Abstract
Objective:
To examine depressive symptoms during postmenopause and the contribution of depressive symptom trajectories before the final menstrual period (FMP) and psychosocial/health factors to postmenopause depressive symptoms.
Methods:
Longitudinal analysis of depressive symptoms (Center for Epidemiologic Studies-Depression scale) collected every 1–2 years from 1996–2017 from 1,551 midlife women in the Study of Women’s Health Across the Nation for a median follow-up of 19.0 years. Latent class growth analysis identified depression trajectories from baseline to FMP. Multivariable random effects (woman as random effect) linear or logistic regression models were conducted.
Results:
Women had higher odds of reporting high depressive symptom score (≥16) during postmenopause than when they were premenopausal (OR=1.49, 95%CI, 1.09–2.04), but not when perimenopausal. Three pre-FMP trajectories were identified: Group 1 (47.7%), consistently low scores, Group 2 (39.9%), moderate scores below the high depressive symptom threshold, and Group 3 (12.4%), consistently high scores. Both the moderate (OR=2.62, 95%CI, 1.89–3.66) and high score (OR=6.88, 95%CI, 4.72–10.02) groups, compared with the consistently low group, had significantly higher postmenopausal depressive symptom scores. Other pre-FMP variables associated with high postmenopausal depressive symptoms were: higher odds of childhood trauma/maltreatment, poor role physical, high anxiety symptoms, sleep problems, high vasomotor symptoms and lower odds for chronological aging and lower social support.
Conclusions:
Compared to premenopause, postmenopause remains a period of increased risk for higher depressive symptoms, especially for women with pre-FMP depressive symptoms. Pre-FMP depressive symptom trajectories are highly predictive of postmenopause depressive symptoms independent of health and psychosocial factors.
Keywords: Depressive symptoms, Center for Epidemiologic Studies-Depression scale (CES-D), final menstrual period, longitudinal, regression models, postmenopause
Introduction
The menopause transition (MT, a period from peri- to post-menopause) has been the focus of most research on depression among women during midlife. Overall, studies indicate that the MT is a period of greater risk for high depressive symptoms compared to premenopause.1–4 However, few longitudinal studies have examined depression risk after the final menstrual period (FMP), i.e., postmenopause relative to the late reproductive period.
Previous longitudinal multivariable analyses from The Study of Women’s Health Across the Nation (SWAN) reported that women are more vulnerable to high depressive symptoms during the MT than before.1,2 We also found that over the first five years1 and then eight years of SWAN2 odds of high depressive symptom levels (Center for Epidemiologic Studies-Depression scale [CES-D]5 scores ≥ 166,7 were significantly greater in postmenopause relative to premenopause, OR=1.57 and 1.87, respectively. However, these earlier SWAN publications were limited to 2–3 years of postmenopause data and only included just over half of the cohort who had reached their FMP.
Several other investigators have conducted longitudinal studies to examine the risk for high depressive symptoms during postmenopause, with mixed results.3,4,8 However, no study has had sufficient data to evaluate the risk for high levels of depressive symptom levels during the post-FMP years. Also, limitations of other studies include ascertainment of depression history by yes/no response to ‘ever had depression’ at baseline or diagnosed by interview using the PRIME-MD (Primary Care Evaluation of Mental Disorders),9 small sample sizes, limited assessment of and/or statistical adjustment for other risk factors and relevant covariates and confounders.
In addition to menopause status, very stressful life events, low social support, sociodemographic factors (e.g., less than a college education, financial strain), and health-related concerns (fair perceived health, sleep problems, vasomotor symptoms, higher body mass index (BMI)) were found to predict high depressive symptoms.1,2 Compared to White women, Chinese, Hispanic and Japanese women had greater odds of high depressive symptoms over time, but Black women were no more likely than White women to report high depressive symptoms. However, currently we lack knowledge of how long this vulnerability for high depressive symptoms persists in the postmenopause and what factors are associated with potentially clinically significant postmenopausal depressive symptoms.
The current analysis evaluates the risk for high levels of depressive symptoms several years into the postmenopause, as assessed with the CES-D, in our large and diverse SWAN sample, and extends the work of Bromberger et al.1,2 as women age and continue in the postmenopause. Specifically, our three hypotheses are: (1) postmenopause is a period of (a) increased risk for high depressive symptoms (CES-D score ≥ 16) as compared to premenopause, and (b) a greater risk for high depressive symptoms as is the menopausal transition (early/late perimenopause), even after adjusting for concurrent covariates; (2) pre-FMP CES-D symptom trajectories will predict CES-D scores after the FMP (i.e., depressive symptoms in postmenopause), after adjusting for concurrent covariates during postmenopause; and (3) pre-FMP psychosocial and health-related variables will be additional risk factors for postmenopausal depression. Multiple limitations in previous work on peri-/post-menopausal depression will be overcome with SWAN’s data on more than 3,000 participants, wide array of potential covariates, and clarity on length of time followed postmenopausally, i.e., after the FMP.
Method
Study Design and Participants
SWAN is a multi-ethnic/multi-racial, community-based, cohort study of the menopausal transition initiated in 1996. The inception cohort comprised 3,302 women who were enrolled at seven sites. Study design and recruitment of the cohort have been described in detail.10 Briefly, each site recruited White women and a racial/ethnic minority group: Black women in Boston, MA, Chicago, IL, southeast MI, and Pittsburgh, PA, Japanese women in Los Angeles, CA, Chinese women in Oakland, CA, and Hispanic women in Newark, NJ. Eligible women were 42–52 years, premenopausal or early perimenopausal, had an intact uterus and at least one ovary, had at least one menstrual period in the previous three months, and were not pregnant/lactating or using any sex steroid hormones in the three months preceding the baseline interview. At baseline, extensive data on psychosocial and health parameters were collected, and follow-up visits were conducted annually or biennially. Institutional review board approval was obtained at each SWAN study site, and written informed consent was obtained from participants.
Procedure and Measures
Participant selection for inclusion in the analytic sample is shown in Figure 1 (STROBE [Strengthening the Reporting of Observational Studies in Epidemiology] diagram). Of the 3,302 women enrolled in SWAN, our analytic sample consisted of 1,551 women who had an observed FMP, a baseline CES-D, and completed at least one CES-D from follow-up assessments 01 through 15 (1997 – 2017), for a total of 20,446 records. Women who were missing all follow-up CES-D scores were excluded. We excluded data collected during pregnancy/breast feeding from two pre- and perimenopausal women (n = 2 observations), which left N=20,444 observations for the analysis. Table 1 displays the baseline comparisons between the women in the analytic sample (n = 1,551) and excluded women (n = 1,751). Women in the analytic sample generally had better baseline physical and mental health.
Figure 1:
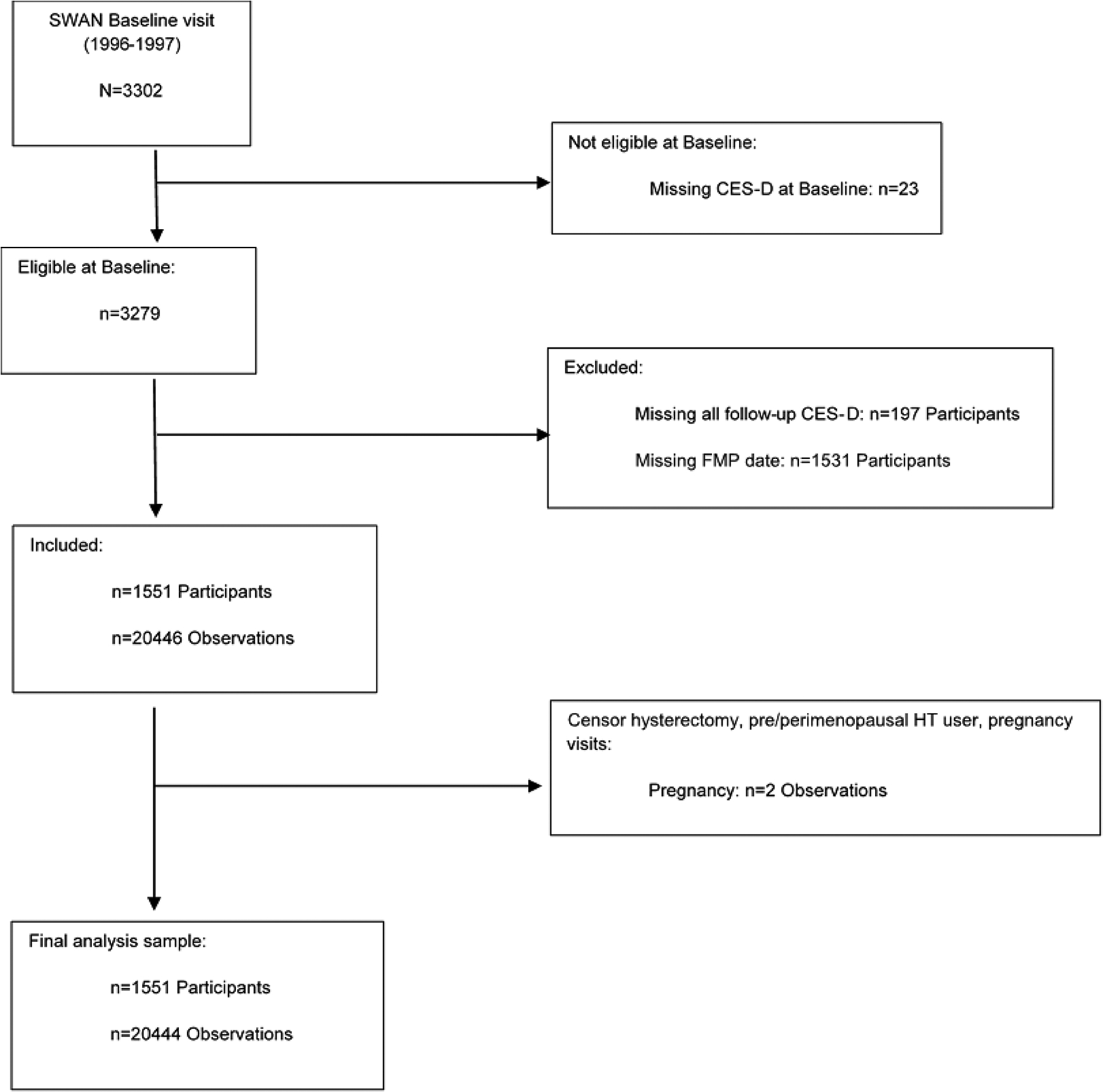
Participant Flow Chart.
CES-D, Center for Epidemiologic Studies-Depression Scale; FMP, Final Menstrual Period; HT, Hormone Therapy
Table 1.
Baseline (1996–1997) characteristics by inclusion statusa
| Baseline Characteristics | Analytic sample N=1551 |
Excluded N=1751 |
p-value |
|---|---|---|---|
| CES-D≥16, n (%) | 354 (22.82) | 450 (25.77) | 0.05 |
| CES-D score, mean±SD | 10.14±10.07 | 11.25±9.32 | 0.001 |
| Age (years), mean±SD | 45.79±2.68 | 45.91±2.70 | 0.20 |
| Race/Ethnicity, n (%) | <.0001 | ||
| White | 667 (43.00) | 884 (50.49) | |
| Black | 457 (29.46) | 477 (27.24) | |
| Chinese | 147 (9.48) | 103 (5.88) | |
| Hispanic | 108 (6.96) | 178 (10.17) | |
| Japanese | 172 (11.09) | 109 (6.23) | |
| Education, n (%) | 0.16 | ||
| Less than High school | 123 (8.00) | 115 (6.63) | |
| High school/Some college | 745 (48.47) | 887 (51.15) | |
| College/More than college | 669 (43.53) | 732 (42.21) | |
| Body Mass Index (kg/m2), mean±SD | 28.21±7.38 | 28.32±7.06 | 0.66 |
| Financial Strainb, n (%) | 617 (40.06) | 695 (39.94) | 0.94 |
| Medication for nervous conditionc, n (%) | 114 (7.37) | 214 (12.27) | <.0001 |
| Poor role emotional score, n (%) | 496 (32.04) | 626 (35.81) | 0.02 |
| Poor role physical score, n (%) | 393 (25.39) | 543 (31.05) | 0.0003 |
| Poor social function score, n (%) | 376 (24.26) | 518 (29.63) | 0.0005 |
| Anxiety symptom score≥4, n (%) | 309 (20.13) | 434 (25.16) | 0.0006 |
| Social support, mean±SD | 12.29±3.34 | 12.34±3.37 | 0.67 |
| Stressful life events, n (%) | 0.003 | ||
| 0 | 821 (53.17) | 823 (47.30) | |
| 1 | 307 (19.88) | 372 (21.38) | |
| 2+ | 416 (26.94) | 545 (31.32) | |
| Number of comorbiditiesd, n (%) | 0.03 | ||
| 0 | 802 (53.04) | 834 (48.49) | |
| 1 | 453 (29.96) | 556 (32.33) | |
| 2+ | 257 (17.00) | 330 (19.19) | |
| Sleep problems, n (%) | 436 (28.29) | 587 (33.64) | 0.001 |
| Vasomotor symptoms, n (%) | 545 (35.32) | 745 (42.79) | <.0001 |
| Menopause status, n (%) | 0.0001 | ||
| Early perimenopause | 662 (42.88) | 839 (48.41) | |
| Pre-menopause | 882 (57.12) | 888 (51.24) | |
| Symptom Sensitivity, mean±SD | 10.10±3.54 | 10.29±3.66 | 0.18 |
| C-reactive protein, median (Q1–Q3)e | 1.73(0.53–5.66) | 1.98(0.64–5.93) | 0.23 |
To be included in the analytic sample women had to have a baseline measure of the CES-D (Center for Epidemiologic Studies-Depression) scale, at least one follow-up CES-D measure and an observed FMP (final menstrual period). SD, standard deviation. Significant p-values in bold font.
Financial strain: very or somewhat difficult paying for basics (n, % shown) versus not difficult at all.
Medication for nervous conditions includes antidepressants, tranquilizers, sedatives, and sleeping pills.
Comorbidities included diabetes, hypertension, arthritis/osteoarthritis, under/overactive thyroid, osteoporosis, high cholesterol, heart attack, angina, stroke, or cancer.
Q1–Q3, interquartile range.
Dependent variable:
The outcome variable was the dichotomized CES-D score (≥ 16 (high symptoms) versus < 16).6,7 The CES-D asks for depressive symptom reporting with a time frame of within the past 1 week. All available CES-D measures were used in the analysis for first hypothesis, and all available CES-D measures during the postmenopause were used to address the remaining hypotheses, which focused on depressive symptoms during postmenopause.
Independent variables:
At each annual visit, participants reported the date of their most recent menstrual period, and any changes in cycle regularity. These data were used to obtain menopausal status and the final menstrual period date (FMP). Menopausal status classification was based on menstrual bleeding patterns in the previous 12 months and was categorized as: a) premenopausal (menses in the past 3 months with no change in menstrual bleeding regularity); b) early perimenopausal (menses in the past 3 months with change in regularity; c) late perimenopausal (no menses within the past 3 months, but some menstrual bleeding within the past 12 months); 4) postmenopausal (no menses within the past 12 months). The classifications are similar to those recommended by the World Health Organization11 and STRAW+10.12 FMP date was assigned retrospectively as the date of the participant’s last menstrual period before 12 consecutive months of amenorrhea. FMP could not be determined for 1,531 women due to the following: lost to follow-up (36%), hormone therapy use (36%), hysterectomy/bilateral salpingo-oophorectomy (17%), or three or more consecutive missed visits (12%). FMP is a marker of the divide in time between pre-/early peri- and post-menopause. Time since FMP (postmenopause) was calculated from the FMP date and study visit dates, and was used 1) as the time anchor to develop pre-FMP CES-D trajectories, and 2) to assess whether number of years in postmenopause was a significant predictor of high postmenopausal depressive symptom scores.
Subgroups of participants following similar trajectories of pre-FMP depressive symptoms were identified using latent class growth modeling and these pre-FMP CES-D trajectory groups were evaluated as predictors of depressive symptoms in the postmenopause. Our approach to identifying these trajectory groups is described in Data Analysis.
Covariates:
The primary covariates were time varying from FMP through postmenopause (except study site, race/ethnicity, education, and baseline age), and were selected based on previously identified associations with depressive symptoms.
Sociodemogaphics:
These included age, self-reported race/ethnicity, education (less than high school, completed high school/some college, completed college/more than college), marital status (married/living with a partner versus unpartnered), and financial strain (difficulty paying for basics, scored as very or somewhat difficult versus not difficult at all).13
Health-related Factors:
Height and weight were measured annually by trained staff according to a standard protocol and were used to calculate body mass index (BMI) as weight (kg)/height (m)2. Chronic medical conditions were assessed annually by asking participants whether a medical professional had ever told them that they had any of the following: diabetes, hypertension, arthritis/osteoarthritis, under/overactive thyroid, osteoporosis, high cholesterol, heart attack, angina, stroke, or cancer. The total number of chronic medical conditions reported was categorized into none, one, or 2 or more conditions. Vasomotor symptoms (VMS; hot flashes/flushes and/or night sweats) were reported at each visit as part of a symptom checklist that has been used in several menopause studies14,15 and were scored as reporting any or none. We examined three domains from the SF-3616 which assesses the impact of health in various life areas: role physical, role emotional, and social function. For all three domains we used the previously used cut-point of scoring in the lowest 25% to indicate poor functioning.17 Sleep problems were assessed using self-reported frequency of trouble falling asleep, waking several times, or early morning awakening in the past 2 weeks, dichotomized as any one sleep problem occurring <3 times a week or ≥3 times a week.18 Menopausal hormone therapy (MHT) use and medications for nervous conditions (antidepressants, tranquilizers/antipsychotics, sedatives, and/or sleeping pills) were obtained through participant self-report. High sensitivity C-reactive protein (hs-CRP) (higher hs-CRP has been associated with higher depressive symptom levels19) was measured on plasma, using different assay methods over time; a calibration equation was developed and applied to convert all values from different assays to a high sensitivity assay (ELISA),20 which also reports assay sensitivity and inter- and intra-assay coefficients of variation (CV).
Psychosocial Variables:
Life stress was assessed using a modified version of the Psychiatric Epidemiology Research Interview Life Events Scale (PERI).21 Women were asked whether they had 1) experienced any of 18 negative life events across eight domains (school, work, romantic relationships, children, family, criminal/legal matters, finances, and health) in the past year and 2) how upsetting each of the events was for them (not at all upsetting, somewhat upsetting, very upsetting, very upsetting and still upsetting) We included events relevant to midlife women or those living in low socioeconomic environments.22 Women were categorized as having experienced none, one or two or more very upsetting life events in the past year. Responses from the 4-item Medical Outcomes Study Social Support Survey23 were summed to create a social support score (range 0–16), with higher scores indicating more social support. An anxiety symptoms score was created by summing 4 items (irritability, nervousness or tension, feeling fearful for no reason, heart pounding or racing during the previous 2 weeks) derived from a symptom checklist consisting of 15 items similar to those used in numerous studies of menopause.24 Each item was scored 0 (not at all) to 4 (every day) (range 0 – 16), with a total score ≥4 indicating high anxiety. Symptom sensitivity25 was only measured at the first follow-up visit and is a summed score of degree of awareness of loud noise, hot or cold, hunger, pain, and things happening in one’s body; sensitivity ratings ranged from not at all true to extremely true.
The Childhood Trauma Questionnaire26 was administered at follow-up 15. This 28-item self-report instrument assesses five types of maltreatment experienced during childhood (emotional abuse, physical abuse, emotional neglect, physical neglect, and sexual abuse). For each type of maltreatment, validated clinical cutoff points were used to determine exposure to abuse/neglect.26,27 Participants exposed to at least one type of maltreatment were categorized as experiencing any childhood trauma vs. participants reporting no exposure.
Data Analysis
Analyses were conducted using SAS 9.3 (SAS, Cary, NC), and two-tailed P values < 0.05 were considered statistically significant. Odds ratios (OR) and 95% confidence intervals (95%CI) are presented.
Differences in baseline characteristics between excluded women (n=1,751) and women in the analytic sample (n=1,551) were assessed using chi-square tests for comparison of categorical variables and t-tests or Wilcoxon rank-sum tests, as appropriate, for comparisons of continuous variables.
To test the first hypothesis, multivariable longitudinal random effects logistic regression was used to run a base model assessing the relationship between menopausal status and depressive symptoms over the course of the study. Models included a woman-specific random intercept, allowing us to assess whether women had higher odds of reporting high depressive symptoms when women were postmenopausal compared to when they were in early or late perimenopause or when they were premenopausal. This model included time since baseline, baseline age, race/ethnicity, study site, and menopausal status. Visits from baseline through visit 15 were included in the longitudinal modeling. Multivariable random effects logistic regression models were then used to construct a final multivariable model of predictors of depressive symptoms. Bivariate analyses of the following variables were then conducted: education, number of chronic medical conditions, stressful life events, social support, medications for nervous condition, menopausal hormone therapy, financial strain, anxiety symptom score, sleep problems, vasomotor symptoms, poor role emotional and role physical, and poor social functioning.The remaining significant predictors (at p < 0.10) from the bivariate analyses were included in the model building process. The base model variables were forced into the model. Variables were assessed for significance and removed using a manual backwards elimination process (based on p-values and changes in estimates), until all variables in the model (with the exception of forced variables) were at p < 0.05. This process was also used to build final adjusted models for hypotheses 2 and 3.
To test the second hypothesis (trajectories of high depressive symptoms prior to the FMP predict high depressive symptoms in the postmenopause), latent class growth modeling (PROC TRAJ in SAS)28–31 was used to identify subgroups of participants following similar trajectories of depressive symptoms prior to the FMP. Women had to have at least 3 CES-D measurements within the specified time frame to be included in the trajectory analyses. Out of our overall study sample of 1,551 women, 53 did not meet this criteria and thus are not included in the trajectory analysis or in the analyses for the remaining hypothesis. Continuous CES-D scores (sum of the 20 items, range 0 – 60)5 were used to create the CES-D trajectories, and model selection was based on scientific plausibility and Bayesian Information Criteria (BIC) to evaluate goodness of fit. The chosen solution needed to have >.70 average posterior probabilities and ≥ 5% of the total sample for each group. Based on these criteria, a 3 group (consistently low CES-D scores, moderate CES-D scores, and consistently high CES-D scores) model was selected. After the trajectory classes were determined, participants were assigned to the trajectory class that reflected their highest posterior probability. The trajectory analysis represents the patterns of CES-D reporting from visit to visit, and we categorized women according to the trajectories. Longitudinal random effects logistic regression was used to model the dichotomized CES-D score and assess the associations between pre-FMP depressive symptoms trajectory grouping and CES-D scores during postmenopause, adjusted for relevant covariates from the postmenopausal period (i.e. measured at visits after the FMP).
To examine the third hypothesis, relevant pre-FMP covariates represented by psychosocial and health-related variables of interest were assessed with postmenopausal CES-D using longitudinal random effects logistic regression to model the dichotomized CES-D score. Education, ethnicity, and childhood trauma were assessed as non-time-varying variables. The remaining covariates of interest were time-varying and taken from all available visits prior to the FMP. Cumulative pre-FMP values were obtained using area under the curve (AUC)32 analysis for continuous variables; proportion of visits reported at a meaningful cut-point (e.g., the proportion of visits at which the participant repored high anxiety scores prior to the FMP) for dichotomous/categorical variables was created.
The New Jersey (NJ) site did not complete in-person follow-up visits 7 and 8, but in-person assessments resumed at visit 9. Because sensitivity analyses excluding NJ women (N = 162) showed almost identical results, they were included in the primary analysis.
Results
Baseline characteristics
The mean age of the sample was 45.8 ± 2.7 years, and women were followed for 17.5 years on average (median=19.0 years) (Table 1). Women attended an average of 13 study visits (range = 2–15 visits). At baseline, 57.1% were premenopausal and 42.9% were early perimenopausal. Racial/ethnic distribution was almost 50% White by design. Mean CES-D score was 10.1 ± 10.1 (SD), and baseline prevalence of high depressive symptoms (i.e., CES-D ≥ 16) was 22.8%.
Hypothesis 1a:
postmenopause compared to premenopause is a period of increased risk for high depressive symptoms (CES-D score ≥ 16). In the final adjusted model, compared to their premenopausal symptom levels, women had higher odds of reporting high depressive symptoms when postmenopausal. The OR was 1.49 (95% CI, 1.09–2.04).
Hypothesis 1b:
postmenopause compared to the MT is a period of greater risk for high depressive symptoms. None of the adjusted comparisons of postmenopause with early (OR=1.19, 95% CI, 0.93–1.52) and late perimenopause (OR=1.15, 95% CI, 0.85–1.56) were statistically significantly different.
Hypothesis 2:
CES-D symptom trajectories up until the FMP will predict CES-D scores after the FMP, even after adjusting for concurrent (i.e. during postmenopause) covariates. Figure 2 displays the three distinct pre-FMP trajectory groups that were identified: Group 1 (n=715, 47.7%), consistently low CES-D scores throughout the time period prior to the FMP; Group 2 (n=598, 39.9%), moderate CES-D scores, with some fluctuation early on but CES-D scores remain below 16 (threshold for high depressive symptoms) throughout this time period; Group 3 (n=185, 12.4%), high CES-D scores, which stay at a relatively high level throughout this time period.
Figure 2:
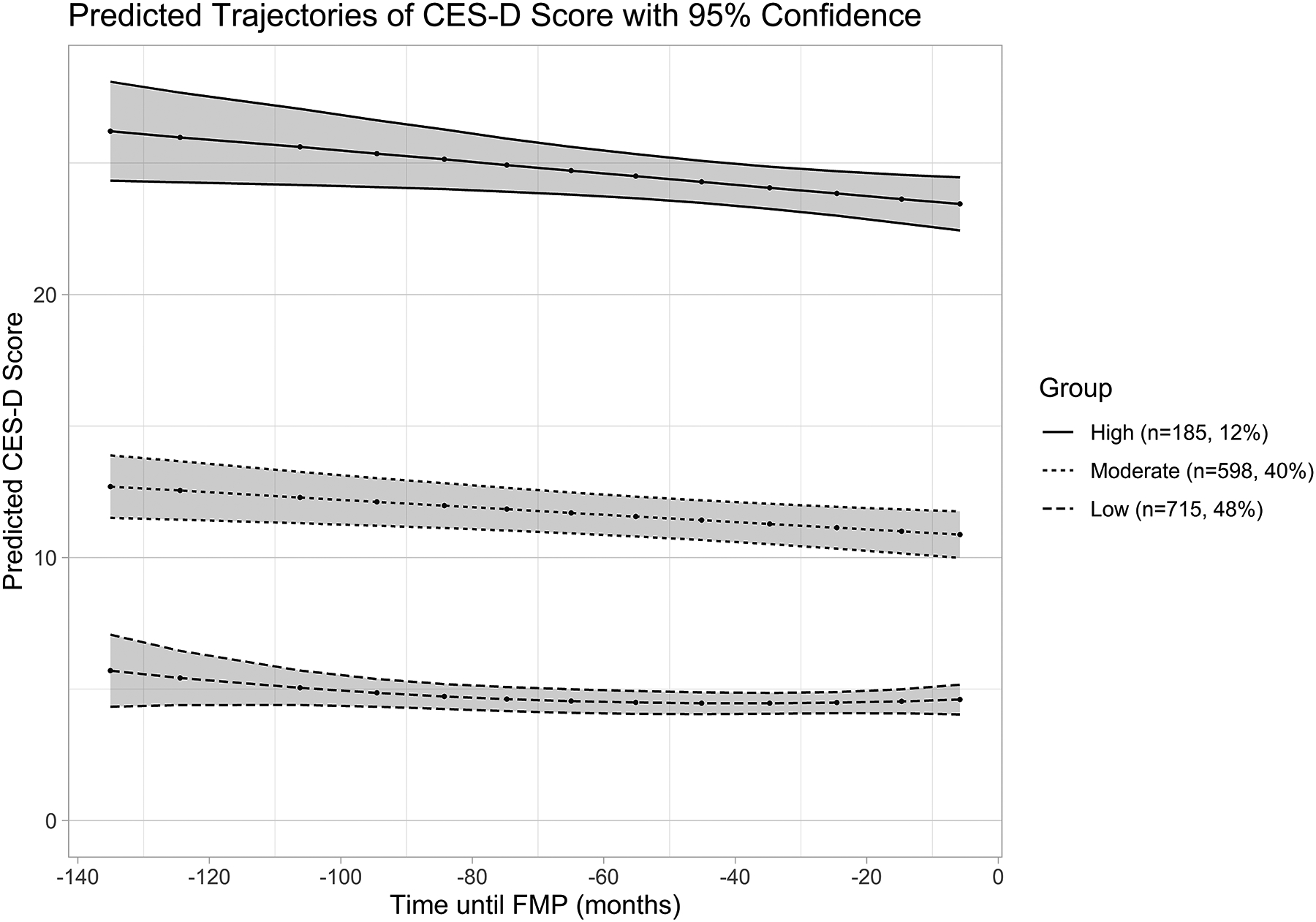
Pre-FMP predicted trajectories of depressive symptoms (N=1,498)a
FMP, Final Menstrual Period; CES-D, Center for Epidemiologic Studies-Depression scale.
Low CES-D Group n=715 (48%), Moderate CES-D Group n=598 (40%), High CES-D Group n=185 (12%)
a 53 women from the overall sample were excluded from the Pre-FMP trajectory analyses because they had fewer than three Pre-FMP CES-D measures.
We then assessed the relationship of pre-FMP CES-D trajectories to CES-D summary scores post-FMP, during postmenopause, adjusted for relevant postmenopause concurrent covariates. At last visit, women were an average of 11.9 (SD=4.4) years since FMP. Table 2 focuses on CES-D during the postmenopause as our outcome, and the covariates are concurrent and from the postmenopause period. Race/ethnicity was included as a covariate in all of the models, with White women as the reference group for statistical comparisons. Even after adjustment for other concurrent factors, the consistently low pre-FMP CES-D score trajectory group was significantly associated with lower depressive symptom scores during postmenopause. Comparing trajectory groups, with the consistently low CES-D scores group as the referent, both the moderate (OR=2.62, 95%CI, 1.89–3.66) and high CES-D score (OR=6.88, 95%CI, 4.72–10.02) groups were significantly associated with higher depressive symptom scores. The number of years in postmenopause (years since FMP) was included in the model building but was not statistically significant and was dropped from the final model. No statistically significant associations of depressive symptoms and racial/ethnic group were found.
Table 2.
Adjusted Association of Pre-FMP CES-D Trajectories with High Depressive Symptoms (CES-D≥16) during the Post-FMP (Postmenopause) (N=1,551, Number of observations=5,232)
| Modela | CES-D≥16 during Postmenopause OR (95% CI) | p-value |
|---|---|---|
| Pre-FMP CES-D trajectory group | ||
| Consistently low CES-D scores (REF) | --- | --- |
| Mid-level CES-D scores | 2.62 (1.89–3.66) | <.0001 |
| High CES-D scores | 6.88 (4.72–10.02) | <.0001 |
FMP, Final Menstrual Period; CES-D, Center for Epidemiologic Studies-Depression scale; OR, Odds Ratio; CI, Confidence Interval; REF, referent group.
Adjusted for site, time, age at baseline, race/ethnicity, education at baseline, stressful life events, medications for nervous conditions, social support, poor role emotional score, poor social function score, significant anxiety symptoms, and sleep problems. Covariates are time-varying from the FMP forward unless described as baseline variables
Hypothesis 3:
Examine whether selected pre-FMP psychosocial and health-related variables will be additional risk factors for high CES-D scores in postmenopause. As shown in Table 3, compared to the consistently low pre-FMP CES-D scores group, both the moderate and the high CES-D score groups still had higher odds of reporting high depressive symptoms during the post-FMP (postmenopause), even after adjusting for pre-FMP psychosocial and health-related factors. In all adjusted analyses, when compared with White women, African-American, Chinese, Hispanic, and Japanese women did not differ in their reporting of high depressive symptoms during postmenopause. Variables significantly associated with higher odds of depressive symptoms during postmenopause were: any childhood trauma/maltreatment and pre-FMP factors: poor role physical score, high anxiety symptom score, sleep problems, and high VMS symptoms. Variables significantly associated with lower odds of depressive symptoms during postmenopause were time from baseline (i.e., chronological aging) and social support prior to FMP. Both years in postmenopause and log transformed CRP were added into the model building but neither was statistically significant.
Table 3.
Adjusted Associations between Pre-FMP Psychosocial and Health-Related Predictors and High Depressive Symptoms during the Post-FMP (Postmenopause) (N=924, Number of observations=6,240)
| Model* | CES-D≥16 during postmenopause OR (95% CI) |
p-value |
|---|---|---|
| Pre-FMP CES-D trajectory group | ||
| Consistently low CES-D scores (REF) | --- | --- |
| Mid-level CES-D scores | 3.59 (2.64–4.88) | <.0001 |
| High CES-D scores | 14.73 (9.26–23.42) | <.0001 |
| Time (years) | 0.94 (0.92–0.96) | <.0001 |
| Age at baseline (years) | 0.95 (0.90–1.00) | 0.05 |
| Race/Ethnicity | ||
| White (REF) | --- | --- |
| Black | 1.37 (0.95–1.98) | 0.09 |
| Chinese | 1.21 (0.62–2.39) | 0.58 |
| Hispanic | 0.76 (0.24–2.37) | 0.63 |
| Japanese | 1.69 (0.81–3.51) | 0.16 |
| Education at baseline | ||
| Less than high school | 1.45 (0.80–2.63) | 0.22 |
| High school/some college | 0.86 (0.64–1.16) | 0.34 |
| College/more than college (REF) | --- | --- |
| Anxiety symptom score ≥ 4 at ≥ 50% of visits prior to FMP | 1.46 (1.02–2.10) | 0.04 |
| Social support (AUC) prior to FMP | 0.94 (0.90–0.98) | 0.004 |
| Sleep problems reported at ≥ 50% of visits prior to FMP | 1.35 (1.01–1.81) | 0.04 |
| Poor role physical score at ≥ 50% of visits prior to FMP | 1.78 (1.30–2.44) | 0.0003 |
| VMS at ≥ 50% of visits prior to FMP | 1.67 (1.26–2.23) | 0.0004 |
| History of any childhood maltreatment | 1.40 (1.07–1.83) | 0.01 |
FMP, Final Menstrual Period, CES-D, Center for Epidemiologic Studies-Depression scale; OR, Odds Ratio; CI, Confidence Interval; REF, referent group; AUC, Area Under the Curve; VMS, Vasomotor symptoms.
Adjusted for site. Significant p-values in bold font.
Secondary analysis:
We also examined the effects of transitioning into menopause and the risk of depression in our analytic sample. The proportion of women in our sample with high CES-D at baseline is included in Table 1 (n=354, 22.82%). Comparisons of the trajectory of symptom reporting across the entire transition of those who did/did not have high CES-D at baseline indicates that women with high CES-D at baseline significantly differ from women without high CES-D at baseline in their reporting of depressive symptoms across the transition (χ2=482.29, df=2, p<0.0001). Among women with high CES-D at baseline (N=354), 34 (10%) reported low depressive symptoms, 164 (46%) reported moderate depressive symptoms, and 156 (44%) reported high depressive symptoms. Among women without high CES-D at baseline (N=1197), 707 (59%) reported low depressive symptoms, 443 (37%) reported moderate depressive symptoms, and 47 (4%) report high depressive symptoms.
Sensitivity analysis:
We conducted two sensitivity analyses to examine the impact of sleep and anxiety-related variables on results because both of these symptoms overlap with CES-D items. We (1) removed the sleep problems and anxiety score variables from the model building process for all hypotheses, and (2) removed the sleep and anxiety-related items that are included in the CES-D from the scoring and updated all analyses using the new CES-D score. There were no significant changes in variables included in the final models or in the estimates or p values in either of these analyses. The associations of menopausal status and high depressive symptoms from baseline through visit 15 remained the same, associations of pre-FMP CES-D trajectories with high depressive symptoms during postmenopause were similar, and the associations of psychosocial and health-related predictors and high depressive symptoms during postmenopause did not change significantly.
Discussion
In this longitudinal analysis of depressive symptom self-reports ascertained over a 21-year period during which midlife women transitioned from pre-/early peri-menopause to postmenopause, the postmenopause was associated with a greater odds of reporting depressive symptoms than was premenopause (Hypothesis 1a). However, for Hypothesis 1b, the odds of reporting high depressive symptoms did not differ significantly between postmenopause and early/late perimenopause (i.e., the menopausal transition).
For Hypothesis 2, we identified three CES-D score trajectory patterns preceding the FMP: consistently low (47.7%), moderate with mean scores below the threshold (CES-D < 16) for high depressive symptoms 39.9%), and high CES-D score trajectory (12.4%). These trajectory patterns predicted post-FMP CES-D scores: both the moderate and high CES-D score groups, had significantly higher odds of reporting higher postmenopausal depressive symptom scores than did the consistently low group, even after adjusting for key concurrent (i.e. during postmenopause) factors. Our finding that pre-FMP depressive symptoms was such a strong predictor of post-FMP depressive symptoms also suggests that most women with low depressive symptom levels prior to the FMP may be protected from developing severe levels of depressive symptoms. Consistent with this finding, when we compared the trajectories across the entire transition of women who did/did not have baseline high CES-D, as anticipated, women with high CES-D at baseline tended to continue to report high or moderate symptoms throughout the transition. For women with lower CES-D at baseline, the majority continued to report low depressive symptoms through the transition and very few reported high depressive symptoms. However, more than one-third of these women go on to report moderate depressive symptoms across the transition.
Other investigators have reported higher depressive symptoms during both peri- and post-menopause compared to premenopause,4 lower depressive symptoms in postmenopause compared to perimenopause,8 and higher depressive symptoms before the FMP than after the FMP.3 These discrepant findings among studies can be attributed to different lengths of follow-up, analytic strategies, and control for important covariates. Our comprehensive approach, using trajectory analysis and comparing pre- versus post-FMP CES-D scores in a within women analysis, and adjusting for known covariates/confounders such as stressful events, social support, and medical conditions, differs from the analytic approach taken by these other investigators. The trajectory analyses, however, and the lack of association of postmenopausal CES-D with years since FMP, suggest that CES-D prevalence tracks within woman over time and may be fairly constant, in which case duration of follow-up might not explain the discrepancies between studies. Alternatively, perhaps the Hypothesis 1b results indicate a higher risk for depression during the MT compared with postmenopause but the stage-related differences are not statistically significant, in which case the discrepancies with prior research are with statistical significance rather than with the direction of the stage-related differences. Importantly, although our findings that the postmenopause may not be a period of worsening depressive symptoms relative to the MT are consistent with those of investigators, depressive symptoms do not necessarily improve. In contrast, Freeman reported that reaching the FMP was a key factor in reducing the prevalence of depressive symptoms.3
In addition to the pre-FMP CES-D trajectory groups, we also examined in Hypothesis 3 the importance of select pre-FMP health and psychosocial risk factors and found the following to be associated with greater odds of high postmenopausal depressive symptom scores: any childhood trauma/maltreatment, poor role physical, high anxiety symptoms, sleep problems, and high VMS symptoms. Chronological aging and higher social support pre- FMP were associated with lower odds. Perhaps most noteworthy is the enduring impact of childhood trauma. In the Seattle Midlife Women’s Health Study, Mitchell and Woods33 greater perceived stress, having a history of sexual abuse, and sleep difficulties were associated with more severe depressed mood, during postmenopause. Exercising more, and having a partner were associated with lower levels of depressed mood. Freeman et al34 found that stages in the MT (based on bleeding patterns) were associated with VMS, physical symptoms, and depressed mood.
Although BMI was significantly and positively related to high depressive symptoms during postmenopause in the bivariate analysis, and was included in multivariable models for all hypotheses, in adjusted analyses its association no longer was significant during postmenopause and was dropped out of the final models. This could be due to the correlation between BMI and other variables included in the model. The variables most highly correlated with BMI in our models were study site, education, role emotional, and social function, and their addition to the models changed the BMI estimate. The remaining variables included in our models did not have much impact on BMI when added/removed. We also looked separately at thyroid illness due to its occurrence during the menopausal transition and its association with depressive symptoms. This information was collected approximately annually and was one of the comorbidities included in the total number of comorbidities variable we assessed. Within our sample, the prevalence of thyroid illness increased from approximately 10% at SWAN baseline to 25% by Visit 15; however, thyroid illness was not significantly associated with high depressive symptoms in postmenopause (OR=1.12, 95%CI, 0.87–1.42, p=0.38). Its non-association with postmenopausal depression may be an important clinically translatable observation.
Strengths of the SWAN cohort and our longitudinal analyses include a long follow-up in a large community sample of women unselected for depressive symptoms, which enhances the generalizability of our sample and decreases the risk of selection bias. The large sample and number of observations provided sufficient statistical power to control for the effects of a number of covariates. Group-based trajectory modeling accounted for heterogeneity within the sample and identification of statistically sound and scientifically valid groups.35
Our longitudinal data and analytic approach permitted us to examine the effects of different patterns of depressive symptoms before and during the menopausal transition on postmenopausal symptom self-reports. Although this analysis does not provide individual level results, these data do identify subgroups that share similar courses of depressive symptoms and demonstrate their patterns and persistence over time, and allowed us to assess the relative contribution of chronological age that may be associated with health, lifestyle, and/or environmental factors versus the role of reproductive aging/reproductive hormones associated with the menopausal transition.
Hormonal data through the visit 15 assessment (2015 through 2017) were not available at the time of this writing and will be presented in a subsequent report. Nevertheless, our observations are supported by complementary data examining the contribution of female reproductive hormone changes and depressive symptoms during the menopausal transition. Real-time concurrent serial measures of gonadal steroids and depressive symptoms in perimenopausal women show that greater variability in estradiol and the absence of ovulatory levels of progesterone associate in real-time with more severe depressive symptoms.36 In addition, increasing levels of follicle stimulating hormone and greater variability in estradiol during perimenopause predict subsequent emergence of depression.37 Together these data show that the changing hormone dynamics of perimenopause may contribute to the depression vulnerability observed in our study. However, in contrast to the perimenopause, resolution of these hormonal changes in the postmenopause would predict that hormonally-driven depression risk should subside after the menopause transition. Therefore, aside from the absence of ovulatory levels of progesterone, this endocrine profile would not explain the persistence of depression risk into the postmenopause in our study.
It was not surprising that chronological aging was associated with lower odds of postmenopausal depression. A recent article reports data from several large studies showing that depressive symptoms decreased from midlife to late adulthood,38 which is consistent with other studies showing depressive symptoms increasing up to mid-age, but then decreasing until late age. We have also previously shown that chronological aging is associated with better mental component scores on the SF-3639 and others40,41 have also shown that mental health improves with age.
Several limitations of these data should be considered. In particular, among the excluded women, most were missing the FMP because of drop-out (39%) or their FMP could not be correctly identified due to hormone use (38%). In both cases, we might expect these women to be worse off in terms of both menopausal symptoms and higher CES-D scores. Thus, our results may be limited to women who are relatively healthy. Drop-outs were less healthy and of lower socioeconomic status. This may have contributed to our inability to find statistically significant associations in depressive symptoms between White and racial/ethnic minority groups. Clinicians should consider that it may be likely that women who are less healthy, exposed to greater stress, etc. when premenopausal and early perimenopausal may be at greater risk for elevated depressive symptoms when they are postmenopausal and should be assessed for depressive symptoms.
Use of a self-report measure of depressive symptoms may be considered a limitation because it carries a risk of information bias, but it is a clinical criterion standard for clinical management of depressive symptoms, including antidepressant clinical trials. The CES-D is a valid and reliable self-report measure routinely used in epidemiological studies and in various racial/ethnic groups. In SWAN, the CES-D has been administered at each clinical assessment since the study inception. Women were not screened for depressive disorders. Although the CES-D includes a sleep item, which also was included as a separate covariate/risk factor, sensitivity analyses revealed no change in results with this item removed from the CES-D score.
Other limitations also should be considered. Although SWAN has collected a substantial number of variables related to depression, it was not designed to assess all potential correlates of depressive symptoms in this large community sample with its primary focus on various aspects of the menopausal transition. SWAN has extensive information, mostly on subjective assessments rather than objective diagnostic assessments of depression by clinicians (as noted above), and not more detailed descriptions of the nature, severity, duration/course, timing, and comorbid psychiatric illness, or details regarding the effects of antidepressants, psychotherapy, or estradiol or progesterone on participants’ depressive symptom trajectories. Thus, SWAN has CES-D scores from each visit but no information on previous number, duration, type or severity of personal and family history of depressive illness, the timing of depressive and sleep/circadian rhythm symptoms, chronotype, and when they occurred in relation to the FMP. Due to the episodic data collection in community-based epidemiological studies and the limitations of subjective recall bias we do not truly know whether pre-FMP depressive symptoms were episodic or chronic. Although we cannot say for certain how symptoms wax and wane in the interim, trajectory analysis allowed us to look at how women fall into different patterns in their visit-to-visit reporting over time, the main objective of this analysis.
Conclusions
Pre FMP patterns of depressive symptoms were highly predictive of postmenopause depressive symptoms, especially among women who had consistently high depressive symptoms prior to the FMP, even after adjusting for a number of other risk factors, including psychosocial and health-related variables. The consistently low pre-FMP CES-D score trajectory group was significantly associated with lower depressive symptom scores during postmenopause; both the moderate and high CES-D score groups had significantly higher level of symptoms. These findings suggest that the years after the final menstrual period do not necessarily bring a decrease in depressive mood for women who have high or moderate depressive symptoms prior to the FMP, as has been previously thought.3 This analysis shows that depressive symptoms tend to be highly consistent over midlife.
Acknowledgments:
The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants U01NR004061; U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
Clinical Centers: University of Michigan, Ann Arbor – Siobán Harlow, PI 2011 – present, MaryFran Sowers, PI 1994–2011; Massachusetts General Hospital, Boston, MA – Joel Finkelstein, PI 1999 – present; Robert Neer, PI 1994 – 1999; Rush University, Rush University Medical Center, Chicago, IL – Howard Kravitz, PI 2009 – present; Lynda Powell, PI 1994 – 2009; University of California, Davis/Kaiser – Ellen Gold, PI; University of California, Los Angeles – Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY – Carol Derby, PI 2011 – present, Rachel Wildman, PI 2010 – 2011; Nanette Santoro, PI 2004 – 2010; University of Medicine and Dentistry – New Jersey Medical School, Newark – Gerson Weiss, PI 1994 – 2004; and the University of Pittsburgh, Pittsburgh, PA – Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD – Chhanda Dutta 2016- present; Winifred Rossi 2012–2016; Sherry Sherman 1994 – 2012; Marcia Ory 1994 – 2001; National Institute of Nursing Research, Bethesda, MD – Program Officers.
Central Laboratory: University of Michigan, Ann Arbor – Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center: University of Pittsburgh, Pittsburgh, PA – Maria Mori Brooks, PI 2012 - present; Kim Sutton-Tyrrell, PI 2001 – 2012; New England Research Institutes, Watertown, MA - Sonja McKinlay, PI 1995 – 2001.
Steering Committee: Susan Johnson, Current Chair
Chris Gallagher, Former Chair
We thank the study staff at each site and all the women who participated in SWAN.
Sources of funding:
National Institutes of Health (National Institute on Aging and National Institute of Nursing Research)/DHHS Grants U01NR004061, U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495.
Conflicts of interest/financial disclosures:
Drs. Kravitz (NIA), Joffe (NIA, NCI), Bromberger (NIA. NIMH), and Avis (NIA, NCI) report grants from NIH during the period of the study. Dr. Joffe reports the following additional disclosures for the past 12 months: Grants: Merck, Pfizer; Consulting: NeRRe/KaNDy, Eisai, Jazz, Bayer, Que-Oncology; Spouse: Merck Research Labs employee; Arsenal Biosciences consulting fees and equity; Tango equity. Dr. Colvin and Ms. Chen declared no conflicts of interest or financial involvement (including employment, fees, share ownership) or affiliation with any organization whose financial interests may be affected by material in the manuscript, or any other conflicts of interest which might potentially bias it.
References
- 1.Bromberger JT, Matthews KA, Schott LL, et al. Depressive symptoms during the menopausal transition: The Study of Women’s Health Across the Nation (SWAN). J Affect Disord 2007;103:267–272. (doi: 10.1016/j.jad.2007.01.034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bromberger JT, Schott LL, Kravitz HM, et al. Longitudinal change in reproductive hormones and depressive symptoms across the menopausal transition: results from the Study of Women’s Health Across the Nation (SWAN). Arch Gen Psychiatry 2010;67:598–607. (doi: 10.1001/archgenpsychiatry.2010.55) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freeman EW, Sammel MD, Boorman DW, Zhang R. Longitudinal pattern of depressive symptoms around natural menopause. JAMA Psychiatry 2014;71:36–43. (doi: 10.1001/jamapsychiatry.2013.2819) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woods NF, Smith-DiJulio K, Percival DB, Tao EY, Mariella A, Mitchell ES. (2008). Depressed mood during the menopausal transition and early postmenopause: observations from the Seattle Midlife Women’s Health Study. Menopause 2008;15:223–232. (doi: 10.1097/gme.0b013e3181450fc2) [DOI] [PubMed] [Google Scholar]
- 5.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977;1:385–401. (doi: 10.1177/014662167700100306) [DOI] [Google Scholar]
- 6.Boyd JH, Weissman MM, Thompson WD, Myers JK. Screening for depression in a community sample: understanding the discrepancies between depression symptom and diagnostic scales. Arch Gen Psychiatry 1982;39:1195–1200. (doi: 10.1001/archpsyc.1982.04290100059010) [DOI] [PubMed] [Google Scholar]
- 7.Weissman MM, Sholomskas D, Pottenger M, Prusoff BA, Locke BZ. Assessing depressive symptoms in five psychiatric populations: a validation study. Am J Epidemiol 1977;106:203–214. (doi: 10.1093/oxfordjournals.aje.a112455) [DOI] [PubMed] [Google Scholar]
- 8.Hickey M, Schoenaker DA, Joffe H, Mishra GD. Depressive symptoms across the menopause transition: findings from a large population-based cohort study. Menopause 2016;23:1287–1293. (doi: 10.1097/GME.0000000000000712) [DOI] [PubMed] [Google Scholar]
- 9.Spitzer RL, Williams JB, Kroenke K, et al. Utility of a new procedure for diagnosing mental disorders in primary care: the PRIME-MD 1000 study. JAMA 1994;272:1749–1756. (Accessed from https://pubmed.ncbi.nlm.nih.gov/7474219/) [PubMed] [Google Scholar]
- 10.Sowers MF, Crawford SL, Sternfeld B, et al. SWAN: a multicenter, multiethnic, community-based cohort study of women and the menopausal transition. In: Lobo RA, Kelsey J, Marcus R, editors. Menopause: Biology and Pathobiology. San Diego, CA: Academic Press, 2000:175–188. [Google Scholar]
- 11.WHO Scientific Group on Research on the Menopause in the 1990s. Research on the menopause in the 1990s: report of a WHO scientific group. World Health Organ Tech Rep Ser 1996;866:1–107. (Accessed from https://apps.who.int/iris/handle/10665/41841) [PubMed] [Google Scholar]
- 12.Harlow SD, Gass M, Hall JE, et al. , for the STRAW + 10 Collaborative Group. Executive summary of the stages of reproductive aging workshop + 10: Addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metabol 2012;97:1159–1168. (doi: 10.1210/jc.2011-3362) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kahn JR, Pearlin LI. Financial strain over the life course and health among older adults. J Health Soc Behav 2006;47:17–31. (doi: 10.1177/002214650604700102) [DOI] [PubMed] [Google Scholar]
- 14.Matthews KA, Wing RR, Kuller LH, et al. Influences of natural menopause on psychological characteristics and symptoms of middle-aged healthy women. J Consult Clin Psychol 1990;58:345–351. (doi: 10.1037//0022-006x.58.3.345) [DOI] [PubMed] [Google Scholar]
- 15.Neugarten BL, Kraines RJ. “Menopausal symptoms” in women of various ages. Psychosom Med 1965;27:266–273. (doi: 10.1097/00006842-196505000-00009) [DOI] [PubMed] [Google Scholar]
- 16.Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473–483. (Accessed from https://pubmed.ncbi.nlm.nih.gov/1593914/) [PubMed] [Google Scholar]
- 17.Avis NE, Colvin A, Bromberger JT, et al. Change in health-related quality of life over the menopausal transition in a multiethnic cohort of middle-aged women: Study of Women’s Health Across the Nation (SWAN). Menopause 2009;16:860–869. (doi: 10.1097/gme.0b013e3181a3cdaf) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levine DW, Dailey ME, Rockhill B, Tipping D, Naughton MJ, Shumaker SA. Validation of the Women’s Health Initiative Insomnia Rating Scale in a multicenter controlled clinical trial. Psychosom Med 2005;67:98–104. (doi: 10.1097/01.psy.0000151743.58067.f0) [DOI] [PubMed] [Google Scholar]
- 19.Matthews KA, Schott LL, Bromberger JT, Cyranowski JM, Everson-Rose SA, Sowers MF. Are there bi-directional associations between depressive symptoms and C-reactive protein in mid-life women? Brain Behav Immun 2010;24:96–101. (doi: 10.1016/j.bbi.2009.08.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swabe G, Matthews K, Brooks M, Janssen I, Wang N, El Khoudary SR. High-density lipoprotein cholesterol and arterial calcification in midlife women: the contribution of estradiol and C-reactive protein. Menopause 2021;28:237–246. (doi: 10.1097/GME.0000000000001706) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dohrenwend BS, Krasnoff L, Askenasy AR, Dohrenwend BP. Exemplification of a method for scaling life events: The PERI life events scale. J Health Soc Behav 1978;19:205–229. (Accessed from https://pubmed.ncbi.nlm.nih.gov/681735/) [PubMed] [Google Scholar]
- 22.Bromberger JT, Chang Y, Colvin AB, Kravitz HM, Matthews KA. Does childhood maltreatment or current stress contribute to increased risk for major depression during the menopause transition? Psychol Med. Advance online publication. (doi.org: 10.1017/S0033291720004456) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med 1991;32:705–714. (doi: 10.1016/0277-9536(91)90150-b) [DOI] [PubMed] [Google Scholar]
- 24.Bromberger JT, Kravitz HM, Chang Y, et al. Does risk for anxiety increase during the menopausal transition? Study of Women’s Health Across the Nation (SWAN). Menopause 2013;20:488–495. (doi: 10.1097/GME.0b013e3182730599) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barsky AJ, Goodson JD, Lane RS, Cleary PD (1988). The amplification of somatic symptoms. Psychosom Med 1988;50:510–519. (doi: 10.1097/00006842-198809000-00007) [DOI] [PubMed] [Google Scholar]
- 26.Bernstein DP, Stein JA, Newcomb MD, et al. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl 2003;27:169–190. (doi: 10.1016/s0145-2134(02)00541-0) [DOI] [PubMed] [Google Scholar]
- 27.Walker EA, Gelfand A, Katon WJ, et al. Adult health status of women with histories of childhood abuse and neglect. Am J Med 1999;107:332–339. (doi: 10.1016/s0002-9343(99)00235-1) [DOI] [PubMed] [Google Scholar]
- 28.Andruff H, Carraro N, Thompson A, Gaudreau P, Louvet B. Latent class growth modelling: a tutorial. Tutor Quant Methods Psychol 2009;5:11–24. (doi: 10.20982/tqmp.05.1.p011) [DOI] [Google Scholar]
- 29.Jones BL, Nagin DS. Advances in group-based trajectory modeling and an SAS procedure for estimating them. Sociol Methods Res 2007;35:542–571. (doi: 10.1177/0049124106292364) [DOI] [Google Scholar]
- 30.Nagin DS. Group-based Modeling of Development. Cambridge, MA: Harvard University Press, 2005. [Google Scholar]
- 31.Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol 2010;6:109–138. (doi: 10.1146/annurev.clinpsy.121208.131413) [DOI] [PubMed] [Google Scholar]
- 32.Herbers PM, Elder DA, Woo JG. Area under a curve: Calculation and visualization. Paper DG12–2011. Cincinnati Children’s Hospital Medical Center, Cincinnati, OH. (Accessed from www.mwsug.org›2011›dataviz›MWSUG-2011-DG12) [Google Scholar]
- 33.Mitchell ES, Woods NF. Depressed mood during the menopausal transition: is it reproductive aging or is it life? Women’s Midlife Health 2017;3:11. (doi: 10.1186/s40695-017-0030-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Freeman EW, Sammel MD, Lin H, et al. Symptoms associated with menopausal transition and reproductive hormones in midlife women. Obstet Gynecol 2007;110:230–240. (doi: 10.1097/01.AOG.0000270153.59102.40) [DOI] [PubMed] [Google Scholar]
- 35.Tepper PG, Brooks MM, Randolph JF Jr, et al. Characterizing the trajectories of vasomotor symptoms across the menopausal transition. Menopause 2016;23:1067–1074. (doi: 10.1097/GME.0000000000000676) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joffe H, de Wit A, Coborn J, et al. Impact of estradiol variability and progesterone on mood in perimenopausal women with depressive symptoms. J Clin Endocrinol Metab 2020;105:e642–e650. (doi: 10.1210/clinem/dgz181) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Freeman EW, Sammel MD, Lin H, Nelson DB. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Arch Gen Psychiatry 2006;63:375–382. (doi: 10.1001/archpsyc.63.4.375.) [DOI] [PubMed] [Google Scholar]
- 38.Best JR, Gan DRY, Wister AV, Cosco TD. Age and sex trends in depressive symptoms across middle and older adulthood: Comparison of the Canadian Longitudinal Study on Aging to American and European cohorts. J Affect Disord 2021;295:1169–1176. (doi: 10.1016/j.jad.2021.08.109) [DOI] [PubMed] [Google Scholar]
- 39.Avis NE, Colvin A, Bromberger JT, Hess R. Midlife predictors of health-related quality of life in older women. J Gerontol A Biol Sci Med Sci 2018;73:1574–1580. (doi: 10.1093/gerona/gly062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40., Mishra GD, Hockey R, Dobson AJ. A comparison of SF-36 summary measures of physical and mental health for women across the life course. Qual Life Res 2014;23:1515–1521. (doi: 10.1007/s11136-013-0586-3) [DOI] [PubMed] [Google Scholar]
- 41.Jokela M, Ferrie JE, Gimeno D, et al. From midlife to early old age: Health trajectories associated with retirement. Epidemiology 2010;21:284–290. (doi: 10.1097/EDE.0b013e3181d61f53) [DOI] [PMC free article] [PubMed] [Google Scholar]


