Abstract
Alginate is a polysaccharide with the property of forming hydrogels, which is economic production, zero toxicity, and biocompatibility. In the agro-industry, alginate is used as a super absorbent polymer, coating seeds, fruits, and vegetables and as a carrier of bacteria and fungi as plant-growth promoters and biocontrol. The latter has a high impact on agriculture since the implementation of microorganisms in a polymer matrix improves soil quality; plant nutrition, and is functional as a preventive measure for the appearance of phytopathogenic. Additionally, it minimizes losses of foods due to wrong post-harvest handling. In this review, we provide an overview of physicochemical properties of alginate, some methods for preparation and modification of capsules and coatings, to finally describe its application in agro-industry as a matrix of plant-growth-promoting microorganisms, its effectiveness in cultivation and post-harvest, and its effect on the environment, as well as the prospects for future agro-industrial applications.
Keywords: sodium alginate, coating, encapsulation, microorganisms, biocontrol, plant-growth promotion
1. Introduction
Sodium alginate is a very versatile polysaccharide with many applications due to its high-tech functionality, it is economical to produce, and it can be obtained in bulk; it is not toxic, it has continuos quality, it is practically sterile, and it is biocompatible with microorganisms [1]. It is currently functional in the food, textile, agrotechnological, biomedical, and pharmaceutical industries since it can be easily modified through chemical and physical reactions to create matrices such as hydrogels, microspheres, microcapsules, sponges, and fibers [2].
The use of microorganisms arises as an alternative to replace the use of agrochemicals partially or totally [3], and they have been supplied in the form of inoculum, in the soil, or directly in the plant, these are preparation of beneficial microorganisms, phytostimulants, fertilizers, and biocontrol agents, without a matrix that protects them bride [4]. However, the results obtained are not as expected since these microorganisms can be negatively affected by the competition of native microorganisms or by unfavorable environmental, physical, and chemical conditions [5].
Therefore, it is relevant to implement a matrix, either in the form of spheres or film, that provides optimal conditions to improve the life of microorganisms and improve their application, to obtain the desired benefits [6], considering the economic competitiveness based on general operating costs of the formulation [7].
The purpose of this review is to know the characteristics of alginate, its applications in agriculture, its benefits, and its possible deficiencies to improve how beneficial microorganisms are applied in the soil and to provide innovative technology solutions that allow obtaining the desired results in the field.
This review article contains specific information on the use of alginate in agroindustrial processes, the different methods to obtain alginate products such as films, coatings, matrices, hydrogels, capsules, and others that contain active agents of interest to be used as fertilizers, biological controls, growth promoters, plant health preservatives, among others. The differentiator of this review is the concept of exploring a single specific compound for various processes related to agroindustrial, from plant growth and health situations to methods to extend and preserve the shelf life of fresh products.
2. Alginate Overview
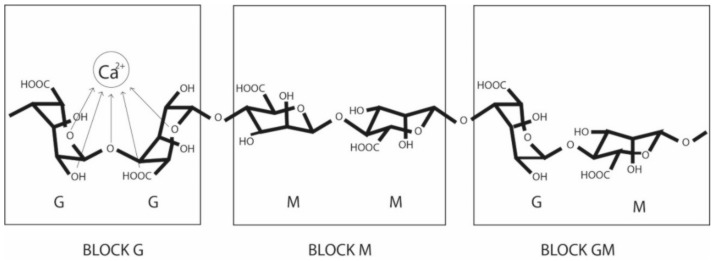
Sodium alginate is a polysaccharide obtained from marine algae and bacteria. The chemical structure is a copolymer of blocks, which are formed from β-D-manuronic acid (M) and α-L-guluronic acid (G) linked through 1,4-glucosidic bonds (Figure 1). Its structure is heteropolymeric, that is, a combination of manuronic (M)/guluronic (G) residues, and its sequence varies according to the source from which it is obtained [8,9]. The composition, extension, and molecular weight of the sequences establish the physical properties of the alginate [10].
Figure 1.
Chemical structure of alginate differentiating G-blocks and M-blocks. The selective binding of G-block with divalent cations such as calcium is represented, which produces the formation of a hydrogel.
The main characteristic of sodium alginate is gelation with calcium ions [11]; this characteristic is responsible for its wide use in the food industry since it can be used as a thickener [12], stabilizer, and binder [13,14].
Sodium alginate has properties that allow the use of this compound in food and products, such as the capacity for water retention, gelling, as a thickener, and forming capsules and films [14]. In addition, its consumption does not present toxicity, and it is a biocompatible and human-degradable compound [15].
Together, the physical and chemical properties of the alginate determine the base of the product to be manufactured and the application method for a specific sector. As previously mentioned, the proportion of the MM, GG, and MG blocks influences the behavior of the material. A high G content provides a high gelling capacity; however, the higher the M content, the higher the viscosity. This implies that the interactions between M/G are decisive in the behavior of the gel obtained, a high ratio causes greater elasticity, while a low ratio generates weak products [16].
The strength of the interaction is another factor that is modified by the M/G ratio. This property determines the mechanical behavior of the resulting gel through the tensile strength, the breaking point, and the viscoelasticity. In addition, there are properties such as solubility, pH, and particle size that depend directly on the type of algae from which the alginate is extracted [10].
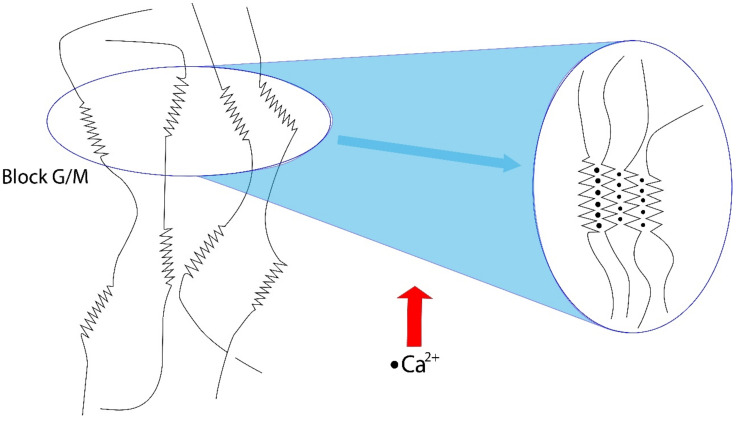
Sodium alginate can form a hydrogel, which presents a cross-linked three-dimensional network of hydrophilic polymers with a high quantity of water by ionic crosslinking and covalent crosslinking. Ionic crosslinking is the most common method to form a hydrogel and is carried out from an aqueous alginate solution with divalent cations, mainly Ca2+ (Figure 2) [17].
Figure 2.
Alginate egg box fix in the presence of Ca2+ ions.
Homogeneous alginate beads are formed by the controlled introduction of divalent crosslinking cations and can be carried out by two methods: diffusion or internal configuration. The diffusion method consists of dripping a solution of sodium alginate into an aqueous solution of CaCl2. This process has fast kinetics and a non-homogeneous distribution of alginate, high concentration on the surface, and gradually decreases to the center of the pearl of the gel formed [18]. In the internal configuration method, the cation source is found inside the alginate solution, and its release is controlled by the pH or solubility of the cation source, and the gradual release allows the formation of gels with a homogeneous concentration of cations [19].
The main applications of alginate capsules are biomedical and pharmaceutical: to make a slow and controlled release of drugs and enzymes [20,21]; in the food industry: to microencapsulate probiotics, prebiotics [22], nutrients [23], as well as microorganisms that benefit the intestinal flora [24]; and in agriculture, for the treatment of wastewater, adsorption of heavy metals [25], for the encapsulation of bioactive substances [26], eliminate some organic pollutants [27], encapsulation of compounds to remove pathogenic bacteria [28] and to encapsulate bacteria beneficial for plant growth [29].
3. Formation of Alginate Coatings and Films
Sodium alginate is used as a coating, which is defined as a layer of material to maintain quality, reduce deterioration processes by microorganisms, as well as extend the shelf life of food [30]. The generation of an edible film or coating can be obtained by immersion or by spraying, by different methods such as solvent removal, microfluidization, thermal gelation, lipid solidification, casting, and electrospraying. The first technique consists of a solution with the additives, where the solvent is evaporated from the solution, resulting in a plasticizing layer of material on the food or product. Microfluidization is a process where microchannels are generated to form a network of nanoparticles that form the coating, improving the physicochemical characteristics of the food or product [31,32].
Another method is thermal gelation, which is a simple and inexpensive process, where a protein compound is subjected to heat treatment to form a structured gel as a covering for a food or product [33]. Lipid solidification is a technique for making films and coatings that consists of a mixture of components, including an oil where layers of the material are formed by immersion, which solidifies with each immersion in the mixture of components [34]. Among these methods, there is also casting; in this process it is necessary to have a previously formed layer with some method of immersion or spraying, to form an elastic and plastic layer around the food or product under controlled conditions of humidity and temperature to achieve a continuous and complete thermoforming [35].
One of the most widely used methods for its high efficiency, low cost, and versatility in the film-forming elements and coatings is electrospraying; this process has the fundamentals of spray formation. However, electromagnetism is used for training when the attraction of the solution begins between two magnetic poles. The application of a potential difference is the outside by which a continuous wire is generated while the solvent evaporates; there are variants of this technique according to the desired result so they can be capsules or fibers of different sizes from millimetric to nanometric [36,37].
Benefits of Alginate Coatings and Films
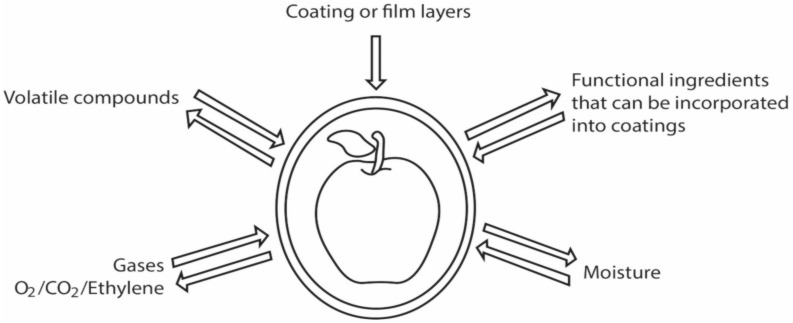
A coating or film of sodium alginate has the particularity of being a semi-permeable layer that reduces the loss of water and solutes, in addition to keeping O2 and CO2 gases in balance controlling biochemical changes, and delays organoleptic damage such as sight and texture [38]. In addition to these characteristics as shown in Figure 3, this type of coating or film must cover some quality aspects such as versatility and ease of processing, compliance with the sensory qualities by not being noticeable in consumption, improve physicochemical properties of the feed, and regulate conditions through the addition of additives [39].
Figure 3.
Types of transfers controlled by edible barriers in food.
The properties of the coatings or films can be divided into two parameters to guarantee their quality and performance. The first is the permeability to water vapor, which can predict water loss; this parameter can be modified according to the composition of the coating, the interaction with the food or product, the thickness, and the flexibility of the layers. Although these last two attributes are related because the higher the thickness of the layer, the lower the flexibility. However, the thicker the coating, the greater the permeability to water vapor [40].
The mechanism of water transfer between the environment and the humidity of the food is carried out in three stages: the first stage is the condensation of the vapor that is stored in the film; the second stage is the concentration of water by activity or effect, and the third stage is evaporation or transfers to the other side of the barrier. What involves a process of equilibrium between water and the film or coating material, this balance is the adsorption and desorption mechanisms [41].
The second parameter is the mechanical properties of the coating or the film, among them are tensile strength, flexibility, stiffness, and compaction force. These properties are mainly influenced by the viscosity of the coating suspension. This is due to changes at a structural level in the composition of the alginates [30,42].
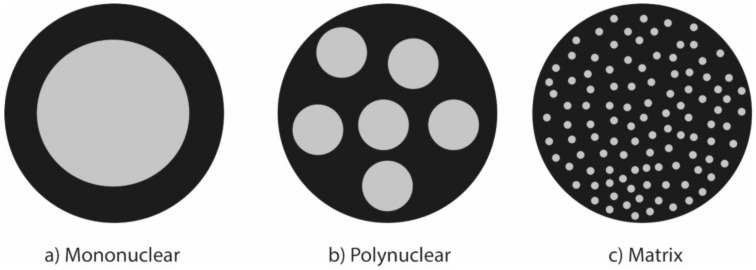
The resulting forms of a covering are shown in Figure 4, which will depend to a great extent on the relationship between the materials, which are the core and the wall, both materials must be compatible to guarantee the viability of the covering. The nature of the core or internal material determines the use of the resulting product [43].
Figure 4.
Structures resulting from a coating. (a) mononuclear: a single cluster of material within the capsule; (b) polynuclear: different large cluster of material within the capsule; (c) matrix: small centers or dispersed material within the capsules.
4. Use of Alginate in Agro-Industry
Alginate has had different applications in agriculture in recent decades: as a matrix of microbial formulations to improve plant productivity [44], for wastewater treatments [45], as a polymer super absorbent with eliciting properties [46,47], seed coating to increase germination [15], fruit and vegetable coating to increase post-harvest quality [48], a formulation for controlled release of agrochemicals [49] and carriers of fungi and bacteria, promoters of plant growth and antagonists for biological control [7,50,51].
The use of alginate as a microbial carrier arose because conventional solid or liquid formulations have a relatively short shelf life, and the transport and storage costs are very high, without ensuring the conservation of the microbial strains [52]. The encapsulation of plant growth-promoting bacteria in polymers such as alginate is a proposed alternative in the last 30 years. Since they ensure protection and a controlled and gradual release of the inoculant in the soil, they improve the growth of plants, they have good adhesion to seeds, the material is biodegradable in the soil, and they have no toxicity. The capsules can be dried and transported easily [53]. Additionally, with a suitable method of industrial microencapsulation, it is possible to maximize yield and produce alginate-based inoculants that may be suitable for agricultural application [29]. In addition to encapsulation, microorganisms can be applied in the form of a coating, mainly antimicrobial, when the confrontation occurs on the surface of the product or food [32].
4.1. Biocontrol Effect through the Mechanisms of Action in Coatings
The mechanism of action of a coating can be divided into two ways: the first is the partial or total transfer of antimicrobial agents to the surface of the product or food; however, when the antimicrobial effect occurs directly on the surface or in the layers of the coating, constitutes the second form [32].
The use of sodium alginate as a coating has been used against the effect of pathogens. The encapsulation of microorganisms is an effective measure of biological control [54]. The effectiveness of fungi is due to different factors typical of their biologies, such as their life cycle, the effect on specific organisms, the production of spores, or the antagonistic activity of other fungi [55], while some bacteria produce metabolites that work for the same purpose [56].
The mechanisms for biological control by microorganisms are antibiosis, antagonism, and mycoparasitism. Table 1 shows the organisms that have been used in the study of biological control. Where each type of mechanism is used, and the type of pathogen is treated are shown.
Table 1.
Microorganisms used for the biocontrol of pathogens.
| Microorganism (Antagonistic) | Pathogen or Causative Agent | Host | Mechanism of Action | Reference |
|---|---|---|---|---|
|
Aureimonas altamirensis
Bacillus toyonensis Herbaspirillum huttiense Micronospora marítima |
Phytophthora nicotianae | Pineapple | Antagonism | [57] |
| Bacillus amyloliquefaciens | Burkholderia glumae | Rice | Antibiosis | [58] |
| Bacillus amyloliquefaciens | Penicillium italicum | Lime | Antagonism | [14] |
| Bacillus subtilis | Penicillium digitatum | Orange | ||
| Cryptococcus diffluens | Fusarium oxysporum | Chilli pepper | ||
| Debaryomyces hansenii | Alternaria solani | Tomato | ||
| Rhodotorula minuta | Neoscytalidium dimidiatum | Fig tree | ||
| Stenotrophomonas rhizophila | Colletotrichum gloeosporioides | Papaya | ||
| Alternaria alternata | Basil | |||
| Fusarium solani | Chickpea | |||
| Curvularia sp. | Palm | |||
| Bacillus aryabhattai | Burkholderia glumae | Rice | Antibiosis | [59] |
| Burkholderia vietnamiensis | ||||
| Bacillus foraminis | Alternaria alternata | Tomato | Antagonism | [60] |
| Bacillus subtilis | Corynespora cassiicola | |||
| Bacillus thuringiensis | Stemphylium lycopersici | |||
| Bacillus thioparans | ||||
| Micrococcus yunnanensis | ||||
| Paenibacillus polymyxa | ||||
| Bacillus subtilis | Pythium aphanidermatum | Cucumber | Mycoparasitism | [61] |
| Trichoderma asperellum | ||||
| Trichoderma fertile | ||||
| Beauveria bassiana | Diaphorina citri | Citrus | Mycoparasitism | [62] |
| Hirsutella citriformis | ||||
| Isaria javanica | ||||
| Simplicillium lanosoniveum | ||||
| Rhodotorula mucilaginosa | Botrytis cinérea | Roses | Antagonism | [63] |
| Trichoderma asperellum | Phytophthora capsici | In vitro | Non-volatile metabolites | [64] |
| Trichoderma hamatum | ||||
| Trichoderma harzianum | Zymoseptoria tritici | Bean | Mycoparasitism | [65] |
Antibiosis is a process by which an organism generates toxic substances for another organism without direct contact between them. When the plant pathogen is a fungus, the effects produced include inhibition in sporulation, mycelial growth, and delayed activation of conidia [66]. At the cellular level, antibiosis involves a breakdown in the cell wall of pathogens, causing a loss of material it is also known that it can cause vacuolation, disintegration, and coagulation inside the cell [67]. Martínez-Padrón, et al. [64] mention the volatile and non-volatile metabolic compounds produced such as trichodermine, gliotoxin, viridine, isonitrine, and trichozianine that can produce the antibiosis effect between the antagonist and the pathogen.
The antagonistic effect is the confrontation with the action, growth, and development of another organism, by different means such as competition. Some characteristics that favor the antagonistic effect are adaptability and the capacity for development and growth. The antagonistic effect has a greater presence in the soil and the rhizosphere, where recognition between antagonist and pathogen occurs, through reactions of lecithin and carbohydrates, due to the specific action that an antagonist has on a pathogen or group of them [68,69]. The competition for nutrients is mainly for nitrogen and carbohydrates [64].
Mycoparasitism is a complex antagonist-pathogen mechanism the way it is carried out can be divided into stages. The process begins with the identification of the pathogen by the antagonist, then the interaction of the hyphae of the antagonist occurs, it is when lytic activity occurs, where enzymes that degrade the pathogen cell wall are produced, and it ends with the degradation of cytoplasmic content [70].
4.2. Encapsulation of Microorganisms Promoting Plant Growth and Biocontrol
Inoculation of plant growth-promoting microorganisms emerges as an alternative to replace or minimize the use of agrochemicals and clean soils affected by contamination [71], and also improves plant growth by increasing the availability of nutrients in the soil through its different functions as biostimulants, biofertilizers, and biocontrollers, leading to sustainable agricultural production and ensuring food security in a changing climate [72].
However, one of the main problems in applying bacterial inocula is that sometimes they cannot perform their specific function because the bacterial population of the inoculum progressively decreases shortly after inoculation, due to the heterogeneity and unpredictable nature of the environment, the physicochemical characteristics of the soil, and nutritional factors [7,51,73].
That is why the research is directed to the development of an inoculant formulation with a suitable microenvironment to prevent the rapid decrease in the bacterial population, during storage before use, or once it is applied to the soil [74]. In recent years, several experimental polymer-based formulations have been successful as possible bacteria-bearing matrices; these carriers offer advantages over peat by protecting bacteria from environmental stresses and gradually releasing them into the soil once the polymers are degraded by the microorganisms present in the soil [6].
One of the most used polymers in the agricultural industry is alginate, due to its physical and chemical properties, its water-holding capacity, and being a soft and non-toxic method, it allows better handling, less dust, high fluidity, high service life, less abrasion and better soil establishment [75]. Furthermore, its degradation products have been found to influence the plant’s physiological activities as elicitors, promote germination, elongation of shoots, and increase root growth [76,77].
How this polymer is used as a matrix of bacterial inocula is encapsulation, which is a very versatile technology that protects bacteria from biotic and abiotic factors since it provides a beneficial physical barrier [78]; also, it can be modified with nutrients to improve the short-term survival of bacteria after inoculation [79]. Furthermore, encapsulation allows bacteria to be released into the target medium in a slow and controlled manner, thus increasing long-term effectiveness without losing their ability to stimulate plant growth [80]. Table 2 shows some microorganisms that have been encapsulated in alginate-based formulations for agricultural purposes.
Table 2.
Plant growth-promoting microorganisms encapsulated in alginate-based formulations to increase microbial viability and efficacy tested in different plants and vegetables.
| Microorganism | Formulation Material | Plant | Reference |
|---|---|---|---|
| Azospirillum brasilense | Alginate and skimmed milk | Wheat | [81] |
| Alginate | Tomate | [82] | |
| Alginate | Desert trees | [83] | |
| Alginate and starch | In vitro | [84] | |
| Azospirillum lipoferum | Alginate | Corn | [85] |
| Alginate and humic acid | Rice | [86] | |
|
Azospirillum sp. and Methylobacterium sp. |
Alginate | Tomato | [87] |
| A. brasilense and Bacillus pumilus | Alginate | Legume trees | [88] |
| A. brasilense and Chlorella sorokiniana | Alginate | Tomato | [89] |
| Alginate | Sorghum | [83] | |
| A. brasilense and Pantoea dispersa | Alginate and organic olive waste | Pinus halepensis | [90] |
| A. brasilense and P. fluorescens | Alginate | In vitro | [91] |
| Alginate and skimmed milk | Wheat | [92] | |
| A. lipoferum, Bacillus polymyxa, and Nostoc muscorum | Alginate, carboxymethyl cellulose, and talc | Bread wheat | [93] |
| Nitrogen-fixing bacteria | Alginate y maltodextrin | In vitro | [94] |
| Bacillus megaterium | Alginate | Corn | [95] |
| Alginate and humic acid | Rice | [96] | |
| Bacillus subtilis | Alginate and pea protein | Brachypodium distachyon and Phleum pretense | [97] |
| Alginate and jelly | In vitro | [98] | |
| Alginate and humic acid | Lettuce | [99] | |
| Beauveria bassiana | Alginate and wheat bran | Cattle pasture | [100] |
| B. subtilis and Pseudomonas corrugata | Alginate | Wheat | [101] |
| Alginate and skimmed milk | Corn | [102] | |
| Enterobacter sp. | Alginate and skimmed milk | Lettuce | [103] |
| Glomus desertícola (AM mycorrhizae) | Alginate | Tomato | [104] |
| Klebsiella oxytoca | Alginate | Cotton seeds | [105] |
| Methylobacterium oryzae, Methylobacterium suomiense y Azospirillum brasilense | Alginate | Tomato | [87] |
| Pantoea agglomerans | Alginate, glycerol, and chitin | In vitro | [106] |
| Pseudomonas sp. | Alginate and attapulgite | In vitro | [107] |
| Pseudomonas fluorescens | Alginate, skimmed milk, and clay | Wheat | [108] |
| Alginate | Sugar cane | [109] | |
| Alginate | Corn | [110] | |
| Pseudomonas plecoglossicida | Alginate | Potato | [111] |
| Pseudomonas putida | Alginate | Corn | [112] |
| Pseudomonas striata | Alginate | In vitro | [113] |
| Pseudomonas putida y B. subtilis | Alginate and humic acid | Lettuce | [114] |
| Raoultella planticola | Alginate and bentonite | In vitro | [115] |
| Alginate, chitin and bran | Cotton | [116] | |
| Raoultella terrigena | Alginate and starch | In vitro | [84] |
| Rhizobium spp. | Alginate | Leucaena leucocephala | [117] |
| Serratia marcescens | Alginate | Corn | [118] |
| Streptomyces sp. | Alginate, starch, and talc | Tomato | [119] |
| Trichoderma asperellum | Alginate | In vitro | [120] |
| Trichoderma harzianum | Alginate and chitosan | In vitro | [121] |
| Trichoderma viride | Chitosan and alginate | In vitro | [122] |
| Yarrowia lipolytica | Alginate | Bean | [123] |
4.3. The Potential Effect of Alginate Encapsulation on Plant Growth
Fages [91] began using alginate to encapsulate beneficial bacteria from Azospirillum brasilense and Pseudomonas fluorescens. His experiment was successful in wheat plants under field conditions; he found that polymer-protected bacteria survive in the field long enough to have a beneficial effect on plants; in addition, the colonization in roots by the bacteria released in alginate capsules was higher than that obtained by direct inoculation. These results provide strong evidence of the efficiency of alginate encapsulation in achieving the controlled release and protection of bacteria from the environment. From that moment, different investigations to obtain a suitable formulation for different types of plant growth-promoting bacteria at a low cost [6].
A study where the bacteria Methylobacterium oryzae and Methylobacterium suomiense co-added with Azospirillum brasilense were encapsulated in alginate shows that, from an initial concentration of viable cells of 109 CFU/g, after 12 months at room temperature 108 CFU/g of M. oryzae and 106 CFU/g of M. suomiense were conserved, which conferred stress reduction in inoculated tomato plants [87]. Likewise, the immobilization of Pseudomonas plecoglossicida in sodium alginate promotes the symbiotic development of an arbuscular mycorrhizal fungus in potato seedlings [111].
Although alginate has many advantages to be used as a matrix to encapsulate bacterial inocula, it has a relevant limitation: the loss of bacteria during the preparation of the capsules, and also the presence of macrospores in the alginate matrix facilitates the diffusion of hydrophilic molecules. Therefore, the admixture of a filling material in the alginate matrix, such as starch, is a profitable solution strategy, since this material is abundant, cheap, renewable, and fully biodegradable [80].
Some studies show that encapsulation with both compounds improves the properties of the capsules. Additionally, the degradation and release can be controlled by changing the amount of addition of the components [124]. Wu, et al. [116] developed biodegradable and controlled release formulations of the Raoultella planticola bacteria, and they determined that the properties of the capsules can be modulated by varying the amounts of starch and alginate, without the bacteria losing their properties to improve plant growth, which has application to meet the needs of agricultural production.
For their part, He, et al. [80], carried out a study to evaluate the survival and colonization efficiency of Pseudomonas putida encapsulated in a sodium alginate-based formulation, on cotton plants under saline conditions. They found that the survival rates were 89.67, almost 9% higher than in the free cells. Additionally, on day 49 of the experiment, an increase was observed in the encapsulated bacterial population. Regarding cotton, an increase in biomass was found for the encapsulated strain, which can be attributed to the increase in the number of bacteria and the high production of indole-3-acetic acid and gibberellin, which is why microencapsulation with sodium alginate, inexpensive starch, and bentonite is recommended.
In addition to starch, sodium bentonite has been used in combination with alginate to develop effective biofertilizer formulations that minimize production costs. The mixture encapsulation efficiency is almost 100, 88.9% of Raoultella planticola bacteria survived after 6 months of storage and swelling, biodegradability, and release rate were found to increase with increasing alginate content, presenting a first-order release, which proves a slow-release, ideal for farmland [115].
Previous studies show that alginate is a very useful polymer for the encapsulation of bacteria. However, there are some experiments with contradictory results. Those indicate that when encapsulating bacteria in sodium alginate, populations are diminished due to inert carrier, which also minimizes bacterial viability. Additionally, the irregular surface of the capsules can negatively influence the release of bacteria, which reduces the colony-forming units present in the soil and the roots of plants, modifying inoculum effectiveness [125].
In a study by Bashan, et al. [126], it was demonstrated that the alginate capsule structure has low mechanical resistance, which produces an unstable and uncontrolled bacterial release. In addition, the cellular mortality during the drying of the capsules is a critical point to improve. Without losing sight of the fact that the large-scale production of alginate capsules and their application in the field are still limited, in addition to the fact that the cost of production is relatively high [7].
4.4. Alginate Capsule Shelf Life and Microbial Viability
One of the main reasons why the use of a matrix to contain plant growth promoter and biocontrol microorganisms is implemented is to increase their viability and survival; that is, to allow microorganisms introduced into the soil or plant to have a greater resistance to biotic and abiotic factors that can decrease its viability. Therefore, the evaluation of the survival of the microorganisms in preparation based on a matrix or carrier in storage for a certain period is essential to decide on suitability [101]. Furthermore, it has been established that a quality formulation must supply enough cells for effective colonization of the plant rhizosphere to improve plant growth [127].
Some studies suggest that the survival of alginate-encapsulated microbial cells is mainly related to the type of microorganism used [1], as is the case of Pseudomonas corrugata, deformation, and degradation of capsules, which can be attributed to the release of certain acids by the bacteria [101]. Therefore, the use of alginate will depend on the type of microorganism to be encapsulated, it is necessary to know its physical–chemical properties and the nature of the compounds it releases.
The viability and survival of microorganisms are also affected by compounds added as additives. The use of calcium gluconate in alginate capsules as a matrix of the fungi Metarhizium brunneum and Saccharomyces cerevisiae improves the viability after drying and rehydration, the hygroscopic properties, the shelf life, and the supply of nutrients, for which it is recommended to use this compound to increase microbial viability after drying, which is a crucial step for the survival of encapsulated microorganisms [128].
In a study by Trivedi and Pandey [101], they measured the survival rate over time to determine the ability of the formulations to improve the survival of B. subtilis and P. corrugata, which, being in alginate capsules was higher when compared to a char or broth matrix. They also found that the bacterial population in alginate capsules was above 106 CFU/g after three years, suggesting that plant growth-promoting bacteria may survive in capsules of alginate for long periods.
Furthermore, alginate capsules can stably preserve Mesorhizobium ciceri and Bradyrhizobium japonicum during long storage periods, longer than one year, maintaining a stable concentration of colony-forming units [129].
The viability and survival of the cells encapsulated in alginate depend on the characteristics of the microorganism, the release of compounds that damage the structure of the capsule, and the compounds that are used as additives. These characteristics will affect the quality of the capsule and the time it will remain in good condition. However, according to the studies indicated above, alginate can be considered a viable alternative to preserve microorganisms in storage for approximately one year, but having an adequate estimation is necessary to evaluate cell viability with the microbial strain under storage conditions.
4.5. Environmental Evaluation after the Application of Encapsulated Microorganisms
The microbial and fermentative activity of the soil is an important factor in the hydrolytic and degradation processes of alginate capsules. It is well known that there are many microorganisms that degrade natural polymers and use them as a growth substrate [130], in addition to other substances present in the soil that bind to Ca2+ ions and also cause the degradation of the alginate capsule [131].
A biodegradation process study by Shcherbakova et al. [129] shows that the presence of alginate capsules in the soil activates microorganisms, making the mineralization processes of organic matter more efficient compared to soils without alginate, increases the total number of soil microorganisms, increases hydrolytic and oligotrophic microorganisms, which indicates that the processes of destruction of organic substances increase. Therefore, soil microorganisms actively react to the introduction of alginate, breaking it down and using it as an additional nutritional substrate. The changes in the capsules are observed 7 days after the addition with a reduction in size; however, until 14 days it is indicated that the degradation process begins.
Exudates from the roots of plants rich in organic acids stimulate the multiplication of microorganisms present in the rhizosphere and accelerate the destruction of the polymer matrix, facilitating the release of encapsulated microorganisms into the environment. The increase in the number of microorganisms coincides with the period of active growth and development of the radicle of the plants; therefore, the introduction of microorganisms in alginate provides a controlled release and retains them for successful colonization, preventing their death before they manage to colonize the root of the plant or vegetable [132].
Belaid, et al. [133] evaluated the survival and proliferation of Azospirillum brasilense immobilized in alginate in the soil at different humidity levels, and they found that the strain survived for a period of 75 days in the soil without plant roots, being relevant humidity and the amount of organic matter, without negative interaction effect with native microbial strains.
In addition to the release and colonization capacity of the encapsulated microorganisms, another important aspect to consider is that the characteristics of the strain are conserved. Trivedi and Pandey [101] made qualitative estimates for the characteristics related to plant growth promotion and biocontrol of bacteria B. subtilis and P. corrugata recovered from the alginate capsules and found that the bacteria encapsulated in alginate positively affected the growth parameters of wheat, increased colonization capacity, and fresh root weight when comparing the results with the broth-based formulation.
The ability of alginate-encapsulated Pseudomonas fluorescens to produce 2,4-diacetylfloroglucinol, an antifungal metabolite, is not affected after 12 months in storage at 4 °C and 28 °C ± 2 °C. In addition, the bacteria showed colonization of effective roots and protection against pathogenic fungi in sugar beets [109]. Encapsulation of Mesorhizobium ciceri and Bradyrhizobium japonicum increases the number of nodules in chickpea and soybean roots, as well as the weight of the nodules compared to non-encapsulated bacteria [129].
Likewise, a study shows that the inoculation of wheat plants with bacteria recovered from dry pearls after 14 years of storage of Azospirillum brasilense and Pseudomonas fluorescens had the same effect on colonization and increase in plant growth when compared with contemporary cultivated strains [92].
As well as these, there are many studies that demonstrate the effectiveness of encapsulation with alginate when applied in the soil, preserving, and sometimes increasing the effect of encapsulated microorganisms as promoters of plant growth and biocontrol. This considers the alginate as an alternative for inoculation of microorganisms into the soil without losing effectiveness in the final product. However, it is necessary to make pertinent evaluations for each microbial strain.
In resume, the encapsulation of plant growth-promoting microorganisms has several advantages compared to cell-free formulations. The encapsulation allows a gradual release, improves the physiological activity of microorganisms, reduces the risk of contamination in storage and transport, and significantly increases cell viability due to protection against adverse environmental factors. Encapsulated products can be stored over a wide temperature range. However, some encapsulation techniques are hard to scale industrially and are expensive.
4.6. The Economic Aspect of the Use of Alginate in Agriculture
Sodium alginate is a widely used biopolymer due to its biocompatibility, biodegradability, ability to form hydrogels, and water-solubility properties. Currently, the demand for the manufacture of alginates is expected to enhance due to its current and future biomedical, bioengineering, and food applications. As mentioned above, alginate can be obtained from numerous brown algae. A large amount of biomass of the brown alga Sargassum horneri, known as “golden tide” has recently caused a severe ecological impact on coastal ecosystems in many countries [134]. Massive algal blooms are a menace to the economy and tourist attractions of affected countries. Therefore, governments and researchers are exploring the uses of S. horneri to obtain natural products and useful materials for the golden tides’ control and sustainable management.
The principal industrial use of marine macroalgal residues is related to the extraction and purification of the polysaccharide fraction, which can range between 4 and 76% dry weight, being the alginate in brown algae. The extraction of alginate in the industry based on marine algae residues is well examined, and following an adequate recovery route, compounds with a higher value and a lower production cost can be obtained [135]. A study by Fernando, et al. [136] suggests extracting alginate from S. horneri to produce alginate microparticles for drug delivery. The previous study is aligned with the principles of circular economy, where a residue, in this case, the excess coastal biomass of brown algae, can be used as a resource.
Microencapsulation and film formation with sodium alginate as a vehicle for plant-growth-promoting and biocontrol microorganisms can help to prolong their useful life, facilitate their incorporation into agricultural systems and allow controlled release. The objective of having a controlled release is to reduce the amount of product that is added to the soil, which decreases operating costs and ensures the implementation of a constant and correct amount of the bioactive. Therefore, the product is not released into the environment, avoiding environmental problems [137].
There are different methods for microencapsulation with alginate: extrusion, which is easy to apply and industrialize but is not compatible with thermosensitive microorganisms; coextrusion, which is a process for a large payload but has high costs; spray-drying, it is an economical technology and equipment is widely available, it has good encapsulation efficiency, and it is a fast process, but it has a low production volume; spray-cooling is an economical technology for encapsulation but requires high energy and a long process time, which makes it more expensive; and complex coacervation, it has a high payload and no specific equipment is required; however, it is a costly process due to the complexity of the technique [138].
In general, laboratory-scale processes for microencapsulation and alginate film formation are hard to scale industrially; however, equipment such as electrostatic extruders, mechanical jet cutters, and impact spray method, among others, are currently available. To assess whether an industrial process is techno-economically viable, it is essential to carry out an analysis that includes the mass and energy balance around the unit operations included in the process and add an accounting of the costs and potential income associated with the entire installation at industrial scale.
Strobe, et al. [139] performed a techno-economic analysis to evaluate two industrial encapsulation techniques with sodium alginate: a traditional external gelation process and a process that achieves in situ alginate cross-linking during spray drying. They found that the cross-linking alginate microcapsule process exhibited lower investment and annual operating costs than the external gelation process, and the process required less water and utility usage. Dimitrellou, et al. [140] used the extrusion technique for the encapsulation of Lactobacillus casei and, according to their analysis of production costs, they determined that the introduction of the encapsulation is profitable if an increase in the sale prices of the new product range between 0.04 and 0.05 €/L, depending on the volume of production.
In economic terms, the most important thing is to choose the encapsulation technique or film production according to cost–benefit, which allows obtaining high efficiency, with few steps in the process at a low cost. The application of the different products in the agroindustry reduces the amount of product to be used (biofertilizer, biostimulant, biocontrol), operating costs and minimizes the environmental damage caused by the excessive use of agrochemicals.
4.7. Technology Used in the Large-Scale Production of Alginate Products in Agriculture
The large-scale production of alginate-derived products is influenced by the conditions of the alginate used, the nature of the model used in the products, and the technology used for its preparation. Cross-linked alginate is used in the production of microcapsules and hydrogels on a large scale due to the advantages presented during the production process. The main advantage is the simplification of unit operations during gelation and drying that decreases the resources used and therefore, the cost of investment and operating expenses are reduced [139].
The implementation of different unit operations depends on the type of model retained in the alginate products, these compounds can be enzymes, concentrates, bacteria, vitamins, and oils, among others [141]. Therefore, the nature of the additive or active agent added to the alginate products modifies the stages of the production process. Thus, a hydrophilic material requires fewer unit operations, unlike a hydrophobic material that requires a prior emulsification stage [139].
Emulsion technology is the most efficient method for the large-scale production of viable bacteria in alginate products for use in agro-industrial processes, this is due to the absence of heat sources in the method that damage the active agent [142]. However, the spray-drying technology to obtain microcapsules and particles enriched with the active agent has been reported to lower production costs and higher quality in terms of the content retained in the alginate product; therefore, it is the technology best suited for industrial production [143]. In addition, there are other techniques such as extrusion that are more expensive and difficult to scale to higher production even when the model of additive or active agent is microorganisms [144].
Currently, alginate products added with retained models for agroindustrial use are marketed mainly in fertilizers, the most common uses are fertilizers of plant extracts, leachates, fungi, and bacteria that promote plant health [145].
5. Perspectives for the Use of Alginate in Agro-Industry
Alginate is considered a very useful polymer in the agro-industry due to its great versatility and qualities, zero toxicity, and compatibility with many compounds; it has been used with wide benefits as a matrix for beneficial microorganisms. In the form of capsules to be implemented during cultivation obtaining improvements in yield, quality, and pest control, thus promoting sustainable agriculture practices, which contribute to increased agricultural productivity, decreased use of agrochemicals, and less environmental impact [146]. In addition, in the form of a coating, to reduce contamination by pathogenic microorganisms and improve post-harvest quality [32]. However, there are still unsolved problems, so a future frontier in the field of microbial encapsulation and coatings is the development of matrices with polymeric nanoparticles as an additive or microencapsulated formulation, to solve the main problems of technology and improve the stability of microorganisms concerning environmental conditions.
The implementation of low-cost additives or substances necessary for the inoculum can be beneficial to increase the useful life of the product or control the release profile of microorganisms [147,148]. With this, there is also the need to carry out a more in-depth analysis of the alginate–additives–microorganisms–soil–plant system relationship; to obtain important information that allows us to understand the functional characteristics of the encapsulated microorganisms and establish strategies for their application [7]. On the other hand, in the preparation of microbial coatings for food, it is necessary to make a complete analysis of the effect that the coating has on the quality of food and its by-products, as well as on the consumer. The previous is to understand the mechanisms of action of microbial inoculate, not only to contain contamination by pathogens but also their relationship with food and with the final consumer.
The future trends are directed towards the development of microenvironmental conditions to facilitate the reproduction, growth, and functional activities of microorganisms encapsulated or disposed of in the coating. Finally, it is imperative to do a comprehensive assessment of environmental and health safety issues before the technology is moved to the industrial level, to provide complex, efficient, safe, economically acceptable, and easy-to-apply biotech products that ensure health and plant growth as well as consumer acceptability.
Another perspective for alginate application is the encapsulation of microalgae. This technology increases biomass and protects the microalgae from predators and toxic contaminants, which reduces production costs. The application of encapsulated microalgae can be used in different areas of the agro-industry. Dębowski, et al. [149] found that by immobilizing and encapsulating Chlorella vulgaris it is possible to increase biomass, and CO2 sequestration also increases. On the other hand, Cheirsilp, et al. [150] found that immobilizing the microalgae Nannochloropsis sp. in alginate beads for use in phytoremediation of industrial effluents significant increases biomass and lipid production, as well as nitrogen and phosphorus removal and CO2 mitigation. Therefore, it is relevant to increase research on the immobilization of microalgae in sodium alginate since it has multiple applications for the removal of pollutants and wastewater treatment.
In response to the need to improve the implementation of microorganisms that promote plant growth and biocontrol in agriculture, our work team proposes using alginate as a matrix and adding compounds that allow improving the physicochemical characteristics of capsules and coatings; through multidisciplinary studies that allow evaluating the viability and effectiveness from the ecological, biological, technological, and economic aspects, to obtain a product that can be transferred to the industrial level and subsequently used successfully by agricultural producers.
6. Conclusions
Alginate is a polymer that has allowed significant progress in the technology of formulations for microorganisms that promote plant growth and biocontrol due to its physicochemical properties, biocompatibility, and non-toxicity. Likewise, some studies suggest that the use of certain additives positively affects its protection capacity, increasing microbial viability, and survival, perceived as a beneficial effect on plant growth and biological control. However, in some cases, some compounds have been shown to exert a negative effect on the microbial encapsulation system and coatings; therefore, it is necessary to analyze the relationship between alginate, additives, and microorganisms to be implemented. As well as the effect they have on the soil and plant, food, and consumer systems to obtain the necessary information to propose a quality formulation, safe, effective, and easy to apply both for agricultural producers and for workers in the food industry, which confers benefits on crop quality and health.
Acknowledgments
The authors thank the Consejo Nacional de Ciencia y Tecnología (CONACyT, B. M-C 804133; C.J. M-M 796218) for the scholarships awarded for postgraduate studies.
Author Contributions
Conceptualization—B.M.-C., C.J.M.-M., A.A.F.-P.; preparing the original draft—B.M.-C., C.J.M.-M.; reviewing and editing—J.F.G.-T., G.M.-B., H.A.-B., A.A.F.-P., G.M.S.-Z.; supervising—A.A.F.-P., G.M.S.-Z. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Szczech M., Maciorowski R. Microencapsulation technique with organic additives for biocontrol agents. J. Hortic. Res. 2016;24:111–122. doi: 10.1515/johr-2016-0013. [DOI] [Google Scholar]
- 2.Pawar S.N., Edgar K.J. Alginate derivatization: A review of chemistry, properties and applications. Biomaterials. 2012;33:3279–3305. doi: 10.1016/j.biomaterials.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 3.Grobelak A., Kokot P., Hutchison D., Grosser A., Kacprzak M. Plant growth-promoting rhizobacteria as an alternative to mineral fertilizers in assisted bioremediation-sustainable land and waste management. J. Environ. Manag. 2018;227:1–9. doi: 10.1016/j.jenvman.2018.08.075. [DOI] [PubMed] [Google Scholar]
- 4.Creus C.M. Inoculantes microbianos: Piezas de un rompecabezas que aún requiere ser ensamblado. Rev. Argent. Microbiol. 2017;49:207–209. doi: 10.1016/j.ram.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Calabi-Floody M., Medina J., Rumpel C., Condron L.M., Hernandez M., Dumont M., de la Luz Mora M. Smart fertilizers as a strategy for sustainable agriculture. Adv. Agron. 2018;147:119–157. [Google Scholar]
- 6.Sahu P.K., Brahmaprakash G.P. Formulations of biofertilizers–approaches and advances. In: Singh D., Singh H., Prabha R., editors. Microbial Inoculants in Sustainable Agricultural Productivity. Springer; New Delhi, India: 2016. pp. 179–198. [Google Scholar]
- 7.Vassilev N., Vassileva M., Martos V., Garcia del Moral L.F., Kowalska J., Tylkowski B., Malusá E. Formulation of microbial inoculants by encapsulation in natural polysaccharides: Focus on beneficial properties of carrier additives and derivatives. Front. Plant Sci. 2020;11:270. doi: 10.3389/fpls.2020.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barros A.P.H., Morantes M.T.A., Hoyos M.I.C., Ospino L.J.M. Preparación de nanopartículas de quitosano modificadas con alginato de sodio con potencial para la liberación controlada de medicamentos. Rev. EIA. 2016;3:75–83. doi: 10.24050/reia.v12i2.965. [DOI] [Google Scholar]
- 9.de Jesús Hernández-Torres C., Ilina A., Ventura-Sobrevilla J.M., Belmares-Cerda R.E., Contreras-Esquivel J.C., Álvarez G.M., Martínez-Hernández J.L. La microencapsulación de bioactivos para su aplicación en la industria. ICIDCA. Sobre Los Deriv. Caña Azúcar. 2016;50:12–19. [Google Scholar]
- 10.Moradali M.F., Ghods S., Rehm B.H. Alginate biosynthesis and biotechnological production. In: Rehm B., Moradali M., editors. Alginates and Their Biomedical Applications. Volume 11. Springer; Singapore: 2018. pp. 1–25. [Google Scholar]
- 11.Avendaño-Romero G., López-Malo A., Paolu E. Propiedades del alginato y aplicaciones en alimentos. Temas Sel. Ing. Aliment. 2013;7:87–96. [Google Scholar]
- 12.Armas Jara E.S., Guevara Lezama A.G. Bachelor’s Thesis. Universidad Nacional de Trujillo; Trujillo, Perú: 2017. Biosorción de Cromo Total en Soluciones Ideales Utilizando una Matriz de Microalgas Nativas Inmovilizadas en Alginato Cálcico. [Google Scholar]
- 13.Reyes-Avalos M.C., Minjares-Fuentes R., Esparza-Rivera J.R., Contreras-Esquivel J.C., Montañez Sáenz J.C., Meza-Velázquez J.A. Calidad de melón cantaloupe (Cucumis melo) cubierto con una película comestible de alginato-hpmc-parafina. Nova Sci. 2017;9:222–238. doi: 10.21640/ns.v9i18.797. [DOI] [Google Scholar]
- 14.Hernández Montiel L.G., Chiquito Contreras R.G., Castillo Rocha D.G., Chiquito Contreras C.J., Vidal Hernández L., Beltrán Morales F.A. Efecto de microcápsulas de Pseudomonas putida sobre crecimiento y rendimiento de pimiento morrón. Rev. Mex. Cienc. Agríc. 2018;9:4223–4233. doi: 10.29312/remexca.v0i20.992. [DOI] [Google Scholar]
- 15.Peña-Datoli M., Hidalgo-Moreno C.M., González-Hernández V.A., Alcántar-González E.G., Etchevers-Barra J.D. Recubrimiento de semillas de maíz (Zea mays L.) con quitosano y alginato de sodio y su efecto en el desarrollo radical. Agrociencia. 2016;50:1091–1106. [Google Scholar]
- 16.Łabowska M., Izabela M., Jerzy D. Methods of Extraction, Physicochemical Properties of Alginates and Their Applications in Biomedical Field–a Review. Open Chem. 2019;17:738–762. doi: 10.1515/chem-2019-0077. [DOI] [Google Scholar]
- 17.Petzold G., Rodríguez A., Valenzuela R., Moreno J., Mella K. Alginate as a versatile polymer matrix with biomedical and food applications. In: Grumezescu V., Grumezescu A.M., editors. Materials for Biomedical Engineering. Elsevier; Amsterdam, The Netherlands: 2019. pp. 323–350. [Google Scholar]
- 18.Liling G., Di Z., Jiachao X., Xin G., Xiaoting F., Qing Z. Effects of ionic crosslinking on physical and mechanical properties of alginate mulching films. Carbohydr. Polym. 2016;136:259–265. doi: 10.1016/j.carbpol.2015.09.034. [DOI] [PubMed] [Google Scholar]
- 19.Kuo C.K., Ma P.X. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: Part 1. Structure, gelation rate and mechanical properties. Biomaterials. 2001;22:511–521. doi: 10.1016/S0142-9612(00)00201-5. [DOI] [PubMed] [Google Scholar]
- 20.Anal A.K., Stevens W.F. Chitosan–alginate multilayer beads for controlled release of ampicillin. Int. J. Pharm. 2005;290:45–54. doi: 10.1016/j.ijpharm.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 21.Xu Y., Zhan C., Fan L., Wang L., Zheng H. Preparation of dual crosslinked alginate–chitosan blend gel beads and in vitro controlled release in oral site-specific drug delivery system. Int. J. Pharm. 2007;336:329–337. doi: 10.1016/j.ijpharm.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 22.Chávarri M., Marañón I., Ares R., Ibáñez F.C., Marzo F., del Carmen Villarán M. Microencapsulation of a probiotic and prebiotic in alginate-chitosan capsules improves survival in simulated gastro-intestinal conditions. Int. J. Food Microbiol. 2010;142:185–189. doi: 10.1016/j.ijfoodmicro.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 23.Desbrieres J., Peptu C., Ochiuz L., Savin C., Popa M., Vasiliu S. Application of chitosan-based formulations in controlled drug delivery. In: Crini G., Lichtfouse E., editors. Sustainable Agriculture Reviews. Volume 36. Springer; Cham, Switzerland: 2019. pp. 241–314. [Google Scholar]
- 24.Vaziri A.S., Alemzadeh I., Vossoughi M. Improving survivability of Lactobacillus plantarum in alginate-chitosan beads reinforced by Na-tripolyphosphate dual cross-linking. LWT. 2018;97:440–447. doi: 10.1016/j.lwt.2018.07.037. [DOI] [Google Scholar]
- 25.Mende M., Schwarz D., Steinbach C., Boldt R., Schwarz S. The influence of salt anions on heavy metal ion adsorption on the example of nickel. Materials. 2018;11:373. doi: 10.3390/ma11030373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu Q., Wang T., Zhou M., Xue J., Luo Y. Formation of redispersible polyelectrolyte complex nanoparticles from gallic acid-chitosan conjugate and gum arabic. Int. J. Biol. Macromol. 2016;92:812–819. doi: 10.1016/j.ijbiomac.2016.07.089. [DOI] [PubMed] [Google Scholar]
- 27.Ngo H.H., Guo W., Zhang J., Liang S., Ton-That C., Zhang X. Typical low cost biosorbents for adsorptive removal of specific organic pollutants from water. Bioresour. Technol. 2015;182:353–363. doi: 10.1016/j.biortech.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 28.Motshekga S.C., Ray S.S., Onyango M.S., Momba M.N. Preparation and antibacterial activity of chitosan-based nanocomposites containing bentonite-supported silver and zinc oxide nanoparticles for water disinfection. Appl. Clay Sci. 2015;114:330–339. doi: 10.1016/j.clay.2015.06.010. [DOI] [Google Scholar]
- 29.Strobel S.A., Allen K., Roberts C., Jimenez D., Scher H.B., Jeoh T. Industrially-scalable microencapsulation of plant beneficial bacteria in dry cross-linked alginate matrix. Ind. Biotechnol. 2018;14:138–147. doi: 10.1089/ind.2017.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fernández N., Echeverria D.C., Mosquera S.A., Paz S.P. Estado actual del uso de recubrimientos comestibles en frutas y hortalizas. Biotecnol. Sect. Agropecu. Agroind. 2017;15:134–141. doi: 10.18684/BSAA(15)134-141. [DOI] [Google Scholar]
- 31.Morales Gomero J.C., Corzo Lucioni A., Alarcón Cavero H., Lazo Hoyos D. Síntesis y caracterización de películas de (WO3) n vía sol-gel mediante técnica de recubrimiento por inmersión. Rev. Soc. Quím. Perú. 2017;83:420–436. doi: 10.37761/rsqp.v83i4.215. [DOI] [Google Scholar]
- 32.Solano-Doblado L.G., Alamilla-Beltrán L., Jiménez-Martínez C. Functionalized edible films and coatings. Tip Rev. Espec. Cienc. Quím. Biol. 2019;21:30–42. [Google Scholar]
- 33.Aguilar J.M., López-Castejón M.L., Cordobés F., Guerrero A. Efecto del pH y del procesado térmico sobre las propiedades viscoelásticas lineales de la yema de huevo acidulada con ácido clorhídrico. Afinidad. 2020;77:22–30. [Google Scholar]
- 34.Pyo S.M., Müller R.H., Keck C.M. Encapsulation by nanostructured lipid carriers. In: Jafari S.M., editor. Nanoencapsulation Technologies for the Food and Nutraceutical Industries. Academic Press; Cambridge, MA, USA: 2017. pp. 114–137. [Google Scholar]
- 35.Cota Leal M.A., Sotelo Lerma M., Quevedo López M. Fabricación de películas de perovskita (CH3NH3PbI3-XClX) por drop casting. Biotecnia. 2020;19:34–37. doi: 10.18633/biotecnia.v19i0.408. [DOI] [Google Scholar]
- 36.Gaona-Sánchez V.A., Calderón-Domínguez G., Morales-Sánchez E., Chanona-Pérez J.J., Arzate-Vázquez I., Terrés-Rojas E. Pectin-based films produced by electrospraying. J. Appl. Polym. Sci. 2016;133:43779. doi: 10.1002/app.43779. [DOI] [Google Scholar]
- 37.Liu Z.P., Zhang Y.Y., Yu D.G., Wu D., Li H.L. Fabrication of sustained-release zein nanoparticles via modified coaxial electrospraying. Chem. Eng. J. 2018;334:807–816. doi: 10.1016/j.cej.2017.10.098. [DOI] [Google Scholar]
- 38.Sánchez-Moreno C., González-Peña D., Colina-Coca C., Ancos B.D. Métodos físicos no tradicionales de control microbiológico aplicables al proceso de elaboración de hortalizas de IV Gama. Agrocienc. Urug. 2018;22:26–36. [Google Scholar]
- 39.Fernández Valdés D., Bautista Baños S., Fernández Valdés D., Ocampo Ramírez A., García Pereira A., Falcón Rodríguez A. Películas y recubrimientos comestibles: Una alternativa favorable en la conservación poscosecha de frutas y hortalizas. Rev. Cie Téc. Agríc. 2015;24:52–57. [Google Scholar]
- 40.Ramos-García M.D.L., Bautista-Baños S., Barrera-Necha L.L., Bosquez-Molina E., Alia-Tejacal I., Estrada-Carrillo M. Compuestos antimicrobianos adicionados en recubrimientos comestibles para uso en productos hortofrutícolas. Rev. Mex. Fitopatol. 2010;28:44–57. [Google Scholar]
- 41.García M., Delgado F., Escamilla M., García B., Regalado C. Métodos modernos para la caracterización de películas y recubrimientos comestibles. BioTecnología. 2018;22:37–54. [Google Scholar]
- 42.López D.F., Osorio O., Checa O.E. Propiedades mecánicas de un material de pectina para revestimiento de fibras naturales utilizadas en aplicaciones agrícolas. Inf. Tecnol. 2019;30:189–198. doi: 10.4067/S0718-07642019000300189. [DOI] [Google Scholar]
- 43.Costamagna M.S., Gómez-Mascaraque L.G., Zampini I.C., Alberto M.R., Pérez J., López-Rubio A., Isla M.I. Microencapsulated chañar phenolics: A potential ingredient for functional foods development. J. Funct. Foods. 2017;37:523–530. doi: 10.1016/j.jff.2017.08.018. [DOI] [Google Scholar]
- 44.Saxena J. Efficacy of rhizobacterial strains encapsulated in nontoxic biodegradable gel matrices to promote growth and yield of wheat plants. Appl. Soil Ecol. 2011;48:301–308. [Google Scholar]
- 45.Cassidy M.B., Lee H., Trevors J.T. Environmental applications of immobilized microbial cells: A review. J. Ind. Microbiol. 1996;16:79–101. doi: 10.1007/BF01570068. [DOI] [Google Scholar]
- 46.Abd El-Rehim H.A. Characterization and possible agricultural application of polyacrylamide/sodium alginate crosslinked hydrogels prepared by ionizing radiation. J. Appl. Polym. Sci. 2006;101:3572–3580. doi: 10.1002/app.22487. [DOI] [Google Scholar]
- 47.Peng Q., Zhang M., Gao L., Eromosele O., Qiao Y., Shi B. Effects of alginate oligosaccharides with different molecular weights and guluronic to mannuronic acid ratios on glyceollin induction and accumulation in soybeans. J. Food Sci. Technol. 2018;55:1850–1858. doi: 10.1007/s13197-018-3101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ayala L.C., Valenzuela C.P., Bohorquez Y. Efecto de un recubrimiento comestible a base de alginato de sodio y iones de calcio sobre la calidad de mora de castilla (Rubus glaucus Benth) Vitae. 2012;19:S129–S131. [Google Scholar]
- 49.Dubey S., Jhelum V., Patanjali P.K. Controlled release agrochemicals formulations: A review. J. Sci. Ind. Res. 2011;70:105–112. [Google Scholar]
- 50.Bora T., Özaktan H., Göre E., Aslan E.M.E.K. Biological control of Fusarium oxysporum f. sp. melonis by wettable powder formulations of the two strains of Pseudomonas putida. J. Phytopathol. 2004;152:471–475. doi: 10.1111/j.1439-0434.2004.00877.x. [DOI] [Google Scholar]
- 51.Joe M.M., Karthikeyan B., Chauhan P.S., Shagol C., Islam M.R., Deiveekasundaram M., Sa T. Survival of Azospirillum brasilense flocculated cells in alginate and its inoculation effect on growth and yield of maize under water deficit conditions. Eur. J. Soil Biol. 2012;50:198–206. doi: 10.1016/j.ejsobi.2012.03.002. [DOI] [Google Scholar]
- 52.John R.P., Tyagi R.D., Brar S.K., Surampalli R.Y., Prévost D. Bio-encapsulation of microbial cells for targeted agricultural delivery. Crit. Rev. Biotechnol. 2011;31:211–226. doi: 10.3109/07388551.2010.513327. [DOI] [PubMed] [Google Scholar]
- 53.Schoebitz M., López M.D., Roldán A. Bioencapsulation of microbial inoculants for better soil–plant fertilization: A review. Agron. Sustain. Dev. 2013;33:751–765. doi: 10.1007/s13593-013-0142-0. [DOI] [Google Scholar]
- 54.Sosa-del Castillo D., Álvarez-Barreto J., Pérez-Martínez S. Encapsulación de Trichoderma en micropartículas de alginato para el control de patógenos de cacao. Rev. Prot. Veg. 2015;30:80. [Google Scholar]
- 55.Fernández-Jiménez M.A., Bulla-Castañeda D.M., Sanabria-Villate A.M., Pulido-Medellín M.O. Implementación de hongos nematófagos para el control de parásitos gastrointestinales. Rev. Pensam. Acción. 2019;27:7–20. [Google Scholar]
- 56.Alviz L., Pérez A., Pérez-Cordero A. Efecto inhibitorio de compuestos tipo metabolitos de bacterias endófitas contra Colletotrichum gloeosporioides y Burkholderia glumae. Rev. Colomb. Cienc. Anim. RECIA. 2017;9:18–25. doi: 10.24188/recia.v9.nS.2017.516. [DOI] [Google Scholar]
- 57.Gonzalez Rodriguez R.M., Mendoza Rodriguez J., Pérez V., Soler A. Evaluación de la actividad antimicrobiana de bacterias endógenas contra Phytophthora nicotianae var. parasitica. In: Bartholomew D.P., Reinhardt D.H., Duarte Souza F.V., editors. Proceedings of the IX International Pineapple Symposium 1239; Havana, Cuba. 19 October 2017; Havana, Cuba: Acta Hortic. 1239; 2019. pp. 195–202. [Google Scholar]
- 58.Shrestha B.K., Karki H.S., Groth D.E., Jungkhun N., Ham J.H. Biological control activities of rice-associated Bacillus sp. strains against sheath blight and bacterial panicle blight of rice. PLoS ONE. 2016;11:e0146764. doi: 10.1371/journal.pone.0146764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nuñez R.A.V., Ruiz W.F.R., Orrillo E.O., Chávez E.E.T., Delgado J.T. Caracterización genética de bacterias endofíticas de arroz (Oryza sativa L.) con actividad antimicrobiana contra Burkholderia glumae. Rev. Argent. Microbiol. 2020;52:51–60. doi: 10.1016/j.ram.2019.12.002. [DOI] [PubMed] [Google Scholar]
- 60.López S.M.Y., Franco M.E.E., Pastorino G.N., Saparrat M., Ronco B.L., Balatti P.A. Bacterias endófitas de tomate y su acción antagonista contra hongos fitopatógenos; Proceedings of the IV Congreso Argentino de Fitopatología; Mendoza, Argentina. 19–21 April 2017; p. 133. [Google Scholar]
- 61.Villanueva D.M.L. Eficacia de biofungicidas frente a la caída de plántula de pepino, inducida por Pythium aphanidermatum. Rev. Investig. Agroprod. Sustent. 2018;2:72–78. doi: 10.25127/aps.20181.387. [DOI] [Google Scholar]
- 62.Berlanga-Padilla A.M., Gallou A., Ayala-Zermeño M.A., Serna-Domínguez M.G., Montesinos-Matías R., Rodríguez-Rodríguez J.C., Arredondo-Bernal H.C. Entomopathogenic fungi associated to Diaphorina citri (Hemiptera: Liviidae) in Colima, Mexico. Rev. Mex. Biodivers. 2018;89:986–1001. [Google Scholar]
- 63.Bautista J.P., Barbosa H., Uribe-Vélez D. Prototipo de formulación a base de Rhodotorula mucilaginosa para el control de Botrytis cinerea en Rosas. Rev. Colomb. Biotecnol. 2016;18:13–23. doi: 10.15446/rev.colomb.biote.v18n2.55826. [DOI] [Google Scholar]
- 64.Martínez-Padrón H.Y., Osorio-Hernández E.O., Estrada-Drouaillet B., López-Santillán J.A., Varela-Fuentes S.E., Torres-Castillo J.A., Victoria T. Control biológico de fitopatógenos mediante aislados de Trichoderma spp. AGRO. 2017;10:9–14. [Google Scholar]
- 65.Stocco M., Lampugnani G., Zuluaga S., Abramoff C., Cordo C., Mónaco C. Fungicida biológico a base de una cepa del hongo Trichoderma harzianum: Su supervivencia en el suelo. Rev. Fac. Agron. Univ. Nac. Plata. 2019;118:020. doi: 10.24215/16699513e020. [DOI] [Google Scholar]
- 66.Osorio-Hernández E., Conde-Martínez V., Michel-Aceves A.C., Lopez-Santillan J.A., Torres-Castillo J.A. In vitro activities of Trichoderma species against Phytophthora parasitica and Fusarium oxysporum. Afr. J. Microbiol. Res. 2016;10:521–527. [Google Scholar]
- 67.Cubilla-Ríos A.A., Ruíz-Díaz-Mendoza D.D., Romero-Rodríguez M.C., Flores-Giubi M.E., Barúa-Chamorro J.E. Antibiosis de proteínas y metabolitos en especies de Trichoderma contra aislamientos paraguayos de Macrophomina phaseolina. Agron. Mesoam. 2019;30:63–77. doi: 10.15517/am.v30i1.34423. [DOI] [Google Scholar]
- 68.Das M.M., Haridas M., Sabu A. Biological control of black pepper and ginger pathogens, Fusarium oxysporum, Rhizoctonia solani and Phytophthora capsici, using Trichoderma spp. Biocatal. Agric. Biotechnol. 2019;17:177–183. [Google Scholar]
- 69.Andrade-Hoyos P., Luna-Cruz A., Osorio-Hernández E., Molina-Gayosso E., Landero-Valenzuela N., Barrales-Cureño H.J. Antagonismo de Trichoderma spp. vs hongos asociados a la marchitez de chile. Rev. Mex. Cienc. Agric. 2019;10:1259–1272. doi: 10.29312/remexca.v10i6.1326. [DOI] [Google Scholar]
- 70.Martínez B., Infante D., Reyes Y. Trichoderma spp. y su función en el control de plagas en los cultivos. Rev. Prot. Veg. 2013;28:1–11. [Google Scholar]
- 71.Finley S.D., Broadbelt L.J., Hatzimanikatis V. In silico feasibility of novel biodegradation pathways for 1, 2, 4-trichlorobenzene. BMC Syst. Biol. 2010;4:7. doi: 10.1186/1752-0509-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Timmusk S., Behers L., Muthoni J., Muraya A., Aronsson A.C. Perspectives and challenges of microbial application for crop improvement. Front. Plant Sci. 2017;8:49. doi: 10.3389/fpls.2017.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bashan Y. Inoculants of plant growth-promoting bacteria for use in agriculture. Biotechnol. Adv. 1998;16:729–770. doi: 10.1016/S0734-9750(98)00003-2. [DOI] [Google Scholar]
- 74.Pacheco-Aguirre J.A., Ruíz-Sánchez E., Ballina-Gómez H.S., Alvarado-López C.J. Does polymer-based encapsulation enhance performance of plant growth promoting microorganisms? A meta-analysis view. Agrociencia. 2017;51:173–187. [Google Scholar]
- 75.Przyklenk M., Vemmer M., Hanitzsch M., Patel A. A bioencapsulation and drying method increases shelf life and efficacy of Metarhizium brunneum conidia. J. Microencapsul. 2017;34:498–512. doi: 10.1080/02652048.2017.1354941. [DOI] [PubMed] [Google Scholar]
- 76.Hien N.Q., Nagasawa N., Tham L.X., Yoshii F., Dang V.H., Mitomo H., Kume T. Growth-promotion of plants with depolymerized alginates by irradiation. Radiat. Phys. Chem. 2000;59:97–101. doi: 10.1016/S0969-806X(99)00522-8. [DOI] [Google Scholar]
- 77.Olatunji O. Understanding Their Industrial Significance and Environmental Implications. 1st ed. Springer International Publishing; Cham, Switzerland: 2020. Aquatic biopolymers. [Google Scholar]
- 78.Covarrubias S.A., de-Bashan L.E., Moreno M., Bashan Y. Alginate beads provide a beneficial physical barrier against native microorganisms in wastewater treated with immobilized bacteria and microalgae. Appl. Microbiol. Biotechnol. 2012;93:2669–2680. doi: 10.1007/s00253-011-3585-8. [DOI] [PubMed] [Google Scholar]
- 79.Bashan Y., Holguin G. Azospirillum–plant relationships: Environmental and physiological advances (1990–1996) Can. J. Microbiol. 1997;43:103–121. doi: 10.1139/m97-015. [DOI] [PubMed] [Google Scholar]
- 80.He Y., Wu Z., Ye B.C., Wang J., Guan X., Zhang J. Viability evaluation of alginate-encapsulated Pseudomonas putida Rs-198 under simulated salt-stress conditions and its effect on cottonágrowth. Eur. J. Soil Biol. 2016;75:135–141. doi: 10.1016/j.ejsobi.2016.05.002. [DOI] [Google Scholar]
- 81.Bashan Y. Alginate beads as synthetic inoculant carriers for slow release of bacteria that affect plant growth. Appl. Environ. Microbiol. 1986;51:1089–1098. doi: 10.1128/aem.51.5.1089-1098.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bashan Y., Hernandez J.P., Leyva L.A., Bacilio M. Alginate microbeads as inoculant carriers for plant growth-promoting bacteria. Biol. Fertil. Soils. 2002;35:359–368. doi: 10.1007/s00374-002-0481-5. [DOI] [Google Scholar]
- 83.Trejo A., De-Bashan L.E., Hartmann A., Hernandez J.P., Rothballer M., Schmid M., Bashan Y. Recycling waste debris of immobilized microalgae and plant growth-promoting bacteria from wastewater treatment as a resource to improve fertility of eroded desert soil. Environ. Exp. Bot. 2012;75:65–73. doi: 10.1016/j.envexpbot.2011.08.007. [DOI] [Google Scholar]
- 84.Schoebitz M., Simonin H., Poncelet D. Starch filler and osmoprotectants improve the survival of rhizobacteria in dried alginate beads. J. Microencapsul. 2012;29:532–538. doi: 10.3109/02652048.2012.665090. [DOI] [PubMed] [Google Scholar]
- 85.Bacilio M., Rodriguez H., Moreno M., Hernandez J.P., Bashan Y. Mitigation of salt stress in wheat seedlings by a gfp-tagged Azospirillum lipoferum. Biol. Fertil. Soils. 2004;40:188–193. doi: 10.1007/s00374-004-0757-z. [DOI] [Google Scholar]
- 86.Sivakumar P.K., Parthasarthi R., Lakshmipriya V.P. Encapsulation of plant growth promoting inoculant in bacterial alginate beads enriched with humic acid. Int. J. Curr. Microbiol. Appl. Sci. 2014;3:415–422. [Google Scholar]
- 87.Joe M.M., Saravanan V.S., Islam M.R., Sa T. Development of alginate-based aggregate inoculants of Methylobacterium sp. and Azospirillum brasilense tested under in vitro conditions to promote plant growth. J. Appl. Microbiol. 2014;116:408–423. doi: 10.1111/jam.12384. [DOI] [PubMed] [Google Scholar]
- 88.Bashan Y., Salazar B., Puente M.E. Responses of native legume desert trees used for reforestation in the Sonoran Desert to plant growth-promoting microorganisms in screen house. Biol. Fertil. Soils. 2009;45:655–662. doi: 10.1007/s00374-009-0368-9. [DOI] [Google Scholar]
- 89.Yabur R., Bashan Y., Hernández-Carmona G. Alginate from the macroalgae Sargassum sinicola as a novel source for microbial immobilization material in wastewater treatment and plant growth promotion. J. Appl. Phycol. 2007;19:43–53. doi: 10.1007/s10811-006-9109-8. [DOI] [Google Scholar]
- 90.Mengual C., Roldán A., Caravaca F., Schoebitz M. Advantages of inoculation with immobilized rhizobacteria versus amendment with olive-mill waste in the afforestation of a semiarid area with Pinus halepensis Mill. Ecol. Eng. 2014;73:1–8. doi: 10.1016/j.ecoleng.2014.09.007. [DOI] [Google Scholar]
- 91.Fages J. An industrial view of Azospirillum inoculants: Formulation and application technology. Symbiosis. 1992;13:15–26. [Google Scholar]
- 92.Bashan Y., González L.E. Long-term survival of the plant-growth-promoting bacteria Azospirillum brasilense and Pseudomonas fluorescens in dry alginate inoculant. Appl. Microbiol. Biotechnol. 1999;51:262–266. doi: 10.1007/s002530051391. [DOI] [Google Scholar]
- 93.El-Gamal M.A., Abo-Kora H.A., Massoud O.N. Impact of formulated Azospirillum lipoferum, Bacillus polymyxa and Nostoc muscorum on wheat productivity. Int. J. Chemtech Res. 2015;8:100–113. [Google Scholar]
- 94.Campos D.C., Acevedo F., Morales E., Aravena J., Amiard V., Jorquera M.A., Rubilar M. Microencapsulation by spray drying of nitrogen-fixing bacteria associated with lupin nodules. World J. Microbiol. Biotechnol. 2014;30:2371–2378. doi: 10.1007/s11274-014-1662-8. [DOI] [PubMed] [Google Scholar]
- 95.Schoebitz M., Ceballos C., Ciamp L. Effect of immobilized phosphate solubilizing bacteria on wheat growth and phosphate uptake. J. Soil Sci. Plant Nutr. 2013;13:1–10. doi: 10.4067/S0718-95162013005000001. [DOI] [Google Scholar]
- 96.Reetha D., Kumaresan G., John Milton D. Studies to improve the shelf life of Azospirillum lipoferum immobilized in alginate beads. Int. J. Recent Sci. Res. 2014;5:2178–2182. [Google Scholar]
- 97.Gagné-Bourque F., Xu M., Dumont M.J., Jabaji S. Pea protein alginate encapsulated Bacillus subtilis B26, a plant biostimulant, provides controlled release and increased storage survival. J Fertil. Pestic. 2015;6:2–9. doi: 10.4172/jbfbp.1000157. [DOI] [Google Scholar]
- 98.Tu L., He Y., Yang H., Wu Z., Yi L. Preparation and characterization of alginate–gelatin microencapsulated Bacillus subtilis SL-13 by emulsification/internal gelation. J. Biomater. Sci. Polym. Ed. 2015;26:735–749. doi: 10.1080/09205063.2015.1056075. [DOI] [PubMed] [Google Scholar]
- 99.Young C.C., Rekha P.D., Lai W.A., Arun A.B. Encapsulation of plant growth-promoting bacteria in alginate beads enriched with humic acid. Biotechnol. Bioeng. 2006;95:76–83. doi: 10.1002/bit.20957. [DOI] [PubMed] [Google Scholar]
- 100.Bextine B.R., Thorvilson H.G. Field applications of bait-formulated Beauveria bassiana alginate pellets for biological control of the red imported fire ant (Hymenoptera: Formicidae) Environ. Entomol. 2002;31:746–752. doi: 10.1603/0046-225X-31.4.746. [DOI] [Google Scholar]
- 101.Trivedi P., Pandey A. Recovery of plant growth-promoting rhizobacteria from sodium alginate beads after 3 years following storage at 4 C. J. Ind. Microbiol. Biotechnol. 2008;35:205–209. doi: 10.1007/s10295-007-0284-7. [DOI] [PubMed] [Google Scholar]
- 102.Trivedi P., Pandey A., Palni L.M.S. Carrier-based preparations of plant growth-promoting bacterial inoculants suitable for use in cooler regions. World J. Microbiol. Biotechnol. 2005;21:941–945. doi: 10.1007/s11274-004-6820-y. [DOI] [Google Scholar]
- 103.Vassileva M., Azcon R., Barea J.M., Vassilev N. Effect of encapsulated cells of Enterobacter sp on plant growth and phosphate uptake. Bioresour. Technol. 1999;67:229–232. doi: 10.1016/S0960-8524(98)00130-8. [DOI] [Google Scholar]
- 104.Vassilev N., Vassileva M., Azcon R., Medina A. Application of free and Ca-alginate-entrapped Glomus deserticola and Yarowia lipolytica in a soil–plant system. J. Biotechnol. 2001;91:237–242. doi: 10.1016/S0168-1656(01)00341-8. [DOI] [PubMed] [Google Scholar]
- 105.Wu Z., Zhao Y., Kaleem I., Li C. Preparation of calcium–alginate microcapsuled microbial fertilizer coating Klebsiella oxytoca Rs-5 and its performance under salinity stress. Eur. J. Soil Biol. 2011;47:152–159. doi: 10.1016/j.ejsobi.2010.11.008. [DOI] [Google Scholar]
- 106.Sarrocco S., Raeta R., Vannacci G. Seeds encapsulation in calcium alginate pellets. Seed Sci. Technol. 2004;32:649–661. doi: 10.15258/sst.2004.32.3.01. [DOI] [Google Scholar]
- 107.Wang H.Q., Hua F., Zhao Y.C., Li Y., Wang X. Immobilization of Pseudomonas sp. DG17 onto sodium alginate–attapulgite–calcium carbonate. Biotechnol. Biotechnol. Equip. 2014;28:834–842. doi: 10.1080/13102818.2014.961123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Van Elsas J.D., Trevors J.T., Jain D., Wolters A.C., Heijnen C.E., Van Overbeek L.S. Survival of, and root colonization by, alginate-encapsulated Pseudomonas fluorescens cells following introduction into soil. Biol. Fertil. Soils. 1992;14:14–22. doi: 10.1007/BF00336297. [DOI] [Google Scholar]
- 109.Russo A., Basaglia M., Tola E., Casella S. Survival, root colonisation and biocontrol capacities of Pseudomonas fluorescens F113 LacZY in dry alginate microbeads. J. Ind. Microbiol. Biotechnol. 2001;27:337–342. doi: 10.1038/sj.jim.7000154. [DOI] [PubMed] [Google Scholar]
- 110.El-Komy H. Coimmobilization of Azospirillum lipoferum and Bacillus megaterium for successful phosphorus and nitrogen nutrition of wheat plants. Food Technol. Biotechnol. 2005;43:19–27. [Google Scholar]
- 111.Loján P., Demortier M., Velivelli S.L., Pfeiffer S., Suárez J.P., De Vos P., Declerck S. Impact of plant growth-promoting rhizobacteria on root colonization potential and life cycle of Rhizophagus irregularis following co-entrapment into alginate beads. J. Appl. Microbiol. 2017;122:429–440. doi: 10.1111/jam.13355. [DOI] [PubMed] [Google Scholar]
- 112.Gurley H.G., Zdor R.E. Differential rhizosphere establishment and cyanide production by alginate-formulated weed-deleterious rhizobacteria. Curr. Microbiol. 2005;50:167–171. doi: 10.1007/s00284-004-4422-4. [DOI] [PubMed] [Google Scholar]
- 113.Viveganandan G., Jauhri K.S. Growth and survival of phosphate-solubilizing bacteria in calcium alginate. Microbiol. Res. 2000;155:205–207. doi: 10.1016/S0944-5013(00)80033-6. [DOI] [PubMed] [Google Scholar]
- 114.Rekha P.D., Lai W.A., Arun A.B., Young C.C. Effect of free and encapsulated Pseudomonas putida CC-FR2-4 and Bacillus subtilis CC-pg104 on plant growth under gnotobiotic conditions. Bioresour. Technol. 2007;98:447–451. doi: 10.1016/j.biortech.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 115.He Y., Wu Z., Tu L., Han Y., Zhang G., Li C. Encapsulation and characterization of slow-release microbial fertilizer from the composites of bentonite and alginate. Appl. Clay Sci. 2015;109:68–75. doi: 10.1016/j.clay.2015.02.001. [DOI] [Google Scholar]
- 116.Wu Z., Guo L., Zhao Y., Li C. Effect of free and encapsulated Raoultella planticola Rs-2 on cotton growth promotion under salt stress. J. Plant Nutr. 2014;37:1187–1201. doi: 10.1080/01904167.2014.881865. [DOI] [Google Scholar]
- 117.Forestier S., Alvarado G., Badjel Badjel S., Lesueur D. Effect of Rhizobium inoculation methodologies on nodulation and growth of Leucaena leucocephala. World J. Microbiol. Biotechnol. 2001;17:359–362. doi: 10.1023/A:1016627012296. [DOI] [Google Scholar]
- 118.Farhat M.B., Taktek S., Chouayekh H. Encapsulation in alginate enhanced the plant growth promoting activities of two phosphate solubilizing bacteria isolated from the phosphate mine of Gafsa. Net J. Agric. Sci. 2014;2:131–139. [Google Scholar]
- 119.Sabaratnam S., Traquair J.A. Formulation of a Streptomyces biocontrol agent for the suppression of Rhizoctonia damping-off in tomato transplants. Biol. Control. 2002;23:245–253. doi: 10.1006/bcon.2001.1014. [DOI] [Google Scholar]
- 120.Lopes A.R.D.O., Locatelli G.O., Barbosa R.D.M., Lobo Junior M., Moura Mascarin G., Lamenha Luna Finkler C. Preparation, characterisation and cell viability of encapsulated Trichoderma asperellum in alginate beads. J. Microencapsul. 2020;37:270–282. doi: 10.1080/02652048.2020.1729884. [DOI] [PubMed] [Google Scholar]
- 121.Bagewadi Z.K., Mulla S.I., Ninnekar H.Z. Purification and immobilization of laccase from Trichoderma harzianum strain HZN10 and its application in dye decolorization. J. Genet. Eng. Biotechnol. 2017;15:139–150. doi: 10.1016/j.jgeb.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Vinceković M., Jalsenjak N., Topolovec-Pintarić S., Đermić E., Bujan M., Juric S. Encapsulation of biological and chemical agents for plant nutrition and protection: Chitosan/alginate microcapsules loaded with copper cations and Trichoderma viride. J. Agric. Food Chem. 2016;64:8073–8083. doi: 10.1021/acs.jafc.6b02879. [DOI] [PubMed] [Google Scholar]
- 123.Morsy E.M. Efficiency of free and encapsulated yeast strains as PGRs producers on faba bean plants. Middle East J. Appl. Sci. 2015;5:534–542. [Google Scholar]
- 124.Martin M.J., Lara-Villoslada F., Ruiz M.A., Morales M.E. Effect of unmodified starch on viability of alginate-encapsulated Lactobacillus fermentum CECT5716. LWT Food Sci. Technol. 2013;53:480–486. doi: 10.1016/j.lwt.2013.03.019. [DOI] [Google Scholar]
- 125.Wong C.K.F., Saidi N.B., Vadamalai G., Teh C.Y., Zulperi D. Effect of bioformulations on the biocontrol efficacy, microbial viability and storage stability of a consortium of biocontrol agents against Fusarium wilt of banana. J. Appl. Microbiol. 2019;127:544–555. doi: 10.1111/jam.14310. [DOI] [PubMed] [Google Scholar]
- 126.Bashan Y., de-Bashan L.E., Prabhu S.R. Superior polymeric formulations and emerging innovative products of bacterial inoculants for sustainable agriculture and the environment. In: Singh H.B., Sarma B.K., Keswani C., editors. Agriculturally Important Microorganisms. Springer; Singapore: 2016. pp. 15–46. [Google Scholar]
- 127.Glick B.R., Bashan Y. Genetic manipulation of plant growth-promoting bacteria to enhance biocontrol of phytopathogens. Biotechnol. Adv. 1997;15:353–378. doi: 10.1016/S0734-9750(97)00004-9. [DOI] [PubMed] [Google Scholar]
- 128.Humbert P., Przyklenk M., Vemmer M., Patel A.V. Calcium gluconate as cross-linker improves survival and shelf life of encapsulated and dried Metarhizium brunneum and Saccharomyces cerevisiae for the application as biological control agents. J. Microencapsul. 2017;34:47–56. doi: 10.1080/02652048.2017.1282550. [DOI] [PubMed] [Google Scholar]
- 129.Shcherbakova E.N., Shcherbakov A.V., Rots P.Y., Gonchar L.N., Mulina S.A., Yahina L.M., Chebotar V.K. Inoculation technology for legumes based on alginate encapsulation. Agron. Res. 2018;16:2156–2168. [Google Scholar]
- 130.Vassilev N., Toro M., Vassileva M., Azcon R., Barea J.M. Rock phosphate solubilization by immobilized cells of Enterobacter sp. in fermentation and soil conditions. Bioresour. Technol. 1997;61:29–32. doi: 10.1016/S0960-8524(97)84694-9. [DOI] [Google Scholar]
- 131.Roger S., Talbot D., Bee A. Preparation and effect of Ca2+ on water solubility, particle release and swelling properties of magnetic alginate films. J. Magn. Magn. Mater. 2006;305:221–227. doi: 10.1016/j.jmmm.2006.01.005. [DOI] [Google Scholar]
- 132.Shcherbakova E.N., Shcherbakov A.V., Andronov E.E., Gonchar L.N., Kalenskaya S.M., Chebotar V.K. Combined pre-seed treatment with microbial inoculants and Mo nanoparticles changes composition of root exudates and rhizosphere microbiome structure of chickpea (Cicer arietinum L.) plants. Symbiosis. 2017;73:57–69. doi: 10.1007/s13199-016-0472-1. [DOI] [Google Scholar]
- 133.Belaid F.M., Abubaker N.S., El-Komy H.M. Survival of Azospirillum brasilense under different moisture content levels. EPH-Int. J. Appl. Sci. 2019;1:545–552. [Google Scholar]
- 134.Byeon S.Y., Cheon K.S., Kim S., Yun S.H., Oh H.J., Park S.R., Lee H.J. Comparative Analysis of Sequence Polymorphism in Complete Organelle Genomes of the ‘Golden Tide’ Seaweed Sargassum horneri between Korean and Chinese Forms. Sustainability. 2020;12:7280. doi: 10.3390/su12187280. [DOI] [Google Scholar]
- 135.Pardilhó S., Cotas J., Pereira L., Oliveira M.B., Dias J.M. Marine macroalgae in a circular economy context: A comprehensive analysis focused on residual biomass. Biotechnol. Adv. 2022;60:107987. doi: 10.1016/j.biotechadv.2022.107987. [DOI] [PubMed] [Google Scholar]
- 136.Fernando I.P.S., Kirindage K.G.I.S., Jeon H.N., Han E.J., Jayasinghe A.M.K., Ahn G. Preparation of microspheres by alginate purified from Sargassum horneri and study of pH-responsive behavior and drug release. Int. J. Biol. Macromol. 2022;202:681–690. doi: 10.1016/j.ijbiomac.2022.01.171. [DOI] [PubMed] [Google Scholar]
- 137.Cristina G., Camelin E., Ottone C., Garofalo S.F., Jorquera L., Castro M., Tommasi T. Recovery of humic acids from anaerobic sewage sludge: Extraction, characterization and encapsulation in alginate beads. Int. J. Biol. Macromol. 2020;164:277–285. doi: 10.1016/j.ijbiomac.2020.07.097. [DOI] [PubMed] [Google Scholar]
- 138.Bennacef C., Desobry-Banon S., Probst L., Desobry S. Advances on alginate use for spherification to encapsulate biomolecules. Food Hydrocoll. 2021;118:106782. doi: 10.1016/j.foodhyd.2021.106782. [DOI] [Google Scholar]
- 139.Strobel S.A., Knowles L., Nitin N., Scher H.B., Jeoh T. Comparative technoeconomic process analysis of industrial-scale microencapsulation of bioactives in cross-linked alginate. J. Food Eng. 2020;266:109695. doi: 10.1016/j.jfoodeng.2019.109695. [DOI] [Google Scholar]
- 140.Dimitrellou D., Kandylis P., Lević S., Petrović T., Ivanović S., Nedović V., Kourkoutas Y. Encapsulation of Lactobacillus casei ATCC 393 in alginate capsules for probiotic fermented milk production. LWT. 2019;116:108501. doi: 10.1016/j.lwt.2019.108501. [DOI] [Google Scholar]
- 141.Dhakal S., He J. Microencapsulation of Vitamins in Food Applications to Prevent Losses in Processing and Storage: A Review. Food Res. Int. 2020;137:109326. doi: 10.1016/j.foodres.2020.109326. [DOI] [PubMed] [Google Scholar]
- 142.Saberi-Riseh R., Moradi-Pour M., Mohammadinejad R., Kumar V. Biopolymers for Biological Control of Plant Pathogens: Advances in Microencapsulation of Beneficial Microorganisms. Polymers. 2021;13:1938. doi: 10.3390/polym13121938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yang M., Liang Z., Wang L., Qi M., Luo Z., Li L. Microencapsulation Delivery System in Food Industry—Challenge and the Way Forward. Adv. Polym. Technol. 2020;2020:e7531810. doi: 10.1155/2020/7531810. [DOI] [Google Scholar]
- 144.Panghal A., Jaglan S., Sindhu N., Manga A., Surendran V., Chhikara N. Nanobiotechnology in Bioformulation. Springer; Cham, Switzerland: 2019. Microencapsulation for delivery of probiotic bacteria; pp. 135–160. [Google Scholar]
- 145.Procuraduría Federal del Consumidor Biofertilizantes. [(accessed on 7 May 2022)];2021 Available online: http://www.gob.mx/profeco/articulos/biofertilizantes?idiom=es.
- 146.Yooyongwech S., Suriyan C.U., Tisarum R., Therawitaya C., Samphumphung T., Aumtong S., Phisalaphong M. Influence of different encapsulation types of arbuscular mycorrhizal fungi on physiological adaptation and growth promotion of maize (Zea mays L.) subjected to water deficit. Not. Bot. Horti Agrobot. Cluj-Napoca. 2019;47:213–220. doi: 10.15835/nbha47111249. [DOI] [Google Scholar]
- 147.Prasad R., Bhattacharyya A., Nguyen Q.D. Nanotechnology in sustainable agriculture: Recent developments, challenges, and perspectives. Front. Microbiol. 2017;8:1014. doi: 10.3389/fmicb.2017.01014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pirzada T., de Farias B.V., Mathew R., Guenther R.H., Byrd M.V., Sit T.L., Khan S.A. Recent advances in biodegradable matrices for active ingredient release in crop protection: Towards attaining sustainability in agriculture. Curr. Opin. Colloid Interface Sci. 2020;48:121–136. doi: 10.1016/j.cocis.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Dębowski M., Krzemieniewski M., Zieliński M., Kazimierowicz J. Immobilized microalgae-based photobioreactor for CO2 capture (IMC-CO2PBR): Efficiency estimation, technological parameters, and prototype concept. Atmosphere. 2021;12:1031. doi: 10.3390/atmos12081031. [DOI] [Google Scholar]
- 150.Cheirsilp B., Thawechai T., Prasertsan P. Immobilized oleaginous microalgae for production of lipid and phytoremediation of secondary effluent from palm oil mill in fluidized bed photobioreactor. Bioresour. Technol. 2017;241:787–794. doi: 10.1016/j.biortech.2017.06.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.