Abstract
OBJECTIVE
Combining intranasal fentanyl (IN FENT) with inhaled nitrous oxide (N2O) seems to have good properties for pediatric procedural sedation and analgesia (PSA). This study aims to assess the side effect rate of the combined use of IN FENT and N2O.
METHODS
We performed a retrospective, single-center study. Patients treated in either the pediatric emergency department (PED) or the pediatric surgery outpatient clinic (PSOC) were included, if they received PSA with IN FENT and nitrous oxide with 50% oxygen (N2O 50%).
RESULTS
Three hundred seventy-five patients were included over a period of 4 years. Median age was 9.4 years (range, 3.1 to 15.9) and 39% of patients were female. Overall side effect rate was 30% (114 patients). Most frequent was dizziness (n = 63, 17%; 95% CI, 13–21), followed by nausea (n = 23, 6%; 95% CI, 4–9) and emesis (n = 14, 4%; 95% CI, 2–6), with 35 patients having either nausea and/or emesis (9%; 95% CI, 7–13). No serious side effects were recorded (0%; 95% CI, 0–0.1). Of 298 patients with information regarding satisfaction, 280 patients would like the same sedation for a similar procedure in the future (94%; 95% CI, 90–96). We found no relation between previously described risk factors and emesis and/or nausea.
CONCLUSIONS
N2O 50% combined with IN FENT can be recommended as an effective and safe treatment in the PED and the PSOC. While the side effect rate, primarily dizziness, nausea and emesis was substantial, antiemetic prophylaxis is not indicated owing to the overall low incidence of nausea and emesis.
Keywords: analgesia, conscious sedation, drug-related side effects, fentanyl, intranasal administration, nitrous oxide, pediatrics
Introduction
Procedural sedation and analgesia (PSA) is defined by the use of anxiolytic, sedative, analgesic, or dissociative drugs.1 Its goal is to attenuate pain, anxiety, and motion, to facilitate performance of necessary diagnostic or therapeutic procedures, and to ensure patient safety.2,3 For decades, different medications have been used outside the pediatric operating room to reduce stress and pain in the setting of elective procedures, or at pediatric emergency departments (PEDs),4,5 for example, for placing intravenous (IV) lines, radiographic imaging, wound dressing, fracture reduction, joint relocation, or lumbar puncture.6,7
Nitrous oxide (N2O) is a tasteless, colorless gas acting via the N-methyl-D-aspartate receptor, the glutamate receptor, and via opioid agonism, as well as having gamma-aminobutyric acid effects.3,8 Owing to its low blood-gas partition coefficient, it has both a short onset and quick offset time of effect (around 5 minutes).1 Together with the fact that no IV line is required for its application, this is an excellent agent for PSA in children.8 Furthermore, only few side effects (5%–10%) were found in pediatrics, mainly emesis and nausea.9,10 No association was found between pre-procedural fasting and side effects.5,11 Nitrous oxide is applied as a mixture with oxygen (e.g., N2O 50% is combined with 50% oxygen). Although N2O concentrations vary from N2O 30% up to N2O 70%, concentrations of N2O 50% to N2O 70% are used in PEDs.8 Depending on concentration, it has sedative, anxiolytic, analgesic, and slight amnestic effects.8,9 However, the main disadvantage is its limited analgesic effect.12
Fentanyl is a highly potent selective opioid agonist at the μ-receptor.13 Intranasal fentanyl (IN FENT) has an onset, peak, and offset time each within a few minutes.14,15 Its effect is comparable to IV fentanyl and IV morphine.16–18 Added to the advantages of no influence via first-pass metabolism, and no need for an IV line, it has very good analgesic properties for children.1 Intranasal fentanyl for the treatment of acute pain has been studied.18–22 Variable rates of nausea and emesis have been described in detail for pediatric patients.15,22–27
Combining the 2 medications for the purpose of having additional analgesic effect in pediatric procedures seems very attractive. However, this could lead to a significant rise in side effect rate due to the proemetic and centrally effective properties of both agents.
To date only 5 pediatric studies have reported side effect rates of IN FENT combined with N2O.28–32 Four were prospective studies. Two larger studies with patient numbers of around 200 used only nitrous oxide 70%,30,31 the other smaller studies reported on a mixed population treated with either N2O 50% or 70%.28,29 They found a highly variable side effect rate (22%–70%), mainly emesis and nausea. One retrospective study using N2O 50% in 52 patients found a very low side effect rate of 3.8%.32 As of the first study published in 2012,28 PSA with IN FENT and N2O 50% has been used frequently in our PED since 2013, and in the pediatric surgery outpatient clinic (PSOC) since 2015.
The aim of this study was to assess proportion and details of side effects of this combination in a large retrospective study to see whether data found in the smaller studies are accurate and whether prevention of side effects is recommended for N2O 50%.
Methods
Study Design. This was a single-center retrospective study. Procedures and thereby patients were identified by institutional mandatory N2O PSA evaluation sheets, and by the legally required documentation of opioid use for IN FENT. Procedures were included if patients were treated with IN FENT and N2O 50% in the PED (since March 2013) or the PSOC (since September 2015) until June 2017, irrespective of patient age. We excluded all patients with documented refusal of general consent (Figure). Of note, internal guidelines required a minimal age of 3 years for N2O application, and we rarely treat children older than 16 years in our PED or PSOC.
Figure.
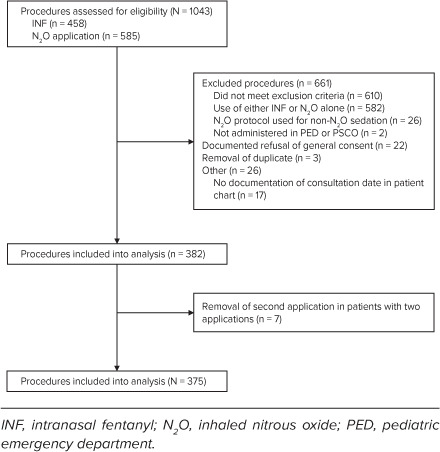
Study flowchart.
Data Collection and Quality Control. Patients were identified by codes (e.g., “A001”). Data including baseline characteristics, side effects, additional medication, and details on course of treatment were extracted from electronic or paper patient charts into paper case report forms, then transferred into a REDCap (Research Electronic Data Capture; Vanderbilt University, Nashville, TN) database.
Data extraction was done by one of the authors (RGV). Full data extraction was repeated by random sample of 50 patients and checked by the senior author (JH). Discrepancies were resolved by consensus. No systematic errors were detected. Extensive automated checks for plausibility and missing data were done in REDCap.
Definitions. The N2O PSA evaluation sheets contained questions regarding general information (e.g., first application of N2O, additional medication), duration of N2O application, details of surveillance (including sedation depth and vital parameters), and side effects. According to internal guidelines, patients and/or parents were questioned about side effects and satisfaction by health care professionals after procedure.
Primary Outcomes: Side Effects. The primary outcome was the proportion of patients with any side effect reported in N2O PSA evaluation sheets and patient charts.
Definition of side effect was taken from our institutional N2O PSA evaluation sheets. Side effects mentioned on the N2O PSA evaluation sheet were emesis, nausea, dizziness, dysphoria, excitation, hyperventilation, headache, as well as free space for documenting other side effects. This information had been specifically asked for and recorded after each application by the treating health care personnel.
Classification of interventions as well as outcomes of side effects were taken from the suggestions of Mason et al33 for reporting side effects in PSA.33 They used an international task force for creating a tool applicable to sedation in any location for reporting side effects.
Secondary Outcomes. Secondary outcomes were details on side effects (occurrence in relation to type of procedure), efficacy (success rate and satisfaction), pain, and use of additional medication.
Sedation depth was correlated with the validated University of Michigan Sedation Scale (UMSS).34 This scale has 5 levels of sedation from 0 to 4 (0 = awake and alert; 1 = minimally sedated; 2 = moderately sedated; 3 = deep sedation; 4 = unarousable).
Efficacy was defined by successful completion of the procedure.
Satisfaction was assessed by the following question on the N2O PSA evaluation sheets: Would the parents, and, if applicable, the patient, agree to have this PSA with IN FENT and N2O in the future?
Pain scales were chosen age appropriately, because no single scale is validated for all age groups.35 For comparison purposes in the analysis they were coded into 4 categories, namely A (no or mild pain) to D (severe pain) (Table 1). Information on pain was extracted from patient charts before and after the procedure and from N2O PSA evaluation sheets for pain during procedure.
Table 1.
Coding of Pain Scales *
| Code | KUSS | Knopf | FPS-R | VAS |
|---|---|---|---|---|
| A | 0–1 | 0 | 0 | 0–9 |
| B | 2–3 | 1 | 2–4 | 10–35 |
| C | 3–7 | 2–3 | 6 | 36–69 |
| D | 8–10 | 4 | 8–10 | 70–100 |
Statistical Analysis. Statistical analysis was done in R version 3.4.4.37 Assuming not normally distributed data, median and IQR were calculated. Five potential risk factors were identified from the literature7,28,29: age, application time of N2O, time between IN FENT application and start of N2O application, sedation depth, and additional centrally effective analgesics.
The association of potential risk factors of nausea and/or emesis was analyzed by non-parametric tests (Wilcoxon test or chi-square test). A p value < 0.05 was considered statistically significant.
Results
In total, 1043 patient procedures were assessed for eligibility. Of these, 611 did not meet inclusion criteria. Of the remaining 432 procedures, 51 (12%) were excluded (Figure). Of the remaining 381 (88%) procedures in 375 patients, 7 procedures were excluded because 7 patients received 2 applications on 2 different dates. For these 7 patients only the first procedure with application of IN FENT and N2O was included. Baseline characteristics are listed in Table 2. In 294 (91%) of 322 procedures with the respective information, patients received N2O for the first time.
Table 2.
Baseline Patient Characteristics *
| Characteristic | All Patients (n = 375) | Patients With Any Side Effect (n = 113) | Patients With Nausea and/or Emesis (n = 35) |
|---|---|---|---|
| Age, median (IQR), yr | 9.4 (3.1–15.9) | 9.9 (3.8–15.3) | 9.0 (3.8–15.0) |
| Sex, n (%) | |||
| Male | 230 (61) | 69 (61) | 22 (63) |
| Female | 145 (39) | 46 (39) | 13 (37) |
| Location of treatment, n (%) | |||
| PED | 271 (72) | 72 (64) | 27 (77) |
| PSOC | 104 (28) | 41 (36) | 8 (23) |
| Indication, n (%) | |||
| Reduction of fracture or luxation | 154 (41) | 42 (37) | 9 (26) |
| Pin removal | 95 (25) | 36 (32) | 7 (20) |
| Burn dressing | 35 (9) | 6 (5) | 5 (14) |
| Wound management | 24 (6) | 5 (4) | 3 (9) |
| Immobilization | 22 (6) | 7 (6) | 4 (12) |
| Laceration repair | 18 (5) | 7 (6) | 4 (11) |
| Abscess drainage | 11 (3) | 3 (4) | 0 |
| Other | 16 (4) | 7 (6) | 3 (9) |
INF, intranasal fentanyl; N2O, inhaled nitrous oxide; PED, pediatric emergency department; PSA, procedural sedation and analgesia; PSOC, pediatric surgery outpatient clinic
* Baseline characteristics of patients receiving N2O and INF for PSA.
In the PED the combination of IN FENT and N2O was mainly used for reduction of fracture or luxation (n = 154, 57%; 95% CI, 51–63), whereas at the PSOC it was mainly used for transcutaneous removal of pins (n = 93, 89%; 95% CI, 82–95).
Side Effects. Of 375 patients, 114 (30%) experienced some kind of side effect, mostly dizziness (n = 63, 17%), followed by nausea (n = 23, 6%) and emesis (n = 14, 4%) (Table 3). There were no serious side effects: no patient required assisted ventilation; none had clinically apparent pulmonary aspiration, laryngospasm or bronchospasm, cardiovascular instability, or permanent complications; none had an unplanned hospital admission or death.
Table 3.
Treatment Associated Side Effects
| Number | Proportion (95% CI)* | |
|---|---|---|
| Any side effect | 114 | 30 (26–35) |
| Dizziness | 63 | 17 (13–21) |
| Nausea | 23 | 6 (4–9) |
| Emesis | 14 | 4 (2–6) |
| Nausea and emesis | 2 | 0.5 (0.1–2) |
| Nausea and/or emesis | 35 | 9 (7–13) |
| Bradycardia | 7 | 2 (1–3) |
| Hyperventilation | 6 | 2 (1–3) |
| Excitation | 4 | 1 (0.2–3) |
| Headache | 4 | 1 (0.2–3) |
| O2 saturation <93% | 4 | 1 (0.2–3) |
| Apnea | 1 | 0.3 (0–1) |
| Other† | 32 | 9 (6–12) |
* Data are reported as percentage of 375 patients with 95% CI. Multiple side effects per patient (and thereby procedure) were possible.
† Other side effects mainly included fear, pain, sweating, and insufficient analgesia. See text for details
Most patients did not need any specific treatment for side effects, because they were self-resolving. Fourteen side effects in 12 patients needed minor intervention, mainly sweet drinks or glucose (3%; 95% CI, 2–6). Three patients with nausea (0.8%; 95% CI, 0.2–2.3) received antiemetics. Two patients received additional medication for a total of 3 side effects (lorazepam for additional sedation, and acetaminophen for pain relief) (0.5%; 95% CI, 0.06 to 1.9) after the intervention. Two patients needed tactile stimulation or administration of additional oxygen (1 patient with apnea and 1 patient with 02 saturation <93%) (0.5%; 95% CI, 0.06–1.9). In 11 patients the sedation was aborted owing to side effects (3%; 95% CI, 1–5). These side effects were nausea (2 patients), emesis (1 patient), malaise (1 patient), nightmare (1 patient), headache (1 patient), and irregularity in respiration (1 patient).
Of the 14 patients with emesis, 1 patient vomited during the procedure. On discontinuation of N2O, emesis resolved. No complication was noted in this case. One patient needed ketamine for successful completion of the procedure and vomited afterwards. Of the remaining patients, 9 vomited after the procedure. In 3 patients the time of emesis was not recorded.
Secondary Outcomes. Median IN FENT dosage was 1.5 μg/kg (1.45–1.55) (n = 347, 93%). Median duration of N2O application in the PED was 10.5 minutes (3.5–17.5) (n = 244, 90%), whereas in the PSOC it was 5.0 minutes (2.0–8.0) (n = 88, 85%). Median time between first IN FENT application and start of N2O was 3.0 minutes (−11 to 17) (n = 227, 61%). Sedation depth was documented in 194 patients (52%), and median sedation depth was UMSS 1 for these. Seven patients were deeply sedated (UMSS 4) (4%), 6 patients with UMSS 3 (3%).
One hundred thirty-seven patients received at least 1 additional centrally effective analgesic (37%); all but 1 were treated in the PED. In most cases (n = 97, 72%), this was more than 90 minutes before start of PSA for the initial pain treatment.
In 96 patients, information on pain both before and during the procedure was noted (26%). Medium pain code was B (mild to moderate pain) for both times.
In 349 patients the sedation was successfully concluded (93%; 95% CI, 90–95). Reasons for non-success were non-compliance or intolerance (n = 7, 1.9%), side effects (n = 7, 1.9%), too much pain or agitation (n = 5, 1.3%), unsuccessful procedure (n = 4, 1.1%), and other or unknown (n = 3, 0.8%).
Two hundred eighty patients were satisfied and would like the same sedation for similar procedures in the future (n = 299, 94%; 95% CI, 90–96). Nineteen patients were not satisfied (6%; 95% CI, 4–10), mainly because of pain or “strange sensation.”
Discussion
In our study we focused on the side effect rate of IN FENT when combined with inhaled N2O 50%. We found a side effect rate of 30% (114 patients), mainly dizziness (17%), followed by nausea (6%), emesis (4%), and a combined rate of nausea and/or emesis of 9%. No serious side effects were found. Median sedation depth was 1, using UMSS. Fourteen patients were deeply sedated with UMSS 3 or 4. One of the latter experienced emesis, but without further complications.
Previous prospective studies found a widely differing side effect rate for this combination in pediatric settings, most using a higher N2O concentration of 70%: In a single-center study in Australia, Seith et al28 found a side effect rate of 22% (n = 41), mainly emesis (19.5%), when using N2O 70% in 98% of patients. In a bicentric study in Australia and Canada, Hoeffe et al29 found an in-hospital side effect rate of 62% (n = 85), mainly nausea (19%), vertigo (23%), and emesis (13%), when using N2O 70% in 32% of patients. In a single-center study in Switzerland, Seiler et al30 found an in-hospital side effect rate of 23.8% (n = 206), mainly emesis (8.7%), nausea (8.3%), and vertigo (6.8%), when using only N2O 70%. In a second single-center study in Switzerland, the same authors31 found a side effect rate of 50% (n = 201), mainly nausea (25%), vertigo (16%), and emesis (15%), when using only N2O 70%. The main difference between the 2 studies by Seiler and colleagues was how the side effect rate was evaluated, as well as the study design (observational prospective collected data30 vs randomized double-blind study31).
In a single-center retrospective study in Spain, Miguez et al32 found a side effect rate of 3.8% (n = 52). They reported no cases of emesis. Information for assessment of side effects (mentioned by patient or explicitly asked) was not reported. They also did not mention whether they inquired about nausea as a side effect, thereby making comparison with our results difficult.
In comparison to the prospective studies, we found a similar overall side effect rate (30% vs 22% to 62%), but a lower rate of emesis (4% vs 8.7%–19.5%) and nausea (6% vs 8.3%–25%). Except for our N2O concentration (N2O 50% instead of N2O 70%), we had comparable baseline characteristics. Reasons for the difference in nausea and emesis could thus relate to the different N2O concentration. Inconsistency in the assessment of side effects can be found. For example, in the first study of Seiler et al,30 emesis was counted only if a patient had explicit complaints about emesis, whereas in the study of Hoeffe et al,29 in the second study by Seiler et al,31 and in our study, side effects including emesis were specifically noted for every patient. In the study of Seith et al,28 by contrary, no information for acquiring emesis cases is given. Another relevant difference is the concentration of N2O: All prospective studies used N2O 70%, at least for a substantial fraction,28–31 whereas we used N2O 50% exclusively. The consistent use of lower-concentration N2O could be a reason for our finding of a lower rate of nausea and emesis, as previous studies have suggested.10,11
Consistent with the previous findings of a vertigo rate of 23% in the study of Hoeffe et al29 and a vertigo rate of 16% in the study of Seiler at al,31 we found a dizziness rate of 17%. Compared with our rate of nausea and emesis, this rate seems elevated. This might be due to an inconsistent definition of dizziness: Because N2O changes the perception of our environment, dizziness in our study could be comparable to a “light-headedness” and not “vertigo.” If one assumes that dizziness is an expected effect of N2O instead of a side effect, this would reduce our side effect rate to a total of 16%.
Consistent with previous findings, we found no serious side effects or complications for a PSA with N2O and IN FENT.28–30 Additional sedative medication might have an effect on rate of nausea and/or emesis, but our data preclude a definitive statement on this (see Table 4). We can corroborate the low sedation depth already described.
Table 4.
Relationship of Treatment-Associated Side Effects to Potential Factors of Influence *
| Patients With Nausea and/or Emesis | Patients With Neither Nausea nor Emesis | p value | |
|---|---|---|---|
| Factor, median (IQR) | |||
| Age, yr | 9.0 (5.0 to 13.0) | 9.5 (4.1 to 14.8) | 0.75* |
| Dose of INF, μg/kg | 1.5 (1.4 to 1.5) | 1.5 (1.5 to 1.6) | 0.90* |
| NO2 total application time, min | 10.0 (0.8 to 19.3) | 10.0 (2 to 18) | 0.71* |
| Time from INF to start N2O, min | 0 (−5 to +5) | 4 (−10 to +18) | 0.11* |
| Depth of sedation | 1 (0 to 2) | 1 (0 to 2) | 0.38* |
|
| |||
| Additional centrally effective analgesics, X | n = 35 | n = 340 | |
| Independent time of application | 19 of 35 | 120 of 340 | 0.03† |
| Added <1.5 hr before N2O | 6 of 35 | 38 of 340 | 0.30† |
| Unknown added time and <1.5 hr before N2O | 9 of 35 | 53 of 340 | 0.12† |
INF, intranasal fentanyl; N2O, inhaled nitrous oxide; X= opioids or ketamine
* Wilcoxon test;
† Chi-square test.
We found that additional centrally effective analgesics had been given in 4 of 14 patients with emesis. Three of 7 deeply sedated patients (UMSS 4) received additional centrally effective analgesics less than 90 minutes before start of procedure. Data for additional medication were mentioned in only 1 prospective study.30 In this study other administrated medications included only paracetamol (62%), ibuprofen (65%), and—as the only additional centrally effective analgesic—midazolam (2.4%). No data were found for when midazolam was administrated.
We found no change in the medium pain code during the procedure compared with before the procedure. This result suggests good pain efficiency of this combination. However, pain information was sparse in our database and therefore no final conclusions regarding pain can be made.
Efficacy, defined as successful completion of the procedure, was high in our study (94%). The satisfaction rate reported by Seiler et al30 (95.6%) and Hoeffe et al29 (88%) was similar to ours. There is no clear consensus in pediatric sedation literature on what is actually considered efficacious. As previously done in literature, we used the one that is clearest.38
Frequent side effects of sedation can sometimes be mitigated by the use of another medication, in this case by the prophylactic administration of an antiemetic. We found a number needed to treat of 25 to prevent emesis (4%). For preventing emesis and nausea (9%), number needed to treat would be 11. We therefore found no indication for a recommendation of adding preventive antiemetics as suggested by Seith et al28 and Hoeffe et al.29 We therefore conclude and recommend IN FENT and N2O 50% as an effective and safe treatment in the PED and the PSOC.
The main limitation of our study is its retrospective design. However, prospective use of N2O PSA evaluation sheets was compulsory throughout the period studied, thus side effects had been prospectively collected. Further strength is a high number of patients in comparison to previous studies. We expect only a few missed cases and complete information on side effect and patient satisfaction, as data collection has been done prospectively because of mandatory N2O PSA evaluation sheets. However, our N2O PSA evaluation sheet had no option to explicitly choose “no side effects.”
Conclusion
In this retrospective study, we found a very low rate of nausea and/or emesis (n = 35, 9%) in children receiving IN FENT and inhaled N2O 50% for PSA. Combined with our high satisfaction rate of 94%, we conclude that this combination can be recommended as an effective and safe treatment in the PED and the PSOC. Primary prophylaxis with antiemetics is not indicated owing to the low incidence of nausea and emesis.
ABBREVIATIONS
- IN FENT
intranasal fentanyl
- IV
intravenous
- N2O
inhaled nitrous oxide
- PED
pediatric emergency department
- PSA
procedural sedation and analgesia
- PSOC
pediatric surgery outpatient clinic
- UMSS
University of Michigan Sedation Scale
Footnotes
Disclosures. The authors report no conflict of interest. Supported by a grant from the Stiftung Batzebaer of the University Children's Hospital, Bern, Switzerland, and the Notfallzentrum fuer Kinder und Jugendliche, University Hospital, Bern, Switzerland.
Ethical Approval and Informed Consent. Ethical consent by the Cantonal Ethical Committee of Berne was granted for this study. Given the nature of this study, informed consent was not required.
References
- 1.Sahyoun C, Krauss B. Clinical implications of pharmacokinetics and pharmacodynamics of procedural sedation agents in children. Curr Opin Pediatr . 2012;24(2):225–232. doi: 10.1097/MOP.0b013e3283504f88. [DOI] [PubMed] [Google Scholar]
- 2.Bhatt M, Kennedy RM, Osmond M et al. Consensus-based recommendations for standardizing terminology and reporting adverse events for emergency department procedural sedation and analgesia in children. Ann Emerg Med . 2009;53(4):426–435.e4. doi: 10.1016/j.annemergmed.2008.09.030. [DOI] [PubMed] [Google Scholar]
- 3.Roback MG, Carlson DW, Babl FE, Kennedy RM. Update on pharmacological management of procedural sedation for children. Curr Opin Anaesthesiol . 2016;29(suppl 1):S21–S35. doi: 10.1097/ACO.0000000000000316. [DOI] [PubMed] [Google Scholar]
- 4.Krauss B, Green SM. Procedural sedation and analgesia in children. Lancet . 2006;367(9512):766–780. doi: 10.1016/S0140-6736(06)68230-5. [DOI] [PubMed] [Google Scholar]
- 5.Ramaiah R, Bhananker S. Pediatric procedural sedation and analgesia outside the operating room: anticipating, avoiding and managing complications. Expert Rev Neurother . 2011;11(5):755–763. doi: 10.1586/ern.11.52. [DOI] [PubMed] [Google Scholar]
- 6.Shavit I, Keidan I, Augarten A. The practice of pediatric procedural sedation and analgesia in the emergency department. Eur J Emerg Med . 2006;13(5):270–275. doi: 10.1097/00063110-200610000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Borland M, Esson A, Babl F, Krieser D. Procedural sedation in children in the emergency department: a PREDICT study. Emerg Med Australas . 2009;21(1):71–79. doi: 10.1111/j.1742-6723.2008.01150.x. [DOI] [PubMed] [Google Scholar]
- 8.Tobias JD. Applications of nitrous oxide for procedural sedation in the pediatric population. Pediatr Emerg Care . 2013;29(2):245–265. doi: 10.1097/PEC.0b013e318280d824. [DOI] [PubMed] [Google Scholar]
- 9.Zier JL, Tarrago R, Liu M. Level of sedation with nitrous oxide for pediatric medical procedures. Anesth Analg . 2010;110(5):1399–1405. doi: 10.1213/ANE.0b013e3181d539cf. [DOI] [PubMed] [Google Scholar]
- 10.Babl F, Oakley E, Seaman C et al. High-concentration nitrous oxide for procedural sedation in children: adverse events and depth of sedation. Pediatrics . 2008;121(3):e528–e532. doi: 10.1542/peds.2007-1044. [DOI] [PubMed] [Google Scholar]
- 11.Babl F, Barnett P, Oakley E, Spicer M. Preprocedural fasting state and adverse events in children receiving nitrous oxide for procedural sedation and analgesia. Pediatr Emerg Care . 2005;21(11):8. doi: 10.1097/01.pec.0000186427.07636.fc. [DOI] [PubMed] [Google Scholar]
- 12.Babl F, Oakley E, Puspitadewi A, Sharwood L N. Limited analgesic efficacy of nitrous oxide for painful procedures in children. Emerg Med J . 2008;25(11):717–721. doi: 10.1136/emj.2007.053751. [DOI] [PubMed] [Google Scholar]
- 13.Grape S, Schug SA, Lauer S, Schug BS. Formulations of fentanyl for the management of pain. Drugs . 2010;70(1):57–72. doi: 10.2165/11531740-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 14.Foster D, Upton R, Christrup L, Popper L. Pharmacokinetics and pharmacodynamics of intranasal versus intravenous fentanyl in patients with pain after oral surgery. Ann Pharmacother . 2008;42(10):1380–1387. doi: 10.1345/aph.1L168. [DOI] [PubMed] [Google Scholar]
- 15.Shelley K. The clinical applications of intranasal opioids. Current Drug Delivery . 2008;5(1):4. doi: 10.2174/156720108783330989. [DOI] [PubMed] [Google Scholar]
- 16.Hansen MS, Mathiesen O, Trautner S, Dahl JB. Intranasal fentanyl in the treatment of acute pain—a systematic review. Acta Anaesthesiol Scand . 2012;56(4):407–419. doi: 10.1111/j.1399-6576.2011.02613.x. [DOI] [PubMed] [Google Scholar]
- 17.Schug S A, Ting S. Fentanyl formulations in the management of pain: an update. Drugs . 2017;77(7):747–763. doi: 10.1007/s40265-017-0727-z. [DOI] [PubMed] [Google Scholar]
- 18.Borland ML, Clark LJ, Esson A. Comparative review of the clinical use of intranasal fentanyl versus morphine in a paediatric emergency department. Emerg Med Australas . 2008;20(6):515–520. doi: 10.1111/j.1742-6723.2008.01138.x. [DOI] [PubMed] [Google Scholar]
- 19.Saunders M, Adelgais K, Nelson D. Use of intranasal fentanyl for the relief of pediatric orthopedic trauma pain. Acad Emerg Med . 2010;17(11):1155–1161. doi: 10.1111/j.1553-2712.2010.00905.x. [DOI] [PubMed] [Google Scholar]
- 20.Christrup LL, Foster D, Popper LD et al. Pharmacokinetics, efficacy, and tolerability of fentanyl following intranasal versus intravenous administration in adults undergoing third-molar extraction: a randomized, double-blind, double-dummy, two-way, crossover study. Clin Ther . 2008;30(3):469–481. doi: 10.1016/j.clinthera.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Crellin D, Ling RX, Babl FE. Does the standard intravenous solution of fentanyl (50 microg/mL) administered intra-nasally have analgesic efficacy. Emerg Med Australas . 2010;22(1):62–67. doi: 10.1111/j.1742-6723.2010.01257.x. [DOI] [PubMed] [Google Scholar]
- 22.Murphy A, O'Sullivan R, Wakai A et al. Intranasal fentanyl for the management of acute pain in children [review] Cochrane Database Syst Rev . 2014;2014(10):CD009942. doi: 10.1002/14651858.CD009942.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borland M, Milsom S, Esson A. Equivalency of two concentrations of fentanyl administered by the intranasal route for acute analgesia in children in a paediatric emergency department: a randomized controlled trial. Emerg Med Australas . 2011;23(2):202–208. doi: 10.1111/j.1742-6723.2011.01391.x. [DOI] [PubMed] [Google Scholar]
- 24.Reynolds J, Rogers A, Medellin E et al. A prospective, randomized, double-blind trial of intranasal dexmedetomidine and oral chloral hydrate for sedated auditory brainstem response (ABR) testing. Paediatr Anaesth . 2016;26(3):286–293. doi: 10.1111/pan.12854. [DOI] [PubMed] [Google Scholar]
- 25.Borland M, Jacobs I, King B, O'Brien D. A randomized controlled trial comparing intranasal fentanyl to intravenous morphine for managing acute pain in children in the emergency department. Ann Emerg Med . 2007;49(3):335–340. doi: 10.1016/j.annemergmed.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 26.Graudins A, Meek R, Egerton-Warburton D et al. The PICHFORK (Pain in Children Fentanyl or Ketamine) Trial: a randomized controlled trial comparing intranasal ketamine and fentanyl for the relief of moderate to severe pain in children with limb injuries. Ann Emerg Med . 2015;65(3):248–254.e1. doi: 10.1016/j.annemergmed.2014.09.024. [DOI] [PubMed] [Google Scholar]
- 27.Roback MG, Wathen JE, Bajaj L, Bothner JP. Adverse events associated with procedural sedation and analgesia in a pediatric emergency department: a comparison of common parenteral drugs. Acad Emerg Med . 2005;12(6):508–513. doi: 10.1197/j.aem.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 28.Seith RW, Theophilos T, Babl FE. Intranasal fentanyl and high-concentration inhaled nitrous oxide for procedural sedation: a prospective observational pilot study of adverse events and depth of sedation. Acad Emerg Med . 2012;19(1):31–36. doi: 10.1111/j.1553-2712.2011.01241.x. [DOI] [PubMed] [Google Scholar]
- 29.Hoeffe J, Doyon Trottier E, Bailey B et al. Intranasal fentanyl and inhaled nitrous oxide for fracture reduction: The FAN observational study. Am J Emerg Med . 2017;35(5):710–715. doi: 10.1016/j.ajem.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 30.Seiler M, Landolt MA, Staubli G. Nitrous oxide 70% for procedural analgosedation in a pediatric emergency department with or without intranasal fentanyl. Pediatr Emerg Care . 2019;35(11):755–759. doi: 10.1097/PEC.0000000000001213. [DOI] [PubMed] [Google Scholar]
- 31.Seiler M, Staubli G, Landolt MA. Combined nitrous oxide 70% with intranasal fentanyl for procedural analgosedation in children: a prospective, randomised, double-blind, placebo-controlled trial. Emerg Med J . 2019;36(3):142–147. doi: 10.1136/emermed-2018-207892. [DOI] [PubMed] [Google Scholar]
- 32.Miguez MC, Ferrero C, Rivas A et al. Retrospective comparison of intranasal fentanyl and inhaled nitrous oxide to intravenous ketamine and midazolam for painful orthopedic procedures in a pediatric emergency department. Pediatr Emerg Care . 2021;37(3):e136–e140. doi: 10.1097/PEC.0000000000001788. [DOI] [PubMed] [Google Scholar]
- 33.Mason KP, Green SM, Piacevoli Q. Adverse event reporting tool to standardize the reporting and tracking of adverse events during procedural sedation: a consensus document from the World SIVA International Sedation Task Force. Br J Anaesth . 2012;108(1):13–20. doi: 10.1093/bja/aer407. [DOI] [PubMed] [Google Scholar]
- 34.Malviya S, Voepel-Lewis T, Tait AR et al. Depth of sedation in children undergoing computed tomography: validity and reliability of the University of Michigan Sedation Scale (UMSS) Br J Anaesth . 2002;88(2):241–245. doi: 10.1093/bja/88.2.241. [DOI] [PubMed] [Google Scholar]
- 35.Birnie KA, Hundert AS, Lalloo C et al. Recommendations for selection of self-report pain intensity measures in children and adolescents: a systematic review and quality assessment of measurement properties. Pain . 2019;160(1):5–18. doi: 10.1097/j.pain.0000000000001377. [DOI] [PubMed] [Google Scholar]
- 37.R Core Team Vienna, Austria: R Foundation for Statistical Computing; 2018. R: a language and environment for statistical computing. Accessed 05 02, 2018. https://www.R-project.org/ [Google Scholar]
- 38.Sirimontakan T, Artprom N, Anantasit N. Efficacy and safety of pediatric procedural sedation outside the operating room. Anesth Pain Med . 2020;10(4):e106493. doi: 10.5812/aapm.106493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hicks CL, von Baeyer CL, Spafford P et al. The Faces Pain Scale - Revised: Toward a common metric in pediatric pain measurement. Pain . 2001;93:173–183. doi: 10.1016/S0304-3959(01)00314-1. [DOI] [PubMed] [Google Scholar]
- 40.Hester N. O. Pediatric pain . 1979. “Measurements of pain in children: generalizability and validity of the pain ladder and the poker chip tool.”; pp. 79–94. [Google Scholar]
- 41.Unbehagens Kindliche, Schmerzskala K.U. S. S. Schmerztherapie bei Kindern . 2005. «Anhang B: Schmerzbeurteilung.»; p. 371. [Google Scholar]
- 42.Benini F, Griffith P, Lago P et al. Evaluating pain in children: experience with a “do-it-yourself” visual analogue scale. Acta Pædiatrica . 1996;1996;85:762–762. doi: 10.1111/j.1651-2227.1996.tb14147.x. [DOI] [PubMed] [Google Scholar]


