Abstract
Obesity is associated with a higher risk of several types of cancer, grouped as obesity-related cancers (ORC). Vitamin D deficiency is more prevalent in obese subjects, and it has been suggested to play a role in the association between obesity and cancer risk. The aim of the study was to analyze the association between vitamin D intake and the subsequent risk of ORC in a prospective Spanish cohort of university graduates. The SUN Project, initiated in 1999, is a prospective dynamic multipurpose cohort. Participants answered a 556-item lifestyle baseline questionnaire that included a validated food-frequency questionnaire. We performed Cox regression models to estimate the hazard ratios (HRs) of ORC according to quartiles of energy-adjusted vitamin D intake (diet and supplements). We included 18,017 participants (mean age = 38 years, SD = 12 years), with a median follow-up of 12 years. Among 206,783 person-years of follow-up, we identified 225 cases of ORC. We found no significant associations between vitamin D intake and ORC risk after adjusting for potential confounders: HRQ2vsQ1 = 1.19 (95% CI 0.81–1.75), HRQ3vsQ1 = 1.20 (95% CI 0.81–1.78), and HRQ4vsQ1 = 1.02 (95% CI 0.69–1.51). Dietary and supplemented vitamin D do not seem to be associated with ORC prevention in the middle-aged Spanish population.
Keywords: obesity, cancer, vitamin D, cohort
1. Introduction
Over recent decades, obesity has become a major public health issue. The prevalence of overweight and obesity has increased in almost all developing and developed countries, reaching nearly 60–70% of the adult population, and being more frequent in women and in urban areas [1,2]. Obesity is commonly defined as a body mass index (BMI) of at least 30 kg/m2. The accumulation of excessive fat tissue has been associated with the development of many chronic diseases, most notably hypertension, dyslipidemia, non-alcoholic fatty liver disease, type 2 diabetes, cardiovascular disease, and several types of cancer [3,4,5,6,7]. Obesity constitutes a major determinant for the increasing incidence of cancer, and it could even surpass tobacco as the main preventable cause of cancer [8].
The International Agency for Research on Cancer (IARC) has identified 13 cancers associated with overweight and obesity (grouped under the term of “obesity-related cancers” (ORC)): esophageal adenocarcinoma, postmenopausal breast carcinoma, colon and rectum, uterus, gallbladder, stomach, kidney, liver, ovary, pancreas, thyroid, meningioma, and multiple myeloma [9]. Despite growing evidence, the role of obesity in cancer etiopathogenesis has not been fully elucidated. The main mechanisms that seem to be implicated in the association between obesity and cancer are hyperinsulinemia, subclinical chronic low-grade inflammation, alterations in adipocytokine pathophysiology, and hormonal imbalance [10,11].
Vitamin D deficiency is a global health problem. For adults aged 19–80 years, the recommended dietary intake of vitamin D is between 10 and 20 µg/day [12]. Approximately 60% of adults worldwide are vitamin D deficient. Although several factors may explain the high prevalence of low vitamin D levels, inadequate sun exposure (i.e., indoor environment, excess of sun avoidance, air pollution) and low vitamin D intake (i.e., dietary lifestyles, lactose intolerance, and even socio-economic status) are the most common causes [13]. Dietary sources of vitamin D include oily fish (such as salmon and tuna fish), red meat, liver, egg yolks, dairy products, and cereals.
Vitamin D is one of many factors suggested to play a role in the obesity-cancer pathway. Vitamin D can be considered as a mediator, an effect modifier, or a confounder in the association between obesity and higher risk of these cancers. Previous studies have found an association between obesity and vitamin D deficiency, although it remains unclear whether vitamin D deficiency leads to an altered metabolism, or the altered metabolic state of obesity leads to vitamin D deficiency. A higher prevalence of vitamin D deficiency in the obese population may be explained through different mechanisms. One mechanism may combine lower dietary intake with lower sunlight exposure or impaired cutaneous vitamin D synthesis. Another mechanism may be influenced by differences in protein binding and metabolic clearance in obese people that could cause lower levels of circulating vitamin D [14,15]. Some observational studies have also suggested that deficient vitamin D levels contribute to a higher risk of malignant neoplasia, such as breast and colorectal cancer [16,17]. However, the question of whether vitamin D influences the association of obesity with cancer (i.e., whether it acts as an effect modifier) has not been prospectively addressed. The available evidence is currently insufficient to be able to support the supplementation of vitamin D as a treatment strategy to mitigate the negative effects its deficiency may have on cancer incidence and survival.
In this study, we aimed to assess the impact of vitamin D intake on the subsequent risk of obesity-related cancers in a prospective Spanish cohort of university graduates.
2. Materials and Methods
2.1. Study Population
The ‘Seguimiento Universidad de Navarra’ (SUN) Project is an ongoing multipurpose cohort study composed of university graduates in Spain [18]. The cohort recruitment began in 1999 and is ongoing. In the entire cohort, the median age at recruitment was 34.7 years (interquartile range: 26–42 years) and 61% of the participants are female. When participants were recruited, they completed a baseline 556-item questionnaire, collecting information about lifestyle, sociodemographic, anthropometric, and medical variables. After completing the baseline questionnaire, participants were contacted biennially through follow-up questionnaires to collect information on lifestyle changes and incident medical conditions.
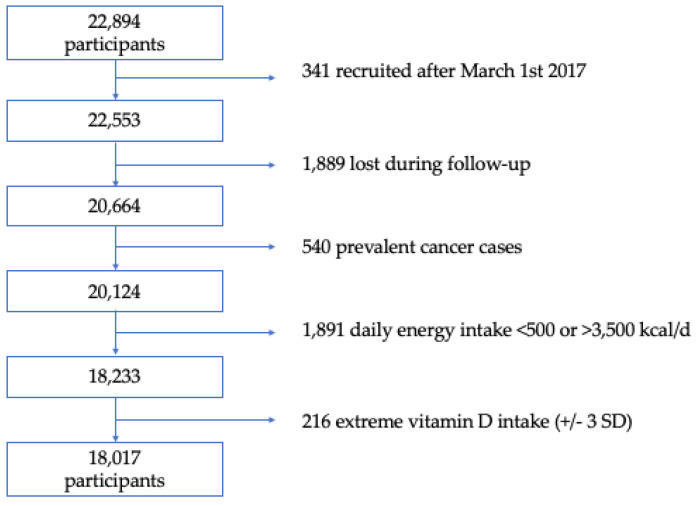
Through December 2019, a total of 22,894 participants completed the baseline questionnaire. For this analysis, we used the following exclusion criteria: 341 participants who answered the baseline questionnaire after 1 March 2017 were excluded to assure a minimum 2-year follow-up period; we further excluded 1889 participants lost in follow-up (overall retention 92%); we also excluded 540 participants with a previous cancer diagnosis at the time of enrollment. Lastly, we excluded 1891 participants with energy intake outside of predefined limits (a daily energy intake below 500 kcal/d or above 3500 kcal/d for women and below 800 kcal/d or above 4000 kcal/d for men) [19], and 216 participants with extreme intake of vitamin D (+/– 3 standard deviations). Finally, a total of 18,017 participants were included (Figure 1). The Institutional Review Board of the University of Navarra approved this study.
Figure 1.
Flowchart of participants in the SUN Project, 1999–2019. Kcal/d: kilocalorie per day. SD: standard deviation.
2.2. Assessment of Vitamin D Intake
At baseline, participants completed a validated 136-item semi-quantitative food-frequency questionnaire (FFQ). The reproducibility and validity of this FFQ has been previously published by our group [20]. The questionnaire gathers information from a wide variety of food groups, such as high-fat dairy products, eggs, meat, fish, seafood, vegetables, fruits, cereals, legumes, processed pastries, or fast food, among others. For each item, a commonly used portion size is defined. Participants were asked to provide the information in terms of long-term dietary exposures. Information was also gathered on the regular use of supplements or multivitamins, including brand, dosage, and frequency. For each subject, energy and nutrient intakes were calculated using food composition tables [21,22]. The total estimated vitamin D intake combined both diet and supplements. Total and dietary vitamin D intake were adjusted for total energy intake with the residual method [19]. As previously demonstrated for our cohort, the FFQ provides a good reproducible assessment of the usual diet and a reasonable validity in relation to vitamin D (intraclass correlation coefficient (ICC) = 0.69, using repeated 3-day dietary records as reference) [23]. For these analyses, participants were categorized into quartiles according to dietary or total (dietary plus supplemented) vitamin D intake.
2.3. Ascertainment of Obesity-Related Cancer Cases
In our study, we considered the outcome of interest to be all the incident cases of any of the following cancer diagnoses: esophageal adenocarcinoma, postmenopausal breast carcinoma, colon and rectum, uterus, gallbladder, stomach, kidney, liver, cholangiocarcinoma, ovary, pancreas, thyroid, meningioma, and multiple myeloma. Initially, cancer cases were self-reported. Participants who reported a diagnosis of any tumor were then asked to provide a copy of their medical records. Subsequently, an independent expert oncologist, who was blinded to the exposure, confirmed the cases by reviewing these records. If any participant did not submit a medical record, they were asked to consent to be contacted via telephone by an expert to confirm malignancy. Deaths due to any cancer identified by reviewing the National Death Index (NDI) were also included as confirmed cases.
2.4. Covariate Assessment
The baseline questionnaire collected information on sociodemographic, lifestyle, and medical variables. The self-reported accuracy of height and weight to estimate the BMI has been previously validated in this cohort [24]. Physical activity was also assessed through a validated questionnaire [25]. The Mediterranean Diet Score proposed by Trichopoulou et al. [26] was used to evaluate the adherence to the Mediterranean dietary pattern, excluding alcohol intake. We considered alcohol consumption as a separate covariate given the growing evidence on its association with several ORC [27,28]. The questionnaire also gathered information on the participants’ average time of sunlight exposure. Participants were inquired about the hours/day of sunlight exposure during the week and for a typical day during the weekend in winter and during the summer. In order to estimate a proxy of solar irradiation intensity in the location of residence, we consulted the Global Horizontal Irradiance (GHI, kWh/m2/day) in a given postal area between 1994 and 2015 and linked this to the participants’ postal codes. Information about the GHI can be obtained from the Global Solar Atlas satellite-derived dataset [29]. Missing values were imputed (simple imputation) using the Stata built-in command impute, based on multivariable linear regression models for continuous variables and multivariable logistic or multinomial regression models for categorical variables. Imputations represented <5% of missing covariates, except for tobacco consumption (pack-years) with 10% of missing values.
2.5. Statistical Analysis
Baseline characteristics of participants were described according to quartiles of total vitamin D intake. Quantitative variables were summarized with means and standard deviations, and qualitative variables with proportions. We verified the normality of distribution with the Shapiro–Wilk test. We used the ANOVA test to compare quantitative traits across quartiles of total vitamin D intake, and we reran these analyses using Kruskal–Wallis tests for those variables with a non-parametric distribution. We also used the Chi-squared test to compare qualitative traits across quartiles of total vitamin D intake.
To examine the association of vitamin D intake (diet or diet and supplements) and the risk of ORC, we fitted Cox regression models, with the lowest quartile as the reference category. The models included age as an underlying time variable and were additionally stratified by recruitment period and age (decades) at recruitment. The time at entry was defined as the date of completion of the baseline questionnaire. The outcome was defined as the date of ORC diagnosis. For exit time, we considered the age of cancer diagnosis for cases and the date of death due to a non-ORC-related cause, or lost to follow-up, for non-cases. We adjusted a first multivariable model, including the following potential confounders: sex, height (cm), family history of breast or colorectal cancer (yes/no), smoking habit (never, current, or former smoker), lifetime tobacco consumption (pack-years), years of university studies, physical activity (METs-h/week), alcohol consumption (g/day), total energy intake (kcal/day), BMI (kg/m2), consumption of sugar-sweetened beverages (servings/day), coffee consumption (servings/day), TV-watching (h/day), sunlight exposure (h/year), and intensity of solar irradiation in the residential area (kWh/m2/day). In a second multivariable model, we additionally adjusted for adherence to Mediterranean Diet Score (0–8 points). We selected potential confounders based on existing evidence and previous results of our cohort studies on cancer [30,31,32,33,34,35]. We estimated the association with ORC risk for both total vitamin D intake (dietary and supplemented) and dietary vitamin D as main exposures. Additionally, to analyze the potential modification of the effect of vitamin D intake by BMI categories (normal weight and overweight/obesity), we performed the multivariable Cox regression model according to BMI strata. We studied multiplicative interaction between vitamin D intake quartiles and BMI using the likelihood-ratio test to assess for the statistical significance of a product term. As sensitivity analysis, we repeated our analyses excluding those tumors which accounted for at least 10% of overall cases.
Analyses were performed using STATA/SE version 15.0 (StataCorp). A two-sided p value < 0.05 was deemed as statistically significant.
3. Results
For the analysis, we included 18,017 participants, with a median follow-up of 12.2 years. Table 1 describes baseline characteristics of participants according to quartiles of total (dietary and supplemented) energy-adjusted vitamin D intake. The median BMI was 23.1 kg/m2 (interquartile range: 20.9–25.6 kg/m2). In our cohort, only 25% of participants met the recommended dietary intake of vitamin D for adults 19–80 years. Participants in the highest quartile of vitamin D intake tended to be more physically active, to have a slightly higher adherence to the Mediterranean diet, and to have a higher sunlight exposure (in h/year). Other important characteristics such as sex, BMI, total energy intake, family history of breast or colorectal cancer, and sunlight exposure were very similar across groups.
Table 1.
Baseline characteristics of participants in the SUN Project, according to energy-adjusted quartiles of vitamin D (diet and supplemented).
| Variable | Q1 | Q2 | Q3 | Q4 | p Value + |
|---|---|---|---|---|---|
| n | 4505 | 4504 | 4504 | 4504 | |
| Total vitamin D intake (µg/day) * | 2.7 (2.0–3.2) | 4.4 (4.0–4.7) | 5.8 (5.4–6.5) | 11.7 (11.0–12.5) | <0.001 |
| Age (years) | 35 (27–45) | 36 (27–46) | 35 (27–46) | 37 (28–49) | <0.001 |
| Sex (% women) | 62.1 | 59.3 | 58.4 | 59.6 | 0.003 |
| Body-mass index (kg/m2) | 22.8 (20.7–25.4) | 23.1 (20.9–25.6) | 23.1 (20.9–25.5) | 23.4 (21.0–25.9) | <0.001 |
| Height (cm) | 168 (162–174) | 168 (162–175) | 168 (162–175) | 168 (162–174) | 0.174 |
| Physical activity (METs-h/week) | 14.2 (4.2–27.5) | 14.8 (5.1–29.1) | 15.9 (5.7–29.8) | 18.9 (7.4–34.4) | <0.001 |
| Total energy intake (kcal/day) | 2517 (2048–2997) | 2362 (1976–2715) | 2041 (1682–2503) | 2358 (1975–2767) | <0.001 |
| Alcohol intake (g/day) | 2.6 (0.6–8.8) | 3.2 (0.9–8.8) | 3.1 (0.9–8.4) | 3.2 (0.9–9.0) | <0.001 |
| Sugar-sweetened beverages (servings/day) | 0.1 (0.0–0.4) | 0.1 (0.0–0.1) | 0.1 (0.0–0.1) | 0.1 (0.0–0.1) | <0.001 |
| Coffee (servings/day) | 1.0 (0.4–2.5) | 1.0 (0.4–2.5) | 1.0 (0.4–2.5) | 1.0 (0.4–2.5) | <0.001 |
| Adherence to Mediterranean Diet Score | 4 (3–5) | 4 (3–5) | 4 (3–5) | 5 (3–6) | <0.001 |
| Time of university education (years) | 5 (4–5) | 5 (4–5) | 5 (4–5) | 5 (4–5) | 0.047 |
| Smoking habit (%) | <0.001 | ||||
| Never | 47.6 | 48.7 | 50.6 | 47.7 | |
| Current | 23.8 | 22.6 | 21.5 | 20.2 | |
| Former | 28.6 | 28.7 | 27.9 | 32.1 | |
| Tobacco consumption (pack-years) | 0.5 (0.0–10.0) | 0.5 (0.0–10.0) | 0.0 (0.0–9.0) | 0.5 (0.0–11.0) | <0.001 |
| TV watching (h/day) | 1.5 (0.8–2.0) | 1.5 (0.8–2.0) | 1.4 (0.7–2.0) | 1.4 (0.8–2.0) | 0.101 |
| Solar irradiation (kWh/m2/day) | 4.0 (3.9–4.5) | 4.0 (3.7–4.6) | 4.1 (3.8–4.6) | 4.2 (3.7–4.7) | 0.192 |
| Sunlight exposure (h/year) | 1984 (1866–2816) | 1984 (1866–2822) | 1984 (1866–2887) | 2279 (1866–2887) | <0.001 |
| Family history (%) | |||||
| Breast cancer | 27.0 | 25.6 | 27.4 | 27.1 | 0.21 |
| Colorectal cancer | 14.6 | 14.6 | 15.1 | 16.3 | 0.09 |
* Values represent medians (interquartile range), unless otherwise stated. + Chi-squared test for comparisons of proportions and ANOVA test or Kruskal–Wallis test for quantitative traits.
During a total follow-up of 206,783 person-years, we identified 225 ORC cases (59 postmenopausal breast, 49 colon and 21 rectum, 11 uterus, 6 ovarian, 22 pancreatic, 5 esophageal, 11 stomach, 2 gallbladder, 7 cholangiocarcinoma, 4 hepatocellular carcinoma, 4 multiple myeloma, 1 meningioma, 9 renal cell carcinomas, and 21 thyroid carcinomas) (7 participants developed two ORC).
When we compared quartiles of total vitamin D intake, we found no significant associations for ORC risk after adjusting for potential confounders (Table 2). Compared to the lowest quartile, the HRQ2vsQ1 was 1.19 (95% CI 0.81–1.75), the HRQ3vsQ1 was 1.20 (95% CI 0.81–1.78), and the HRQ4vsQ1 was 1.02 (95% CI 0.69–1.51).
Table 2.
Hazard ratio (95% CI) of obesity-related cancer according to energy-adjusted quartiles of total vitamin D intake (dietary and supplemented).
| Obesity-Related Cancer Cases | Q1 | Q2 | Q3 | Q4 |
|---|---|---|---|---|
| Cases/person-years | 50/52657 | 57/52666 | 58/51328 | 60/50131 |
| Age adjusted | 1 (Ref.) | 1.14 (0.78–1.67) | 1.14 (0.78–1.67) | 1.01 (0.69–1.48) |
| Multivar. adjusted * | 1 (Ref.) | 1.19 (0.81–1.76) | 1.21 (0.82–1.79) | 1.06 (0.72–1.55) |
| Multivar. adjusted † | 1 (Ref.) | 1.19 (0.81–1.75) | 1.20 (0.81–1.78) | 1.02 (0.69–1.51) |
* Adjusted for sex, height (cm), family history of breast or colorectal cancer (yes/no), smoking habit (never, current, or former smoker), lifetime tobacco consumption (pack-years), years of university studies, physical activity (MET-h/week), alcohol consumption (g/day), total energy intake (kcal/day), BMI (kg/m2), consumption of sugar-sweetened beverages (servings/day), coffee consumption (servings/day), TV-watching (h/day), sunlight exposure (h/year), and intensity of solar irradiation (kWh/m2/day). † Additionally adjusted for adherence to Mediterranean Diet Score (0–8 points).
When comparing the risk of ORC across quartiles of dietary vitamin D intake (using the lowest quartile of vitamin D intake as the reference category), we found no significant association (Table 3): HRQ2vsQ1 = 1.14 (95% CI 0.77–1.69), the HRQ3vsQ1 = 1.25 (95% CI 0.84–1.87), and the HRQ4vsQ1 = 1.08 (95% CI 0.73–1.61).
Table 3.
Hazard ratio (95% CI) of obesity-related cancer according to energy-adjusted quartiles of dietary vitamin D intake.
| Obesity-Related Cancer Cases | Q1 | Q2 | Q3 | Q4 |
|---|---|---|---|---|
| Cases/person-years | 48/52523 | 55/52640 | 59/51412 | 63/50205 |
| Age adjusted | 1 (Ref.) | 1.12 (0.76–1.66) | 1.19 (0.81–1.75) | 1.07 (0.73–1.56) |
| Multivar. adjusted * | 1 (Ref.) | 1.15 (0.78–1.71) | 1.27 (0.85–1.89) | 1.12 (0.76–1.65) |
| Multivar. adjusted † | 1 (Ref.) | 1.14 (0.77–1.69) | 1.25 (0.84–1.87) | 1.08 (0.73–1.61) |
* Adjusted for sex, height (cm), family history of breast or colorectal cancer (yes/no), smoking habit (never, current, or former smoker), lifetime tobacco consumption (pack-years), years of university studies, physical activity (MET-h/week), alcohol consumption (g/day), total energy intake (kcal/day), BMI (kg/m2), consumption of sugar-sweetened beverages (servings/day), coffee consumption (servings/day), TV-watching (h/day), sunlight exposure (h/year), and intensity of solar irradiation (kWh/m2/day). † Additionally adjusted for adherence to Mediterranean Diet Score (0–8 points).
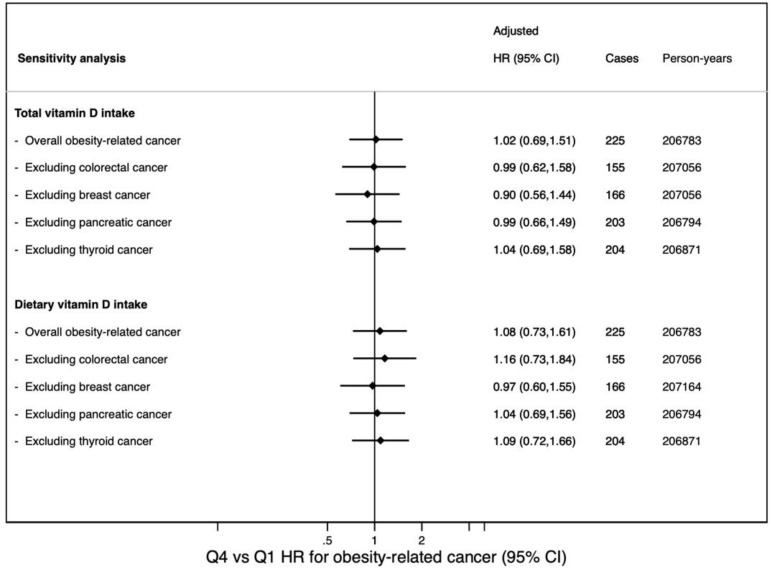
When we excluded tumors representing at least 10% of cases (colorectal, breast, pancreatic, and thyroid carcinomas) results did not change substantially (Figure 2).
Figure 2.
Hazard ratio (95% CI) for the comparison across extreme quartiles of overall obesity-related cancer and excluding tumors which accounted for at least 10% of cases.
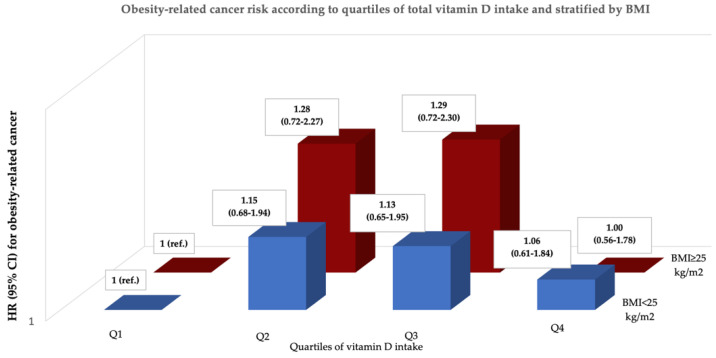
The effect of total vitamin D intake in the ORC risk did not vary across BMI strata (Figure 3). We found no interaction between quartiles of vitamin D intake and BMI categories (p for interaction = 1.00) in the subsequent risk of ORC.
Figure 3.
Hazard ratio (95% CI) of obesity-related cancer according to quartiles of total vitamin D intake and BMI strata (normal weight and overweight/obesity). Adjusted for sex, height (cm), family history of breast or colorectal cancer (yes/no), smoking habit (never, current, or former smoker), lifetime tobacco consumption (pack-years), years of university studies, physical activity (MET-h/week), alcohol consumption (g/day), total energy intake (kcal/day), consumption of sugar-sweetened beverages (servings/day), coffee consumption (servings/day), TV-watching (h/day), sunlight exposure (h/year), intensity of solar irradiation (kWh/m2/day), and adherence to Mediterranean Diet Score (0–8 points).
4. Discussion
In this prospective Spanish cohort, the risk of ORC did not significantly change across quartiles of vitamin D intake, regardless of considering total or dietary intake.
Evidence supporting a link between obesity and cancer, and additionally between low vitamin D and obesity, is consistent across studies. A pooled analysis of 12 observational studies that evaluated the association between vitamin D status and obesity showed an overall relative risk of 1.52 (95% CI 1.33–1.73) for low vitamin D status and obesity [36]. This inverse association may be attributed to an increase in metabolic clearance in the excess adipose tissue characteristic in obese states [37]. Another explanation could be that obese individuals are less likely to engage in outdoor physical activity than non-obese individuals, decreasing sun exposure [38]. The benefit of leisure-time physical activity in reducing the risk of breast cancer has been previously explored in the SUN cohort [35]. In terms of physical activity, the SUN cohort participants have a median of 16.1 METs-h/week. This represents approximately an equivalent of 1 h/day of daily walking. In addition, the mean BMI of the participants included in our analysis was 23.5 kg/m2 (SD = 3.5 kg/m2). Some baseline characteristics of the population included in our study (moderate/high physical activity, middle-aged participants, and mean BMI in the normal weight range) could outweigh the potential role of vitamin D intake in reducing the risk of cancer.
In the present study, 131 out of 225 cases (58%) of ORC identified in the SUN cohort were composed of postmenopausal breast cancer and colorectal cancer. Among all types of ORC, colorectal cancer has shown more consistent results for an inverse association between vitamin D levels and cancer risk. Observational studies performed in different populations worldwide have found a relative decrease in colorectal cancer risk varying from 4 to 50% for the comparison of extreme categories [39,40,41]. As for breast cancer risk, while some observational studies have reported an inverse association between vitamin D intake and breast cancer, others have reported null associations, leading to inconclusive evidence concerning vitamin D intake [42,43]. Previously in our cohort, we specifically analyzed the association between dietary calcium, vitamin D, and breast cancer risk [44]. We found a non-linear association between total calcium intake and breast cancer, with risk reductions associated with higher intake up to approximately 1400 mg/day. No evidence for any association between vitamin D intake and breast cancer was found (HRT3vsT1 = 0.87, 95% CI 0.54–1.41).
The potential role of vitamin D intake in cancer prevention was hypothesized several decades ago [45]. Results from observational studies led to the idea that vitamin D deficiency may increase cancer risk. However, vitamin D supplementation in interventional studies has not shown cancer preventive qualities. In the VITAL trial, conducted in the United States, more than 25,000 participants received a daily vitamin D3 supplementation of 2000 IU (50 mcg) for 5 years, and no significant reduction in overall cancer risk was found [46]. Other randomized controlled trials have also reported no effect of supplementing vitamin D3 in cancer risk [47,48,49]. A differential effect of vitamin D intake across populations has been suggested through studies analyzing single nucleotide polymorphisms associated with 25(OH)D3 levels [50]. Studies performed in the Finnish population have suggested that the possible beneficial effects of vitamin D3 on cancer may not be consistent in the general population. Hence, there could exist ‘low vitamin D responders’—individuals who need to increase their dose of daily vitamin D supplementation to reach full clinical benefit—in contrast with ‘high vitamin D responders’—those who can have a vitamin D deficiency and might be less affected by diseases, such as malignant neoplasms, against which vitamin D may have a preventive role [51,52].
Dietary intake and supplementation of vitamin D may prevent pro-inflammatory processes, such as metabolic syndrome and carcinomas. Despite promising results from previous studies, vitamin D cannot be considered an anti-cancer agent yet, as its potential anti-tumoral activity has not been fully confirmed. Similar to many other nutritional co-factors, such as vitamins or polyphenols, vitamin D can exert a pleiotropic role within cellular machinery with the ability to promote cell response to stress [53]. The active form of vitamin D (1α,25(OH)2vitD or calcitriol) can be considered a dietary-derived immune cytokine. It may interact with immune system, as lymphocytes express the vitamin D receptor (VDR) [54]. However, it should be noted that most of the immunological activity exerted by calcitriol depends on cellular VDR expression. The VDR polymorphisms must be considered when assessing the efficacy of vitamin D in counteracting cancer risk in a given population [55]. In our analyses, we included many potential confounders because any research on the nutritional value expected from vitamin D supplementation has to consider many factors, including lifestyle, different dietary habits from diverse regions, and metabolic state [56].
Some limitations must be noted. First, our cohort participants are relatively young and physically active and show a mean BMI mostly in the normal weight range. These characteristics may partially explain the low incidence of cancer. Consequently, this may limit the statistical power, especially when analyzing the association with each specific type of neoplasia separately. Additionally, to be noted is the fact that only 25% of participants included in the analyses met the recommended dietary intake of vitamin D (10–20 µg/day). This may have limited the capacity to observe the influence of this nutrient (being consumed in adequate amounts). Second, it is important to note that, under the concept of ORC, we include tumors with different pathobiology, carcinogenesis, and cellular pathways. Hence, the biological heterogeneity may also limit the capacity to draw solid conclusions. Third, as the exposure is self-reported and participants may misreport their nutritional pattern in the questionnaire, this potential bias could result in the observed association being underestimated. Fourth, the exposure period considered may not be the most etiologically relevant for participants, as dietary patterns may differ from the early adulthood. Fifth, since the outcome assessment was self-reported, this may have resulted in an underreporting of incident cases and thus a lower sensitivity. Nevertheless, cancer cases were blindly confirmed—with high specificity—by an oncologist. Sixth, since the socio-economic status of the participants was not available, years of university studies were included in the multivariable analysis. As our study sample exclusively involved university graduates, it is homogeneous in this aspect, which may reduce the potential confounding effect of educational and socioeconomic status.
To the best of our knowledge, this is the first study to assess the relationship between vitamin D intake and ORC risk in a middle-aged Spanish population. The prospective nature of the SUN Project ensures the temporal sequence between exposure and outcome, including a large sample size with a long follow-up and a good retention rate. Moreover, the adjustment for a wide number of potential confounders and the sensitivity analyses assure the robustness of our findings. Self- reported cancer cases were confirmed via medical reports to ensure that the final diagnosis was an invasive carcinoma and not a benign lesion.
5. Conclusions
In summary, our study did not find any association between vitamin D intake and risk of ORC. Dietary and supplemental vitamin D do not seem to be associated with ORC prevention in the middle-aged Spanish population. Current guidelines for reducing cancer risk should focus on the detrimental effect of obesity or smoking, and promote other healthy habits such as regular exercise, weight loss, and adherence to the Mediterranean diet, as they have demonstrated more consistent evidence as preventive measures.
Acknowledgments
We thank other members of the SUN Group: Alonso A, Álvarez-Álvarez I, Balaguer A, Barbagallo M, Barrientos I, Barbería-Latasa M, Barrio-López MT, Basterra-Gortari FJ, Battezzati A, Bazal P, Benito S, Bertoli S, Beulen Y, Beunza JJ, Buil-Cosiales P, Canales M, Carlos S, Carmona L, Cervantes S, Cristobo C, de Irala J, de la Fuente-Arrillaga C, de la O V, de la Rosa PA, Delgado-Rodríguez M, Díaz-Gutiérrez J, Díez Espino J, Domínguez L, Donat-Vargas C, Donazar M, Eguaras S, Fernández-Montero A, Fresán U, Galbete C, García-Arellano A, García López M, GutiérrezBedmar M, Gomes-Domingos AL, Gómez-Donoso C, Gómez-Gracia E, Goñi E, Goñi L, Guillén F, Henríquez P, Hernández A, Hershey MS, Hidalgo-Santamaría M, Hu E, Lahortiga F, Leone A, Llorca J, López del Burgo C, Marí A, Marques I, Martí A, Martín Calvo N, Martín-Moreno JM, Martínez JA, Martínez-Lapiscina EH, Mendonça R, Menéndez C, Molendijk M, Molero P, Murphy K, Muñoz M, NúñezCórdoba JM, Pajares R, Papadaki A, Parletta N, Pérez de Ciriza P, Pérez Cornago A, Pérez de Rojas J, Pimenta AM, Pons J, Ramallal R, Razquin C, Rico-Campà A, Ruano C, Ruiz L, Ruiz-Canela M, Ruiz Zambrana A, Salgado E, San Julián B, Sánchez D, Sánchez-Tainta A, Sánchez-Villegas A, Santiago S, Sayón-Orea C, Schlatter J, Serrano-Martinez M, Toledo J, Tortosa A, Valencia F, Vázquez Z, Zarnowiecki D, Zazpe I. We thank very specially all participants in the SUN cohort for their long-standing and enthusiastic collaboration and our advisors from Harvard TH Chan School of Public Health Walter Willett, Alberto Ascherio, Frank B. Hu and Meir J. Stampfer who helped us to design the SUN Project, the PREDIMED study and the PREDIMED-PLUS ongoing trial.
Author Contributions
Conceptualization, E.T., M.A.M.-G. and R.S.-B.; methodology, M.A.M.-G.; validation, R.S.-B. formal analysis, M.B., R.S.-B. and E.T.; resources, E.T., M.A.M.-G. and M.B.-R.; data curation, R.S.-B., M.B., J.J.P. and E.T.; writing—original draft preparation, R.S.-B. and E.T.; writing—review and editing, R.S.-B., M.B.-R., C.I.F.-L., M.B., A.M., J.J.P., M.A.M.-G. and E.T.; supervision, M.A.M.-G. and E.T.; project administration, M.B., M.A.M.-G.; funding acquisition, M.A.M.-G., M.B.-R. and E.T. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The present study was approved by the Institutional Review Board of the University of Navarra.
Informed Consent Statement
Voluntarily given informed consent through free fulfilment of the baseline questionnaire was gathered from all participants.
Data Availability Statement
The data that support the findings of this study are available from the SUN Project at sun@unav.es, upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Funding Statement
The project SUN has received funding from the Spanish Government—Instituto de Salud Carlos III, the European Regional Development Fund (FEDER) (RD 06/0045, CIBER-OBN, Grants PI10/02658, PI10/02293, PI13/00615, PI14/01668, PI14/01798, PI14/01764, PI17/01795, PI20/00564, and G03/140), the Government of Navarra (27/2011, 45/2011, 122/2014), the National Plan on Drugs (2020/021), and the University of Navarra.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet. 2017;16:2627–2642. doi: 10.1016/S0140-6736(17)32129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blüher M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019;15:288–298. doi: 10.1038/s41574-019-0176-8. [DOI] [PubMed] [Google Scholar]
- 3.Vekic J., Zeljkovic A., Stefanovic A., Jelic-Ivanovic Z., Spasojevic-Kalimanovska V. Obesity and dyslipidemia. Metabolism. 2019;92:71–81. doi: 10.1016/j.metabol.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Polyzos S.A., Kountouras J., Mantzoros C.S. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metabolism. 2019;92:82–97. doi: 10.1016/j.metabol.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 5.Piché M.E., Tchernof A., Després J.P. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circ. Res. 2020;126:1477–1500. doi: 10.1161/CIRCRESAHA.120.316101. [DOI] [PubMed] [Google Scholar]
- 6.Seravalle G., Grassi G. Obesity and hypertension. Pharmacol Res. 2017;122:1–7. doi: 10.1016/j.phrs.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 7.Avgerinos K.I., Spyrou N., Mantzoros C.S., Dalamaga M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism. 2019;92:121–135. doi: 10.1016/j.metabol.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Global Burden of Disease 2019 Cancer Collaboration Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life Years for 29 Cancer Groups From 2010 to 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. JAMA Oncol. 2022;8:420–444. doi: 10.1001/jamaoncol.2021.6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lauby-Secretan B., Scoccianti C., Loomis D., Grosse Y., Bianchini F., Straif K., International Agency for Research on Cancer Handbook Working Group Body Fatness and Cancer—Viewpoint of the IARC Working Group. N. Engl. J. Med. 2016;375:794–798. doi: 10.1056/NEJMsr1606602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hopkins B.D., Goncalves M.D., Cantley L.C. Obesity and Cancer Mechanisms: Cancer Metabolism. J. Clin. Oncol. 2016;34:4277–4283. doi: 10.1200/JCO.2016.67.9712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang A.M.Y., Wellberg E.A., Kopp J.L., Johnson J.D. Hyperinsulinemia in Obesity, Inflammation, and Cancer. Diabetes Metab. J. 2021;45:285–311. doi: 10.4093/dmj.2020.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Institutes of Health Office of Dietary Supplements. Vitamin D Fact Sheet for Consumers. [(accessed on 12 June 2022)]; Available online: https://ods.od.nih.gov/factsheets/VitaminD-Consumer/
- 13.Holick M.F. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017;18:153–165. doi: 10.1007/s11154-017-9424-1. [DOI] [PubMed] [Google Scholar]
- 14.Walsh J.S., Bowles S., Evans A.L. Vitamin D in obesity. Curr. Opin. Endocrinol. Diabetes Obes. 2017;24:389–394. doi: 10.1097/MED.0000000000000371. [DOI] [PubMed] [Google Scholar]
- 15.Oussaada S.M., van Galen K.A., Cooiman M.I., Kleinendorst L., Hazebroek E.J., van Haelst M.M., Ter Horst K.W., Serlie M.J. The pathogenesis of obesity. Metabolism. 2019;92:26–36. doi: 10.1016/j.metabol.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 16.Estébanez N., Gómez-Acebo I., Palazuelos C., Llorca J., Dierssen-Sotos T. Vitamin D exposure and Risk of Breast Cancer: A meta-analysis. Sci. Rep. 2018;8:9039. doi: 10.1038/s41598-018-27297-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou J., Ge X., Fan X., Wang J., Miao L., Hang D. Associations of vitamin D status with colorectal cancer risk and survival. Int. J. Cancer. 2021;149:606–614. doi: 10.1002/ijc.33580. [DOI] [PubMed] [Google Scholar]
- 18.Carlos S., De La Fuente-Arrillaga C., Bes-Rastrollo M., Razquin C., Rico-Campá A., Martínez-González M.A., Ruiz-Canela M. Mediterranean Diet and Health Outcomes in the SUN Cohort. Nutrients. 2018;10:439. doi: 10.3390/nu10040439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willett W.C. Nutritional Epidemiology. 3rd ed. Oxford University Press; New York, NY, USA: 2013. [Google Scholar]
- 20.De la Fuente-Arrillaga C., Vázquez Ruiz Z., Bes-Rastrollo M., Sampson L., Martinez-González M. Reproducibility of an FFQ validated in Spain. Public Health Nutr. 2010;13:1364–1372. doi: 10.1017/S1368980009993065. [DOI] [PubMed] [Google Scholar]
- 21.Moreiras O., Carbajal A., Cabrera L., Cuadrado C. Tablas de Composición de Alimentos (Food Composition Tables) 15th ed. Pirámide; Madrid, Spain: 2011. [Google Scholar]
- 22.Mataix-Verdú J., García-Diz L., Mañas M., Martínez-Victoria E., Llopis González J. Tabla de Composición de Alimentos (Spanish Food Composition Tables) 4th ed. Universidad de Granada Press; Granada, Spain: 2003. [Google Scholar]
- 23.Fernández-Ballart J.D., Piñol J.L., Zazpe I., Corella D., Carrasco P., Toledo E., Perez-Bauer M., Martínez-González M.Á., Salas-Salvadó J., Martín-Moreno J.M. Relative validity of a semi-quantitative food-frequency questionnaire in an elderly Mediterranean population of Spain. Br. J. Nutr. 2010;103:1808–1816. doi: 10.1017/S0007114509993837. [DOI] [PubMed] [Google Scholar]
- 24.Bes-Rastrollo M., Valdivieso J.R.P., Sánchez-Villegas A., Alonso Á., Martínez-González M.Á. Validación del peso e índice de masa corporal autodeclarados de los participantes de una cohorte de graduados universitarios (validation of self-reported weight and body mass index among participants of a cohort of university graduates) Rev. Esp. Obes. 2005;3:352–358. [Google Scholar]
- 25.Martínez-González M.A., López-Fontana C., Varo J.J., Sánchez-Villegas A., Martinez J.A. Validation of the Spanish version of the physical activity questionnaire used in the nurses’ health study and the health professionals’ follow-up study. Public Health Nutr. 2005;8:920–927. doi: 10.1079/PHN2005745. [DOI] [PubMed] [Google Scholar]
- 26.Trichopoulou A., Costacou T., Bamia C., Trichopoulos D. Adherence to a Mediterranean dieat and survival in a Greek population. N. Engl. J. Med. 2003;348:2599–2608. doi: 10.1056/NEJMoa025039. [DOI] [PubMed] [Google Scholar]
- 27.Sánchez-Bayona R., Gea A., Gardeazabal I., Romanos-Nanclares A., Martínez-González M.A., Bes-Rastrollo M., Santisteban M., Toledo E. Binge drinking and risk of breast cancer: Results from the SUN (‘Seguimiento Universidad de Navarra’) Project. Nutrients. 2020;12:731. doi: 10.3390/nu12030731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scoccianti C., Cecchini M., Anderson A.S., Berrino F., Boutron-Ruault M.C., Espina C., Key T.J., Leitzmann M., Norath T., Powersi H., et al. European Code against Cancer 4th Edition: Alcohol drinking and cancer. Cancer Epidemiol. 2016;45:181–188. doi: 10.1016/j.canep.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 29.World Bank Group Global Solar Atlas. Global horizontal irradiance (GHI) in Spain 1994–2015. 2020. [(accessed on 1 February 2020)]. Available online: https://globalsolaratlas.info.
- 30.Gardeazabal I., Romanos-Nanclares A., Martínez-González M.Á., Sánchez-Bayona R., Vitelli-Storelli F., Gaforio J.J., Aramendía-Beitia J.M., Toledo E. Total polyphenol intake and breast cancer risk in the Seguimiento Universidad de Navarra (SUN) cohort. Br. J. Nutr. 2019;122:542–551. doi: 10.1017/S0007114518003811. [DOI] [PubMed] [Google Scholar]
- 31.Romanos-Nanclares A., Sánchez-Quesada C., Gardeazábal I., Martínez-González M.Á., Gea A., Toledo E. Phenolic acid subclasses, individual compounds, and breast cancer risk in a Mediterranean cohort: The SUN project. J. Acad. Nutr. Diet. 2020;120:1002–1015.e5. doi: 10.1016/j.jand.2019.11.007. [DOI] [PubMed] [Google Scholar]
- 32.Sánchez-Quesada C., Romanos-Nanclares A., Navarro A.M., Gea A., Cervantes S., Martínez-González M., Toledo E. Coffee consumption and breast cancer risk in the SUN project. Eur. J. Nutr. 2020;59:3461–3471. doi: 10.1007/s00394-020-02180-w. [DOI] [PubMed] [Google Scholar]
- 33.Romanos-Nanclares A., Toledo E., Gardeazabal I., Jiménez-Moleón J.J., Martínez-González M.A., Gea A. Sugar-sweetened beverage consumption and incidence of breast cancer: The Seguimiento Universidad de Navarra (SUN) project. Eur. J. Nutr. 2019;58:2875–2886. doi: 10.1007/s00394-018-1839-2. [DOI] [PubMed] [Google Scholar]
- 34.Romanos-Nanclares A., Gea A., Martínez-González M.Á, Zazpe I., Gardeazabal I., Fernández-Lazaro C.I., Toledo E. Carbohydrate quality index and breast cancer risk in a Mediterranean cohort: The SUN project. Clin. Nutr. 2020;40:137–145. doi: 10.1016/j.clnu.2020.04.037. [DOI] [PubMed] [Google Scholar]
- 35.Sánchez-Bayona R., Gardeazabal I., Romanos-Nanclares A., Fernandez-Lazaro C.I., Alvarez-Alvarez I., Ruiz-Canela M., Gea A., Martinez-Gonzalez M.A., Santisteban M., Toledo E. Leisure-time physical activity, sedentary behavior, and risk of breast cancer: Results from the SUN (‘Seguimiento Universidad De Navarra’) project. Prev. Med. 2021;148:106535. doi: 10.1016/j.ypmed.2021.106535. [DOI] [PubMed] [Google Scholar]
- 36.Shanmugalingam T., Crawley D., Bosco C., Melvin J., Rohrmann S., Chowdhury S., Holmberg L., Van Hemelrijck M. Obesity and cancer: The role of vitamin D. BMC Cancer. 2014;14:712. doi: 10.1186/1471-2407-14-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blum M., Dolnikowski G., Seyoum E., Harris S.S., Booth S.L., Peterson J., Saltzman E., Dawson-Hughes B. Vitamin D(3) in fat tissue. Endocrine. 2008;33:90–94. doi: 10.1007/s12020-008-9051-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kull M., Kallikorm R., Lember M. Body mass index determines sunbathing habits: Implications on vitamin D levels. Intern. Med. J. 2009;39:256–258. doi: 10.1111/j.1445-5994.2009.01900.x. [DOI] [PubMed] [Google Scholar]
- 39.Gorham E.D., Garland C.F., Garland F.C., Grant W., Mohr S.B., Lipkin M., Newmark H.L., Giovannucci E., Wei M., Holick M. Optimal vitamin D status for colorectal cancer prevention: A quantitative meta analysis. Am. J. Prev. Med. 2007;32:210–216. doi: 10.1016/j.amepre.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 40.Touvier M., Chan D.S., Lau R., Aune D., Vieira R., Greenwood D.C., Kampman E., Riboli E., Hercberg S., Norat T. Meta-analyses of vitamin D intake, 25-hydroxyvitamin D status, vitamin D receptor polymorphisms, and colorectal cancer risk. Cancer Epidemiol. Biomark. Prev. 2011;20:1003–1016. doi: 10.1158/1055-9965.EPI-10-1141. [DOI] [PubMed] [Google Scholar]
- 41.Yin L., Grandi N., Raum E., Haug U., Arndt V., Brenner H. Meta-analysis: Longitudinal studies of serum vitamin D and colorectal cancer risk. Aliment. Pharm. 2009;30:113–125. doi: 10.1111/j.1365-2036.2009.04022.x. [DOI] [PubMed] [Google Scholar]
- 42.Rossi M., McLaughlin J.K., Lagiou P., Bosetti C., Talamini R., Lipworth L., Giacosa A., Montella M., Franceschi S., Negri E., et al. Vitamin D intake and breast cancer risk: A case-control study in Italy. Ann. Oncol. 2009;20:374–378. doi: 10.1093/annonc/mdn550. [DOI] [PubMed] [Google Scholar]
- 43.Abbas S., Linseisen J., Rohrmann S., Chang-Claude J., Peeters P.H., Engel P., Brustad M., Lund E., Skeie G., Olsen A., et al. Dietary intake of vitamin D and calcium and breast cancer risk in the European Prospective Investigation into Cancer and Nutrition. Nutr. Cancer. 2013;65:178–187. doi: 10.1080/01635581.2013.752018. [DOI] [PubMed] [Google Scholar]
- 44.Fernandez-Lazaro C.I., Romanos-Nanclares A., Sánchez-Bayona R., Gea A., Sayon-Orea C., Martinez-Gonzalez M.A., Toledo E. Dietary calcium, vitamin D, and breast cancer risk in women: Findings from the SUN cohort. Eur. J. Nutr. 2021;60:3783–3797. doi: 10.1007/s00394-021-02549-5. [DOI] [PubMed] [Google Scholar]
- 45.Garland C.F., Garland F.C. Do sunlight and vitamin D reduce the likelihood of colon cancer? Int. J. Epidemiol. 1980;9:227–231. doi: 10.1093/ije/9.3.227. [DOI] [PubMed] [Google Scholar]
- 46.Manson J.E., Cook N.R., Lee I.M., Christen W., Bassuk S.S., Mora S., Gibson H., Gordon D., Copeland T., D’Agostino D., et al. Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. N. Engl. J. Med. 2019;380:33–44. doi: 10.1056/NEJMoa1809944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wactawski-Wende J., Kotchen J.M., Anderson G.L., Assaf A.R., Brunner R.L., O’Sullivan M.J., Margolis K.L., Ockene J.K., Phillips L., Pottern L., et al. Women’s Health Initiative, Calcium plus vitamin D supplementation and the risk of colorectal cancer. N. Engl. J. Med. 2006;354:684–696. doi: 10.1056/NEJMoa055222. [DOI] [PubMed] [Google Scholar]
- 48.Avenell A., MacLennan G.S., Jenkinson D.J., McPherson G.C., McDonald A.M., Pant P.R., Grant A.M., Campbell M.K., Anderson F.H., Cooper C., et al. Long-term follow-up for mortality and cancer in a randomized placebo-controlled trial of vitamin D3 and/or calcium (RECORD trial) J. Clin. Endocrinol. Metab. 2012;97:614–622. doi: 10.1210/jc.2011-1309. [DOI] [PubMed] [Google Scholar]
- 49.Trivedi D.P., Doll R., Khaw K.T. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: Randomised double blind controlled trial. BMJ. 2003;326:469. doi: 10.1136/bmj.326.7387.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ong J.S., Dixon-Suen S.C., Han X., An J., Esophageal Cancer Consortium. 23 and Me Research Team. Liyanage U., Dusingize J.C., Schumacher J., Gockel I., et al. A comprehensive re-assessment of the association between vitamin D and cancer susceptibility using Mendelian randomization. Nat. Commun. 2021;12:246. doi: 10.1038/s41467-020-20368-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vukic M., Neme A., Seuter S., Saksa N., de Mello V.D., Nurmi T., Uusitupa M., Tuomainen T.P., Virtanen J.K., Carlberg C. Relevance of vitamin D receptor target genes for monitoring the vitamin D responsiveness of primary human cells. PLoS ONE. 2015;10:e0124339. doi: 10.1371/journal.pone.0124339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seuter S., Virtanen J.K., Nurmi T., Pihlajamäki J.P., Mursu J., Voutilainen S., Tuomainen T.-P., Neme A., Carlberg C. Molecularevaluation of vitamin D responsiveness of healthy young adults. J. Steroid. Biochem. Mol. Biol. 2017;174:314–321. doi: 10.1016/j.jsbmb.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 53.Feldman D., Krishnan A.V., Swami S., Giovannucci E., Feldman B.J. The role of vitamin D in reducing cancer risk and progression. Nat. Rev. Cancer. 2014;14:342–357. doi: 10.1038/nrc3691. [DOI] [PubMed] [Google Scholar]
- 54.Charoenngam N., Holick M.F. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients. 2020;12:2097. doi: 10.3390/nu12072097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gnagnarella P., Raimondi S., Aristarco V., Johansson H.A., Bellerba F., Corso F., Gandini S. Vitamin D Receptor Polymorphisms and Cancer. Adv. Exp. Med. Biol. 2020;1268:53–114. doi: 10.1007/978-3-030-46227-7_4. [DOI] [PubMed] [Google Scholar]
- 56.Cuevas-Sierra A., Ramos-Lopez O., Riezu-Boj J.I., Milagro F.I., Martinez J.A. Diet, Gut Microbiota, and Obesity: Links with Host Genetics and Epigenetics and Potential Applications. Adv. Nutr. 2019;10((Suppl. 1)):S17–S30. doi: 10.1093/advances/nmy078. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the SUN Project at sun@unav.es, upon reasonable request.