Abstract
OBJECTIVE
Literature in pediatric patients suggests dosing sirolimus 1.6 mg/m2/day divided twice daily for lymphatic disorders with limited evidence available for dosing in neonates and infants. The objective of this research was to determine the sirolimus dose required to achieve therapeutic trough concentrations in infants with lymphatic disorders at Children's Hospital of Philadelphia.
METHODS
This retrospective review included patients <1 year of age at Children's Hospital of Philadelphia who were initiated on sirolimus for lymphatic disorder. Patients were included if they received at least 5 days of consecutive sirolimus therapy prior to trough concentration monitoring. Measures of central tendency and variability were used for statistical analysis.
RESULTS
A total of 16 patients met criteria for inclusion. The median initial sirolimus dose was 1 mg/m2/day (IQR, 0.5–1.6 mg/m2/day). Fourteen patients (87.5%) achieved therapeutic trough concentrations on a median sirolimus dose of 0.5 mg/m2/day. Dosing frequency to achieve therapeutic trough concentrations included 1 patient (6.25%) on twice daily dosing, 12 patients (75%) on once daily dosing, and 1 patient (6.25%) requiring every 48-hour dosing. The median time to first therapeutic trough was 15.5 days (IQR, 5.5–18.5 days), and patients required a median of 1 dose adjustment.
CONCLUSIONS
A median sirolimus dose to achieve therapeutic sirolimus trough concentrations in infants with lymphatic disorders was 0.5 mg/m2/day with a median of 1 dose adjustment. Sirolimus was well tolerated in the study population.
Keywords: infant, lymphatic disorder, neonate, sirolimus, trough
Introduction
The lymphatic system is a complex network of vessels that run parallel to the venous system, extending to all body systems with exception of the brain and spinal cord.1 The lymphatic system has many roles, most notably maintenance of homeostasis and clearance of pathogens.2 Congenital malformations within the lymphatic system have the propensity to cause serious complications leading to morbidity and mortality. The type and degree of these sequalae vary based on the size and location of the lymphatic malformation. Complications include but are not limited to physical disfigurement, chronic pain, coagulopathies, organ dysfunction, and death.3,4 Lymphatic malformations are related in part to dysregulation of the phosphatidylinositol 3-kinase (PI3K)/AKT signaling pathway, which is involved in cell mortality, proliferation, angiogenesis, and lymphangiogenesis.5
Sirolimus binds the FK Binding Protein-12 (FKBP-12), creating an immunosuppressive complex that inhibits the activation of mammalian target of rapamycin (mTOR),6 a protein kinase involved in the regulation of the PI3K/AKT pathway.5 Inhibition of this pathway by sirolimus exhibits antiproliferative properties, providing benefit in lymphatic vascular malformations.1–3 Sirolimus is approved by the US Food and Drug Administration for pediatric patients 13 years of age or older for the management of renal transplant rejection.6 It exhibits nonlinear kinetics and is monitored through serum trough concentrations.7 Institutional practice at the Children's Hospital of Philadelphia (CHOP) is to target sirolimus trough concentrations of 5 to 15 ng/mL in patients with lymphatic malformations.
The purpose of this study is to describe sirolimus dosing that resulted in therapeutic concentrations in neonates and infants with lymphangiomas or vascular lymphatic malformations. The primary objective of this study was to determine the sirolimus dose required to reach therapeutic trough concentrations. Secondary objectives included the number of dose adjustments required to achieve goal trough concentrations, time to first therapeutic trough concentration, and the frequency of sirolimus-related adverse effects.
Materials and Methods
Study Population. This was a single-center, retrospective study that evaluated the sirolimus doses required to achieve a therapeutic trough concentration (5–15 ng/mL) in neonates and infants. Patients were included if they were younger than 1 year of age, admitted to CHOP, received a diagnosis of a lymphatic malformation, and initiated on sirolimus between January 1, 2012, and September 13, 2020. Patients had to receive at least 5 days of sirolimus prior to a trough concentration, and trough concentrations had to be obtained within 2 hours of the next dose to be included for analysis.
Outcome Measures. Baseline demographics included age (gestational, postmenstrual, and postnatal), sex, race, weight, and height. Sirolimus characteristics included initial dose, trough concentrations, and number of dose adjustments. Factors collected that may impact serum trough concentrations included percentage of goal feeds at the time of trough concentration, stool output 24 hours prior to trough concentration, drug interactions (category D or X), and hepatic impairment based on Child-Pugh scores. Safety data, with respective definitions, included elevations in liver function tests (3 times baseline) thrombocytopenia (<50,000 platelets/μL), anemia (< 9 g/dL), neutropenia (absolute neutrophil count <1000/μL), and hypertriglyceridemia (2 times baseline).
Statistical Analysis. Measures of central tendency, variability, and frequency were used for all data points. Discrete data were presented as number (percentage), and continuous, nonparametric data were presented as median (IQR).
Results
A total of 38 patients younger than 1 year received sirolimus during the study period. Of those 38 patients, 20 were excluded for an indication other than lymphatic malformation, and 2 were excluded for absence of a trough concentration drawn within 2 hours of the next scheduled dose. A total of 16 patients were included for analysis. Patient demographics and clinical characteristics are presented in Table 1. All patients were at goal feeds at the time of trough concentrations, and no patients had hepatic impairment classified as Child-Pugh score C, nor did they have concomitant administration of medications considered category D or X drug interactions with sirolimus.
Table 1.
Patient Demographics and Clinical Characteristics (N = 16) *
| Characteristic | |
|---|---|
| Sex (male), n (%) | 11 (69) |
| Race (white), n (%) | 9 (56) |
| Age, median (IQR), wk | |
| Gestational age | 37. 9 (37–38.7) |
| Postmenstrual age | 42.1 (40.3–48.1) |
| Postnatal age | 4.4 (2.9–9.1) |
| Dosing weight, median (IQR), kg | 4 (3.5–4.9) |
| BSA, median (IQR), m2 | 0.24 (0.22–0.28) |
| Starting frequency (q12), n (%) | 3 (18.8) |
| Stool output 24 hr prior to therapeutic concentration, median (IQR), mL/kg/hr (n = 14) | 2.6 (2.2–3.6) |
BSA, body surface area
* Nominal data reported as n (%). Continuous, non-parametric data reported as median (IQR).
Sirolimus dosing characteristics by patient are reported in Table 2. The median starting dose was 1 mg/m2/day (IQR, 0.5–1.6 mg/m2/day). Three patients (19%) were initiated on sirolimus twice daily. The median dose required to achieve the first therapeutic trough concentration was 0.5 mg/m2/day (IQR, 0.4–1 mg/m2/day). The most common dosing frequency to achieve therapeutic trough was once daily dosing in 12 patients (75%). Two patients (13%) did not achieve therapeutic sirolimus trough concentrations prior to hospital discharge or discontinuation of therapy. Of the 14 patients (88%) that had therapeutic trough concentrations the median time to first therapeutic trough was 15.5 days (IQR, 5.5–18.5 days). A total of 6 patients (38%) did not require dose adjustment, 4 (25%) required 1 dose adjustment, 3 (19%) required 2 dose adjustments, and 1 patient (6%) required 4 dose adjustments. For those patients that achieved therapeutic trough concentrations, a median of 1 dose adjustment was required.
Table 2.
Individual Patient Sirolimus Dosing Characteristics (N=16)
| Patient no. | Postmenstrual Age, wk | Starting Sirolimus Dose, mg/m2/day | Sirolimus Dose Achieving First Therapeutic Trough, mg/m2/day | No. of Days to Therapeutic | No. of Dose Adjustments |
|---|---|---|---|---|---|
| 1 | 53.3 | 1.9 | 1.9 | 5 | 0 |
| 2 | 39.3 | 0.5 | 0.5 | 5 | 0 |
| 3 | 77.4 | 3.1 | 3.5 | 15 | 1 |
| 4 | 40.7 | 0.4 | 0.3 | 17 | 2 |
| 5 | 42.1 | 1.0 | 1.0 | 7 | 0 |
| 6 | 48.1 | 0.4 | 0.4 | 7 | 0 |
| 7 | 40.1 | 1.6 | 0.6 | 27 | 2 |
| 8 | 41.6 | 0.4 | 0.4 | 5 | 0 |
| 9 | 50.6 | 1.7 | 0.9 | 19 | 1 |
| 10 | 53.7 | 0.5 | N/A | N/A | N/A |
| 11 | 39.9 | 1* | 0.2† | 17 | 1 |
| 12 | 44 | 1.7 | 0.3 | 29 | 4 |
| 13 | 42.3 | 1.6* | 1* | 16 | 1 |
| 14 | 40.1 | 1.3 | 0.9 | 22 | 2 |
| 15 | 42.1 | 0.5 | 0.5 | 5 | 0 |
| 16 | 40.3 | 1* | N/A | N/A | N/A |
| Median (IQR) | 42.1 (40.3–48.1) | 1 (0.5–1.6) | 0.5 (0.4–1) | 15.5 (5.5–18.5) | 1 (0–1.8) |
* Total daily dose divided twice daily.
† Sirolimus given every 48 hr.
Figure 1 represents all sirolimus trough concentrations and Figure 2 includes trough concentrations within the 25th to 75th percentiles. Using a logarithmic regression, trough concentrations in the IQR had an r2 value of 0.51, showing a moderate positive correlation between dose and trough concentrations.
Figure 1.
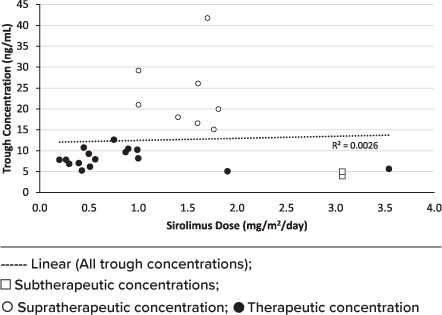
Sirolimus serum trough concentrations.
Figure 2.

Sirolimus serum trough concentrations within the 25th–75th percentile.
Patient 11 started on 0.5 mg/m2 per dose and required dose reduction of sirolimus to 0.4 mg/m2 per dose every 48 hours (0.2 mg/m2/day) to decrease supratherapeutic trough concentrations to therapeutic concentrations. Patient 13 remained on twice daily dosing and achieved therapeutic concentrations after 1 dose adjustment to sirolimus 1 mg/m2/day (0.5 mg/m2 per dose every 12 hours). Patient 16 had supratherapeutic trough concentrations, required holding of sirolimus for 3 days, and did not achieve therapeutic trough concentrations at steady state before discharge. In addition to patient 16 not achieving goal trough concentrations, patient 10 had supratherapeutic trough concentrations, required dose reductions, and was transitioned to outpatient prior to reaching therapeutic concentrations.
Metabolic side effects, including hypertriglyceridemia and elevations in liver enzymes, were observed in 8 (50%) and 3 (19%) patients, respectively. Hematologic side effects, including thrombocytopenia, anemia, and neutropenia, were observed in 1 patient (6%), 5 patients (31%), and 1 patient (6%), respectively.
Discussion
Sirolimus is used for targeted management of lymphatic disorders in neonates and infants despite limited literature to support initial dosing recommendations. Published literature for sirolimus' use in the pediatric population recommends a starting dose of sirolimus 1.6 mg/m2/day divided twice daily. Because of developmental differences in pharmacokinetic and pharmacodynamics in neonates and young infants, including body size, volume of distribution, albumin concentration, and organ maturation and development, dosing extrapolation from pediatric patients can be complex and multifaceted.4,5,7–11 In addition to pharmacokinetic and pharmacodynamic differences, there can also be substantial intrapatient variability regarding feeds, stool output, and gastrointestinal tolerance. These variables have been theorized to affect sirolimus absorption and impact p-glycoprotein efflux pumps.12 Overall, there is a lack of consensus in the literature for target trough concentrations with ranges between 5 and 15 ng/mL suggested.
Case reports and small case series have demonstrated the benefit of sirolimus for lymphatic malformations with starting doses of sirolimus 1.6 mg/m2/day divided twice daily for pediatric patients. Efficacy was assessed based on symptomatic improvement, quality of life, and radiologic improvement.4,5,7–11 A systematic review of sirolimus use in lymphatic malformations included 20 studies with a total of 71 patients, 14 of whom were neonates. Each study varied in its dosing regimens, target trough concentrations, and duration of treatment. A total of 60 patients (85%) demonstrated partial disease remission on sirolimus therapy.8 Additionally, a case series of pediatric patients, ages 2 months to 16 years, started on sirolimus 1.6 mg/m2/day divided twice daily and titrated dose based on goal trough concentrations of 10 to 15 ng/mL, concluded that sirolimus was effective in 89% of patients with no serious adverse effects.9 In a retrospective evaluation 6 patients ranging from ages 7 months to 14 years were treated with sirolimus and showed significant improvement following treatment. The initial sirolimus dose was 1.6 mg/m2/day divided twice daily with titrations to maintain goal trough concentrations of 10 to 15 ng/mL.10 Adams et al5 and Hammer et al11 started sirolimus at 1.6 mg/m2/day divided twice daily with trough guided titrations. Adams et al5 and Hammer et al11 reported a median age of 8.1 years, with age ranges of 21 days to 28.5 years and 3 to 64 years, respectively. Both studies described symptomatic and quality of life improvement with minimal adverse effects.5,11 Finally, a dosing model simulation was developed based on clearance estimates in patients ages 0 to 24 months to provide dosing regimens based on varying trough concentrations. Although this study provides dosing recommendations based on varying age ranges and trough goals, this model has not been validated in patients with lymphatic disorders.13
Our study sought to describe an appropriate starting dose for sirolimus in neonates and infants based on the doses required to reach first therapeutic trough concentration and determine the time required to reach therapeutic concentrations. Because of a lack of institutional protocol at CHOP, there was no consensus regarding the starting dose of sirolimus and the target trough concentrations in neonates and infants being treated for lymphatic malformations. Starting regimens varied widely and were determined based on provider preference and institutional experience. This study found a median starting dose of 1 mg/m2/day (IQR, 0.5–1.6 mg/m2/day), with most patients (81%) initiated on once daily dosing. The median dose for first therapeutic trough concentration was 0.5 mg/m2/day, half of the initial starting dose and lower than starting doses reported in current literature for pediatric patients. In contrast to twice daily dosing reported previously, daily sirolimus administration was the most common dosing frequency to achieve therapeutic trough concentrations in this patient population, indicating that once daily dosing may be an option for this population. All 3 patients started on twice daily dosing had supratherapeutic trough concentrations, requiring dose reduction or interval extension. The 2 major differences of dose and frequency can likely be attributed to the difference in pharmacokinetics and pharmacodynamics during growth and development.
Our study population achieved therapeutic trough concentrations after 15.5 days (IQR, 5.5–18.5 days) of sirolimus therapy. Subtherapeutic/supratherapeutic trough concentrations for 2 weeks may delay lymphatic malformation improvement and increase the risk of adverse effects, respectively. Nearly all patients required less than or equal to 2 dose adjustments, but 1 patient required 4 dose adjustments, taking 29 days to achieve a therapeutic trough concentration. Time to therapeutic trough concentration and the inclusion criteria of at least 5 days of sirolimus therapy prior to trough concentration may have been delayed because of sirolimus' long half-life of approximately 14 hours in children, and it is common practice to wait for sirolimus to reach steady state prior to obtaining a trough concentration.6 Thus, a median of 2 weeks to achieve therapeutic trough concentrations coincides with a median of 1 dose adjustment. Trough concentrations drawn prior to steady state may be considered as a safety measure to prevent supratherapeutic trough concentrations at steady state. Provider education would be required to ensure dose adjustments are not made for subtherapeutic trough concentrations prior to steady state.
All adverse effects reviewed for this study were observed at least once, with triglyceride elevations being the most frequent. This is consistent with an incidence of 45% to 57% reported for sirolimus use.6 Of note, 1 patient had multiple comorbid conditions and experienced nearly all side effects which could not be definitively attributed to sirolimus administration.
There are several limitations to this review that are important to note. The small sample size and requirement of steady-state, appropriately drawn trough concentrations may have limited the number of patients eligible for inclusion and the number of trough concentrations available for analysis. Lastly, sirolimus concentrations were not being assessed daily, therefore time to therapeutic trough concentration may have been falsely prolonged.
Conclusion
This retrospective study included the largest number of neonates and infants administered sirolimus for lymphatic malformations to date. The results showed that a median sirolimus dose to achieve therapeutic sirolimus trough concentrations in patients <1 year of age was 0.5 mg/m2/day given as a once daily dose with a median of 1 dose adjustment. The median number of days to therapeutic trough concentrations was 15.5 days. Overall, sirolimus was well tolerated, but hypertriglyceridemia was the most frequent side effect in this patient population. Larger, prospective studies are needed to determine an appropriate starting dose for sirolimus in neonates and infants with lymphatic malformations.
ABBREVIATIONS
- CHOP
Children's Hospital of Philadelphia
- mTOR
mammalian target of rapamycin
- PI3K/AKT
phosphatidylinositol 3-kinase/AKT
Footnotes
Disclosures. The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria. The authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Ethical Approval and Informed Consent. This study was approved by the Children's Hospital of Philadelphia Institutional Review Board and informed consent was waived.
References
- 1.Moore JE, Bertram CD. Lymphatic system flows. Annu Rev Fluid Mech . 2018;50:459–482. doi: 10.1146/annurev-fluid-122316-045259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santambrogio L. The lymphatic fluid. Int Rev Cell Mol Biol . 2018;337:111–133. doi: 10.1016/bs.ircmb.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Mahady K, Thust S, Berkeley R et al. Vascular anomalies of the head and neck in children. Quant Imaging Med Surg . 2015;5(6):886–897. doi: 10.3978/j.issn.2223-4292.2015.04.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elluru RG, Balakrishnan K, Padua HM. Lymphatic malformations: diagnosis and management. Semin Pediatr Surg . 2014;23(4):178–185. doi: 10.1053/j.sempedsurg.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Adams DM, Trenor CC, Hammill AM et al. Efficacy and safety of sirolimus in the treatment of complicated vascular anomalies. Pediatrics . 2016;137(2):e20153257. doi: 10.1542/peds.2015-3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parsippany, NJ: Ascend Laboratories LLC; 2020. Sirolimus [package insert] [Google Scholar]
- 7.Wang D, Chen X, Li Z. Population pharmacokinetics of sirolimus in pediatric patients with kaposiform hemangioendothelioma: a retrospective study. Oncol Lett . 2019;18(3):2412–2419. doi: 10.3892/ol.2019.10562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiegand S, Wichmann G, Dietz A. Treatment of lymphatic malformations with the mTOR inhibitor sirolimus: a systematic review. Lymphat Res Biol . 2018;16(4):330–339. doi: 10.1089/lrb.2017.0062. [DOI] [PubMed] [Google Scholar]
- 9.Tian R, Liang Y, Zhang W et al. Effectiveness of sirolimus in the treatment of complex lymphatic malformations: single center report of 56 cases. J Pediatr Surg . 2020;55(11):2454–2458. doi: 10.1016/j.jpedsurg.2019.12.021. [DOI] [PubMed] [Google Scholar]
- 10.Hammill AM, Wentzel M, Gupta A et al. Sirolimus for the treatment of complicated vascular anomalies in children. Pediatr Blood Cancer . 2011;57(6):1018–1024. doi: 10.1002/pbc.23124. [DOI] [PubMed] [Google Scholar]
- 11.Hammer J, Seront E, Duez S et al. Sirolimus is efficacious in treatment for extensive and/or complex slow-flow vascular malformations: a monocentric prospective phase II study. Orphanet J Rare Dis . 2018;13(1):191. doi: 10.1186/s13023-018-0934-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lemahieu W, Maes B, Verbeke K et al. Cytochrome p450 3a4 and p-glycoprotein activity and assimilation of tacrolimus in transplant patients with persistent diarrhea. Am J Transplant . 2005;5(6):1383–1391. doi: 10.1111/j.1600-6143.2005.00844.x. [DOI] [PubMed] [Google Scholar]
- 13.Mizuno T, Fukuda T, Emoto C et al. Developmental pharmacokinetics of sirolimus: implications for precision dosing in neonates and infants with complicated vascular anomalies. Pediatr Blood Cancer . 2017;64(8) doi: 10.1002/pbc.26470. [DOI] [PubMed] [Google Scholar]


