.
the French-COVID and the FROG-ICU Investigators:
Laurent Abel Amal Abrous, Claire Andrejak, François Angoulvant, Delphine Bachelet, Marie Bartoli, Sylvie Behilill, Marine Beluze, Krishna Bhavsar, Lila Bouadma, Minerva Cervantes-Gonzalez, Anissa Chair, Charlotte Charpentier, Léo Chenard, Catherine Chirouze, Sandrine Couffin-Cadiergues, Camille Couffignal, Marie-Pierre Debray, Dominique Deplanque, Diane Descamps, Alpha Diallo, Fernanda Dias Da Silva, Céline Dorival, Xavier Duval, Philippine Eloy, Vincent Enouf, Hélène Esperou, Marina Esposito-Farese, Manuel Etienne, Aline-Marie Florence, Alexandre Gaymard, Jade Ghosn, Tristan Gigante, Morgane Gilg, François Goehringer, Jérémie Guedj, Ikram Houas, Isabelle Hoffmann, Jean-Sébastien Hulot, Salma Jaafoura, Ouifiya Kafif, Antoine Khalil, Nadhem Lafhej, Cédric Laouénan, Samira Laribi, Minh Le, Quentin Le Hingrat, Soizic Le Mestre, Sophie Letrou, Yves Levy, Bruno Lina, Guillaume Lingas, Denis Malvy, France Mentré, Hugo Mouquet, Nadège Neant, Christelle Paul, Aurélie Papadopoulos, Christelle Paul, Ventzislava Petrov-Sanchez, Gilles Peytavin, Valentine Piquard, Olivier Picone, Manuel Rosa-Calatrava, Bénédicte Rossignol, Patrick Rossignol, Carine Roy, Marion Schneider, Richa Su, Coralie Tardivon, Jean-François Timsit, Sarah Tubiana, Sylvie Van Der Werf, Benoit Visseaux, Aurélie Wiedemann, N. Deye, C. Fauvaux, A. Mebazaa, C. Damoisel, D. Payen, E. Gayat, E. Azoulay, A. S. Moreau, L. Jacob, O. Marie, M. Legrand, M. Wolf, R. Sonneville, R. Bronchard, I. Rennuit, C. Paugam, J. P. Mira, A. Cariou, A. Tesnieres, N. Dufour, N. Anguel, L. Guerin, J. Duranteau, C. Ract, M. Leone, B. Pastene, T. Sharshar, A. Fayssoyl, J.-L. Baudel, B. Guidet, Q. Lu, WJie Gu, N. Brechot, A. Combes, S. Jaber, A. Pradel, Y. Coisel, M. Conseil, AVeillard Baron, L. Bodson, Jy Lefrant, L. Elotmani, A. Ayral, S. Lloret, S. Pily-Flouri, Jb Pretalli, Pf Laterre, V. Montiel, Mf Dujardin, and C. Berghe
References
- 1.Sigfrid L, Drake TM, Pauley E, et al. Long Covid in adults discharged from UK hospitals after Covid-19: a prospective, multicentre cohort study using the ISARIC WHO Clinical Characterisation Protocol. Lancet Reg Health Europe. 2021;8:100186. doi: 10.1016/j.lanepe.2021.100186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.For the SAPRIS study group. Carrat F, Touvier M, et al. Incidence and risk factors of COVID-19-like symptoms in the French general population during the lockdown period: a multi-cohort study. BMC Infect Dis. 2021;21:169. doi: 10.1186/s12879-021-05864-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McPeake J, Shaw M, MacTavish P, et al. Long-term outcomes following severe COVID-19 infection: a propensity matched cohort study. BMJ Open Resp Res. 2021;8:e001080. doi: 10.1136/bmjresp-2021-001080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghosn J, Piroth L, Epaulard O, et al. Persistent COVID-19 symptoms are highly prevalent 6 months after hospitalization: results from a large prospective cohort. Clin Microbiol Infect. 2021;27:1041.e1–1041.e4. doi: 10.1016/j.cmi.2021.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gayat E, Cariou A, Deye N, et al. Determinants of long-term outcome in ICU survivors: results from the FROG-ICU study. Crit Care. 2018;22:8. doi: 10.1186/s13054-017-1922-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.