Abstract
Cystic fibrosis transmembrane conductance regulator (CFTR) modulators have revolutionized care for patients with cystic fibrosis (CF). The triple combination product elexacaftor/tezacaftor/ivacaftor is a highly effective CFTR modulator that is generally well tolerated. However, in clinical trials of pediatric and adult patients, 4% to 12% developed rash after initiation of therapy. Few reports have described approaches to management of this adverse effect. In this report, we describe 2 children with CF who developed a pruritic, maculopapular rash after initiating elexacaftor/tezacaftor/ivacaftor. These patients were successfully rechallenged after rash resolution with a practical titration schedule.
Keywords: CFTR modulator, cystic fibrosis, rash
Introduction
The treatment of cystic fibrosis (CF) has been transformed with the introduction of CF transmembrane conductance regulator (CFTR) modulators. Most recently, elexacaftor/tezacaftor/ivacaftor (ELX/TEZ/IVA) was approved for use in patients at least 6 years of age who have at least 1 F508del or a responsive mutation in the CFTR gene based on in vitro data. Studies have demonstrated that ELX/TEZ/IVA significantly increases percent predicted forced expiratory volume in 1 second (ppFEV1) by >10% across all age groups, reduces pulmonary exacerbation rates, decreases sweat chloride concentrations, and increases body mass index.1–3 With this approval, approximately 90% of patients with CF now have access to CFTR modulator therapy.
Upon initiation, ELX/TEZ/IVA is generally well tolerated for most patients. However, an overall greater percentage of patients being treated with ELX/TEZ/IVA have experienced rash compared with patients receiving other CFTR modulators. Approximately 11% (n = 22 of 202) of patients in the phase 3 trial of patients ages 12 years and older with a single F508del mutation reported experiencing generalized, erythematous, macular, or pruritic rashes, compared with 6.5% (n = 13 of 201) in the placebo group.1 One patient receiving ELX/TEZ/IVA discontinued therapy because of development of a rash. In the phase 3 trial of patients with homozygous F508del mutations, 2 of 55 patients in the treatment group and 2 of 52 patients in the comparison group being treated with tezacaftor/ivacaftor experienced rashes.2 In both studies, investigators indicated that this adverse effect occurred more frequently in female patients, especially those who used hormonal contraceptives. Approximately 24% (n = 16 of 66) of patients in the phase 3 trial of patients aged 6 to 11 years with a single F508del mutation reported experiencing erythematous, maculopapular, popular, or pruritic rashes or skin exfoliation. Ultimately, 8 of these patients (12% of total) had rashes thought to be directly caused by ELX/TEZ/IVA, and 1 patient discontinued therapy as a result.3 In addition to rash, common adverse effects include headache, upper respiratory tract infection, abdominal pain, diarrhea, and elevated liver function tests.4
Depending on severity of drug rash, discontinuation and symptom management may be necessary in clinical practice. However, permanent discontinuation of ELX/TEZ/IVA could be detrimental to patients because of the significant positive impact this medication can have on their disease. Currently, there are limited published data or manufacturer recommendations describing reinitiation of therapy after CFTR modulator-induced rash. Herein, we describe 2 patients who developed pruritic, maculopapular rash upon initiation of ELX/TEZ/IVA and our approach to acute management and rechallenge with a practical titration schedule.
Case 1
A 12-year-old, 52-kg female CF patient (F508del/5T and M470V) began elexacaftor 100 mg/tezacaftor 50 mg/ivacaftor 75 mg two (orange) tablets every morning and ivacaftor 150 mg one (blue) tablet every evening. This was her first lifetime exposure to any CFTR modulator, and she was not on any interacting medications. Her baseline ppFEV1 was 83%. On the first day of therapy, she developed a pruritic, maculopapular rash on her lower back, eyelids, ears, knees, hands, and head 30 minutes after the first morning dose that progressively worsened throughout the day (Image 1). She also reported right hand and knee pain and the sensation of swelling in the throat without lip swelling, tongue swelling, or shortness of breath. The patient received the evening dose along with 25 mg (0.48 mg/kg/dose) of diphenhydramine. Upon contacting the provider team on day 2 of therapy to report these symptoms, the patient was instructed to stop taking ELX/TEZ/IVA and start taking diphenhydramine 50 mg (0.98 mg/kg/dose) every 4 hours as needed and once-daily fexofenadine 180 mg (3.46 mg/kg/dose). The patient experienced no relief after 24 hours of diphenhydramine and was prescribed a 3-day course of prednisone 60 mg (1.15 mg/kg/dose) once daily. Additionally, the patient used oatmeal baths, oatmeal lotion, and aloe vera with no relief. The rash started to improve after starting prednisone. Ultimately, the rash and other symptoms completely resolved 17 days after ELX/TEZ/IVA discontinuation. All other causes of the skin rash were ruled out. This patient's Naranjo Adverse Drug Reaction (ADR) Probability Scale score can be found in Supplemental Table S1. Based on the score the ADR was probable.
Image 1.
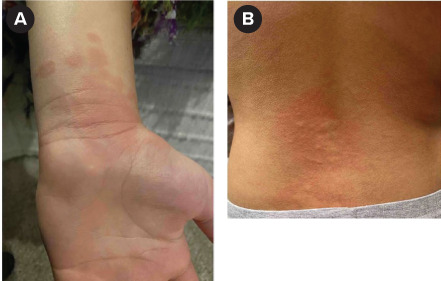
(a) Left palm and (b) lower back of case 1 on first day of elexacaftor/tezacaftor/ivacaftor therapy.
Approximately 43 days after discontinuation, the patient's ppFEV1 had declined to 71%, and the decision was made to reinitiate ELX/TEZ/IVA in hopes of helping her declining lung function. Therapy was titrated by one-quarter tablet every 2 days for a total of 22 days (Table). The patient was instructed to take fexofenadine 180 mg daily beginning on day 1 of the titration. On day 14 of reinitiation, the patient experienced a brief episode of upper back pain and shortness of breath while walking and mild pruritus of the neck, back, and lower extremity with no visible rash. The patient was instructed to take an additional 2 days of three-quarters orange tablet and 1 blue tablet daily. These symptoms ultimately resolved after 1 dose of diphenhydramine 50 mg daily. She continued the remainder of the titration schedule without adverse effects and continued daily fexofenadine administration for 7 days after reaching full dose. After the patient had been maintained on full-dose ELX/TEZ/IVA for approximately 2 months, her ppFEV1 had dramatically improved to 107%. The patient has since remained on full-dose ELX/TEZ/IVA for 6 months and is tolerating well.
Table.
Elexacaftor/Tezacaftor/Ivacaftor Dose Titration Schedule
| Day | Morning Dose (With Breakfast) | Evening Dose (With Dinner) |
|---|---|---|
| 1 | ¼ blue* tab | None |
| 2 | ¼ blue tab | None |
| 3 | ½ blue tab | None |
| 4 | ½ blue tab | None |
| 5 | ¾ blue tab | None |
| 6 | ¾ blue tab | None |
| 7 | 1 blue tab | None |
| 8 | 1 blue tab | None |
| 9 | ¼ orange† tab | 1 blue tab |
| 10 | ¼ orange tab | 1 blue tab |
| 11 | ½ orange tab | 1 blue tab |
| 12 | ½ orange tab | 1 blue tab |
| 13 | ¾ orange tab | 1 blue tab |
| 14 | ¾ orange tab | 1 blue tab |
| 15 | 1 orange tab | 1 blue tab |
| 16 | 1 and ¼ orange tab | 1 blue tab |
| 17 | 1 and ¼ orange tab | 1 blue tab |
| 18 | 1 and ½ orange tab | 1 blue tab |
| 19 | 1 and ½ orange tab | 1 blue tab |
| 20 | 1 and ¾ orange tab | 1 blue tab |
| 21 | 1 and ¾ orange tab | 1 blue tab |
| 22 | 2 orange tabs | 1 blue tab |
| 23 | 2 orange tabs | 1 blue tab |
* Ivacaftor 150 mg.
† Elexacaftor 100 mg/tezacaftor 50 mg/ivacaftor 75 mg.
Case 2
A 14-year-old, 56-kg male patient with CF (Y569D/Y569D) was initiated on elexacaftor 100 mg/tezacaftor 50 mg/ivacaftor 75 mg two (orange) tablets every morning and ivacaftor 150 mg one (blue) tablet every evening after his nasal epithelial cells responded to ELX/TEZ/IVA in the laboratory. This was his first lifetime exposure to any CFTR modulator, and he was not on any interacting medications. His baseline ppFEV1 was 95%. On day 8 of therapy, the patient developed a pruritic, maculopapular rash on his hands that spread to his feet, palms, knee, and face (Image 2). He was instructed to stop taking ELX/TEZ/IVA and take diphenhydramine 50 mg (0.89 mg/kg/dose) once followed by 25 mg (0.45 mg/kg/dose) every 4 to 6 hours as needed. The patient was seen in clinic the following day and other causes of skin rash were ruled out. This patient's Naranjo ADR Probability Scale score can be found in Supplemental Table S2. Based on the score the ADR was probable . He was instructed to reinitiate ELX/TEZ/IVA 7 days after resolution of the rash (10 days after stopping ELX/TEZ/IVA) following the titration schedule in the Table. At this visit, his ppFEV1 was 87%. He successfully completed the rechallenge with no adverse effects. Approximately 2 months after reinitiation, ppFEV1 had fallen to 77%, although this reading was taken at a different center. He has been on full-dose elexacaftor/tezacaftor/ivacaftor for 4 months and is tolerating it well.
Image 2.
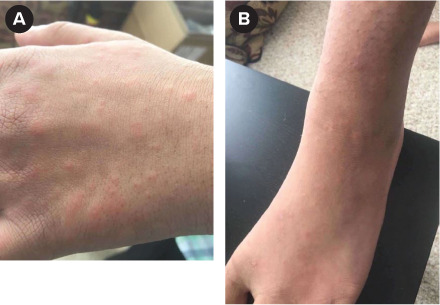
(a) Left hand and (b) left foot of case 2 on eighth day of elexacaftor/tezacaftor/ivacaftor therapy.
Discussion
Given the significant clinical benefit of CFTR modulators in improving lung function and the limited number of treatment options for patients with CF, complete discontinuation of ELX/TEZ/IVA after rash development may be an unacceptable option. Currently, there is limited guidance in both management of this adverse effect and rechallenge after rash resolution.
There are no published reports of pediatric patients who received ELX/TEZ/IVA rechallenge after developing rash. There have been 2 case series5,6 that described adult patients who had successful outpatient rechallenge using commercially available tablets. One case series described a desensitization protocol for 2 adult patients.7 Using aliquots of ELX/TEZ/IVA prepared by a pharmacy using trituration methods, the drug was reintroduced in increasing amounts during a 15-day period to both patients without recurrence of rash. A similar strategy was described by Patterson et al8 in an adult patient who experienced a morbilliform drug eruption with ivacaftor therapy. Although these approaches were successful, specialty pharmaceutical compounding may not be available, affordable, feasible, or necessary for all patients. However, it is important to note that our approach of requesting families to split non-scored ELX/TEZ/IVA tablets is not recommended by the US Pharmacopeia.9 This may be especially difficult if attempting to divide the smaller elexacaftor 50 mg/tezacaftor 25 mg/ivacaftor 37.5 mg tablets. Splitting and weighing tablets in a controlled environment (e.g., preparation by the CF pharmacist) would ensure the most accurate dose is delivered.
Not all drug-induced rash phenotypes may be appropriate for rechallenge. Traditional drug desensitization differs from our approach in that increasing doses beginning at 1/10,000 to 1/1,000,000 of the therapeutic dose are administered to the patient. The patients presented here were thought to have experienced a mild to moderate, non-allergic, ADR, and therefore deemed to be appropriate by our CF physicians and pharmacist for a less intense drug rechallenge. If considering rechallenging patients after development of rash due to ELX/TEZ/IVA, providers should consider rash severity, time to resolution, lung function and severity of CF, likelihood of patient follow-up, interacting medications, and availability and experience with alternative modulators. Some situations might require more frequent follow-up, such as the rash development on day 14 of rechallenge in case 1. Additionally, referral to allergy and immunology might be warranted in some cases based on severity of the reaction.
The patients described in this report completed successful ELX/TEZ/IVA rechallenge with a practical titration schedule. The administration of antihistamines with or without a short course of glucocorticosteroids might have aided in the resolution of rash. If attempting to rechallenge ELX/TEZ/IVA after the development of rash, careful consideration should be made for severity and duration of the reaction and the feasibility of careful monitoring by the provider team in partnership with the patient and/or caregiver. As ELX/TEZ/IVA becomes available to more age groups, we expect additional patients will experience rash. Additional reports and studies are needed to help guide clinicians in managing CFTR modulator drug rash and the best methods for drug rechallenge.
Supplementary Material
ABBREVIATIONS
- ADR
adverse drug reaction
- CF
cystic fibrosis
- CFTR
cystic fibrosis transmembrane conductance regulator
- ELX/TEZ/IVA
elexacaftor/tezacaftor/ivacaftor
- ppFEV1
percent predicted forced expiratory volume in 1 second
Footnotes
Disclosures. The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment gifts, and honoraria. PJM receives grant support from the Cystic Fibrosis Foundation, Eloxx, and Vertex Pharmaceuticals. Authors had full access to all patient information in this report and take responsibility for the integrity and accuracy of the report.
Ethical Approval and Informed Consent. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and have been approved by the appropriate committees at our institution. However, given the nature of this study, informed consent was not required by our institution.
Supplemental Material. DOI: 10.5863/1551-6776-27.5.463.S1, DOI: 10.5863/1551-6776-27.5.463.S2
References
- 1.Middleton PG, Mall MA, Dřevínek P et al. Elexacaftor-tezacaftor-ivacaftor for cystic fibrosis with a single Phe-508del allele. N Engl J Med . 2019;381(19):1809–1819. doi: 10.1056/NEJMoa1908639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heijerman HGM, McKone EF, Downey DG et al. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet . 2019;394(10212):1940–1948. doi: 10.1016/S0140-6736(19)32597-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zemanick E, Taylor-Cousar J, Davies J et al. A phase 3 open-label study of ELX/TEZ/IVA in children 6 through 11 years of age with CF and at least one F508del allele. Am J Respir Crit Care Med . 2021;203(12):1522–1532. doi: 10.1164/rccm.202102-0509OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boston, MA: Vertex Pharmaceuticals Inc; Oct, 2021. TRIKAFTA (elexacaftor, tezacaftor and ivacaftor tablets) [package insert] [Google Scholar]
- 5.Hu MK, Wood G, Dempsey O. “Triple therapy” (elexacaftor, tezacaftor, ivacaftor) skin rash in patients with cystic fibrosis. Postgrad Med J . 2022;98(1156):86. doi: 10.1136/postgradmedj-2020-139264. [DOI] [PubMed] [Google Scholar]
- 6.Balijepally R, Kwong D, Zhu L et al. Elexacaftor/tezacaftor/ivacaftor outpatient desensitization. Ann Allergy Asthma Immunol . 2022;128(1):104–105. doi: 10.1016/j.anai.2021.08.010. [DOI] [PubMed] [Google Scholar]
- 7.Leonhardt K, Autry E, Kuhn R, Wurth M. CFTR modulator drug desensitization: preserving the hope of long term improvement. Pediatr Pulmonol . 2021;56(8):2546–2552. doi: 10.1002/ppul.25437. [DOI] [PubMed] [Google Scholar]
- 8.Patterson A, Autry E, Kuhn R et al. Ivacaftor drug desensitization. Pediatr Pulmonol . 2019;54(6):672–674. doi: 10.1002/ppul.24319. [DOI] [PubMed] [Google Scholar]
- 9.Green G, Berg C, Polli J et al. Pharmacopeial Standards for the Subdivision Characteristics of Scored Tablets. Pharmacopeial Forum . 2009;35(6):1598–1612. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


