Abstract
Variable responses to medications complicates perioperative care. As a potential solution, we evaluated and synthesized pharmacogenomic evidence that may inform anesthesia and pain prescribing to identify clinically actionable drug/gene pairs. Clinical decision support (CDS) summaries were developed and were evaluated using Appraisal of Guidelines for Research and Evaluation (AGREE) II. We found that 93/180 (51%) of commonly-used perioperative medications had some published pharmacogenomic information, with 18 having actionable evidence: celecoxib/diclofenac/flurbiprofen/ibuprofen/piroxicam/CYP2C9, codeine/oxycodone/tramadol CYP2D6, desflurane/enflurane/halothane/isoflurane/sevoflurane/succinylcholine/RYR1/CACNA1S, diazepam/CYP2C19, phenytoin/CYP2C9, succinylcholine/mivacurium/BCHE, and morphine/OPRM1. Novel CDS summaries were developed for these 18 medications. AGREE II mean±standard deviation scores were high for Scope and Purpose(95.0±2.8), Rigor of Development(93.2±2.8), Clarity of Presentation(87.3±3.0), and Applicability(86.5±3.7) (maximum score=100). Overall mean guideline quality score was 6.7±0.2 (maximum score=7). All summaries were recommended for clinical implementation. A critical mass of pharmacogenomic evidence exists for select medications commonly used in the perioperative setting, warranting prospective examination for clinical utility.
INTRODUCTION
Adverse drug events (ADEs) and drug inefficacy remain challenging problems within the perioperative setting.1–4 Patients’ fears surrounding receiving anesthesia are one of the greatest contributors to perioperative anxiety5, and providers are acutely aware of unintended anesthetic and pain medication complications. Unpredictability is affected by a complex interplay of heterogeneous diseases being treated, rapidly changing states of organ function, critical illness, and patient factors, including genetic factors.
Probably the best-known perioperative pharmacogenetic example is malignant hyperthermia—a syndrome recognized since the 1960s6. Various genetic polymorphisms in the RYR1 and CACNA1S genes predispose individuals to this syndrome, which presents as a life-threatening hypermetabolic response to succinylcholine and certain volatile anesthetics7. Identifying a patient at increased risk for this condition through attaining a family history, and if necessary, additional testing, is standard practice and essential for medication decisions, suggesting preemptive pharmacogenomic testing may prove beneficial. Outside of anesthesiology, many additional examples of genetic-related medication risk stratification have been recently identified and incorporated into clinical practice, including HLA-B*57:01 testing for hypersensitivity to abacavir and HLA-B*1502 testing for risk of Stevens-Johnson Syndrome with carbamazepine use8–14. Despite implementation of genetic information to inform prescribing in these other medical settings, the routine use of such information within anesthesiology and critical care remains almost nonexistent.
While a number of potential barriers may explain this15, 16, one of the most frequently-cited reasons is the paucity of guidance around available evidence to support clinical pharmacogenomic actionability for most common medications used by anesthesiologists and critical care physicians. This means that any effort to consider whether a translational gap exists between discovery and clinical practice for anesthesia requires an appraisal and integration of the evidence, and development of straightforward decision supports to enable clinical consideration. Using a comprehensive appraisal and clinical decision-support development methodology that our group has applied in other subspecialty settings, including cardiology and oncology17–19, we sought to interrogate the clinical relevance of current pharmacogenomic evidence and enable potential clinical translation of such knowledge for anesthesia, critical care, and acute pain medicine in this original research study. We hypothesized that the clinical relevance of pharmacogenomic evidence for perioperative medications will be considerable and will comprise an evidence base that justifies future prospective clinical examination of pharmacogenomics in this field.
METHODS
DATA ACQUISITION
A comprehensive list of commonly prescribed perioperative medications was first compiled using publicly available Anesthesia, Critical Care, and Acute Pain Medicine clinical practice guidelines and texts (Supplemental File 1). The goal was to assemble an expansive list, including not only medications that might be used for primary anesthesia, critical care, or pain treatment, but also supportive medications that are used in these contexts (e.g., antibiotics, gastroprotectants). Medications listed as common treatment options by any of the source texts were included. Two individuals (E.H.J. and P.H.O.) reviewed and approved the resulting list. In total, 180 medications were included for appraisal.
Pharmacogenomic articles related to these medications were identified through a custom PubMed search query which has been previously successfully tested and utilized to comprehensively identify clinically relevant published pharmacogenomic evidence: ‘((“Polymorphism, Genetic”[Mesh] OR “Genotype” [Mesh]) AND “Humans”[Mesh] and (“drug” OR “Pharmacologic Actions”[Mesh])) OR (polymorphism AND drug)’20. All abstracts from articles assessing the association between a germline genetic variant and a pharmacogenomic outcome (i.e. toxicity, response) resulting from this search were manually reviewed by at least two independent reviewers for relevance and subsequently catalogued in the University of Chicago pharmacogenomic research and implementation database. Inclusion and exclusion criteria have been previously published17, 18. Briefly, disease risk genetic markers were excluded to focus exclusively on pharmacogenomics. Studies examining animal models and in vitro experiments, review articles, case studies, and those not written in English were also excluded. For articles deemed to assess the relationship between a pharmacogenomic marker and clinical outcome(s), the following study characteristics were entered into the database: PubMed ID, medication(s), genetic variant(s) (as denoted by dbSNP rs number), and common gene name. For each article, a preliminary designation (based on abstract review) of whether the article reported a “positive” or “negative” genetic association was also assigned. Each article for which the full paper was subsequently reviewed was critically assessed to confirm this designation, and the “positive” vs ”negative” associations reported by the authors were not simply accepted at face value but instead were evaluated and ultimately denoted by the review team.
Distinct from the above, a separate literature search was conducted to identify any additional articles, using drug-annotated references listed in PharmGKB (www.pharmgkb.org), reference lists within relevant CPIC guidelines (when available; www.cpicpgx.org), and reference lists assembled for medications with pharmacogenomic recommendations by the Dutch Pharmacogenetics Working Group (DPWG) (www.pharmgkb.org/page/dpwg).
Finally, for each medication we conducted a final PubMed search using the terms “[medication name]” and “polymorphism” to ensure that no remaining critical articles were missed (see Supplemental File 2, tab 2 for articles attained through this search). Data were collected through January 31, 2018. All articles captured by these three various search methods were included. Notably, newly published guidance from CPIC and DPWG was periodically reviewed and incorporated into our analyses through January 2021.
PHARMACOGENOMIC ASSESSMENT
Publications identified via the above searches were assembled into an MS Excel spreadsheet arranged by medication. Sub-groupings for each medication were created to organize all studies together that evaluated the same drug/variant or drug/gene pair. All drug/variant or drug/gene pair groupings for each medication were then evaluated first at the group level, with all articles in each group assessed first at the abstract level (by E.H.J). Each was assessed for eligibility to be taken forward for full article review, with the eligibility assessment performed based on study design, quality, sample size, and the presence of replication (including within the group). Importantly, this included manual inspection of both as-reported ‘positive’ and as-reported ‘negative’ studies within a group. The last author also independently triaged articles for eligibility at the abstract level using similar criteria, with any disagreement between the two assessors automatically triggering a given article to be taken forward for full review. Finally, all articles within a given drug/gene or drug/variant pair group with an existing published clinical pharmacogenomic guideline (CPIC, DPWG) or with pharmacogenomic information in the FDA label were automatically eligible and taken forward for full review.
ASSESSMENT FOR CLINICAL ACTIONABILITY
Articles selected for full review were then rigorously evaluated for scientific, genetic, statistical, and clinical methodological rigor using a formal framework for pharmacogenomic studies that follows state-of-the-art consensus guidelines (see Table 1)21, 22. Methodology from the assessed articles was required to meet multiple criteria described in Table 1, all at least at the “Lower Level of Support Evidence” designation or higher, in order to qualify as “potentially clinically actionable” and thus be further considered. Large cohort sizes, high-quality phenotype measurements (well-defined, prospectively measured, rigorously assessed, and are objectively reproducible), assessment for genetic Hardy-Weinberg equilibrium, large magnitude of effect size, high clinical relevance (i.e. medications that carry serious risk of harm to the patient, and not having genetic information could greatly increase risk), inclusion of key alleles23, and appropriate statistical analyses (including correction for multiple testing) increased support for clinical actionability. Detailed information for each of the publications supporting replicated, consistent and strong-evidence drug/variant and drug/gene pairs were recorded, with the following parameters collected from each study: year of publication, first author, medication(s) studied, diseases under study, genetic variants studied, sample sizes (cases/controls), dosing regimens, follow-up period, and outcomes measured.
Table 1.
Scientific, methodologic, and clinical criteria used to critically evaluate pharmacogenomic articles via systematic review. These criteria are applied at the article level, to each article being evaluated. These criteria follow formal, accepted standards in the field of pharmacogenomics.See also Ratain et al 201321 and Thorn et al 201821.
| Criterion | High Level of Supporting Evidence | Lower Level of Supporting Evidence | Inappropriate Supporting Evidence |
|---|---|---|---|
|
| |||
| Cohort size | Large | Medium or small studies | Case reports* |
|
| |||
| Disease(s) Being Studied/ Clinical Setting | Homogeneous | Mixed, but with reasonable overlap | Heterogeneous |
|
| |||
| Subject Age(s) | Present | Present | Absent |
|
| |||
| Race/ethnicity information | Present | Present | Absent |
|
| |||
| Sex/gender | Present | Present | Absent |
|
| |||
| Possible population stratification | Considered and excluded | No consideration, but population homogenous | Heterogeneous population without appropriate analysis |
|
| |||
| Comedications and comorbidities | Provided | Provided | Absent |
|
| |||
| Source of DNA | Blood or buccal swab | Peritumoral tissue | Tumor |
|
| |||
| Genotyping Methodology | Standard methods, with appropriate quality controls, and excellent coverage of all key (actionable) alleles | Standard methods, quality controls not explicitly stated, allele coverage represents the minimum acceptable alleles | Non-standard methods, failed quality controls, key allele(s) missing*** |
|
| |||
| Haplotype definition (if haplotypes studied) | Present | Present | Undefined |
|
| |||
| Hardy-Weinberg equilibrium | Present | Present | Deviation from HWE, or not tested |
|
| |||
| Variants where no effect was seen | Included | Included | Not included |
|
| |||
| Phenotype measurement | Well-defined, prospectively measured, rigorously assessed, objectively reproducible | Well-defined, but potentially retrospectively collected | Adequate description of phenotype lacking |
|
| |||
| Data analysis | Genetic effect tested alongside or after controlling for other clinical factors, and remains independently associated with the phenotype | Genetic effect rigorously tested against phenotype and is statistically associated, but other potential clinical factors not included / not tested in conjunction | Genetic association is lost after inclusion of other clinical or confounding factors |
|
| |||
| Gene / Disease Association Testing | Variant/gene(s) of interest do not confer disease susceptibility, and there is no association between the variant/gene(s) of interest and baseline disease factors nor disease prognostic classifiers/groups | Formal testing of variant/gene(s) of interest against disease/prognostic classifiers is not performed, but respective baseline characteristics are fully provided so that comparison of each diplotype groups can be performed, with no differences by diplotype group observed | Variant/gene(s) of interest confer disease susceptibility, and/or diplotype groups are imbalanced for key baseline disease characteristics/prognostic factors |
|
| |||
| Statistical analysis** | Careful correction for multiple testing | Exploratory analysis | No attention to multiple testing |
|
| |||
| Clinical relevance of association | Highly relevant (drug would be avoided, or dose would be changed, based on the result) | Potentially relevant (clinician may not avoid or dose-alter the drug, but might monitor the patient differently; or information might help inform prescribing in settings where there is otherwise equipoise about several treatment options) | Irrelevant (i.e., genetic variant is statistically associated but the information would not alter the clinical decision calculus; provides no additional information that would impact dosing, monitoring, or likelihood of response/toxicity) |
|
| |||
| Odds ratios with confidence intervals | Present | Present | Absent |
|
| |||
| Effect size | Large (OR > 5) | Moderate (OR 2–5) | Modest (OR <2) |
|
| |||
| Direction of effect | Consistent | Consistent | Divergent |
|
| |||
| Supporting Pharmacokinetic Data**** | Drug levels provide biologic explanation for observed clinical effect | Not applicable (e.g., for pharmacodynamic genes), or not obtained | Absent |
|
| |||
| Functional / Biologic Rationale | Functional studies are performed and provide a credible explanation for the observed genetic relationship | Variant/gene has clear biologic relevance to the observed phenotype (alters known enzyme activity, is in relevant pathway, or affects drug target), but functional studies were not directly performed | Absent |
In pharmacogenomics, there is a history of case reports (especially those reporting drug-related deaths) being the provoking cause for more formal, larger investigations or for performing subsequent formal studies of a drug/gene relationship; in these instances, case reports might be considered supportive of an association, but case reports would generally not provide sufficient evidence in isolation.
Gene by treatment interaction analyses were not required to be performed, but were considered as a potential feature of high quality studies.
Key alleles were chosen based on a minimum set of variants that should be included in genotyping assays, as set forth by the Association for Molecular Pathology Clinical Practice Committee (Pratt et al 201823). Studies of CYP2D6 where copy number assessment is not included, or studies of CYP2C19 lacking inclusion of *17, would be examples that fall into this category. For RYR1/CACNA1S, we utilized the list endorsed by the EMHG (https://www.emhg.org/diagnostic-mutations). For genes where no consensus allele list is yet published (e.g., CYP2D6), we used a proposed standard of requiring all alleles having known frequencies of at least 5% in the population being studied.
Applies to studies where the primary phenotype of interest is a clinical endpoint (e.g., toxicity).
OR = odds ratio of effect (carrier of actionable genotype vs non-carrier).
Evidence synthesis for the resulting studies was conducted by at least two reviewers independently, with disagreement resolved through discussions until consensus. Drug/variant or drug/gene pairs identified as potentially clinically actionable through this process were taken forward for Clinical Decision Support (CDS) summary development.
CLINICAL DECISION SUPPORT SUMMARY DEVELOPMENT
For medications that emerged from the above primary data assessment, CDS summaries were developed by two members of the evidence evaluation team (E.H.J. and P.H.O.) using methods described previously17, 18, 20. Summaries included point-of-care guidance and specific prescribing recommendations, assignment of a “traffic signal” designation denoting genomic risk (high-risk=red light, caution=yellow light, favorable=green light), references to available external pharmacogenomic guidelines where available (e.g. CPIC, FDA label), and individual annotations of the key supporting primary publications. Only for those ultimately deemed clinically actionable (ultimately deployed as CDS due to unanimous support after AGREE assessment, described below), a level of evidence designation (level 1, 2, or 3) for each CDS was assigned and shown for the clinician using the following published criteria24–26, which closely mirror criteria set forth by PharmGKB13: Level 1 indicates the evidence is supported by a well-performed, large study that either includes replication or has been externally replicated by other well-performed, large studies. Additionally, only those drug/variant or drug/gene associations with existing published clinical guidelines or with pharmacogenomic information in the FDA label are eligible for a Level 1 designation. Level 2 indicates the evidence is based on at least one well-performed study of at least 100 patients with additional separate studies replicating the same result in the same direction. Level 3 evidence consists of a relatively smaller well-performed primary study (<100 patients) with biological relevance or an aggregate signal from several similarly-executed studies but for which other contradictory studies exist. Pharmacokinetic (PK) evidence can be supportive for assigning studies into Levels 2 or 3, but PK data alone are not adequate for solely supporting a CDS. Rather, all CDS are based on clinical studies having a primary clinical endpoint (e.g., toxicity or disease response) as the chief analyzed outcome. Light colors are assigned based on specific results (i.e. effect size of clinical outcome) combined with the potential risk to the patient (i.e., death, severe toxicity, severe risk of non-response).
AGREE II SCORING
After development of each proposed, potentially clinically actionable CDS summary, each CDS was subjected to formal evaluation using the Appraisal of Guidelines for Research and Evaluation (AGREE) II framework in order to assess its quality and to determine clinical use/appropriateness for prospective clinical evaluation or utilization.18, 27 The AGREE II instrument is a standardized, validated tool used to assess the quality and reporting of practice guidelines27–31. The modified AGREE II scoring system used in this study encompasses domains of Scope and Purpose, Rigor of Development, Clarity of Presentation, and Applicability. It is modified from the original AGREE II scoring system by removal of domain 2 (Stakeholder Involvement) and domain 6 (Editorial Independence), as these were not applicable to our study. In accordance with AGREE II specifications (which suggests the use of at least two but preferably four reviewers), and in an effort to include all key stakeholder groups, five independent appraisers with specific credentials and expertise in the fields of anesthesia and critical care, pain management, and pharmacogenomics (J.L.A., M.A., S.S., R.K., T.M.T.) applied the AGREE II scoring framework to the proposed CDS summaries. Each appraiser received detailed information on the scoring framework and AGREE II instrument prior to reviewing any of the summaries. None of those who conducted the evidence integration and developed the proposed CDSs were appraisers. The appraisers represented a purposefully-sampled group of key stakeholders (three anesthesiologists, including one Pain specialist and one Critical Care specialist, plus two pharmacists, one with advanced training in pharmacogenomics and one who oversees Acute Pain Service inpatient pharmacist support). Each appraiser rated each draft summary on all four included domains, in addition to giving each summary an overall quality score. Finally, each appraiser independently voted on whether the summary deserved deployment as a clinical guideline (that is, was “clinically actionable”).
RESULTS
STUDY DEMOGRAPHICS
For the 180 included medications, over 1,900 publications were initially identified and assessed (Supplemental File 2, tab 1). The article evaluation process is depicted in Figure 1. In total, 93 medications (51.1%) were found to have at least 1 published positive pharmacogenomic study. A total of 66 medications had associated drug/variant or drug/gene pair groups containing individual articles that were eligible for full article-level review (Supplemental File 3). Pharmacogenomic evidence had been previously formally evaluated by our group (in prior studies) for 15 of these medications17, 18, 26. The remaining 51 medications (encompassing 200 unique drug/variant or drug/gene pairs) were supported by 382 publications that were fully appraised at the publication level (sent for full review). Of these 51 medications, 18 were deemed to have rigorous, replicated, high quality pharmacogenomic evidence in the literature.
Figure 1. Article Evaluation Process.
For the 180 included medications, over 1,900 publications were initially identified and assessed. In total, 93 medications (51.1%) were found to have at least 1 published positive pharmacogenomic study. A total of 66 medications had associated drug/variant or drug/gene pair groups containing individual articles that were eligible for full article-level review. Pharmacogenomic evidence had been previously formally evaluated by our group (in prior studies) for 15 of these medications. The remaining 51 medications (encompassing 200 unique drug/variant or drug/gene pairs) were supported by 382 publications that were fully appraised at the publication level (sent for full review). After assessment of these publications, 18 medications were deemed potentially clinically actionable, and thus CDS were developed and subjected to AGREE II scoring. All CDS were unanimously recommended for clinical implementation.
CLINICALLY ACTIONABLE ASSOCIATIONS
Table 2 shows details for the 18 medications with high quality, replicated pharmacogenomic evidence supporting clinical actionability. Of note, the Table highlights only the positive studies for each gene-drug pair, though both negative and positive studies were considered when determining clinical actionability, and negative studies were cited in our CDS. Publication-level evidentiary information for the key studies supporting the replicated, consistent and strong-evidence drug/variant and drug/gene pairs are provided in Table 3. There did not appear to be any pattern based on year of FDA drug approval that predicted medication-specific clinical actionability (Figure 2). Almost all of the 18 medications determined to be clinically actionable have similar CPIC, DPWG, and/or FDA label prescribing guidance.
Table 2.
Perioperative and Pain Medications with Actionable Pharmacogenomic Evidence.
| Medication | Gene | Variant or Phenotype | Implication | CPIC/DPWG/FDA PGx Info* | # of Positive Studies | Top Supporting Publications† | Total # of Study Subjects in Supporting Publications | Clinical Effects | Recommended Clinical Action | Actionable Genotype/Phenotype Frequencies§ |
|---|---|---|---|---|---|---|---|---|---|---|
| Analgesia | ||||||||||
| Codeine# | CYP2D6 | UM/NM/IM/PM | UM: risk of CNS depression and death; IM: decreased analgesic effect with standard dosing; PM: high risk of lack of analgesic effect | Y/Y/Y | 27 | 1–11 | 362 | Undetectable active metabolite in 36% of patients given codeine4; Undetectable active metabolite in all 12 PMs6; 50% higher plasma concentration of active metabolite w/ more sedation in UMs than in NMs9. | Avoid codeine in UM and PM individuals. Monitor closely in IM individuals. | UM: 1–2% IM: 2–11% PM: 5–10% |
| Tramadol | CYP2D6 | UM/NM/IM/PM | UM: risk of serious adverse drug effects (respiratory depression) and toxicity (nausea/vomiting); IM: decreased analgesic effect with standard dosing; PM: high risk of inadequate analgesia | Y/Y/Y | 28 | 1,12–16 | 536 | Response rate of 78.4% vs. 53.3% (NM vs. PM)16; 10 of 18 PMs required rescue medication, significantly more than other phenotypes15. | Avoid tramadol in PM individuals. Reduce initial dose by 30% in UM individuals. Monitor closely in IM individuals. | UM: 1–2% IM: 2–11% PM: 5–10% |
| Oxycodone | CYP2D6 | UM/NM/IM/PM | UM: narcotic-related toxicity; IM and PM: decreased analgesic effect with standard dosing |
N/Y/Y | 8 | 17–19 | 141 | Cumulative postoperative doses higher for PMs & IMs, lower for UMs, compared to NMs (25 mg, 22mg, 18mg versus 20 mg)17; UMs experience more side effects than NMs18. | Monitor closely in UM, IM, and PM individuals. | UM: 1–2% IM: 2–11% PM: 5–10% |
| Morphine | OPRM1 | A118G | Inadequate Analgesia | Y‖/N/N | 28 | 20–23 | 8,462 | Each additional copy of the G allele increases morphine intake by 1.87 mg and pain score by 0.51 units20. | Monitor closely in individuals with A/G or G/G genotypes. | AG: 40–49% GG: 14–15% |
| Celecoxib¶ Diclofenac Flurbiprofen Ibuprofen Piroxicam |
CYP2C9 |
*2 allele *3 allele |
Gastrointestinal Bleeding | Y/N/Y‡ | 10 | 24–30, 58 | 685 | NSAID treatment associated with bleeding compared to aspirin (OR=15.7)24; 2 to 8-fold higher plasma concentrations with the risk allele27. | Reduce initial dose by at least 50% for *3/*3 individuals. Reduce initial dose by 25–50% for *2/*3 and *2/*2 individuals. Reduce initial dose by 25–40% for *1/*3 individuals. Monitor closely in *1/*2 individuals. |
*3 heterozygote: 2–7% *3 homozygote: <1% *2 heterozygote: <1%−20% *2 homozygote: <1% |
| Anesthesia | ||||||||||
| Mivacurium | BCHE | K-variant A-variant |
Prolonged Apnea | N/N/Y | 5 | 31–35 | 114 | Patients have been seen to have paralysis for up to 12 hours after standard doses of mivacurium. | Avoid mivacurium in those with the A/A, AK/A, and AK/AK genotypes. Use with caution in those with the A/U, AK/U, A/K, and AK/K genotypes. Monitor closely in those with the K/K and K/U genotypes. | A/U: 4% AK/U: 10% A/K: 22% AK/K: 4% K/K: 8% K/U: 18% |
| Desflurane Enflurane Halothane Isoflurane Sevoflurane Succinylcholine |
RYR1
CACNA1S |
40 RYR1 mutations, 2 CACNA1S mutations | Malignant Hyperthermia | Y/N/Y | 41 | 36–40, 57 | 200 | 101 of 196 of malignant hyperthermia patients carry a risk allele41. | Avoid succinylcholine & volatile anesthetic use in individuals carrying any risk alleles. | RYR1 variants: <1% |
| Succinylcholine | BCHE | A-variant | Prolonged Apnea | N/N/Y | 14 | 31,32,35,41,42 | 1,312 | Prolonged apnea of 1 to 6 hours in homozygous genotypes, and 6 to 20 min. in heterozygous carriers (normal 4 to 6 min)41. | Avoid succinylcholine in homozygous carriers of the A-variant. Administer cautiously in heterozygous carriers. | A allele frequency: <4% |
| Antiepilepsy | ||||||||||
| Phenytoin | CYP2C9 | NM/IM/PM | IM and PM: Neurotoxicity or Severe Cutaneous Adverse Reactions (SCAR) | Y/Y/Y | 40 | 2,43–53 | 5,704 | OR=11 for SCAR43; OR=15.3 for neurotoxicity44. | Reduce initial maintenance dose by 50% in PM individuals and 25% in IM individuals. | IM: 8% PM: 1% |
| Antianxiety | ||||||||||
| Diazepam | CYP2C19 | NM/IM/PM | IM and PM: Increased Emergence Time from Anesthesia | N/N/Y | 5 | 54–56 | 102 | General anesthesia median emergence time of 18, 13, 10 min in PM, IM, & NMs, respectively54. | Monitor closely in PM and IM individuals. | IM: 18–45% PM: 2–15% |
Y=yes, N=no; CPIC and DPWG both provide actionable recommendations, whereas FDA may provide general information about genetic alleles without specific prescribing guidance.
Details of supporting publications are reported in Table 3. Complete references available in Supplemental File 5.
Dosing and/or caution information provided for celecoxib, piroxicam, and flurbiprofen.
See Discussion for specific details about CPIC evaluation of this drug/gene pair
Only the NSAIDs that were specifically included in pharmacogenomic clinical outcome studies and/or pharmacogenomic-pharmacokinetic studies that demonstrated the genetic association were specifically developed into Clinical Decision Support summaries.
Also addresses tramadol and oxycodone in guideline.
As reported in supporting publications or CPIC guidelines
CPIC= Clinical Pharmacogenetics Implementation Consortium, DPWG= Dutch Pharmacogenomics Working Group, FDA=Food and Drug Administration; PGx information from CPIC and DPWG is indicated as “yes” if clinical guidelines are available.
UM=Ultrarapid Metabolizer; NM=Normal Metabolizer; IM=Intermediate Metabolizer; PM=Poor Metabolizer
Table 3.
Publication-level evidentiary information for the key studies supporting the replicated, consistent and strong-evidence drug/variant and drug/gene pairs.
| Author (Year) | Study Design | Population and Diseases | Follow-Up | Genotype/Phenotypes/Outcome Measure | Medication and Dosing Regimens | Results of Reviewed Markers |
|---|---|---|---|---|---|---|
| Lotsch et al 2009 | Open randomized cross-over design in which CYP2D6 activity score was tested in comparison to genotype-based classification and plasma dextromethorphan metabolic ratio | 57 healthy Caucasian subjects genotyped for CYP2D6 receiving either dextromethorphan or codeine | Codeine, codeine metabolites, morphine, and morphine metabolites were measured after extraction of plasma samples. | CYP2D6 activity score; plasma concentration | 50 mg oral codeine or 30 mg oral dextromethorphan | Most subjects at the lower 15% of morphine formation from codeine were correctly identified by CYP2D6 genotype- or phenotype-based systems, while CYP2D6 genotyping predicted only the 50% who carried gene duplications in subjects at the upper 15% of morphine formation. Dextromethorphan-based phenotyping identified 67.5% of subjects with high morphine formation. |
| Williams et al 2002 | Randomized double-blind study | 96 children undergoing adenotonsillectomy | Blood was drawn 1 hour after induction for the measurement of plasma morphine and morphine metabolites. | CYP2D6 PM, IM/PM, IM, NM; plasma concentration | Codeine 1.5 mg/kg or morphine 0.15 mg/kg | Plasma morphine concentrations were related to phenotype (p<0.02). Plasma morphine metabolite concentrations, as measured by the M3G:M6G ratio, were not significant (EM group: 4.5, IM group: 3.4, IM/PM group: 2.95) p>0.05. |
| Eckhardt et al 1998 | Randomized placebo-controlled double-blind trial | Pain tolerance was assessed in 18 adults undergoing the cold pressor test. | Codeine and morphine metabolites were measured in serum and urine. | CYP2D6 EM, PM; response and adverse events | Codeine 170 mg or morphine 20 mg | Following administration of codeine, analgesia was observed in EM but not PM - EM: 54.9 +/− 42.2 vs 1.7 +/− 4.2 p<0.01; PM: 9.6 +/− 10.9 vs. 3.3 +/− 23.7 p>0.05); No differences in adverse effects among phenotype groups were observed; Morphine concentrations after codeine administration comparable to after administration of morphine were only observed in EM; Percentage of codeine dose converted to morphine and metabolites was 3.9% in EM compared to 0.17% in PM. |
| Sindrup et al 1990 | Double-blind, placebo-controlled crossover study | Pain tolerance to laser stimuli was assessed in 24 adults. | Pain threshold measurements and medication level in plasma was measured before ingestion of codeine or placebo and then 90, 150, and 210 minutes after ingestion. | CYP2D6 EM, PM; efficacy | Codeine 75 mg; placebo | In EM, there was a statistically significant increase in pain thresholds 90 and 150 minutes after codeine with no difference after placebo. In PM, neither codeine nor placebo resulted in significant changes in pain threshold. Codeine concentrations were significantly higher in EM than in PM but did not differ 150 and 210 minutes after codeine administration. In EM, there was a significant correlation between the plasma concentration of morphine and pain threshold difference after codeine and after placebo after 90 minutes. |
| Poulsen et al 1996 | Randomized, double-blind, three-way, crossover study | Pain tolerance was assessed via the cold pressor test in addition to heat and pressure stimulation in 28 adults. | Pain tests were performed before and 1, 2, 3, and 4 hours after medication administration. | CYP2D6 EM, PM; adverse effects | Codeine 75 mg or 100 mg; Morphine 20 mg or 30 mg; placebo | After codeine administration, neither morphine nor morphine-6-glucoronide could be detected in 13 of the 14 PMs, whereas at least one of the compounds could be detected in all EM. Codeine only reduced pain measures significantly in EM. In PMs, adverse effects were more pronounced on morphine as opposed to codeine, and a slight difference was observed between codeine and placebo. In EM, there was no difference between codeine and morphine and more pronounced adverse effects on both drugs as compared to placebo. |
| Sistonen et al 2012 | Telephone interviews for self-reported adverse effects | 111 mothers who used codeine during pregnancy were assessed for potential genetic association with adverse effects, specifically CNS depression. | Mothers were initially called after giving birth. A second follow-up call was conducted within one year of the original call. | CYP2D6 PM, EM, UM; ABCB1 rs1128503; ABCB1 rs2032582; ABCB1 rs1045642; UGT2B7 rs62298861; OPRM1 rs1799971; OPRM1 rs563649; COMT rs4633; COMT rs4818; COMT rs4680; toxicities | Codeine use during pregnancy | Genetic model combining the maternal risk genotypes in CYP2D6 and ABCB1 was significantly associated with adverse outcomes in infants (OR: 2.68; 95% CI 1.61–4.48, p=0.0002) and their mothers (OR: 2.74; 95% CI 1.55–4.84, p=0.0005). |
| Kirchheiner et al 2007 | Pharmacokinetic/pharmacodynamic study | 26 healthy Caucasian volunteers | Blood samples were obtained before codeine was administered and at 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12 and 24 hours after administration. Pupil diameter measured as a pharmacodynamic parameter. | CYP2D6 EM, PM, UM; plasma metabolite levels | Single dose of 30 mg codeine | Median morphine and M3G AUCs were significantly different among EM, PM, and UM (p=0.02 and p=0.02, respectively). Higher O-demethylated codeine metabolites with increasing CYP2D6 activity was detected (p<0.001). 50% higher plasma concentration of active metabolite in UM compared to NM. Influence of genotype on pupil diameter not significant. |
| Kirchheiner et al 2008 | Pharmacokinetic/pharmacodynamic study | 22 healthy volunteers | Pharmacokinetic parameters measured were total clearance, renal clearance and maximum concentration. Pharmacodynamics were measured using cold pressor test, pupillometry, and standardized adverse event recording. | CYP2D6 EM, UM; drug plasma concentrations and adverse events | Single dose of 100 mg tramadol | Maximum plasma concentrations of the active metabolite were significantly higher in the UM group than the EM group (p=0.005). Median tramadol AUC was 786 and 587 mug.h.L in EM and UM, respectively, and the corresponding median metabolite AUC was 416 and 448 mug.h.L (p=0.005). UM experienced increased pain threshold and tolerance and a stronger miosis after tramadol. Nearly half of the UM group experienced nausea compared to only 9% of the EM group. |
| Pedersen et al 2006 | Open-label crossover trial with different formulations | 16 healthy volunteers | Urine and plasma concentrations of tramadol and metabolite (M1) were measured 48 hours after administration. | CYP2D6 EM, PM; drug plasma concentration | 150 mg single dose oral racemic tramadol, 50 mg single oral racemic tramadol every 8 hours for 48 hours, 100 mg intravenous racemic tramadol | In all three phases, significant differences existed between EM and PM in AUC and half life of (+) tramadol (p<0.0015), (−) tramadol (p<0.0062), (+)-M1 (p<0.0001) and (−)-M1 (p<0.0370). EM and PM also showed significant differences for Cmax of (+)-M1 (p<0.0001) and (−)-M1 (p<0.001). No significant differences between absolute bioavailability of tramadol in EM and PM. Urinary recoveries of (+) tramadol and (−) tramadol, in addition to (+) M1 and (−) M1 were significantly different in EM and PM (p<0.05). |
| Garcia-Quetglas et al 2007 | Pharmacokinetic study | 24 healthy volunteers | Blood samples were collected at 30, 60, 90, 120, 150, 180 and 210 minutes and 4, 5, 6, 8, 10, 12, 24, 36, and 48 hours after oral administration of tramadol. Tramadol and metabolites (M1 and M2) were measured. | CYP2D6 EM, PM; drug plasma concentration | 100 mg racemic tramadol | Plasma concentrations of tramadol enantiomers were consistently higher in PM than in EM, with 1.98 and 1.74-fold differences in mean AUC, respectively. Oral clearance of (+) and (−) tramadol ere 1.91- and 1.71-fold greater in PM. The mean AUC values of (+)-M1 and (−)-M1 were 4.33 and 0.89-fold greater in EM. Differences in AUC for M2 enantiomers were 7.40 and 8.69-fold greater in PM. |
| Stamer et al 2007 | Pharmacokinetic study | 174 patients receiving intravenous tramadol for postoperative analgesia | Blood samples were drawn 30, 90, and 180 minutes after administration and were analyzed for plasma concentrations of (+) and (−) tramadol and (+) and (−) O-desmethyltramadol. Efficacy was also measured. | CYP2D6 PM, IM, EM, UM; drug plasma concentrations and efficacy | Intravenous tramadol 3 mg/kg | Median AUC-time curves for (+)O-desmethyltramadol were 0, 38.6, 66.5, and 149.7 ng x h/ml for PM, IM, EM, and UM (p<0.001). In PM, non-response rates to tramadol increased fourfold compared to other genotypes (p<0.001). |
| Stamer et al 2003 | Prospective cohort study | 271 patients recovering from abdominal surgery | Pain scores, analgesic consumption, and need for rescue medication was collected. | CYP2D6 EM, PM; response and dose | After titration of individual loading dose, patients could self-administer 1 ml bolus doses of the drug combination tramadol 20 mg/ml, dipyrone 200 mg/ml and metoclopramide 0.4 mg/ml via patient-controlled analgesia. | Percentage of non-responders was significantly higher in the PM group (46.7%) compared with the EM group (21.6%, p=0.005). Tramadol loading dose differed between EM and PM (108.2 +/− 56.9 and 144.7 +/− 22.6 mg, p<0.001). More PM patients needed rescue medication in the recovery room and during PCA period (21.6 vs. 43.3%, p=0.02). |
| Stamer et al 2013 | Pharmacokinetic study | 121 patients receiving oxycodone before emerging from anesthesia and patient-controlled anesthesia for 48 hours postoperatively. | Blood samples were drawn at 30, 90, and 180 minutes after initial oxycodone dose. Plasma concentrations of oxycodone, oxymorphone, noroxycodone and noroxymorphine were analyzed. Pain scores were also obtained. | CYP2D6 PM, IM, EM, UM; drug plasma concentrations | Oxycodone 0.05 mg/kg before emerging from anesthesia and for use as patient-controlled analgesia. | Mean oxymorphone/oxycodone ratios were 0.10, 0.13, 0.18, and 0.28 in PM, IM, EM, and UM (p-0.005). Oxycodone consumption within the first 12 hours postoperatively was highest in PM (p=0.005). Pain scores did not differ between genotypes. |
| Samer et al 2010 | Randomized crossover (five arms) double-blind placebo-controlled study | 10 healthy volunteers | Experimental pain (cold pressor test, electrical stimulation, thermode), pupil size, psychomotor effects and toxicity were assessed after oral oxycodone administration. | CYP2D6 UM, PM, EM, IM; toxicities and response | On five occasions, patients randomly received oxycodone (0.2 mg/kg) and placebo; oxycodone and quinidine; oxycodone and ketoconazole; oxycodone and quinidine + ketoconzaole; placebo | UM experienced increased pharmacodynamic effects compared to EM. This effect was not seen in PM. Side effects were observed after CYP2D6 and/or CYP3A4 blockade in UM. |
| Samer et al 2010 | Randomized crossover (five arms) double-blind placebo-controlled study | 10 healthy volunteers | Blood samples for plasma concentrations of oxycodone and metabolites oxymorphone, noroxycodone, and noroxymorphine were collected for 24 hours after dosing. | CYP2D6 UM, PM, EM; drug plasma concentration | On five occasions, patients randomly received oxycodone (0.2 mg/kg) and placebo; oxycodone and quinidine; oxycodone and ketoconazole; oxycodone and quinidine + ketoconzaole; placebo | Oxymorphone C(max) was 62% and 75% lower in PM than EM and UM. Noroxymorphone C(max) was reduced by 90% in PM. In UM, oxymorphone and noroxymorphone concentrations increased and noroxycodone exposure was halved. |
| Sia et al 2008 | Pharmacodynamic study | 586 women receiving morphine for postcesaerean analgesia | Pain scores, severity of nausea and vomiting, incidence of pruritis, and self-administered morphine were recorded for the first 24 postoperative hours. | OPRM1 A118G; adverse events | Bolus dose of 1 mg morphine, lockout of 5 minutes, and total hourly dose of 10 mg for treatment of postoperative pain (patient-controlled analgesia). | The 24 hour self-administered intravenous morphine consumption was lowest in the AA group (p=0.001). Pain scores were lowest in the AA group and highest in the G group (p=0.049). The AA group had the highest incidence of nausea (p=0.02). |
| Sia et al 2013 | Prospective cohort study | 973 patients undergoing scheduled total hysterectomy under general anesthesia | The association of a common polymorphism in the OPRM1 gene with patient-rated pain scores and amount of morphine use. | mu-opioid receptor gene OPRM1; response and dose | The PCA was set to deliver 1 mg IV bolus of morphine per demand with a lockout time of 5 minutes, without continuous background infusion. The maximum amount of morphine allowed was 10 mg/h. For the next 24 hours, the cumulative dose of morphine administered by each patient within every 4-hour period was recorded. Patients were monitored and could also request for additional IV morphine in 1-mg boluses. | There was no statistically significant association with OPRM1 118A>G for either pain threshold or pain tolerance. There was a statistically significant association of genotype with total morphine and morphine self-administered through PCA, with the GG group using the most and the AA group the least (p=.006). |
| Hwang et al 2014 | Systematic review and meta-analysis | 346 articles were retrieved from databases, and 18 studies involving 4,607 participants were included in the final analyses. | The standardized mean difference (SMD) of required amounts of opioids between AA homozygotes and G-allele carriers was calculated. | OPRM1 A118G polymorphism; opioid dose | post-operative opioid response | In a random-effect meta-analysis, G-allele carriers required a higher mean opioid dose than AA homozygotes (SMD, −0.18; P = 0.003). Although there was no evidence of publication bias, heterogeneity was present among studies (I(2) = 66.8%). In the subgroup meta-analyses, significance remained robust in Asian patients (SMD, −0.21; P = 0.001), morphine users (SMD, −0.29; P <0.001), and patients who received surgery for a viscus (SMD, −0.20; P = 0.008). |
| Klepstad et al 2011 | Cohort study including a development and validation analysis | A total of 2294 cancer pain patients from 17 centres located in 11 countries were recruited to the study. Participants were adult patients (>18 years of age) with a malignant disease who were using an opioid for moderate to severe pain. | The dose and routes of opioids, both scheduled and rescue doses, for the last 24 hours, the duration of opioid treatment and previous number of unsuccessful trials with other opioids were recorded. Oral opioid equivalent morphine doses were calculated using standard tables. | 112 SNPs in the 25 candidate genes OPRM1, OPRD1, OPRK1, ARRB2, GNAZ, HINT1, Stat6, ABCB1, COMT, HRH1, ADRA2A, MC1R, TACR1, GCH1, DRD2, DRD3, HTR3A, HTR3B, HTR2A, HTR3C, HTR3D, HTR3E, HTR1, or CNR1; opioid efficacy and dose | Morphine (n = 830), oxycodone (n = 446), fentanyl (n = 699), or other opioids (n = 234). | None of 112 SNPs in the 25 candidate genes showed significant associations with opioid dose in both the development and the validation analyses. |
| Carbonell et al 2010 | Prospective, multicenter, case–case study | Patients hospitalized for acute upper gastrointestinal bleeding (AUGIB) related to the use of NSAIDs. A total of 131 patients had been treated with aspirin and 57 patients had been treated with an NSAID other than aspirin. | Any hospitalization for AUGIB related to NSAIDs. | CYP2C9 359Leu (CYP2C9*3) loss-of-function allele | 131 patients were treated with aspirin and 57 were treated with other types of NSAIDs. Aspirin had been given as an antiaggregant treatment (<325 mg/day) in 78 patients, including in 2 patients who were on a chronic regimen of low-dose aspirin in addition to a short course of high-dose aspirin (1 g twice a day). In the group taking non-ASP NSAIDs, 18 were on ketoprofen, 12 were on diclofenac, 11 were on ibuprofen, 10 were on piroxicam, 4 were on naproxen, 4 were on celecoxib, 1 was on flurbiprofen, 1 was on meloxicam, 1 was on tenoxicam, and 1 was on rofecoxib; 6 of these patients were taking 2 non-ASP NSAIDs concomitantly. | In the aspirin group, 12 patients (9.2%) had the CYP2C9 359Leu allele as compared with 19 (33.3%) in the non-ASP group (odds ratio (OR) = 5.0; 95% confidence interval 2.2–11.1, P < 0.0001). In a multivariate analysis, CYP2C9 359Leu remained associated with the non-ASP group (OR = 7.2 (2.6–20.3), P = 0.0002) even though 40% of these patients were under treatment with antiulcer drugs at the time of admission. |
| Garcia-Martin et al 2004 | Cohort pharmacokinetic study | 130 healthy volunteers | Plasma samples were collected at 0, 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 10, and 12 hours after administration and immediately frozen until analysis. | CYP2C8 and CYP2C9; ibuprofen clearance | All participants received a single oral dose of a solution of 400 mg racemic ibuprofen. | Ibuprofen clearance values were 4.04 L/h (95% confidence interval [CI], 3.61–4.47 L/h), 2.79 L/h (95% CI, 2.07–3.52 L/h), and 0.40 L/h (95% CI, 0.37–0.43 L/h) for carriers of CYP2C8 genotypes *1/*1, *1/*3, and *3/*3, respectively, and 4.43 L/h (95% CI, 3.94–4.92 L/h), 3.26 L/h (95% CI, 2.53–3.99 L/h), 2.91 L/h (95% CI, 1.52–4.30 L/h), 2.05 L/h (95% CI, 0–6.37 L/h), 1.83 L/h (95% CI, 1.24–2.41 L/h), and 1.13 L/h (95% CI, 0.58–1.66 L/h) for carriers of the CYP2C9 genotypes *1/*1, *1/*2, *1/*3, *2/*2, *2/*3, and *3/*3, respectively. The P values for comparison across nonmutated, heterozygous, and homozygous genotypes were as follows: P < .001 for CYP2C8*3, P < .005 for CYP2C9*2, and P < .001 for CYP2C9*3. |
| Vogl et al 2015 | Cohort pharmacokinetic study | 283 healthy young adults | The urinary metabolic ratio MR (concentration of CYP2C9-dependent metabolite divided by concentration of flurbiprofen) determined two hours after flurbiprofen administration served as phenotyping metric. | CYP2C9*1, *2, *3;metabolic ratios | 8.75 mg of flurbiprofen | Linear statistical models correlating genotype and phenotype provided highly significant allele-specific MR estimates of 0.596 for the wild type allele CYP2C9*1, 0.405 for CYP2C9*2 (68 % of wild type), and 0.113 for CYP2C9*3 (19 % of wild type). If these estimates were used for flurbiprofen dose adjustment, taking 100% for genotype *1/*1, an average reduction to 84%, 60%, 68%, 43%, and 19% would result for genotype *1/*2, *1/*3, *2/*2, *2/*3, and *3/*3, respectively. |
| Prieto-Pérez et al 2013 | Crossover pharmacokinetic trial | 24 healthy volunteers | Blood samples were collected at the following times: baseline(before receiving the drug), 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 4.5,5, 6, 7, 8, 10, 12, 24, 48, and 72 hours after administration. The maximum plasma concentration (Cmax) and the time to reach Cmax(Tmax) were the actual observed values. | CYP2C8*2, CYP2C8*3, CYP2C8*4, CYP2C9*2, and CYP2C9*3; clearance values | 200 mg single-dose celecoxib with 240 mL of water | Subjects carrying CYP2C9 *1/*3 and CYP2C9 *3/*3 had a higher AUC (2-and 7.7-fold, respectively) and Cmax (1.5-and 1.8-fold, respectively) and lower clearance (2.3-and 10-fold, respectively) than those carrying CYP2C9 *1/*1. Half-life was 2.7-fold higher in subjects with CYP2C9 *3/*3 than in those with the wild type but not in those with CYP2C9 *1/*3. |
| Lundblad et al 2006 | Open-label pharmacokinetic study | 13 healthy volunteers | On days 1 and 7, blood samples were collected before and up to 24 hours after celecoxib intake. | CYP2C9*1/*1, CYP2C9*1/*3, and CYP2C9*3/*3; drug and metabolite accumulation | Daily dose of celecoxib, 200 mg, was administered orally each morning for 7 days | A marked drug accumulation over the 7-day period was noticed in subjects genotyped as CYP2C9 *3/*3, with median trough values of 5.1 μmol/L, as compared with 0.2 and 0.3 μmol/L in subjects genotyped as CYP2C9 *1/*1 and CYP2C9*1/*3, respectively. Significantly lower levels of both metabolites were found in subjects genotyped as CYP2C9*3/*3. |
| Pilotto et al 2007 | Non-randomized, case-control study | 26 patients with endoscopically documented NSAID-related gastroduodenal bleeding lesions and 52 age-, sex- and NSAID use-matched controls with no lesions at endoscopy | N/A | CYP2C9*2 and *3; adverse events | Treatment with an NSAID that undergoes CYP2C9 metabolism | Setting the CYP2C9 *1/*1 wild type as reference, significantly higher frequencies of CYP2C9 *1/*3 (34.6% vs 5.8%; P < .001; odds ratio [OR], 12.9; 95% confidence interval [CI], 2.917–57.922) and CYP2C9*1/*2 (26.9% vs 15.4%; P = .036; OR, 3.8; 95% CI, 1.090–13.190) were identified in bleeding versus control patients, whereas no differences between bleeding and controls were observed in the distribution of CYP2C9 *2/*3 heterozygotes. |
| Kirchheiner et al 2002 | Pharmacokinetic, genetic association study | 21 healthy volunteers | Plasma samples were taken at 0,0.5, 1, 1.5, 2, 2.5, 3, 4, 5, 6, 8, 10, 24, 28, 34, and 48hours after administration. | CYP2C9*1/*1, CYP2C9*1/*2, CYP2C9*1/*3, CYP2C9*2/*2, CYP2C9*2/*3, and CYP2C9*3/*3; drug clearance | Oral dose of 600 mg racemic ibuprofen | The pharmacokinetics of racemic and of S-ibuprofen depended on the CYP2C9Leu359 polymorphism: population mean S-ibuprofen clearances were 3.25 L/h (95% confidence interval[CI], 2.84 to 3.73), 2.38 L/h (95% CI, 2.09 to 2.73), and 1.52 L/h (95% CI, 1.33 to 1.74) in carriers of the CYP2C9 genotypes*1/*1, *1/*3,and*3/*3, respectively. The CYP2C9 variant*2 exhibited no significant effect. |
| Gätke et al 2005 | Prospective, multi-center study | 58 adult patients who had previously been issued with warning cards by the Danish Cholinesterase Research Unit, requesting them and the anesthesiologist to contact the Research Unit if they were to undergo surgery | After induction of anesthesia, the ulnar nerve was stimulated supramaximally every 12 seconds using train-of-four (TOF) nerve stimulation. The evoked response from the adductor pollicis muscle was measured using mechanomyography. | A, U, and K variants of the BCHE gene; response | Patients who were homozygous for the A variant, whether linked with the K variant or not (A/A, AK/A, and AK/AK), were given 0.03 mg/kg intravenous mivacurium. Patients carrying the wild type (U/U) and patients with heterozygous occurrence of the A variant or with heterozygous or homozygous occurrence of the K variant (U/K, K/K, U/A, U/AK, and K/AK) received 0.2 mg/kg intravenous mivacurium. | Heterozygosity of the K variant prolonged the time to train-of-four 0.70 from 26.6 to 34.5 min (30%; not significant) as compared with the wild type. Heterozygosity of the K variant linked to the A variant prolonged the corresponding time from 32 to 42.7 min (33%; P 0.03) as compared with patients who were heterozygous for solely an A allele. For eight patients who were homozygous for both the A and K variants, the time to 25% recovery was 78 – 89 min as compared with 44 –57 min in patients who were homozygous for the A variant or had only one linked K variant. |
| Cerf et al 2002 | Prospective, multi-center cohort study | 36 patients from different institutions in France exhibiting a prolonged response to mivacurium or succinylcholine | Blood samples were withdrawn within 72 hours after the event except in one patient, in whom a blood sample was obtained 5 days after anesthesia. | A and U variants of the BCHE gene; response | The mivacurium or succinylcholine dose varied per each patient in the study | Thirty-two patients had a BCHE deficiency of genetic origin: 20 were homozygous (AA), 10 were heterozygous (UA) for the A variant, and 2 did not have the A mutation (UU). One heterozygous UA patient had normal BCHE activity. Nine among the heterozygous UA and the two homozygous UU patients probably carried a not-screened variant. |
| Klinger et al 2015 | Multi-center, genetic association study | 200 patient cases of malignant hyperthermia were included | N/A | RYR1 mutations of all 106 RYR1 exons and additionally for known mutations of CACNA1S; adverse events | Halothane, isoflurane and enflurane (varied per patient). | Crises triggered by enflurane had a significantly higher clinical grading scale (CGS) compared to halothane, isoflurane and sevoflurane. Of the 200 patients, 103 carried RyR1 variants, of which 14 were novel. CGS varied depending on the location of the mutation within the RYR1 gene. |
| Jensen and Viby-Mogensen 1995 | Prospective familial cohort study with purposeful sampling of individuals with abnormal clinical responses | A total of 6,688 individuals from 2,081 families were investigated. 1,247 were referred because of a suspected abnormal response to succinylcholine. | Monitoring post-succinylcholine administration | J, A, F, S, K, J, and H variant of BCHE; response | Succinylcholine 1.0–1.5 mg/kg | The time to sufficient recovery of neuromuscular function following succinylcholine 1.0–1.5 mg/kg was 15–30 min in patients heterozygous for one abnormal gene, 35–45 min in patients heterozygous for two abnormal genes and 90–180 min in patients homozygous for the atypical gene. Patients with two newly discovered genotypes (AK (5 patients) and AH (1 patient) showed slightly prolonged (20 min) and markedly prolonged (90 min) duration of action of succinylcholine, respectively. |
| Levano et al 2005 | Abnormal responder study | Nine patients with a neuromuscular block of 14 min to 5 hours | Patients were contacted 24–48 h after administration of succinylcholine. | A, F, S, H, J, K variants of BCHE; response | Succinylcholine | Seven of nine patients were mutation carriers. Five of these had more than one mutation. The A and K variants were the most frequent variations. Three of four patients who were homozygous for the A variant were also carriers of the K allele. The authors identified one novel mutation (G1294T) introducing a stop codon at amino acid position 432. The duration of neuromuscular block was substantially different between patients with identical BCHE genotypes. |
| Chung et al 2014 | Case-control, genome-wide association study with a validation cohort | 105 cases with phenytoin-related severe cutaneous adverse reactions, 78 cases with maculopapular exanthema, 130 phenytoin-tolerant control participants, and 3655 population controls from Taiwan, Japan, and Malaysia | Plasma samples of controls who received the maintenance dosage were collected within 24 hours after the last dose of phenytoin. Available samples from phenytoin-tolerant controls and patients with severe cutaneous adverse reactions were obtained before or after withdrawal of phenytoin. | GWAS was performed which is composed of 909,622 single-nucleotide polymorphisms (SNPs). | phenytoin | Direct sequencing of CYP2C identified missense variant rs1057910 (CYP2C9*3) that showed significant association with phenytoin-related severe cutaneous adverse reactions (odds ratio, 12; 95% CI, 6.6–20; P=1.1 × 10−17). A meta-analysis using the data from the 3 populations showed an overall odds ratio of 11 (95% CI, 6.2–18; z=8.58; P < .00001) for CYP2C9*3 association with phenytoin-related severe cutaneous adverse reactions. |
| Kesavan et al 2010 | Case-control, pharmacogenomic association study | 292 Tamilian patients who were taking phenytoin for the treatment of various epileptic seizures; 58 with PHT toxicity and 234 controls without toxicity | Blood samples (6 ml) for measurement of phenytoin level were obtained from all subjects within 4–14 hours after the last dose of phenytoin. | CYP2C9*1, CYP2C9*2, CYP2C9*3, CYP2C19*1, CYP2C19*2, and CYP2C19*3 alleles; adverse effects | These patients had been receiving oral phenytoin for more than 2 months and were on a stable drug regimen at the time of the clinical and drug level assessments | When risk ratios were calculated for each mutant CYP2C9 genotype separately, the adjusted odds ratio for CYP2C9*1/*3 was found to be 15.3 (95% confidence interval 5.8–40.3, P < 0.0001) for the cases compared to controls. When the four single nucleotide polymorphisms of CYP2C9 and CYP2C19 were analyzed using a haplotype approach, significant difference in the distribution of the C-C-G-G haplotype was observed between the cases and controls. |
| Depondt et al 2011 | Retrospective, candidate gene study with replication cohort | 495 patients with epilepsy | Clinical data were extracted from medical records and entered in a web-based clinical database. For each patient, the following clinical data were recorded: (i) presence or absence of any adverse drug reaction (ADR) attributed by the clinician to CBZ, sodium valproate (VPA) and phenytoin (PHT) therapy, (ii) efficacy of VPA and (iii) overall efficacy of AEDs with a major action on sodium channels. | EPHX1 and CBZ adverse drug reactions; GSS, GSR, GSTA3, GSTA4, GSTA5, GSTM3, GSTM4, UGT1A6, UGT2B7, CYP2A6, CYP2C9 and VPA adverse drug reactions and efficacy; SCN1A, SCN2A, SCN3A, SCN8Aand overall AED efficacy; CYP2C9 and PHT adverse drug reactions; GSTM1 and CBZ adverse drug reactions | phenytoin, carbamazepine, valproic acid (drug and dose varied among patients) | After correction for multiple comparisons, two associations remained significant: CYP2C9*2 and *3 alleles and PHT ADRs (Pc 0.008); and GSTM1 CNV and CBZ ADRs (Pc 0.009). Replication of the association of GSTM1 CNV with CBZ ADRs in the second patient cohort failed to show a significant association. |
| Hung et al 2012 | Case-control, candidate gene study examining pharmacokinetics and pharmacodynamics | 269 epileptic patients under maintenance phenytoin monotherapy and 190 healthy volunteer controls | Compliance was monitored over the course of the study period. | SCN1A IVS5– 91G>A (rs3812718), c.3184A>G (rs2298771), SCN2A c.56G>A (rs17183814), CYP2C9*3 (rs1057910), CYP2C19*2 (rs4244285), CYP2C19*3 (rs4986893), ABCB1 c.1236C>T (rs1128503), c.2677G>T/A (rs2032582), c.3435C>T (rs1045642), ABCC2 c.−24C>T (rs717620) and c.1249G>A (rs2273697); efficacy | Patients reached a maintenance dose for at least 1 year (phenytoin dose: 315.48 ± 86.47 mg/day; concentration: 15.13 ± 6.62 mg/l) | Results of a bivariate analysis demonstrated that among tested polymorphisms, carriers of the variant CYP2C9*3 tended to require significantly lower maintenance phenytoin dosages than wild-type carriers (p < 0.0001); on the other hand, carriers of the variants CYP2C9*3 or CYP2C19*3 revealed significantly higher concentration-dose ratio (CDR) than wild-type carriers (p < 0.004). In a further multivariate analysis, variants in SCN1A, CYP2C9, CYP2C19 and ABCB1 genes were significantly associated with CDRs of phenytoin under adjustment of age, gender and epilepsy classifications. |
| Aynacioglu et al 1999 | Mixed pharmacokinetic cohort study including healthy volunteers | 499 unrelated Turkish subjects; 280 outpatients with various trivial diagnoses and 218 healthy volunteers | Blood sample was drawn and trough levels taken 12 hours after phenytoin was administered. | Cysteine144 (CYP2C9*2 ) and leucine359 (CYP2C9*3 ); drug plasma concentration | After at least 4 hours of fasting, each subject took a 300 mg phenytoin tablet with tap water at around 23.00 hour | Mean phenytoin serum concentrations at 12 h after dosage were 4.16 mg (95% CI 3.86–4.46) in carriers of the genotype CYP2C9*1/1, 5.52 mg (4.66–6.39) in CYP2C9*1/2, and 5.65 mg (4.86–6.43) in CYP2C9*1/3. These differences were significant and accounted for 31% of total variability in phenytoin trough levels. |
| Mamiya et al 1998 | Retrospective, population-defined pharmacokinetic study | 134 Japanese adult patients with epilepsy | Serum phenytoin concentration data at steady state | CYP2C9 (Arg144/Cys, Ile359/Leu) and CYP2C19 (*1, *2 or *3), EM and PM; elimination rates | Routine treatment with oral administration of the tablet or granule of phenytoin | The mean maximal elimination rate (Vmax) was 42% lower in the heterozygote for Leu359 allele in CYP2C9, and the mean Michaelis-Menten constants (K,) in the heterozygous extensive metabolizers and the poor metabolizers of CYP2C19 were 22 and 54%, respectively, higher than those without the mutations in CYP2C9/19 genes. |
| Odani et al 1997 | Retrospective pharmacokinetic study | 44 Japanese patients with epilepsy | Most serum samples had been obtained for measurement of approximate peak levels 2 to 5 hours after dosing. | CYP2C9 (Arg144 → Cys and Ile359 → Leu) and CYP2C19 (m1 and m2); elimination rates | Phenytoin had been administered at 12-hour intervals to most patients, and the mean daily dose was 5.18 mg/kg/day phenytoin. | The maximal elimination rate (V-max) of phenytoin among patients with heterozygous wild type/Leu359 in CYP2C9 was 33% lower than that among patients with normal CYP2C9. The V-max values of phenytoin were slightly decreased (up to 14%) among patients with CYP2C19 mutations compared with patients with normal CYP2C19. |
| Inomato et al 2005 | Prospective, correlational pharmacokinetic study | 63 native Japanese patients who were scheduled for either a mastectomy or leg surgery | Blood was drawn from the indwelling arterial catheter before and at 15 and 30 minutes and 1, 2, 3, and 24 hours after administration of diazepam. | CYP2C19, EM, IM, and PM; drug plasma concentration | Received 0.1 mg/kg diazepam intravenously on entering the operating room | The PM subjects showed a larger area under the curve representing the concentration of diazepam over a 24-hour period ( P = .0259), lower clearance of diazepam (P = .0287), and longer emergence time (median, 18 minutes; 25th-75th percentile range, 13–21 minutes; P < .001) in comparison with subjects in the EM group. The IM group also showed a longer emergence time (median, 13 minutes; 25th-75th percentile range, 9–20 minutes; P < .001) and a larger variation in this parameter in comparison with the EM group. |
| Wan et al 1996 | Pharmacokinetic study | 21 healthy male Chinese subjects | 10 mL venous blood samples were collected at 0, 1, 2, 4, 8, 12, and 24 hours and 2, 3, 6, 12, 18, and 24 days after dosing. | CYP2C19, PM and EM; drug plasma concentration | A single oral dose of 5 mg diazepam. | The plasma elimination half-lives of diazepam (100.8 +/− 32.3 h) and desmethyldiazepam (219.9 +/− 62.7 h) in PMs were significantly longer than those (34.7 +/− 23.0 h for diazepam, 103.1 +/− 25.9 h for desmethyldiazepam) of the 17 phenotyped extensive metabolizers (EM), and those (30.8 +/− 24.9 h for diazepam, 103.1 +/− 27.5 h for desmethyldiazepam) of the five genotyped EMs. |
| Qin et al 1999 | Pharmacokinetic study | 18 unrelated healthy Chinese men | 10 mL venous blood samples were collected at 1, 2, 4, 8, 12, and 24 hours and then 2, 3, 6, and 12 days after administration. | CYP2C19 wild type (wt) and m1; elimination rates | A single oral dose of 5 mg diazepam with 100 mL water was given to the subjects in the morning after overnight fasting | The plasma elimination half-life values of diazepam (84.0 +/− 13.7 hours) and desmethyldiazepam (176.0 +/− 28.9 hours) in subjects of ml/ml were significantly longer than those (62.9 +/− 9.8 hours for diazepam; 132.1 +/− 24.9 hours for desmethyldiazepam; both P < .01) in subjects of wt/ml or those (20.0 +/− 10.8 hours for diazepam; 99.2.+/− 21.7 hours for desmethyldiazepam; both P < .01) in subjects of wt/wt. A significant difference in the corresponding half-life values existed between the wt/ml and wt/wt subjects (P < .01). As expected, the slowest mean clearance of diazepam was observed in the ml/ml subjects (2.8 +/− 0.9 mL/min) and the fastest in the wt/wt subjects (19.5 +/− 9.8 mL/min), with the wt/ml heterozygotes having an intermediate value (7.2 +/− 2.6 mL/min). |
UM=ultrarapid metabolizer/NM=normal metabolizer/EM=extensive metabolizer/IM=intermediate metabolizer/PM=poor metabolizer
Figure 2. Published Pharmacogenomic Articles per Perioperative Medication by FDA Approval Year.
The total number of published pharmacogenomic studies per perioperative medication are listed in order of U.S. Food and Drug Administration (FDA) approval year. Medications included in the figure are those meeting at least one of the following three criteria: (1) the medication had ≥3 separate published articles describing a positive pharmacogenomic association with the same genetic variant or gene, (2) the medication had at least 1 positive pharmacogenomic association with a given genetic variant or gene described in a journal with an impact factor of at least 10, or (3) the medication has published clinical pharmacogenomic guidance from Clinical Pharmacogenetics Implementation Consortium (CPIC), Dutch Pharmacogenetics Working Group (DPWG), and/or within its FDA label. These data suggest that varying amounts of pharmacogenomic studies have been performed on many medications relevant to the perioperative setting, regardless of FDA approval year. The 18 clinically actionable medications with pharmacogenomic evidence warranting clinical evaluation as identified through the current analysis are indicated in green. (Clinically actionable pharmacogenomic medications that have been previously already implemented for clinical delivery are shown in gray)17, 18, 20.
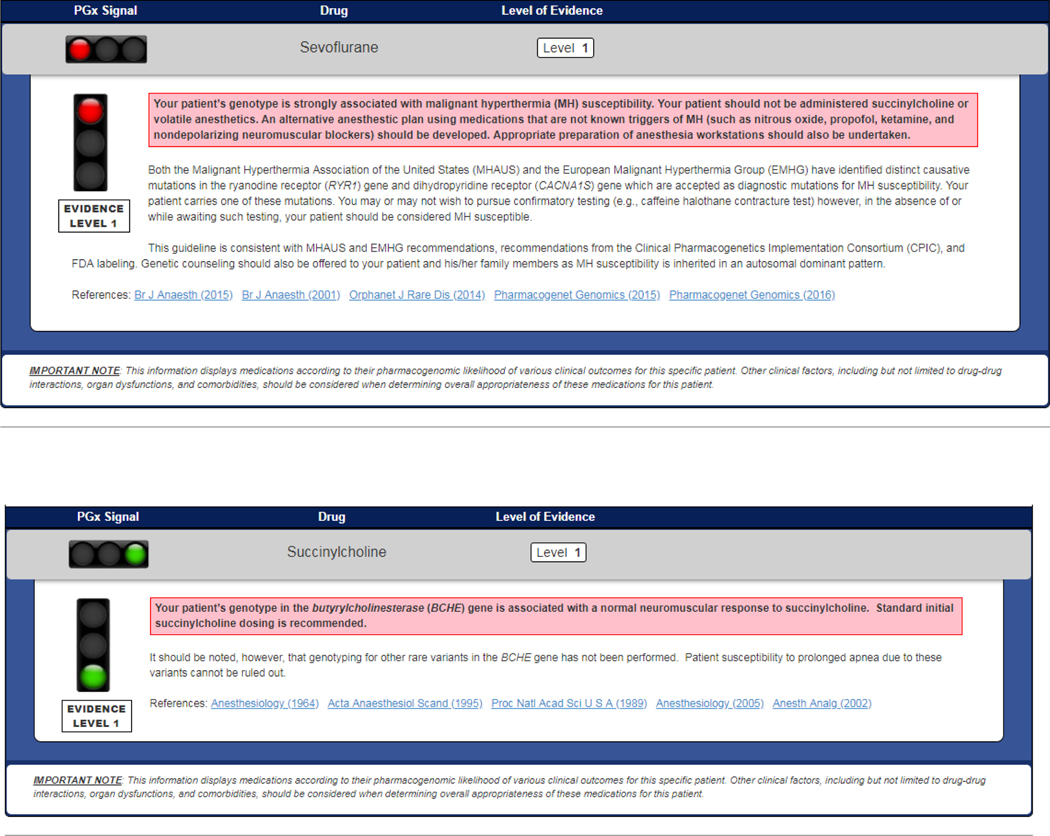
Original CDS summaries for each potential genotype associated with each potential clinical consequence were then developed for each of the 18 medications. Screen shots of genotype-specific CDS summaries for sevoflurane and succinylcholine, as examples, are shown in Figure 3. The remaining CDS summaries are available in Supplemental File 4. One composite summary was written for all of the NSAIDs (celecoxib, diclofenac, flurbiprofen, ibuprofen, and piroxicam as associated with CYP2C9), and one composite summary was written for the six anesthetics (desflurane, enflurane, halothane, isoflurane, sevoflurane, and succinylcholine as associated with RYR1 and CACNA1S mutations).
Figure 3. Clinical Decision Support Summaries for Sevoflurane and Succinylcholine.
These are examples of the clinical decision support (CDS) summaries written for sevoflurane with CACNA1S/RYR1 variants, and for succinylcholine with BCHE.
AGREE II RESULTS
Four domains were assessed for scoring the newly-developed proposed clinical summaries, with scores summed and scaled to a total percentage of the maximum possible score (100) (Table 4). For the 11 summaries encompassing the 18 potentially clinically actionable medications, the Scope & Purpose domain received an average score of 95.0±2.8 (mean ± standard deviation) (range 90.0–100), and the Rigor of Development domain scored 93.2±2.8 (range 90.0–96.7). The Clarity of Presentation domain scored an 87.3±3.0 (range 83.3–93.3), and the Applicability domain an 86.5±3.7 (81.7–91.7). The average overall quality score for all guidelines was a 6.7±0.2 (out of 7) with a range of 6.5–7.0. All potentially clinically actionable clinical summaries were unanimously recommended for implementation and thus deemed clinically actionable.
Table 4.
Pharmacogenomic Decision-Support Guideline AGREE II Scores & Recommendations for Implementation in the Perioperative Setting.
| Medication | Gene | Variants | Domains* | Overall Quality | Recommended for Implementation | |||
|---|---|---|---|---|---|---|---|---|
| Scope & Purpose | Rigor of Development | Clarity of Presentation | Applicability | |||||
| Analgesia | ||||||||
| Codeine | CYP2D6 | UM/NM/IM/PM | 92.2 | 93.3 | 84.4 | 80.0 | 6.5 | YES |
| Tramadol | CYP2D6 | UM/NM/IM/PM | 96.7 | 92.2 | 85.6 | 86.7 | 6.8 | YES |
| Oxycodone | CYP2D6 | UM/NM/IM/PM | 96.7 | 94.4 | 86.7 | 85.0 | 7.0 | YES |
| Morphine | OPRM1 | A118G | 90.0 | 91.1 | 83.3 | 85.0 | 6.5 | YES |
| Celecoxib Diclofenac Flurbiprofen Ibuprofen Piroxicam |
CYP2C9 | *3 allele | 100.0 | 96.7 | 93.3 | 91.7 | 7.0 | YES |
| Anesthesia | ||||||||
| Mivacurium | BCHE | K-variant A-variant |
93.3 | 90.0 | 87.8 | 88.3 | 6.5 | YES |
| Desflurane Enflurane Halothane Isoflurane Sevoflurane Succinylcholine |
RYR1
CACNA1S |
40 RYR1 mutations, 2 CACNA1S mutations | 93.3 | 90.0 | 90.0 | 90.0 | 6.8 | YES |
| Succinylcholine | BCHE | A-variant | 94.4 | 91.1 | 85.6 | 86.7 | 6.8 | YES |
| Antiepilepsy | ||||||||
| Phenytoin | CYP2C9 | NM/IM/PM | 96.7 | 96.7 | 90.0 | 90.0 | 7.0 | YES |
| Antianxiety | ||||||||
| Diazepam | CYP2C19 | NM/IM/PM | 96.7 | 96.7 | 86.7 | 81.7 | 6.8 | YES |
| Overall mean ± SD | 95.0±2.8 | 93.2±2.8 | 87.3±3.0 | 86.5±3.7 | 6.7±0.2 | |||
Scores in this table represent the average of the individual scores from 5 independent expert appraisers. The exception is the overall quality scores, which were calculated as the averages of the individual scores from 4 of the 5 appraisers, as 1 appraiser did not submit Overall Quality scores.
For each of the four Domains, the maximum score=100.0. For Overall Quality, the maximum score=7.0.
UM=Ultrarapid Metabolizer; NM=Normal Metabolizer; IM=Intermediate Metabolizer; PM=Poor Metabolizer
SD=standard deviation
DISCUSSION
Our study comprehensively identified high-quality replicated pharmacogenomic evidence supporting clinical actionability for 18 medications commonly-used in the perioperative setting, and we proposed and appraised for these medications CDS summaries with actionable prescribing recommendations. We thus observed a critical mass of medications for which clinically actionable pharmacogenomic associations exist. Given the large number of these medications that a patient may be exposed to when undergoing anesthesia and post-operative care, and the high stakes of perioperative drug-related morbidities2, our findings argue that these 18 medications deserve formal consideration for clinical implementation in developing pharmacogenomic programs, or for prospective testing in clinical utility evaluations/clinical trials. One such immediate evaluation—at our institution—is their deployment in our electronic medical record-linked pharmacogenomic software tool to support our recently-launched prospective clinical pharmacogenomic study which will examine clinical utility (clinicaltrials.gov NCT#03729180)32 among research subjects consenting to preemptive pharmacogenomic testing in advance of their surgery. This randomized study will evaluate the actual impact of the presence of preemptively-known pharmacogenomic results prior to anesthesia and perioperative care, and will allow examination of whether knowledge of clinically ‘actionable’ patient-specific results alters clinical outcomes like adverse events and/or non-response. As such, this current work lays the important foundation for future prospective testing of the potential clinical impact of pharmacogenomic genotyping and CDS delivery during perioperative care.
Until now, pharmacogenomic results have been infrequently utilized in anesthesia and critical care clinical settings33, 34. Barriers to clinical use not only included prior skepticism about the readiness of evidence for clinical utility examinations, but also lack of available genetic testing and reimbursement, concerns about test turnaround times, lack of integration into clinical workflows/electronic medical records, and inadequate decision support for providers unfamiliar with genomics15, 16, 35, 36. This latter point—including confusion around recommendations for many pharmacogenomic drug/gene pairs—has likely slowed the adoption of pharmacogenomic testing in anesthesia, as it has in other areas. For example, preemptive RYR1 screening is not endorsed by the Malignant Hyperthermia Association of the U.S. (MHAUS) for the general population, yet it is endorsed if there is a pre-test probability for MH-susceptibility37. Separately, CPIC guidelines clearly recommend against using triggering medications if an implicated genetic alteration in RYR1 or CACNA1S is known. Indeed, it would be difficult to find a clinician who would proceed with use of a trigger medication without at least confirmatory (e.g., contracture) testing, if a genetic alteration were known.
Recent prospective studies are beginning to address and overcome these evidence/guideline uncertainties, especially in other areas of medicine26,38–40. Of particular relevance to post-operative pain management, Smith et al. recently showed that pain scores could be improved by the use of CYP2D6-informed analgesic drug guidance in intermediate and poor metabolizer chronic pain patients41. While it is not known whether these findings would also extend to patients receiving analgesia in the post-operative setting, these data as well as those of other emerging studies32, 42 may begin to assert a mandate for genomic medicine/precision medicine considerations. Our study thus creates an evidence-driven decision-support framework to enable prospective evaluation of pharmacogenomic testing in the perioperative setting (i.e., to examine the potential clinical utility of having pharmacogenomic results for key perioperative medications in advance of a patient’s surgery date).
This study took an approach to evidence appraisal that included a recognized rubric (AGREE) for considering clinical guidelines because the ultimate goal was to synthesize current pharmacogenomic evidence into CDS summaries that could be clinically deployed, embedded within EMR clinical workflows, and applied—especially to support prospective clinical utility evaluation contexts. We importantly wanted to harmonize the ultimate CDS drug/gene recommendations (when possible) with those of other available bodies, most importantly including FDA and CPIC (the latter being the world’s best-recognized pharmacogenomic guidance body). Consistently, our guidance does harmonize, reflecting the fact that like conclusions are reached by our process as those of other consensus bodies like FDA and CPIC which reflects collective agreement about the available current evidence. Small differences, such as the list of individual NSAIDs being implicated as actionable related to CYP2C943, likely reflect our process’ more stringent requirement for existence of studies showing clinical outcomes differences, not just pharmacokinetic phenotypes. In other instances (e.g. BCHE/mivacurium and BCHE/succinylcholine as actionable in our rubric and included in the FDA labels44, 45, but without a current CPIC guideline), there is not necessarily disagreement (in fact CPIC grades this pair as “B/C”)46 but rather that this pair has not yet risen to the level of a published guideline by CPIC. It should also be noted that CPIC recently evaluated morphine/OPRM1 and acknowledged the presence of evidence for a small increase in post-operative morphine dose requirements based on genotype, but concluded that the alteration in morphine dose was “so modest as to not be clinically actionable47.” Our process and our AGREE reviewers instead chose to call this morphine dose difference – which has been repeatedly statistically associated with rs1799971 within this gene – as ‘actionable’, because clinical knowledge of pharmacogenomic information for morphine/OPRM1 at worst would be non-inferior to blinded prescribing48 and at best could benefit prescribers especially in the critical post-operative period where pain control is so essential. Finally, within the same recent CPIC guideline47, CYP2D6 and both hydrocodone and oxycodone were assessed. Similar to morphine and OPRM1, CYP2D6 and oxycodone rose to the level of clinical actionability in our rigorous analysis, though CPIC did not publish guidance for this gene-drug pair. Specifically, CPIC stated that it was “difficult to conclude” whether CYP2D6 affects analgesia or risk of toxicity for oxycodone. Despite a surface level difference in our recommendations, our CDS summaries for oxycodone, indeed, echo what has been suggested by CPIC. The oxycodone CDS (available in Supplemental File 4) states that there is a “potential” association between CYP2D6 and oxycodone analgesic effect, and the recommendation to providers is to “closely monitor”. In our synthesis, we particularly valued the evidence from a well-performed positive prospective study that was focused specifically on post-operative analgesia with oxycodone49, along with several other smaller positive supporting studies50–52. Notably, our CDS also cites the same negative studies cited by CPIC53, 54. Hence, the ultimate recommendations from both parties are harmonious. Separately, CPIC recently published prescribing recommendations for hydrocodone based on CYP2D6 phenotype, but this gene-drug association did not reach actionability through our analyses. It is noted that the ultimate CPIC recommendation for hydrocodone for CYP2D6 intermediate and poor metabolizers is “optional”, and the guidance states that there is “insufficient evidence” to determine whether the varying pharmacokinetic effects for hydrocodone observed between CYP2D6 metabolizer groups translate into decreased analgesia or adverse effects. Through our analysis, we concluded hydrocodone was not yet ready for clinical implementation via this same rationale, although we are actively and constantly evaluating emerging evidence.
Our study had limitations. It is possible that our searches may have missed articles with relevant drug/variant or drug/gene associations, although we conducted three separate searches in order to minimize this chance. It is also likely that some degree of publication bias exists, although we carefully considered both positive and negative studies. We guarded against the possibilities of false positive findings and studies with poor methodologic quality by applying rigorous criteria when evaluating each article, and by examining for independent replication of the same result across separate studies—a characteristic that was present for all of our ultimately-included, clinically actionable findings. We acknowledge, however, that candidate gene studies, most of what we uncovered in our comprehensive analysis, may bias research towards certain parts of the genome55. As more genome-wide association studies (GWAS) surrounding pharmacogenomic markers are published, we will ensure necessary updates to our CDS occur. Separately, our CDS were written with guidance to consider deviation from a “standard dose”, however, it is acknowledged that in anesthesia and perioperative analgesia many medications are typically titrated to effect. This fact will be important to keep in mind during clinical applications. An additional important consideration is that many phenotypes studied in the perioperative setting have a complex background, where both genetic and non-genetic factors interact, potentially limiting clinical utility of pharmacogenomic results. The heritability of some of these genetic factors is largely unknown, and we acknowledge the fact that patients’ genetic backgrounds may differ from the population in which pharmacogenomic studies were conducted. In the future, even more complex decision supports will likely be required to integrate multiple genetic loci, in addition to non-genetic factors. Finally, we acknowledge that the clinical guidance for some medications in our CDS is to closely monitor the patient, which should be done in the absence of genomic results as well. This calls into question the definition and use of the word “actionable”, which may be context-specific56. The idea, however, is that an “actionable” result may not require immediate action on the part of the provider. Rather, the provider may ultimately and eventually act sooner than he or she otherwise would without pharmacogenomic results.
In summary, we found that actionable pharmacogenomic evidence for perioperative medications is considerable, justifying development of evidence-integrating CDS-based implementation tools to enable future prospective investigations of the utility of pharmacogenomic information in the perioperative setting. Such subsequent work will ultimately determine the potential impact on clinical decision making and patient outcomes.
Supplementary Material
FUNDING
This work was supported by National Institutes of Health (NIH)/NIGMS 5T32GM007019-41 (for E.H.J. and T.M.T. as trainees), NIH/NHGRI 1R01HG009938-01A1 (P.H.O.), by an Innovations Grant from the University of Chicago Medicine Office of Clinical Effectiveness (P.H.O.), and the Benjamin McAllister Research Fellowship (T.M.T.).
Footnotes
COMPETING INTERESTS
Dr. Jhun is currently an employee of OneOme. Dr. Ratain is a co-inventor holding patents related to pharmacogenetic diagnostics and receives royalties related to UGT1A1 genotyping outside of this work. All other authors declared no competing interests.
REFERENCES
- 1.Zheng SL, Sun J, Wiklund F, Gao Z, Stattin P, Purcell LD, et al. Genetic variants and family history predict prostate cancer similar to prostate-specific antigen. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15(3):1105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nanji KC, Patel A, Shaikh S, Seger DL, Bates DW. Evaluation of Perioperative Medication Errors and Adverse Drug Events. Anesthesiology. 2016;124(1):25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiss AJ, Elixhauser A, Bae J, Encinosa W. Origin of Adverse Drug Events in U.S. Hospitals, 2011: Statistical Brief #158. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD) 2006. [PubMed] [Google Scholar]
- 4.Wu CL, Raja SN. Treatment of acute postoperative pain. The Lancet. 2011;377(9784):2215–25. [DOI] [PubMed] [Google Scholar]
- 5.Mavridou P, Dimitriou V, Manataki A, Arnaoutoglou E, Papadopoulos G. Patient’s anxiety and fear of anesthesia: effect of gender, age, education, and previous experience of anesthesia. A survey of 400 patients. J Anesth. 2013;27(1):104–8. [DOI] [PubMed] [Google Scholar]
- 6.Denborough MA. Malignant hyperthermia. 1962. Anesthesiology. 2008;108(1):156–7. [DOI] [PubMed] [Google Scholar]
- 7.Sangkuhl K, Dirksen RT, Alvarellos ML, Altman RB, Klein TE. PharmGKB summary: very important pharmacogene information for CACNA1S. Pharmacogenet Genomics. 2020;30(2):34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Relling MV, Evans WE. Pharmacogenomics in the clinic. Nature. 2015;526(7573):343–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pharmacogenetics Kalow W. and Anesthesia. The Journal of the American Society of Anesthesiologists. 1964;25(3):377–87. [Google Scholar]
- 10.Hopkins PM, Ruffert H, Snoeck MM, Girard T, Glahn KP, Ellis FR, et al. European Malignant Hyperthermia Group guidelines for investigation of malignant hyperthermia susceptibility. Br J Anaesth. 2015;115(4):531–9. [DOI] [PubMed] [Google Scholar]
- 11.Relling MV, Klein TE. CPIC: Clinical Pharmacogenetics Implementation Consortium of the Pharmacogenomics Research Network. Clin Pharmacol Ther. 2011;89(3):464–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Wouden CH, Cambon-Thomsen A, Cecchin E, Cheung KC, Davila-Fajardo CL, Deneer VH, et al. Implementing Pharmacogenomics in Europe: Design and Implementation Strategy of the Ubiquitous Pharmacogenomics Consortium. Clin Pharmacol Ther. 2017;101(3):341–58. [DOI] [PubMed] [Google Scholar]
- 13.Whirl-Carrillo M, McDonagh EM, Hebert JM, Gong L, Sangkuhl K, Thorn CF, et al. Pharmacogenomics knowledge for personalized medicine. Clin Pharmacol Ther. 2012;92(4):414–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swen JJ, Wilting I, de Goede AL, Grandia L, Mulder H, Touw DJ, et al. Pharmacogenetics: from bench to byte. Clin Pharmacol Ther. 2008;83(5):781–7. [DOI] [PubMed] [Google Scholar]
- 15.Borden BA, Galecki P, Wellmann R, Danahey K, Lee SM, Patrick-Miller L, et al. Assessment of provider-perceived barriers to clinical use of pharmacogenomics during participation in an institutional implementation study. Pharmacogenet Genomics. 2019;29(2):31–8. [DOI] [PubMed] [Google Scholar]
- 16.McKinnon RA, Ward MB, Sorich MJ. A critical analysis of barriers to the clinical implementation of pharmacogenomics. Ther Clin Risk Manag. 2007;3(5):751–9. [PMC free article] [PubMed] [Google Scholar]
- 17.Wellmann R, Borden BA, Danahey K, Nanda R, Polite BN, Stadler WM, et al. Analyzing the clinical actionability of germline pharmacogenomic findings in oncology. Cancer. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaufman AL, Spitz J, Jacobs M, Sorrentino M, Yuen S, Danahey K, et al. Evidence for Clinical Implementation of Pharmacogenomics in Cardiac Drugs. Mayo Clin Proc. 2015;90(6):716–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hussain S, Kenigsberg BB, Danahey K, Lee YM, Galecki PM, Ratain MJ, et al. Disease-drug database for pharmacogenomic-based prescribing. Clin Pharmacol Ther. 2016;100(2):179–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Danahey K, Borden BA, Furner B, Yukman P, Hussain S, Saner D, et al. Simplifying the use of pharmacogenomics in clinical practice: Building the genomic prescribing system. J Biomed Inform. 2017;75:110–21. [DOI] [PubMed] [Google Scholar]
- 21.Ratain MJ, Nakamura Y, Cox NJ. CYP2D6 genotype and tamoxifen activity: understanding interstudy variability in methodological quality. Clin Pharmacol Ther. 2013;94(2):185–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thorn CF, Whirl-Carrillo M, Hachad H, Johnson JA, McDonagh EM, Ratain MJ, et al. Essential Characteristics of Pharmacogenomics Study Publications. Clin Pharmacol Ther. 2019;105(1):86–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pratt VM, Del Tredici AL, Hachad H, Ji Y, Kalman LV, Scott SA, et al. Recommendations for Clinical CYP2C19 Genotyping Allele Selection: A Report of the Association for Molecular Pathology. J Mol Diagn. 2018;20(3):269–76. [DOI] [PubMed] [Google Scholar]
- 24.O’Donnell PH, Bush A, Spitz J, Danahey K, Saner D, Das S, et al. The 1200 patients project: creating a new medical model system for clinical implementation of pharmacogenomics. Clin Pharmacol Ther. 2012;92(4):446–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Donnell PH, Danahey K, Jacobs M, Wadhwa NR, Yuen S, Bush A, et al. Adoption of a clinical pharmacogenomics implementation program during outpatient care--initial results of the University of Chicago “1,200 Patients Project”. American Journal of Medical Genetics Part C, Seminars in Medical Genetics. 2014;166C(1):68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Donnell PH, Wadhwa N, Danahey K, Borden BA, Lee SM, Hall JP, et al. Pharmacogenomics-Based Point-of-Care Clinical Decision Support Significantly Alters Drug Prescribing. Clin Pharmacol Ther. 2017;102(5):859–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010;182(18):E839–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Development Collaboration A. and validation of an international appraisal instrument for assessing the quality of clinical practice guidelines: the AGREE project. Qual Saf Health Care. 2003;12(1):18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, et al. Development of the AGREE II, part 1: performance, usefulness and areas for improvement. CMAJ. 2010;182(10):1045–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, et al. Development of the AGREE II, part 2: assessment of validity of items and tools to support application. CMAJ. 2010;182(10):E472–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vlayen J, Aertgeerts B, Hannes K, Sermeus W, Ramaekers D. A systematic review of appraisal tools for clinical practice guidelines: multiple similarities and one common deficit. Int J Qual Health Care. 2005;17(3):235–42. [DOI] [PubMed] [Google Scholar]
- 32.Truong TM, Apfelbaum J, Shahul S, Anitescu M, Danahey K, Knoebel RW, et al. The ImPreSS Trial: Implementation of Point-of-Care Pharmacogenomic Decision Support in Perioperative Care. Clin Pharmacol Ther. 2019;106(6):1179–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chidambaran V, Ngamprasertwong P, Vinks AA, Sadhasivam S. Pharmacogenetics and anesthetic drugs. Curr Clin Pharmacol. 2012;7(2):78–101. [DOI] [PubMed] [Google Scholar]
- 34.Zhou S, Skaar DJ, Jacobson PA, Huang RS. Pharmacogenomics of Medications Commonly Used in the Intensive Care Unit. Front Pharmacol. 2018;9:1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lam YW. Scientific challenges and implementation barriers to translation of pharmacogenomics in clinical practice. ISRN Pharmacol. 2013;2013:641089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson JA. Pharmacogenetics in clinical practice: how far have we come and where are we going? Pharmacogenomics. 2013;14(7):835–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malignant Hyperthermia Association of the United States. www.mhaus.org.
- 38.Henricks LM, Lunenburg C, de Man FM, Meulendijks D, Frederix GWJ, Kienhuis E, et al. DPYD genotype-guided dose individualisation of fluoropyrimidine therapy in patients with cancer: a prospective safety analysis. Lancet Oncol. 2018;19(11):1459–67. [DOI] [PubMed] [Google Scholar]
- 39.Bank PCD, Swen JJ, Schaap RD, Klootwijk DB, Baak-Pablo R, Guchelaar HJ. A pilot study of the implementation of pharmacogenomic pharmacist initiated pre-emptive testing in primary care. Eur J Hum Genet. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greden JF, Parikh SV, Rothschild AJ, Thase ME, Dunlop BW, DeBattista C, et al. Impact of pharmacogenomics on clinical outcomes in major depressive disorder in the GUIDED trial: A large, patient- and rater-blinded, randomized, controlled study. J Psychiatr Res. 2019;111:59–67. [DOI] [PubMed] [Google Scholar]
- 41.Smith DM, Weitzel KW, Elsey AR, Langaee T, Gong Y, Wake DT, et al. CYP2D6-guided opioid therapy improves pain control in CYP2D6 intermediate and poor metabolizers: a pragmatic clinical trial. Genet Med. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ignite II - Genomic Medicine Knowledge Base www.gmkb.org. [Google Scholar]
- 43.Theken KN, Lee CR, Gong L, Caudle KE, Formea CM, Gaedigk A, et al. Clinical Pharmacogenetics Implementation Consortium Guideline (CPIC) for CYP2C9 and Nonsteroidal Anti-Inflammatory Drugs. Clin Pharmacol Ther. 2020;108(2):191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Food and Drug Administration. Drug Label: Mivacurium 2015. [Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/020098s018lbl.pdf.
- 45.Food and Drug Administration. Drug Label: Succinylcholine 2010. [Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/008453s027lbl.pdf.
- 46.Clinical Pharmacogenetics Implementation Consortium. Genes-Drugs. 2020. [Available from: https://cpicpgx.org/genes-drugs/. [Google Scholar]
- 47.Crews KR, Monte AA, Huddart R, Caudle KE, Kharasch ED, Gaedigk A, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for CYP2D6, OPRM1, and COMT genotype and select opioid therapy. Clin Pharmacol Ther. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Altman RB. Pharmacogenomics: “noninferiority” is sufficient for initial implementation. Clin Pharmacol Ther. 2011;89(3):348–50. [DOI] [PubMed] [Google Scholar]
- 49.Stamer UM, Zhang L, Book M, Lehmann LE, Stuber F, Musshoff F. CYP2D6 genotype dependent oxycodone metabolism in postoperative patients. PLoS One. 2013;8(3):e60239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Samer CF, Daali Y, Wagner M, Hopfgartner G, Eap CB, Rebsamen MC, et al. Genetic polymorphisms and drug interactions modulating CYP2D6 and CYP3A activities have a major effect on oxycodone analgesic efficacy and safety. Br J Pharmacol. 2010;160(4):919–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Samer CF, Daali Y, Wagner M, Hopfgartner G, Eap CB, Rebsamen MC, et al. The effects of CYP2D6 and CYP3A activities on the pharmacokinetics of immediate release oxycodone. Br J Pharmacol. 2010;160(4):907–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zwisler ST, Enggaard TP, Noehr-Jensen L, Pedersen RS, Mikkelsen S, Nielsen F, et al. The hypoalgesic effect of oxycodone in human experimental pain models in relation to the CYP2D6 oxidation polymorphism. Basic Clin Pharmacol Toxicol. 2009;104(4):335–44. [DOI] [PubMed] [Google Scholar]
- 53.Zwisler ST, Enggaard TP, Mikkelsen S, Brosen K, Sindrup SH. Impact of the CYP2D6 genotype on post-operative intravenous oxycodone analgesia. Acta Anaesthesiol Scand. 2010;54(2):232–40. [DOI] [PubMed] [Google Scholar]
- 54.Andreassen TN, Eftedal I, Klepstad P, Davies A, Bjordal K, Lundstrom S, et al. Do CYP2D6 genotypes reflect oxycodone requirements for cancer patients treated for cancer pain? A cross-sectional multicentre study. Eur J Clin Pharmacol. 2012;68(1):55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Duncan LE, Ostacher M, Ballon J. How genome-wide association studies (GWAS) made traditional candidate gene studies obsolete. Neuropsychopharmacology. 2019;44(9):1518–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hendricks-Sturrup RM, Linsky A, Lu CY, Vassy JL. Genomic testing is best integrated into clinical practice when it is actionable. Per Med. 2020;17(1):5–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.