Abstract
Characterization of two genetically distinct groups of marine Synechococcus sp. strains shows that one, but not the other, increases its phycourobilin/phycoerythrobilin chromophore ratio when growing in blue light. This ability of at least some marine Synechococcus strains to chromatically adapt may help explain their greater abundance in particular ocean environments than cyanobacteria of the genus Prochlorococcus.
The cyanobacterial community of the oceans is dominated by the small unicellular forms of the genera Synechococcus and Prochlorococcus. Prochlorococcus cells are often numerically dominant in highly oligotrophic ocean waters, while Synechococcus cells dominate in coastal waters, although the two are frequently found together (15). In some marine environments, such as the Sargasso Sea, the relative importance of each group changes seasonally, with Prochlorococcus strains dominant in the summer and Synechococcus strains dominant in the winter. It has been suggested that these two microorganisms compete for a similar ecological niche, such that the sum of the biomass of the two genera in the Sargasso Sea is relatively constant (3).
We are only just beginning to understand the genetic diversity of marine cyanobacterial populations. In the California Current off the coast of California, seven or more genetically distinct groups of Synechococcus can be identified based on DNA-dependent RNA polymerase—rpoC1—sequence data (4, 18, 19). As part of an effort to uncover the physiological differences between these groups that might reflect their ecological niches, we are examining the pigment characteristics and other properties of isolates from these groups. For example, one of these groups appears to represent strains that exhibit nonflagellar swimming motility (19).
Several cyanobacterial genera (not phylogenetically closely related to marine Synechococcus) are known to acclimate to light quality, especially green-to-red light ratios, through a process traditionally called complementary chromatic adaptation (5, 17), although this is an acclimation phenomenon. These cyanobacteria make more of the light-harvesting protein phycocyanin in red light and more of the protein phycoerythrin in green light. Photoreceptors similar to plant phytochromes may control this process in cyanobacteria (7). Interestingly, because of their widespread use of PUB (phycourobilin) as a light-harvesting chromophore in the protein phycoerythrin, it has been suggested that marine Synechococcus strains are evolutionarily adapted to the blue light that dominates the light field of the open oceans (26). The ratio of PUB to the chromophore PEB (phycoerythrobilin, a chromophore found mostly in phycoerythrin but potentially also in phycocyanin) has been thought to be constant for marine Synechococcus strains, and it has been reported that strains do not alter their PUB/PEB ratio in response to light quality (1, 20). Thus, it has been thought that this major group of primary producers does not acclimate to light quality on physiological time scales (hours to days) through complementary chromatic adaptation.
The PUB/PEB ratio of Synechococcus cultures (19, 27) and environmental samples (8, 28) can be readily measured using fluorescence excitation spectra, using emission at 570 nm from the terminal PEB energy acceptor of phycoerythrin. The peak at approximately 495 nm represents excitation of PUB, and the peak at approximately 545 nm represents excitation of PEB. When the PUB/PEB ratios of cultures grown under different light conditions were measured, it was found, as shown below, that these ratios changed in response to light quality for some recently reported strains of marine Synechococcus but not for others.
All strains except WH8020, WH8103, and WH8113 were grown at 17°C in f/4 (without silica) nutrient-enriched sterile seawater medium (6) with additional 10 μM EDTA. In some cases, 100 μM ammonia or urea was provided in the absence of nitrate. For the strains WH8020, WH8103, and WH8113 which did not grow well in f/4, SN medium was used (21). White light was provided during growth by 40-W cool white fluorescent bulbs and neutral density screening. Blue light (0.12 × 1016 quanta sec−1 cm−2) or green light (0.04 × 1016 quanta sec−1 cm−2) was provided by 500-W halogen lamps and blue (no. 862; maximum transmission at 450 nm) or green (no. 874; maximum transmission at 520 nm) filters from Edmund Scientific. Spectroscopic methods are described in more detail elsewhere (19). In Table 1, experiments analyzing live whole cells are reported, regardless of the nitrogen source.
TABLE 1.
Range of PUB/PEB ratios measured using whole-cell fluorescence excitation spectra of Synechococcus strains
| Groupa | Strain | PUB/PEB ratio (no. of cultures analyzed)b
|
|
|---|---|---|---|
| White light | Blue light | ||
| 1 | CC9311 | 0.66–0.71 (6) | 0.96–1.8 (5) |
| 1 | CC9617 | 0.75 (1) | 1.8 (1) |
| 1 | WH8020 | 0.7 (1) | 1.7–1.9 (2) |
| 5 | CC9317 | 1.8–1.9 (4) | 1.7–2.0 (2) |
| 5 | CC9318 | 1.8–1.9 (4) | 1.7–2.0 (2) |
| 5 | CC9305-3 | 1.7–1.9 (5) | 1.7–1.8 (3) |
| 3 | WH8103 | 1.2 (1) | 1.1 (1) |
| 3 | WH8113 | 1.0 (1) | 1.8–1.9 (2) |
Groups are defined by Ferris and Palenik (4).
The range of measured ratios (Ex 495:545) is reported. Results of all experiments are reported, regardless of the nitrogen source.
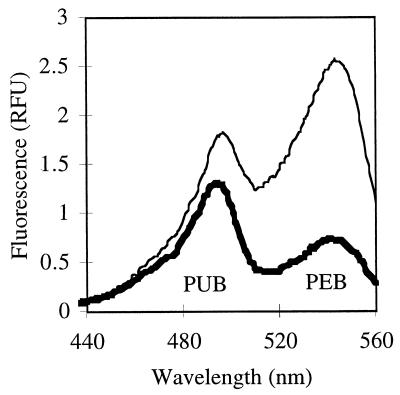
For a genetically related cluster of strains with high PUB/PEB ratios (CC9317, CC9318, and CC9305-3) previously isolated from one site in the California Current (18) and grown under white light, the ratio of the first excitation peak to the second (excitation [Ex] 495:545) was typically 1.8 (Table 1). In contrast, a strain from the same site but from a different genetic cluster of strains, CC9311, had a lower PUB/PEB ratio, with an Ex 495:545 ratio of 0.7 under white or green light (Table 1; Fig. 1).
FIG. 1.
Fluorescence excitation spectra (emission at 570 nm) for Synechococcus strain CC9311 culture grown under white (thin line) or blue (thick line) light. The ratio of the two chromophores changes under different light conditions.
When different strains were grown under blue light (0.12 × 1016 quanta sec−1 cm−2), cells from the first group (CC9317, CC9318, and CC9305-3) did not significantly change their PUB/PEB ratio (Table 1). However, CC9311 increased its PUB/PEB ratio such that the Ex 495:545 was measured at 0.96 to 1.8 (Fig. 1; Table 1). The measured ratio was later shown to depend on the acclimation time, with longer times resulting in higher ratios and incomplete acclimation times (shorter than a week) giving lower ratios (data not shown, but see Fig. 2). To emphasize this point we have reported all data in Table 1. Similar differences in the PUB/PEB ratio for cells of CC9311 grown in blue and white light were also seen using UV-Vis absorbance spectra of whole cells (data not shown).
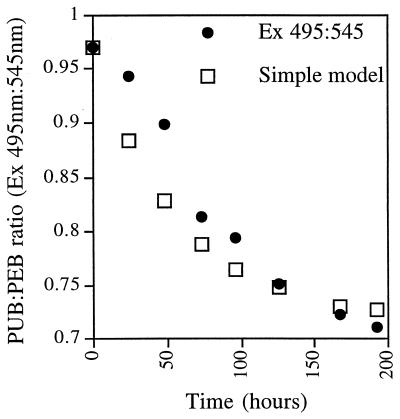
FIG. 2.
Kinetics of adaptation of the PUB/PEB ratio (Ex 495:545) after transfer of a Synechococcus strain CC9311 culture from blue light to green light. Green light was chosen to see if cells would reach a PUB/PEB ratio lower than 0.7. Cells adapted more slowly than was predicted by a simple dilution model in which existing phycoerythrin with the original PUB/PEB ratio was diluted with newly synthesized phycoerythrin that had the final PUB/PEB ratio.
To investigate the trigger for PUB/PEB ratio changes, CC9311 was grown at three white light levels (0.06 × 1016, 0.18 × 1016, and 0.33 × 1016 quanta sec−1 cm−2), resulting in Ex 495:545 ratios of 0.69, 0.66, and 0.71, respectively. The lowest white light intensity was lower than the light intensity for growth under the blue light conditions described above. In addition, the Ex 495:545 ratio for CC9311 during white light growth (0.18 × 1016 quanta sec−1 cm−2) under conditions of ammonia, nitrate, or nitrogen starvation remained about 0.7 (0.66, 0.66, and 0.71, respectively) Thus, these strains did not change their PUB/PEB ratio in response to low light intensity or nitrogen nutrition, only in response to light quality.
A CC9311 culture growing under blue light was transferred to green light. Aliquots were removed daily and fixed with 0.25% glutaraldehyde (in contrast to other experiments) and frozen for later analysis of cell number, using flow cytometry (FACSORT; Becton Dickinson) and fluorescence. Fixation does not significantly change PUB/PEB ratios (data not shown). Green light was chosen in order to push the culture to its lowest PUB/PEB ratio. This could have been low in the absence of PUB, since strains without PUB show an Ex 495:545 ratio of 0.2 (data not shown). However, the ratio under green light was still found to be about 0.7 (Fig. 2).
The transition to the new ratio was slower than a model curve for which it was assumed that the cells instantly began making chromophores at the new ratio (Ex 495:545 of 0.71) but retained any preexisting chromophores at the starting ratio (Ex 495:545 of 0.97 in this experiment). The growth rate of the culture under green light was 0.015 h−1 (one doubling per 46 h). It was assumed that the phycoerythrin content per cell was roughly constant. We did not measure the amount of phycoerythrin per cell, but this may have increased slightly according to the fluorescence excitation spectra normalized to the cell number. If this were the case, the predicted model curve would reach 0.71 faster than the model curve of Fig. 2.
The conclusion from this kinetics experiment is that the cells did not adapt rapidly to ambient light quality. Synechococcus cells likely average light intensity and quality over more than 1 day. An examination of the factors influencing the rate of chromatic adaptation, such as the growth rate, nutrient status, light quality and intensity, or temperature, will be needed to relate this physiological process to water column mixing rates in marine environments.
Several other strains have been screened for chromatic adaptation. Strain CC9617 was tested, since it was previously found to be phylogenetically related to CC9311 (Fig. 3). WH8020, a model strain whose phycobilisome has been characterized genetically and biochemically by Glazer and colleagues (14, 23, 24, 25), was also tested. Under blue light conditions as described above, CC9617 and WH8020 increased their PUB/PEB ratios (Table 1).
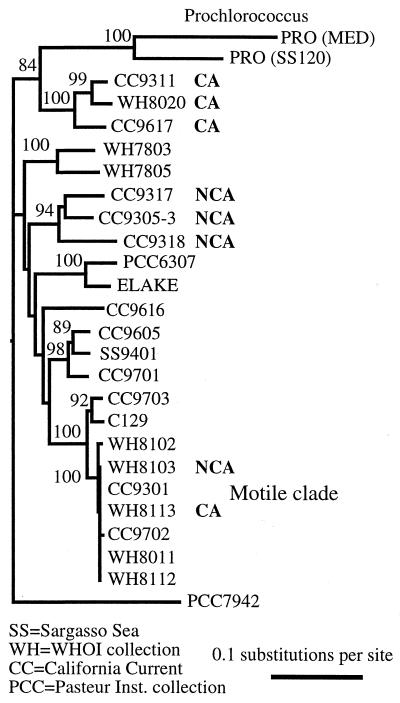
FIG. 3.
Phylogenetic tree of marine Synechococcus isolates based on sequence data from a 612-bp fragment of RNA polymerase (rpoCl). CA indicates chromatic adaptation (an increased PUB/PEB ratio) in blue light relative to white light conditions. NCA indicates no chromatic adaptation. The tree suggests that some but not all clades of marine Synechococcus may have evolved or maintained the ability to chromatically adapt in order to better compete in changing environmental conditions. Numbers at the nodes are the numbers of times out of 100 bootstrap trees that the taxa grouped together.
Because it chromatically adapted, we sequenced a fragment of the rpoCl gene from strain WH8020 in order to examine its relatedness to CC9311 and CC9617. Sequence data were obtained, and phylogenetic trees were constructed using Neighbor Joining phylogenetic analysis with Jukes-Cantor distances as described previously (19). GenBank nucleotide sequence accession numbers for strains listed in Fig. 3 are as follows: for PRO-MED, accession no. Z11159; for PRO-SS120, Z11160; for CC9311, AF013607; for WH8020, AF323594; for CC9617, AF154562; for WH7803, L34061; for WH7805, L34062; for CC9317, AF013609; for CC9305-3, AF013610; for CC9318, AF013608; for PCC6307, U52342; for ELAKE, U52343; for CC9616, AF154561; for CC9605, AF154560; for SS9401, AF154563; for CC9701, AF155131; for CC9703, AF153338; for C129, AF153339; for WH8102, AF153336; for WH8103, L34063; for CC9301, AF153332; for WH8113, AF153335; for CC9702, AF153337; for WH8011, AF153334; for WH8112, AF153333; and for CC7942, Z11155.
Despite being isolated at different times from different oceans, strains CC9311 (isolated in 1993 from the California Current), CC9617 (isolated in 1996 from the California Current), and WH8020 (isolated in 1980 from water near Woods Hole, Mass.) are phylogenetically related with high bootstrap values (Fig. 3). This clade thus appears to have evolved or maintained an ability to respond to changing light conditions in comparison to the clade containing strains CC9317, CC9318, and CC9305-3 observed in the same water samples. Interestingly, we found the cluster of strains containing CC9311 and CC9617 (designated clade 1) in rpoC1 PCR clone libraries when DNA was obtained from a California Current site influenced by coastal water or recent mixing as indicated by a shallower than usual depth in maximum chlorophyll concentration. These strains were not found in clone libraries when the site showed typical highly stratified oligotrophic conditions (4).
Results using the same blue light conditions suggested that some of the motile Synechococcus strains that were recently found to form a phylogenetic cluster (19) may perform this kind of chromatic adaptation (Table 1). Strain WH8113 but not WH8103 increased its PUB/PEB ratio in blue light. Thus, in contrast to the previous two clades, this clade contains adapters and nonadapters. This may be due to microspeciation within the motile clade in response to environmental conditions. Two motile strains (WH8113 and WH8112) had been found to alter their PUB/PEB ratios somewhat in response to light intensity (20). Thus, for WH8113 and the motile strains in general, the effects of light intensity and/or nutrient conditions as triggers of PUB/PEB acclimation need further study.
All marine Synechococcus strains examined to date, including strain WH8020, have a phycobilisome containing two phycoerythrin proteins (PEI and PEII) with different PUB/PEB ratios (14). Differential expression of the two phycoerythrin proteins could explain the phenomenon reported here. For example, the PEII protein, containing higher levels of PUB, would be expressed at higher levels under blue light. Alternatively, it is possible that the PUB/PEB ratio could be changed by enzymatically converting PEB to PUB on existing proteins as has been proposed for light intensity effects on the PUB/PEB ratio in the nitrogen-fixing cyanobacterium Trichodesmium (16).
Several observations have been made of changes in the PUB/PEB ratio in relation to depth in the water column. These observations have been made by flow cytometery (13) and by fluorometry with natural samples (8, 28). Fluorometrically measured PUB/PEB ratios of 0.66 (surface) to 1.08 (66 m) have been found in the Arabian Sea (28). In mesotrophic sites in the tropical northeastern Atlantic, ratios varied from 0.53 (surface) to 1.33 (60 m) (8). These ratio changes are similar to the changes found in Table 1. Typically the explanation for these observations has been the presence of distinct strains with different PUB/PEB ratios, but the process of chromatic adaptation reported here is equally likely.
Synechococcus strains are as abundant (or more so) than Prochlorococcus strains during the winter mixed period in the Northern Sargasso Sea but are not as successful during the summer (12). A similar seasonal cycle of Synechococcus and Prochlorococcus abundance is found in the Red Sea (9). In the Arabian Sea, which experiences large dramatic seasonal mixing due to monsoon cycles, Synechococcus strains are remarkably abundant relative to Prochlorococcus strains during the Southwest and Northeast Monsoon periods (2). The current set of Prochlorococcus isolates seems to lack chromatic adaptation, and this genus appears to have speciated into high- and low-light clades (4, 10, 11, 15, 22). Although several water column properties change during mixing, Synechococcus cells possessing the ability to chromatically adapt to ambient light intensity and quality during mixed conditions in either coastal or open ocean regimes will do better than Prochlorococcus cells finding themselves in their suboptimal light niche, such as high-light-clade cells at low light and low-light-clade cells at toxic light levels. Thus the ability to chromatically adapt may help explain the global comparative distributions of strains of Synechococcus and Prochlorococcus, the two major unicellular marine cyanobacterial genera.
Acknowledgments
This work was funded by the Biological Oceanography program of the U.S. National Science Foundation (OCE9633111).
Synechococcus strain CC9617 was isolated and provided by G. Toledo (SIO). WH8020 was provided by J. Waterbury (WHOI). R. Chastain (SIO) helped with the rpoCl sequence determination. I also thank V. Vacquier (SIO) for use of the spectrofluorometer and B. Brahamsha (SIO) and several anonymous reviewers for comments on the manuscript.
REFERENCES
- 1.Alberte R S, Wood A M, Kursar T A, Guillard R R L. Novel phycoerythrins in marine Synechococcus spp. Plant Physiol. 1984;75:732–739. doi: 10.1104/pp.75.3.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campbell L, Landry M R, Constantinou J, Nolla H A, Brown S L, Liu H, Caron D A. Response of microbial community structure to environmental forcing in the Arabian Sea. Deep-Sea Res II. 1998;45:2301–2325. [Google Scholar]
- 3.Chisholm S W. Phytoplankton size. In: Falkowski P G, Woodhead A D, editors. Primary productivity and biogeochemical cycles. New York, N.Y: Plenum Press; 1992. pp. 213–237. [Google Scholar]
- 4.Ferris M J, Palenik B. Niche adaptation in ocean cyanobacteria. Nature. 1998;396:226–228. [Google Scholar]
- 5.Grossman A R, Schaefer M R, Chiang G G, Collier J L. The responses of cyanobacteria to environmental conditions: light and nutrients. In: Bryant D A, editor. The molecular biology of cyanobacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 641–675. [Google Scholar]
- 6.Guillard R R L. Culture of phytoplankton for feeding marine invertebrates. In: Smith W L, Chanley M H, editors. Culture of marine invertebrate animals. New York, N.Y: Plenum; 1975. pp. 29–60. [Google Scholar]
- 7.Kehoe D M, Grossman A R. Similarity of a chromatic adaptation sensor to phytochrome and ethylene receptors. Science. 1996;273:1409–1412. doi: 10.1126/science.273.5280.1409. [DOI] [PubMed] [Google Scholar]
- 8.Lantoine F, Neveux J. Spatial and seasonal variations in abundance and spectral characteristics of phycoerythrins in the tropical northeastern Atlantic Ocean. Deep-Sea Res I. 1997;44:223–246. [Google Scholar]
- 9.Lindell D, Post A F. Ultraphytoplankton succession is triggered by deep winter mixing in the Gulf of Aqaba (Eilat), Red Sea. Limnol Oceanogr. 1995;40:1130–1141. [Google Scholar]
- 10.Moore L R, Goericke R, Chisholm S W. Comparative physiology of Synechococcus and Prochlorococcus—influence of light and temperature on growth, pigments, fluorescence and absorptive properties. Mar Eco Prog Ser. 1995;116:259–275. [Google Scholar]
- 11.Moore L R, Rocap G, Chisholm S W. Physiology and molecular phylogeny of coexisting Prochlorococcus ecotypes. Nature. 1998;393:464–467. doi: 10.1038/30965. [DOI] [PubMed] [Google Scholar]
- 12.Olson R J, Chisholm S W, Zettler E R, Altabet M A, Dusenberry J A. Spatial and temporal distributions of prochlorophyte picoplankton in the North Atlantic Ocean. Deep-Sea Res. 1990;37:1033–1051. [Google Scholar]
- 13.Olson R J, Chisholm S W, Zettler E R, Armbrust E V. Analysis of Synechococcus pigment types in the sea using single and dual beam flow cytometry. Deep-Sea Res. 1988;35:425–440. [Google Scholar]
- 14.Ong L J, Glazer A N. Phycoerythrins of marine unicellular cyanobacteria. J Biol Chem. 1991;266:9515–9527. [PubMed] [Google Scholar]
- 15.Partensky F, Hess W R, Vaulot D. Prochlorococcus, a marine photosynthetic prokaryote of global significance. Microbiol Mol Biol Rev. 1999;63:106–127. doi: 10.1128/mmbr.63.1.106-127.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Subramaniam A, Carpenter E J, Karentz D, Falkowski P G. Bio-optical properties of the marine diazotroph cyanobacteria Trichodesmium spp. I. Absorption and photosynthetic action spectra. Limnol Oceanogr. 1999;44:608–617. [Google Scholar]
- 17.Tandeau de Marsac N. Occurrence and nature of chromatic adaption in cyanobacteria. J Bacteriol. 1977;130:82–91. doi: 10.1128/jb.130.1.82-91.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toledo G, Palenik B. Synechococcus diversity in the California Current as seen by RNA polymerase (rpoC1) gene sequences of isolated strains. Appl Environ Microbiol. 1997;63:4298–4303. doi: 10.1128/aem.63.11.4298-4303.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toledo G, Palenik B, Brahamsha B. Swimming strains of marine Synechococcus with widely different photosynthetic pigment ratios form a monophyletic group. Appl Environ Microbiol. 1999;65:5247–5251. doi: 10.1128/aem.65.12.5247-5251.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waterbury J B, Watson F W, Valois F W, Franks D G. Biological and ecological characterization of the marine unicellular cyanobacterium Synechococcus. In: Platt T, Li W K W, editors. Photosynthetic picoplankton. Ottawa: Canadian Department of Fisheries and Oceans; 1986. pp. 71–120. [Google Scholar]
- 21.Waterbury J B, Willey J M. Isolation and growth of marine planktonic cyanobacteria. In: Packer L, Glazer A N, editors. Methods in enzymology—cyanobacteria. San Diego, Calif: Academic Press; 1988. pp. 100–105. [Google Scholar]
- 22.West N J, Scanlan D J. Niche-partitioning of Prochlorococcus populations in a stratified water column in the eastern North Atlantic Ocean. Appl Environ Microbiol. 1999;65:2585–2591. doi: 10.1128/aem.65.6.2585-2591.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilbanks S M, de Lorimier R, Glazer A N. Phycoerythrins of marine unicellular cyanobacteria III. J Biol Chem. 1991;266:9535–9539. [PubMed] [Google Scholar]
- 24.Wilbanks S M, Glazer A N. Rod structure of a phycoerythrin II-containing phycobilisome. I. Organization and sequence of the gene cluster encoding the major phycobiliprotein rod components in the genome of marine Synechococcus sp. WH8020. J Biol Chem. 1993;268:1226–1235. [PubMed] [Google Scholar]
- 25.Wilbanks S M, Glazer A N. Rod structure of a phycoerythrin II-containing phycobilisome. II. Complete sequence and bilin attachment site of a phycoerythrin gamma subunit. J Biol Chem. 1993;268:1236–1241. [PubMed] [Google Scholar]
- 26.Wood A M. Adaptation of photosynthetic apparatus of marine ultraphytoplankton to natural light fields. Nature. 1985;316:253–255. [Google Scholar]
- 27.Wood A M, Horan P K, Muirhead K, Phinney D A, Yentsch C M, Waterbury J B. Discrimination between types of pigments in marine Synechococcus spp. by scanning spectroscopy, epifluorescence microscopy, and flow cytometry. Limnol Oceanogr. 1985;30:1303–1315. [Google Scholar]
- 28.Wood A M, Lipsen M, Coble P. Fluorescence-based characterization of phycoerythrin-containing cyanobacterial communities in the Arabian Sea during the Northeast and early Southwest Monsoon (1994–1995) Deep-Sea Res II. 1999;46:1769–1790. [Google Scholar]