Abstract
Introduction: Vertebral body tethering (VBT) is gaining popularity for the management of selected AIS patients. The most frequent non-mechanical complications after VBT are pulmonary complications, with a reported incidence of up to 8% for recurrent pleural effusion. However, only trace data have been published on this topic. We aimed to analyze the incidence, timing, treatment, outcomes and risk factors of pulmonary complications after VBT. Materials and Methods: All patients who underwent VBT between September 2018 and September 2022 were retrospectively reviewed. The rate of pulmonary complications was analyzed and the symptoms, timing of onset, treatment and outcomes were recorded. An analysis of demographic, radiographic, surgical and pulmonary function data was conducted to explore possible risk factors for pulmonary complications. Results: Data from 140 patients were available: 14 experienced a pulmonary complication 1 day to 6 weeks after VBT, with 9 presenting a recurrent pleural effusion. A total of 13 patients required invasive treatment. All recovered without sequelae. The risk factor analysis did not result in any significant observations. However, 11/14 patients had had a diaphragm split. Conclusion: Pulmonary complications were observed in 10% of patients. The timing, symptoms and required treatment were heterogeneous. Pleural effusion seems to be more common after diaphragm crossing, but evidence is not yet conclusive.
Keywords: vertebral body tethering, pleural effusion, pulmonary complications, complication management, adolescent idiopathic scoliosis, risk factor analysis
1. Introduction
Vertebral Body Tethering (VBT) is increasingly and rapidly becoming popular as a non-fusion surgical alternative for selected patients with adolescent idiopathic scoliosis (AIS). While VBT was originally developed as a growth-modulating technique for skeletally immature patients [1,2], recent evidence shows its efficacy as a correction technique also in patients approaching skeletal maturity [3,4]. Several studies have observed success rates above 80% when success is defined as a patient with a controlled scoliosis, ideally below 30°, two or more years after VBT or at skeletal maturity [5,6,7,8]. However, the complication rate after VBT is still high. Many complications are mechanical and possibly related to patient selection [3,5,6,7,8,9,10,11,12]. Non-mechanical perioperative complications, though not uncommon [5,6,9], are currently less enthusiastically discussed. Non-mechanical perioperative complications include rarities such as ureteral injury or screw misplacement resulting in penetration of the spinal canal (liquor deficiency syndrome) [6,13,14,15], but the most frequent non-mechanical complications are intrathoracic and often represented by pleural effusion [6]. Although several authors have observed pleural effusions after VBT, no study has yet focused on this complication. With an incidence of up to 8% [16], it is not unlikely that surgeons who are planning to start VBT will observe pleural effusions or other pulmonary complications. We therefore sought to analyze data on pulmonary complications among our patients and to report details about the incidence, diagnostic tools, treatment options, risk factors and outcomes that we experienced.
2. Methods
This single-center, single-surgeon retrospective study was conducted according to the STROBE statement [17].
2.1. Patient Recruitment
All consecutive patients who underwent VBT at our institution from September 2018 to September 2021 were considered for inclusion. Only patients who presented a complete pre- and perioperative diagnostic including preoperative and 1st standing X-ray and preoperative pulmonary function were included in this study.
2.2. Surgical Technique and Postoperative Care
Thoracic curves were usually performed in a combination of one or two mini-open intercostal approaches (5 to 6 cm each) and one or two thoracoscopic portals for video-assisted thoracic surgery (VATS). Thoracolumbar/lumbar curves were instrumented by using two mini-open approaches: a retroperitoneal one to L2 and below and an intercostal one to L1 and above. The diaphragm was usually not detached but punctured to let the tether pass through (diaphragm split). Double curves were all operated via a single-staged approach with the thoracolumbar/lumbar curve being instrumented first.
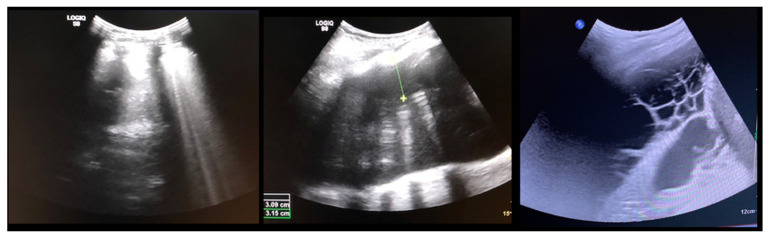
All patients received a postoperative chest tube, one for each side for double instrumentation. The tube was removed once the output decreased to less than 100 mL per 24 h. Once this threshold was reached, as an extra safety measure, we performed ultrasounds to quantify the remaining pleural effusion (Figure 1): the chest-tube was removed if less than 200 mL residual effusion was measured. The patients were monitored at the Intermediate Care Unit (ICU) until the chest tubes were removed.
Figure 1.
(Left) normal pleural ultrasound with the lung-shadow attached to the ribs; (Middle) Pleural effusion shown by a black/empty area between the ribs and the lung tissue; (Right) Cavernous hematoma shown by multiple cystic formations.
From the first postoperative day, patients were encouraged to train with a tri-flow multiple times per day and were asked to continue with the training for 4–6 weeks after hospital discharge. If patients presented symptoms such as dyspnea or cough, a chest X-ray was performed along with a pleural sonography, which was repeated daily until symptoms receded or to control the efficacy of the adopted therapy. Patients with acute symptoms were taken back to the ICU or to surgery. Patients with respiratory symptoms were discharged after symptoms subsided and when the remaining pleural effusion was less than 100 mL in the sonograph.
While there is no established protocol for the treatment of pleural effusion, at our institution we tend to treat these patients conservatively (ultrasound controls) when symptoms are absent or mild, such as a light cough or minor dyspnea, when the fluid collection is less than 200 mL as measured in pleural ultrasounds and when the collection is regredient within 24–48 h. Otherwise, invasive treatment is indicated. This usually consist in the reinsertion of the chest tube and dietary restriction in case of chylothorax. When an active bleeding is suspected, a surgical revision is usually performed.
Routine follow-up visits are conducted at 6 weeks, 6 months and at 1, 2 and 5 years postoperatively. At each timepoint, along with a clinical assessment, whole spine standing X-rays and pulmonary function are taken. At the 1-, 2- and 5-year follow-ups, the SRS-22 questionnaire is given to the patient as well. The level of physical activity is controlled at the 1-year follow-up with the sport activity questionnaire [18].
2.3. Outcomes of Interest
Although pulmonary complications usually occur within the first three months after surgery, patients were monitored for this complication throughout the follow-up (e.g., pulmonary function tests, specific questions at follow-up visits). The rate, type and timing of pulmonary complications were recorded, along with the correlated symptoms and therapy.
Demographic, radiographic (curve type, coronal and sagittal parameters from the preoperative and 1st standing X-ray) and intraoperative (anesthesia time and surgical time, instrumented levels) data were collected, along with the preoperative pulmonary function data, to seek possible risk factors for the development of a pulmonary complication. The curve type was defined following a previously published algorithm [19]. Regarding the instrumented levels, the lowest instrumented vertebra (LIV) for thoracic curves and the upper instrumented vertebra (UIV) for lumbar curves were noted. Regarding the pulmonary function parameters, a clinically significant difference was defined as a change of ±10% [20].
2.4. Statistical Analysis
The statistical analyses were performed using the software IBM SPSS 25. Regarding the analysis of the risk factors, the mean difference (MD) effect measure was evaluated with the t-test to assess statistical significance for continuous data. For binary data, the odds ratio (OR) effect measure was performed with the χ2 test to assess statistical significance. The confidence interval (CI) was set at 95% in all comparisons. Values of p < 0.05 were considered statistically significant.
3. Results
3.1. Patient Recruitment and Baseline Data
During the observation period, VBT was performed on 140 patients. The pre- and perioperative records were complete and available for all subjects, so that 140 patients were included in the study. The mean follow-up was 18.6 months.
Twenty-two patients were male (15%) and the mean age was 15.7 ± 3.9 years old. The mean Risser grade was 2.9 ± 3.9, and the mean Sanders score was 5.9 ± 1.8. Preoperatively, the thoracic curves measured averagely 54.4 ± 17.6° and bent down to 34.2 ± 17.5° on side-bending X-rays. The lumbar curves measured 48.3 ± 14.3° and bent to 19.7 ± 15.6°. The mean thoracic kyphosis was 32.9 ± 13.4° and the mean lumbar lordosis was 52.7 ± 11.6°. At the first standing X-ray after surgery, the mean thoracic Cobb angle measured 25.2 ± 10° (mean correction 50.7%) and the lumbar Cobb angle measured 16.7 ± 10° (mean correction 64%).
Regarding the pulmonary function, the mean Total Lung Capacity (TLC) was 102 ± 18%, the mean Forced Expiratory Pressure in 1 Second (FEV1) was 88.9 ± 16.3% and the Forced Vital Capacity (FVC) was 102.1 ± 20.5%.
3.2. Pulmonary Complications
Pulmonary complications were observed in 14 out of 140 included patients (10%). The most observed complication was a recurrent pleural effusion (nine cases, 64%), followed by haematothorax (two cases, 15%), pleural empyema (one case, 7%), chylothorax (one case, 7%) and contralateral atelectasis after right thoracic surgery (one case, 7%). The pulmonary complication was diagnosed one day to six weeks after index surgery.
For four patients, the pulmonary complication occurred while they were still inpatients after VBT. The remaining 10 patients were diagnosed after discharge: two were re-admitted at our hospital, and the other eight were treated in hospitals nearby their residence. The treatment of pleural effusions differed between these institutions, but all patients kept updating us and we therefore have a 100% follow-up rate.
All patients fully recovered without any remaining complaints. A preoperative and postoperative pulmonary function test was available for 7 of the 14 patients. The mean preoperative TLC was in line with the expected values for healthy patients of the same age (107.9 ± 17.2%), both the FEV1 and FVC were reduced (83 ± 9% and 80.7 ± 3.8%, respectively). One year after surgery, however, there was no clinically significant reduction in the pulmonary function parameters in comparison to the preoperative values (TLC 98.7 ± 7.1%, FEV1 104.5 ± 8.8% and FVC 81.5 ± 4.5%).
A detailed description of the patients who presented a pulmonary complication is shown in Table 1.
Table 1.
Overview of the characteristics of the patients who presented pulmonary complications, with details regarding presentation symptoms, time to diagnosis and treatment. CRP = C reactive protein.
| Patient | Age (Years) | Sex | Curve Type | Instrumented Levels | Complication | Time to Diagnosis | Side of the Complication | Symptoms | Treatment |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 13.3 | F | 2 | T5-T12 right T12-L4 left |
Pleural effusion | 2 weeks | Right | Minor dyspnea | Ultrasound, conservative treatment |
| 2 | 17.6 | F | 4 | T6-L1 right | Pleural effusion | 2 weeks | Right | Dyspnea, fatigue | 2 × aspiration |
| 3 | 16.2 | F | 4 | T5-T12 right | Contralateral atelectasis | 2 days | Left | Severe dyspnea | Re-intubation for 3 days, 3 bronchoscopies and removal of a mucus plug |
| 4 | 17.6 | M | 1 | T9-L3 right | Pleural effusion | 4 weeks | Right | Chest pressure | Chest tube reinsertion |
| 5 | 16.2 | F | 2 | T5-T11 right T11-L3 left |
Pleural effusion | 3 weeks | Bilateral | Chest pain and elevated CRP levels | Bilateral aspiration, forced diuresis and i.v. albumin treatment |
| 6 | 17.7 | F | 2 | T5-T11 right T11-L3 left |
Chylothorax | 3 days | Right | None effusion, diagnosed on routine post-op X-ray | Chest tube reinsertion and dietary restriction |
| 7 | 16.8 | M | 2 | T5-T11 right T11-L4 left |
Pleural effusion | 3 weeks | Left | Unknown | Explorative thoracoscopy |
| 8 | 17.9 | F | 1 | T10-L3 left | Pleural effusion | 4 days | Left | None, effusion diagnosed on routine post-op X-ray | Aspiration followed by chest tube reinsertion for recurrent effusion |
| 9 | 14.6 | F | 2 | T5-T11 right T11-L4 left |
Pleural effusion | 3 weeks | Right | Fatigue, dyspnea | Chest tube reinsertion, antibiotics for co-existing pyelonephritis |
| 10 | 14.3 | F | 1 | T11-L4 left | Pleural effusion with concomitant infection | 3 weeks | Left | Sudden sharp pain in the left chest and dyspnea | Attempted aspiration and chest tube without output. VATS and six weeks antibiotitcs because of postivie culture for staph epidermidis |
| 11 | 12 | F | 4 | T5-T11 right | Haematothorax | 1 day | Right | No symptoms, significant blood loss noticed after declamping the chest tube and drop of haemoglobin levels | Emergency explorative thoracotomy using the same surgical approach. No active bleeding found but clotted hematoma |
| 12 | 13 | F | 2 | T6-T12 right T12-L4 left |
Haematothorax | 6 weeks | Right | Acute chest pain | Emergency explorative thoracotomy |
| 13 | 16.5 | M | 1 | T10-L4 left | Pleural empyema | 5 weeks | Left | Dyspnea, elevated CRP levels | VATS and antibiotic therapy |
| 14 | 16.3 | F | 2 | T5-T11 right T11-L4 left |
Pleural effusion | 5 weeks | Right | Dyspnea | Aspiration |
Examples of the X-rays of the two asymptomatic patients are shown in Figure 2 and Figure 3. Figure 4 shows the evolution of patient n. 6, who presented a chylothorax.
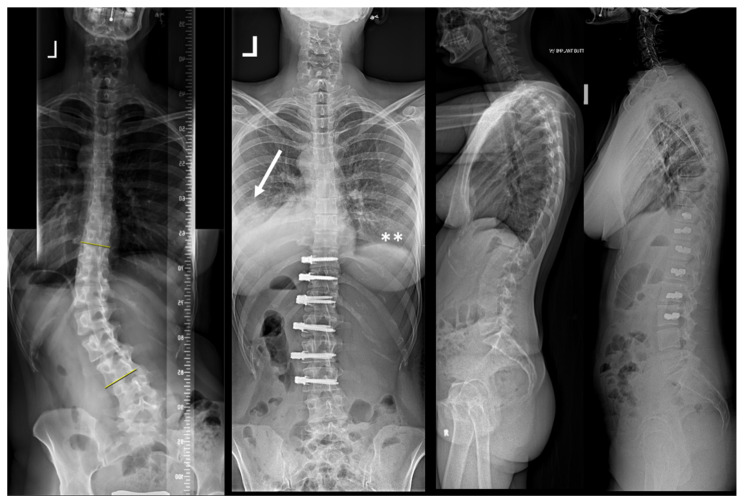
Figure 2.
The patient (n. 8 in the series) presented with a lumbar curve that measured 41° but resulted in a severe coronal imbalance and the patient suffered from daily pain. Surgery was able to almost completely correct her deformity. Pleural effusion on the left was diagnosed on routine post-operative radiographs with a vanished lateral recess (white arrow; ** shows a visible right lateral recess for comparison).
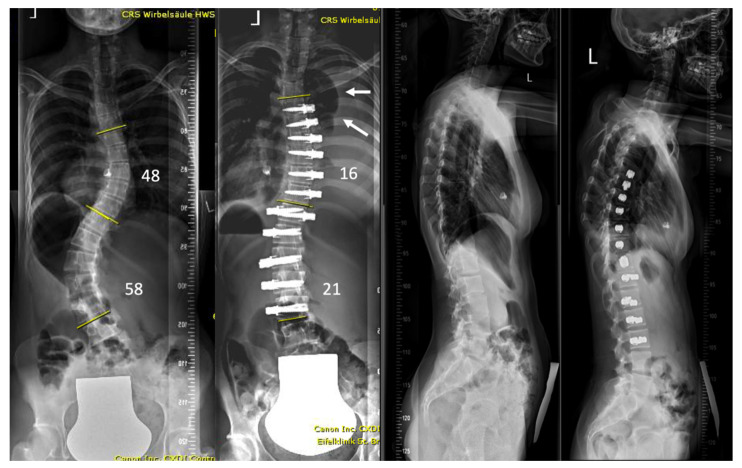
Figure 3.
17-year-old but very small patient (34 kg) with mild form of Di-George syndrome, which is known to present with vessel anomalies. Post-operative recovery was uneventful with no symptoms of fatigue or shortness of breath. Severe pleural effusion was noticed on first erect postoperative spine radiograph (arrows).
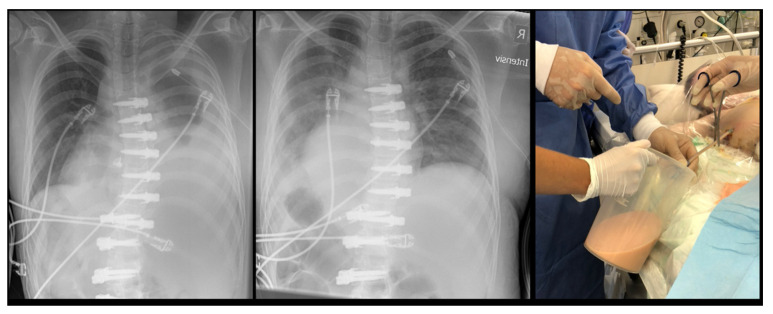
Figure 4.
Patient from Figure 3. (Left) Chest radiograph after drainage of 1 L; (Middle) Chest radiograph after drainage of 2.5 L; (Right) Macroscopic appearance of orange-milky chylos.
3.3. Risk Factor Analysis
The overview of all considered risk factors is shown in Table 2. The only parameters that showed a significant association with the risk of developing a pulmonary complication were the lumbar lordosis and the forced vital capacity.
Table 2.
Summary of the risk factor analysis for developing a pulmonary complication after VBT. MD = mean difference; OR = odds ration; SE = standard error, CI = confidence interval.
| Endpoint | No Pulmonary Complications (N = 126) |
Pulmonary Complications (N = 14) |
MD/OR | SE | 95% CI | p |
|---|---|---|---|---|---|---|
| Demographic data | ||||||
| Age | 15.8 ± 4.8 | 15.7 ± 1.8 | 2.6 | 1.4 | −0.2 to 5.4 | 0.1 |
| Gender (male) | 15% (19 of 126) | 21.4% (3 of 14) | 0.6 | 0.9 | 0.1 to 2.3 | 0.5 |
| Risser | 2.8 ± 1.9 | 3.3 ± 1.9 | −1.5 | 0.6 | −2.6 to −0.4 | 0.007 |
| Sanders | 5.9 ± 1.9 | 6.2 ± 2.0 | 0.3 | 0.6 | −0.7 to 1.3 | 0.6 |
| Preoperative data | ||||||
| Thoracic Cobb angle (°) | 53.8 ± 17.7 | 47.9 ± 16.1 | −5.9 | 5.0 | −15.7 to 3.9 | 0.2 |
| Thoracic bending (°) | 35.0 ± 17.6 | 26.0 ± 13.7 | −9.0 | 5.0 | −18.9 to 0.9 | 0.08 |
| Thoracic flexibility (%) | 39.0 ± 20.8 | 48.2 ± 16.2 | 9.2 | 6.0 | −2.5 to 20.9 | 0.1 |
| Lumbar Cobb angle (°) | 48.3 ± 14.1 | 48.0 ± 15.3 | −0.3 | 4.1 | −8.4 to 7.8 | 0.9 |
| Lumbar bending (°) | 19.9 ± 15.6 | 19.5 ± 14.6 | −0.4 | 4.5 | −9.3 to 8.5 | 0.9 |
| Lumbar flexibility (%) | 61.2 ± 37.1 | 64.1 ± 24.9 | 2.9 | 10.5 | −17.9 to 23.7 | 0.8 |
| Thoracic kyphosis (°) | 33.0 ± 13.7 | 31.2 ± 9.8 | −1.8 | 3.9 | −9.5 to 5.9 | 0.6 |
| Lumbar lordosis (°) | 53.0 ± 11.5 | 48.8 ± 12.2 | −4.2 | 3.4 | −10.8 to 2.4 | 0.2 |
| Sagittal vertical axis (mm) | 4.8 ± 27.1 | 6.2 ± 23.2 | 1.4 | 7.8 | −14 to 16.8 | 0.9 |
| Coronal balance (mm) | 9.2 ± 18.9 | 8.9 ± 24.0 | −0.3 | 5.6 | −11.4 to 10.8 | 0.9 |
| Pelvic incidence (°) | 50.3 ± 13.7 | 45.7 ± 15.3 | −4.6 | 4.0 | −12.5 to 3.3 | 0.3 |
| Pelvic tilt (°) | 9.4 ± 7.5 | 13.3 ± 15.5 | 3.9 | 2.5 | −0.9 to 8.7 | 0.1 |
| Intraoperative data | ||||||
| Double tether | 53% (67 of 126) | 42.8% (6 of 14) | 1.3 | 0.5 | 0.4 to 4.1 | 0.7 |
| Disk release | 66% (84 of 126) | 57% (8 of 14) | 1.2 | 0.3 | 0.3 to 3.9 | 0.7 |
| Anaesthesia time (min) | 334.9 ± 93.3 | 335.0 ± 87.2 | 0.1 | 2.7 | −53.3 to 53.5 | 0.9 |
| Surgical time (min) | 236.0 ± 28.8 | 232.5 ± 71.3 | −3.5 | 10.1 | −23.4 to 16.4 | 0.7 |
| 1st erect X-ray | ||||||
| Thoracic Cobb angle (°) | 25.7 ± 10.2 | 21.1 ± 6.0 | −4.6 | 2.9 | −10.3 to 1.1 | 0.1 |
| Thoracic correction (%) | 50.7 ± 15.6 | 50.5 ± 17.9 | −0.2 | 4.6 | −9.3 to 8.9 | 0.9 |
| Lumbar Cobb angle (°) | 16.6 ± 9.9 | 17.7 ± 11.2 | 1.1 | 2.9 | −4.6 to 6.8 | 0.7 |
| Lumbar correction (%) | 64.5 ± 20.7 | 63.9 ± 16.1 | −0.6 | 5.9 | −12.3 to 11.1 | 0.9 |
| Thoracic kyphosis (°) | 34.0 ± 11.7 | 36.2 ± 7.9 | 2.2 | 3.3 | −4.3 to 8.7 | 0.5 |
| Lumbar lordosis (°) | 46.5 ± 10.4 | 39.9 ± 12.2 | −6.6 | 3.1 | −12.6 to −0.5 | 0.03 |
| Sagittal vertical axis (mm) | 26.2 ± 27.3 | 35.1 ± 27.0 | 8.9 | 3.1 | −6.8 to 24.6 | 0.3 |
| Coronal balance (mm) | 15.5 ± 22.2 | 19.7 ± 20.9 | 4.2 | 6.4 | −8.5 to 16.9 | 0.5 |
| Pulmonary function | ||||||
| Total lung capacity | 101.3 ± 17.6 | 109.1 ± 19.8 | 7.8 | 5.2 | −2.4 to 18 | 0.1 |
| Forced expiratory volume 1 s | 89.0 ± 16.8 | 87.3 ± 10.2 | −1.7 | 4.8 | −11.1 to 7.7 | 0.7 |
| Forced vital capacity | 101.0 ± 19.7 | 113.2 ± 23.8 | 12.2 | 5.9 | 0.6 to 23.7 | 0.04 |
The number of patients who developed a pulmonary complication according to the curve type or to the level of the thoracic UIV/lumbar LIV are reported in Table 3 and Table 4.
Table 3.
Summary of the events (pulmonary complication) according to the curve type (1 = thoracolumbar/lumbar curves, 2 = double curves, 3 = long thoracic curves, 4 = short thoracic curves, 5 = presence of a rigid, high thoracic curve).
| Curve Type | Patients (N) | Pulmonary Complication |
|---|---|---|
| 1 | 20.7% (29 of 140) | 10.3% (3 of 29) |
| 2 | 50% (70 of 140) | 10% (7 of 70) |
| 3 | 10.7% (15 of 140) | 6.6% (1 of 15) |
| 4 | 15% (21 of 140) | 4.7% (1 of 21) |
| 5 | 3.4% (5 of 140) | 40% (2 of 5) |
Table 4.
Summary of the events (pulmonary complication) according to the lumbar upper instrumented vertebra (UIV) and/or thoracic lowest instrumented vertebra (LIV).
| UIV/LIV | Patients (N) | Pulmonary Complication |
|---|---|---|
| T10 | 18.5% (26 of 140) | 7.6% (2 of 26) |
| T11 | 34.3% (48 of 140) | 14.5% (7 of 48) |
| T12 | 28.5% (40 of 140) | 7.5% (3 of 40) |
| L1 | 11.3% (13 of 140) | 7.7% (1 of 13) |
4. Discussion
This is the first study to offer a detailed analysis of pulmonary complications after VBT, a not-so-rare occurrence after this kind of surgery. We reported a 10% incidence of pulmonary complications and observed that the majority were diagnosed after discharge from the hospital, usually between 2 and 6 weeks postoperatively. As the number of VBT cases is increasing worldwide, we believe this study presents important data from which surgeons could benefit.
Just two of the considered risk factors, the lumbar lordosis and the FVC, showed a significant association with the development of pulmonary complications. However, it is unlikely that these parameters have any clinical significance and are thus interpreted as mathematical associations only. While the Risser grade was higher among patients who presented a pulmonary complication, this finding is not coherent with data on age and Sanders stage. Thus, we believe that this is likely to be a spurious correlation.
Despite the high rate of pulmonary complications, it is important to highlight that the affected patients recovered well and did not suffer any long-term complications. As many of our patients come from abroad, some of them did not have the opportunity to obtain pulmonary function tests and some have not yet reached a 1-year follow-up. For these reasons, the number of longer-term follow-ups is limited. However, the available data are in line with the postoperative pulmonary function values observed one year after VBT (TLC 99%, FEV1 89% and FVC 86%) [21].
The observation interval excludes the learning curve, as the surgeon performing all procedures had already performed over 50 VBT cases before September 2018. Cases prior to this date were not considered for the present study, as we had not yet routinely obtained a preoperative pulmonary function test for our patients. Thus, it is safe to say that the learning curve did not have any effect on the incidence of pulmonary complications in this cohort. Over time, the surgical technique was modified in an effort to reduce the rate of pulmonary complications. These modifications included a very limited opening of the pleura (only for the screw entry point), suturing of the diaphragm when a split is performed, and changes in postoperative chest tube management. In fact, at the beginning of our experience with VBT, the tubes were removed when the output was below 200 mL per 24/h. However, data are not sufficient to say whether these modifications had any influence on the rate of pulmonary complications.
The exact pathophysiology of this complication still raises questions. While hemo- or chylothorax seem to be directly related to the surgical procedure, a delayed pleural effusion may be related to postoperative activity. In our practice, we do not restrict postoperative activity and it has been observed that 94% of patients were able to resume their pre-operative activity level within three months after VBT [22]. However, most pleural effusions were diagnosed within the first month after surgery, when patients had probably not yet returned to their normal level of activity. Thus, limited mobility may play a role and patients should be advised to continue pulmonary training with the tri-flow even after hospital discharge.
Interestingly, only four cases of pulmonary complications occurred after a thoracic-only VBT (one in type 3 and 4 curves, respectively, and two in type 5 curves). Bypassing the diaphragm with a tether (double and thoracolumbar curves) may therefore represent a risk factor, as 10 cases out of 14 (71%) presented a pulmonary complication after a lumbar or bilateral procedure. However, we do not have an explanation as to why the majority of pleural effusions in double curves were diagnosed on the right side, where the diaphragm usually remained intact. Furthermore, the number of observations is still too small to reach a definitive conclusion on this point.
Similar considerations can be made for the analysis of the UIV/LIV. The majority of the patients presenting a pulmonary complication had the UIV/LIV at T11, but numbers are too small to reach a definitive conclusion and we do not have an explanation for this finding.
Alanay et al. observed 4 pulmonary complications in 31 analyzed patients, i.e., an incidence of 12% [9]. All patients in their series had a thoracic curve but six patients received VBT for a long thoracic curve, which usually requires splitting or dissection of the diaphragm. None of the patients had a bilateral VBT. One patient in their series was found to have a chylothorax, which required non-invasive dietary precautions, and one patient with a delayed effusion required readmission to the hospital and non-invasive medical treatment. In other similar-sized cohorts, Hoernschmeyer et al. reported 1 case of pneumothorax among 29 treated patients (3%) [10], while Samdani et al. observed 1 case of persistent atelectasis in 32 patients (3%) [23]. In smaller-sized groups, Newton observed 1 atelectasis case in 23 operated patients (4%) [24] and Pehlivanoglu reported 1 case of chylothorax in 21 treated subjects (5%) [25]. Wong et al. observed 5 pulmonary complications in 3/5 patients (60%) [26]. Conversely, Boudissa and colleagues did not observe any pulmonary complications in six operated patients. While many of the studies available in the literature showed a lower rate of pulmonary complications than the one presented in this study, other authors mainly performed thoracic VBT and the patient cohorts are thus not directly comparable.
The Humanitarian Use Device Exemption study, which was published by the US Food and Drug Administration, and which resulted in approval of the first VBT device in the US, reported a 5.3% incidence (3/57 patients) in a very homogeneous patient cohort consisting of only thoracic Lenke type 1 curves. No information was provided with respect to the treatment or outcomes [27]. Comparing these data to the ones of the presented cohort supports the hypothesis that diaphragm splitting may represent a risk factor for pulmonary complications. Further studies on a larger patient cohort will be required to clarify this point.
Rushton et al. reported 2 out of 112 patients with a pleural effusion after VBT [15]. While their calculated incidence was significantly lower compared to our patient population, most patients in this study received a single curve VBT. Only four patients from their cohort had a double curve VBT and all these four patients were operated on in a staged manner. Therefore, no patient had a single-staged double curve VBT. However, the number of observations for double instrumentation presented in Rushton’s study is too small to support the hypothesis that single-staged surgery represents a risk factor for the development of pulmonary complications. Both patients in Rushton’s series required invasive treatment; one had a chest drainage and the other patient required surgical revision for bleeding control [15].
With 140 analyzed patients, to our best knowledge, this study has the highest number of included patients who have had VBT. Additionally, the study reviews a single surgeon’s patients and therefore the surgical technique confounder can be limited to a minimum. On the other hand, this study does not come without limitations, which are not only related to the retrospective methodology. While we are able to calculate the incidence of pleural effusions after VBT and hypothesize a few risk factors, we still have no insight into the exact pathophysiology and therefore have not been able to eradicate this complication in our practice. Nevertheless, with this analysis of a very heterogeneous patient cohort we are able to add valuable information about a not-so-rare complication after VBT.
5. Conclusions
In the presented cohort, an incidence of pulmonary complications after VBT of 10% was observed. The timing, symptoms and required treatment were heterogeneous. However, none of the presented patients showed long-term consequences. Pleural effusion seems to be a common complication after diaphragm crossing VBT, but evidence is not yet conclusive. Patients need to be informed that this complication can occur after discharge from the hospital and that it may require re-admission. However, patients can be reassured that long-term consequences are unlikely.
Author Contributions
All authors made substantial contributions to the design of the work; or the acquisition/analysis/interpretation of data; Draft of the work or critical revision for important intellectual content; Approval of the version to be published; Agreement to be accountable for all aspects of the work. A.B.: Conception, data analysis and interpretation, draft and revision. P.T.: Conception, data interpretation, draft and revision. T.V.: Data collection, critical revision of the manuscript. F.M.: Conception, data analysis, critical revision of the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
RWTH Aachen, Faculty of Medicine, approval EK 130/19.
Informed Consent Statement
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent for being included in the study was waived as it is not required by local law for retrospective studies.
Data Availability Statement
Data can be made available upon reasonable request.
Conflicts of Interest
P.D.T.: Globus Medical (personal fees), Zimmer Biomet (personal fees). A.B., F.M. and T.V.: none.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Parent S., Shen J. Anterior Vertebral Body Growth-Modulation Tethering in Idiopathic Scoliosis: Surgical Technique. J. Am. Acad. Orthop. Surg. 2020;28:693–699. doi: 10.5435/JAAOS-D-19-00849. [DOI] [PubMed] [Google Scholar]
- 2.McDonald T.C., Shah S.A., Hargiss J.B., Varghese J., Boeyer M.E., Pompliano M., Neal K., Lonner B.S., Larson A.N., Yaszay B., et al. When successful, anterior vertebral body tethering (VBT) induces differential segmental growth of vertebrae: An in vivo study of 51 patients and 764 vertebrae. Spine Deform. 2022;10:791–797. doi: 10.1007/s43390-022-00471-2. [DOI] [PubMed] [Google Scholar]
- 3.Bernard J., Bishop T., Herzog J., Haleem S., Lupu C., Ajayi B., Lui D.F. Dual modality of vertebral body tethering: Anterior scoliosis correction versus growth modulation with mean follow-up of five years. Bone Jt. Open. 2022;3:123–129. doi: 10.1302/2633-1462.32.BJO-2021-0120.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hegde S.K., Venkatesan M., Akbari K.K., Badikillaya V.M. Efficacy of Anterior Vertebral Body Tethering in Skeletally Mature Children with Adolescent Idiopathic Scoliosis: A Preliminary Report. Int. J. Spine Surg. 2021;15:995–1003. doi: 10.14444/8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker C.E., Milbrandt T.A., Larson A.N. Anterior Vertebral Body Tethering for Adolescent Idiopathic Scoliosis: Early Results and Future Directions. Orthop. Clin. North Am. 2021;52:137–147. doi: 10.1016/j.ocl.2021.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Baroncini A., Trobisch P.D., Birkenmaier C., Da Paz S., Migliorini F. Radiologische Ergebnisse nach Vertebral Body Tethering. Zentrum für Orthopädie und Unfallchirurgie. 2021 doi: 10.1055/a-1387-8334. [DOI] [PubMed] [Google Scholar]
- 7.Samdani A.F., Pahys J.M., Ames R.J., Grewal H., Pelletier G.J., Hwang S.W., Betz R.R. Prospective Follow-up Report on Anterior Vertebral Body Tethering for Idiopathic Scoliosis: Interim Results from an FDA IDE Study. J. Bone Jt. Surg. 2021;103:1611–1619. doi: 10.2106/JBJS.20.01503. [DOI] [PubMed] [Google Scholar]
- 8.Trobisch P.D., Kobbe P., Baroncini A. Die dynamische Skoliosekorrektur als Therapieoption bei adoleszenter idiopathischer Skoliose. Zentrum für Orthopädie und Unfallchirurgie. 2020;158:641–646. doi: 10.1055/a-0983-1265. [DOI] [PubMed] [Google Scholar]
- 9.Alanay A., Yucekul A., Abul K., Ergene G., Senay S., Ay B., Cebeci B.O., Yalinay D., Pinar Z.T., Yavuz Y., et al. Thoracoscopic Vertebral Body Tethering for Adolescent Idiopathic Scoliosis: Follow-up Curve Behavior According to Sanders Skeletal Maturity Staging. Spine. 2020;45:E1483–E1492. doi: 10.1097/BRS.0000000000003643. [DOI] [PubMed] [Google Scholar]
- 10.Hoernschemeyer D.G., Boeyer M.E., Robertson M.E., Loftis C.M., Worley J.R., Tweedy N.M., Gupta S.U., Duren D.L., Holzhauser C.M., Ramachandran V.M. Anterior Vertebral Body Tethering for Adolescent Scoliosis with Growth Remaining: A Retrospective Review of 2 to 5-Year Postoperative Results. J. Bone Jt. Surg. 2020;102:1169–1176. doi: 10.2106/JBJS.19.00980. [DOI] [PubMed] [Google Scholar]
- 11.Newton P.O., Kluck D.G., Saito W., Yaszay B., Bartley C.E., Bastrom T.P. Anterior Spinal Growth Tethering for Skeletally Immature Patients with Scoliosis: A Retrospective Look Two to Four Years Postoperatively. J. Bone Jt. Surg. 2018;100:1691–1697. doi: 10.2106/JBJS.18.00287. [DOI] [PubMed] [Google Scholar]
- 12.Samdani A.F., Ames R.J., Kimball J.S., Pahys J.M., Grewal H., Pelletier G.J., Betz R.R. Anterior vertebral body tethering for idiopathic scoliosis: Two-year results. Spine. 2014;39:1688–1693. doi: 10.1097/BRS.0000000000000472. [DOI] [PubMed] [Google Scholar]
- 13.Rathbun J.R., Hoernschemeyer D.S., Wakefield M.R., Malm-Buatsi E.A., Murray K.S., Ramachandran V. Ureteral injury following vertebral body tethering for adolescent idiopathic scoliosis. J. Pediatric Surg. Case Rep. 2019;46:101219. doi: 10.1016/j.epsc.2019.101219. [DOI] [Google Scholar]
- 14.Shen J., Parent S. Iatrogenic dural tear after growth modulation in AIS: An unusual complication and its management. Spine Deform. 2021;9:1699–1703. doi: 10.1007/s43390-021-00368-6. [DOI] [PubMed] [Google Scholar]
- 15.Rushton P.R.P., Nasto L., Parent S., Turgeon I., Aldebeyan S., Miyanji F. Anterior Vertebral Body Tethering for Treatment of Idiopathic Scoliosis in the Skeletally Immature: Results of 112 Cases. Spine. 2021;46:1461–1467. doi: 10.1097/BRS.0000000000004061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baroncini A., Rodriguez L., Verma K., Trobisch P.D. Feasibility of Single-Staged Bilateral Anterior Scoliosis Correction in Growing Patients. Glob. Spine J. 2021;11:76–80. doi: 10.1177/2192568219892904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Epidemiology. 2007;18:800–804. doi: 10.1097/EDE.0b013e3181577654. [DOI] [PubMed] [Google Scholar]
- 18.Sarwahi V., Wendolowski S., Gecelter R., Maguire K., Gambassi M., Orlando D., Lo Y., Amaral T. When Do Patients Return to Physical Activities and Athletics After Scoliosis Surgery?: A Validated Patient Questionnaire Based Study. Spine. 2018;43:167–171. doi: 10.1097/BRS.0000000000002284. [DOI] [PubMed] [Google Scholar]
- 19.Baroncini A., Trobisch P.D., Migliorini F. Learning curve for vertebral body tethering: Analysis on 90 consecutive patients. Spine Deform. 2021;9:141–147. doi: 10.1007/s43390-020-00191-5. [DOI] [PubMed] [Google Scholar]
- 20.Kim Y.J., Lenke L.G., Bridwell K.H., Cheh G., Whorton J., Sides B. Prospective pulmonary function comparison following posterior segmental spinal instrumentation and fusion of adolescent idiopathic scoliosis: Is there a relationship between major thoracic curve correction and pulmonary function test improvement? Spine. 2007;32:2685–2693. doi: 10.1097/BRS.0b013e31815a7b17. [DOI] [PubMed] [Google Scholar]
- 21.Baroncini A., Trobisch P., Blau C., Golias C., Kobbe P., Eschweiler J., Tingart M., Migliorini F. Analysis of the pulmonary function in patients undergoing vertebral body tethering for adolescent idiopathic scoliosis. Eur. Spine J. 2021;31:1022–1027. doi: 10.1007/s00586-021-07029-2. [DOI] [PubMed] [Google Scholar]
- 22.Baroncini A., Trobisch P.D., Berrer A., Kobbe P., Tingart M., Eschweiler J., Da Paz S., Migliorini F. Return to sport and daily life activities after vertebral body tethering for AIS: Analysis of the sport activity questionnaire. Eur. Spine J. 2021;30:1998–2006. doi: 10.1007/s00586-021-06768-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samdani A.F., Ames R.J., Kimball J.S., Pahys J.M., Grewal H., Pelletier G.J., Betz R.R. Anterior vertebral body tethering for immature adolescent idiopathic scoliosis: One-year results on the first 32 patients. Eur. Spine J. 2015;24:1533–1539. doi: 10.1007/s00586-014-3706-z. [DOI] [PubMed] [Google Scholar]
- 24.Newton P.O., E Bartley C., Bastrom T.P., Kluck D.G., Saito W., Yaszay B. Anterior Spinal Growth Modulation in Skeletally Immature Patients with Idiopathic Scoliosis: A Comparison with Posterior Spinal Fusion at 2 to 5 Years Postoperatively. J. Bone Jt. Surg. 2020;102:769–777. doi: 10.2106/JBJS.19.01176. [DOI] [PubMed] [Google Scholar]
- 25.Pehlivanoglu T., Oltulu I., Ofluoglu E., Sarioglu E., Altun G., Korkmaz M., Yildirim K., Aydogan M. Thoracoscopic Vertebral Body Tethering for Adolescent Idiopathic Scoliosis: A Minimum of 2 Years’ Results of 21 Patients. J. Pediatr. Orthop. 2020;40:575–580. doi: 10.1097/BPO.0000000000001590. [DOI] [PubMed] [Google Scholar]
- 26.Wong H.-K., Ruiz J.N.M., Newton P.O., Liu K.-P.G. Non-Fusion Surgical Correction of Thoracic Idiopathic Scoliosis Using a Novel, Braided Vertebral Body Tethering Device: Minimum Follow-up of 4 Years. JBJS Open Access. 2019;4:e0026. doi: 10.2106/JBJS.OA.19.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Humanitarian Device Exemption (HDE) Number: H190005. [(accessed on 3 September 2021)]; Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf19/H190005b.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data can be made available upon reasonable request.