Abstract
Osteoarthritis (OA) is an age-related disorder that affects the joints and causes functional disability. Hericium erinaceus is a large edible mushroom with several known medicinal functions. However, the therapeutic effects of H. erinaceus in OA are unknown. In this study, data from Sprague-Dawley rats with knee OA induced by anterior cruciate ligament transection (ACLT) indicated that H. erinaceus mycelium improves ACLT-induced weight-bearing asymmetry and minimizes pain. ACLT-induced increases in articular cartilage degradation and bone erosion were significantly reduced by treatment with H. erinaceus mycelium. In addition, H. erinaceus mycelium reduced the synthesis of proinflammatory cytokines interleukin-1β and tumor necrosis factor-α in OA cartilage and synovium. H. erinaceus mycelium shows promise as a functional food in the treatment of OA.
Keywords: osteoarthritis, Hericium erinaceus, mycelium, anterior cruciate ligament transection, interleukin 1 beta, tumor necrosis factor-alpha
1. Introduction
Osteoarthritis (OA) is an age-related disorder that affects the joints and causes functional disability [1]. During OA development, low-grade inflammatory reactions progressively degrade the joints [2]. Typical OA symptoms include joint swelling and deformities that are associated with constant pain and consequent interference with normal daily life activities [2]. Around 80% of OA patients face movement disorders, 20% cannot perform basic activities and 10% require daily care [3]. The ever-growing numbers of elderly people worldwide are confounding the already large healthcare and economic burdens imposed by patients with OA [2,4].
Two major proinflammatory cytokines, interleukin 1 beta (IL-1β) and tumor necrosis factor-alpha (TNF-α), facilitate the development of OA by increasing catabolic enzyme formation that degrades the cartilage extracellular matrix [5,6]. Levels of IL-1β and TNF-α expression are higher in human OA serum and synovial fluid than in samples from healthy individuals [7,8], and they are targeted by therapies such as the anti-IL-1β antibody canakinumab and the TNF-α-blocking agent adalimumab [7]. Inhibiting proinflammatory cytokine expression successfully inhibits OA progression [5,9].
Nonsteroidal anti-inflammatory drugs (NSAIDs) and corticosteroids are commonly applied to inhibit ongoing inflammation and reduce the pain associated with OA [10,11]. However, the undesirable side effects of these synthetic agents make the discovery of anti-OA ingredients from natural products an attractive proposition. Hericium erinaceus is a large edible mushroom that is popularly consumed in Asian countries and is accepted as a dietary supplement or functional food [12,13]. H. erinaceus is rich in bioactive compounds including glycoproteins, polysaccharides and ketones [14]. In addition, the mushroom fruiting bodies, mycelium and bioactive pure compounds of H. erinaceus exhibit several medicinal functions including antitumor, anti-inflammatory, nephroprotective, neuroprotective effects, antimicrobial, antioxidant, immunomodulatory and antihyperglycemic properties [12,15,16,17,18,19,20]. A standardized extract containing H. erinaceus (Bull.) Persoon, Kalopanax pictus Castor-Aralia and Astragalus membranaceus Schischkin has shown in vitro and in vivo chondroprotective effects in OA models [21]. However, the therapeutic effects of H. erinaceus in human OA remain unknown. Here, we found that H. erinaceus mycelium prevents disease progression in an anterior cruciate ligament transection (ACLT) model of OA, suggesting that H. erinaceus mycelium has therapeutic utility for OA.
2. Materials and Methods
2.1. Preparation of Hericium erinaceus Mycelium
The Bioresource Collection and Research Center (BCRC, Food Industry Research and Development Institute, Hsinchu, Taiwan) supplied H. erinaceus mycelium (BCRC strain no. 35669) [22]. The strain was first grown in a potato dextrose agar plate at 25 °C for 15 days. The H. erinaceus mycelium cultures were transferred to 1.3 L of liquid medium in 2 L flasks and cultured for five days at 25 °C in a shaking incubator at 120 rpm. The cultures were scaled-up to a 500-L bioreactor for a further five days, then to a 20-ton fermenter for 12 days, under the same conditions described above. The culture liquid medium used for scaling-up was adjusted to pH 4.5 and contained 4.5% glucose, 0.5% soybean powder, 0.25% yeast extract, 0.25% peptone and 0.05% MgSO4. Finally, H. erinaceus mycelium from the 20-ton fermentation process were harvested, lyophilized and ground into powder. The dosage of H. erinaceus mycelium applied in the OA animal model was equivalent to a 60 kg adult consuming 1 g of H. erinaceus powder daily.
2.2. Anterior Cruciate Ligament Transection (ACLT) Animal Model
Male Sprague-Dawley (SD) rats (eight weeks of age; 300–350 g) were purchased from the National Laboratory Animal Center (Taipei, Taiwan) and randomly divided into three groups: sham surgery (controls), ACLT only and ACLT with H. erinaceus (100 mg/kg). ACLT surgery was performed according to the procedure mentioned in our previous reports [6,23]. Briefly, the rats were anesthetized and underwent arthrotomy to expose the right knee joint, and the ACL was severed. Controls underwent arthrotomy only. Two days after surgery, the rats started to receive H. erinaceus mycelium. The static weight-bearing incapacitance test (Bioseb, Paris, France) evaluated spontaneous pain after ACLT, as previously described [24]. The left and right hind limbs were placed on separate sensor plates to measure between-limb differences in dynamic weight bearing (expressed as g) over a 10-s period. The mean score of three consecutive measurements was recorded for each animal on every test day.
2.3. Micro-Computed Tomography (μ-CT) Measurements
Micro-CT analysis was performed at six weeks after ACLT surgery. The rats were sacrificed and the right knee joints were collected and fixed in 4% formaldehyde and then 70% ethanol at room temperature, as previously described [17,25]. The knee joints were scanned by a SkyScan 2211 micro-CT system (Bruker, Kontich, Belgium), using a voxel resolution of 10.5 µm over 180° of rotation, a voltage of 70 kVp, a current of 290 µA and a 0.5-mm aluminum filter to prevent beam-hardening artifacts. Image reconstruction of coronal and transverse images used InstaRecon® software (Bruker micro-CT, Kontich, Belgium). Reconstructed cross-sections were reorientated and 59 slices (0.5 mm) were selected, then manual regions of interest (ROI) were drawn. Bone mineral density (BMD), bone mineral content (BMC), bone volume/total volume (BV/TV), bone surface/total volume (BS/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N) and trabecular separation (Tb.Sp) were analyzed by Bruker micro-CT software (CTAn, version 1.7.1, Bruker, Kontich, Belgium), as previously detailed [26,27].
2.4. Immunohistochemistry (IHC)
The right knee joints were decalcified in 10% EDTA of phosphate-buffered saline for two weeks after μ-CT scanning. The knee samples were then dehydrated with ethanol (from 70% to 100%) and embedded in paraffin blocks to prepare slices of 5-µm thicknesses. Hematoxylin & Eosin (H&E) and Safranin-O/Fast Green staining enabled us to analyze histopathological changes under an optical microscope, as previously described [27,28]. For analysis of IL-1β and TNF-α expression, the tissue sections were stained with primary antibodies against IL-1β or TNF-α (GeneTex; Hsinchu, Taiwan) at 4 °C overnight, followed by incubation with secondary antibody (1:200) at room temperature for 1 h. The sections were stained with diaminobenzidine and observed under a light microscope, as previously described [29,30]. The sum of the intensity and percentage scores was used as the final staining score [25].
2.5. Statistical Analysis
All values are given as the mean ± standard deviation (SD). The statistical calculations were analyzed by using PRISM 5.0 software (GraphPad, San Diego, CA, USA). The paired sample t-test was selected to compare results from two groups. One-way ANOVA followed by Bonferroni post-hoc testing for multiple comparisons was used to analyze more than two groups. Student’s t-test assessed between-group differences. A p-value of <0.05 was considered statistically significant.
3. Results
3.1. H. erinaceus Mycelium Reduces ACLT-Induced Weight-Bearing Asymmetry and Pain
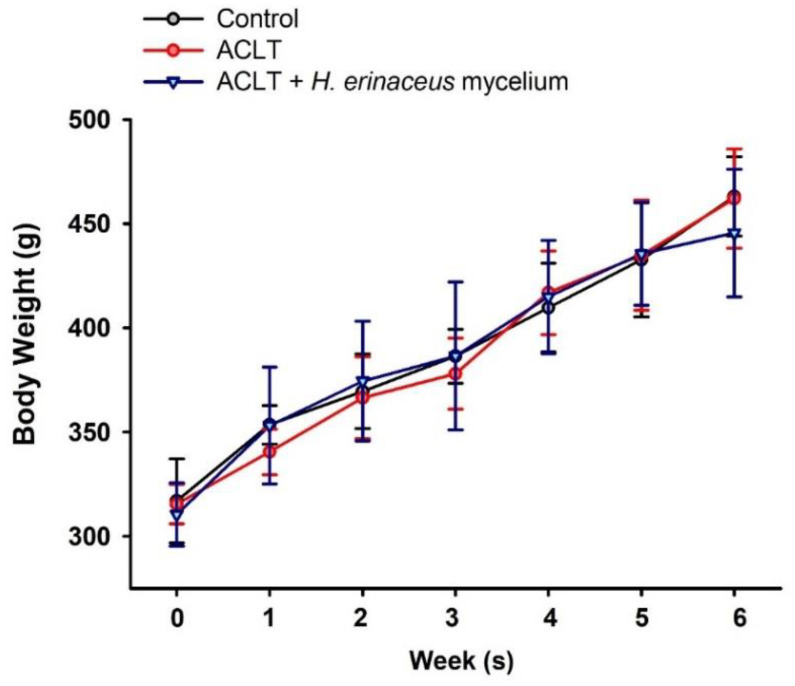
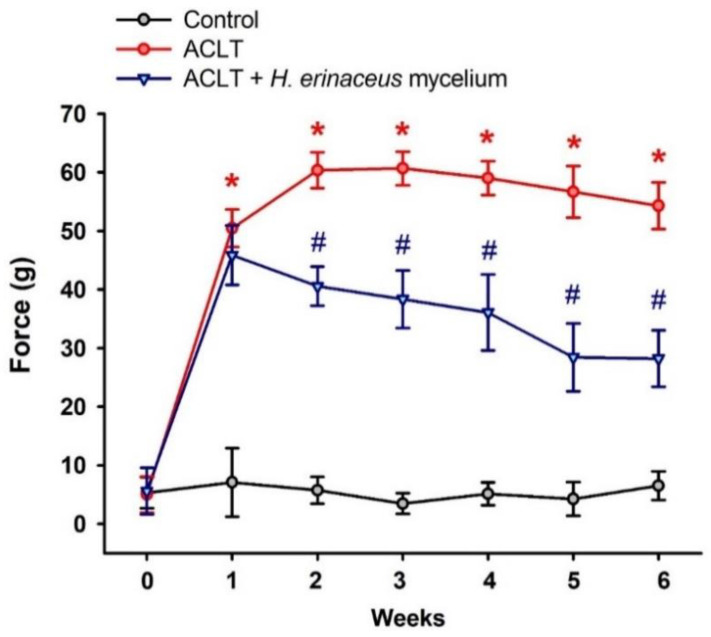
We examined the effects of H. erinaceus mycelium in a rat model of ACLT [6,23]. As shown in Figure 1, no changes in body weight were observed in the ACLT-only and ACLT + H. erinaceus groups. At six weeks, rats fed with H. erinaceus mycelium exhibited significant improvements in ACLT-induced weight-bearing asymmetry and pain compared to the ACLT-only group (Figure 2).
Figure 1.
Body weight gain during the experimental period. Body weight was measured during the experimental period. (n = 6 for each group).
Figure 2.
H. erinaceus mycelium improves ACLT-induced weight-bearing asymmetry. Deficits in weight-bearing forces were examined every week by weight-bearing behavioral testing (n = 6 for each group). * p < 0.05 compared to the control group; # p < 0.05 compared to the ACLT-only group.
3.2. H. erinaceus Mycelium Improves Bone and Cartilage Architecture in ACLT Rats
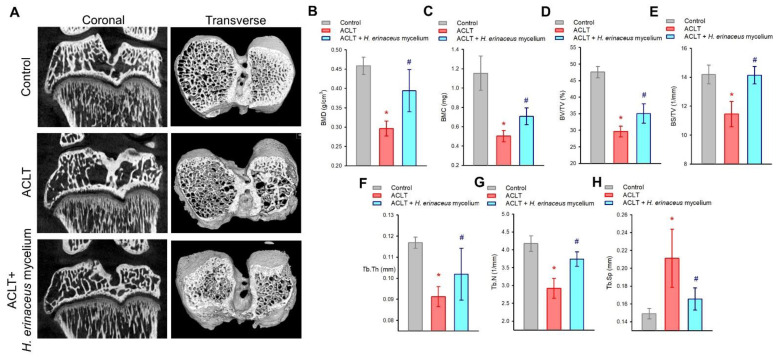
Next, we used μ-CT to analyze in detail the changes in bone and cartilage architecture after H. erinaceus mycelium application. Marked improvements were seen in bone mineral density (BMD), bone mineral content (BMC), bone volume/tissue volume (BV/TV), bone surface/TV (BS/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N) and trabecular separation (Tb.Sp), compared to the ACLT-only group (Figure 3).
Figure 3.
Micro-CT analysis of the effects of H. erinaceus mycelium on the ACLT bone architecture. (A) Representative micro-CT images from knee subchondral bone. Quantitative analyses of (B) BMD, (C) BMC, (D) BV/TV, (E) BS/TV, (F) Tb.Th, (G) Tb.N and (H) Tb.Sp (n = for each group). * p < 0.05 compared to the control group; # p < 0.05 compared to the ACLT-only group.
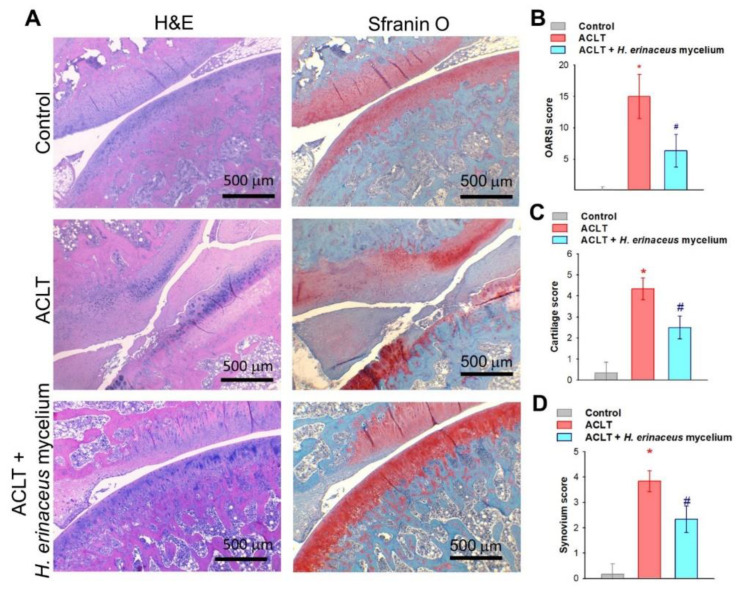
H&E and Safranin-O staining revealed that H. erinaceus mycelium dramatically prevented ACLT-induced increases in Osteoarthritis Research Society International (OARSI) scores, cartilage and synovium scores and cartilage damage (Figure 4).
Figure 4.
H. erinaceus mycelium ameliorates ACLT-induced cartilage degradation and synovial inflammation. (A) Histological sections from knees stained with H&E and Safranin-O. (B) OARSI scores, (C) cartilage scores and (D) synovium scores (n = 6 for each group). * p < 0.05 compared to the control group; # p < 0.05 compared to the ACLT-only group.
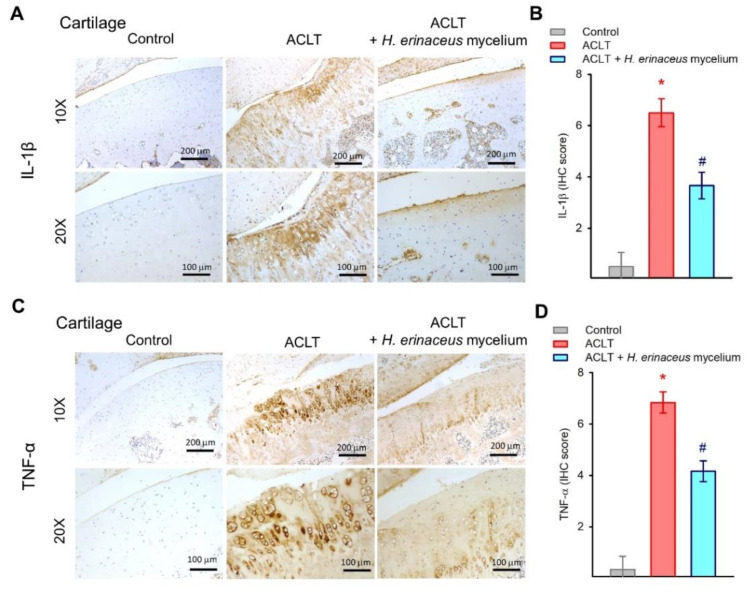
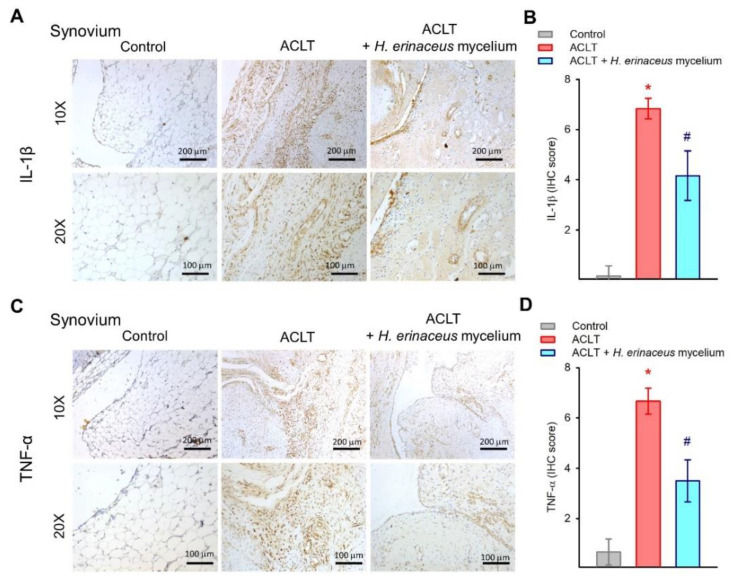
3.3. H. erinaceus Mycelium Suppresses Proinflammatory Cytokine Upregulation
IL-1β and TNF-α are critical proinflammatory cytokines during OA progression [5,6]. IHC staining revealed that while IL-1β and TNF-α synthesis was significantly elevated in the cartilage and synovium of the ACLT-only group, the expression of both cytokines in both tissues was lowered by H. erinaceus mycelium (Figure 5 and Figure 6).
Figure 5.
H. erinaceus mycelium suppresses IL-1β and TNF-α expression in the cartilage. (A,C) Representative images of IL-1β and TNF-α staining. (B,D) Quantification of IHC scores (n = 6 for each group). * p < 0.05 compared to the control group; # p < 0.05 compared to the ACLT-only group.
Figure 6.
H. erinaceus mycelium inhibits IL-1β and TNF-α expression in synovial tissue. (A,C) Representative images of IL-1β and TNF-α staining. (B,D) Quantification of IHC scores (n = 6 for each group). * p < 0.05 compared to the control group; # p < 0.05 compared to the ACLT-only group.
4. Discussion
OA causes great physical disability [31]. Much remains unknown about the pathogenesis of OA, although synovial inflammation is a well-recognized factor [32], so treating synovial inflammation is favored as an effective means of inhibiting the progression of OA [33,34]. Elevated levels of proinflammatory cytokine expression are found in OA joints [35]. The ACLT animal model for surgical initiation of OA erodes the knee joint cartilage, leading to OA-like disease [6,23]. Here, we found that H. erinaceus mycelium antagonized ACLT-induced promotion of weight-bearing asymmetry, bone loss, synovial inflammation and degradation of articular cartilage. In addition, H. erinaceus mycelium effectively reduced IL-1β and TNF-α levels in cartilage and synovial tissue, suggesting promising therapeutic effects for OA.
Numerous proinflammatory mediators are produced during the progression of OA, including IL-1β and TNF-α, leading to the activation of catabolic factors, resulting in cartilage degradation and bone erosion [36,37]. The levels of IL-1β and TNF-α expression in serum and synovial tissue are associated with the pathological process of OA [38,39]. Our ACLT-induced OA model demonstrated that ACLT surgery mimics clinical features, increasing IL-1β and TNF-α synthesis in cartilage and synovial tissue. H. erinaceus mycelium administration clearly downregulated the IL-1β and TNF-α expression in both cartilage and synovial tissues. The anti-OA effects of H. erinaceus mycelium are due to its ability to inhibit IL-1β and TNF-α production.
Nonsurgical treatment OARSI guidelines issued in 2019 suggest that exercises such as balance training and muscle strengthening are important components in the control of OA [4]. In regard to pharmacological therapy, the OARSI guidelines strongly recommend (Level 1A evidence) topical NSAIDs for patients with knee OA, while intra-articular hyaluronic acid or intra-articular corticosteroids are recommended (Level 1B/Level 2) treatments for knee OA dependent on comorbidities. Oral NSAIDs are conditionally not recommended (Levels 4A and 4B) and oral or transdermal opioids are strongly not recommended (Level 5) [4]. Although NSAIDs are commonly used for OA patients, these agents have unwanted side effects including substantial damage to the gastrointestinal and cardiovascular systems [40]. Our results indicate that H. erinaceus mycelium prevents cartilage damage by inhibiting ACLT-facilitated promotion of OARSI, cartilage and synovium scores. Our study data show that OA-induced damage to bone microarchitectural parameters BMD, BMC, BV/TV, BS/TV, Tb.Th and Tb.N was rescued by treatment with H. erinaceus mycelium. Thus, H. erinaceus mycelium protects against cartilage degradation and bone erosion.
Natural products have been used to remedy human disorders for millennia, and H. erinaceus is a well-known component of traditional Chinese medicine [41,42]. The constituents of H. erinaceus have been examined, and their functions have been documented for different body systems, particularly the nervous system [43,44]. H. erinaceus contains many bioactive components such as polysaccharides, secondary metabolites and nutritional components [45]. Several important polyphenol oxidase inhibitors (adenosine, ergosterol, ergothioneine and glutathione) have been found in H. erinaceus mycelium [46]. Other research has also identified that the diterpenoids Erinacine A and Erinacine S show high levels of bioactivity in H. erinaceus extract, after analysis by HPLC and LC-MS methods [47,48,49,50]. However, whether any of these bioactive compounds possess anti-arthritic functions remains to be investigated. We have identified a novel function for H. erinaceus mycelium as an effective inhibitor of ACLT-induced facilitation of weight-bearing asymmetry and pain, cartilage degradation, bone erosion and proinflammatory cytokine production. Thus, H. erinaceus mycelium may serve as a functional food that is beneficial in OA therapy.
Acknowledgments
We would like to thank Iona J. MacDonald from China Medical University for her English language revision of this manuscript.
Author Contributions
S.-Y.Y., C.-J.F., C.-C.C. and C.-H.T. (Chih-Hsin Tang), conceptualization, supervision and investigation. C.-J.F., Y.-Y.L., S.-C.L. and C.-H.T. (Chun-Hao Tsai), data curation, investigation and methodology. Y.-W.C. and W.-P.C., resources. L.-Y.L., W.-C.H. and C.-H.T. (Chih-Hsin Tang), project administration. S.-Y.Y., Y.-Y.L., Y.-C.W. and C.-H.T. (Chih-Hsin Tang), writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
All experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of China Medical University (approval number CMUIACUC-2021-291).
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data for this study are available from the corresponding authors on reasonable request.
Conflicts of Interest
No financial or personal relationships with other individuals or organizations inappropriately influenced this work.
Funding Statement
This work was supported by a grant from China Medical University (CMU110-ASIA-02), China Medical University Hospital (DMR-111-117, DMR-111-235) and An Nan Hospital, China Medical University (ANHRF111-04).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cui A., Li H., Wang D., Zhong J., Chen Y., Lu H. Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. EClinicalMedicine. 2020;29:100587. doi: 10.1016/j.eclinm.2020.100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martel-Pelletier J., Barr A.J., Cicuttini F.M., Conaghan P.G., Cooper C., Goldring M.B., Goldring S.R., Jones G., Teichtahl A.J., Pelletier J.P. Osteoarthritis. Nat. Rev. Dis. Primers. 2016;2:16072. doi: 10.1038/nrdp.2016.72. [DOI] [PubMed] [Google Scholar]
- 3.Abolhasani M., Halabchi F., Afsharnia E., Moradi V., Ingle L., Shariat A., Hakakzadeh A. Effects of kinesiotaping on knee osteoarthritis: A literature review. J. Exerc. Rehabil. 2019;15:498–503. doi: 10.12965/jer.1938364.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bannuru R.R., Osani M.C., Vaysbrot E.E., Arden N.K., Bennell K., Bierma-Zeinstra S.M.A., Kraus V.B., Lohmander L.S., Abbott J.H., Bhandari M., et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr. Cartil. 2019;27:1578–1589. doi: 10.1016/j.joca.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 5.Chevalier X., Eymard F., Richette P. Biologic agents in osteoarthritis: Hopes and disappointments. Nat. Rev. Rheumatol. 2013;9:400–410. doi: 10.1038/nrrheum.2013.44. [DOI] [PubMed] [Google Scholar]
- 6.Su C.-H., Lin C.-Y., Tsai C.-H., Lee H.-P., Lo L.-C., Huang W.-C., Wu Y.-C., Hsieh C.-L., Tang C.-H. Betulin suppresses TNF-α and IL-1β production in osteoarthritis synovial fibroblasts by inhibiting the MEK/ERK/NF-κB pathway. J. Funct. Foods. 2021;86:104729. doi: 10.1016/j.jff.2021.104729. [DOI] [Google Scholar]
- 7.Dinarello C.A. The IL-1 family of cytokines and receptors in rheumatic diseases. Nat. Rev. Rheumatol. 2019;15:612–632. doi: 10.1038/s41584-019-0277-8. [DOI] [PubMed] [Google Scholar]
- 8.Law Y.Y., Lee W.F., Hsu C.J., Lin Y.Y., Tsai C.H., Huang C.C., Wu M.H., Tang C.H., Liu J.F. miR-let-7c-5p and miR-149-5p inhibit proinflammatory cytokine production in osteoarthritis and rheumatoid arthritis synovial fibroblasts. Aging. 2021;13:17227–17236. doi: 10.18632/aging.203201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu S.C., Tsai C.H., Wu T.Y., Tsai C.H., Tsai F.J., Chung J.G., Huang C.Y., Yang J.S., Hsu Y.M., Yin M.C., et al. Soya-cerebroside reduces IL-1 beta-induced MMP-1 production in chondrocytes and inhibits cartilage degradation: Implications for the treatment of osteoarthritis. Food Agr. Immunol. 2019;30:620–632. doi: 10.1080/09540105.2019.1611745. [DOI] [Google Scholar]
- 10.Dorais M., Martel-Pelletier J., Raynauld J.P., Delorme P., Pelletier J.P. Impact of oral osteoarthritis therapy usage among other risk factors on knee replacement: A nested case-control study using the Osteoarthritis Initiative cohort. Arthritis Res. Ther. 2018;20:172. doi: 10.1186/s13075-018-1656-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sinusas K. Osteoarthritis: Diagnosis and treatment. Am. Fam. Physician. 2012;85:49–56. [PubMed] [Google Scholar]
- 12.Khan M.A., Tania M., Liu R., Rahman M.M. Hericium erinaceus: An edible mushroom with medicinal values. J. Complementary Integr. Med. 2013;10:253–258. doi: 10.1515/jcim-2013-0001. [DOI] [PubMed] [Google Scholar]
- 13.Tian B., Geng Y., Xu T., Zou X., Mao R., Pi X., Wu W., Huang L., Yang K., Zeng X., et al. Digestive Characteristics of Hericium erinaceus Polysaccharides and Their Positive Effects on Fecal Microbiota of Male and Female Volunteers During in vitro Fermentation. Front. Nutr. 2022;9:858585. doi: 10.3389/fnut.2022.858585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedman M. Chemistry, Nutrition, and Health-Promoting Properties of Hericium erinaceus (Lion’s Mane) Mushroom Fruiting Bodies and Mycelia and Their Bioactive Compounds. J. Agric. Food Chem. 2015;63:7108–7123. doi: 10.1021/acs.jafc.5b02914. [DOI] [PubMed] [Google Scholar]
- 15.He X., Wang X., Fang J., Chang Y., Ning N., Guo H., Huang L., Huang X., Zhao Z. Structures, biological activities, and industrial applications of the polysaccharides from Hericium erinaceus (Lion’s Mane) mushroom: A review. Int. J. Biol. Macromol. 2017;97:228–237. doi: 10.1016/j.ijbiomac.2017.01.040. [DOI] [PubMed] [Google Scholar]
- 16.Lee S.L., Hsu J.Y., Chen T.C., Huang C.C., Wu T.Y., Chin T.Y. Erinacine A Prevents Lipopolysaccharide-Mediated Glial Cell Activation to Protect Dopaminergic Neurons against Inflammatory Factor-Induced Cell Death In Vitro and In Vivo. Int. J. Mol. Sci. 2022;23:810. doi: 10.3390/ijms23020810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Darmasiwi S., Aramsirirujiwet Y., Kimkong I. Antibiofilm activity and bioactive phenolic compounds of ethanol extract from the Hericium erinaceus basidiome. J. Adv. Pharm. Technol. Res. 2022;13:111–116. doi: 10.4103/japtr.japtr_1_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang S., Liu S., Qin M. Effects of Extraction Conditions on Crude Polysaccharides and Antioxidant Activities of the Lion’s Mane Medicinal Mushroom, Hericium erinaceus (Agaricomycetes) Int. J. Med. Mushrooms. 2019;21:1007–1018. doi: 10.1615/IntJMedMushrooms.2019032566. [DOI] [PubMed] [Google Scholar]
- 19.Liang B., Guo Z., Xie F., Zhao A. Antihyperglycemic and antihyperlipidemic activities of aqueous extract of Hericium erinaceus in experimental diabetic rats. BMC Complement. Altern. Med. 2013;13:253. doi: 10.1186/1472-6882-13-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu D., Tang C., Liu Y., Li Q., Wang W., Zhou S., Zhang Z., Cui F., Yang Y. Structural elucidation and immunomodulatory activity of a beta-D-glucan prepared by freeze-thawing from Hericium erinaceus. Carbohydr. Polym. 2019;222:114996. doi: 10.1016/j.carbpol.2019.114996. [DOI] [PubMed] [Google Scholar]
- 21.Rahman M.M., Kim H.K., Kim S.E., Kim M.J., Kim D.H., Lee H.S. Chondroprotective Effects of a Standardized Extract (KBH-JP-040) from Kalopanax pictus, Hericium erinaceus, and Astragalus membranaceus in Experimentally Induced In Vitro and In Vivo Osteoarthritis Models. Nutrients. 2018;10:356. doi: 10.3390/nu10030356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu P.S., Chueh S.H., Chen C.C., Lee L.Y., Shiu L.Y. Lion’s Mane Medicinal Mushroom, Hericium erinaceus (Agaricomycetes), Modulates Purinoceptor-Coupled Calcium Signaling and Murine Nociceptive Behavior. Int. J. Med. Mushrooms. 2017;19:499–507. doi: 10.1615/IntJMedMushrooms.v19.i6.20. [DOI] [PubMed] [Google Scholar]
- 23.Lee K.T., Su C.H., Liu S.C., Chen B.C., Chang J.W., Tsai C.H., Huang W.C., Hsu C.J., Chen W.C., Wu Y.C., et al. Cordycerebroside A inhibits ICAM-1-dependent M1 monocyte adhesion to osteoarthritis synovial fibroblasts. J. Food Biochem. 2022:e14108. doi: 10.1111/jfbc.14108. [DOI] [PubMed] [Google Scholar]
- 24.Hou P.W., Liu S.C., Tsay G.J., Tang C.H., Chang H.H. The Traditional Chinese Medicine “Hu-Qian-Wan” Attenuates Osteoarthritis-Induced Signs and Symptoms in an Experimental Rat Model of Knee Osteoarthritis. Evid. Based Complement. Alternat. Med. 2022;2022:5367494. doi: 10.1155/2022/5367494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Achudhan D., Liu S.C., Lin Y.Y., Lee H.P., Wang S.W., Huang W.C., Wu Y.C., Kuo Y.H., Tang C.H. Antcin K inhibits VEGF-dependent angiogenesis in human rheumatoid arthritis synovial fibroblasts. J. Food Biochem. 2022;46:e14022. doi: 10.1111/jfbc.14022. [DOI] [PubMed] [Google Scholar]
- 26.Liu S.C., Tsai C.H., Wang Y.H., Su C.M., Wu H.C., Fong Y.C., Yang S.F., Tang C.H. Melatonin abolished proinflammatory factor expression and antagonized osteoarthritis progression in vivo. Cell Death Dis. 2022;13:215. doi: 10.1038/s41419-022-04656-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin Y.Y., Ko C.Y., Liu S.C., Wang Y.H., Hsu C.J., Tsai C.H., Wu T.J., Tang C.H. miR-144-3p ameliorates the progression of osteoarthritis by targeting IL-1beta: Potential therapeutic implications. J. Cell Physiol. 2021;236:6988–7000. doi: 10.1002/jcp.30361. [DOI] [PubMed] [Google Scholar]
- 28.Chen W.C., Lu Y.C., Kuo S.J., Lin C.Y., Tsai C.H., Liu S.C., Chen Y.L., Wang S.W., Tang C.H. Resistin enhances IL-1beta and TNF-alpha expression in human osteoarthritis synovial fibroblasts by inhibiting miR-149 expression via the MEK and ERK pathways. FASEB J. 2020;34:13671–13684. doi: 10.1096/fj.202001071R. [DOI] [PubMed] [Google Scholar]
- 29.Lee H.P., Wang S.W., Wu Y.C., Lin L.W., Tsai F.J., Yang J.S., Li T.M., Tang C.H. Soya-cerebroside inhibits VEGF-facilitated angiogenesis in endothelial progenitor cells. Food Agr. Immunol. 2020;31:193–204. doi: 10.1080/09540105.2020.1713055. [DOI] [Google Scholar]
- 30.Lee H.P., Wang S.W., Wu Y.C., Tsai C.H., Tsai F.J., Chung J.G., Huang C.Y., Yang J.S., Hsu Y.M., Yin M.C., et al. Glucocerebroside reduces endothelial progenitor cell-induced angiogenesis. Food Agr. Immunol. 2019;30:1033–1045. doi: 10.1080/09540105.2019.1660623. [DOI] [Google Scholar]
- 31.Yuan X., Meng H., Wang Y., Peng J., Guo Q., Wang A., Lu S. Bone–cartilage interface crosstalk in osteoarthritis: Potential pathways and future therapeutic strategies. Osteoarthr. Cartil. 2014;22:1077–1089. doi: 10.1016/j.joca.2014.05.023. [DOI] [PubMed] [Google Scholar]
- 32.Gao B., Gao W., Wu Z., Zhou T., Qiu X., Wang X., Lian C., Peng Y., Liang A., Qiu J., et al. Melatonin rescued interleukin 1beta-impaired chondrogenesis of human mesenchymal stem cells. Stem Cell Res. Ther. 2018;9:162. doi: 10.1186/s13287-018-0892-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sellam J., Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat. Rev. Rheumatol. 2010;6:625–635. doi: 10.1038/nrrheum.2010.159. [DOI] [PubMed] [Google Scholar]
- 34.Mathiessen A., Conaghan P.G. Synovitis in osteoarthritis: Current understanding with therapeutic implications. Arthritis Res. Ther. 2017;19:18. doi: 10.1186/s13075-017-1229-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu X., Lee C.W., Xu H., Wang Y.F., Yung P.S.H., Jiang Y., Lee O.K. Phenotypic alteration of macrophages during osteoarthritis: A systematic review. Arthritis Res. Ther. 2021;23:110. doi: 10.1186/s13075-021-02457-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schulze-Tanzil G. Experimental Therapeutics for the Treatment of Osteoarthritis. J. Exp. Pharmacol. 2021;13:101–125. doi: 10.2147/JEP.S237479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liyis B.G.de, Nolan J., Maharjana M.A. Fibroblast Growth Factor Receptor 1-Bound Extracellular Vesicle as Novel Therapy for Osteoarthritis. BioMedicine. 2022;12:1. doi: 10.37796/2211-8039.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.MacDonald I.J., Huang C.C., Liu S.C., Lin Y.Y., Tang C.H. Targeting CCN Proteins in Rheumatoid Arthritis and Osteoarthritis. Int. J. Mol. Sci. 2021;22:4340. doi: 10.3390/ijms22094340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.MacDonald I.J., Liu S.C., Su C.M., Wang Y.H., Tsai C.H., Tang C.H. Implications of Angiogenesis Involvement in Arthritis. Int. J. Mol. Sci. 2018;19:2012. doi: 10.3390/ijms19072012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bindu S., Mazumder S., Bandyopadhyay U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol. 2020;180:114147. doi: 10.1016/j.bcp.2020.114147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Geng Y., Zhu S., Lu Z., Xu H., Shi J.S., Xu Z.H. Anti-inflammatory activity of mycelial extracts from medicinal mushrooms. Int. J. Med. Mushrooms. 2014;16:319–325. doi: 10.1615/IntJMedMushrooms.v16.i4.20. [DOI] [PubMed] [Google Scholar]
- 42.Ulziijargal E., Mau J.L. Nutrient compositions of culinary-medicinal mushroom fruiting bodies and mycelia. Int. J. Med. Mushrooms. 2011;13:343–349. doi: 10.1615/IntJMedMushr.v13.i4.40. [DOI] [PubMed] [Google Scholar]
- 43.Lai P.L., Naidu M., Sabaratnam V., Wong K.H., David R.P., Kuppusamy U.R., Abdullah N., Malek S.N. Neurotrophic properties of the Lion’s mane medicinal mushroom, Hericium erinaceus (Higher Basidiomycetes) from Malaysia. Int. J. Med. Mushrooms. 2013;15:539–554. doi: 10.1615/IntJMedMushr.v15.i6.30. [DOI] [PubMed] [Google Scholar]
- 44.Wong K.H., Naidu M., David R.P., Bakar R., Sabaratnam V. Neuroregenerative potential of lion’s mane mushroom, Hericium erinaceus (Bull.: Fr.) Pers. (higher Basidiomycetes), in the treatment of peripheral nerve injury (review) Int. J. Med. Mushrooms. 2012;14:427–446. doi: 10.1615/IntJMedMushr.v14.i5.10. [DOI] [PubMed] [Google Scholar]
- 45.Zhang C.C., Yin X., Cao C.Y., Wei J., Zhang Q., Gao J.M. Chemical constituents from Hericium erinaceus and their ability to stimulate NGF-mediated neurite outgrowth on PC12 cells. Bioorganic Med. Chem. Lett. 2015;25:5078–5082. doi: 10.1016/j.bmcl.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 46.Kim S. Antioxidant Compounds for the Inhibition of Enzymatic Browning by Polyphenol Oxidases in the Fruiting Body Extract of the Edible Mushroom Hericium erinaceus. Foods. 2020;9:951. doi: 10.3390/foods9070951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee K.F., Tung S.Y., Teng C.C., Shen C.H., Hsieh M.C., Huang C.Y., Lee K.C., Lee L.Y., Chen W.P., Chen C.C., et al. Post-Treatment with Erinacine A, a Derived Diterpenoid of H. erinaceus, Attenuates Neurotoxicity in MPTP Model of Parkinson’s Disease. Antioxidants. 2020;9:137. doi: 10.3390/antiox9020137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee K.C., Lee K.F., Tung S.Y., Huang W.S., Lee L.Y., Chen W.P., Chen C.C., Teng C.C., Shen C.H., Hsieh M.C., et al. Induction Apoptosis of Erinacine A in Human Colorectal Cancer Cells Involving the Expression of TNFR, Fas, and Fas Ligand via the JNK/p300/p50 Signaling Pathway with Histone Acetylation. Front. Pharmacol. 2019;10:1174. doi: 10.3389/fphar.2019.01174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang H.T., Ho C.H., Sung H.Y., Lee L.Y., Chen W.P., Chen Y.W., Chen C.C., Yang C.S., Tzeng S.F. Hericium erinaceus mycelium and its small bioactive compounds promote oligodendrocyte maturation with an increase in myelin basic protein. Sci. Rep. 2021;11:6551. doi: 10.1038/s41598-021-85972-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen C.C., Tzeng T.T., Chen C.C., Ni C.L., Lee L.Y., Chen W.P., Shiao Y.J., Shen C.C. Erinacine S, a Rare Sesterterpene from the Mycelia of Hericium erinaceus. J. Nat. Prod. 2016;79:438–441. doi: 10.1021/acs.jnatprod.5b00474. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data for this study are available from the corresponding authors on reasonable request.