Abstract
Proteins are functional building blocks of living organisms that exert a wide variety of functions, but their synthesis and industrial production can be cumbersome and expensive. By contrast, short peptides are very convenient to prepare at a low cost on a large scale, and their self-assembly into nanostructures and gels is a popular avenue for protein biomimicry. In this Review, we will analyze the last 5-year progress on the incorporation of bioactive motifs into self-assembling peptides to mimic functional proteins of the extracellular matrix (ECM) and guide cell fate inside hydrogel scaffolds.
Keywords: self-assembly, peptides, proteins, RGD, collagen, ECM, hydrogels, biomaterials, biomimicry, nanofibrils
1. Introduction
Proteins exert a wide variety of functions in cells and living organisms and can be considered one of the key building blocks of life. It is thus not surprising that they are very popular components in the development of new solutions as biomaterials for applications in medicine [1,2,3,4], cosmetics [5,6] and food science [7], including highly biotechnological products, such as cultured meat [8]. Collagen [9,10], gelatin [11,12,13,14], keratin [15,16,17], and silk fibroin [18,19] are amongst the most widely used proteins to develop biomaterials.
However, proteins present also limitations, such as low oral bioavailability, for which new nanotechnological carriers are continuously being developed [20]. Furthermore, their production on a large scale can be costly, inefficient, and present batch-to-batch high variability, also in terms of purity, correct folding, and, thus, activity. For all these reasons, new nanotechnological alternatives for protein biomimicry are highly sought after, to reduce costs and increase efficiency both for the production process and for the final product performance and lifetime [21]. Amongst the various substitutes for their mimicry, short peptide sequences based on bioactive motifs certainly play an elected role. Peptide-protein interactions are indeed crucial for the design of biomaterials [22]. In particular, the use of minimalistic bioactive motifs to this end was exhaustively reviewed in 2017 [23], and for this reason, here we will cover the recent progress in the field made since then.
2. Self-Assembling Short Peptides with Bioactive Motifs for Hydrogel Biomaterials
Over the last three decades, great efforts have been devoted to the design of self-assembling peptides to attain nanostructured biomaterial gels. Readers interested in the details of their various types of design are recommended to read a recent book chapter that provides a comprehensive overview of the topic [24]. Several recent reviews also cover this area [25,26], as well as the use of enzymes to control self-assembly [27], applications for the delivery of drugs and therapeutics [28,29,30,31,32,33], proteins [34,35], and, more generally biomedical uses [36], with a specific focus on antimicrobials [37], cancer [38], and wound healing too [39].
Briefly, the vast majority of peptides for self-assembly into hydrogels are amphipathic in nature. Self-assembly in water is hydrophobically driven, and aromatic components play an elected role in the stabilization of steric zippers and hydrophobic interactions that hold together the peptide superstructures. Hydrophilic components are crucial to ensure good water solubility and hydrogelation ability, and often are involved in weak interactions, such as H-bonding, binding peptides together [24]. Popular approaches for their design use complementary charges alternated with hydrophobic amino acids [40,41], polyaromatic N-caps [42], peptide amphiphiles (PA) [43], or heterochirality [44], to attain an amphipathic character for correct self-assembly and hydrogelation (Figure 1).
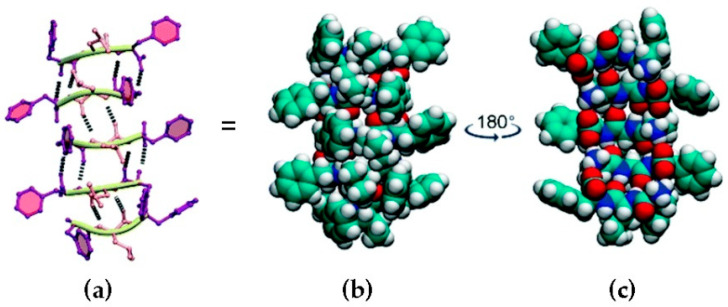
Figure 1.
Heterochiral peptide self-assembly. l-Phe-d-Leu-l-Phe forms stacks held together by H-bonding between amides (a), and space-fill representations (b,c) show the amphipathic character of the stacks, which display a hydrophobic face with the peptide sidechains (b) and a hydrophilic face with amide bonds (c). Carbon atoms are shown in green, hydrogen in white, nitrogen in blue, and oxygen in red. Reproduced from [45].
Considering that the shorter the motif, the lower and easier will be the cost of production, it is thus not surprising that ultra-short peptides are amongst the most attractive supramolecular peptide gelators [46]. In particular, cyclic [47,48,49] or linear dipeptides [50,51,52,53,54,55] are amongst the simplest options as building blocks for hydrogels, however, tripeptides are better positioned for bioactivity. Indeed, it was shown that 25 atoms other than hydrogen constitute the ideal size for drugs and drug-like molecules for maximal ligand-binding efficacy and bioactivity, and this number corresponds to the average number for a tripeptide [56]. Indeed, there are several tripeptides, or slightly longer sequences, that are used as bioactive motifs for protein mimicry in nanostructured hydrogel biomaterials [23], and the examples reported in the last 5 years are summarized in Table 1.
Table 1.
Latest reported supramolecular peptide-based hydrogels with bioactive motifs.
| Bioactive Sequence | Gelator | Function | Model | Ref. |
|---|---|---|---|---|
| RGD(S) and mimic | KFE-RGD KFE-RDG KFE-8 |
Cell adhesion | hMSC | [58] |
| RADA16 | Cell adhesion | 3T3 cells | [59] | |
| G-Y sequence | Cell adhesion | L929 cells | [60] | |
| RGDSGAITIGC | Cell proliferation | 3T3 cells | [61] | |
| E3-PA E3G3Ada-PA |
Cell adhesion | 3T3 cells | [62] | |
| Fmoc-FF Fmoc-RGD |
Cell adhesion and Differentiation |
3A6 cells Mice |
[63] | |
| Fmoc-FF Fmoc-RGD |
Cell delivery | Osteoblast Fibroblast Mice |
[64] | |
| Fmoc-F5-Phe Fmoc-K(Fmoc)-RGD |
Antimicrobial Cell adhesion |
3T3 cells | [65] | |
| Silk fibroin Nap-FFRGD |
Cell adhesion Angiogenesis |
HUVEC Mice |
[66] | |
| Silk fibroin Nap-FFRGD |
Cell adhesion Osteogenesis |
mBMSC Mice |
[67] | |
| Collagen-like peptide | Neuronal cell maturation | Neuronal-glial cells | [68] | |
| Fmoc-FFβAR(K)βA-OH Fmoc-FFβAR(K)βA-NH2 |
Cell adhesion | MSC-P5, N2a, A549 cells | [69] | |
| Fmoc-FFGGRGD | Inhibition of β1-integrin, FAK and Akt expression | Tenon’s capsule fibroblasts | [70] | |
| Fmoc-FRGDF Agarose |
Laminin and fibronectin mimic | - | [71] | |
| C16-V3A3E3E3RGDS C16-V3A3K3SVVYGLR C16-V3E3DGEA |
Osteogenesis and angiogenesis | hAMSC, HUVEC | [72] | |
| Fmoc-FRGDF Fmoc-PHSRN |
Cell adhesion | HMFC | [73] | |
| E1Y9-ALK E1Y9-RGDS E1Y9-DGR E1Y9-PRG |
Osteogenesis | MC3T3-E1 cells | [74] | |
| LDV | fFL and fFLDV | Cell adhesion | L929 cells | [75] |
| PHSRN | Fmoc-FRGDF Fmoc-PHSRN |
Cell adhesion | HMFC | [73] |
| IKVAV | RADA4GGSIKVAV | Neuronal stem-cell delivery Anti-inflammatory |
hMgSC Mice |
[76] |
| Fmoc-DIKVAV | Neuronal cell differentiation | Mice | [77] | |
| Fmoc-DDIKVAV | Neuronal cell differentiation | hPSC Mice |
[78] | |
| IKVAV-PA | Neuronal cell differentiation | hESC Mice Human temporal bone |
[79] | |
| IKVAV-PA | Neuronal cell differentiation | BMSC | [80] | |
| IKVAV-PA YRSRKYSSWYVALKR |
Spinal cord injury repair (laminin and FGF2 mimicry) |
Mice | [81] | |
| Fmoc-DIKVAV Agarose |
Laminin and fibronectin mimic | - | [71] | |
| Fmoc-IKVAV Fmoc-YIGSR |
Neuronal cell growth | C6 cells SHSY5Y cells |
[82] | |
| YIGSR | KLD-IKVAV KLD-YIGSR |
Vasculogenesis | HUVEC, hMS cells | [83] |
| Nap-GFF(p)YIGSR | Anticancer Self-assembly directly on cells |
HeLa cells | [84] | |
| Fmoc-IKVAVFmoc-YIGSR | Neuronal cell growth | C6 cells SHSY5Y cells |
[82] | |
| YSV | FKFEYYSV | Anticancer | A549 cancer cells | [85] |
| Taxol-EYSV | Anticancer | HeLa, A2780 cells Mice |
[86] | |
| Nap-GffyGYSV | Anticancer | BEL-7402, HeLa, MCF-7 cells Mice |
[87] | |
| Nap-Gff(p)YSV | Anticancer Self-assembly directly on cells |
HeLa, A549 cells | [88] | |
| Nap-GFF(p)YSV | Anticancer Self-assembly directly on cells |
HeLa cells | [84] | |
| HAV | Fmoc/Nap-HAVDI | Cell adhesion | C6, L929 cells | [89] |
| HAV-PA E-PA |
Chondrogenesis | rMSC | [90] | |
| KLD-12 | Chondrogenesis | hMSC | [91] | |
| SVVYGLR | RADA16 | Angiogenesis | HCN-A94-2 cells Zebrafish |
[92] |
| C16-V3A3E3E3RGDS C16-V3A3K3SVVYGLR C16-V3E3DGEA |
Osteogenesis and angiogenesis | hAMSC, HUVEC | [72] | |
| DGEA | C16-V3A3E3E3RGDS C16-V3A3K3SVVYGLR C16-V3E3DGEA |
Osteogenesis and angiogenesis | hAMSC, HUVEC | [72] |
| KTT | C16KTTβAH | Collagen production | MCF-7, MDA-MB-231, HDFa cells |
[93] |
| βAH | C16KTTβAH | Anticancer | MCF-7, MDA-MB-231, HDFa cells |
[93] |
| ALKRQGRTLYGF | E1Y9-ALK E1Y9-RGDS E1Y9-DGR E1Y9-PRG |
Osteogenesis | MC3T3-E1 cells | [74] |
| DGRDSVAYG | E1Y9-ALK E1Y9-RGDS E1Y9-DGR E1Y9-PRG |
Osteogenesis | MC3T3-E1 cells | [74] |
| PRGDSGYRGDS | E1Y9-ALK E1Y9-RGDS E1Y9-DGR E1Y9-PRG |
Osteogenesis | MC3T3-E1 cells | [74] |
In particular, self-assembling peptides that form nanofibrillar hydrogels are ideally suited to mimic the extracellular matrix (ECM), which comprises a structurally similar nanofibrous network. The ECM is composed of a complex and dynamic mixture of proteins (e.g., laminin, fibronectin, collagen), glycosaminoglycans (e.g., hyaluronic acid and heparin), and growth factors, that altogether allow a continuous remodeling to respond to the requirements of resident cells to sustain their growth. It is thus not surprising that peptide-based nanofibrous hydrogels are often used in regenerative medicine as ECM mimics, as described in the examples below, to foster cell differentiation and proliferation and facilitate tissue-repair processes [57].
2.1. RGD
The RGD motif is by far the most widely applied bioactive motif in biomaterials science. It originates from fibronectin and it is well known to promote cell adhesion because of its affinity for integrins expressed on cells’ membranes, and hydrogels containing the RGD motifs can mimic the extracellular matrix (ECM) [94]. For this reason, the RGD motif is studied as a scaffold for cell cultures, or for biomedical devices and drug delivery. As the αIIbβ3 integrin is expressed on platelets’ surfaces, RGD was also considered an antithrombotic drug. However, due to its peptidic nature, RGD is easily degraded in biological environments, thus prompting research to move towards the design of RGD mimetics [95].
Mechanical and adhesion properties of hydrogels based on the self-assembly of RGD-modified peptides can be tuned by varying the hydrogel composition. Recently, a hydrogel was obtained using the gelator peptide KFE-8 (i.e., Ac-FKFEFKFE-NH2) modified with either the bioactive RGD sequence (i.e., Ac-GRGDSPGGFKFEFKFE-NH2) or the inactive, scrambled RDG sequence (i.e., Ac-GRDGSPGGFKFEFKFE-NH2) [58]. The adhesion properties of this hydrogel could be modified by varying the composition in terms of active KFE-RGD and inactive KFE-RDG whilst keeping constant the total concentration of both peptides, thus constant mechanical properties. Conversely, increasing the concentration of the gelator KFE-8 resulted in an increase of gel stiffness. In this manner, it was possible to tailor the differentiation of human mesenchymal stem cells, with adipogenesis being favored by softer gels with lower concentrations of RGD. In another recent work, RGD-modified hydrogels were obtained by simply changing an alanine with glycine in different positions of the well-known gelator peptide RADA16 [59]. This study showed that the substitution position has a great impact on the gelation ability and properties of the system, as well as on the cell adhesiveness. In particular, the substitution A6G inhibited β-sheet formation, whilst A10G and A14G resulted in twisted molecular alignment along with the sheets, and overall higher viscoelasticity and bioadhesiveness of the resulting gels. A similar approach was reported using the R-Y peptide sequence (i.e., RRKSYSGILGDLIQAVIRYY) from cp-52k to form a hydrogel and inserting the RGDS sequence in different positions of the chain [60]. In this case, too, it was demonstrated that the position of the RGDS sequence influenced the ability of R-Y to form β-sheets and, thus, the mechanical properties of the hydrogel. Specifically, the introduction of the RGD motif at the N-terminus resulted in higher β-sheet content, while the secondary conformation was not significantly changed upon inclusion of RGD in the middle of the sequence, and was reduced when positioned at the C-terminus, with consequently reduced gelation ability.
Recently, the self-assembling RGDSGAITIGC sequence containing the RGD motif was discovered via computational methods and tested for fibroblast proliferation [61]. This sequence preserved the ability to form a hydrogel with β-sheet amyloid structure and carried the RGD adhesion motif at one end, while at the other end there was a cysteine for further functionalization with other bioactive molecules or binding to metal surfaces.
A non-covalent approach via host-guest interactions was reported too [62]. Briefly, a hydrogel based on PA and PA functionalized with adamantane (ada) was formed, and host-guest interactions occurred between ada and a β-cyclodextrin (βCD) functionalized with the RGDS peptide. This hydrogel was studied for fibroblasts adhesion and growth, and it was observed that the host-guest interaction was critical in the epitope presentation, as control hydrogels without ada or without βCD showed a cell morphology and spreading comparable to the PA hydrogel alone.
The Fmoc protecting group is an established promoter of short peptide self-assembly via π-π stacking interactions, as is the FF sequence for amyloid proteins. Furthermore, the Fmoc-F sequence is also known to display antibacterial properties [96]. A hydrogel based on Fmoc-FF and Fmoc-RGD was developed to induce mesenchymal stem cells’ proliferation and to enhance their induced differentiation compared to the Fmoc-RGE non-bioactive hydrogel [63]. A similar injectable hydrogel based on Fmoc-FF and Fmoc-RGD was enriched with magnetic nanoparticles in order to increase the mechanical properties of the gel and also to allow for a magnetically targeted cell delivery for tissue regeneration [64]. The Fmoc-based self-assembly strategy allowed to obtain a hydrogel based on Fmoc-F5-Phe and a Fmoc-RGD derivative suitable for cell cultures, due to the adhesion properties of RGD combined with antimicrobial properties of Fmoc-F5-Phe [65]. Interestingly, fluorescence measurements allowed to establish the cooperative co-assembly of the different peptide sequences.
Another gelation strategy is the functionalization of peptides with the naphthyl group (Nap) that can perform π-π stacking interactions and lead to self-assembly [42]. In two recent works, this strategy was used to obtain hydrogels based on silk fibroin (SF) and Nap-FFRGD that were studied as scaffolds for regenerative medicine to promote angiogenesis [66] and osteogenesis [67] (Figure 2). The presence of the Nap-FFRGD gelator was key to obtaining gels at lower concentrations of SF. In another work, a PEGylated collagen-like peptide functionalized with the RGD motif (PEG-CLP-RGD) allowed for better maturation and structural organization of neuronal cells, relative to controls with PEG-CLP hydrogel or poly-l-lysine [68]. Finally, a hybrid polysaccharide-peptide hydrogel based on the co-assembly of agarose and Fmoc-FRGDF was found to better mimic the physical and chemical properties of ECM compared to hydrogels based on the peptide alone [71]. Indeed, the ECM matrix is rich in proteoglycans, besides fibrous proteins, thus the inclusion of polysaccharides offers better biomimicry of the natural scaffold.
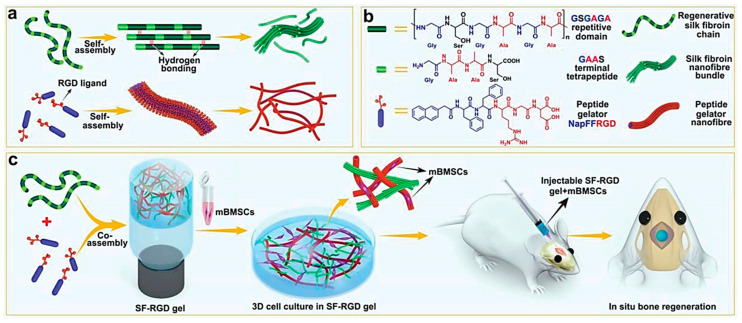
Figure 2.
(a,b) Molecular structures and self-assembling properties of peptide gelator (Nap-FFRGD) and silk fibroin (SF) for the formation of nanofiber and nanofibril bundle structures individually; (c) illustration of the preparation process for SF-RGD gel from Nap-FFRGD and SF, and its biological functions to enhance osteogenesis of encapsulated mBMSCs for bone regeneration in calvarial defect areas of mice. Reprinted with permission from [67], © 2022 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany.
A problem in the use of peptide-based systems for biological applications is their rapid enzymatic degradation. This problem can be solved using β- or d-amino acids or designing peptidomimetics. A recent work reported the preparation of thixotropic hydrogels based on Fmoc-FFβAR(K)βA-OH peptide and the amidated Fmoc-FFβAR(K)βA-NH2 containing the RGD-mimetic R(K) motif. Both hydrogels were tested on various types of cells (including neuronal cells) with good results in terms of cell adhesion and growth, and the amidated peptide also showed antimicrobial activity suitable for cell cultures [69].
Finally, a Fmoc-FFGGRGD-based hydrogel proved that the RGD sequence also interferes with gene expression in Tenon’s capsule fibroblasts, in particular reducing the β1-integrin, FAK, and Akt expression in order to inhibit fibrogenesis and scar formation that limits the success of glaucoma filtration surgery [70].
2.2. LDV
LDV is another fibronectin-derived tripeptide discovered in 1991 as an activator of β1 integrins and a cell adhesion promoter [97,98]. It was also discovered to have anti-inflammatory [99] and anti-metastatic activities [100]. The first incorporation of this bioactive tripeptide in a supramolecular peptide hydrogel was reported by our group using the self-assembling d-Phe-l-Phe-l-Leu (i.e., fFL) together with the bioactive fFLDV [75]. The so-obtained hydrogel successfully acted as a scaffold for cell adhesion and spreading, and the active engagement of integrins was demonstrated (Figure 3). In this case, the choice for LDV over RGD was determined also by the increased hydrophobicity of the former sequence, in order to avoid possible hindrance of the hydrophobically-driven peptide self-assembly in water that enables hydrogelation.
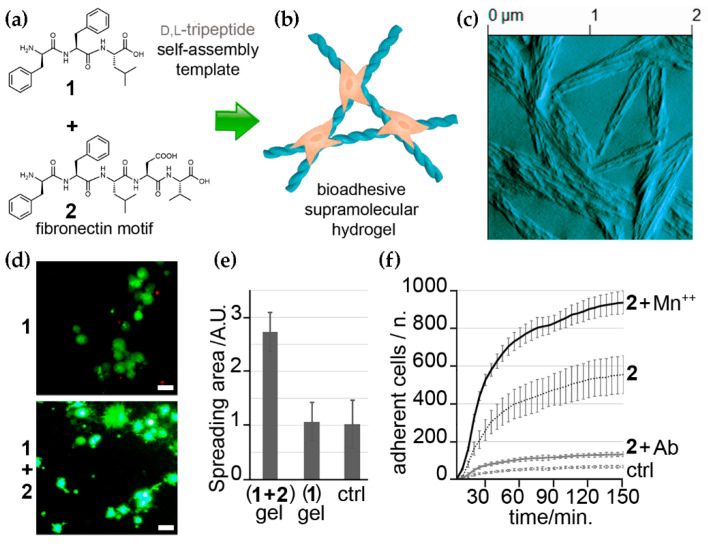
Figure 3.
(a) Chemical structures of two self-assembling short peptides with structural (1) and bioactive (2) roles for the co-assembly into bioadhesive hydrogels (b). (c) AFM image of the co-assembled gel. (d) live-dead cell-microscopy images of the control (1) and the bioadhesive (1 + 2) hydrogels with fibroblasts. (e) Quantification of cell spreading. (f) Adherent cells’ count in the presence of Mn++ and a β1 integrin-blocking antibody (Ab) demonstrates integrin engagement for cell adhesion on the biomaterial and successful ECM mimicry. Adapted from [75].
2.3. PHSRN
PHSRN is another fibronectin-derived bioactive motif, which acts in synergy with RGD for the binding of β1 integrins and was found to accelerate cell invasion and wound healing [101,102,103]. However, most researches focus only on RGD because the synergistic effect requires a precise spatial disposition of the two bioactive fragments, and so in most cases, the combination of RGD and PHSRN does not show significant improvements in cell adhesion [104,105]. Recently, a hydrogel based on Fmoc-FRGDF and Fmoc-PHSRN was successfully obtained, with increased cell adhesion compared to the Fmoc-FRGDF peptide alone [73]. It was also noted that, while Fmoc-FRGDF alone could self-assemble, Fmoc-PHSRN alone could not, and Fmoc-FRGDFPHSRN combined peptide gave a weak gel. This was due to the disruptive presence of the rigid proline, so mixing the two Fmoc-peptides was necessary to get a hydrogel with synergistic bioadhesiveness. Indeed, proline is well-known for its β-breaker role, which can be exploited to modulate the self-assembling behavior of short-peptide gelators based on the β-sheet prone FF motif [106].
2.4. IKVAV
IKVAV is another integrin-binding peptide that originates from laminin, so that it mimics the ECM, it promotes cell adhesion and growth, and differentiation of stem cells too [107,108]. For these reasons, IKVAV-containing peptides are widely studied to yield cell cultures and medical scaffolds for regenerative medicine. One of the hardest challenges in this field is to repair nerve tissues after damage to the peripheral [109] or central systems [110]. The IKVAV motif plays an elected role to this end, thanks to its favorable interactions with neurons and stem cells [111].
In a recent work, a hydrogel-based on the self-assembling and bioactive RADA4GGSIKVAV peptide was tested on mice [76]. It led to good results in improving brain injuries via neuronal stem cell delivery and it was also found to inhibit the molecular inflammatory pathway. Similar results were obtained with Fmoc-DIKVAV-based hydrogels used to treat Parkinson’s in a mouse model [77]. In this case, the hydrogel was loaded with a glial cell line-derived neurotrophic factor to induce the differentiation of both delivered and endogenous stem cells. This scaffold showed a good ability in enhancing neural-cell survival and differentiation, as well as in inhibiting the formation of glial scars. In another recent work, a hydrogel based on the very similar Fmoc-DDIKVAV was used to treat stroke-affected mice, resulting in the recovery of motor function, thanks to the enhanced cell differentiation, growth, and incorporation [78]. The reason for choosing the sequence with the additional two aspartic acid residues was to modulate the self-assembly behavior and obtain hydrogels at the physiological pH of 7.4 [78].
PA-based hydrogels with incorporated IKVAV sequences were successfully used to create a niche in mice inner ear to increase the survival and support the differentiation of neuron progenitors to regenerate the spiral ganglion [79]. While in all other previous cases the differentiation of stem cells was externally induced, and only supported and enhanced by IKVAV, a recent work reported the successful differentiation of BMSCs induced only by this peptide sequence contained in a PA-based hydrogel [80]. Remarkably, the combination of IKVAV-PA with another PA functionalized with an FGF-2 mimetic sequence, allowed for locomotor recovery after spinal cord injury in a mouse model [81]. Indeed, the use of more than one biomolecule is a promising strategy for regeneration, as demonstrated for the co-assembly of Fmoc-DIKVAV and agarose [71], as well as for the concomitant use of two laminin-derived motifs in Fmoc-IKVAV and Fmoc-YIGSR [82].
2.5. YIGSR
YIGSR is another cell adhesion domain found in the ECM protein laminin [112,113], and it is often used in combination with other peptides. A recent work reported the formation of a supramolecular hydrogel based on the self-assembling KLD peptide (i.e., KLDLKLDLKLDL) elongated with the bioactive YIGSR sequence (i.e., KLDLKLDLKLDLYIGSR) [83]. This hydrogel showed better vascularization ability on hMSC/HUVEC cell cultures, than the analogue KLD-IKVAV (i.e., KLDLKLDLKLDL-IKVAV) hydrogel.
The enzyme-instructed self-assembly (EISA) strategy was also applied to YIGSR-containing peptides, to form a self-assembled hydrogel directly on HeLa cells overexpressing alkaline phosphatase (ALP), so that a phosphorylated gelator precursor (that was too hydrophilic to self-assemble) could be dephosphorylated in situ and gel [84]. Importantly, direct use of the non-phosphorylated Nap-GFFYIGSR was not effective due to its poor solubility in water, thus confirming the effectiveness of the EISA approach. The co-assembly of Fmoc-YIGSR and Fmoc-IKVAV, both cell adhesion segments from laminin, produced a supramolecular hydrogel that displayed a synergistic effect in controlling neuronal cell adhesion and growth [82].
2.6. YSV
YSV is a bioactive tripeptide that attracted considerable attention in the field of drug discovery due to its anticancer properties against various types of cancer. The action mechanism involves the inhibition of both P-glycoprotein and histone deacetylase [114]. However, a millimolar concentration of peptide is needed to see any effect, and its peptidic nature limits its bioavailability. For this reason, different approaches are needed in order to enhance its anticancer effect and use this peptide as a drug, including the use of d-amino acids and the formation of hydrogels that also allow for the simultaneous delivery of other anticancer drugs, for a synergic treatment to overcome drug resistance. It was previously demonstrated that the formation of hydrogels on cancer cells via self-assembly of d-peptides interrupts intercellular exchanges leading to apoptosis, and that the EISA approach is applicable as phosphatases can dephosphorylate d-peptides too (e.g., Nap-ffy phosphorylated on y) [115].
For example, a peptide-sequence screening for hydrogelation yielded the octapeptide FKFEYYSV, composed of the bioactive tripeptide YSV and the gelation moiety FKFEY which also increased the anticancer activity of YSV. In addition, hydroxycamptothecin was loaded on the hydrogel for simultaneous drug delivery, and the whole system showed good anticancer activity, without toxicity for non-cancerous cells [85]. A similar combined approach was developed using an EYSV peptide linked to taxol (taxol-EYSV) [86]. In this case, the hydrogel formation was driven by the auto-hydrolysis of taxol-EYSV in biological environments that led to the co-assembly of the resulting two components, showing good anticancer properties also in vivo in mice.
Another approach consisted of the use of Nap-GffyGYSV peptide, containing the Nap gelation moiety and d-amino acids to increase the gelation ability and resistance of the gel in biological environments [87]. A smarter strategy using the similar peptides Nap-Gff(p)YSV [88] and Nap-GFF(p)YSV [84] with a phosphorylated tyrosine was developed to selectively form a hydrogel via dephosphorylation of tyrosine with EISA, directly on cancer cells overexpressing alkaline phosphatase.
2.7. HAV
HAV is a tripeptide that originates from cadherins, which are calcium-dependent glycoproteins expressed on cell membranes and involved in cell-to-cell adhesion, cell differentiation, morphogenesis, and many other processes [116,117,118]. This motif has cell recognition and adhesion functions in cadherins [119], but also acts as a cadherin antagonist, as it binds tyrosine-kinase receptors but it is too small to cause the receptor dimerization [120].
In a recent work, the bioactive peptide HAVDI was linked to Fmoc and Nap gelling moieties [89]. In both cases, an ECM-mimic hydrogel was obtained with good cell adhesion, viability, and proliferation, and promoted normal cellular functions in both neuronal and non-neuronal cells. The use of the aromatic N-caps was key to enabling gelation, as it allowed to significantly increase the hydrophobicity (C logP ≥ 1.4) of the peptide sequence relative to the uncapped analog (C logP −3.9) that was too hydrophilic to gel. The chondrogenic properties of HAV were also proven using a hydrogel based on oppositely charged HAV-PA and E-PA [90]. Differentiation of mesenchymal stem cells into chondrocytes was artificially induced, but the hydrogel bioactive support enhanced the differentiation and organization of cells stimulating the expression of cartilage-specific markers. In another similar work, in which an HAV-modified KLD-12 self-assembling peptide was used to obtain a hydrogel, it was demonstrated that the chondrogenic abilities of this kind of support involved the inhibition of canonical Wnt/β-catenin signaling. This gel was used for the successful encapsulation of stem cells (Figure 4) [91].
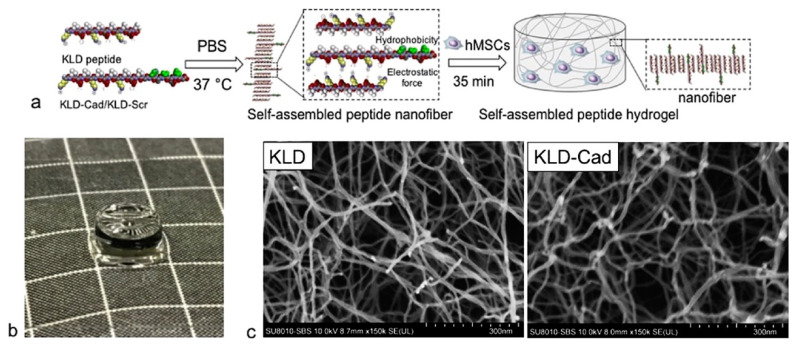
Figure 4.
(a) Scheme of self-assembly of hMSC-encapsulated KLD-Cad/KLD-Scr hydrogels. (b) Photograph of KLD-Cad self-assembled hydrogel (d = 5 mm, h = 2.2 mm) (c) SEM images of KLD (left) and KLD-Cad (right) hydrogels after critical point drying show that the average diameter of self-assembled fiber in the KLD and KLD-Cad hydrogels are approximately 17.6 nm and 20.4 nm, respectively. Reprinted from [91], copyright © 2022, with permission from Elsevier.
2.8. SVVYGLR
SVVYGLR is a bioactive peptide motif derived from osteopontin that shows high avidity for α9β1 and α4β1 integrins, promoting endothelial cells adhesion and migration, and angiogenesis, with subsequently enhanced neurogenesis too. A recent work reported the formation of a hydrogel based on the RADA16 gelator peptide linked to SVVYGLR bioactive motif (i.e., Ac-RADARADARADARADASVVYGLR-NH2) [92]. This novel hydrogel scaffold showed excellent angiogenesis and neurogenesis in vitro and in vivo when tested on zebrafish, and could potentially act as a bioactive scaffold for the regeneration of damaged brain tissues. Furthermore, the viscoelastic properties could be tuned with peptide concentration, as the elastic modulus G’ could be increased 15–20 times as the amount of gelator increased from 1% w/v to 2% w/v.
2.9. DGEA
DGEA is a collagen-I mimetic motif that showed to promote adhesion and osteogenic differentiation of stem cells [121]. A combined approach involving PAs containing SVVYGLR, RGDSm, and DGEA sequences was recently reported to create a hydrogel scaffold for the formation of vascularized bone-like constructs in vitro [72]. Co-assembly was favored by electrostatic interactions between oppositely charged sequences, for which the zeta potential was indeed confirmed to be positive for the former, and negative for the latter two. The gels, which also contained tyramine-functionalized hyaluronic acid for better ECM biomimicry, could be attained with elastic moduli in the range of 0.6–3.2 kPa upon inclusion of Ca++ salts to favor crosslinking and gelation.
2.10. βAH and KTT
Carnosine is a dipeptide (βAH) with various biological functions [122,123], especially as an antioxidant due to the presence of β-alanine. It has also been proposed as a treatment for Alzheimer’s disease [124], and it was shown to have anticancer properties [125]. A recent work reported a dual-component hydrogel based on the lipopeptide C16KTTβAH, that displayed anticancer activity against breast cancer, and lower toxicity on non-cancerous cells, thanks to the presence of the KTT motif [93]. The KTT motif is derived from procollagen I and it is widely used in cosmetic products due to its ability to induce collagen production [126]. Interestingly, the cytotoxicity of C16KTTβAH was manifest also at concentrations below the critical aggregation concentration, thus suggesting that it was not related to the nanofibrillation [93]. Furthermore, this gelator displayed promising potential also in terms of biomaterial viscoelastic properties, which could be fine-tuned over a wide range (e.g., G’ from 1 kPa to 1 MPa), depending on the gelation protocol and ionic strength used.
2.11. Other Bioactive Motifs Combined for Osteogenesis
Finally, a hydrogel based on various bioactive peptides was reported and acted as a potential scaffold for bone regeneration due to its adhesion properties and differentiation support toward MC3T3-E1 cells [74]. In particular, the hydrogel was based on the E1Y9 peptide (Ac-EYEYKYEYKY-NH2), which self-assembled in the presence of Ca2+ ions. This was functionalized with RGDS as a cell adhesion motif, ALK (ALKRQGRTLYGF) as an osteogenic growth peptide [127], DGR (DGRDSVAYG) as a cell adhesion motif of osteopontin that is involved in many bioactivities of osteoblasts and osteoclasts [128,129], and PRG (PRGDSGYRGDS) as a cell adhesion motif from type IV collagen that promotes adhesion of osteoblast cells [130]. The gelation ability of Y1E9 relies on a β-sheet conformation, which is partially disrupted by the introduction of positive charges, as in the bioactive motifs. Despite the peptides’ ability to respond to the introduction of CaCl2 as a self-assembly trigger, nanofibrillation could not always be rescued, as in the case of the cationic peptide sequence with the ALK motif. This study provided further evidence that the mere introduction of bioactive sequences to self-assembling peptides requires careful design, as it can hinder peptide conformation and gelation ability, depending on the sequence physicochemical properties.
3. Conclusions
The ECM offers a complex environment to sustain cell growth and differentiation that over the last few decades scientists began to unravel, for applications in regenerative medicine. In particular, we have witnessed a plethora of studies that confirmed short, self-assembling peptides as ideal building blocks to mimic the structural features of the ECM nanofibrous hydrogel. In parallel, several scientists have been decoding the minimalistic peptide sequences of the ECM components that bear bioactivity, often through integrin-engagement to promote cell adhesion and migration. As the two fields keep advancing, in the last 5 years we have been witnessing the combination of self-assembling and bioactive motifs into functional hydrogel scaffolds, through covalent and non-covalent approaches, also in some cases exploiting enzymes as triggers for assembly and/or disassembly.
The multi-component approach is promising not only to better recapitulate the complexity of the ECM, e.g., through the inclusion of both peptides and polysaccharides, but also to offer the means to fine-tune the bioactive, gelling, and viscoelastic properties of the systems. Indeed, a key challenge that is often encountered as mentioned in this Review, is the fact that bioactive sequences are often hydrophilic and present ionizable groups that can interfere with the hydrophobically-driven self-assembly of short peptides. To this end, a successful approach has been the combination of positively and negatively charged sequences through co-assembly promoted by electrostatic interactions, or the careful design of peptide sequences with additional amino acids to better control the overall charge and self-assembly at physiological pH.
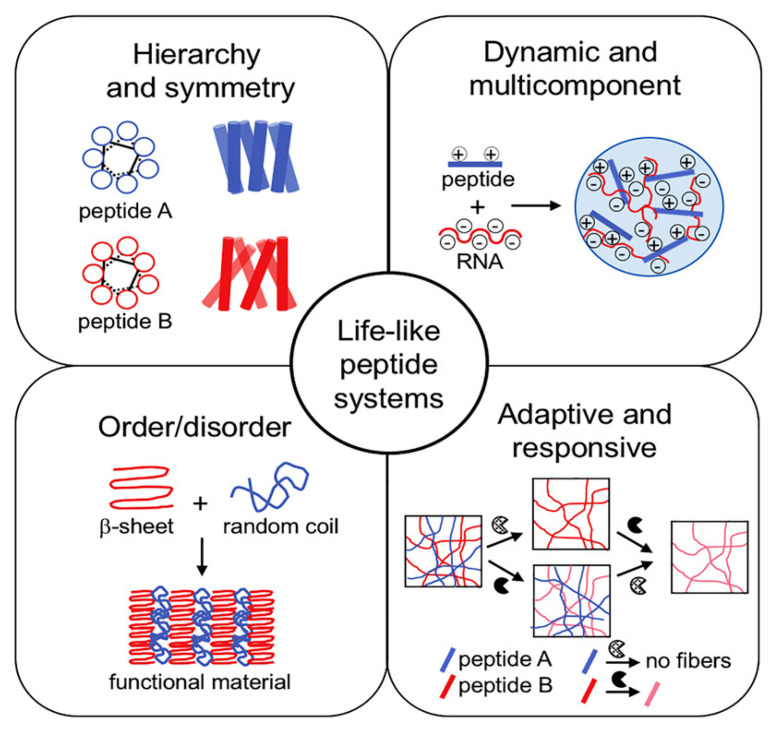
Despite the many successes, clearly, the ECM mimetics available today are still rudimental, when compared to the natural ECM fine complexity and dynamism. In particular, recent studies have revealed how not only the surface availability, but also the mobility, of bioactive motifs is key to attaining the desired effects [81]. Other studies are pointing to out-of-equilibrium self-assembled hydrogels to better mimic the dynamic nature of natural tissues, together with elements of hierarchical assembly, order/disorder, and so on (Figure 5) [131]. Furthermore, the inclusion of different types of biomolecules offers net advantages in terms of biomaterial performance relative to peptide-only systems, as we have mentioned in this review. Lastly, the next-generation scaffolds need to feature structural elements to increase their lifetime and slow down the biodegradation rate by enzymes. Different avenues are possible to this end, spanning from the inclusion of d-amino acids [132] or non-natural amino acids [133], to chemical crosslinking ideally with biocompatible agents [134]. Now is the time to raise the bar for the biomaterials scientists and take on the challenge to combine together all these elements, as well as structural features to slow down biodegradation rates by endogenous enzymes, to realize the awaited promise of full repair of human tissues through fine-level regenerative medicine.
Figure 5.
Life-like systems are out-of-equilibrium and present several useful features to mimic living tissues. Reproduced from [131], copyright © 2022, with permission from Elsevier.
Acknowledgments
The authors acknowledge Manuela Bisiacchi for her kind technical assistance.
Author Contributions
Writing—original draft preparation, D.M.; writing—review and editing, S.M.; visualization, D.M. and S.M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the University of Trieste (FRA2022).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kaur G., Narayanan G., Garg D., Sachdev A., Matai I. Biomaterials-based regenerative strategies for skin tissue wound healing. ACS Appl. Bio Mater. 2022;5:2069–2106. doi: 10.1021/acsabm.2c00035. [DOI] [PubMed] [Google Scholar]
- 2.Kojima M., Abe S., Ueno T. Engineering of protein crystals for use as solid biomaterials. Biomater. Sci. 2022;10:354–367. doi: 10.1039/D1BM01752G. [DOI] [PubMed] [Google Scholar]
- 3.Stie M.B., Kalouta K., Vetri V., Foderà V. Protein materials as sustainable non- and minimally invasive strategies for biomedical applications. J. Control. Release. 2022;344:12–25. doi: 10.1016/j.jconrel.2022.02.016. [DOI] [PubMed] [Google Scholar]
- 4.Yi J., Liu Q., Zhang Q., Chew T.G., Ouyang H. Modular protein engineering-based biomaterials for skeletal tissue engineering. Biomaterials. 2022;282:121414. doi: 10.1016/j.biomaterials.2022.121414. [DOI] [PubMed] [Google Scholar]
- 5.Tinoco A., Martins M., Cavaco-Paulo A., Ribeiro A. Biotechnology of functional proteins and peptides for hair cosmetic formulations. Trends Biotechnol. 2022;40:591–605. doi: 10.1016/j.tibtech.2021.09.010. [DOI] [PubMed] [Google Scholar]
- 6.Kazimierska K., Kalinowska-Lis U. Milk proteins-their biological activities and use in cosmetics and dermatology. Molecules. 2021;26:3253. doi: 10.3390/molecules26113253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen H., Ma L., Dai H., Fu Y., Wang H., Zhang Y. Advances in rational protein engineering toward functional architectures and their applications in food science. J. Agric. Food Chem. 2022;70:4522–4533. doi: 10.1021/acs.jafc.2c00232. [DOI] [PubMed] [Google Scholar]
- 8.Seah J.S.H., Singh S., Tan L.P., Choudhury D. Scaffolds for the manufacture of cultured meat. Crit. Rev. Biotechnol. 2022;42:311–323. doi: 10.1080/07388551.2021.1931803. [DOI] [PubMed] [Google Scholar]
- 9.Binlateh T., Thammanichanon P., Rittipakorn P., Thinsathid N., Jitprasertwong P. Collagen-based biomaterials in periodontal regeneration: Current applications and future perspectives of plant-based collagen. Biomimetics. 2022;7:34. doi: 10.3390/biomimetics7020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qin D., Wang N., You X.G., Zhang A.D., Chen X.G., Liu Y. Collagen-based biocomposites inspired by bone hierarchical structures for advanced bone regeneration: Ongoing research and perspectives. Biomater. Sci. 2022;10:318–353. doi: 10.1039/D1BM01294K. [DOI] [PubMed] [Google Scholar]
- 11.Salahuddin B., Wang S., Sangian D., Aziz S., Gu Q. Hybrid gelatin hydrogels in nanomedicine applications. ACS Appl. Bio Mater. 2021;4:2886–2906. doi: 10.1021/acsabm.0c01630. [DOI] [PubMed] [Google Scholar]
- 12.Kang J.I., Park K.M. Advances in gelatin-based hydrogels for wound management. J. Mater. Chem. B. 2021;9:1503–1520. doi: 10.1039/D0TB02582H. [DOI] [PubMed] [Google Scholar]
- 13.Łabowska M.B., Cierluk K., Jankowska A.M., Kulbacka J., Detyna J., Michalak I. A review on the adaption of alginate-gelatin hydrogels for 3d cultures and bioprinting. Materials. 2021;14:858. doi: 10.3390/ma14040858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zulkiflee I., Fauzi M.B. Gelatin-polyvinyl alcohol film for tissue engineering: A concise review. Biomedicines. 2021;9:979. doi: 10.3390/biomedicines9080979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giannelli M., Guerrini A., Ballestri M., Aluigi A., Zamboni R., Sotgiu G., Posati T. Bioactive keratin and fibroin nanoparticles: An overview of their preparation strategies. Nanomaterials. 2022;12:1406. doi: 10.3390/nano12091406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan R.R., Gong J.S., Su C., Liu Y.L., Qian J.Y., Xu Z.H., Shi J.S. Preparation and applications of keratin biomaterials from natural keratin wastes. Appl. Microbiol. Biotechnol. 2022;106:2349–2366. doi: 10.1007/s00253-022-11882-6. [DOI] [PubMed] [Google Scholar]
- 17.Ye W., Qin M., Qiu R., Li J. Keratin-based wound dressings: From waste to wealth. Int. J. Biol. Macromol. 2022;211:183–197. doi: 10.1016/j.ijbiomac.2022.04.216. [DOI] [PubMed] [Google Scholar]
- 18.Lehmann T., Vaughn A.E., Seal S., Liechty K.W., Zgheib C. Silk fibroin-based therapeutics for impaired wound healing. Pharmaceutics. 2022;14:651. doi: 10.3390/pharmaceutics14030651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li G., Sun S. Silk fibroin-based biomaterials for tissue engineering applications. Molecules. 2022;27:2757. doi: 10.3390/molecules27092757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haddadzadegan S., Dorkoosh F., Bernkop-Schnürch A. Oral delivery of therapeutic peptides and proteins: Technology landscape of lipid-based nanocarriers. Adv. Drug Deliv. Rev. 2022;182:114097. doi: 10.1016/j.addr.2021.114097. [DOI] [PubMed] [Google Scholar]
- 21.Ji X., Li Q., Song H., Fan C. Protein-mimicking nanoparticles in biosystems. Adv. Mater. 2022:e202201562. doi: 10.1002/adma.202201562. [DOI] [PubMed] [Google Scholar]
- 22.Caporale A., Adorinni S., Lamba D., Saviano M. Peptide-protein interactions: From drug design to supramolecular biomaterials. Molecules. 2021;26:1219. doi: 10.3390/molecules26051219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamley I.W. Small bioactive peptides for biomaterials design and therapeutics. Chem. Rev. 2017;117:14015–14041. doi: 10.1021/acs.chemrev.7b00522. [DOI] [PubMed] [Google Scholar]
- 24.Cringoli M.C., Fornasiero P., Marchesan S. Soft Matter for Biomedical Applications. The Royal Society of Chemistry; London, UK: 2021. Chapter 10 Minimalistic peptide self-assembly into supramolecular biomaterials; pp. 236–263. [Google Scholar]
- 25.La Manna S., Di Natale C., Onesto V., Marasco D. Self-assembling peptides: From design to biomedical applications. Int. J. Mol. Sci. 2021;22:12662. doi: 10.3390/ijms222312662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lombardo D., Calandra P., Pasqua L., Magazù S. Self-assembly of organic nanomaterials and biomaterials: The bottom-up approach for functional nanostructures formation and advanced applications. Materials. 2020;13:1048. doi: 10.3390/ma13051048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao J., Zhan J., Yang Z. Enzyme-instructed self-assembly (EISA) and hydrogelation of peptides. Adv. Mater. 2020;32:e1805798. doi: 10.1002/adma.201805798. [DOI] [PubMed] [Google Scholar]
- 28.Webber M.J., Pashuck E.T. (Macro)molecular self-assembly for hydrogel drug delivery. Adv. Drug Deliv. Rev. 2021;172:275–295. doi: 10.1016/j.addr.2021.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peng F., Zhang W., Qiu F. Self-assembling peptides in current nanomedicine: Versatile nanomaterials for drug delivery. Curr. Med. Chem. 2020;27:4855–4881. doi: 10.2174/0929867326666190712154021. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Z., Ai S., Yang Z., Li X. Peptide-based supramolecular hydrogels for local drug delivery. Adv. Drug Deliv. Rev. 2021;174:482–503. doi: 10.1016/j.addr.2021.05.010. [DOI] [PubMed] [Google Scholar]
- 31.Yang J., An H.W., Wang H. Self-assembled peptide drug delivery systems. ACS Appl. Bio Mater. 2021;4:24–46. doi: 10.1021/acsabm.0c00707. [DOI] [PubMed] [Google Scholar]
- 32.Pentlavalli S., Coulter S., Laverty G. Peptide nanomaterials for drug delivery applications. Curr. Prot. Peptide Sci. 2020;21:401–412. doi: 10.2174/1389203721666200101091834. [DOI] [PubMed] [Google Scholar]
- 33.Nambiar M., Schneider J.P. Peptide hydrogels for affinity-controlled release of therapeutic cargo: Current and potential strategies. J. Pept. Sci. 2022;28:e3377. doi: 10.1002/psc.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uzunalli G., Guler M.O. Peptide gels for controlled release of proteins. Therap. Deliv. 2020;11:193–211. doi: 10.4155/tde-2020-0011. [DOI] [PubMed] [Google Scholar]
- 35.Lyu Y., Azevedo H.S. Supramolecular hydrogels for protein delivery in tissue engineering. Molecules. 2021;26:873. doi: 10.3390/molecules26040873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cai L., Liu S., Guo J., Jia Y.G. Polypeptide-based self-healing hydrogels: Design and biomedical applications. Acta Biomater. 2020;113:84–100. doi: 10.1016/j.actbio.2020.07.001. [DOI] [PubMed] [Google Scholar]
- 37.Kurbasic M., Parisi E., Garcia A.M., Marchesan S. Self-assembling, ultrashort peptide gels as antimicrobial biomaterials. Curr. Top. Med. Chem. 2020;20:1300–1309. doi: 10.2174/1568026620666200316150221. [DOI] [PubMed] [Google Scholar]
- 38.Cai Y., Zheng C., Xiong F., Ran W., Zhai Y., Zhu H.H., Wang H., Li Y., Zhang P. Recent progress in the design and application of supramolecular peptide hydrogels in cancer therapy. Adv. Healthc. Mater. 2021;10:e2001239. doi: 10.1002/adhm.202001239. [DOI] [PubMed] [Google Scholar]
- 39.Guan T., Li J., Chen C., Liu Y. Self-assembling peptide-based hydrogels for wound tissue repair. Adv. Sci. 2022;9:e2104165. doi: 10.1002/advs.202104165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wychowaniec J.K., Smith A.M., Ligorio C., Mykhaylyk O.O., Miller A.F., Saiani A. Role of sheet-edge interactions in β-sheet self-assembling peptide hydrogels. Biomacromolecules. 2020;21:2285–2297. doi: 10.1021/acs.biomac.0c00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wychowaniec J.K., Patel R., Leach J., Mathomes R., Chhabria V., Patil-Sen Y., Hidalgo-Bastida A., Forbes R.T., Hayes J.M., Elsawy M.A. Aromatic stacking facilitated self-assembly of ultrashort ionic complementary peptide sequence: β-sheet nanofibers with remarkable gelation and interfacial properties. Biomacromolecules. 2020;21:2670–2680. doi: 10.1021/acs.biomac.0c00366. [DOI] [PubMed] [Google Scholar]
- 42.Martin A.D., Thordarson P. Beyond Fmoc: A review of aromatic peptide capping groups. J. Mater. Chem. B. 2020;8:863–877. doi: 10.1039/C9TB02539A. [DOI] [PubMed] [Google Scholar]
- 43.Dasgupta A., Das D. Designer peptide amphiphiles: Self-assembly to applications. Langmuir. 2019;35:10704–10724. doi: 10.1021/acs.langmuir.9b01837. [DOI] [PubMed] [Google Scholar]
- 44.Cringoli M.C., Marchesan S. Peptide-Based Biomaterials. The Royal Society of Chemistry; London, UK: 2021. Chapter 6 The use of D-amino acids for peptide self-assembled systems; pp. 174–216. [Google Scholar]
- 45.Vargiu A.V., Iglesias D., Styan K.E., Waddington L.J., Easton C.D., Marchesan S. Design of a hydrophobic tripeptide that self-assembles into amphiphilic superstructures forming a hydrogel biomaterial. Chem. Commun. 2016;52:5912–5915. doi: 10.1039/C5CC10531E. [DOI] [PubMed] [Google Scholar]
- 46.Yadav N., Chauhan M.K., Chauhan V.S. Short to ultrashort peptide-based hydrogels as a platform for biomedical applications. Biomater. Sci. 2020;8:84–100. doi: 10.1039/C9BM01304K. [DOI] [PubMed] [Google Scholar]
- 47.Scarel M., Marchesan S. Diketopiperazine gels: New horizons from the self-assembly of cyclic dipeptides. Molecules. 2021;26:3376. doi: 10.3390/molecules26113376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Manchineella S., Govindaraju T. Molecular self-assembly of cyclic dipeptide derivatives and their applications. ChemPlusChem. 2017;82:88–106. doi: 10.1002/cplu.201600450. [DOI] [PubMed] [Google Scholar]
- 49.Kurbasic M., Semeraro S., Garcia A.M., Kralj S., Parisi E., Deganutti C., De Zorzi R., Marchesan S. Microwave-assisted cyclization of unprotected dipeptides in water to 2,5-piperazinediones and self-assembly study of products and reagents. Synthesis. 2019;51:2829–2838. [Google Scholar]
- 50.Li L., Xie L., Zheng R., Sun R. Self-assembly dipeptide hydrogel: The structures and properties. Front. Chem. 2021;9:739791. doi: 10.3389/fchem.2021.739791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bellotto O., Kralj S., Melchionna M., Pengo P., Kisovec M., Podobnik M., De Zorzi R., Marchesan S. Self-assembly of unprotected dipeptides into hydrogels: Water-channels make the difference. ChemBioChem. 2022;23:e202100518. doi: 10.1002/cbic.202100518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bellotto O., Pierri G., Rozhin P., Polentarutti M., Kralj S., D’Andrea P., Tedesco C., Marchesan S. Dipeptide self-assembly into water-channels and gel biomaterial. Org. Biomol. Chem. 2022 doi: 10.1039/D2OB00622G. [DOI] [PubMed] [Google Scholar]
- 53.Scarel E., Bellotto O., Rozhin P., Kralj S., Tortora M., Vargiu A.V., De Zorzi R., Rossi B., Marchesan S. Single-atom substitution enables supramolecular diversity from dipeptide building blocks. Soft Matter. 2022;18:2129–2136. doi: 10.1039/D1SM01824H. [DOI] [PubMed] [Google Scholar]
- 54.Kralj S., Bellotto O., Parisi E., Garcia A.M., Iglesias D., Semeraro S., Deganutti C., D’Andrea P., Vargiu A.V., Geremia S., et al. Heterochirality and halogenation control Phe-Phe hierarchical assembly. ACS Nano. 2020;14:16951–16961. doi: 10.1021/acsnano.0c06041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bellotto O., Kralj S., De Zorzi R., Geremia S., Marchesan S. Supramolecular hydrogels from unprotected dipeptides: A comparative study on stereoisomers and structural isomers. Soft Matter. 2020;16:10151–10157. doi: 10.1039/D0SM01191F. [DOI] [PubMed] [Google Scholar]
- 56.Ung P., Winkler D.A. Tripeptide motifs in biology: Targets for peptidomimetic design. J. Med. Chem. 2011;54:1111–1125. doi: 10.1021/jm1012984. [DOI] [PubMed] [Google Scholar]
- 57.Hellmund K.S., Koksch B. Self-assembling peptides as extracellular matrix mimics to influence stem cell’s fate. Front. Chem. 2019;7:172. doi: 10.3389/fchem.2019.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hogrebe N.J., Reinhardt J.W., Tram N.K., Debski A.C., Agarwal G., Reilly M.A., Gooch K.J. Independent control of matrix adhesiveness and stiffness within a 3D self-assembling peptide hydrogel. Acta Biomater. 2018;70:110–119. doi: 10.1016/j.actbio.2018.01.031. [DOI] [PubMed] [Google Scholar]
- 59.Ishida A., Oshikawa M., Ajioka I., Muraoka T. Sequence-dependent bioactivity and self-assembling properties of RGD-containing amphiphilic peptides as extracellular scaffolds. ACS Appl. Bio Mater. 2020;3:3605–3611. doi: 10.1021/acsabm.0c00240. [DOI] [PubMed] [Google Scholar]
- 60.Fujii D., Takase K., Takagi A., Kamino K., Hirano Y. Design of RGDS peptide-immobilized self-assembling β-strand peptide from barnacle protein. Int. J. Mol. Sci. 2021;22:1240. doi: 10.3390/ijms22031240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Deidda G., Jonnalagadda S.V.R., Spies J.W., Ranella A., Mossou E., Forsyth V.T., Mitchell E.P., Bowler M.W., Tamamis P., Mitraki A. Self-assembled amyloid peptides with Arg-Gly-Asp (RGD) motifs as scaffolds for tissue engineering. ACS Biomater. Sci. Eng. 2017;3:1404–1416. doi: 10.1021/acsbiomaterials.6b00570. [DOI] [PubMed] [Google Scholar]
- 62.Redondo-Gómez C., Padilla-Lopategui S., Azevedo H.S., Mata A. Host–guest-mediated epitope presentation on self-assembled peptide amphiphile hydrogels. ACS Biomater. Sci. Eng. 2020;6:4870–4880. doi: 10.1021/acsbiomaterials.0c00549. [DOI] [PubMed] [Google Scholar]
- 63.Wang Y.-L., Lin S.-P., Nelli S.R., Zhan F.-K., Cheng H., Lai T.-S., Yeh M.-Y., Lin H.-C., Hung S.-C. Self-assembled peptide-based hydrogels as scaffolds for proliferation and multi-differentiation of mesenchymal stem cells. Macromol. Biosci. 2017;17:1600192. doi: 10.1002/mabi.201600192. [DOI] [PubMed] [Google Scholar]
- 64.Mañas-Torres M.C., Gila-Vilchez C., Vazquez-Perez F.J., Kuzhir P., Momier D., Scimeca J.-C., Borderie A., Goracci M., Burel-Vandenbos F., Blanco-Elices C., et al. Injectable magnetic-responsive short-peptide supramolecular hydrogels: Ex vivo and in vivo evaluation. ACS Appl. Mater. Interfaces. 2021;13:49692–49704. doi: 10.1021/acsami.1c13972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chakraborty P., Aviv M., Netti F., Cohen-Gerassi D., Adler-Abramovich L. Molecular co-assembly of two building blocks harnesses both their attributes into a functional supramolecular hydrogel. Macromol. Biosci. 2022;22:2100439. doi: 10.1002/mabi.202100439. [DOI] [PubMed] [Google Scholar]
- 66.Cheng B., Yan Y., Qi J., Deng L., Shao Z.-W., Zhang K.-Q., Li B., Sun Z., Li X. Cooperative assembly of a peptide gelator and silk fibroin afford an injectable hydrogel for tissue engineering. ACS Appl. Mater. Interfaces. 2018;10:12474–12484. doi: 10.1021/acsami.8b01725. [DOI] [PubMed] [Google Scholar]
- 67.Yan Y., Cheng B., Chen K., Cui W., Qi J., Li X., Deng L. Enhanced osteogenesis of bone marrow-derived mesenchymal stem cells by a functionalized silk fibroin hydrogel for bone defect repair. Adv. Healthc. Mater. 2019;8:1801043. doi: 10.1002/adhm.201801043. [DOI] [PubMed] [Google Scholar]
- 68.Balion Z., Cėpla V., Svirskiene N., Svirskis G., Druceikaitė K., Inokaitis H., Rusteikaitė J., Masilionis I., Stankevičienė G., Jelinskas T., et al. Cerebellar cells self-assemble into functional organoids on synthetic, chemically crosslinked ECM-mimicking peptide hydrogels. Biomolecules. 2020;10:754. doi: 10.3390/biom10050754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ahmadi Z., Yadav S., Kar A.K., Jha D., Gautam H.K., Patnaik S., Kumar P., Sharma A.K. An injectable self-assembling hydrogel based on RGD peptidomimetic β-sheets as multifunctional biomaterials. Mater. Sci. Eng. C. 2021;133:112633. doi: 10.1016/j.msec.2021.112633. [DOI] [PubMed] [Google Scholar]
- 70.Chen B., Wu P., Liang L., Zhao C., Wang Z., He L., Zhang R., Xu N. Inhibited effect of an RGD peptide hydrogel on the expression of β1-integrin, FAK, and Akt in Tenon’s capsule fibroblasts. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021;109:1857–1865. doi: 10.1002/jbm.b.34847. [DOI] [PubMed] [Google Scholar]
- 71.Firipis K., Boyd-Moss M., Long B., Dekiwadia C., Hoskin W., Pirogova E., Nisbet D.R., Kapsa R.M.I., Quigley A.F., Williams R.J. Tuneable hybrid hydrogels via complementary self-assembly of a bioactive peptide with a robust polysaccharide. ACS Biomater. Sci. Eng. 2021;7:3340–3350. doi: 10.1021/acsbiomaterials.1c00675. [DOI] [PubMed] [Google Scholar]
- 72.Derkus B., Okesola B.O., Barrett D.W., D’Este M., Chowdhury T.T., Eglin D., Mata A. Multicomponent hydrogels for the formation of vascularized bone-like constructs in vitro. Acta Biomater. 2020;109:82–94. doi: 10.1016/j.actbio.2020.03.025. [DOI] [PubMed] [Google Scholar]
- 73.Aye S.-S.S., Li R., Boyd-Moss M., Long B., Pavuluri S., Bruggeman K., Wang Y., Barrow C.R., Nisbet D.R., Williams R.J. Scaffolds formed via the non-equilibrium supramolecular assembly of the synergistic ECM peptides RGD and PHSRN demonstrate improved cell attachment in 3D. Polymers. 2018;10:690. doi: 10.3390/polym10070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tsutsumi H., Kawamura M., Mihara H. Osteoblastic differentiation on hydrogels fabricated from Ca2+-responsive self-assembling peptides functionalized with bioactive peptides. Bioorg. Med. Chem. 2018;26:3126–3132. doi: 10.1016/j.bmc.2018.04.039. [DOI] [PubMed] [Google Scholar]
- 75.Cringoli M.C., Romano C., Parisi E., Waddington L.J., Melchionna M., Semeraro S., De Zorzi R., Grönholm M., Marchesan S. Bioadhesive supramolecular hydrogel from unprotected, short D,L-peptides with Phe-Phe and Leu-Asp-Val motifs. Chem. Commun. 2020;56:3015–3018. doi: 10.1039/C9CC09947F. [DOI] [PubMed] [Google Scholar]
- 76.Sahab Negah S., Oliazadeh P., Jahanbazi Jahan-Abad A., Eshaghabadi A., Samini F., Ghasemi S., Asghari A., Gorji A. Transplantation of human meningioma stem cells loaded on a self-assembling peptide nanoscaffold containing IKVAV improves traumatic brain injury in rats. Acta Biomater. 2019;92:132–144. doi: 10.1016/j.actbio.2019.05.010. [DOI] [PubMed] [Google Scholar]
- 77.Rodriguez A.L., Bruggeman K.F., Wang Y., Wang T.Y., Williams R.J., Parish C.L., Nisbet D.R. Using minimalist self-assembling peptides as hierarchical scaffolds to stabilise growth factors and promote stem cell integration in the injured brain. J. Tissue Eng. Regen. Med. 2018;12:e1571–e1579. doi: 10.1002/term.2582. [DOI] [PubMed] [Google Scholar]
- 78.Somaa F.A., Wang T.-Y., Niclis J.C., Bruggeman K.F., Kauhausen J.A., Guo H., McDougall S., Williams R.J., Nisbet D.R., Thompson L.H., et al. Peptide-based scaffolds support human cortical progenitor graft integration to reduce atrophy and promote functional repair in a model of stroke. Cell Rep. 2017;20:1964–1977. doi: 10.1016/j.celrep.2017.07.069. [DOI] [PubMed] [Google Scholar]
- 79.Matsuoka A.J., Sayed Z.A., Stephanopoulos N., Berns E.J., Wadhwani A.R., Morrissey Z.D., Chadly D.M., Kobayashi S., Edelbrock A.N., Mashimo T., et al. Creating a stem cell niche in the inner ear using self-assembling peptide amphiphiles. PLoS ONE. 2017;12:e0190150. doi: 10.1371/journal.pone.0190150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ruan H., Xiao R., Jiang X., Zhao B., Wu K., Shao Z., Zhang Z., Duan H., Song Y. Biofunctionalized self-assembly of peptide amphiphile induces the differentiation of bone marrow mesenchymal stem cells into neural cells. Mol. Cell. Biochem. 2019;450:199–207. doi: 10.1007/s11010-018-3386-9. [DOI] [PubMed] [Google Scholar]
- 81.Álvarez Z., Kolberg-Edelbrock A.N., Sasselli I.R., Ortega J.A., Qiu R., Syrgiannis Z., Mirau P.A., Chen F., Chin S.M., Weigand S., et al. Bioactive scaffolds with enhanced supramolecular motion promote recovery from spinal cord injury. Science. 2021;374:848–856. doi: 10.1126/science.abh3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jain R., Roy S. Controlling neuronal cell growth through composite laminin supramolecular hydrogels. ACS Biomater. Sci. Eng. 2020;6:2832–2846. doi: 10.1021/acsbiomaterials.9b01998. [DOI] [PubMed] [Google Scholar]
- 83.Onak Pulat G., Gökmen O., Çevik Z.B.Y., Karaman O. Role of functionalized self-assembled peptide hydrogels in in vitro vasculogenesis. Soft Matter. 2021;17:6616–6626. doi: 10.1039/D1SM00680K. [DOI] [PubMed] [Google Scholar]
- 84.Li X., Wang Y., Zhang Y., Yang Z., Gao J., Shi Y. Enzyme-instructed self-assembly (EISA) assists the self-assembly and hydrogelation of hydrophobic peptides. J. Mater. Chem. B. 2022;10:3242–3247. doi: 10.1039/D2TB00182A. [DOI] [PubMed] [Google Scholar]
- 85.Liu J., Wu C., Dai G., Feng F., Chi Y., Xu K., Zhong W. Molecular self-assembly of a tyroservatide-derived octapeptide and hydroxycamptothecin for enhanced therapeutic efficacy. Nanoscale. 2021;13:5094–5102. doi: 10.1039/D0NR08741F. [DOI] [PubMed] [Google Scholar]
- 86.Ren C., Gao Y., Guan Y., Wang Z., Yang L., Gao J., Fan H., Liu J. Carrier-free supramolecular hydrogel composed of dual drugs for conquering drug resistance. ACS Appl. Mater. Interfaces. 2019;11:33706–33715. doi: 10.1021/acsami.9b12530. [DOI] [PubMed] [Google Scholar]
- 87.Ren C., Gao Y., Liu J., Zhang Y., Pu G., Yang L., Huang F., Yang C., Yang Z., Liu J. Anticancer supramolecular hydrogel of D/L-peptide with enhanced stability and bioactivity. J. Biomed. Nanotechnol. 2018;14:1125–1134. doi: 10.1166/jbn.2018.2564. [DOI] [PubMed] [Google Scholar]
- 88.Gao Y., Zhang C., Chang J., Yang C., Liu J., Fan S., Ren C. Enzyme-instructed self-assembly of a novel histone deacetylase inhibitor with enhanced selectivity and anticancer efficiency. Biomater. Sci. 2019;7:1477–1485. doi: 10.1039/C8BM01422A. [DOI] [PubMed] [Google Scholar]
- 89.Kaur H., Roy S. Designing aromatic N-cadherin mimetic short-peptide-based bioactive scaffolds for controlling cellular behaviour. J. Mater. Chem. B. 2021;9:5898–5913. doi: 10.1039/D1TB00598G. [DOI] [PubMed] [Google Scholar]
- 90.Eren Cimenci C., Kurtulus G.U., Caliskan O.S., Guler M.O., Tekinay A.B. N-cadherin mimetic peptide nanofiber system induces chondrogenic differentiation of mesenchymal stem cells. Bioconjug. Chem. 2019;30:2417–2426. doi: 10.1021/acs.bioconjchem.9b00514. [DOI] [PubMed] [Google Scholar]
- 91.Li R., Xu J., Wong D.S.H., Li J., Zhao P., Bian L. Self-assembled N-cadherin mimetic peptide hydrogels promote the chondrogenesis of mesenchymal stem cells through inhibition of canonical Wnt/β-catenin signaling. Biomaterials. 2017;145:33–43. doi: 10.1016/j.biomaterials.2017.08.031. [DOI] [PubMed] [Google Scholar]
- 92.Wang T.-W., Chang K.-C., Chen L.-H., Liao S.-Y., Yeh C.-W., Chuang Y.-J. Effects of an injectable functionalized self-assembling nanopeptide hydrogel on angiogenesis and neurogenesis for regeneration of the central nervous system. Nanoscale. 2017;9:16281–16292. doi: 10.1039/C7NR06528K. [DOI] [PubMed] [Google Scholar]
- 93.Castelletto V., Edwards-Gayle C.J.C., Greco F., Hamley I.W., Seitsonen J., Ruokolainen J. Self-assembly, tunable hydrogel properties, and selective anti-cancer activity of a carnosine-derived lipidated peptide. ACS Appl. Mater. Interfaces. 2019;11:33573–33580. doi: 10.1021/acsami.9b09065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pierschbacher M.D., Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984;309:30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- 95.Wang W., Borchardt T.R., Wang B. Orally active peptidomimetic RGD analogs that are glycoprotein IIb/IIa antagonists. Curr. Med. Chem. 2000;7:437–453. doi: 10.2174/0929867003375074. [DOI] [PubMed] [Google Scholar]
- 96.Gahane A.Y., Ranjan P., Singh V., Sharma R.K., Sinha N., Sharma M., Chaudhry R., Thakur A.K. Fmoc-phenylalanine displays antibacterial activity against Gram-positive bacteria in gel and solution phases. Soft Matter. 2018;14:2234–2244. doi: 10.1039/C7SM02317K. [DOI] [PubMed] [Google Scholar]
- 97.Mould A.P., Komoriya A., Yamada K.M., Humphries M.J. The CS5 peptide is a second site in the IIICS region of fibronectin recognized by the integrin α4β1. Inhibition of α4β1 function by RGD peptide homologues. J. Biol. Chem. 1991;266:3579–3585. doi: 10.1016/S0021-9258(19)67834-8. [DOI] [PubMed] [Google Scholar]
- 98.Komoriya A., Green L.J., Mervic M., Yamada S.S., Yamada K.M., Humphries M.J. The minimal essential sequence for a major cell type-specific adhesion site (CS1) within the alternatively spliced type III connecting segment domain of fibronectin is leucine-aspartic acid-valine. J. Biol. Chem. 1991;266:15075–15079. doi: 10.1016/S0021-9258(18)98588-1. [DOI] [PubMed] [Google Scholar]
- 99.Singh J., Adams S., Carter M.B., Cuervo H., Lee W.-C., Lobb R.R., Pepinsky R.B., Petter R., Scott D. Rational design of potent and selective VLA-4 inhibitors and their utility in the treatment of asthma. Curr. Top. Med. Chem. 2004;4:1497–1507. doi: 10.2174/1568026043387520. [DOI] [PubMed] [Google Scholar]
- 100.Kaneda Y., Yamamoto Y., Okada N., Tsutsumi Y., Nakagawa S., Kakiuch M., Maeda M., Kawasaki K., Mayumi T. Antimetastatic effect of synthetic Glu-Ile-Leu-Asp-Val peptide derivatives containing D-amino acids. Anti-Cancer Drugs. 1997;8:702–707. doi: 10.1097/00001813-199708000-00009. [DOI] [PubMed] [Google Scholar]
- 101.Hattori A., Hozumi K., Ko J.A., Chikama T., Oomikawa K., Kato J., Ishida K., Hoshi N., Katagiri F., Kikkawa Y., et al. Sequence specificity of the PHSRN peptide from fibronectin on corneal epithelial migration. Biochem. Biophys. Res. Commun. 2009;379:346–350. doi: 10.1016/j.bbrc.2008.12.057. [DOI] [PubMed] [Google Scholar]
- 102.Aota S., Nomizu M., Yamada K.M. The short amino acid sequence Pro-His-Ser-Arg-Asn in human fibronectin enhances cell-adhesive function. J. Biol. Chem. 1994;269:24756–24761. doi: 10.1016/S0021-9258(17)31456-4. [DOI] [PubMed] [Google Scholar]
- 103.Aucoin L., Griffith C.M., Pleizier G., Deslandes Y., Sheardown H. Interactions of corneal epithelial cells and surfaces modified with cell adhesion peptide combinations. J. Biomater. Sci. Polym. Ed. 2002;13:447–462. doi: 10.1163/156856202320253956. [DOI] [PubMed] [Google Scholar]
- 104.Shroff K., Pearce T.R., Kokkoli E. Enhanced integrin mediated signaling and cell cycle progression on fibronectin mimetic peptide amphiphile monolayers. Langmuir. 2012;28:1858–1865. doi: 10.1021/la203322t. [DOI] [PubMed] [Google Scholar]
- 105.Mardilovich A., Craig J.A., McCammon M.Q., Garg A., Kokkoli E. Design of a novel fibronectin-mimetic peptide−amphiphile for functionalized biomaterials. Langmuir. 2006;22:3259–3264. doi: 10.1021/la052756n. [DOI] [PubMed] [Google Scholar]
- 106.Garcia A.M., Melchionna M., Bellotto O., Kralj S., Semeraro S., Parisi E., Iglesias D., D’Andrea P., De Zorzi R., Vargiu A.V., et al. Nanoscale Assembly of Functional Peptides with Divergent Programming Elements. ACS Nano. 2021;15:3015–3025. doi: 10.1021/acsnano.0c09386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tashiro K., Sephel G.C., Weeks B., Sasaki M., Martin G.R., Kleinman H.K., Yamada Y. A synthetic peptide containing the IKVAV sequence from the A chain of laminin mediates cell attachment, migration, and neurite outgrowth. J. Biol. Cem. 1989;264:16174–16182. doi: 10.1016/S0021-9258(18)71604-9. [DOI] [PubMed] [Google Scholar]
- 108.Frith J.E., Mills R.J., Hudson J.E., Cooper-White J.J. Tailored integrin-extracellular matrix interactions to direct human mesenchymal stem cell differentiation. Stem Cells Dev. 2012;21:2442–2456. doi: 10.1089/scd.2011.0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu K., Yan L., Li R., Song Z., Ding J., Liu B., Chen X. 3D printed personalized nerve guide conduits for precision repair of peripheral nerve defects. Adv. Sci. 2022;9:e2103875. doi: 10.1002/advs.202103875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Varadarajan S.G., Hunyara J.L., Hamilton N.R., Kolodkin A.L., Huberman A.D. Central nervous system regeneration. Cell. 2022;185:77–94. doi: 10.1016/j.cell.2021.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sahab Negah S., Khooei A., Samini F., Gorji A. Laminin-derived Ile-Lys-Val-Ala-Val: A promising bioactive peptide in neural tissue engineering in traumatic brain injury. Cell Tissue Res. 2018;371:223–236. doi: 10.1007/s00441-017-2717-6. [DOI] [PubMed] [Google Scholar]
- 112.Graf J., Iwamoto Y., Sasaki M., Martin G.R., Kleinman H.K., Robey F.A., Yamada Y. Identification of an amino acid sequence in laminin mediating cell attachment, chemotaxis, and receptor binding. Cell. 1987;48:989–996. doi: 10.1016/0092-8674(87)90707-0. [DOI] [PubMed] [Google Scholar]
- 113.Sasaki M., Yamada Y. The laminin B2 chain has a multidomain structure homologous to the B1 chain. J. Biol. Chem. 1987;262:17111–17117. doi: 10.1016/S0021-9258(18)45498-1. [DOI] [PubMed] [Google Scholar]
- 114.Pan X., Xu J., Jia X. Research progress evaluating the function and mechanism of anti-tumor peptides. Cancer Manag. Res. 2020;12:397–409. doi: 10.2147/CMAR.S232708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kuang Y., Shi J., Li J., Yuan D., Alberti K.A., Xu Q., Xu B. Pericellular hydrogel/nanonets inhibit cancer cells. Angew. Chem. Int. Ed. 2014;53:8104–8107. doi: 10.1002/anie.201402216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Halbleib J.M., Nelson W.J. Cadherins in development: Cell adhesion, sorting, and tissue morphogenesis. Genes Dev. 2006;20:3199–3214. doi: 10.1101/gad.1486806. [DOI] [PubMed] [Google Scholar]
- 117.Pokutta S., Weis W.I. Structure and mechanism of cadherins and catenins in cell-cell contacts. Annu. Rev. Cell. Dev. Biol. 2007;23:237–261. doi: 10.1146/annurev.cellbio.22.010305.104241. [DOI] [PubMed] [Google Scholar]
- 118.Gumbiner B.M. Regulation of cadherin-mediated adhesion in morphogenesis. Nat. Rev. Mol. Cell Biol. 2005;6:622–634. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- 119.Blaschuk O.W., Sullivan R., David S., Pouliot Y. Identification of a cadherin cell adhesion recognition sequence. Dev. Biol. 1990;139:227–229. doi: 10.1016/0012-1606(90)90290-Y. [DOI] [PubMed] [Google Scholar]
- 120.Williams E.-J., Williams G., Gour B., Blaschuk O., Doherty P. INP, a novel N-cadherin antagonist targeted to the amino acids that flank the HAV motif. Mol. Cell. Neurosci. 2000;15:456–464. doi: 10.1006/mcne.2000.0847. [DOI] [PubMed] [Google Scholar]
- 121.Yoo S.Y., Kobayashi M., Lee P.P., Lee S.-W. Early osteogenic differentiation of mouse preosteoblasts induced by collagen-derived DGEA-peptide on nanofibrous phage tissue matrices. Biomacromolecules. 2011;12:987–996. doi: 10.1021/bm1013475. [DOI] [PubMed] [Google Scholar]
- 122.Gariballa S.E., Sinclair A.J. Carnosine: Physiological properties and therapeutic potential. Age Ageing. 2000;29:207–210. doi: 10.1093/ageing/29.3.207. [DOI] [PubMed] [Google Scholar]
- 123.Quinn P.J., Boldyrev A.A., Formazuyk V.E. Carnosine: Its properties, functions and potential therapeutic applications. Mol. Asp. Med. 1992;13:379–444. doi: 10.1016/0098-2997(92)90006-L. [DOI] [PubMed] [Google Scholar]
- 124.Hobart L.J., Seibel I., Yeargans G.S., Seidler N.W. Anti-crosslinking properties of carnosine: Significance of histidine. Life Sci. 2004;75:1379–1389. doi: 10.1016/j.lfs.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 125.Gaunitz F., Hipkiss A.R. Carnosine and cancer: A perspective. Amino Acids. 2012;43:135–142. doi: 10.1007/s00726-012-1271-5. [DOI] [PubMed] [Google Scholar]
- 126.Jones R.R., Castelletto V., Connon C.J., Hamley I.W. Collagen stimulating effect of peptide amphiphile C16–KTTKS on human fibroblasts. Mol. Pharm. 2013;10:1063–1069. doi: 10.1021/mp300549d. [DOI] [PubMed] [Google Scholar]
- 127.Bab I., Gazit D., Chorev M., Muhlrad A., Shteyer A., Greenberg Z., Namdar M., Kahn A. Histone H4-related osteogenic growth peptide (OGP): A novel circulating stimulator of osteoblastic activity. EMBO J. 1992;11:1867–1873. doi: 10.1002/j.1460-2075.1992.tb05238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Horii A., Wang X., Gelain F., Zhang S. Biological designer self-assembling peptide nanofiber scaffolds significantly enhance osteoblast proliferation, differentiation and 3-D migration. PLoS ONE. 2007;2:e190. doi: 10.1371/journal.pone.0000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lu T., Chen T., Zhai Y., Ma Y., Xiao Y. Designer functionalized self-assembling peptide scaffolds for adhesion, proliferation, and differentiation of MC3T3-E1. Soft Mater. 2014;12:79–87. doi: 10.1080/1539445X.2012.756018. [DOI] [Google Scholar]
- 130.Paralkar V.M., Vukicevic S., Reddi A.H. Transforming growth factor β type 1 binds to collagen IV of basement membrane matrix: Implications for development. Dev. Biol. 1991;143:303–308. doi: 10.1016/0012-1606(91)90081-D. [DOI] [PubMed] [Google Scholar]
- 131.Lampel A. Biology-inspired supramolecular peptide systems. Chem. 2020;6:1222–1236. doi: 10.1016/j.chempr.2020.03.005. [DOI] [Google Scholar]
- 132.Melchionna M., Styan K.E., Marchesan S. The unexpected advantages of using D-amino acids for peptide self- assembly into nanostructured hydrogels for medicine. Curr. Top. Med. Chem. 2016;16:2009–2018. doi: 10.2174/1568026616999160212120302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Clerici F., Erba E., Gelmi M.L., Pellegrino S. Non-standard amino acids and peptides: From self-assembly to nanomaterials. Tetrahedron Lett. 2016;57:5540–5550. doi: 10.1016/j.tetlet.2016.11.022. [DOI] [Google Scholar]
- 134.Chronopoulou L., Daniele M., Perez V., Gentili A., Gasperi T., Lupi S., Palocci C. A physico-chemical approach to the study of genipin crosslinking of biofabricated peptide hydrogels. Process Biochem. 2018;70:110–116. doi: 10.1016/j.procbio.2018.04.005. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.