Abstract
Monoamine oxidase inhibitors (MAOIs) are an important class of drugs prescribed for treatment of depression and other neurological disorders. Evidence has suggested that patients with atypical depression preferentially respond to natural product MAOIs. This review presents a comprehensive survey of the natural products, predominantly from plant sources, as potential new MAOI drug leads. The psychoactive properties of several traditionally used plants and herbal formulations were attributed to their MAOI constituents. MAO inhibitory constituents may also be responsible for neuroprotective effects of natural products. Different classes of MAOIs were identified from the natural product sources with non-selective as well as selective inhibition of MAO-A and -B. Selective reversible natural product MAOIs may be safer alternatives to the conventional MAOI drugs. Characterization of MAO inhibitory constituents of natural products traditionally used as psychoactive preparations or for treatment of neurological disorders may help in understanding the mechanism of action, optimization of these preparations for desired bioactive properties, and improvement of the therapeutic potential. Potential therapeutic application of natural product MAOIs for treatment of neuroblastoma is also discussed.
Keywords: natural products, monoamine oxidases (MAO-A and -B), antidepressant, monoamine oxidase inhibitors, neurological disorders, neuroprotection, Parkinson’s disease, neuroblastoma
1. Introduction
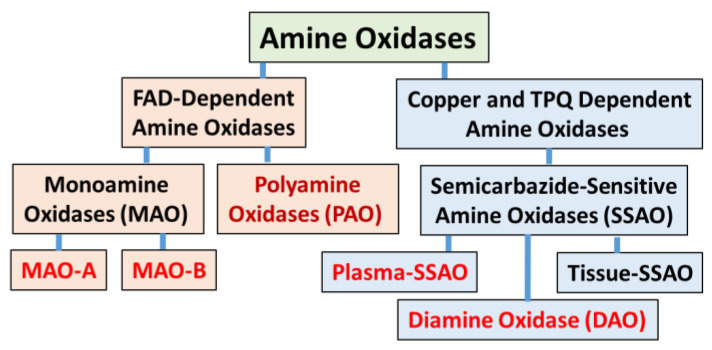
Amine oxidases are a heterogenous group of enzymes that metabolize various monoamines, diamines, and polyamines produced endogenously for physiological functions or exogenous xenobiotic substances absorbed through dietary intake [1]. The amine oxidases are distinguished by their co-factor requirements and substrate specificities [2]. The flavin adenine dinucleotide (FAD)-dependent amine oxidases include mitochondrial monoamine oxidase A (MAO-A), monoamine oxidase B (MAO-B), and cytosolic polyamine oxidases (PAOs). Copper and topoquinone (TPQ)-dependent amine oxidases include plasma and tissue enzymes, also referred to as semicarbazide-sensitive amine oxidases (SSAOs) [3] (Figure 1). This review primarily focused on MAO-A and MAO-B due to their predominant role in oxidative deamination of endogenous neurotransmitter biogenic monoamines such as dopamine, epinephrine (EPI), and norepinephrine (NE) [4]. Changes in the physiological levels of these monoamines have been implicated in the pathophysiology of several neurological disorders.
Figure 1.
General classification of mammalian amine oxidases.
Differential localization of MAO-A and -B in tissues determines their physiological functions. MAO-A and -B play an important role in deamination of biogenic amines in neural and peripheral tissues [1,5]. MAO-A is more predominant in peripheral tissues such as the intestine, liver, lungs, and placenta and protects the body by oxidation of biogenic monoamines amines in the blood or by preventing the entry of dietary monoamines into circulation [6,7]. MAO-B plays a similar protective role in the micro vessels, acting as a metabolic barrier. In the peripheral and central nervous systems, intra-neuronal MAO-A and -B protect neurons from exogenous amines, regulate the contents of intracellular amine stores, and control pharmacological actions of amine neurotransmitters.
2. Structural Difference of MAO-A and MAO-B
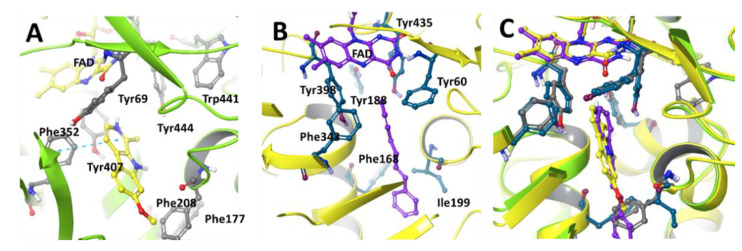
MAOs, mitochondrial FAD-containing enzymes, exist as one of two functionally and structurally distinguishable isoforms, either MAO-A or MAO-B. Both isoforms are expressed in all tissues in different ratios. MAO-A is predominantly expressed in the heart, adipose tissue, and skin fibroblast and MAO-B is mainly found in platelets and lymphocytes, but the kidney, liver, and brain express both type of isozymes [8,9]. MAO-A and -B are distinguished by different substrate specificities and inhibitor sensitivities. MAO-A, selectively inhibited by clorgylin, preferentially deaminates norepinephrine and serotonin (5-HT). MAO-B, selectively inhibited by deprenyl and pargyline, metabolizes phenylethylamine and benzylamine. The three-dimensional crystal structures of human MAO-A and MAO-B with their specific inhibitors show significant similarities in their crystalline structure forms (Figure 2). However, important differences can be described in the oligomeric states and structures of substrate binding sites of MAO-A and -B [10,11]. In purified protein form, human MAO-B is dimeric, whereas human MAO-A exists in a monomeric form [12]. The active binding site of human MAO-B is a characterized by a hydrophobic dipartite cavity with a substrate entrance cavity (~290 Å3) connected with a larger substrate binding cavity (~420 Å3). The Ile-199 was identified as an important structural component of the enzyme active site because it plays an important role in the accessibility of the second substrate cavity. In the closed conformation, Ile-199 is physically responsible for the separation of two cavities, whereas bulky ligands are present to adapt an open conformation. The binding of clinical inhibitors (R)-deprenyl and rasagiline induces a midspan type of cavity pushing Ile-199 into the open conformation. The active site of human MAO-A differs from human MAO-B in the loop conformation region with residues 108–118 and 210–216. Both these regions are critical components of the enzyme active site [12]. The structural differences incorporating Ile-335 in MAO-A vs. Tyr-326 in MAO-B active sites are responsible for the differential susceptibility of MAO-A and MAO-B toward selective MAO inhibitors [11]. Both human and rat MAO-A contain 16 conserved residues surrounding the substrate and inhibitor cavity. Only 6 of the 16 residues differ between human MAO-A and MAO-B. Substrate and inhibitor selectivity of human MAO-A and MAO-B are determined by Ile-335 and Tyr-326, respectively [13,14]. The selectivity of reversible inhibitors is determined by the size and shape of the substrate and inhibitor cavity, which are related to Ile-335 and Phe-208 in MAO-A and Tyr-326 and Ile-199 in MAO-B. The differential inhibitor susceptibility of human MAO-A and -B is also attributed to their accommodation by the induced fit of Ile-335, which is similar to that observed for Ile-199 in MAO-B [15]. Neither human MAO-A nor MAO-B contain any disulfide bridges as determined by mass spectrometric analysis [8].
Figure 2.
Active site configuration of human (A) MAO-A (PDB structure 2Z5X) with harmine and (B) MAO-B (PDB structure 1OJ9) with 1,4,-diphenyl butene, depicting key amino acids lining the enzyme active site. (C) Overlaid view of MAO-A and MAO-B substrate binding site. Harmine and 1,4,-diphenyl butene are selective reversible inhibitors of MAO-A and MAO-B, respectively.
2.1. MAO Inhibitors (MAOIs)
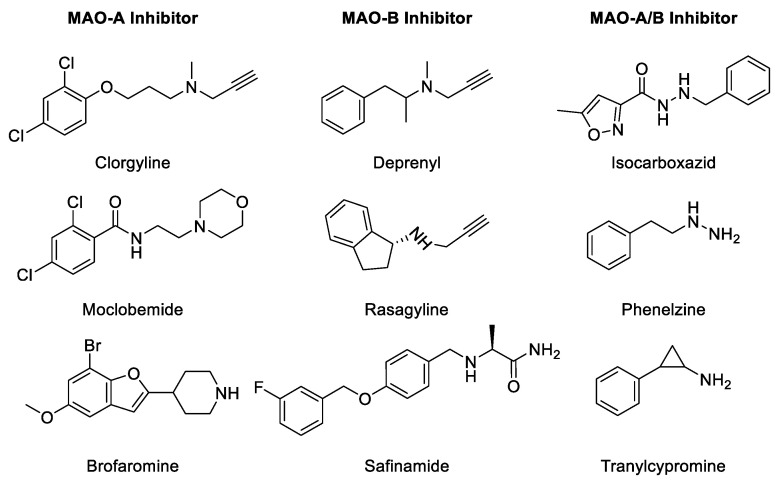
MAO inhibition prevents the degradation of monoamine neurotransmitters in the target cells and reinstates their physiological levels required for normal physiological functions [16]. Derangements in physiological levels and homeostasis of biogenic monoamines lead to pathophysiological consequences. The most important effect of MAO inhibition is a rapid increase in the intracellular concentration of monoamines. A rise in the concentrations of monoamines leads to secondary adaptive consequences, including a reduction in biosynthesis of monoamines via an apparent feedback mechanism, which is most clearly demonstrated for the noradrenergic system [17]. Both reversible as well as irreversible MAOIs have been developed for treatment of disorders caused due to depletion of biogenic amines in the target cells [18,19,20]. The MAOIs are classified into three types (Table 1): (1) irreversible non-selective inhibitors, such as phenelzine and tranylcypromine, (2) irreversible selective MAOI drugs, such as selegiline and rasagiline, and (3) reversible selective MAO-A inhibitors (RIMAs), such as moclobemide [21,22].
Table 1.
| Inhibitor Type | MAO-A | MAO-B | Non-Selective |
|---|---|---|---|
| Irreversible inhibitors | Clorgyline; Lilly 51641 | (-) Deprenyl Lilly 54781 MDL 72145 AGN 1133 AFN 1135 Rasagiline Pargyline Selegiline |
Phenelzine Tranylcypromine Isocarboxazid Nialamide Iproniazid Safrazine Metfendrazine |
| Reversible inhibitors | Harmaline Amiflamine Cimoxatone Moclobemide Brofaromine Ro 11-1163 Toloxatone MD 780515 FLA 336(+) |
Safinamide |
2.2. Therapeutic Applications of MAO Inhibitors
The MAOIs are included in a group of drugs, commonly referred as thymoleptic drugs, that favorably modifies mood in serious mood disorders and is primarily prescribed for the treatment of clinical depression or mania [23]. The MAO-A inhibitors show efficacy for treating anxiety and depression while the inhibition of MAO-B appears to be effective for prevention and treatment of Parkinson’s disease [24,25]. MAOIs are also used as a medicine for controlling hypertension or treating depression and other neurological disorders. Psychiatric disorders, such as obsessive-compulsive disorder, somatoform pain, panic disorder, and schizophrenia, have been reported to occasionally respond to treatment with MAOIs.
The progressive death of dopaminergic neurons results in a deficiency of dopamine during the development of Parkinson’s disease (PD), a neurodegenerative disorder of the brain. PD is characterized by a combination of rigidity movements, lack of movements, tremors, and postural instability. Selegiline, a propargylamine, is an irreversible inhibitor of MAO-B which inhibits dopamine metabolism and has been used in the treatment of PD effectively [26,27]. However, the therapeutic utility of selegiline is compromised due to the generation of potential neurotoxic metabolites [28]. However, the neuroprotective effects of propargylamines in different neuronal models seem to be independent of inhibition of MAO-B. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH), MAO-B, and/or other unknown proteins may be crucial targets in the survival of the injured neurons and may be crucial for the mechanism of neuroprotection by propargylamines. Further analysis of the mechanism(s) involved in the neuroprotective efficacy of MAO-B inhibitors may lead to the development of novel modalities for therapeutic applications. Selected MAO-A and -B inhibitors are listed in Figure 3 and Table 1. Historically, non-selective MAOIs have had in their structure a hydrazine group. The irreversibility of several MAOIs is caused by the triple bond scaffold which interacts directly with the active site and forms a covalent bond, irreversibly inactivating the enzyme (see Table 1) [2,5].
Figure 3.
Chemical structure of selected MAO inhibitors.
3. MAO Inhibitors in Clinical Development
Several new MAOIs are currently under development and clinical evaluation for the treatment of neurological diseases [26]. Impaired monoamine-mediated neurotransmission is directly connected to neurological disorders such as depression and anxiety as well as susceptibility to stress [27]. The majority of the antidepressants presently used are designed to control one or both of the most important neurotransmitters of the brain (DA and serotonin (5HT)). Inhibition of their degradation to raise the concentrations in synaptic regions using MAOIs restores this function [30,31].
CX157 (3-fluoro-7-(2,2,2-trifluoroethoxy)-phenoxanthiin 10, 10-dioxide) is currently under development for the treatment of major neurological disorders. CX157 causes potent and specific MAO-A inhibition in mammalian brain tissue [32]. MAO-A specifically deaminates serotonin, NE, and tyramine and is inhibited selectively at nanomolar concentrations of clorgyline, while MAO-B is insensitive to inhibition by clorgyline [33]. The first selective inhibitor of MAO-B was L-deprenyl (selegiline) [34] patented as an antidepressant and as psychic energizer [35,36]. Advanced basic studies have suggested the therapeutic utility of selegiline for treatment of both Parkinson’s disease and depression [37]. The evidence of selegiline having neuroprotective action is still followed and proven by the follow-up pro-pargylamino derivative, rasagiline, as shown recently in a study [37,38,39]. Researchers discovered isoxazole derivatives that have good MAO-B inhibition in the micromolar range. They also describe the synthesis and formulation of the molecule for MAO inhibition [28].
Limitations of Currently Approved MAOIs and Current Approach for Development of New MAOIs
MAOIs are powerful antidepressant agents and have been proven to reduce depression symptoms. The most commonly used drugs for neurological disorders are phenelzine, tricyclic antidepressants (TCAs), and benzodiazepines [40,41]. MAOIs are not recommended for use in combination with TCAs. MAOIs in clinical use have different side effects which can interfere with the treatment of depression. The most common adverse effects of MAOIs are nausea, diarrhea, insomnia, drowsiness, dizziness, headaches, and constipation, among others. MAOIs can potentially produce interactions including drug–drug, drug–food, and drug–herbal interactions. Use of antidepressant drugs such as bupropion, paroxetine fluoxetine, nortriptyline, and amitriptyline should be avoided with MAOIs. MAOIs also show interactions with pain medications such as cyclobenzaprine, mirtazapine, meperidine, tramadol, and St. John’s wort. Interactions between MAOIs and foods containing tyramine lead to hypertensive crisis. Hypertension is characterized by severe headache, high blood pressure, sweating, and nausea. To minimize the risk of hypertension effects, patients on MOAIs should take a low-tyramine diet and avoid foods which contain degraded protein such as aged cheeses, sauerkraut, tyramine-rich foods, and smoked meats [42,43]. Other side effects of MAOIs reported are weight gain, impaired sexual functioning, insomnia, and anticholinergic effects (dry mouth and constipation), which occur after long-term treatment with antidepressants [31,44,45,46,47,48]. First-generation MAOIs (non-selective and irreversible) may also cause serious side effects such as hepatotoxicity, orthostatic hypotension, and most importantly, hypertensive crisis that occurs following the consumption of tyramine-rich foods such as aged cheeses [42,49]. Patients using selegiline for Parkinson’s disease in combination with levodopa can suffer with the side effects such as anorexia or nausea, dry mouth, dyskinesia, and orthostatic hypotension [49]. Hydrazine derivatives such as phenelzine used as an antidepressant drug have shown serious adverse effects, for example, liver toxicity, hypertensive crises, hemorrhage, and in some cases, death. Liver toxicity has been reported specifically with hydrazine-derived inhibitors. This led to the development of non-hydrazine MAOIs such as tranylcypromine and pargyline. However, hypertensive crises due to treatments with these MAOIs have continued to cause problems [50]. The greatest disadvantages of TCAs and benzodiazepines are overstimulation, heart palpitations, and sweating. Therefore, these drugs have been discontinued for clinical use [50,51].
Antidepressants acting as reversible inhibitors of MAO-A (also referred as RIMAs) have much less impact on the clinical psychopharmacology than other modern classes of medications, such as selective serotonin reuptake inhibitors (SSRIs). RIMAs are distinguished from the previously used MAOIs by their selectivity and reversibility [32]. As suggested with the use of irreversible MAOIs, dietary restrictions are not required during RIMA therapy and hypertensive calamities are relatively less common. Little evidence has emerged to suggest that RIMAs distribute older MAOIs’ efficacy for treatment of depression characterized by prominent reverse neurodegenerative features [52]. Based on the available evidence, RIMAs appear to have a limited but important role in the differential therapeutics of depressive disorders [21].
4. Traditional and Psychoactive Medicinal Plants and Herbal Formulations for Treatment of Neurological Disorders
The ancestral traditional medical systems worldwide have widely used medicinal plants for the treatment of different ailments, including neurological disorders. Recent review reports have described the use of psychoactive medicinal plants and herbal formulations for treatment of different neurological disorders [53,54,55,56,57,58]. Among the medicinal plants with neurological effects, a prominent example is Banisteriopsis caapi, a woody vine plant that generally grows in the Amazonian basin. B. caapi is an ingredient of the hallucinogenic and sacred drink popularly known as ayahuasca “aya”, a Quechua (South American language) word which means “vine of the dead”; “aya” is also known locally as “hoasca, caapi, oasca” (Brazil) and “yage” (Colombia) [59]. For the preparation of the sacred drink in healing purposes or divine exploration, B. caapi is utilized as an auxiliary plant along with primarily Psychotria viridis (chacruna) or Diplopterys cabrerana (oco yage). B. caapi contains beta-carbolines, which have therapeutic properties for neurological disorders [60,61,62]. Other notable examples are Pegnanum harmala (wild rue), Rhodiola rosea (roseroot), and Crocus sativus (saffron) for depression; Passiflora incarnata (passionflower), Scutellaria lateriflora (scullcap), Gingko biloba (gingko), and Zizyphus jujuba (jujube) for early dementia and anxiety disorders; and Piper methysticum (kava-kava) for phobic, panic, and obsessive-compulsive disorders [53,54]. Moreover, many species of mind-altering (psychodysleptic) plants have been used by humans throughout the planet to reach mind distortion states; among those, a few examples have been utilized for therapeutic aims, including Cannabis sativa (cannabis), Tabernanthe iboga (iboga), psychotria viridis (chacruna), and Papaver somniferum (opium poppy) [63].
MAO-A inhibitors have been demonstrated to be effective antidepressants, and MAO-B inhibitors are used in the treatment of neurodegenerative diseases including PD, as mentioned in Section 3 [35]. The mechanistic aspects of the potential therapeutic applications of MAO-B inhibitors in Alzheimer’s disease were reviewed recently [35]. In this context, several medicinal plants have shown pharmacological effects similar to MAOIs. Recent review reports have summarized the medicinal plants as effective as MAOIs [63,64]. This review presents an extensive survey of the chemical properties of MAO inhibitory constituents identified from natural product sources presented as different chemical classes. This may be useful for further follow-up studies with these MAO inhibitory constituents as new drugs leads for structure activity analysis and further optimization of these leads. Utility of the natural product MAO inhibitory constituents as neuroprotective agents and the therapeutic application of natural products for neuroblastoma are also discussed.
Electronic searches were specifically conducted on the literature using major databases including PubMed, Google Scholar, SciFinder®, and Web of Science. The keywords used in the searches were a combination of the words “natural products’, “alkaloids”, “flavonoids”, “phenols”, “terpenes”, “monoamine oxidase inhibitors”, “MAO”, and “MAOI”. We mostly selected published reports that reported natural products or natural product derivatives with inhibition of MAO-A or-B with IC50 < 100 μM. These reports were further grouped into different chemical classes of natural product MAOIs. The natural product constituents tested against MAO-A and/or MAO-B are included in the data tables.
5. Different Classes of Natural Product MAOIs
Natural products are an important class of nature-based MAOIs. In the present scenario, several research labs are working to identify and characterize the class of natural products with medicinal value of identified lead compounds. In this review, we summarized different classes of natural product MAOIs. The natural products are generally classified based on chemical classes of secondary metabolites, such as alkaloids, flavonoids, coumarins, xanthones, terpenoids, sterols, and phenolic compounds. Scientists believe that many more compounds of these classes still can be discovered in nature. These natural products and compounds are responsible for a plethora of therapeutic uses, including for treatment of neurological disorders. Various classes of natural product MAOIs are described below. Table 2, Table 3, Table 4, Table 5, Table 6 and Table 7 include selected natural products, the natural source, their MAO-A or MAO-B IC50 values, Ki (when available), selective index (SI; MAO-A or -B), and the source of the enzyme used for MAO inhibition analysis.
Table 2.
Alkaloid natural product inhibitors of MAO-A and MAO-B.
| Compounds | Source | MAO-A | MAO-B | SI | Enzyme Source | References | ||
|---|---|---|---|---|---|---|---|---|
| IC50 (μM) | Ki (μM) | IC50 (μM) | Ki (μM) | MAOA/B | ||||
| Piperine [1] | Piper longum | 49.3 | 35.8 | 91.3 | 79.9 | 0.54 | A | [68] |
| Piper longum | 20.9 | 19.0 | 7.0 | 3.19 | 2.98 | A | [69] | |
| Methylpiperate [2] | Piper longum | 27.1 | 23.5 | 1.6 | 1.3 | 16.93 | A | [70] |
| piperic acid N-propyl amide [4] | Piperine derivative | 3.66 | 0.045 | 81.3 | B | [71] | ||
| Coptisine [5] | Coptis japonica | 1.8 | 3.3 | A | [72] | |||
| Avicine [8] | Zanthoxylum rigidum | 0.41 | >100 | >0.0041 | F | [76] | ||
| Nitidine [9] | Zanthoxylum rigidum | 1.89 | >300 | >0.0063 | F | [76] | ||
| Chelerythrin [10] | Zanthoxylum rigidum | 0.55 | >20 | >0.0275 | F | [76] | ||
| 1-Methyl-2-undecyl-4-(1H) quinolone [13] | Evodia rutaecarpa | 338.2 | 15.3 | 9.91 | 22.10 | A | [78] | |
| 1-Methyl-2-nonyl-4 (1H)-quinolone [14] | Evodia rutaecarpa | 240.2 | 2.3 | 104.4 | A | [80] | ||
| 1-Methyl-2 [(6Z,9Z)-6,9-pentadecadienyl] -4-(1H) quinolone [15] | Evodia rutaecarpa | >400 | 3.6 | 3.8 | 111.1 | A | [80] | |
| Quinoline derivative [16] | Quinolone derivative | >100 | 0.0053 | >18,867 | F | [81] | ||
| Crinamine [17] | Crossyne guttata | 0.014 | F | [82] | ||||
| Epibuphanisine [18] | Crossyne guttata | 0.039 | F | [82] | ||||
| Haemanthamine [19] | Scadoxus puniceus | 0.112 | F | [82] | ||||
| Haemanthidine [20] | Scadoxus puniceus | 0.017 | F | [82] | ||||
| Harmol [21] | Banisteriopsis caapi | 0.018 | F | [61] | ||||
| Banisteriopsis caapi | 0.5 | F | [84] | |||||
| Peganum harmala | 0.352 | F | [86] | |||||
| Harmine [22] | Banisteriopsis caapi | 0.002 | 20 | 0.0001 | F | [61] | ||
| Diverse vendors | 0.06 | NR | F | [84] | ||||
| Peganum harmala | 0.008 | NR | F | [86] | ||||
| Banisteriopsis caapi | 0.004 | >10 | >0.0004 | A | [89] | |||
| Harmane [23] | Banisteriopsis caapi | 0.64 | NR | F | [84] | |||
| Harmalol [24] | Banisteriopsis caapi | 0.66 | NR | F | [84] | |||
| Peganum harmala | 0.48 | NR | F | [86] | ||||
| Harmaline [25] | Banisteriopsis caapi | 0.002 | 25 | 0.00008 | F | [61] | ||
| Diverse vendors | 0.09 | NR | F | [84] | ||||
| Peganum harmala | 0.012 | NR | F | [86] | ||||
| Tetrahydro harmine [26] | Diverse vendors | 1.52 | NR | F | [84] | |||
| Norharmane [27] | Diverse vendors | 4.29 | NR | F | [84] | |||
| Lyaloside [29] | Psychotria. Laciniata | 182 | >100 | F | [93] | |||
| Strictosamide [30] | Psychotria laciniata | 141 | >100 | F | [93] | |||
| Angustine [31] | Psychotria laciniata | 1.10 | 138 | 0.0079 | F | [93] | ||
| Vallesiachotamine lactone [32] | Psychotria laciniata | 0.87 | 34 | 0.025 | F | [93] | ||
| E-vallesiachotamine [33] | Psychotria laciniata | 2.14 | 120 | 0.017 | F | [93] | ||
| Z-vallesiachotamine [34] | Psychotria laciniata | 0.85 | 126 | 0.0067 | F | [93] | ||
| Cimitrypazepine [35] | Psychotria nemorosa | 22 | 1.4 | 15.71 | F | [94] | ||
| Fargesine [36] | Psychotria nemorosa | 27 | 1.4 | 19.28 | F | [94] | ||
| Nemorosine A [37] | Psychotria nemorosa | 31 | 0.9 | 34.4 | F | [94] | ||
| Desmodeleganine [38] | Desmonium elegans | 9.33 | 10.16 | 0.91 | F | [95] | ||
Note: natural products tested on total MAO are not listed. Enzyme source: A = mouse brain crude mitochondrial fraction; B = rat brain mitochondrial MAOs; C = rat liver mitochondrial MAOs; D = mouse liver MAOs; E = human MAO-A and -B over-expressed; F = recombinant human MAO-A and -B.
Table 3.
Flavonoid natural product inhibitors of MAO-A and MAO-B.
| Compounds | Source | MAO-A | MAO-B | SI | Enzyme Source | References | ||
|---|---|---|---|---|---|---|---|---|
| IC50 (μM) | Ki (μM) | IC50 (μM) | Ki (μM) | MAOA/B | ||||
| Apigenin [39] | Propolis | 0.64 | 0.125 | 1.12 | 0.238 | 0.57 | F | [104] |
| Luteolin [40] | Cirsium maacki | 8.57 | >100 | >0.0857 | F | [106] | ||
| Acacetin [41] | Calea urticifolia | 0.121 | 0.0592 | 0.049 | 0.049 | 2.46 | F | [107] |
| Vetulin [42] | Turnera diffusa | 18.79 | 0.447 | 42.03 | F | [108] | ||
| Acacetin 7-O-methyl ether [43] | Turnera diffusa | >100 | 0.198 | 0.045 | <505.05 | F | [108] | |
| 7-Methoxy-4′-isopropyloxy-5-hydroxyflavone [44] | Acacetin derivative | 30.74 | 0.016 | 0.052 | 1921.25 | F | [101] | |
| 7-Methoxy-4′-isobutyloxy-5-hydroxyflavone [45] | Acacetin derivative | >100 | 0.031 | 0.037 | >3225.8 | F | [101] | |
| Chrysin [46] | Cytisus villosus | 0.25 | 1.04 | NT | 0.24 | F | [109] | |
| Genkwanin [47] | Prunus padus | 0.14 | 0.097 | 0.35 | 0.12 | 0.4 | F | [109] |
| Xanthomicrol [48] | Sideritis spp | 0.76 | 99.54 | 0.0076 | F | [111] | ||
| Salvigenin [49] | Sideritis spp. | 0.54 | 6.27 | 0.086 | F | [111] | ||
| Orientin [50] | Vitex grandiflora | >100 | 11.04 | >9.05 | F | [113] | ||
| Isoorientin [51] | Vitex grandiflora | >100 | 11.08 | >9.02 | F | [113] | ||
| Isovitexin [52] | Vitex grandiflora | >100 | 21.3 | >4.69 | F | [113] | ||
| Acacetin 7-O-(6-O-malonylglucoside) [53] | Agastache rugosa | 2.34 | 1.06 | 1.87 | 0.38 | 1.25 | F | [114] |
| Quercetin [54] | Hypericum hircinum | 0.010 | 20 | 0.0005 | I | [116] | ||
| Hypericum afrum | 1.52 | 0.29 | 28.39 | 0.053 | F | [109] | ||
| Vitis vinifera | 3.98 | >100 | >0.039 | F | [117] | |||
| Kaempferol [55] | Vitis vinifera | 0.525 | >100 | >0.00525 | F | [117] | ||
| Myricetin [56] | Hypericum afrum | 9.93 | 2.24 | 59.34 | 0.167 | F | [109] | |
| Galangin [57] | Propolis | 0.13 | 0.029 | 3.65 | 1.998 | 0.035 | F | [104] |
| Rhamnocitrin [58] | Prunus padus | 0.051 | 0.097 | 2.97 | 0.12 | 0.017 | F | [110] |
| Formononetin [59] | Sophora flavescens | 21.2 | 11.0 | 1.92 | A | [119] | ||
| Maackia amurensis | 4.82 | 0.19 | 25.36 | F | [121] | |||
| Kushenol F [60] | Sophora flavescens | 103.7 | 63.1 | 1.64 | A | [119] | ||
| Biochanin [61] | Psoralea corylifolia | 3.43 | 0.099 | 0.09 | 0.0038 | 38.11 | F | [120] |
| Calycosin [62] | Maackia amurensis | 70.5 | 0.24 | 293.75 | F | [122] | ||
| 8-O-Methylretusin [63] | Maackia amurensis | 18.7 | 0.23 | 81.30 | F | [122] | ||
| Glycitein [64] | Pueraria lobata | 8.3 | 24.9 | 0.33 | F | [123] | ||
| Naringenin [65] | Colvillea racemosa | 8.64 | 0.272 | 31.76 | F | [124] | ||
| Catechin [66] | Uncaria rhynchophylla | 88.6 | 74 | B | [127] | |||
| Epicatechin [67] | Uncaria rhynchophylla | 58.9 | 21 | B | [127] | |||
| Morelloflavone [68] | Garcinia gardneriana | 5.05 | 66.2 | 0.076 | F | [127] | ||
| GB-2a [69] | Garcinia gardneriana | 5.47 | 56.7 | 0.20 | F | [127] | ||
| Bichalcone-derivative [70] | Gentiana lutea | 12.5 | 6.2 | 1.2 | 2.01 | B | [127] | |
| Isoliquiritigenin [71] | Colvillea racemosa | 22.66 | 0.51 | 44.43 | F | [124] | ||
| Colveol A [72] | Colvillea racemosa | 0.62 | 29.90 | 0.020 | F | [124] | ||
| 4-Hydroxyderricin [73] | Angelica keiskei | >3000 | 3.43 | >874.63 | F | [131] | ||
| Hispidol [74] | Glycine max | 0.26 | 0.10 | 2.45 | 0.51 | 0.10 | F | [132] |
| Medicarpin [75] | Maackia amurensis | 10.2 | 0.30 | 34.0 | F | [122] | ||
Note: natural products tested on total MAO are not listed. Enzyme source: A = mouse brain crude mitochondrial fraction; B = rat brain mitochondrial MAOs; F = recombinant human MAO-A and -B.
Table 4.
Coumarin natural product inhibitors of MAO-A and MAO-B.
| Compounds | Source | MAO-A | MAO-B | SI | Enzyme Source | References | ||
|---|---|---|---|---|---|---|---|---|
| IC50 (μM) | Ki (μM) | IC50 (μM) | Ki (μM) | MAOA/B | ||||
| Geiparvarin [84] | Geijera parviflora | 27 | 0.144 | 187.5 | B | [142] | ||
| Desmethylgeiparvarin [85] | Geijera parviflora | 24 | 0.028 | 857.1 | B | [142] | ||
| 2-Methoxy-3-(1,10-dimethylallyl)-6a,10a-dihydrobenzo(1,2-c)chroman-6-one [86] | Gentina lutea | >100 | -- | 2.9 | 1.1 | >34.5 | B | [129] |
| 7-(6′R-Hydroxy-3′,7′-dimethyl-2′E, 7′-octadienyloxy) coumarin [87] | Dictamnus albus | 1.3 | -- | 0.5 | 0.46 | 2.6 | A | [141] |
| Auraptene [88] | Dictamnus albus | 34.6 | -- | 0.6 | 0.83 | 57.6 | A | [141] |
| Decursin [89] | Angelica gigas | 0.6 | 70.5 | 0.0085 | F | [142] | ||
| Anisucoumaramide [90] | Clausena anisum-olens | >100 | 0.143 | 699.3 | F | [143] | ||
| Osthenol [91] | Angelica pubescens | 0.74 | 0.26 | >60 | -- | >0.012 | F | [144] |
| Bakuchicin [92] | Angelica pubescens | 1.78 | 5.44 | 0.32 | F | [144] | ||
| Isopsoralen [93] | Angelica pubescens | 0.88 | 0.46 | 2.73 | 0.32 | F | [144] | |
| Umbelliferone [94] | Angelica decursiva | 39.16 | 147.37 | 0.26 | F | [145] | ||
| 6-Formylumbelliferone [95] | Angelica decursiva | 3.23 | 3.05 | 15.31 | 6.81 | 0.21 | F | [145] |
Note: natural products tested on total MAO are not listed. Enzyme source: A = mouse brain crude mitochondrial fraction; B = rat brain mitochondrial MAOs; F = recombinant human MAO-A and -B.
Table 5.
Xanthone, anthraquinone, and naphthoquinone natural product inhibitors of MAO-A and MAO-B.
| Compounds | Source | MAO-A | MAO-B | SI | Enzyme Source | References | ||
|---|---|---|---|---|---|---|---|---|
| IC50 (μM) | Ki (μM) | IC50 (μM) | Ki (μM) | MAOA/B | ||||
| Bellidolin [98] | Gentiana lactea. | 0.66 | >100 | >0.0066 | C | [157] | ||
| 6-Deoxyjacareubin [99] | Hypericum brasiliense | 12.0 | 47.3 | 0.25 | B | [156] | ||
| 1,5-Dihidroxyxanthone [100] | Hypericum brasiliense | 0.73 | 76.3 | 0.0095 | B | [156] | ||
| 1,5-dihydroxy-3-methoxy xanthone [101] | Chironia krebsii | 0.04 | 33.0 | 0.0012 | C | [157] | ||
| 12b-hydroxy-des-D-garcigerrin A [102] | Garcinia gerrardii | 3.3 | >100 | 0.033 | C | [157] | ||
| Gentiacaulein [103] | Gentiana kochiana | 0.22 | 96.0 | 0.0022 | B | [161] | ||
| Mangiferin [104] | Hypericum aucheri | 410 | >1000 | 0.41 | C | [170] | ||
| Emodin [107] | Polygonaceae Fam. | 35.4 | 15.1 | B | [68] | |||
| Shikonin [108] | Lithospermum erythrorhizon | 16.4 | 12.8 | 13.6 | 13.0 | 1.20 | A | [167] |
| Acetyl shikonin [109] | Lithospermum erythrorhizon | 16.9 | 10.5 | 10.1 | 6.3 | 1.67 | A | [167] |
| 2,3,6-Trimethyl-1,4-naphthoquinone [110] | Nicotiana tabacum | 3.0 | 6.0 | 0.5 | E | [168] | ||
| 1.14 | 7.14 | 1.59 | F | [169] | ||||
| Menadione [111] | Vit K derivative | 26.0 | 0.4 | 65 | F | [169] | ||
| 10.2 | 3.02 | 3.37 | F | [170] | ||||
| Juglone [112] | Juglans spp. | 1.71 | 4.36 | 0.39 | F | [170] | ||
| Plumbagin [113] | Plumbago spp. | 4.91 | 1.09 | 4.50 | F | [170] | ||
| Purpurin [114] | Rubia tinctorum | 2.50 | 0.422 | >40 | >0.062 | F | [172] | |
| Alizarin red [115] | Rubia tinctorum | 30.1 | >60 | >0.50 | F | [173] | ||
| Questin [116] | Cassia obtusifolia | 0.17 | 4.14 | 10.58 | 0.016 | F | [173] | |
| Aloe emodin [117] | Cassia obtusifolia | 2.47 | 0.50 | >400 | >0.0061 | F | [173] | |
| Alaternin [118] | Cassia obtusifolia | 5.35 | 3.97 | 4.55 | 1.17 | F | [173] | |
| Rubrofusarin [119] | Cassia obtusifolia | 5.90 | 4.38 | 91.40 | 0.064 | F | [173] | |
| Toralactone-9-O-β-gentiobioside [120] | Cassia obtusifolia | 7.36 | 4.30 | >400 | >0.0184 | F | [173] | |
Note: natural products tested on total MAO are not listed. Enzyme source: A = mouse brain crude mitochondrial fraction; B = rat brain mitochondrial MAOs; C = rat liver mitochondrial MAOs; F = recombinant human MAO-A and -B.
Table 6.
Phenol and polyphenolic natural product inhibitors of MAO-A and MAO-B.
| Compounds | Source | MAO-A | MAO-B | SI | Enzyme Source | References | ||
|---|---|---|---|---|---|---|---|---|
| IC50 (μM) | Ki (μM) | IC50 (μM) | Ki (μM) | MAOA/B | ||||
| Paeonol [121] | Paeonia spp. | 54.6 | 51.1 | 42.5 | 1.28 | B | [68] | |
| Ferulic acid [122] | 7.55 | 24.0 | 0.31 | F | [175] | |||
| Gallic acid [123] | 9.49 | NR | F | [175] | ||||
| t-Cinnamic acid [124] | 6.47 | 1.21 | 5.34 | A | [175] | |||
| Ellagic acid [125] | 0.40 | B | [175] | |||||
| Caffeic acid [126] | 11.7 | 22.9 | 0.51 | F | [175] | |||
| 3,5-di-O-caffeoylquinic acid [127] | Lonicera macranthoides | 20.04 | B | [176] | ||||
| Isochlorogenic acid A [128] | Lonicera japonica | 29.05 | 9.55 | J | [177] | |||
| Isochlorogenic acid C [129] | Lonicera japonica | 29.77 | 9.53 | J | [177] | |||
| Curcumin [130] | Curcuma longa | 3.64 | 3.36 | 1.08 | F | [180] | ||
| Demethoxycurcumin [132] | Curcuma longa | 3.09 | 0.91 | 2.59 | 0.86 | 1.19 | F | [180] |
| bis-Demethoxycurcumin [133] | Curcuma longa | 3.24 | 1.40 | 2.45 | 0.80 | 1.32 | F | [180] |
| Paleacenins A [134] | Elaphoglossum paleaceum | 31.0 | 4.7 | 6.59 | B | [181] | ||
| Paleacenins C [135] | Elaphoglossum paleaceum | 1.3 | 4.4 | 0.29 | B | [181] | ||
Note: natural products tested on total MAO are not listed. Enzyme source: A = mouse brain crude mitochondrial fraction; B = rat brain mitochondrial MAOs; F = recombinant human MAO-A and -B; J = liver porcine.
Table 7.
Terpenoids, marine sources, and miscellaneous natural product inhibitors of MAO-A and MAO-B.
| Compounds | Source | MAO-A | MAO-B | SI | Enzyme Source | References | ||
|---|---|---|---|---|---|---|---|---|
| IC50 (μM) | Ki (μM) | IC50 (μM) | Ki (μM) | MAOA/B | ||||
| Dihydrotanshinone I [136] | Salvia miltiorrhiza | 23 | F | [183] | ||||
| Cryptotanshinone [137] | Salvia miltiorrhiza | 80 | F | [183] | ||||
| Tanshinone I [138] | Salvia miltiorrhiza | 84 | F | [183] | ||||
| Illudinine [140] | Clitocybe illudens | 18.3 | F | [185] | ||||
| Eckol [143] | Eisenia bicyclis | 7.20 | 20.26 | 83.44 | 162.8 | 0.086 | F | [185] |
| Dieckol [144] | Eisenia bicyclis | 11.43 | 20.28 | 43.42 | 18.50 | 0.26 | F | [190] |
| Phlorofucofuroeckol-A [145] | Ecklonia stolonifera | 9.22 | 5.18 | 4.89 | 2.69 | 1.88 | F | [190] |
| 2,3,6-Tribromo-4,5-dihydroxybenzyl methyl ether [146] | Symphyocladia latiuscula | 63.16 | 25.4 | 105.13 | 40.7 | 0.60 | F | [191] |
| bis-(2,3,6-Tribromo-4,5-dihydroxybenzyl) ether [147] | Symphyocladia latiuscula | 89.31 | 22.8 | 102.53 | 35.5 | 0.87 | F | [191] |
| 5,7-Dihydroxy-2-isopropyl-4H-chromen-4-one [148] | Streptomyces spp. | 2.70 | 27.0 | 0.10 | F | [192] | ||
| 5,7-Dihydroxy-2-(1-methylpropyl)-4H-chromen-4-one [149] | Streptomyces spp. | 6.92 | 3.42 | 2.02 | F | [192] | ||
| Desmethoxyyangonin [150] | Renealmia alpinia | 1.85 | 0.922 | 0.12 | 0.031 | 15.41 | F | [22] |
| (+)-Kavain [151] | Piper methysticum | 19.0 | 5.34 | 3.55 | F | [193] | ||
| (+)-7,8-Dihydrokavain [152] | Piper methysticum | >100 | 8.23 | 12.15 | F | [193] | ||
| (+)-Methysticin [153] | Piper methysticum | 8.12 | 0.42 | 19.33 | F | [193] | ||
| (+)-7,8-Dihydromethysticin [154] | Piper methysticum | 23.2 | 0.855 | 27.13 | F | [193] | ||
| Yangonin [155] | Piper methysticum | 1.29 | 1.12 | 0.085 | 0.226 | 15.17 | F | [193] |
| (S)-5-Methymellein [156] | Rosellinia corticium | 5.31 | 2.45 | 9.15 | 0.58 | F | [194] | |
| (R)-5-Methylmellein [157] | Xylaria nigripes | 4.6 | 38.5 | 0.11 | F | [195] | ||
| (R)-3-Ethyl-8-hydroxy-5-methyl-7-(pyrimidin-5-yl)-3,4- dihydronaphthalen-1(2H)-one [158] | Methylmellein derivative | 0.06 | >50 | >0.012 | F | [195] | ||
| Alternariol [159] | Diaporthe mahothocarpus | 0.020 | 0.0075 | 20.7 | 0.00096 | F | [196] | |
| 5′-Hydroxy-alternariol [160] | Diaporthe mahothocarpus | 0.31 | 0.116 | >40 | 0.00775 | F | [196] | |
| Mycoepoxydiene [161] | Diaporthe mahothocarpus | 8.7 | 3.76 | >40 | >0.21 | F | [196] | |
| Virodhamine [162] | 38.7 | 0.71 | 0.258 | 54.50 | F | [196] | ||
Note: natural products tested on total MAO are not listed. Enzyme source: F = recombinant human MAO-A and -B.
5.1. Alkaloids
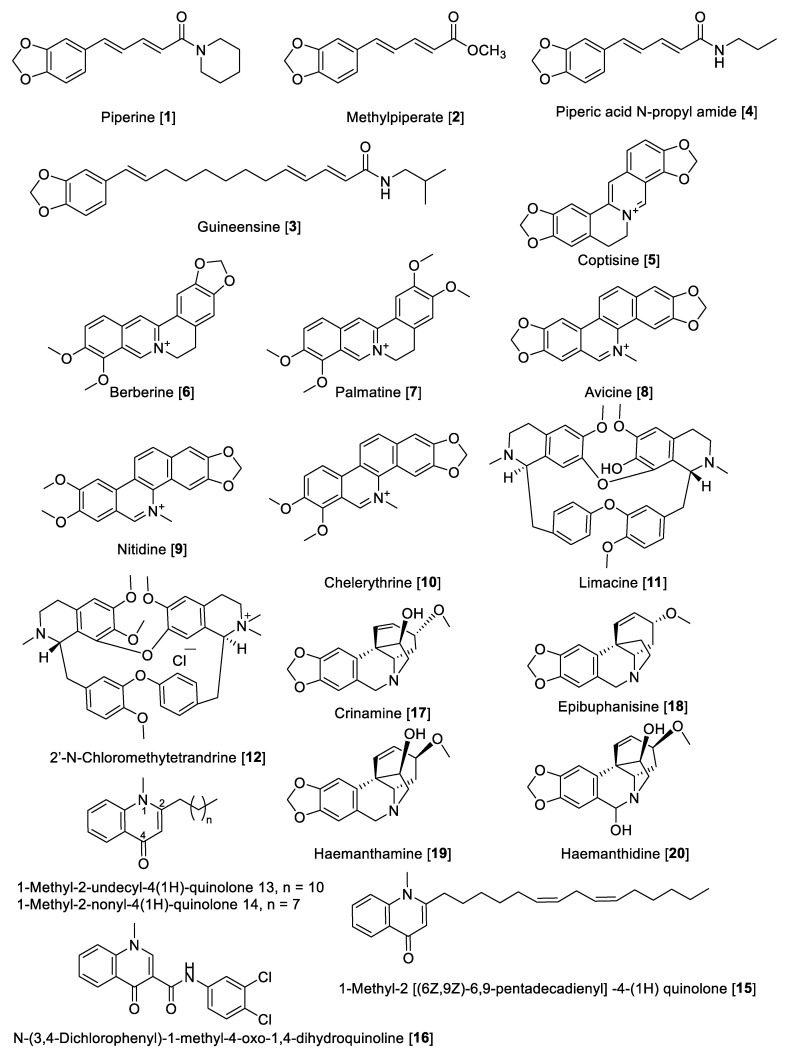
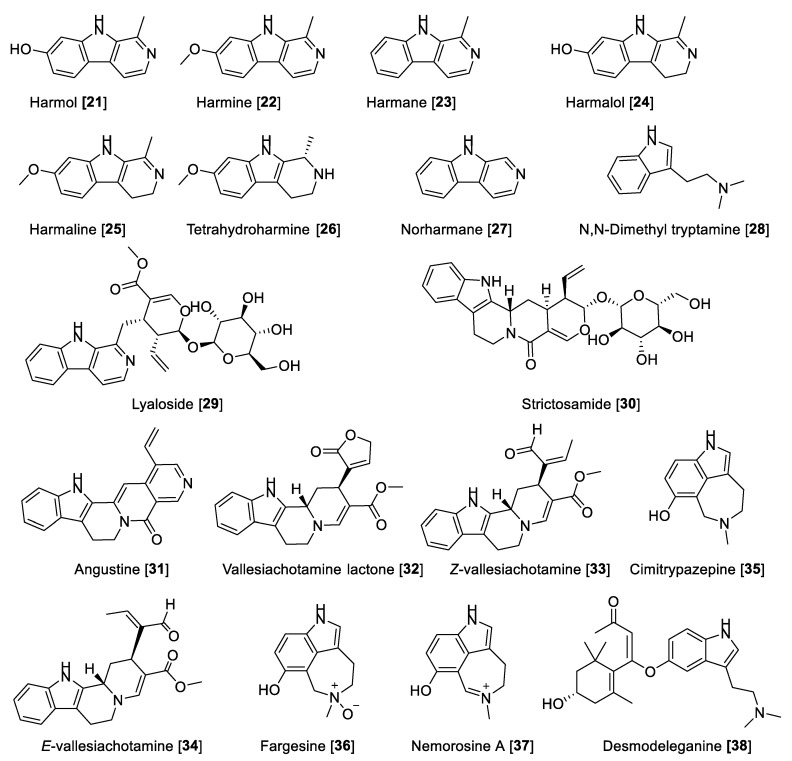
Alkaloids are “cyclic compounds containing nitrogen in a negative oxidative state” that are of limited distribution in living organisms [65]. Alkaloids are natural products of non-peptidic origin containing a nitrogen atom, and their basic characteristic is revealed in the name derived from alkaline that means basic. Being one of the most diverse kind of secondary metabolites discovered in living organisms, alkaloids present an array of chemical structures, biosynthetic pathways, and pharmacological activities. While alkaloids have been conventionally isolated from plants, an increasing amount remains to be found in animals, insects, microorganisms (bacteria and fungi), and marine sources (invertebrates and microorganisms) [66]. Several alkaloids have been reported for MAO-A and -B inhibitory activity. Herein, we describe some notable examples of alkaloid types that inhibit MAO enzymes. The chemical structures for the selected alkaloid MAOIs are shown in Figure 4 and Figure 5. Generally, short alkaloids from Evodia are moderately selective toward MAO-B. Monoterpene alkaloids, tetra and pentacyclic berberine types, have shown to be selective to MAO-A inhibition, and quinolone and quinoline alkaloids are low- and high-selective MAO-B inhibitors, respectively. Crinine-type alkaloids have been proposed as MAO-A inhibitors; however, no MAO-B inhibition has been reported (Table 2, Figure 4). Harmine-type (beta-carbolines) alkaloids are highly selective toward MAO-A isoenzymes, and tetra and pentacyclic indole alkaloids are also highly potent MAO-A inhibitors; on the contrary, azepine-indole alkaloids are relatively selective toward MAO-B (Table 2, Figure 5).
Figure 4.
Chemical structure of selected alkaloid MAO inhibitors with piperine, quinolone, and isoquinoline skeletons.
Figure 5.
Chemical structure of selected alkaloid MAO inhibitors with indole motif.
5.1.1. MAO Inhibitory Activity of Piperine, Quinolone, and Isoquinoline Alkaloids
Piperine (1) alkaloid is the main component in black pepper (Piper nigrum) and in general in the Piper genus, which has a wide spectrum of pharmacological activities, including MAO inhibition. For example, from Piper longum, piperine 1 and methylpiperate 2 were isolated and their MAO inhibition was evaluated using rat brain mitochondrial fraction [67]. The enzyme inhibition kinetics study for piperine 1 showed that the mode of inhibition for MAO-A was mixed type with a Ki value of 35.8 μM and for MAO-B was competitive with a Ki value of 79.9 μM [68]. In another study, piperine 1 showed an inhibitory effect against MAO-A with an IC50 value of 20.9 μM and for MAO-B with an IC50 value of 7.0 μM. The MAO inhibition by piperine 1 was reversible as determined by the recovery of enzyme activity after removal of the inhibitor by dialysis of the incubation mixture [69]. The chemical structure of 1 (Figure 4 and Table 2) consists of a free phenolic –OH group, and it was proposed that the inhibition could probably be initiated by the hydrogen bonding of its amide active protons such as –NH–, –OH, and –SH in the active sites of MAO-A and -B [68]. Methylpiperate 2 was isolated from the fruits of P. longum and evaluated for inhibitory effects on the MAO-A activity on mouse brain homogenates yielding an IC50 value of 3.6 μM; guineesine 3 showed moderate inhibition of total MAO with an IC50 of 139.2 μM [69,70]. Several N-amide derivatives of piperine 1 were synthesized and tested for MAO inhibition. Piperic acid N-propyl amide 4 inhibited MAO isolated from rat brain mitochondrial homogenate with an IC50 value of 45 nM for MAO-B and an IC50 value of 3.66 μM for MAO-A [71].
Coptisine 5, as well as other isoquinoline alkaloids, were isolated from Coptis japonica. The isolated alkaloids from C. japonica were evaluated for their inhibitory effect on MAO from mouse brain fraction. The result showed an inhibitory effect of 5 on MAO-A with an IC50 value of 1.8 μM but none on the MAO-B enzyme [72]. Earlier reports have mentioned that various alkaloids including berberine 6 and palmatine 7 and 5 inhibited MAO enzymes [73,74]. Berberine 6 and palmatine 7 exhibit a non-competitive inhibition of MAO with Ki values of 44.2 μM and 58.9 μM, respectively [75].
Isoquinoline alkaloids including avicine 8, nitidine 9, and chelerythrine 10 (Figure 4) isolated from Zanthoxylum rigidum showed potent inhibition of human MAO-A with IC50 values of 0.41, 1.89, and 0.55 μM, respectively [76]. Limacine 11 and 2′-N-chloromethytetrandrine 12, two benzylisoquinoline dimers isolated from the roots of Stephania tetrandra, were found to have moderate inhibitory effects on total MAO with IC50 values of 37.7 and 29.2 μM, respectively [77].
Quinolone-type alkaloids also have been reported to possess MAO inhibition; in fact, 1-methyl-2-undecyl-4-(1H) quinolone 13 isolated from Evodia rutaecarpa competitively inhibited MAO-B with an IC50 value of 15.3 µM but did not inhibit MAO-A [78]. Evodia rutaecarpa (Rutaceae) is a well-known traditional Chinese medicine with several therapeutic properties, including analgesic, antiemetic, astringent, hemostatic, uterotonic, cardiotonic, and antihypertensive activities [79]. Seven quinolone alkaloids were isolated from the fruits of E. rutaecarpa and their inhibitory effects against MAO enzymes were evaluated using mouse brain mitochondrial fraction. The results found that all compounds were more active against MAO-B compared to MAO-A, with 1-methyl-2-nonyl-4 (1H)-quinolone 14 and 1-methyl-2 [(6Z,9Z)-6,9-pentadecadienyl] -4-(1H) quinolone 15 being the most potent inhibitors for MAO-B with IC50 values of 2.3 µM and 3.6 µM, respectively [80]. Based on the quinolone base structure, efforts were made to modulate the activity; thus, the synthesis of a series of quinolone derivatives found N-(3,4-Dichlorophenyl)-1-methyl-4-oxo-1,4-dihydroquinoline-3 carboxamide 16 as a selective inhibitor of human MAO-B with a selective index (SI) of ~1887 and an IC50 value of 5.3 nM [81]. The crinine-type alkaloids crinamine 17 and epibuphanisine 18 (isolated from Crossyne guttata) and haemanthamine 19 and haemanthidine 20 (isolated from Scadoxus puniceus) showed inhibition of human MAO-B with IC50 values of 14.9, 39.2, 112.0, and 17.20 nM, respectively, with no inhibition of human MAO-A [82].
5.1.2. MAO Inhibitory Activity of Beta-Carbolines and Indole-Type Alkaloids
Beta-carbolines derived from indole are endowed with neuropharmacological and neuromodulating properties. Beta-carbolines have been described as potent MAO-A inhibitors [61]. Fernandez de Arriba et al. [83] described the first studies about the kinetic behavior of beta-carboline derivatives as MAO-A inhibitors in the bovine retina. It has also been found that the beta-carbolines harmol 21, harmine 22, and harmane 23 are the most potent MAO-A inhibitors, followed by the 3,4-dihydro-beta-carbolines harmalol 24, harmaline 25, 1,2,3,4-tetrahydro-beta-carboline (tetrahydro harmine 26), and norharman 27 [84,85,86]. The MAO inhibitory activity of beta-carbolines present in the extract of Banisteriopsis caapi, a component of “Ayahuasca”, and in mixtures with different compounds or plant extracts was evaluated and also compared with other previously reported MAO inhibition data [87,88]. B. caapi extracts and two of its components, 22 and 25, were tested for MAO inhibition using mouse liver homogenate. B. caapi extract and harmaline 25 showed a concentration-dependent inhibition for MAO-A with IC50 values of 1.24 μg/mL and 4.54 nM, respectively, showing moderate inhibition of MAO-B [88,89]. Phytochemical and biological studies with the specimens of B. caapi collected from Hawaii found 22 and 24–26 as the main inhibitors of recombinant human MAO-A with IC50 values of 2.0, 2.5, 18, and 74 nM, respectively. Only 22 and 25 were found to be moderately active toward recombinant human MAO-B with IC50 values of 20 and 25 μM, respectively [60].
Harmane 23 was also isolated from the medicinal plant Uncaria rhynchophylla (Cat’s claw) and tested for its inhibitory effect on the total MAO activity of mouse brain homogenate. Harmane 23 was found to be a MAO-A inhibitor with an IC50 value of 11.1 µM [90]. Structural and mechanistic studies have demonstrated that harmine 22 shows reversible inhibition and bounds with the active site of the enzyme cavity. Compound 22 interacts with Tyr-69, Asn-181, Phe-208, Val-210, Gln-215, Cys-323, Ile-325, Ile-335, Leu-337, Phe-352, Tyr-407, Tyr-444, and FAD. The seven molecules of water occupy the gap between the inhibitor and these groups. The amide groups of the Gln-215 side chain tightly interact with 22 in human MAO-A or Gln-206 in human MAO-B (Gln-215/206). These results are consistent with previously reported structural analyses of human MAO-A and MAO-B by active site-directed mutagenesis studies [11,12]. The selectivity of the reversible inhibitors is caused by the different size and shape of the substrate and inhibitor cavity regulated by Ile-335 and Phe-208 in MAO-A, which corresponds to Tyr-326 and Ile-199 of MAO-B [91].
Psychotria (Rubiaceae) is a complex neotropical genus of remarkable interest due to the high content of alkaloids. Psychotria viridis is a component of the hallucinogenic beverage known as ayahuasca. The metabolite responsible for its hallucinogenic effects is the alkaloid N, N-dimethyl tryptamine (DMT) 28, which is biosynthetically based on the indole scaffold. There are several other alkaloid skeletons produced by the Psychotria species, some of them with notable bioactivities [92]. The monoterpene indole alkaloids lyaloside 29, strictosamide 30, angustine 31, vallesiachotamine lactone 32, E-vallesiachotamine 33, and Z-vallesiachotamine 34 (Figure 5 and Table 2) were isolated from P. laciniata and were found to be MAO inhibitors with a preference toward human MAO-A with IC50 values of 182, 141, 1.10, 0.87, 2.14, and 0.85 μM, respectively, and negligible or low inhibition toward human MAO-B. These alkaloids were also selective inhibitors of butyrylcholinesterase, suggesting that this scaffold can be a multifunctional agent [93]. Recently, the azepine-indole alkaloids cimitrypazepine 35, fargesine 36, and nemorosine A 37 were isolated from Psychotria nemorosa among other alkaloids and found to inhibit both MAOs but preferentially human MAO-A with IC50 values of 1.4, 1.4, and 0.9 μM, respectively [94]. Harmine 22 has been subjected to a clinical trial in combination with DMT 28 to study the network dynamics following the modulation of the serotonin system [95].
Desmodeleganine 38, a derivative of 28, was found to inhibit both MAOs at micromolar concentrations. Compound 38 was isolated from the traditional Chinese plant “Sha MaHuang” [96]. Several other alkaloids have also been tested for their inhibitory effect on MAO but have not shown potency of inhibition for MAO-A and -B. Table 2 presents IC50 values of selected alkaloid metabolites for inhibition of MAO-A and -B.
5.2. Flavonoids
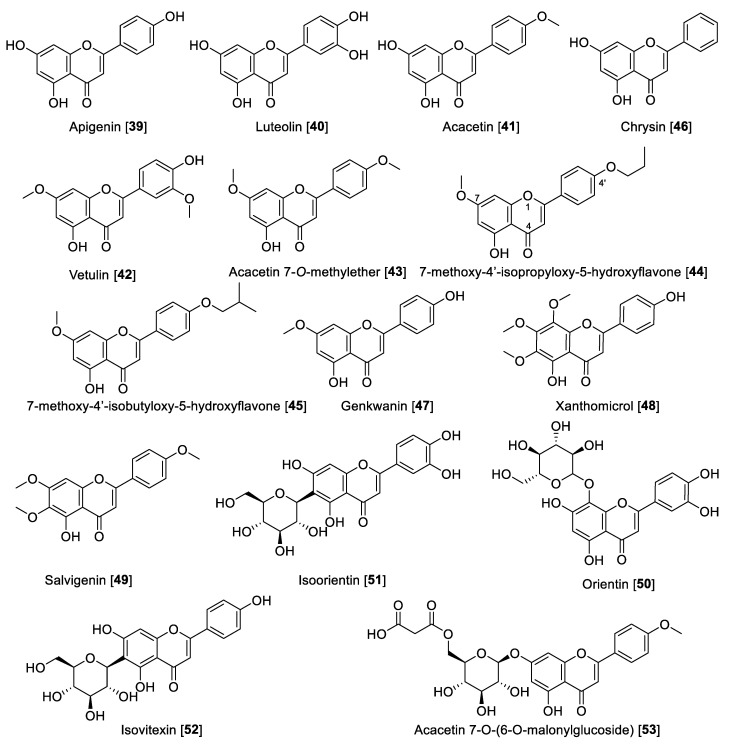
Flavonoids are included in the polyphenol family, which are made up of two benzene rings and merged with a short three-carbon chain. These types of compounds are water-soluble polyphenolics containing 15 carbon atoms. One of the carbons of the short chain is always connected to a carbon of one of the benzene rings, either directly or through an oxygen bridge, thereby forming a third middle ring, which can be five or six-membered. Flavones, flavanols, flavanones, anthocyanins, isoflavones, and flavans are the subgroups in flavonoids [97], also including the precursors chalcones, neoflavonoids, and dimers and oligomers. Some flavonoids are responsible for the coloring in the plants, including herbs, fruits, vegetables, etc. The most important dietary sources of flavonoids are green tea, fruits, and vegetables. Green and black tea contain about 25% flavonoids. Fruits such as apple (quercetin) and citrus fruits (rutin and hesperidin) are rich sources of flavonoids [27,98]. Flavonoids are also known for their strong antioxidant properties; it is believed that flavonoids are integrated in a network in plants, activating their defenses to fight stress conditions [99,100]. Flavonoids have also been reported as MAO inhibitors. A series of reports has listed several flavonoids to possess MAO inhibition, including a comprehensive structure activity relationship (SAR) study [27,101,102]. Herein, we describe notable examples of several flavonoids that inhibit MAO, separated by class. Notably, flavones depending on the substituents can be MAO-A or -B inhibitors. Those substituted at the C-4′ position at ring C are mainly MAO-B inhibitors, and those disubstituted at ring C or per-substituted at ring B-turn are relatively MAO-A selective (Table 3, Figure 6). Flavanols are selective MAO-A inhibitors, while isoflavones are reported to be moderately MAO-B selective (Table 3, Figure 6). The chemical structures for the selected flavonoids are shown in Figure 6, Figure 7 and Figure 8. The MAO inhibition profiles including IC50 values against MAO-A and MAO-B of these metabolites are presented in Table 3. The sources of MAO employed in these studies are also specified.
Figure 6.
Chemical structure of selected flavone MAO inhibitors.
Figure 7.
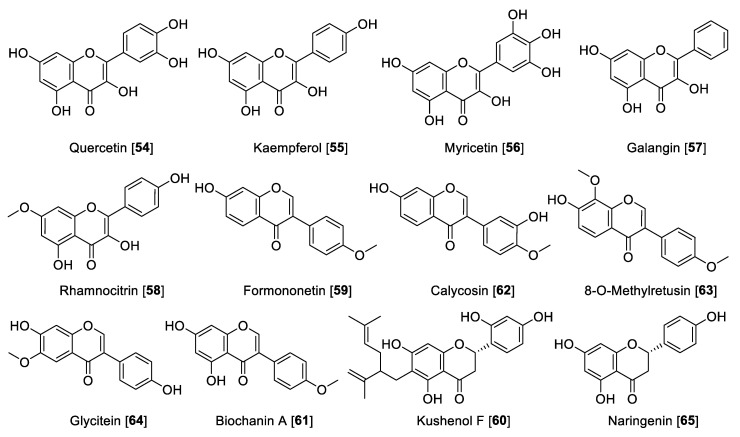
Chemical structure of selected flavanol, isoflavone, and flavanone MAO inhibitors.
Figure 8.
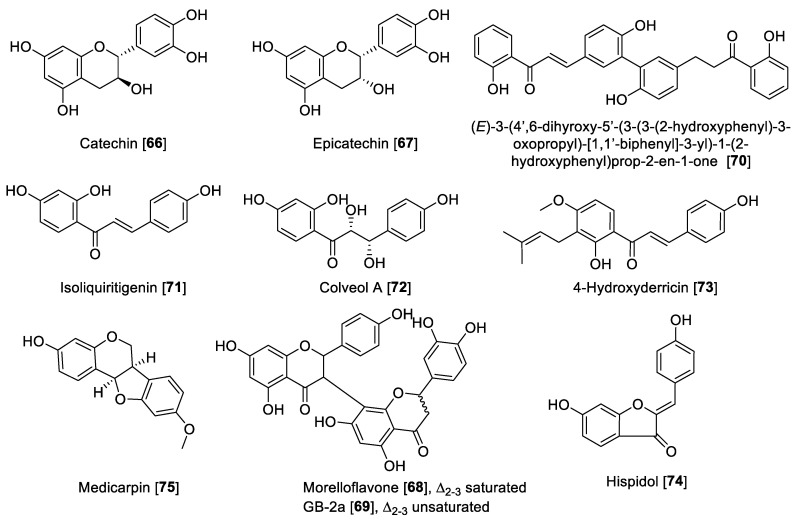
Chemical structure of selected miscellaneous flavonoid MAO inhibitors.
5.2.1. MAO Inhibitory Activity of Flavones
Apigenin 39, one of the most notable examples of dietary flavone, is heavily consumed by humans. It is found in many fruits, vegetables, and herbs [103]. Apigenin 39 is also one of the most renowned flavones with several nutritional and pharmacological properties [104]. Regarding its MAO inhibitory activity, 39 isolated from propolis samples was reported to have moderate inhibition toward MAO enzymes, being slightly preferential to human MAO-A than MAO-B with IC50 values of 0.64 and 1.12 μM, respectively [105]. Luteolin 40 is another remarkable dietary flavonoid with great pharmacological potential including its use to treat diabetes, Alzheimer’s disease, and depression [106]. Luteolin 40, isolated from Cirsium japonicum var. maackii (Compositae) (also known as Korean thistle), was found to be an inhibitor of human MAO-A with an IC50 value of 8.57 μM [107]. Acacetin 41, another interesting dietary flavone, was isolated from the Central American medicinal plant Calea urticifolia in a bioassay guided fractionation paradigm as the active MAO inhibitor with IC50 values of 121 and 49 nM for human MAO-A and MAO-B, respectively [108]. Later, the studies with medicinal plant Turnera diffusa allowed the isolation of the flavones 41, vetulin 42, and acacetin 7-O-methyl ether 43 (Figure 6 and Table 3) as the active bio-components of MAO inhibition. The study found that 42 preferentially inhibited human MAO-B with an IC50 value of 447 nM and an SI of 42. However, most importantly, 43 was found to be a selective and reversible human MAO-B inhibitor with an IC50 value of 198 nM and SI of 505 [109]. The search for highly selective reversible MAO-B inhibitors was accomplished based on the computational study of acacetin 7-O-methyl ether-scaffold and the active sites of the MAO enzymes. Several derivatives of 43 were designed and found to have 1000- to 3000-fold selectivity toward human MAO-B; for example, 7-methoxy-4′-isopropyloxy-5-hydroxyflavone 44 and 7-methoxy-4′-isobutyloxy-5-hydroxyflavone 45 were found to inhibit human MAO-B with IC50 values of 33 and 31 nM, respectively, and an SI of 1921 and 3225, respectively [101]. Several other dietary flavones have been isolated and evaluated by their MAO inhibition. For example, the dietary flavone chrysin 46 isolated from the North African medicinal plant Cytisus villosus showed IC50 values of 0.25 and 1.04 μM for human MAO-A and MAO-B, respectively [110]. The phytochemical study of Prunus padus yielded the flavone genkwanin 47 as a non-selective MAO inhibitor with IC50 values of 0.14 for MAO-A and 0.35 μM for MAO-B [111]. Xanthomicrol 48 and salvigenin 49 are two well-known flavones present in the Sideritis genus. Xanthomicrol 48 and salvigenin 49 were shown to be human MAO-A inhibitors with Ki values of 0.76 and 0.54 μM, respectively [112].
Lately, interest has been growing about the health benefits of glycoside flavones. Flavone O-glycosides or C-glycosides are present in the human diet and are keys to the biosynthesis and functions of plants, several of which have significant reported bioactivities [113]. There are several glycosides reported to display MAO inhibition, for example, orientin 50, isoorientin 51, and isovitexin 52 are well-known flavone glycosides isolated from Vitex grandiflora (grapes). These flavonoid glycosides were reported to be selective human MAO-B inhibitors with IC50 values of 11.04, 11.08, and 21.3 μM, respectively [114]. From Agastache rugosa and using a bioguided assay fractionation, 41 and its glycoside acacetin 7-O-(6-O-malonylglucoside) 53 were isolated and found to be MAO inhibitors. Compound 53 was found to be a non-selective MAO inhibitor with IC50 values of 2.34 and 1.87 μM toward human MAO-A and MAO-B, respectively [115].
5.2.2. MAO Inhibitory Activity of Flavanols, Isoflavones, and Flavanones
Dietary flavanols are mainly referred to quercetin 54, kaempferol 55, and myricetin 56 (Figure 7), are specially found in fruits, vegetables, and beverages, and are also the key components in several plants. Similar to flavones, flavanols are also linked with the antioxidant properties of medicinal plants [116]. Regarding their MAO inhibition property, flavanols have been reported widely. Quercetin 54 isolated from Hypericum hircinum showed inhibitory activity for MAO-B with an IC50 value of 20 µM and for MAO-A with an IC50 value of 10 nM, and the source of the MAO was beef brain mitochondria [117]. Compounds 54 and 55 are common components of wine. A study on the constituents of Vitis vinifera and MAO inhibition found that 55 was a potent and selective human MAO-A inhibitor with an IC50 value of 0.525 μM and no inhibition of MAO-B [118]. This group also found that 54 is a good MAO-A inhibitor with an IC50 value of 3.98 μM and poor inhibition of MAO-B with an IC50 of more than 100 μM [118]. The differences in the results of this study [117] and others may be explained by the different sources of the MAO enzymes used for inhibition studies [119]. Myricetin 56 was found to have a moderate inhibitory effect toward human MAO with an IC50 value of 9.93 μM [110]. Galangin 57, a flavonol found in propolis, was found to be a preferential MAO-A inhibitor with an IC50 value of 0.13 μM compared to an IC50 value of 3.65 μM for human MAO-B [105]. Another interesting flavonol isolated from Prunus padus is rhamnocitrin 58. Rhamnocitrin 58 showed selective reversible inhibition of human MAO-A with an IC50 value of 51 nM [120].
The isoflavonoid formononetin 59 and the flavanone kushenol F 60 were extracted and isolated from the roots of Sophora flavescens and showed significant MAO inhibition in a dose-dependent manner (mouse brain fraction). Formononetin 59 showed IC50 values of 21.1 and 11.0 µM for MAO-A and MAO-B, respectively. Kushenol F 60 showed a moderate inhibition of MAO-B with an IC50 value of 63.1 µM [121]. Seed extracts of Psoralea coryfolia have been used for centuries as chemoprotective and antioxidant agents. The study carried out to find the MAO-inhibiting bioactive components found biochanin A 61 as notable inhibitor. The isoflavone 61 was found to be a selective reversible MAO inhibitor with IC50 values of 3.43 and 0.09 μM for human MAO-A and human MAO-B, respectively [122]. The Asian medicinal plant Maackia amurensis was reported to be a good source of isoflavonoids. The bioassay guided fractionation of M. amurensis bark extract yielded isoflavonoids calycosin 62 and 8-O-methylretusin 63 as reversible and selective inhibitors of human MAO-B with IC50 values of 0.24 and 0.23 μM, respectively [123]. The isoflavone glycitein 64 is one of the major components in Glycine max (soybeans) and showed selective inhibition of human MAO-A with an IC50 value of 8.30 μM [124]. Naringenin 65, a common flavanone isolated from Colvillea racemosae, was identified as a preferential inhibitor of human MAO-B with an IC50 value of 0.272 μM [125].
5.2.3. MAO Inhibitory Activity of Miscellaneous Flavonoids and Related Compounds
Miscellaneous flavonoids and precursors (Figure 8) such as chalcones have been recently gaining attention for their effects of inhibiting MAO enzymes; for example, chalcones have been proposed as novel and effective MAO-B inhibitors [126,127]. Catechin 66 and epicatechin 67 have been reported to inhibit MAO enzymes. Compounds 66 and 67 isolated from Uncaria rhynchophylla and using rat brain homogenate as a source of MAO enzymes showed inhibition of MAO-B with IC50 values of 58.9 and 88.6 μM, respectively [128]. The biflavonoids, morelloflavone 68 and GB-2a 69 isolated from Garcinia gardneriana, were reported as preferential human MAO-A inhibitors with IC50 values of 5.05 and 5.47 μM, respectively [129]. The bichalcone A (3-3″-linked-(2′-hydroxy-4-O-isoprenylchalcone)-(2‴-hydroxy-4″-O-isoprenyldihydrochalcone) isolated from the dried bark of Gentiana lutea was hydrolyzed to form the product (E)-3-(4′,6-dihyroxy-5′-(3-(3-(2-hydroxyphenyl)-3-oxopropyl)-[1,1′-biphenyl]-3-yl)-1-(2-hydroxyphenyl)prop-2-en-1-one 70. This hydrolytic product was found to be a MAO inhibitor with an IC50 value of 48.7 for MAO-A and 6.2 μM for MAO-B [130]. Isoliquiritigenin 71, a natural chalcone, showed preferential inhibition of human MAO-B with an IC50 value of 0.51 μM and an SI of 44.69 compared to human MAO-A [125]. When the chalcone was saturated and hydroxylated as in the case of colveol A 72, the selectivity turned toward MAO-A, as denoted by IC50 values of 0.62 and 29.9 μM for MAO-A and MAO-B, respectively [125]. Isoliquiritigenin 71, besides being a potent MAO inhibitor, was also found to have important bioactivity as an antagonist to D1 dopamine receptors and agonist to dopamine D3 and vasopressin V1A receptors. These finding suggest a potential therapeutic utility of Isoliquiritigenin 71 for neurological disorders [131]. The chalcone 4-hydroxyderricin 73, isolated from Angelica keiskei, was reported to be a selective human MAO-B inhibitor with an IC50 value of 3.43 μM and an SI of 1000-fold difference compared to MAO-A [132]. From Glycine max Merrill (soybean), an aurone hispidol 74 was isolated. Compound 74 exhibited a preferential inhibition of human MAO-A with IC50 values of 0.26 and 2.45 μM for MAO-A and MAO-B, respectively [133]. Medicarpin 75, a flanovol, was recently reported to have a preferential inhibition of human MAO-B with an IC50 value of 0.3 μM [123]. Figure 8 shows the chemical structures and Table 3 presents the IC50 values of selected miscellaneous flavonoids.
5.3. MAO Inhibitory Activity of Coumarins
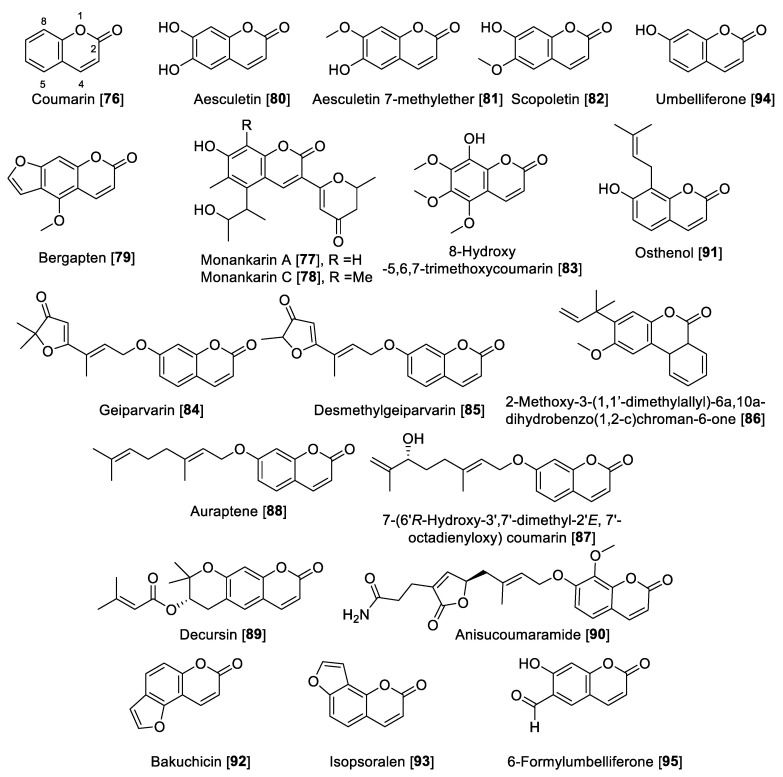
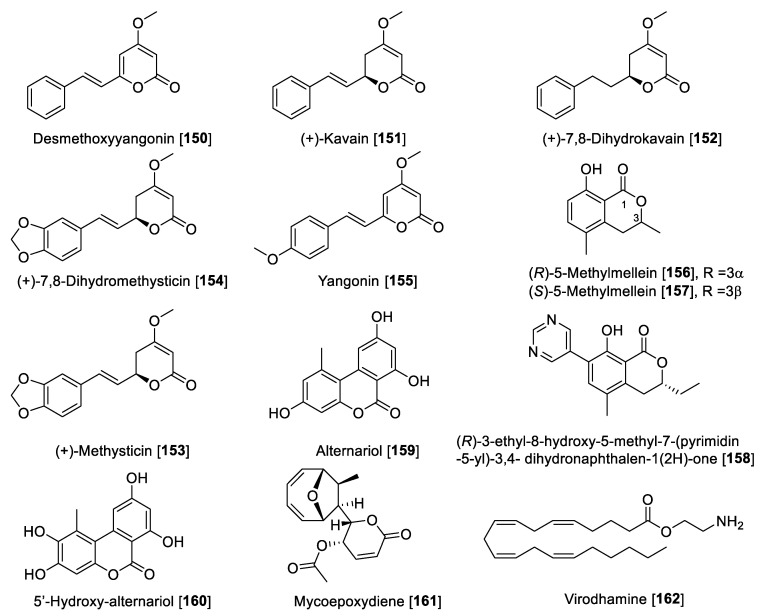
Coumarins are versatile small lactones, constructed by the combination of a benzene and α pyrone rings fused with each other, which are biosynthetically phenylpropanoid derivatives [134]. Compound 76 was the first example of natural coumarins reported from the Dipteryx odorata (Coumarona odoroata Syn) beans (tonka beans) way back in 1820. Currently, coumarins are described as important metabolites in natural sources such as medicinal plants and their different parts [135]. The coumarin scaffold shows wide industrial applications and medicinal uses, of which a notable example is warfarin, the most common anticoagulant prescribed worldwide [135]. Coumarins (Figure 9 and Table 4) have been proposed as privilege scaffold and focused on as MAO inhibitors [136,137].
Figure 9.
Chemical structure of selected coumarin MAO inhibitors.
Several natural coumarins have been reported as MAO inhibitors, including the monankarins, pigments extracted from the fungi Monascus anka [138]. In particular, monankarin A 77 and C 78 were found to exhibit inhibitory activities for MAO in mouse brain homogenate with IC50 values of 15.5 and 1.0.7 μM, respectively [138]. From the roots of Peucedanum japonicum, a series of coumarins were isolated, and among those, bergapten 79 was found to inhibit total MAO in mouse brain homogenate with an IC50 value of 13.8 μM [139]. Additionally, the coumarins aesculetin 80, aesculetin 7-methyl ether 81, and scopoletin 82 showed moderate MAO inhibition with IC50 vales of 30.1, 32.2, and 45.0 µM, respectively, in mouse brain homogenate. Compounds 80–82 were also isolated from Artemisia vulgaris, a well-known European and Asian herb [74]. From the dried flowers of Hibiscus syriacus, several coumarins including 8-hydroxy-5,6,7-trimethoxycoumarin 83 and 82 were found to inhibit MAO (brain mouse homogenates) in a dose-dependent manner with IC50 values of 44.5 and 19.4 μg/mL, respectively [140]. Geiparvarin 84 and desmethylgeiparvarin 85, two coumarins isolated from Geijera parviflora, were found to be selective toward MAO-B. Geiparvarin 84 showed IC50 values of 27 μM and 144 nM for MAO-A and MAO-B, respectively, and desmethylgeiparvarin 85 showed IC50 values of 24 μM and 28 nM for MAO-A and MAO-B, respectively [141].
From Gentiana lutea, the rearranged coumarin 2-methoxy-3-(1,10-dimethylallyl)-6a,10a-dihydrobenzo(1,2-c)chroman-6-one 86 was isolated and found to be a selective inhibitor of MAO-B with an IC50 value of 3.8 μM [130]. Using bioassay guided fractionation of the Asian medicinal plant Dictamnus albus, the coumarins 7-(6′R-hydroxy-3′,7′-dimethyl-2′E, 7′-octadienyloxy) coumarin 87 and auraptene 88 were found to have the highest inhibitory MAO activity. Compound 87 inhibited MAO in a non-selective way with IC50 values of 1.3 and 0.5 μM for MAO-A and MAO-B, respectively, while 88 preferentially inhibited MAO-B with an IC50 value of 0.6 μM compared to an IC50 value of 34.6 μM for MAO-A [142]. Decursin 89, a tetrahydropyrone coumarin isolated from Angelica gigas, was found to be selective toward MAO-A with an IC50 value of 1.89 μM compared to an IC50 value of 70.5 μM for human MAO-B [143]. From the leaves and twigs of the medicinal plant Clausena anisum-olens, anisucoumaramide 90 was isolated. This secondary metabolite 90 showed selective inhibition of human MAO-B with an IC50 value of 144 nM and SI of more than 696 compared to MAO-A [144]. From another Apiaceae, Angelica pubescens, osthenol 91 was isolated and found to be selective toward human MAO-A with an IC50 value of 0.74 μM and SI of >81 compared to MAO-B [145]. The same group also reported that bakuchicin 92 and isopsoralen 93 coumarins isolated from Psoralea corylifolia were non-selective MAO inhibitors and that 92 exhibited IC50 values of 1.78 and 5.44 μM for human MAO-A and MAO-B, respectively. Compound 93 inhibited human MAO-A and MAO-B with IC50 values of 0.88 μM and 2.73 μM, respectively [145]. Umbelliferone 94 and 6-formylumbelliferone 95 isolated from Angelica decursiva were found to inhibit MAO enzymes with moderate preference toward human MAO-B with IC50 values of 39.16 compared to 147 μM for human MAO-A. The metabolite 95 showed IC50 values of 3.23 and 15.31 μM for MAO-A and -B, respectively [145]. The metabolite 95, besides inhibition of MAO-A, also inhibited lipid peroxidation and Aβ self-aggregation and indicated that 6-formylumbelliferone 95 can be a promising lead for the development of treatments for neurodegenerative diseases [145]. The synthetic studies to modulate the MAO inhibition of coumarins prepared hybrid compounds, making these derivatives more flexible in the case of coumarin-N-benzylalkyloxy derivatives [146] and coumarin-chalcones hybrids [147], to mention a few examples. Figure 9 shows the chemical structures for selected coumarins and Table 4 presents the IC50 values of selected coumarin compounds.
5.4. MAO Inhibitory Activity of Xanthones, Anthraquinones, and Naphthoquinones
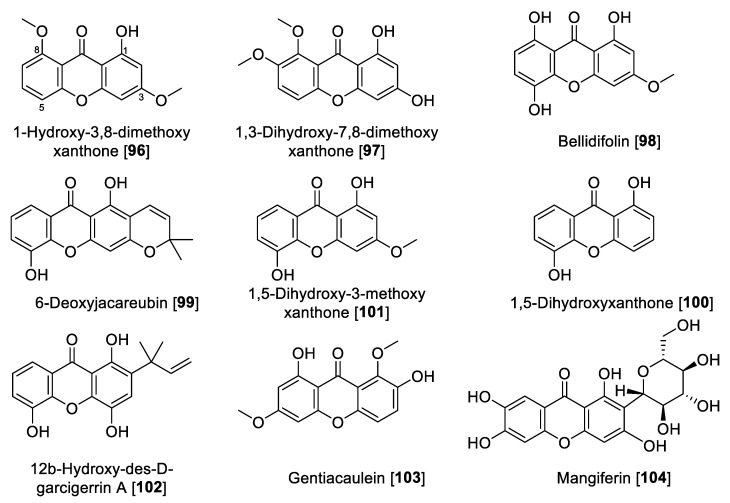
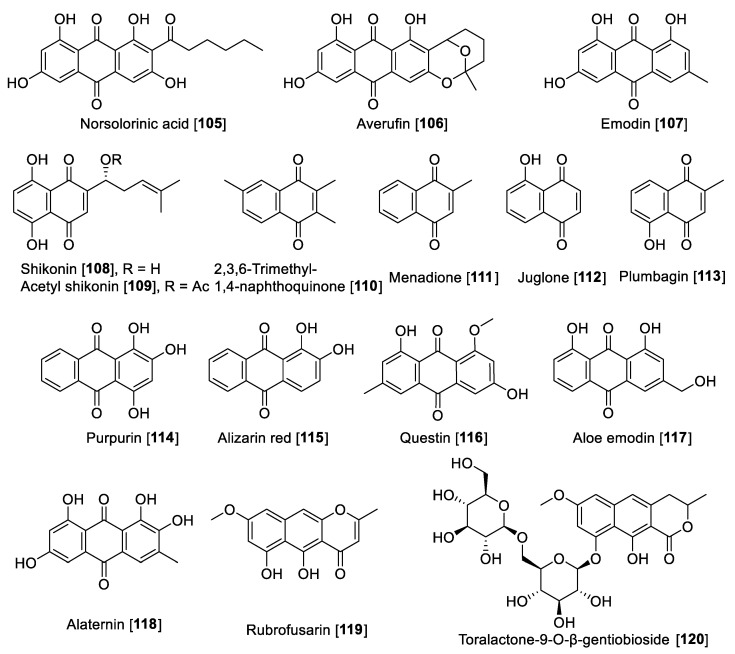
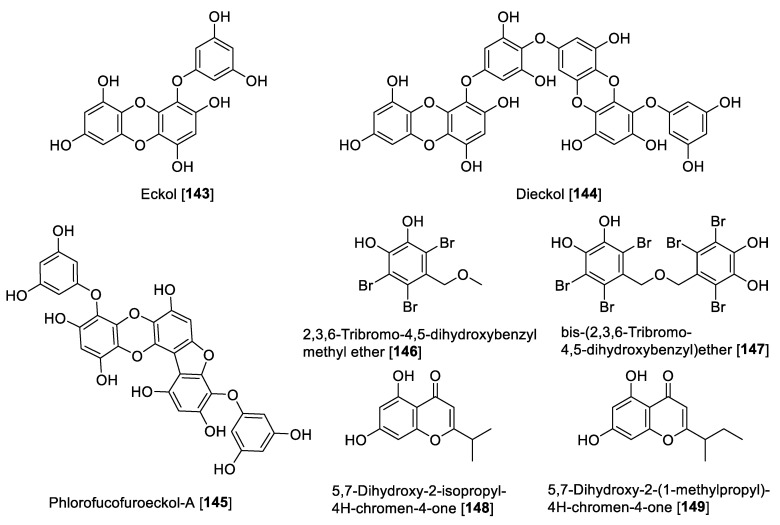
Natural xanthones, anthraquinones, and naphthoquinones are polyphenolic compounds characterized by benzene rings attached to each other by carbonyl and oxygen in the case of xanthones with a C6-C1-C6 skeleton or benzene rings coupled with para-quinones mainly to form naphthoquinone or anthraquinones (C6-C2-C6 sequence), configuring a planar ring structure [148]. The biosynthesis for these secondary metabolites is different depending on the natural source but they can be derived through several pathways. In fact, in plants, anthraquinones are derived mainly by polyketide, mevalonic, and methylerythritol 4-phosphate pathways [149]. On the other hand, in plants, shikimate and fatty acid pathways are predominantly found for xanthones [150]. Recent studies have shown a biosynthetic relationship between xanthones and anthraquinones, especially in bacteria and fungi [151]. Figure 10 and Figure 11 show the structures and Table 5 presents the IC50 values of selected xanthones, anthraquinones, and naphthoquinones against MAOs.
Figure 10.
Chemical structure of selected xanthone MAO inhibitors.
Figure 11.
Chemical structure of selected naphthoquinone and anthraquinone MAO inhibitors.
Xanthones and xanthone glycosides possess numerous biological and pharmacological potentials. Those compounds have been reported to show several therapeutic properties including antiallergic, anti-inflammatory, antituberculotic, antitumor, and antiplatelet [152,153]. In earlier studies, a series of natural xanthones has been evaluated for their activity to inhibit MAO-A and -B [154,155]. Rat brain mitochondrial extract was the source for MAO. 1-Hydroxy-3,8-dimethoxy-xanthone 96, 1,3-dihydroxy-7,8-dimethoxy xanthone 97, and bellidifolin 98 were the potent inhibitors of MAO-A. Xanthones exhibited only moderate inhibition of MAO-B [154,155]. From Hypericum brasiliense, a series of compounds was isolated, and among those, 6-deoxyjacareubin 99 and 1,5-dihidroxyxanthone 100 were found to exhibit the best MAO inhibition, using rat brain mitochondria as source of MAO enzymes. Compounds 99 and 100 exhibited slight preferential inhibition of MAO-A with IC50 values of 12.0 and 0.73 μM, respectively [156].
In a study performed by Gnerre et al., a series of fifty-nine natural and synthetic xanthones was evaluated for inhibition of MAO-A and -B [157]. It was found that the tested xanthones in general were preferential toward MAO-A at low micromolar concentrations. It is important to highlight that the natural xanthone 1,5-dihydroxy-3-methoxy xanthone 101 isolated from Chironia krebsii, a medicinal plant growing in the tropical East Africa country Malawi [158], was the most active within the series. Compound 101 exhibited inhibition of MAO-A and MAO-B with IC50 values of 40 nM and 33 μM, respectively [157]. Another notable example of natural xanthones is 12b-hydroxy-des-D-garcigerrin A 102, found in several Garcinia species [159], which showed an IC50 value of 3.3 μM for MAO-A and no inhibition of MAO-B [157]. Compound 102 also showed antioxidant and antiplasmodial activities [159]. Compound 98 was found to inhibit MAO-A with an IC50 value of 0.66 μM [157]. This natural xanthone is frequently isolated from Gentiana species [160].
Gentiacaulein 103, a natural xanthone isolated from Gentiana kochiana, was found as one of the two main xanthones present in the plant. Compound 103 exhibited MAO-A inhibition with an IC50 value of 0.49 μM [161]. In addition, several other natural xanthones were isolated from Cudrania tricuspidata and Gentianella amarella and evaluated for their ability to inhibit MAO enzymes. The novel xanthones isolated from C. tricuspidata showed poor inhibition of MAO [162], and the only percentage inhibition was reported for the xanthones isolated from G. amarella [163]. Dimitrov et al. reported that mangiferin 104, a glucoside xanthone usually found in the Hypericum species, showed selective inhibition of MAO-A with an IC50 value of 41 μM and negligible inhibition of MAO-B, using rat liver mitochondria as the MAO source. The authors explained that this MAO inhibition plays a key role in the antidepressant effect of the Hypericum aucheri extracts [164].
There are several anthraquinones and naphthoquinones reported to have inhibition toward MAO. Earlier examples are the anthraquinones, norsolorinic acid 105 and averufin 106, isolated from the fungus Emericella navahoensis and which inhibited MAO in mouse liver homogenate. Compound 105 inhibited total MAO with an IC50 value of 0.3 μM [165]. Emodin 107, a common anthraquinone found in plants, exhibited MAO-B inhibition with an IC50 value of 35.4 μM [68]. Lithospermum erythrorhizon roots are a common component in several preparations used in traditional Chinese medicine (TCM) for treatment of wounds and dermatitis [166]. Bioassay guided fractionation studies of L. erythrorhizon-root extracts for MAO inhibition using mouse brain homogenate allowed the isolation of naphthoquinones shikonin 108 and acetyl shikonin 109 as the bioactive components and non-selective MAO inhibitors. Shikonin 108 inhibited MAO-A with an IC50 value of 16.4 μM and MAO-B with an IC50 value of 13.6 μM; 109 had IC50 values of 16.9 and 10.1 μM for MAO-A and MAO-B, respectively [167].
Another notable naphthoquinone, 2,3,6-trimethyl-1,4-naphthoquinone 110, was extracted and isolated from cured tobacco leaves (Nicotiana tabacum) and was found to be a competitive inhibitor of human MAO (human liver mitochondrial preparation) with Ki values of 3 and 6 μM for MAO-A and MAO-B, respectively [168]. Recent studies found using a human MAO assay that 110 inhibited MAOs with IC50 values of 1.14 and 7.14 μM for MAO-A and MAO-B, respectively [169]. The same authors evaluated the anxiolytic effects of 110 on a zebrafish model, describing that the compound induced anxiolytic and anxiogenic-like effects on the model tested [169]. Menadione 111, a naphthoquinone formed by metabolism from the dietary vitamin K (menaquinones), was reported as selective MAO-B inhibitor with a Ki value of 0.4 μM [170]. Mostert et al. in a study with a series of synthetic and natural naphthoquinones reported juglone 112 and plumbagin 113 to inhibit MAO in a non-selective manner. Compound 112 exhibited IC50 values of 1.71 and 4.36 μM for human MAO-A and MAO-B, respectively; plumbagin 113 showed IC50 values of 4.91 and 1.09 μM for human MAO-A and MAO-B, respectively [171]. Two natural dyes anthraquinones purpurin 114 and alizarin red 115 were reported to inhibit MAO, and both anthraquinones showed a selectivity toward MAO-A. Compound 114 exhibited an IC50 value of 2.50 μM and for 115, an IC50 value of 30.1 μM [172]. In a phytochemical study of the components of Cassia obtusifolia seeds, a series of anthraquinones and related compounds were isolated and evaluated for the ability to inhibit human recombinant MAO. The compounds showed selective inhibition of MAO-A, selective and preferential, and notable examples are questin 116 that inhibited human MAO-A with an IC50 value of 0.17 μM, as well as aloe emodin 117, alaternin 118, rubrofusarin 119, and toralactone-9-O-β-gentiobioside 120 which were found to be strong inhibitors with IC50 values of 2.47, 5.35, 5.9, and 7.3 μM, respectively [173].
5.5. MAO Inhibitory Phenols and Polyphenolic Compounds
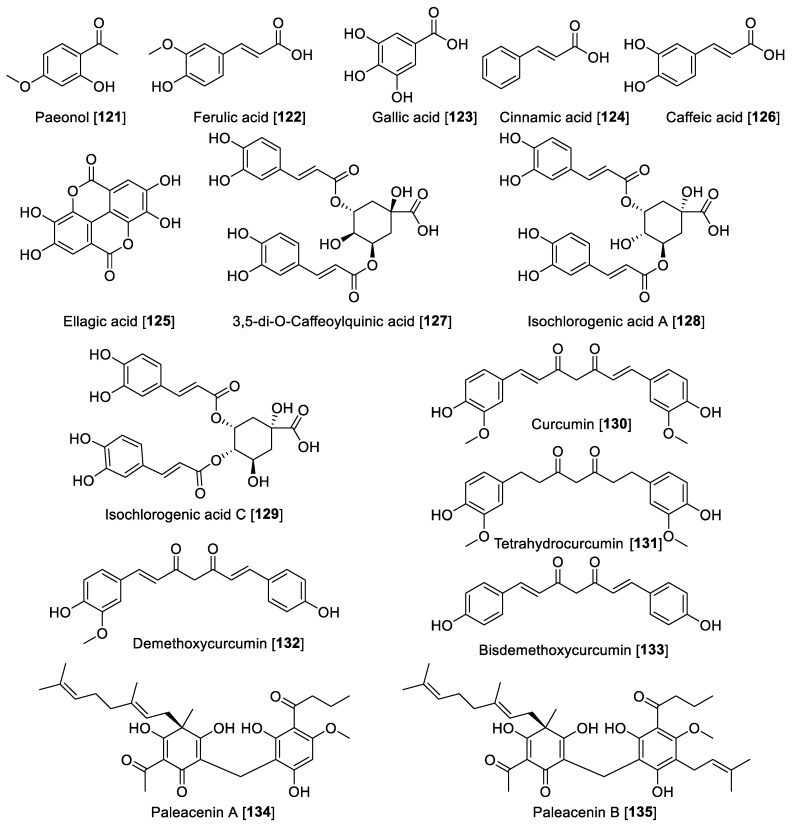
The polyphenolic compounds are simple phenolics and their acids and esters produced predominantly by the shikimate pathway include phenylpropanoids and lignans [174]. Several natural phenols were evaluated on MAO rat brain mitochondrial fraction and only Paeonol 121 showed a moderate inhibition toward MAO-A with a Ki value of 51.1 μM [68]. Ferulic acid 122 showed to be preferential toward MAO-A with an IC50 value of 7.55 μM for human MAO-A and 24.0 μM for MAO-B. Gallic acid 123 exhibited IC50 of 9.49 μM for human MAO-A; trans-cinnamic acid 124, a non-selective MAO inhibitor, exhibited IC50 values of 6.47 and 1.21 μM for MAO-A and -B, respectively; ellagic acid 125 was a selective MAO-B inhibitor with an IC50 value of 0.40 μM; and caffeic acid 126 was shown to be a non-selective MAO inhibitor with IC50 values of 11.72 and 22.88 μM for human MAO-A and MAO-B, respectively [175].
A series of chlorogenic acids was isolated from the flowers of Lonicera macranthoides, and from the isolated compounds, only 3,5-di-O-caffeoylquinic acid 127 exhibited moderate inhibition with an IC50 value of 20.04 μM for MAO-B and no inhibition for MAO-A [176]. From Lonicera japonica, using a magnetic nanoparticle MAO-B immobilized affinity solid-phase extraction method, two new chlorogenic acids isochlorogenic A 128 and C 129 were identified along with 122 as the bioactive component in L. japonica [177]. Isochlorogenic acid A 128 and C 129 were found to be mixed-type inhibitors for MAO-B with IC50 values of 29.0 and 29.77 μM, respectively [177]. Turmeric is a commonly used spice prepared from the rhizomes of Curcuma longa. Several bioactivities are attributed to turmeric, and those responsible for that bioactivity are the curcuminoid-type compounds, and in particular, curcumin 130 [178]. The inhibitory effects of 130 and tetrahydrocurcumin 131 on MAO-B were evaluated in a Parkinson’s disease rodent model induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Depletion of dopamine and 3,4-dihydroxy phenyl acetic acid occurs with increased MAO-B activity. Use of 130 and 131 reversed the decrease in dopamine and 3,4-dihydroxy phenyl acetic acid induced by the model [179]. Recently, the ability of 130, demethoxycurcumin 132, and bisdemethoxycurcumin 133 to inhibit MAO was reported, where the three compounds inhibited MAO moderately in a non-selective manner. Compound 130 exhibited IC50 values of 3.64 and 3.36 μM for human MAO-A and MAO-B, respectively; 131 exhibited IC50 values of 3.09 and 2.59 μM, respectively; and 132 exhibited IC50 values of 3.24 and 2.45 μM for MAO-A and MAO-B, respectively [180]. Paleacenins A 134 and B 135 are two novel acylphloroglucinols isolated from Elaphoglossum paleaceum rhizome, and these two undescribed compounds exhibited high MAO inhibition [181]. Compounds 134 and 135 showed to be a non-selective MAO inhibitor in rat brain mitochondria, and 134 exhibited IC50 values of 31.0 and 4.7 μM for MAO-A and-B, respectively. Meanwhile, 135 showed IC50 values of 1.3 and 4.4 μM for MAO-A and -B, respectively. Besides their MAO inhibition, 134 and 135 also showed activity toward several cancer cell lines including the prostate, cervix, breast, and colon, denoting a multitarget effect for paleacinins [181]. Figure 12 shows the selected additional polyphenolic compound MAO inhibitors and Table 6 presents the IC50 values of selected phenol and polyphenolic compounds.
Figure 12.
Chemical structure of miscellaneous polyphenol MAO inhibitors.
5.6. MAO Inhibitory Terpenes and Terpenoids
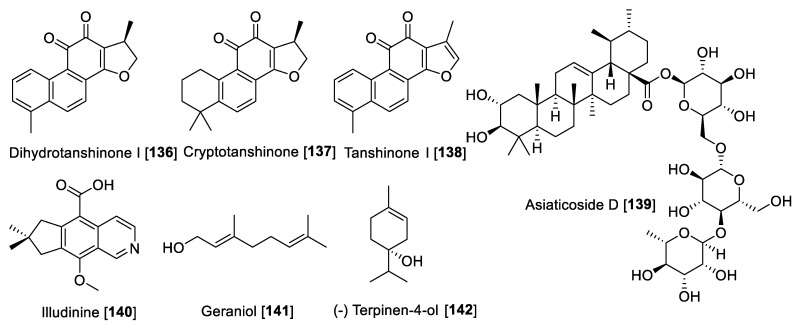
Terpenes or terpenoids are one of the broad families of natural products with uncountable pharmacological and biological uses. These secondary metabolites are built based on isoprene units (five carbon building blocks) [182]. There are few terpenoids that have been reported to exhibit MAO inhibition. In a study exploring the use of HPLC-based bioguided activity profiling, Salvia miltiorrhiza was screened. The extract of S. miltiorrhiza showed a high inhibitory effect on rat liver monoamine oxidase fraction with a preference toward MAO A. From the active fractions based on the HPLC-bioguided assay, the diterpenes dihydrotanshinone I 136, cryptotanshinone 137, and tanshinone I 138 were identified as the bioactive compounds with IC50 values of 23, 80, and 84 μM, respectively [183]. Recently, a triterpene saponin asiaticoside D 139, isolated from Centella asiatica (a herbal product used in Ayurvedic medicine) following a bioassay guided fractionation, exhibited MAO inhibition [184]. Compound 139 was found to be a non-selective inhibitor with IC50 values of 4.0 and 1.3 μg/mL for MAO-A and MAO-B, respectively [184]. Illudinine 140, a sesquiterpene-alkaloid with an illudalane skeleton, was found to be an inhibitor of human MAO-B with an IC50 value of 18.3 μM. Several synthetic derivatives of illudinine have been synthesized; however, none of these compounds were found to be superior to illudinine [185]. In a bioassay guided fractionation study of the Zingiber officinale rhizomes, several monoterpenes showed potential MAO-A inhibitory properties [186]. The monoterpenes geraniol 141 and (-) terpinen-4-ol 142 were found to be inhibitors of MAO-A, but only a percentage of inhibition was reported [186]. Figure 13 shows the chemical structures for selected terpenoid MAO inhibitors and Table 7 presents the IC50 values of selected terpenoid compounds.
Figure 13.
Chemical structure of selected terpenoid MAO inhibitors.
5.7. MAO Inhibitors from Marine Sources
In the last three decades, the study of marine environments has been the source of great chemical diversity, and at least six isolated compounds from marine organisms have been FDA approved, with several others currently in clinical trials [187,188]. Several marine natural products have been reported with inhibitory activity toward MAO enzymes, including aplysinopsins, piloquinones, anthiactins, bromopyrroles, caulerpins, and astaxanthins, which have been compiled in a recent review [189]. As an update of the review mentioned before, two well-known phlorotannins isolated from the edible brown alga Eisenia bicyclis were found to be MAO inhibitors [190]. The two phlorotannins named eckol 143 and dieckol 144 showed a preferential human MAO-A inhibition with IC50 values of 7.2 and 11.43 μM, respectively [190]. Phlorofucofuroeckol-A 145 (PFF-A), another phlorotannin isolated from Ecklonia stolonifera (edible brown alga), showed non-selective MAO inhibition. PFF-A 145 showed IC50 values of 9.22 and 4.89 μM for human MAO-A and MAO-B, respectively [191]. Compounds 144 and 145 also exhibited dopamine D3R and D4R agonism as well as D1, serotonin 5-HT1A, and neurokin NK1 antagonism [191]. From the red alga Symphyocladia latiuscula, a series of bromophenols was isolated and 2,3,6-tribromo-4,5-dihydroxybenzyl methyl ether 146 and bis-(2,3,6-tribromo-4,5-dihydroxybenzyl) ether 147 exhibited moderate inhibition of human MAO-A with IC50 values of 63.2 and 89.3 μM, respectively [192]. Two chromenones isolated from the marine microorganism Streptomyces sp (CNQ-031), 5,7-dihydroxy-2-isopropyl-4H-chromen-4-one 148 and 5,7-dihydroxy-2-(1-methylpropyl)-4H-chromen-4-one 149, were found to be inhibitors for human MAO. Compound 148 inhibited human MAO-A with a 10-fold difference than for MAO-B with IC50 values of 2.7 and 27.0 μM, respectively, while 149 showed a non-selective inhibition with IC50 values of 6.92 and 3.42 μM for human MAO-A and MAO-B, respectively [123]. Figure 14 shows marine compounds with MAO inhibition and Table 7 presents the IC50 values of marine MAO inhibitors.
Figure 14.
Chemical structure of selected marine isolated compound MAO inhibitors.
5.8. MAO Inhibitors from Miscellaneous Classes of Natural Products
Small polyketides have been reported as MAOIs; for example, desmethoxyyangonin 150 isolated from the medicinal plant Renealmia Alpinia was found to be a MAO inhibitor, being selective toward human MAO-B with an IC50 value of 0.12 μM compared to an IC50 value of 1.85 μM for MAO-A [18]. Desmethoxyyangonin 150 belongs to the well-known kavalactone-type secondary metabolites which are the major constituents isolated predominantly from Piper methysticum roots, the principal ingredient of the kava drink [193]. The kava drink has been used for centuries by the natives of the pacific islands of Vanuatu, Fiji, Tonga, Samoa, and Micronesia due to its psychoactive and medicinal properties. The kava drink is also a popular drink in Western countries [194]. Besides 150, the kavalactones: (+)-kavain 151, (+)-7,8-dihydrokavain 152, (+)-methysticin 153, (+)-7,8-dihydromethysticin 154, and yangonin 155 were reported to be selective MAO-B inhibitors with IC50 values of 4.34, 8.23, 0.42, 0.85, and 0.085 μM, respectively. Only 153 and 155 exhibited inhibition of human MAO-A with IC50 values of 8.12 and 1.29 μM, respectively [195]. Another small polyketide, (S)-5-methymellein 156, a small lactone isolated from the endogenous fungus Rosellinia corticium within the lichen Pseudevernia furfuracea, exhibited MAO inhibition. Compound 156 was identified through bioassay guided fractionation and exhibited IC50 values of 5.31 and 9.15 μM for human MAO-A and MAO-B, respectively [196]. Its enantiomer, (R)-5-methylmellein 157, was also isolated from the fermented mycelia of Xylaria nigripes, and 157 showed moderate selectivity toward MAO-A with IC50 values of 4.6 and 38.5 μM for human MAO-A and MAO-B, respectively [197]. The enantiomers were subjected to a synthetic study aiming to find better inhibitory agents, and the study found a selective human MAO-B inhibitor in a pyrimidyl derivative, (R)-3-ethyl-8-hydroxy-5-methyl-7-(pyrimidin-5-yl)-3,4-dihydronaphthalen-1(2H)-one 158, with an IC50 value of 0.06 μM for human MAO-B and SI value of >830 [197]. From another endogenous fungus Diaporthe mahothocarpus isolated from the lichen Cladonia symphycarpia, three polyketides were isolated with potent MAO inhibition. The polyketides alternariol 159, 5′-hydroxy-alternariol 160, and mycoepoxydiene 161 were isolated following a bioassay guided paradigm and were found to be MAO-A inhibitors. Compound 159 exhibited the best value with an IC50 of 0.020 μM, while 160 and 161 exhibited IC50 values of 0.31 and 8.7 μM for human MAO-A, respectively [198]. The in vitro evaluation of several endogenous endocannabinoids found that virodhamine 162 inhibited both MAO-A and -B, being preferential to MAO-B with IC50 values of 38.70 and 0.71 μM for human MAO-A and MAO-B, respectively [199]. Figure 15 shows the structures and Table 7 presents the IC50 values of selected miscellaneous natural product classes on natural product inhibitors.
Figure 15.
Chemical structure of selected miscellaneous compound MAO inhibitors.
6. Natural MAO Inhibitors in Neuroblastoma
Therapeutic applications of MAOIs have been established primarily for the treatment of depression and other neurological disorders. Oxidative deamination of monoamines by MAO produces oxidative stress [200]. Recent studies have suggested the role of MAO-A in antitumor immunity [201]. An enhanced T cell immunity and suppression of tumor growth were reported in MAO-A knockout mice. Treatment with clinically approved MAO-A inhibitors such as phenelzine, moclobemide, and clorgyline produced tumor suppression in preclinical animal models [201]. The role of MAOs in tumor progression and metastasis suggests these enzymes as potential anticancer drug targets [202]. Neuroblastoma, known to originate from the undifferentiated neural crest cells which differentiate into malignant neuroblastoma, is the most common extracranial solid tumor in children. Excess production of catecholamines in neuroblastoma tumor cells has been reported [203]. This may be the cause for severe hypertension associated with neuroblastoma [204,205,206]. Elevations in the levels of urinary metabolites of catecholamines vanillylmandelic acid and homovanillic acid are routinely assessed as prognostic biomarkers of neuroblastoma [207,208]. A report from the children oncology group suggested a correlation between the elevated expression of the vesicular monoamine transporter with clinical features, tumor biology, and metaiodobenzylguanidine (MIBG) avidity in neuroblastoma [84]. MOA-A has been shown to promote protective autophagy in human SH-SY5Y neuroblastoma cells through Bcl-2 phosphorylation [209]. These studies suggest the potential utility of MAO-A inhibitors for treatment of neuroblastoma. Isatin, an endogenous MAO inhibitor, caused a dose-dependent switch from apoptosis to necrosis in human neuroblastoma cells [210]. The natural product MAO inhibitors may also have potential utility for treatment of neuroblastoma tumors. Harmine 22, the potent natural product alkaloid inhibitor of MAO-A, has been shown to induce apoptosis and inhibit cell proliferation of several human cancer cell lines [211,212]. Harmine 22 also induced apoptosis in different neuroblastoma cell lines such as SKNBE and KELLY (MYCN amplified) and SKNAS and SKNFI (MYCN non-amplified) [213]. Induction of progressive apoptosis of human neuroblastoma cells by harmine was also attributed to the activation of caspase-3/7 and caspase-9 [214]. Selective inhibition of dual-specificity tyrosine phosphorylation-regulated kinase (DYRK) family proteins and mitogen-activated protein kinase by harmine [214] led to the investigation of the expression of DYRK family kinases in neuroblastoma tumors [213]. A clinical study suggested the role of DYRK2 in tumorigenesis of neuroblastoma. Inhibition of DYRK2 by 22 [212,214] and activation of caspase-mediated apoptosis in neuroblastoma cells [213] suggest the treatment of neuroblastoma by 22. Norharman 27, a potential MAO-A inhibitor, also induced apoptotic cell death in human neuroblastoma cells [215]. Berberine treatment produced the induction of neuronal cell differentiation, attenuated cancer stemness markers, and potentiated G0/G1 cell cycle arrest in neuro2a (N2a) neuroblastoma cells [216]. Concurrent inhibition of β-adrenergic signaling pathways with a β-blocker and inhibition of MAO-A with berberine 6 produced attenuation of tumor growth and an increase in differentiation of cells [217].
7. Concluding Remarks
This review presents a comprehensive survey of natural products, predominantly isolated or purified from plant sources, which have shown promising MAO inhibitory activity. The natural product MAOIs may be utilized as potential leads for new drug discovery and safer alternatives for treatment of neurological disorders. The neuroactive effects of several traditionally used herbal formulations have been attributed to the presence of the constituents with selective inhibition of MAOs; for example, the potent inhibition of MAO-A by harmala alkaloids is responsible for the hallucinogenic effects of ayahuasca. The survey was further extended to analyze different classes of natural product MAOIs and selectivity of the inhibition by these constituents. Natural products with selective and reversible inhibition of MAOs may be advanced to clinical evaluation for treatment of disorders linked to pathophysiological consequences of the metabolism of biogenic monoamines.
Selective MAO-A and -B inhibitors isolated from natural sources may be safer alternatives for the treatment of neurological disorders. A greater understanding of the natural product inhibitors for MAOs may improve the drug discovery and treatment of neurological disorders. Evaluation of MAOIs as constituents of natural product ingredients used in dietary supplements and traditional herbal preparations may also be important for preventing adverse drug–dietary supplement interactions.
Naturally occurring MAOIs are good alternatives for the treatment of depression, anxiety, Parkinson’s disease, and neurodegenerative diseases. Non-selective and irreversible classical MAOIs are characterized by the risk of inducing hypertensive crisis when foods rich in tyramine are ingested. However, selective MAO-B inhibitors do not show such interactions. Several natural products with selective reversible inhibition of MAO-B have been identified. The selective MAO-B inhibitors and RIMAs are potential and safer alternatives to irreversible MAOIs. A greater understanding of the pharmacodynamic and pharmacokinetic properties of the natural product MAOIs may improve treatment of patients in the future. Another challenge for future basic and clinical research is to examine the potential preventive medications that slow the physiological and other age-related activities. The natural products are also an important source of MAOIs that can slow the age-related behavioral problems and decrease susceptibility to senile depression, Parkinson’s disease, and Alzheimer’s disease. Characterization of MAO inhibitory constituents of natural products traditionally used as psychoactive preparations or for treatment of neurological disorders may help in understanding the mechanisms of action and in optimization of these preparations for desired bioactive properties. Potential therapeutic applications of natural product MAOIs for the treatment of neuroblastoma are also discussed.
Acknowledgments
The authors are thankful to Southern Research, National Center for Natural Products Research, University of Mississippi and Department of Drug Discovery and Biomedical Sciences, College of Pharmacy, University of South Carolina, Columbia SC, 29208 for providing necessary facilities for these surveys. The authors are thankful to Pankaj Pandey, Research Scientist at the National Center for Natural Products Research, School of Pharmacy, University of Mississippi for contributing to the figures (Figure 2) for MAO-A and MAO-B active site structures bound to selective enzyme inhibitors.
Author Contributions
All the authors have contributed equally for the survey of literature and published sources and compilation and analysis of the references. N.D.C. and B.L.T. prepared initial draft of the manuscript. F.L. and I.M. revised the section on different classes of natural products as MAOIs for chemistry and accuracy of the chemical structures presented. All the authors reviewed the manuscript draft, added relevant information, edited the manuscript drafts, and approved the final draft. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No experimental data are reported in the review manuscript.
Conflicts of Interest
Authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Edmondson D.E., Binda C. Monoamine Oxidases. Subcell Biochem. 2018;87:117–139. doi: 10.1007/978-981-10-7757-9_5. [DOI] [PubMed] [Google Scholar]
- 2.Ramsay R.R. Monoamine oxidases: The biochemistry of the proteins as targets in medicinal chemistry and drug discovery. Curr. Top. Med. Chem. 2012;12:2189–2209. doi: 10.2174/156802612805219978. [DOI] [PubMed] [Google Scholar]
- 3.Klinman J.P. The multi-functional topa-quinone copper amine oxidases. Biochim. Biophys. Acta. 2003;1647:131–137. doi: 10.1016/S1570-9639(03)00077-3. [DOI] [PubMed] [Google Scholar]
- 4.Abell C.W., Kwan S.W. Molecular characterization of monoamine oxidases A and B. Prog. Nucleic Acid. Res. Mol. Biol. 2001;65:129–156. doi: 10.1016/s0079-6603(00)65004-3. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein D.S., Castillo G., Sullivan P., Sharabi Y. Differential Susceptibilities of Catecholamines to Metabolism by Monoamine Oxidases. J. Pharmacol. Exp. Ther. 2021;379:253–259. doi: 10.1124/jpet.121.000826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramonet D., Rodriguez M., Saura J., Lizcano J.M., Romera M., Unzeta M., Finch C., Billett E., Mahy N. Localization of monoamine oxidase A and B and semicarbazide-sensitive amine oxidase in human peripheral tissues. Inflammopharmacology. 2003;11:111–117. doi: 10.1163/156856003765764272. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez M.J., Saura J., Billett E.E., Finch C.C., Mahy N. Cellular localization of monoamine oxidase A and B in human tissues outside of the central nervous system. Cell Tissue Res. 2001;304:215–220. doi: 10.1007/s004410100361. [DOI] [PubMed] [Google Scholar]
- 8.Hubalek F., Pohl J., Edmondson D.E. Structural comparison of human monoamine oxidases A and B: Mass spectrometry monitoring of cysteine reactivities. J. Biol. Chem. 2003;278:28612–28618. doi: 10.1074/jbc.M303712200. [DOI] [PubMed] [Google Scholar]
- 9.Bach A.W., Lan N.C., Johnson D.L., Abell C.W., Bembenek M.E., Kwan S.W., Seeburg P.H., Shih J.C. cDNA cloning of human liver monoamine oxidase A and B: Molecular basis of differences in enzymatic properties. Proc. Natl. Acad. Sci. USA. 1988;85:4934–4938. doi: 10.1073/pnas.85.13.4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hubalek F., Binda C., Khalil A., Li M., Mattevi A., Castagnoli N., Edmondson D.E. Demonstration of isoleucine 199 as a structural determinant for the selective inhibition of human monoamine oxidase B by specific reversible inhibitors. J. Biol. Chem. 2005;280:15761–15766. doi: 10.1074/jbc.M500949200. [DOI] [PubMed] [Google Scholar]
- 11.Son S.Y., Ma J., Kondou Y., Yoshimura M., Yamashita E., Tsukihara T. Structure of human monoamine oxidase A at 2.2-A resolution: The control of opening the entry for substrates/inhibitors. Proc. Natl. Acad. Sci. USA. 2008;105:5739–5744. doi: 10.1073/pnas.0710626105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Colibus L., Li M., Binda C., Lustig A., Edmondson D.E., Mattevi A. Three-dimensional structure of human monoamine oxidase A (MAO A): Relation to the structures of rat MAO A and human MAO B. Proc. Natl. Acad. Sci. USA. 2005;102:12684–12689. doi: 10.1073/pnas.0505975102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma J., Yoshimura M., Yamashita E., Nakagawa A., Ito A., Tsukihara T. Structure of rat monoamine oxidase A and its specific recognitions for substrates and inhibitors. J. Mol. Biol. 2004;338:103–114. doi: 10.1016/j.jmb.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 14.Ma J., Kubota F., Yoshimura M., Yamashita E., Nakagawa A., Ito A., Tsukihara T. Crystallization and preliminary crystallographic analysis of rat monoamine oxidase A complexed with clorgyline. Acta Crystallogr. D Biol. Crystallogr. 2004;60:317–319. doi: 10.1107/S0907444903025770. [DOI] [PubMed] [Google Scholar]
- 15.Binda C., Li M., Hubalek F., Restelli N., Edmondson D.E., Mattevi A. Insights into the mode of inhibition of human mitochondrial monoamine oxidase B from high-resolution crystal structures. Proc. Natl. Acad. Sci. USA. 2003;100:9750–9755. doi: 10.1073/pnas.1633804100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones D.N., Raghanti M.A. The role of monoamine oxidase enzymes in the pathophysiology of neurological disorders. J. Chem. Neuroanat. 2021;114:101957. doi: 10.1016/j.jchemneu.2021.101957. [DOI] [PubMed] [Google Scholar]
- 17.Fowler J.S., Volkow N.D., Logan J., Franceschi D., Wang G.J., MacGregor R., Shea C., Garza V., Pappas N., Carter P., et al. Evidence that L-deprenyl treatment for one week does not inhibit MAO A or the dopamine transporter in the human brain. Life Sci. 2001;68:2759–2768. doi: 10.1016/S0024-3205(01)01079-7. [DOI] [PubMed] [Google Scholar]
- 18.Chaurasiya N.D., Leon F., Ding Y., Gomez-Betancur I., Benjumea D., Walker L.A., Cutler S.J., Tekwani B.L. Interactions of Desmethoxyyangonin, a Secondary Metabolite from Renealmia alpinia, with Human Monoamine Oxidase-A and Oxidase-B. Evid. Based Compl. Alternat Med. 2017;2017:1–10. doi: 10.1155/2017/4018724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liebowitz M.R., Hollander E., Schneier F., Campeas R., Welkowitz L., Hatterer J., Fallon B. Reversible and irreversible monoamine oxidase inhibitors in other psychiatric disorders. Acta Psychiatr. Scand. Suppl. 1990;360:29–34. doi: 10.1111/j.1600-0447.1990.tb05321.x. [DOI] [PubMed] [Google Scholar]
- 20.Priest R.G., Gimbrett R., Roberts M., Steinert J. Reversible and selective inhibitors of monoamine oxidase A in mental and other disorders. Acta Psychiatr. Scand. Suppl. 1995;386:40–43. doi: 10.1111/j.1600-0447.1995.tb05923.x. [DOI] [PubMed] [Google Scholar]
- 21.Amrein R., Hetzel W., Stabl M., Schmid-Burgk W. RIMA: A safe concept in the treatment of depression with moclobemide. Can. J. Psychiatry. 1992;37((Suppl. S1)):7–11. [PubMed] [Google Scholar]
- 22.Mann J.J., Aarons S.F., Frances A.J., Brown R.D. Studies of selective and reversible monoamine oxidase inhibitors. J. Clin. Psychiatry. 1984;45:62–66. [PubMed] [Google Scholar]
- 23.Suchting R., Tirumalajaru V., Gareeb R., Bockmann T., de Dios C., Aickareth J., Pinjari O., Soares J.C., Cowen P.J., Selvaraj S. Revisiting monoamine oxidase inhibitors for the treatment of depressive disorders: A systematic review and network meta-analysis. J. Affect Disord. 2021;282:1153–1160. doi: 10.1016/j.jad.2021.01.021. [DOI] [PubMed] [Google Scholar]
- 24.Silverman R.B., Ding C.Z., Gates K.S. Design and meachanism of monoamine oxidase inactivators from an organic chemical perspective. In: Testa B., Kyburz E., Fuhrer W., Giger R., editors. Perspectives in Medicinal Chemistry. Verlag Helvetica Chimica; Basel, Switzerland: 1993. pp. 73–86. [Google Scholar]
- 25.Kanazawa I. Short review on monoamine oxidase and its inhibitors. Eur. Neurol. 1994;34:36–39. doi: 10.1159/000119540. [DOI] [PubMed] [Google Scholar]
- 26.Binda C., Newton-Vinson P., Hubalek F., Edmondson D.E., Mattevi A. Structure of human monoamine oxidase B, a drug target for the treatment of neurological disorders. Nat. Struct. Biol. 2002;9:22–26. doi: 10.1038/nsb732. [DOI] [PubMed] [Google Scholar]
- 27.Dhiman P., Malik N., Sobarzo-Sanchez E., Uriarte E., Khatkar A. Quercetin and Related Chromenone Derivatives as Monoamine Oxidase Inhibitors: Targeting Neurological and Mental Disorders. Molecules. 2019;24:418. doi: 10.3390/molecules24030418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bolasco A., Carradori S., Fioravanti R. Focusing on new monoamine oxidase inhibitors. Exp. Opin. Ther. Pat. 2010;20:909–939. doi: 10.1517/13543776.2010.495716. [DOI] [PubMed] [Google Scholar]
- 29.Riederer P.L.G. MAO-inhibitors in Parkinson’s Disease. Exp. Neurobiol. 2011;20:1–17. doi: 10.5607/en.2011.20.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirwin J.L., Gören J.L. Duloxetine: A dual serotonin-norepinephrinereuptake inhibitor for treatment of major depressive disorder. Pharmacotherapy. 2005;25:396–410. doi: 10.1592/phco.25.3.396.61600. [DOI] [PubMed] [Google Scholar]
- 31.Moret C., Isaac M., Briley M. Problems associated with long-termtreatment with selective serotonin reuptake inhibitors. J. Psychopharmacol. 2009;23:967–974. doi: 10.1177/0269881108093582. [DOI] [PubMed] [Google Scholar]
- 32.Fowler J.S., Logan J., Azzaro A.J., Fielding R.M., Zhu W., Poshusta A.K., Burch D., Brand B., Free J., Asgharnejad M., et al. Reversible inhibitors of monoamine oxidase-A (RIMAs): Robust, reversible inhibition of human brain MAO-A by CX157. Neuropsychopharmacology. 2010;35:623–631. doi: 10.1038/npp.2009.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gleiter C.H., Muhlbauer B., Schulz R.M., Nilsson E., Antonin K.H., Bieck P.R. Monoamine oxidase inhibition by the MAO-A inhibitors brofaromine and clorgyline in healthy volunteers. J. Neural Transm. Gen. Sect. 1994;95:241–245. doi: 10.1007/BF01271570. [DOI] [PubMed] [Google Scholar]
- 34.Ebadi M., Brown-Borg H., Ren J., Sharma S., Shavali S., El ReFaey H., Carlson E.C. Therapeutic efficacy of selegiline in neurodegenerative disorders and neurological diseases. Curr. Drug Targets. 2006;7:1513–1529. doi: 10.2174/1389450110607011513. [DOI] [PubMed] [Google Scholar]
- 35.Riederer P., Lachenmayer L., Laux G. Clinical applications of MAO-inhibitors. Curr. Med. Chem. 2004;11:2033–2043. doi: 10.2174/0929867043364775. [DOI] [PubMed] [Google Scholar]
- 36.Youdim M.B., Bakhle Y.S. Monoamine oxidase: Isoforms and inhibitors in Parkinson’s disease and depressive illness. Br. J. Pharmacol. 2006;147((Suppl. S1)):S287–S296. doi: 10.1038/sj.bjp.0706464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stocchi F. Rasagiline: Defining the role of a novel therapy in the treatment of Parkinson’s disease. Int. J. Clin. Pract. 2006;60:215–221. doi: 10.1111/j.1742-1241.2006.00806.x. [DOI] [PubMed] [Google Scholar]
- 38.Lecht S., Haroutiunian S., Hoffman A., Lazarovici P. Rasagiline-a novel MAO B inhibitor in Parkinson’s disease therapy. Ther. Clin. Risk Manag. 2007;3:467–474. [PMC free article] [PubMed] [Google Scholar]
- 39.Chen J.J., Ly A.V. Rasagiline: A second-generation monoamine oxidase type-B inhibitor for the treatment of Parkinson’s disease. Am. J. Health Syst. Pharm. 2006;63:915–928. doi: 10.2146/ajhp050395. [DOI] [PubMed] [Google Scholar]
- 40.McGrath P.J., Blood D.K., Stewart J.W., Harrison W., Quitkin F.M., Tricamo E., Markowitz J. A comparative study of the electrocardiographic effects of phenelzine, tricyclic antidepressants, mianserin, and placebo. J. Clin. Psychopharmacol. 1987;7:335–339. doi: 10.1097/00004714-198710000-00009. [DOI] [PubMed] [Google Scholar]
- 41.Tibrewal P., Kanigere M.K., Looi J.C., Allison S., Bastiampillai T. The evidence for the use of long-term benzodiazepines in the setting of treatment-refractory anxiety disorders. Aust. N. Z. J. Psychiatry. 2022;56:723–724. doi: 10.1177/00048674221087163. [DOI] [PubMed] [Google Scholar]
- 42.Sathyanarayana Rao T.S., Yeragani V.K. Hypertensive crisis and cheese. Indian J Psychiatry. 2009;51:65–66. doi: 10.4103/0019-5545.44910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Flockhart D.A. Dietary restrictions and drug interactions with monoamine oxidase inhibitors: An update. J. Clin. Psychiatry. 2012;73((Suppl. S1)):17–24. doi: 10.4088/JCP.11096su1c.03. [DOI] [PubMed] [Google Scholar]
- 44.Baldwin D.S. Sexual dysfunction associated with antidepressant drugs. Exp. Opin. Drug Saf. 2004;3:457–470. doi: 10.1517/14740338.3.5.457. [DOI] [PubMed] [Google Scholar]
- 45.Cohen S., Kuhn K.U., Strater B., Scherbaum N., Weig W. Adverse side-effect on sexual function caused by psychotropic drugs and psychotropic substances. Nervenarzt. 2010;81:1129–1137. doi: 10.1007/s00115-010-3074-9. [DOI] [PubMed] [Google Scholar]
- 46.Mago R.T.N., Andrade C. Cardiovascular adverse effects of newer antidepressants. Exp. Rev. Neurother. 2014;14:539–551. doi: 10.1586/14737175.2014.908709. [DOI] [PubMed] [Google Scholar]
- 47.Reichenpfader U.G.G., Morgan L.C., Greenblatt A., Nussbaumer B., Hansen R.A. Sexual dysfunction associated with second-generation antidepressants in patients with major depressive disorder: Results from a systematic review with network meta-analysis. Drug Saf. 2014;37:19–31. doi: 10.1007/s40264-013-0129-4. [DOI] [PubMed] [Google Scholar]
- 48.Cappetta K.B.C., Johnson J.A., Bloch M.H. Meta-analysis: Risk of dry mouth with second generation antidepressants. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2018;84:282–293. doi: 10.1016/j.pnpbp.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 49.Rapaport M.H. Dietary restrictions and drug interactions with monoamine oxidase inhibitors: The state of the art. J. Clin. Psychiatry. 2007;68((Suppl. S8)):42–46. [PubMed] [Google Scholar]
- 50.Remick R.A. Anticholinergic side effects of tricyclic antidepressants and their management. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1988;12:225–231. doi: 10.1016/0278-5846(88)90039-5. [DOI] [PubMed] [Google Scholar]
- 51.Brambilla P.C.A., Hotopf M., Barbui C. Side-effect profile of fluoxetine in comparison with other SSRIs, tricyclic and newer antidepressants: A meta-analysis of clinical trial data. Pharmacopsychiatry. 2005;38:69–77. doi: 10.1055/s-2005-837806. [DOI] [PubMed] [Google Scholar]
- 52.Nair N.P., Ahmed S.K., Kin N.M., West T.E. Reversible and selective inhibitors of monoamine oxidase A in the treatment of depressed elderly patients. Acta Psychiatry Scand. Suppl. 1995;386:28–35. doi: 10.1111/j.1600-0447.1995.tb05921.x. [DOI] [PubMed] [Google Scholar]
- 53.Sarris J. Herbal medicines in the treatment of psychiatric disorders: 10-year updated review. Phytother. Res. 2018;32:1147–1162. doi: 10.1002/ptr.6055. [DOI] [PubMed] [Google Scholar]
- 54.Moragrega I., Rios J.L. Medicinal Plants in the Treatment of Depression: Evidence from Preclinical Studies. Planta Med. 2021;87:656–685. doi: 10.1055/a-1338-1011. [DOI] [PubMed] [Google Scholar]
- 55.Martins J.S.B. Phytochemistry and pharmacology of anti-depressant medicinal plants: A review. Biomed. Pharmacother. 2018;104:343–365. doi: 10.1016/j.biopha.2018.05.044. [DOI] [PubMed] [Google Scholar]
- 56.Geck M.S., Lecca D., Marchese G., Casu L., Leonti M. Ethnomedicine and neuropsychopharmacology in Mesoamerica. J. Ethnopharmacol. 2021;278:114243. doi: 10.1016/j.jep.2021.114243. [DOI] [PubMed] [Google Scholar]
- 57.Rehman M.U., Wali A.F., Ahmad A., Shakeel S., Rasool S., Ali R., Rashid S.M., Madkhali H., Ganaie M.A., Khan R. Neuroprotective Strategies for Neurological Disorders by Natural Products: An update. Curr. Neuropharmacol. 2019;17:247–267. doi: 10.2174/1570159X16666180911124605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ji H.F., Zhang H.Y. Multipotent natural agents to combat Alzheimer’s disease. Functional spectrum and structural features. Acta Pharmacol. Sin. 2008;29:143–151. doi: 10.1111/j.1745-7254.2008.00752.x. [DOI] [PubMed] [Google Scholar]
- 59.Schultes R.E., Raffauf R.F. The Healing Forest: Medicinal and Toxic Plants of the Northwest Amazonia. Dioscorides Press; Portland, OR, USA: 1990. [Google Scholar]
- 60.Samoylenko V., Rahman M.M., Tekwani B.L., Tripathi L.M., Wang Y.H., Khan S.I., Khan I.A., Miller L.S., Joshi V.C., Muhammad I. Banisteriopsis caapi, a unique combination of MAO inhibitory and antioxidative constituents for the activities relevant to neurodegenerative disorders and Parkinson’s disease. J. Ethnopharmacol. 2010;127:357–367. doi: 10.1016/j.jep.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Glover V., Liebowitz J., Armando I., Sandler M. beta-Carbolines as selective monoamine oxidase inhibitors: In vivo implications. J. Neural Transm. 1982;54:209–218. doi: 10.1007/BF01254930. [DOI] [PubMed] [Google Scholar]
- 62.Morales-Garcia J.A., de la Fuente Revenga M., Alonso-Gil S., Rodriguez-Franco M.I., Feilding A., Perez-Castillo A., Riba J. The alkaloids of Banisteriopsis caapi, the plant source of the Amazonian hallucinogen Ayahuasca, stimulate adult neurogenesis in vitro. Sci. Rep. 2017;7:5309. doi: 10.1038/s41598-017-05407-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Das T., Saha S.C., Sunita K., Majumder M., Ghorai M., Mane A.B., Prasanth D.A., Kumar P., Pandey D.K., Al-Tawaha A.R., et al. Promising botanical-derived monoamine oxidase (MAO) inhibitors: Pharmacological aspects and structure-activity studies. S. Afr. J. Bot. 2022;146:127–145. doi: 10.1016/j.sajb.2021.09.019. [DOI] [Google Scholar]
- 64.Ege T.S.H.D. Monoamine oxidase inhibitory effects of medicinal plants in management of Alzheimer’s dis-ease. J. Turk. Chem. Soc. 2021;8:239–248. [Google Scholar]
- 65.Verpoorte R. In: Alkaloids in Encyclopedia of Analytical Sciences. 2nd ed. Worsfold P., Townshend A., Poole C., editors. Elsevier Sciences; London, UK: 2005. [Google Scholar]
- 66.Fattorusso E., Taglialatela-Scafati O. Modern Alkaloids: Structure, Isolation, Synthesis, and Biology. Wiley-VCH Verlag GmbH & Co. KGaA; Weinheim, Germany: 2008. p. 689. [Google Scholar]
- 67.Schurr A., Livne A. Differential inhibition of mitochondrial monoamine oxidase from brain by hashish components. Biochem. Pharmacol. 1976;25:1201–1203. doi: 10.1016/0006-2952(76)90369-5. [DOI] [PubMed] [Google Scholar]
- 68.Kong L.D., Cheng C.H., Tan R.X. Inhibition of MAO A and B by some plant-derived alkaloids, phenols and anthraquinones. J. Ethnopharmacol. 2004;91:351–355. doi: 10.1016/j.jep.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 69.Lee S.A., Hong S.S., Han X.H., Hwang J.S., Oh G.J., Lee K.S., Lee M.K., Hwang B.Y., Ro J.S. Piperine from the fruits of Piper longum with inhibitory effect on monoamine oxidase and antidepressant-like activity. Chem. Pharm. Bull. 2005;53:832–835. doi: 10.1248/cpb.53.832. [DOI] [PubMed] [Google Scholar]
- 70.Lee S.A., Hwang J.S., Han X.H., Lee C., Lee M.H., Choe S.G., Hong S.S., Lee D., Lee M.K., Hwang B.Y. Methylpiperate derivatives from Piper longum and their inhibition of monoamine oxidase. Arch. Pharm. Res. 2008;31:679–683. doi: 10.1007/s12272-001-1212-7. [DOI] [PubMed] [Google Scholar]
- 71.Mu L.H., Wang B., Ren H.Y., Liu P., Guo D.H., Wang F.M., Bai L., Guo Y.S. Synthesis and inhibitory effect of piperine derivates on monoamine oxidase. Bioorg. Med. Chem. Lett. 2012;22:3343–3348. doi: 10.1016/j.bmcl.2012.02.090. [DOI] [PubMed] [Google Scholar]
- 72.Ro J.S., Lee S.S., Lee K.S., Lee M.K. Inhibition of type A monoamine oxidase by coptisine in mouse brain. Life Sci. 2001;70:639–645. doi: 10.1016/S0024-3205(01)01437-0. [DOI] [PubMed] [Google Scholar]
- 73.Lee S.S., Lee M.K. Effects of natural isoquinoline alkaloids on monoamine oxidase activity in mouse brain: Inhibition by berberine and palmatine. Med. Sci. Res. 1999;27:749–751. [Google Scholar]
- 74.Lee S.J., Chung H.Y., Lee I.K., Oh S.U., Yoo I.D. Phenolics with Inhibitory Activity on Mouse Brain Monoamine Oxidase (MAO) from Whole Parts of Artemisia vulgaris L (Mugwort) Food Sci. Biotechnol. 2000;9:179–182. [Google Scholar]
- 75.Lee S.S., Yun-Choi H.S., Kim E.I., Lee M.K. Inhibition of monoamine oxidase by higenamine. Med. Sci. Res. 1999;27:71–72. [Google Scholar]
- 76.Plazas E., Hagenow S., Avila Murillo M., Stark H., Cuca L.E. Isoquinoline alkaloids from the roots of Zanthoxylum rigidum as multi-target inhibitors of cholinesterase, monoamine oxidase A and Abeta1-42 aggregation. Bioorg. Chem. 2020;98:103722. doi: 10.1016/j.bioorg.2020.103722. [DOI] [PubMed] [Google Scholar]
- 77.Yang L.J., Yang Z.D., Li Z.J., Yang S.H., Shu Z.M. Stephtetrandrine A-D, bisbenzylisoquinoline alkaloids from Stephania tetrandra. Nat. Prod. Res. 2021;5:1–12. doi: 10.1080/14786419.2021.1961135. [DOI] [PubMed] [Google Scholar]
- 78.Lee M.K., Hwang B.Y., Lee S.A., Oh G.J., Choi W.H., Hong S.S., Lee K.S., Ro J.S. 1-methyl-2-undecyl-4(1H)-quinolone as an irreversible and selective inhibitor of type B monoamine oxidase. Chem. Pharm. Bull. 2003;51:409–411. doi: 10.1248/cpb.51.409. [DOI] [PubMed] [Google Scholar]
- 79.Chen C.F., Chiou W.F., Chou C.J., Liao J.F., Lin L.C., Wang G.J., Ueng Y.F. Pharmacological effects of Evodia rutaecarpa and its bioactive components. Chin. Pharm. J. 2002;54:419–435. [Google Scholar]
- 80.Han X.H., Hong S.S., Lee D., Lee J.J., Lee M.S., Moon D.C., Han K., Oh K.W., Lee M.K., Ro J.S., et al. Quinolone alkaloids from evodiae fructus and their inhibitory effects on monoamine oxidase. Arch. Pharm. Res. 2007;30:397–401. doi: 10.1007/BF02980210. [DOI] [PubMed] [Google Scholar]
- 81.Mesiti F., Maruca A., Silva V., Rocca R., Fernandes C., Remiao F., Uriarte E., Alcaro S., Gaspar A., Borges F. 4-Oxoquinolines and monoamine oxidase: When tautomerism matters. Eur. J. Med. Chem. 2021;213:113183. doi: 10.1016/j.ejmech.2021.113183. [DOI] [PubMed] [Google Scholar]
- 82.Naidoo D., Roy A., Slavětínská L.P., Chukwujekwu J.C., Gupta S., Van Staden J. New role for crinamine as a potent, safe and selective inhibitor of human monoamine oxidase B: In vitro and in silico pharmacology and modeling. J. Ethnopharmacol. 2020;248:112305. doi: 10.1016/j.jep.2019.112305. [DOI] [PubMed] [Google Scholar]
- 83.Fernandez de Arriba A., Lizcano J.M., Balsa M.D., Unzeta M. Inhibition of monoamine oxidase from bovine retina by beta-carbolines. J. Pharm. Pharmacol. 1994;46:809–813. doi: 10.1111/j.2042-7158.1994.tb03735.x. [DOI] [PubMed] [Google Scholar]
- 84.Temple W., Mendelsohn L., Kim G.E., Nekritz E., Gustafson W.C., Lin L., Giacomini K., Naranjo A., Van Ryn C., Yanik G.A., et al. Vesicular monoamine transporter protein expression correlates with clinical features, tumor biology, and MIBG avidity in neuroblastoma: A report from the Children’s Oncology Group. Eur. J. Nucl. Med. Mol. Imaging. 2016;43:474–481. doi: 10.1007/s00259-015-3179-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Herraiz T., Chaparro C. Analysis of monoamine oxidase enzymatic activity by reversed-phase high performance liquid chromatography and inhibition by beta-carboline alkaloids occurring in foods and plants. J. Chromatogr. 2006;1120:237–243. doi: 10.1016/j.chroma.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 86.Herraiz T., González D., Ancín-Azpilicueta C., Arán V.J., Guillén H. Beta-Carboline alkaloids in Peganum harmala and inhibition of human monoamine oxidase (MAO) Arch. Pharm. Res. 2010;48:839–845. doi: 10.1016/j.fct.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 87.McKenna D.J., Towers G.N., Abbott F. Monoamine oxidase inhibitors in south American Hallucinogenic Plants Part 2: Constituents of orally active myristicaceous hallucinogens. J. Ethanopharmacol. 1984;12:179–211. doi: 10.1016/0378-8741(84)90048-5. [DOI] [PubMed] [Google Scholar]
- 88.Denis J.M., Towers G.H.N., Abbott F.S. Monoamine oxidase inhibitors in south American Hallucinogenic Plants: Tryptamine and beta-carboline constituents of Ayahuasca. J. Ethanopharmacol. 1984;10:195–223. doi: 10.1016/0378-8741(84)90003-5. [DOI] [PubMed] [Google Scholar]
- 89.Schwarz M.J., Houghton P.J., Rose S., Jenner P., Lees A.D. Activities of extract and constituents of Banisteriopsis caapi relevant to parkinsonism. Pharmacol. Biochem. Behav. 2003;75:627–633. doi: 10.1016/S0091-3057(03)00129-1. [DOI] [PubMed] [Google Scholar]
- 90.Hong S.S., Han X.H., Park S.Y., Choi W.H., Lee M.K., Hur J.D., Ro J.S. Monoamine Oxidase inhibitors from Uncaria rhynchophylla. Nat. Prod. Sci. 2005;11:145–149. [Google Scholar]
- 91.Reniers J., Robert S., Frederick R., Masereel B., Vincent S., Wouters J. Synthesis and evaluation of beta-carboline derivatives as potential monoamine oxidase inhibitors. Bioorg. Med. Chem. 2011;19:134–144. doi: 10.1016/j.bmc.2010.11.041. [DOI] [PubMed] [Google Scholar]
- 92.Calixto N.O., Pinto M.E.F., Ramalho S.D., Burger M., Bobey A.F., Young M.C.M., Pinto A.C. The genus Psychotria: Phytochemistry, chemotaxonomy, ethnopharmacology and biological properties. J. Braz. Chem. Soc. 2016;27:1355–1378. [Google Scholar]
- 93.Passos C.S., Simoes-Pires C.A., Nurisso A., Soldi T.C., Kato L., de Oliveira C.M., de Faria E.O., Marcourt L., Gottfried C., Carrupt P.A., et al. Indole alkaloids of Psychotria as multifunctional cholinesterases and monoamine oxidases inhibitors. Phytochemistry. 2013;86:8–20. doi: 10.1016/j.phytochem.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 94.Klein-Junior L.C., Cretton S., Vander Heyden Y., Gasper A.L., Nejad-Ebrahimi S., Christen P., Henriques A.T. Bioactive Azepine-Indole Alkaloids from Psychotria nemorosa. J. Nat. Prod. 2020;83:852–863. doi: 10.1021/acs.jnatprod.9b00469. [DOI] [PubMed] [Google Scholar]
- 95.Milan S. Neurodynamics of Prosocial Emotional Processing Following Serotonergic Stimulation with N,N-Dimethyltryptamine (DMT) and Harmine in Healthy Subjects. [(accessed on 29 April 2022)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04716335.
- 96.Zhi K.K., Yang Z.D., Shi D.F., Yao X.J., Wang M.G. Desmodeleganine, a new alkaloid from the leaves of Desmodium elegans as a potential monoamine oxidase inhibitor. Fitoterapia. 2014;98:160–165. doi: 10.1016/j.fitote.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 97.Kumar S., Pandey A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013;2013:162750. doi: 10.1155/2013/162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nijveldt R.J., van Nood E., van Hoorn D.E., Boelens P.G., van Norren K., van Leeuwen P.A. Flavonoids: A review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001;74:418–425. doi: 10.1093/ajcn/74.4.418. [DOI] [PubMed] [Google Scholar]
- 99.Dias M.C., Pinto D., Silva A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules. 2021;26:5377. doi: 10.3390/molecules26175377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Agati G., Brunetti C., Fini A., Gori A., Guidi L., Landi M., Sebastiani F., Tattini M. Are Flavonoids Effective Antioxidants in Plants? Twenty Years of Our Investigation. Antioxidants. 2020;9:1098. doi: 10.3390/antiox9111098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gogineni V., Nael M.A., Chaurasiya N.D., Elokely K.M., McCurdy C.R., Rimoldi J.M., Cutler S.J., Tekwani B.L., Leon F. Computationally Assisted Lead Optimization of Novel Potent and Selective MAO-B Inhibitors. Biomedicines. 2021;9:1304. doi: 10.3390/biomedicines9101304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Carradori S., Gidaro M.C., Petzer A., Costa G., Guglielmi P., Chimenti P., Alcaro S., Petzer J.P. Inhibition of Human Monoamine Oxidase: Biological and Molecular Modeling Studies on Selected Natural Flavonoids. J. Agric. Food Chem. 2016;64:9004–9011. doi: 10.1021/acs.jafc.6b03529. [DOI] [PubMed] [Google Scholar]
- 103.Wang M., Firrman J., Liu L., Yam K. A Review on Flavonoid Apigenin: Dietary Intake, ADME, Antimicrobial Effects, and Interactions with Human Gut Microbiota. Biomed. Res. Int. 2019;2019:7010467. doi: 10.1155/2019/7010467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Salehi B., Venditti A., Sharifi-Rad M., Kregiel D., Sharifi-Rad J., Durazzo A., Lucarini M., Santini A., Souto E.B., Novellino E., et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019;20:1305. doi: 10.3390/ijms20061305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chaurasiya N.D., Ibrahim M.A., Muhammad I., Walker L.A., Tekwani B.L. Monoamine oxidase inhibitory constituents of propolis: Kinetics and mechanism of inhibition of recombinant human MAO-A and MAO-B. Molecules. 2014;19:18936–18952. doi: 10.3390/molecules191118936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lopez-Lazaro M. Distribution and biological activities of the flavonoid luteolin. Mini Rev. Med. Chem. 2009;9:31–59. doi: 10.2174/138955709787001712. [DOI] [PubMed] [Google Scholar]
- 107.Park S.E., Paudel P., Wagle A., Seong S.H., Kim H.R., Fauzi F.M., Jung H.A., Choi J.S. Luteolin, a Potent Human Monoamine Oxidase-A Inhibitor and Dopamine D4 and Vasopressin V1A Receptor Antagonist. J. Agric. Food Chem. 2020;68:10719–10729. doi: 10.1021/acs.jafc.0c04502. [DOI] [PubMed] [Google Scholar]
- 108.Chaurasiya N.D., Gogineni V., Elokely K.M., Leon F., Nunez M.J., Klein M.L., Walker L.A., Cutler S.J., Tekwani B.L. Isolation of Acacetin from Calea urticifolia with Inhibitory Properties against Human Monoamine Oxidase-A and -B. J. Nat. Prod. 2016;79:2538–2544. doi: 10.1021/acs.jnatprod.6b00440. [DOI] [PubMed] [Google Scholar]
- 109.Chaurasiya N.D., Zhao J., Pandey P., Doerksen R.J., Muhammad I., Tekwani B.L. Selective Inhibition of Human Monoamine Oxidase B by Acacetin 7-Methyl Ether Isolated from Turnera diffusa (Damiana) Molecules. 2019;24:810. doi: 10.3390/molecules24040810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Larit F., Elokely K.M., Chaurasiya N.D., Benyahia S., Nael M.A., Leon F., Abu-Darwish M.S., Efferth T., Wang Y.H., Belouahem-Abed D., et al. Inhibition of human monoamine oxidase A and B by flavonoids isolated from two Algerian medicinal plants. Phytomedicine. 2018;40:27–36. doi: 10.1016/j.phymed.2017.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Baek S.C., Kang M.G., Park J.E., Lee J.P., Lee H., Ryu H.W., Kim H. Osthenol, a prenylated coumarin, as a monoamine oxidase A inhibitor with high selectivity. Bioorg. Med. Chem. Lett. 2019;29:839–843. doi: 10.1016/j.bmcl.2019.01.016. [DOI] [PubMed] [Google Scholar]
- 112.Turkmenoglu F.P., Baysal I., Ciftci-Yabanoglu S., Yelekci K., Temel H., Pasa S., Ezer N., Calis I., Ucar G. Flavonoids from Sideritis Species: Human Monoamine Oxidase (hMAO) Inhibitory Activities, Molecular Docking Studies and Crystal Structure of Xanthomicrol. Molecules. 2015;20:7454–7473. doi: 10.3390/molecules20057454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jiang N., Doseff A.I., Grotewold E. Flavones: From Biosynthesis to Health Benefits. Plants. 2016;5:27. doi: 10.3390/plants5020027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bello O.M., Ogbesejana A.B., Adetunji C.O., Oguntoye S.O. Flavonoids Isolated from Vitex grandifolia, an Underutilized Vegetable, Exert Monoamine A & B Inhibitory and Anti-inflammatory Effects and Their Structure-activity Relationship. Turk J. Pharm. Sci. 2019;16:437–443. doi: 10.4274/tjps.galenos.2018.46036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lee H.W., Ryu H.W., Baek S.C., Kang M.G., Park D., Han H.Y., An J.H., Oh S.R., Kim H. Potent inhibitions of monoamine oxidase A and B by acacetin and its 7-O-(6-O-malonylglucoside) derivative from Agastache rugosa. Int. J. Biol. Macromol. 2017;104:547–553. doi: 10.1016/j.ijbiomac.2017.06.076. [DOI] [PubMed] [Google Scholar]
- 116.Aherne S.A., O’Brien N.M. Dietary flavonols: Chemistry, food content, and metabolism. Nutrition. 2002;18:75–81. doi: 10.1016/S0899-9007(01)00695-5. [DOI] [PubMed] [Google Scholar]
- 117.Chimenti F., Cottiglia F., Bonsignore L., Casu L., Casu M., Floris C., Secci D., Bolasco A., Chimenti P., Granese A., et al. Quercetin as the active principle of Hypericum hircinum exerts a selective inhibitory activity against MAO-A: Extraction, biological analysis, and computational study. J. Nat. Prod. 2006;69:945–949. doi: 10.1021/np060015w. [DOI] [PubMed] [Google Scholar]
- 118.Gidaro M.C., Astorino C., Petzer A., Carradori S., Alcaro F., Costa G., Artese A., Rafele G., Russo F.M., Petzer J.P., et al. Kaempferol as Selective Human MAO-A Inhibitor: Analytical Detection in Calabrian Red Wines, Biological and Molecular Modeling Studies. J. Agric. Food Chem. 2016;64:1394–1400. doi: 10.1021/acs.jafc.5b06043. [DOI] [PubMed] [Google Scholar]
- 119.Novaroli L., Reist M., Favre E., Carotti A., Catto M., Carrupt P.A. Human recombinant monoamine oxidase B as reliable and efficient enzyme source for inhibitor screening. Bioorg. Med. Chem. 2005;13:6212–6217. doi: 10.1016/j.bmc.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 120.Baek S.C., Park M.H., Ryu H.W., Lee J.P., Kang M.G., Park D., Park C.M., Oh S.R., Kim H. Rhamnocitrin isolated from Prunus padus var. seoulensis: A potent and selective reversible inhibitor of human monoamine oxidase A. Bioorg. Chem. 2019;83:317–325. doi: 10.1016/j.bioorg.2018.10.051. [DOI] [PubMed] [Google Scholar]
- 121.Hwang J.S., Lee S.A., Hong S.S., Lee K.S., Lee M.K., Hwang B.Y., Ro J.S. Monoamine oxidase inhibitory components from the roots of Sophora flavescens. Arch. Pharm. Res. 2005;28:190–194. doi: 10.1007/BF02977714. [DOI] [PubMed] [Google Scholar]
- 122.Zarmouh N.O., Eyunni S.K., Soliman K.F. The Benzopyrone Biochanin-A as a reversible, competitive, and selective monoamine oxidase B inhibitor. BMC Compl. Altern Med. 2017;17:34. doi: 10.1186/s12906-016-1525-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Oh J.M., Lee C., Nam S.J., Kim H. Chromenone Derivatives as Monoamine Oxidase Inhibitors from Marine-Derived MAR4 Clade Streptomyces sp. CNQ-031. J. Microbiol. Biotechnol. 2021;31:1022–1027. doi: 10.4014/jmb.2105.05003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Prajapati R., Park S.E., Park H.J., Jung H.A., Choi J.S. Identification of a potent and selective human monoamine oxidase A inhibitor, glycitein, an isoflavone isolated from Pueraria lobata flowers. ACS Food Sci. Technol. 2021;1:538–550. doi: 10.1021/acsfoodscitech.0c00152. [DOI] [Google Scholar]
- 125.Mohamed E.I., Zaki M.A., Chaurasiya N.D., Owis A.I., AbouZid S., Wang Y.H., Avula B., Seida A.A., Tekwani B.L., Ross S.A. Monoamine oxidases inhibitors from Colvillea racemosa: Isolation, biological evaluation, and computational study. Fitoterapia. 2018;124:217–223. doi: 10.1016/j.fitote.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Minders C., Petzer J.P., Petzer A., Lourens A.C. Monoamine oxidase inhibitory activities of heterocyclic chalcones. Bioorg. Med. Chem. Lett. 2015;25:5270–5276. doi: 10.1016/j.bmcl.2015.09.049. [DOI] [PubMed] [Google Scholar]
- 127.Guglielmi P., Mathew B., Secci D., Carradori S. Chalcones: Unearthing their therapeutic possibility as monoamine oxidase B inhibitors. Eur. J. Med. Chem. 2020;205:112650. doi: 10.1016/j.ejmech.2020.112650. [DOI] [PubMed] [Google Scholar]
- 128.Hou W.C., Lin R.D., Chen C.T., Lee M.H. Monoamine oxidase B (MAO-B) inhibition by active principles from Uncaria rhynchophylla. J. Ethnopharmacol. 2005;100:216–220. doi: 10.1016/j.jep.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 129.Recalde-Gil M.A., Klein-Junior L.C., Passos C.D.S., Salton J., Bordignon S.A.L., Monace F.D., Cechinel V., Teresinha Henriquesa A. Monoamine Oxidase Inhibitory Activity of Biflavonoids from Branches of Garcinia gardneriana (Clusiaceae) Nat. Prod. Commun. 2017;12:505–508. doi: 10.1177/1934578X1701200411. [DOI] [PubMed] [Google Scholar]
- 130.Haraguchi H., Tanaka Y., Kabbash A., Fujioka T., Ishizu T., Yagi A. Monoamine oxidase inhibitors from Gentiana lutea. Phytochemistry. 2004;65:2255–2260. doi: 10.1016/j.phytochem.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 131.Prajapati R., Seong S.H., Park S.E., Paudel P., Jung H.A., Choi J.S. Isoliquiritigenin, a potent human monoamine oxidase inhibitor, modulates dopamine D1, D3, and vasopressin V1A receptors. Sci. Rep. 2021;11:23528. doi: 10.1038/s41598-021-02843-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kim J.H., Son Y.K., Kim G.H., Hwang K.H. Xanthoangelol and 4-Hydroxyderricin Are the Major Active Principles of the Inhibitory Activities against Monoamine Oxidases on Angelica keiskei K. Biomol. Ther. 2013;21:234–240. doi: 10.4062/biomolther.2012.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Baek S.C., Lee H.W., Ryu H.W., Kang M.G., Park D., Kim S.H., Cho M.L., Oh S.R., Kim H. Selective inhibition of monoamine oxidase A by hispidol. Bioorg. Med. Chem. Lett. 2018;28:584–588. doi: 10.1016/j.bmcl.2018.01.049. [DOI] [PubMed] [Google Scholar]
- 134.Stefanachi A., Leonetti F., Pisani L., Catto M., Carotti A. Coumarin: A Natural, Privileged and Versatile Scaffold for Bioactive Compounds. Molecules. 2018;23:250. doi: 10.3390/molecules23020250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sharifi-Rad J., Cruz-Martins N., Lopez-Jornet P., Lopez E.P., Harun N., Yeskaliyeva B., Beyatli A., Sytar O., Shaheen S., Sharopov F., et al. Natural Coumarins: Exploring the Pharmacological Complexity and Underlying Molecular Mechanisms. Oxid. Med. Cell Longev. 2021;2021:6492346. doi: 10.1155/2021/6492346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rauhamaki S., Postila P.A., Niinivehmas S., Kortet S., Schildt E., Pasanen M., Manivannan E., Ahinko M., Koskimies P., Nyberg N., et al. Structure-Activity Relationship Analysis of 3-Phenylcoumarin-Based Monoamine Oxidase B Inhibitors. Front. Chem. 2018;6:41. doi: 10.3389/fchem.2018.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Koyiparambath V.P., Prayaga Rajappan K., Rangarajan T.M., Al-Sehemi A.G., Pannipara M., Bhaskar V., Nair A.S., Sudevan S.T., Kumar S., Mathew B. Deciphering the detailed structure-activity relationship of coumarins as Monoamine oxidase enzyme inhibitors-An updated review. Chem. Biol. Drug. Des. 2021;98:655–673. doi: 10.1111/cbdd.13919. [DOI] [PubMed] [Google Scholar]
- 138.Hossain C.F., Okuyama E., Yamazaki M. A new series of coumarin derivatives having monoamine oxidase inhibitory activity from Monascus anka. Chem. Pharm. Bull. 1996;44:1535–1539. doi: 10.1248/cpb.44.1535. [DOI] [PubMed] [Google Scholar]
- 139.Huong D.T., Choi H.C., Rho T.C., Lee H.S., Lee M.K., Kim Y.H. Inhibitory activity of monoamine oxidase by coumarins from Peucedanum japonicum. Arch. Pharm. Res. 1999;22:324–326. doi: 10.1007/BF02976373. [DOI] [PubMed] [Google Scholar]
- 140.Yun B.S., Lee I.K., Ryoo I.J., Yoo I.D. Coumarins with monoamine oxidase inhibitory activity and antioxidative coumarino-lignans from Hibiscus syriacus. J. Nat. Prod. 2001;64:1238–1240. doi: 10.1021/np0100946. [DOI] [PubMed] [Google Scholar]
- 141.Carotti A., Carrieri A., Chimichi S., Boccalini M., Cosimelli B., Gnerre C., Carotti A., Carrupt P.A., Testa B. Natural and synthetic geiparvarins are strong and selective MAO-B inhibitors. Synthesis and SAR studies. Bioorg. Med. Chem. Lett. 2002;12:3551–3555. doi: 10.1016/S0960-894X(02)00798-9. [DOI] [PubMed] [Google Scholar]
- 142.Jeong S.H., Han X.H., Hong S.S., Hwang J.S., Hwang J.H., Lee D., Lee M.K., Ro J.S., Hwang B.Y. Monoamine oxidase inhibitory coumarins from the aerial parts of Dictamnus albus. Arch. Pharm. Res. 2006;29:1119–1124. doi: 10.1007/BF02969302. [DOI] [PubMed] [Google Scholar]
- 143.Lee H.W., Ryu H.W., Kang M.G., Park D., Lee H., Shin H.M., Oh S.R., Kim H. Potent inhibition of monoamine oxidase A by decursin from Angelica gigas Nakai and by wogonin from Scutellaria baicalensis Georgi. Int. J. Biol. Macromol. 2017;97:598–605. doi: 10.1016/j.ijbiomac.2017.01.080. [DOI] [PubMed] [Google Scholar]
- 144.Wang Y.S., Li B.T., Liu S.X., Wen Z.Q., Yang J.H., Zhang H.B., Hao X.J. Anisucoumaramide, a Bioactive Coumarin from Clausena anisum-olens. J. Nat. Prod. 2017;80:798–804. doi: 10.1021/acs.jnatprod.6b00391. [DOI] [PubMed] [Google Scholar]
- 145.Seong S.H., Ali M.Y., Jung H.A., Choi J.S. Umbelliferone derivatives exert neuroprotective effects by inhibiting monoamine oxidase A, self-amyloidbeta aggregation, and lipid peroxidation. Bioorg. Chem. 2019;92:103293. doi: 10.1016/j.bioorg.2019.103293. [DOI] [PubMed] [Google Scholar]
- 146.Farina R., Pisani L., Catto M., Nicolotti O., Gadaleta D., Denora N., Soto-Otero R., Mendez-Alvarez E., Passos C.S., Muncipinto G., et al. Structure-Based Design and Optimization of Multitarget-Directed 2H-Chromen-2-one Derivatives as Potent Inhibitors of Monoamine Oxidase B and Cholinesterases. J. Med. Chem. 2015;58:5561–5578. doi: 10.1021/acs.jmedchem.5b00599. [DOI] [PubMed] [Google Scholar]
- 147.Rehuman N.A., Oh J.M., Nath L.R., Khames A., Abdelgawad M.A., Gambacorta N., Nicolotti O., Jat R.K., Kim H., Mathew B. Halogenated Coumarin-Chalcones as Multifunctional Monoamine Oxidase-B and Butyrylcholinesterase Inhibitors. ACS Omega. 2021;6:28182–28193. doi: 10.1021/acsomega.1c04252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Perveen S., Al-Taweel A.M. Phenolic Compounds from the Natural Sources and Their Cytotoxicity. In: Soto-Hernandez M., Palma-Tenango M., Garcia-Mateos M.d.R., editors. Phenolic Compounds—Natural Sources, Importance and Applications. IntechOpen; London, UK: 2017. [Google Scholar]
- 149.Han Y.S., Van der Heijden R., Verpoorte R. Biosynthesis of anthraquinones in cell cultures of the Rubiaceae. Plant Cell Tissue Organ. Cult. 2001;67:201–220. doi: 10.1023/A:1012758922713. [DOI] [Google Scholar]
- 150.El-Awaad I., Bocola M., Beuerle T., Liu B., Beerhues L. Bifunctional CYP81AA proteins catalyse identical hydroxylations but alternative regioselective phenol couplings in plant xanthone biosynthesis. Nat. Commun. 2016;7:11472. doi: 10.1038/ncomms11472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Matsuda Y., Gotfredsen C.H., Larsen T.O. Genetic Characterization of Neosartorin Biosynthesis Provides Insight into Heterodimeric Natural Product Generation. Org. Lett. 2018;20:7197–7200. doi: 10.1021/acs.orglett.8b03123. [DOI] [PubMed] [Google Scholar]
- 152.Huang Q., Wang Y., Wu H., Yuan M., Zheng C., Xu H. Xanthone Glucosides: Isolation, Bioactivity and Synthesis. Molecules. 2021;26:5575. doi: 10.3390/molecules26185575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ruan J., Zheng C., Liu Y., Qu L., Yu H., Han L., Zhang Y., Wang T. Chemical and Biological Research on Herbal Medicines Rich in Xanthones. Molecules. 2017;22:1698. doi: 10.3390/molecules22101698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Suzuki O., Katsumata Y., Oya M., Chari V.M., Vermes B., Wagner H., Hostettmann K. Inhibition of type A and type B monoamine oxidases by naturally occurring xanthones. Planta Med. 1981;42:17–21. doi: 10.1055/s-2007-971540. [DOI] [PubMed] [Google Scholar]
- 155.Schaufelberger D., Hostettmann K. Chemistry and pharmacology of Gentiana lactea. Planta Med. 1988;54:219–221. doi: 10.1055/s-2006-962408. [DOI] [PubMed] [Google Scholar]
- 156.Rocha L., Marston A., Kaplan M.A., Stoeckli-Evans H., Thull U., Testa B., Hostettmann K. An antifungal gamma-pyrone and xanthones with monoamine oxidase inhibitory activity from Hypericum brasiliense. Phytochemistry. 1994;36:1381–1385. doi: 10.1016/S0031-9422(00)89727-7. [DOI] [PubMed] [Google Scholar]
- 157.Gnerre C., Thull U., Gaillard P., Carrupt P.A., Testa B., Fernandes E., Cruciani G. Natural and synthetic xanthones as monoamine oxidase inhibitors: Biological assay and 3D-QSAR. Helv. Chim. Acta. 2001;84:552–570. doi: 10.1002/1522-2675(20010321)84:3<552::AID-HLCA552>3.0.CO;2-X. [DOI] [Google Scholar]
- 158.Wolfender J.L., Hamburger M., Msonthi J.D., Hostettmann K. Xanthones from Chironia krebsii. Phytochemistry. 1991;30:3625–3629. doi: 10.1016/0031-9422(91)80080-K. [DOI] [Google Scholar]
- 159.Wairata J., Sukandar E.R., Fadlan A., Purnomo A.S., Taher M., Ersam T. Evaluation of the Antioxidant, Antidiabetic, and Antiplasmodial Activities of Xanthones Isolated from Garcinia forbesii and Their In Silico Studies. Biomedicines. 2021;9:1380. doi: 10.3390/biomedicines9101380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yang J.L., Liu L.L., Shi Y.P. Phytochemicals and biological activities of Gentiana species. Nat. Prod. Commun. 2010;5:649–664. doi: 10.1177/1934578X1000500432. [DOI] [PubMed] [Google Scholar]
- 161.Tomic M., Tovilovic G., Butorovic B., Krstic D., Jankovic T., Aljancic I., Menkovic N. Neuropharmacological evaluation of diethylether extract and xanthones of Gentiana kochiana. Pharmacol. Biochem. Behav. 2005;81:535–542. doi: 10.1016/j.pbb.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 162.Hwang J.H., Hong S.S., Han X.H., Hwang J.S., Lee D., Lee H., Yun Y.P., Kim Y., Ro J.S., Hwang B.Y. Prenylated xanthones from the root bark of Cudrania tricuspidata. J. Nat. Prod. 2007;70:1207–1209. doi: 10.1021/np070059k. [DOI] [PubMed] [Google Scholar]
- 163.Urbain A., Marston A., Grilo L.S., Bravo J., Purev O., Purevsuren B., Batsuren D., Reist M., Carrupt P.A., Hostettmann K. Xanthones from Gentianella amarella ssp. acuta with acetylcholinesterase and monoamine oxidase inhibitory activities. J. Nat. Prod. 2008;71:895–897. doi: 10.1021/np070690l. [DOI] [PubMed] [Google Scholar]
- 164.Dimitrov M., Nikolova I., Benbasat N., Kitanov G., Danchev N. Acute toxicity, antidepressive and MAO inhibitory ac-tivity of mangiferin isolated from Hypericum aucheri. Biothechnol. Biotechnol. Equip. 2011;25:2668–2671. doi: 10.5504/BBEQ.2011.0099. [DOI] [Google Scholar]
- 165.Yamazaki M., Satoh Y., Maebayashi Y., Horie Y. Monoamine oxidase inhibitors from a fungus, Emericella navahoensis. Chem. Pharm. Bull. 1988;36:670–675. doi: 10.1248/cpb.36.670. [DOI] [PubMed] [Google Scholar]
- 166.Andujar I., Recio M.C., Giner R.M., Rios J.L. Traditional chinese medicine remedy to jury: The pharmacological basis for the use of shikonin as an anticancer therapy. Curr. Med. Chem. 2013;20:2892–2898. doi: 10.2174/09298673113209990008. [DOI] [PubMed] [Google Scholar]
- 167.Choi W.H., Hong S.S., Lee S.A., Han X.H., Lee K.S., Lee M.K., Hwang B.Y., Ro J.S. Monoamine oxidase inhibitory naphthoquinones from the roots of Lithospermum erythrorhizon. Arch. Pharm. Res. 2005;28:400–404. doi: 10.1007/BF02977668. [DOI] [PubMed] [Google Scholar]
- 168.Khalil A.A., Steyn S., Castagnoli N., Jr. Isolation and characterization of a monoamine oxidase inhibitor from tobacco leaves. Chem. Res. Toxicol. 2000;13:31–35. doi: 10.1021/tx990146f. [DOI] [PubMed] [Google Scholar]
- 169.Jaka O., Iturria I., van der Toorn M., Hurtado de Mendoza J., Latino D., Alzualde A., Peitsch M.C., Hoeng J., Koshibu K. Effects of Natural Monoamine Oxidase Inhibitors on Anxiety-Like Behavior in Zebrafish. Front. Pharmacol. 2021;12:669370. doi: 10.3389/fphar.2021.669370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Coelho Cerqueira E., Netz P.A., Diniz C., Petry do Canto V., Follmer C. Molecular insights into human monoamine oxidase (MAO) inhibition by 1,4-naphthoquinone: Evidences for menadione (vitamin K3) acting as a competitive and reversible inhibitor of MAO. Bioorg. Med. Chem. 2011;19:7416–7424. doi: 10.1016/j.bmc.2011.10.049. [DOI] [PubMed] [Google Scholar]
- 171.Mostert S., Petzer A., Petzer J.P. Evaluation of Natural and Synthetic 1,4-naphthoquinones as Inhibitors of Monoamine Oxidase. Chem. Biol. Drug Des. 2016;87:737–746. doi: 10.1111/cbdd.12708. [DOI] [PubMed] [Google Scholar]
- 172.Lee H.W., Ryu H.W., Kang M.G., Park D., Oh S.R., Kim H. Selective inhibition of monoamine oxidase A by purpurin, an anthraquinone. Bioorg. Med. Chem. Lett. 2017;27:1136–1140. doi: 10.1016/j.bmcl.2017.01.085. [DOI] [PubMed] [Google Scholar]
- 173.Paudel P., Seong S.H., Shrestha S., Jung H.A., Choi J.S. In Vitro and in Silico Human Monoamine Oxidase Inhibitory Potential of Anthraquinones, Naphthopyrones, and Naphthalenic Lactones from Cassia obtusifolia Linn Seeds. ACS Omega. 2019;4:16139–16152. doi: 10.1021/acsomega.9b02328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Tohge T., Watanabe M., Hoefgen R., Fernie A.R. Shikimate and phenylalanine biosynthesis in the green lineage. Front. Plant Sci. 2013;4:62. doi: 10.3389/fpls.2013.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Dhiman P., Malik N., Khatkar A. Hybrid caffeic acid derivatives as monoamine oxidases inhibitors: Synthesis, radical scavenging activity, molecular docking studies and in silico ADMET analysis. Chem. Cent. J. 2018;12:112. doi: 10.1186/s13065-018-0481-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mei Y., Pan D., Jiang Y., Zhang W., Yao X., Dai Y., Yu Y., Yao X. Target discovery of chlorogenic acid derivatives from the flower buds of Lonicera macranthoides and their MAO B inhibitory mechanism. Fitoterapia. 2019;134:297–304. doi: 10.1016/j.fitote.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 177.Wu G.F., Jiang X.L., Gong Y.Z., Hu Y.D., Bai X.L., Liao X. Ligand fishing of anti-neurodegenerative components from Lonicera japonica using magnetic nanoparticles immobilised with monoamine oxidase B. J. Sep. Sci. 2019;42:1289–1298. doi: 10.1002/jssc.201801255. [DOI] [PubMed] [Google Scholar]
- 178.Xu X.Y., Meng X., Li S., Gan R.Y., Li Y., Li H.B. Bioactivity, Health Benefits, and Related Molecular Mechanisms of Curcumin: Current Progress, Challenges, and Perspectives. Nutrients. 2018;10:1553. doi: 10.3390/nu10101553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Rajeswari A., Sabesan M. Inhibition of monoamine oxidase-B by the polyphenolic compound, curcumin and its metabolite tetrahydrocurcumin, in a model of Parkinson’s disease induced by MPTP neurodegeneration in mice. Inflammopharmacology. 2008;16:96–99. doi: 10.1007/s10787-007-1614-0. [DOI] [PubMed] [Google Scholar]
- 180.Baek S.C., Choi B., Nam S.J., Kim H. Inhibition of monoamine oxidase A and B by demethoxycurcumin and bisde-methoxycurcumin. J. Appl. Biol. Chem. 2018;61:187–190. doi: 10.3839/jabc.2018.027. [DOI] [Google Scholar]
- 181.Arvizu-Espinosa M.G., von Poser G.L., Henriques A.T., Mendoza-Ruiz A., Cardador-Martinez A., Gesto-Borroto R., Nunez-Aragon P.N., Villarreal-Ortega M.L., Sharma A., Cardoso-Taketa A. Bioactive Dimeric Acylphloroglucinols from the Mexican Fern Elaphoglossum paleaceum. J. Nat. Prod. 2019;82:785–791. doi: 10.1021/acs.jnatprod.8b00677. [DOI] [PubMed] [Google Scholar]
- 182.Perveen S. In: Introductory Chapter: Terpenes and Terpenoids in Terpenes and Terpenoids. Perveen S., Al-Taweel A., editors. IntechOpen; London, UK: 2018. p. 79683. [Google Scholar]
- 183.Dittmann K., Gerhauser C., Klimo K., Hamburger M. HPLC-based activity profiling of Salvia miltiorrhiza for MAO A and iNOS inhibitory activities. Planta Med. 2004;70:909–913. doi: 10.1055/s-2004-832615. [DOI] [PubMed] [Google Scholar]
- 184.Subaraja M., Vanisree A.J. The novel phytocomponent asiaticoside-D isolated from Centella asiatica exhibits monoamine oxidase-B inhibiting potential in the rotenone degenerated cerebral ganglions of Lumbricus terrestris. Phytomedicine. 2019;58:152833. doi: 10.1016/j.phymed.2019.152833. [DOI] [PubMed] [Google Scholar]
- 185.Gaston R., Jr., Geldenhuys W.J., Dudley G.B. Synthesis of Illudinine from Dimedone and Identification of Activity as a Monoamine Oxidase Inhibitor. J. Org. Chem. 2020;85:13429–13437. doi: 10.1021/acs.joc.0c01301. [DOI] [PubMed] [Google Scholar]
- 186.Kukula-Koch W., Koch W., Czernicka L., Glowniak K., Asakawa Y., Umeyama A., Marzec Z., Kuzuhara T. MAO-A Inhibitory Potential of Terpene Constituents from Ginger Rhizomes-A Bioactivity Guided Fractionation. Molecules. 2018;23:1301. doi: 10.3390/molecules23061301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Jimenez C. Marine Natural Products in Medicinal Chemistry. ACS Med. Chem. Lett. 2018;9:959–961. doi: 10.1021/acsmedchemlett.8b00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lu W.Y., Li H.J., Li Q.Y., Wu Y.C. Application of marine natural products in drug research. Bioorg. Med. Chem. 2021;35:116058. doi: 10.1016/j.bmc.2021.116058. [DOI] [PubMed] [Google Scholar]
- 189.Hong A., Tu L.C., Yang I., Lim K.M., Nam S.J. Marine natural products with monoamine oxidase (MAO) inhibitory activity. Pharm. Biol. 2020;58:716–720. doi: 10.1080/13880209.2020.1790618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Jung H.A., Roy A., Jung J.H., Choi J.S. Evaluation of the inhibitory effects of eckol and dieckol isolated from edible brown alga Eisenia bicyclis on human monoamine oxidases A and B. Arch. Pharm. Res. 2017;40:480–491. doi: 10.1007/s12272-017-0904-3. [DOI] [PubMed] [Google Scholar]
- 191.Seong S.H., Paudel P., Choi J.W., Ahn D.H., Nam T.J., Jung H.A., Choi J.S. Probing Multi-Target Action of Phlorotannins as New Monoamine Oxidase Inhibitors and Dopaminergic Receptor Modulators with the Potential for Treatment of Neuronal Disorders. Mar. Drugs. 2019;17:377. doi: 10.3390/md17060377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Paudel P., Park S.E., Seong S.H., Jung H.A., Choi J.S. Bromophenols from Symphyocladia latiuscula Target Human Monoamine Oxidase and Dopaminergic Receptors for the Management of Neurodegenerative Diseases. J. Agric. Food Chem. 2020;68:2426–2436. doi: 10.1021/acs.jafc.0c00007. [DOI] [PubMed] [Google Scholar]
- 193.Mikell J.R., Schaneberg B.T., Khan I.A. Isolation and purification of Kava lactones by high performance centrifugal partition chromatography. J. Liq. Chromatogr. Relat. Technol. 2003;26:3069–3074. doi: 10.1081/JLC-120025423. [DOI] [Google Scholar]
- 194.Aporosa S.A. De-mythologizing and re-branding of kava as the new world drug of choice. Drug Sci. Pol. Law. 2019;5:1–13. doi: 10.1177/2050324519876131. [DOI] [Google Scholar]
- 195.Prinsloo D., van Dyk S., Petzer A., Petzer J.P. Monoamine Oxidase Inhibition by Kavalactones from Kava (Piper Methysticum) Planta Med. 2019;85:1136–1142. doi: 10.1055/a-1008-9491. [DOI] [PubMed] [Google Scholar]
- 196.Jeong G.S., Lee E.Y., Kang M.G., Nam S.J., Park D., Kim H. (S)-5-Methymellein isolated from an endogenous lichen fungus Rosellinia corticium as a potent inhibitor of human monoamine oxidase A. Processes. 2022;10:166. doi: 10.3390/pr10010166. [DOI] [Google Scholar]
- 197.Huang C., Xiong J., Guan H.D., Wang C.H., Lei X., Hu J.F. Discovery, synthesis, biological evaluation and molecular docking study of (R)-5-methylmellein and its analogs as selective monoamine oxidase A inhibitors. Bioorg. Med. Chem. 2019;27:2027–2040. doi: 10.1016/j.bmc.2019.03.060. [DOI] [PubMed] [Google Scholar]
- 198.Jeong G.S., Hillman P.F., Kang M.G., Hwang S., Park J.E., Nam S.J., Park D., Kim H. Potent and Selective Inhibitors of Human Monoamine Oxidase A from an Endogenous Lichen Fungus Diaporthe mahothocarpus. J. Fungi. 2021;7:876. doi: 10.3390/jof7100876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Pandey P., Chaurasiya N.D., Tekwani B.L., Doerksen R.J. Interactions of endocannabinoid virodhamine and related analogs with human monoamine oxidase-A and -B. Biochem. Pharmacol. 2018;155:82–91. doi: 10.1016/j.bcp.2018.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Fitzgerald J.C., Ugun-Klusek A., Allen G., De Girolamo L.A., Hargreaves I., Ufer C., Abramov A.Y., Billett E.E. Monoamine oxidase-A knockdown in human neuroblastoma cells reveals protection against mitochondrial toxins. FASEB J. 2014;28:218–229. doi: 10.1096/fj.13-235481. [DOI] [PubMed] [Google Scholar]
- 201.Wang X., Li B., Kim Y.J., Wang Y.C., Li Z., Yu J., Zeng S., Ma X., Choi I.Y., Di Biase S., et al. Targeting monoamine oxidase A for T cell-based cancer immunotherapy. Sci. Immunol. 2021;6:2383. doi: 10.1126/sciimmunol.abh2383. [DOI] [PubMed] [Google Scholar]
- 202.Aljanabi R., Alsous L., Sabbah D.A., Gul H.I., Gul M., Bardaweel S.K. Monoamine Oxidase (MAO) as a Potential Target for Anticancer Drug Design and Development. Molecules. 2021;26:6019. doi: 10.3390/molecules26196019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Romero M., Kapur G., Baracco R., Valentini R.P., Mattoo T.K., Jain A. Treatment of Hypertension in Children With Catecholamine-Secreting Tumors: A Systematic Approach. J. Clin. Hypertens. 2015;17:720–725. doi: 10.1111/jch.12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Shinohara M., Shitara T., Hatakeyama S.I., Suzuki N., Maruyama K., Kobayashi T., Tsuchida Y. An infant with systemic hypertension, renal artery stenosis, and neuroblastoma. J. Pediatr. Surg. 2004;39:103–106. doi: 10.1016/j.jpedsurg.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 205.Moon S.B. Childhood neuroblastoma masquerading as pheochromocytoma: Case report. Int. Med. Case Rep. J. 2016;9:65–67. doi: 10.2147/IMCRJ.S100479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Harding M., Deyell R.J., Blydt-Hansen T. Catecholamines in neuroblastoma: Driver of hypertension, or solely a marker of disease? Cancer Rep. 2021:e1569. doi: 10.1002/cnr2.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Laug W.E., Siegel S.E., Shaw K.N., Landing B., Baptista J., Gutenstein M. Initial urinary catecholamine metabolite concentrations and prognosis in neuroblastoma. Pediatrics. 1978;62:77–83. doi: 10.1542/peds.62.1.77. [DOI] [PubMed] [Google Scholar]
- 208.Liu Y.L., Cheng A.T., Chen H.R., Hsu Y.P. Simultaneous HPLC of twelve monoamines and metabolites shows neuroblastoma cell line releases HVA and HIAA. Biomed. Chromatogr. 2000;14:544–548. doi: 10.1002/1099-0801(200012)14:8<544::AID-BMC46>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 209.Ugun-Klusek A., Theodosi T.S., Fitzgerald J.C., Burte F., Ufer C., Boocock D.J., Yu-Wai-Man P., Bedford L., Billett E.E. Monoamine oxidase-A promotes protective autophagy in human SH-SY5Y neuroblastoma cells through Bcl-2 phosphorylation. Redox. Biol. 2019;20:167–181. doi: 10.1016/j.redox.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Igosheva N., Lorz C., O’Conner E., Glover V., Mehmet H. Isatin, an endogenous monoamine oxidase inhibitor, triggers a dose- and time-dependent switch from apoptosis to necrosis in human neuroblastoma cells. Neurochem. Int. 2005;47:216–224. doi: 10.1016/j.neuint.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 211.Matthay K.K., Maris J.M., Schleiermacher G., Nakagawara A., Mackall C.L., Diller L., Weiss W.A. Neuroblastoma. Nat. Rev. Dis. Primers. 2016;2:16078. doi: 10.1038/nrdp.2016.78. [DOI] [PubMed] [Google Scholar]
- 212.Himpel S., Panzer P., Eirmbter K., Czajkowska H., Sayed M., Packman L.C., Blundell T., Kentrup H., Grotzinger J., Joost H.G., et al. Identification of the autophosphorylation sites and characterization of their effects in the protein kinase DYRK1A. Biochem. J. 2001;359:497–505. doi: 10.1042/bj3590497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Uhl K.L., Schultz C.R., Geerts D., Bachmann A.S. Harmine, a dual-specificity tyrosine phosphorylation-regulated kinase (DYRK) inhibitor induces caspase-mediated apoptosis in neuroblastoma. Cancer Cell Int. 2018;18:82. doi: 10.1186/s12935-018-0574-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Gockler N., Jofre G., Papadopoulos C., Soppa U., Tejedor F.J., Becker W. Harmine specifically inhibits protein kinase DYRK1A and interferes with neurite formation. FEBS J. 2009;276:6324–6337. doi: 10.1111/j.1742-4658.2009.07346.x. [DOI] [PubMed] [Google Scholar]
- 215.Uezono T., Maruyama W., Matsubara K., Naoi M., Shimizu K., Saito O., Ogawa K., Mizukami H., Hayase N., Shiono H. Norharman, an indoleamine-derived beta-carboline, but not Trp-P-2, a gamma-carboline, induces apoptotic cell death in human neuroblastoma SH-SY5Y cells. J. Neural Transm. 2001;108:943–953. doi: 10.1007/s007020170014. [DOI] [PubMed] [Google Scholar]
- 216.Naveen C.R., Gaikwad S., Agrawal-Rajput R. Berberine induces neuronal differentiation through inhibition of cancer stemness and epithelial-mesenchymal transition in neuroblastoma cells. Phytomedicine. 2016;23:736–744. doi: 10.1016/j.phymed.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 217.Calvani M., Subbiani A., Bruno G., Favre C. Beta-Blockers and Berberine: A Possible Dual Approach to Contrast Neuroblastoma Growth and Progression. Oxid. Med. Cell Longev. 2020;7:534–693. doi: 10.1155/2020/7534693. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No experimental data are reported in the review manuscript.