Abstract
Background
Brain tumors are the leading cause of cancer death for pediatric patients. Pelareorep, an immunomodulatory oncolytic reovirus, has intravenous efficacy in preclinical glioma models when preconditioned with GM-CSF (sargramostim). We report a phase I trial with the primary goal of evaluating the safety of sargramostim/pelareorep in pediatric patients with recurrent or refractory high-grade brain tumors and a secondary goal of characterizing immunologic responses.
Methods
The trial was open to pediatric patients with recurrent or refractory high-grade brain tumors (3 + 3 cohort design). Each cycle included 3 days of subcutaneous sargramostim followed by 2 days of intravenous pelareorep. Laboratory studies and imaging were acquired upon recruitment and periodically thereafter.
Results
Six patients participated, including three glioblastoma, two diffuse intrinsic pontine glioma, and one medulloblastoma. Two pelareorep dose levels of 3 × 108 and 5 × 108 tissue culture infectious dose 50 (TCID50) were assessed. One patient experienced a dose limiting toxicity of persistent hyponatremia. Common low-grade (1 or 2) adverse events included transient fatigue, hypocalcemia, fever, flu-like symptoms, thrombocytopenia, and leukopenia. High-grade (3 or 4) adverse events included neutropenia, lymphopenia, leukopenia, hypophosphatemia, depressed level of consciousness, and confusion. All patients progressed on therapy after a median of 32.5 days and died a median of 108 days after recruitment. Imaging at progression did not show evidence of pseudoprogression or inflammation. Correlative assays revealed transient but consistent changes in immune cells across patients.
Conclusions
Sargramostim/pelareorep was administered to pediatric patients with recurrent or refractory high-grade brain tumors. Hyponatremia was the only dose limiting toxicity (DLT), though maximum tolerated dose (MTD) was not determined.
Keywords: oncolytic virotherapy, pediatric brain tumors, reovirus, sargramostim
Key Points.
Sargramostim/pelareorep was used to treat six children with recurrent high-grade brain tumors.
Therapy was well-tolerated, with a single DLT (Grade 3 hyponatremia).
Importance of the Study.
Brain tumors are the leading cause of pediatric cancer death in the United States. Therefore, novel therapies are needed to improve survival. Sargramostim/pelareorep is a treatment regimen that has been used preclinically to deliver systemic oncolytic virus across the blood–brain barrier into brain tumors, but has not been tested in pediatric patients with CNS tumors. In this phase I study of pediatric patients with recurrent or refractory high-grade brain tumors, therapy was well tolerated with only one dose limiting toxicity of grade three hyponatremia, but all patients experienced disease progression. Correlative studies revealed transient changes in monocytes, neutrophils, and platelets, and seroconversion with neutralizing antibodies by the end of the first cycle. Future studies may benefit from treating patients earlier in their disease course to allow multiple cycles of therapy, or in combination with other immunomodulatory agents.
Brain tumors are the most common solid tumor in pediatric patients, and are the leading cause of pediatric cancer death in the United States.1,2 In light of this, novel treatments are needed to improve survival.
Pelareorep is a Dearing type 3 strain virus that can selectively replicate in ras mutant cells, inducing oncolysis and generating an anti-tumor immune response.3–6 Phase I, II, and III clinical trials in adults have evaluated intratumoral or intravenous delivery against a variety of tumors including head and neck cancers, sarcoma, melanoma, breast cancer, and prostate cancer, with several complete and partial responses.7 Single agent and combination therapies have been well-tolerated, with frequently reported toxicities of flu-like symptoms, lymphopenia, and neutropenia.7
In two phase I trials treating adult brain tumor patients, intratumoral administration was safe and well-tolerated without reaching a maximum tolerated dose (MTD).8,9 In a third study, adults with brain tumors underwent intravenous administration of reovirus immediately prior to surgical resection, with histological analysis demonstrating that reovirus was successfully delivered into glioma tissue and induced local T-cell recruitment.10
In pediatric patients, a 2015 Children’s Oncology Group study treated 28 patients with a variety of relapsed or refractory non-CNS tumors with pelareorep alone or in combination with cyclophosphamide.11 Treatments were well-tolerated, with a single dose limiting toxicity (DLT) of thromboembolism considered possibly related to pelareorep. Non dose limiting toxicities included anemia, leukopenia, lymphopenia, neutropenia, thrombocytopenia, increased aspartate aminotransferase/alanine aminotransferase (AST/ALT), and flu-like symptoms. MTD was not reached and the recommended phase II dose was determined to be 5 × 108 tissue culture infectious dose 50 (TCID50)/kg/dose. All patients progressed within three cycles of therapy without any complete or partial responses.
Correlate experiments from trials in adults demonstrated that the virus was rapidly cleared from the plasma after intravenous administration, but could be isolated from CD11b + monocyte/macrophages despite the presence of neutralizing antibodies.12 This finding led to a cytokine combination strategy whereby GM-CSF (sargramostim) preconditioning was used to mobilize the CD11b + cellular compartment, enhancing the efficacy of intravenous reovirus against extra-cranial murine melanoma.13 Subsequent studies showed that GM-CSF/intravenous reovirus combination regimens were also efficacious against intracranial murine glioma.10 This combination regimen has demonstrated clinical safety in a trial treating adult glioblastoma (ReoGlio Trial),14 but has not been studied in a pediatric population.
Building on these studies, we tested a GM-CSF/reovirus treatment regimen in pediatric patients with recurrent or refractory high-grade brain tumors. To further understand the interaction between the host immune system and therapy, we also collected blood and serum for correlative analysis. We here report the results from the treatment of six patients.
Methods
Approval
This study (NCT02444546) was designed and approved in accordance with the FDA, Mayo Clinic Institutional Review Board (IRB), Mayo Clinic Institutional Biosafety Committee (IBC), Mayo Clinic Scientific Review Committee-A, and the Mayo Clinic Pediatric and Adolescent Research Committee.
Informed Consent
Written informed consent was obtained from the parents of patients. Additionally, all patients were required to be able to understand and be willing to provide informed assent.
Eligibility Criteria
This study was open to patients between the ages of 10 and 21 years with progressive high-grade primary brain tumors and life expectancy >3 months. Patients were only eligible if baseline laboratory values demonstrated ANC > 800/uL, ALC > 250/uL, platelet count > 70 000/uL without transfusions, hemoglobin > 7.0 g/dL, total bilirubin < 1.5× institutional normal, AST < 3× institutional normal, serum albumin > 2 g/dL, and creatinine < 1.5× institutional normal or GFR > 70 mL/min/1.73 m.2 Karnofsky or Lansky Performance Scores needed to be above 50 within the two weeks prior to trial initiation. Patients were required to be receiving stable or decreasing doses of dexamethasone for at least 1 week prior to initiation of therapy and remain below 0.1 mg/kg/day and a total daily dose of 4 mg/day. Patients were excluded from the trial if they had uncontrolled intercurrent or concurrent illnesses, a history of HIV or TB, vaccinations within the last 14 days, previous viral-based therapy, or other concurrent anti-tumor therapies.
Study Drug and Treatment Plan
Reovirus (pelareorep) was acquired from Oncolytics Biotech Inc. and administered according to Institutional Biosafety Committee recommendations. Each cycle of therapy began with sargramostim (GM-CSF) administered subcutaneously at 250 mcg/m2 on Days 1 and 2, followed by 3 days of pelareorep administered IV over 60 min. Twenty-eight days after the first dose of GM-CSF, if the patient had not exhibited DLTs, a second cycle was initiated, up to a maximum of 12 cycles.
Trial Design
The trial was a dose escalation study with a 3 + 3 cohort design. Briefly, three patients were recruited at each dose level. If a DLT was observed in one of the first three patients at a dose, three more patients would be recruited at the same dose level. If after enrolling six patients at a specific dose level a DLT was observed in two or more patients, then the maximum tolerable dose (MTD) would have been exceeded and would be defined as the previous dose level. If two or more DLTs were experienced at dose level 1, de-escalation to dose level 0 would occur for the next three patients. At the outset of the trial, dose levels 0, 1, and 2 were 3 × 108, 5 × 108, and 8.3 × 108 TCID50/kg/dose, respectively. Any patient experiencing disease progression, unacceptable adverse events or DLTs, intercurrent illness preventing treatment administration, elective withdrawal from the trial, or changes in the patient rendering the patient unacceptable for further treatment in the judgment of the treating physician was transitioned from the active treatment phase to the observation phase of the trial.
Toxicities
Toxicities were graded using the revised NCI CTCAE version 4.0. Blood was collected every week in cycle one and every other week thereafter to assess for toxicities and DLTs. DLTs were defined as Cycle 1 adverse events attributed (definitely, probably, or possibly) to the study treatment, including Grade 4 anemia with life-threatening consequences, myelosuppression causing a > 14 day delay of treatment, Grade 4 neutropenia lasting more than 7 days, Grade 4 thrombocytopenia lasting more than 7 days, or Grade 3 or 4 allergic reactions. DLTs were also defined as any nonhematologic toxicities causing a >14 delay of treatment, nonhematologic Grade 4 adverse events, or nonhematologic Grade 3 adverse events which did not resolve to less than or equal to Grade 1 within 7 days. If a patient experienced a DLT attributable to Pelareorep, they were permanently discontinued from study treatment. DLTs were reported to the Mayo Clinic Cancer Center and to the FDA as required by the IRB protocol.
Response
The primary measure of response was by serial measures of the sum of the product of the two largest cross-sectional diameters (bidirectional product) using the RANO criteria.15 Because virotherapy treatment was likely to result in a higher than normal incidence of treatment-related contrast enhancement (“pseudoprogression”), patients were to continue therapy with close observation if there was a suspicion of pseudoprogression, followed by repeat imaging for reevaluation. MRI imaging was required at baseline, and in the absence of symptomatic progression, was set to occur prior to cycles 3, 6, 9, and 12.
Correlative Studies
Correlative studies were performed using patient blood and urine samples submitted at baseline, weekly during the first cycle, and every other week in subsequent cycles. Because patients were recruited over a period of several years, samples were analyzed in batches of three patients each. Samples in each batch were analyzed using a Luminex Multiplex assay for 46 different cytokines according to the manufacturer’s protocol. Cytokines that were below the limit of detection were considered missing data for subsequent analysis. Cytokine levels at each timepoint were normalized to each patient’s pretreatment baseline, log2 transformed, and clustered with the pheatmap function within RStudio.
Samples were also analyzed for neutralizing antibody titers against pelareorep with a modified neutralizing antibody assay as described previously.4
Statistical Analysis
Graphs were prepared using GraphPad Prism version 9.0.2 or RStudio. Statistical tests were not performed due to low sample size in the trial.
Results
Patient Characteristics
A total of 6 participants were enrolled in the trial between July 2015 and January 2017, with patient characteristics as described in Table 1. Median age was 14.5 (range 10–17), with five females. Two patients were diagnosed with diffuse intrinsic pontine glioma (DIPG), three with glioblastoma (GBM), and one with medulloblastoma, with a median of 11.7 months since diagnosis (range 9.8–75.2). All patients underwent prior radiation therapy, with a median of 7.1 months since RT (range 2.6–35.7). All GBM and medulloblastoma patients underwent prior surgical resection. Patients were heavily pretreated, with a median of 2.5 courses of previous chemotherapy (range 1–5). Three patients received daily dexamethasone prior to and throughout the trial. As established by the inclusion criteria, all patients had progressive disease. One of the four patients with infratentorial primary tumors had supratentorial metastases, and both patients with supratentorial tumors had either infratentorial or spinal metastases at the time of recruitment. Of the patients with glioblastoma, two had thalamic tumors and one had a cerebellar tumor. None of the patients had H3.3K27M testing performed on their tumors. Baseline Karnofsky Performance Scores ranged from 60 to 100.
Table 1.
Patient Characteristics and Course
| Patient | Age | Sex | Diagnosis | Months Since Diagnosis | Surgical Resection | Months Since RT | Number of Previous Chemotherapy Courses | Current Dexamethasone Use | Reolysin Dose Level | Number of Courses | Time to Progression, Days | Overall Survival, Days |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 10 | F | DIPG | 9.8 | N | 7.0 | 1 | Y; 4 mg/day | 1; 5 × 108 TCID50/kg | 1.0 | 28 | 82 |
| 2 | 17 | F | DIPG | 10.0 | N | 4.1 | 3 | Y; 3.5 mg/day | 1; 5 × 108 TCID50/kg | 2.0 | 48 | 60 |
| 3 | 14 | F | GBM | 13.3 | Y | 2.6 | 1 | N | 1; 5 × 108 TCID50/kg | 1.8 | 33 | 134 |
| 4 | 13 | F | GBM | 10.1 | Y | 7.2 | 2 | Y; 3.5 mg/day | 1*; 3 × 108 TCID50/kg | 1.0 | 20 | 26 |
| 5 | 17 | M | MB | 75.2 | Y | 10.6 | 5 | N | 1*; 3 × 108 TCID50/kg | 2.0 | 55 | 529 |
| 6 | 15 | F | GBM | 38.1 | Y | 35.7 | 3 | N | 1*; 3 × 108 TCID50/kg | 1.2 | 32 | 169 |
Reolysin Dose Level 1* refers to the revised dose level 1 following the treatment of the first three patients.
The first three patients received 1, 2, and 1.8 treatment cycles respectively at dose level 1 (5 × 108 TCID50/kg/dose pelareorep). Unfortunately, the third patient experienced Grade 3 depressed level of consciousness and Grade 4 confusion on Cycle 2 Day 4 “possibly but unlikely” related to treatment, and was found to have progressive disease on subsequent imaging. These clinical events occurred later than the initial 28-day cycle, and so did not qualify as a DLT. Additionally, although these symptoms were allocated as only possibly treatment-related, after correspondence with the NCI and Mayo IRB, dose levels were redefined, and the 3 + 3 design was restarted with revised dose levels 0, 1, and 2 of 1 × 108, 3 × 108, and 5 × 108 TCID50/kg/dose. Notably, the revised dose level one was the original dose level zero. The last three patients received 1, 2, and 1.2 treatment cycles, respectively, at the revised dose level 1 (3 × 108 TCID50/kg/dose) (Table 1). Unfortunately, after the trial was paused for analysis of the dose limiting toxicity in patient 6 described below, further patients were unable to be recruited, and study recruitment was closed.
Toxicity
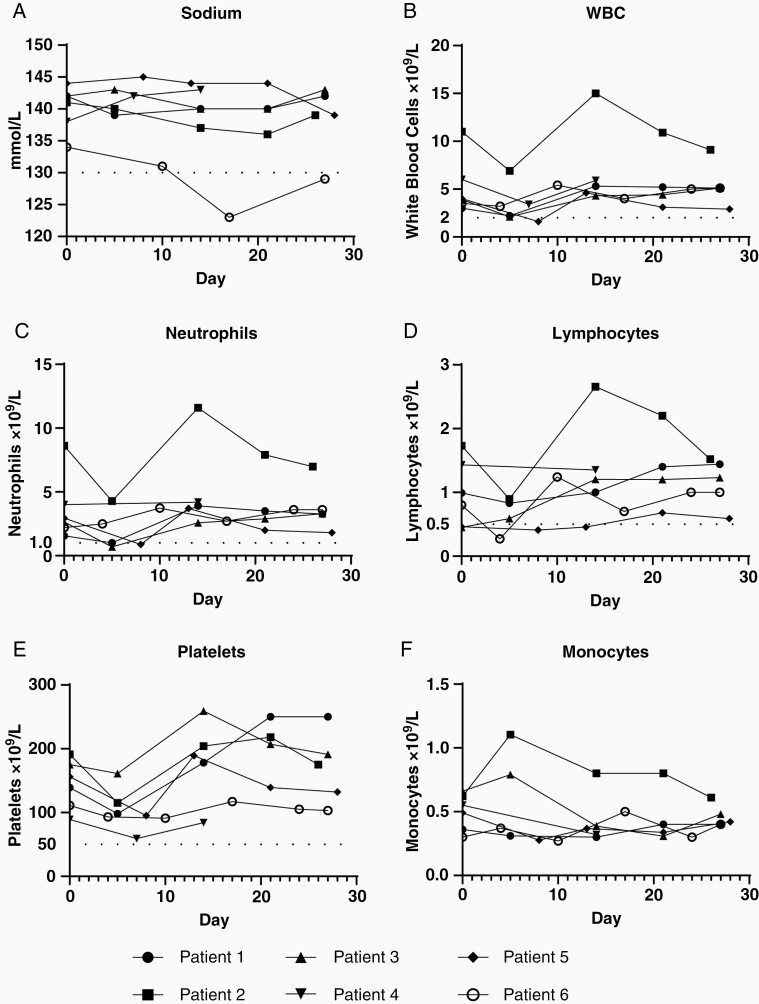
One dose limiting toxicity was experienced in the trial. Patient 6 developed sustained Grade 3 hyponatremia during the first cycle 13 days after study drug, defined as a sodium between 120 and 130 without recovery above 130 within 7 days. This event led to hospitalization for electrolyte management. Contributing factors for this patient include the lowest baseline sodium level among trial participants (Figure 1A) and concurrently taking amitriptyline and oxcarbazepine, both of which are associated with SIADH. Because this was attributed as possibly related to treatment, this was defined as a dose limiting toxicity.
Figure 1.
Complete blood count and chemistry laboratory studies. Laboratory results from all six patients sampled at baseline (Day 0) and weekly until the end of cycle 1 (Day 28). Dashed lines in (A), (B), (C), (D), and (E) represent Grade 3 adverse event cutoffs. Vertical axis values represent (A) sodium, (B) white blood cells (WBCs), (C) neutrophils, (D) lymphocytes, (E) platelets, and (F) monocytes. Samples run on standard clinical equipment.
The most common adverse events during the trial were low-grade (1 or 2) fatigue (67%), hypocalcemia (67%), fever (50%), flu-like symptoms (50%), thrombocytopenia (50%), and leukopenia (50%) which primarily occurred within the first week of treatment (Table 2). Grade 3 leukopenia (17%), neutropenia (33%), and lymphopenia (17%), occurred over the first week with subsequent recovery within 7 days (Figure 1B, C, D). One episode of grade 3 hypophosphatemia occurred immediately prior to the second cycle of therapy.
Table 2.
Adverse Events
| Adverse Event Class | Number of Patients | Grade I | Grade II | Grade III | Grade IV | |
|---|---|---|---|---|---|---|
| Blood and lymphatic system disorders | 2 | |||||
| Anemia | 2 | |||||
| Gastrointestinal | 2 | |||||
| Dysphagia | 1 | |||||
| Nausea | 1 | |||||
| Vomiting | 1 | |||||
| General disorders | 4 | |||||
| Fatigue | 2 | 2 | ||||
| Fever | 3 | |||||
| Flu-like symptoms | 2 | 1 | ||||
| Investigations | 6 | |||||
| ALT increased | 1 | 1 | ||||
| AST increased | 1 | |||||
| Lymphocyte count decreased | 2 | 1 | ||||
| Neutrophil count decreased | 1 | 2 | ||||
| Platelet count decreased | 3 | |||||
| White blood cell count decreased | 3 | 1 | ||||
| Metabolism and nutrition disorders | 4 | |||||
| Hyperglycemia | 1 | |||||
| Hypoalbuminemia | 2 | |||||
| Hypocalcemia | 4 | |||||
| Hypoglycemia | 1 | |||||
| Hypokalemia | 1 | |||||
| Hypomagnesemia | 1 | |||||
| Hyponatremia | 1 | |||||
| Hypophosphatemia | 1 | |||||
| Musculoskeletal and connective tissue disorders | 1 | |||||
| Bone pain | 1 | |||||
| Nervous system disorders | 2 | |||||
| Depressed level of consciousness | 1 | |||||
| Headache | 1 | |||||
| Seizure | 1 | |||||
| Psychiatric disorders | 1 | |||||
| Confusion | 1 |
Several adverse events occurred at the time of or soon after progression, within the observation phase of the trial. Patient one experienced Grade 2 dysphagia and Grade 2 seizure, both possibly related to treatment, 24 and 25 days after last study drug administration, and was found at that time to have progressive disease. Patient 3 experienced Grade 4 confusion and Grade 3 depressed level of consciousness on the last day of cycle two, both possibly related to treatment, but on the same day was also found to have progressive disease. Patient 6 experienced grade 2 confusion and right-sided weakness unlikely to be related to treatment 7 days after progression and 9 days after study drug.
Survival and Imaging
All patients were determined to have progressive disease while on treatment after a median of 32.5 days (range 20–55 days). For patients 1–4, the development of new symptoms prompted radiographic evaluation of progression. Patient 5 was found to have progression during routine imaging prior to initiation of his third cycle of treatment. Patient 6 was removed from the trial following development of dose limiting hyponatremia and was found to have progression on imaging during hospitalization for electrolyte management. Five patients succumbed to their disease within 6 months from recruitment (range 26–169 days), and one patient died after 1.5 years.
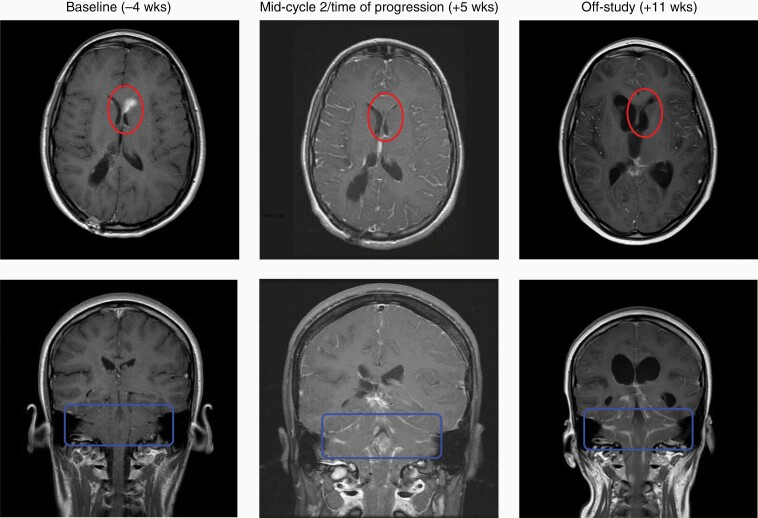
Of these patients, to our knowledge only one underwent autopsy, which did not show evidence of inflammation. Available pre- and post-trial MRI images were reviewed by an independent neuroradiologist who interpreted them as consistent with typical progression without characteristics of pseudoprogression or inflammation. A notable finding occurred in patient 3 (diagnosis of GBM), who had received gamma knife therapy for a left ventricular lesion 2.6 months earlier. This lesion was present on baseline imaging prior to trial initiation but was no longer enhancing when the patient’s progressive symptoms interrupted the second course of therapy (Figure 2, upper panels). Unfortunately, this patient also exhibited diffusely metastatic disease in the brain and spine as demonstrated by meningeal enhancement (Figure 2, lower panels). A follow-up scan 2 months later continued to exhibit minimal left ventricular enhancement and diffuse metastatic disease.
Figure 2.
Patient 3 serial gadolinium-enhanced T1 brain MRIs. Gadolinium-enhanced T1 MRI images acquired at Baseline, Time of Progression, and Off-Study for patient 3. Top series of images represents supratentorial axial slices. Bottom series of images represents coronal slices. Lighter shades within circle or square represent enhancing tumor. Transient regression of left periventricular lesion marked by circles in upper row. Progressive brainstem disease marked by rectangles in lower row. Blinded assessment performed by neuroradiologist (D.R.J.).
Immunologic and Viral Correlates
Throughout the first and second cycles, blood and serum samples were acquired to monitor cell counts, blood chemistries, cytokine profiles, and antiviral neutralizing antibodies. Among the six patients, all experienced transient drops in WBCs and platelets within one week after therapy, with recovery occurring by week two (Figure 1B, E). Four of five patients with neutrophil counts acquired one week posttherapy also demonstrated transient drops. Three of five patients with monocyte counts acquired one week posttherapy exhibited transient increases (Figure 1F).
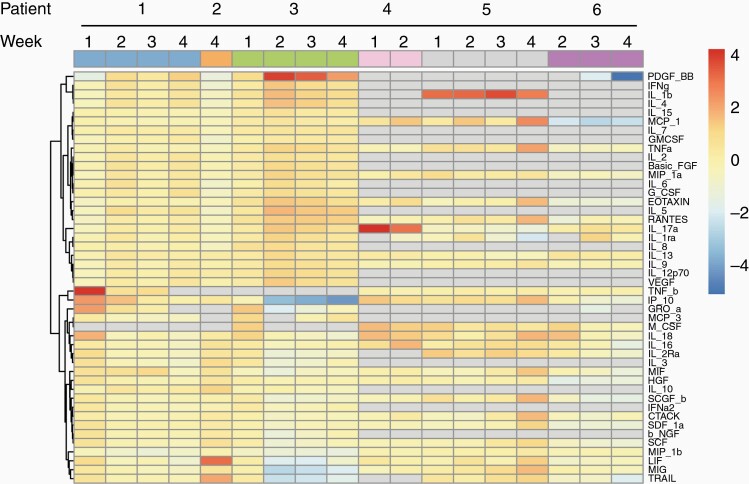
When serial serum samples underwent cytokine analysis by Luminex multiplex assay, no consistent trends between patients were observed at any timepoint. Hierarchical clustering analysis demonstrated more similarity within each patient’s samples than between patients at the same timepoint (Figure 3).
Figure 3.
Clustering analysis of serial cytokine studies. Serum samples from patients were drawn at baseline and weekly throughout cycle one. Cytokine levels were acquired by Luminex Multiplex Assay. Levels from weeks 1 to 4 were normalized to baseline values if available, and log2 transformed for clustering via the pheatmap function in RStudio. Gray boxes represent missing data. Column clustering color labeled by Patient (top line) and Week (bottom line) to aid in visualization.
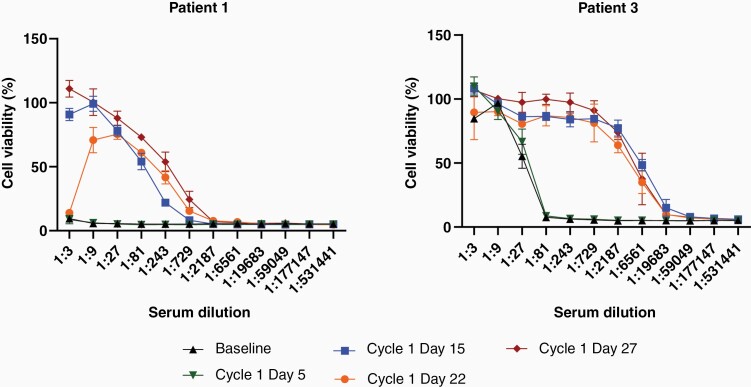
Neutralizing antibody titers were assessed for patients 1 and 3 and demonstrated anti-reovirus seroconversion by Cycle 1 Day 15, which is 12 days postpelareorep administration (Figure 4). Neutralizing antibodies persisted through Cycle 1 Day 27, but unfortunately both assayed patients progressed and were removed from the trial before further samples could be acquired for evaluation of antibody persistence.
Figure 4.
Anti-reovirus neutralizing antibody titers. Patients 1 and 3 had serum samples drawn at baseline and weekly throughout cycle one. Heat-inactivated serum samples were serially diluted and incubated on L929 cells with reovirus at a quantity known to cause 80% cell death. After 48 h, cell survival was measured by MTT assay. High cell viability on the y-axis demonstrates presence of protective, neutralizing antibodies. Dilution on the x-axis represents the concentration of antibodies, with lower dilutions closer to the origin representing higher concentrations of neutralizing antibodies. Error bars represent standard deviation based upon technical quadruplicates.
Discussion
This phase I trial was intended to test the safety of combination GM-CSF and intravenous pelareorep for the treatment of recurrent or refractory high-grade pediatric brain tumors, establish a MTD, and secondarily, to assess immunologic responses to treatment.
Though preclinical studies have demonstrated that preconditioning with GM-CSF enhances pelareorep efficacy via antibody-mediated carriage on CD11b + cells, this regimen has not been widely evaluated.10,12,13 This regimen has been used in a phase I trial for adult gliomas, and while final results have not been published, initial reports suggest treatment is safe and well-tolerated.14 However, prior to our study, GM-CSF and intravenous pelareorep combination therapy had not been used in pediatric patients with CNS or non-CNS tumors.
Six patients participated in this trial, representing a variety of brain tumor types including DIPG, glioblastoma, and medulloblastoma. Patients were heavily pretreated with prior radiation and between one and five previous courses of chemotherapy. All patients received at least one cycle of sargramostim/pelareorep therapy, with two patients receiving two full cycles. One dose limiting toxicity of prolonged grade 3 hyponatremia possibly related to treatment was observed. This resulted in precautionary hospitalization and was responsive to fluid restriction. Hyponatremia was also previously observed in two out of twenty-one patients in a Phase II trial using pelareorep to treat multiple myeloma.16 Among our cohort, the patient with the dose limiting toxicity had the lowest baseline sodium at recruitment (134 mmol/L) and was also taking amitriptyline and oxcarbazine, both of which are associated with SIADH. Other frequent adverse events included fatigue, fever, flu-like symptoms, thrombocytopenia, leukopenia, lymphopenia, and neutropenia, most of which were transient and low grade (I or II).
Unfortunately, given that this patient cohort had recurrent, heavily pretreated, high-grade tumors with a poor prognosis, all patients had disease progression noted within 55 days of the start of treatment. Although there was one DLT, there were no treatment-related deaths. Imaging review by an independent neuroradiologist showed no radiographic changes suggestive of pseudoprogression or inflammation, and findings were consistent with disease progression. Interestingly, patient 3, who was 2.6 months status-post gamma knife therapy for glioblastoma, demonstrated regression of a previously targeted left ventricular lesion in the context of diffusely metastatic disease. While this lesion’s regression could be consistent with a delayed gamma knife response, past studies have demonstrated a radiotherapy/reovirus combination benefit.17
Despite the absence of objective tumor responses, correlative studies suggested that patients did have moderate immunologic responses to therapy, with 60% of patients experiencing transient increases in monocytes following GM-CSF therapy, and both assayed patients generating neutralizing anti-reovirus antibodies within 12 days of pelareorep administration. Interestingly, the patients who did not have increases in monocytes had the lowest levels at baseline, suggesting that they may have benefitted from an extended course of GM-CSF. Patients in our study also had drops in white blood cell counts, platelets, and neutrophils within the first week of therapy, which are consistent with previous studies.18
Regarding cytokine analysis, in vitro and murine ex vivo responses to pelareorep therapy have been well-characterized, with reoviral infection inducing IFN-alpha, IFN-beta, IL28b/IL29, IFN-gamma, TNF-alpha, and TRAIL19,20–22. Two phase I clinical trials using intravenous oncolytic reovirus have also assessed plasma cytokines after treatment. The first administered reovirus to patients with a variety of advanced cancers for 5 days with doses between 1 × 108 and 3 × 108 TCID50 and did not see a statistically significant change in cytokines at any timepoints, though one patient did have increases in IL-2 at Day 2, and IL-5 and IL12p40 at Day 154. A separate trial treated patients with metastatic colon cancer with 5 days of reovirus at 3 × 1010 TCID50 and found statistically increases at Day 8 or Day 15 in IL12p40, GM-CSF, IFN-gamma, IL-6, and IL-12p70, as well as decreases in IL-8 and RANTES-CC523. In our study, we did not see any consistent changes in these cytokines. Our patients received only 3 consecutive days of reovirus at 3 × 108 or 5 × 108 TCID50, so it is possible that our cumulative dose was not high enough to generate systemic cytokine responses in the plasma.
As a phase I trial, there are inherent limitations to our study. First, though our study was initiated in a 3 + 3 design starting at 5 × 108 TCID50/kg/dose, after the symptomatic progression of patient 3 and out of an abundance of caution, the 3 + 3 trial design was restarted with 3 × 108 TCID50/kg/dose as dose level 1. This may have reduced interest in the trial, which was ultimately only able to recruit three more patients. However, although we observed a dose limiting toxicity at 3 × 108 TCID50/kg, no dose limiting toxicities were experienced with the three patients treated previously at 5 × 108 TCID50/kg, supporting results from the COG study that this is a reasonable starting dose for future pediatric trials. Secondly, three of six patients died prior to the three months required for trial eligibility, highlighting the prognostic uncertainty associated with recurrent pediatric high-grade brain tumors and the challenges of performing trials that plan to deliver multiple cycles of therapy.
Future studies may benefit from recruiting patients in earlier phases of therapy, which has been safely done in other virotherapy trials treating pediatric patients with brain tumors.24,25 Our regimen could also be combined with other immunomodulatory agents such as immune checkpoint inhibitors or radiotherapy, particularly given the potential synergy we observed between gamma knife therapy and pelaoreorep therapy. Preclinical studies have demonstrated that GM-CSF/reovirus followed by anti-PD1 antibodies enhances efficacy in syngeneic gliomas,10 and multiple oncolytic viruses are undergoing clinical evaluation in combination with checkpoint inhibitors, with initial results in non-CNS tumors showing increased efficacy.26
Conclusion
In conclusion, we performed a phase I trial of combination GM-CSF and pelareorep therapy for pediatric patients with recurrent or refractory high-grade brain tumors. Six patients participated, with all receiving at least one cycle of therapy. One dose limiting toxicity occurred, but no MTD was identified. No complete or partial responses were observed and all patients experienced progression within 60 days. Treatment was well-tolerated and warrants further clinical evaluation.
Acknowledgments
We thank Toni Woltman for expert editorial assistance and the Mayo Clinic Children’s Research Center staff for their support, especially Deb Schott and Jean Hanson. We also acknowledge Dr. Amulya Nageswararao for her assistance in the trial. Thanks also go out to Dr. John Smestad for education regarding the use of RStudio and the pheatmap function.
Contributor Information
Matthew R Schuelke, Medical Scientist Training Program, Mayo Clinic, Rochester, Minnesota, USA.
Justin H Gundelach, Department of Immunology, Mayo Clinic, Rochester, Minnesota, USA.
Matt Coffey, Oncolytics Biotech, Calgary, Alberta, Canada.
Emma West, Faculty of Medicine and Health, Leeds Institute of Medical Research, University of Leeds, St James’ University Hospital, Leeds, UK.
Karen Scott, Faculty of Medicine and Health, Leeds Institute of Medical Research, University of Leeds, St James’ University Hospital, Leeds, UK.
Derek R Johnson, Department of Radiology, Mayo Clinic, Rochester, Minnesota, USA; Department of Neurology, Mayo Clinic, Rochester, Minnesota, USA.
Adel Samson, Faculty of Medicine and Health, Leeds Institute of Medical Research, University of Leeds, St James’ University Hospital, Leeds, UK.
Alan Melcher, The Institute of Cancer Research/Royal Marsden, National Institute for Health Research Biomedical Research Centre, London, UK.
Richard G Vile, Faculty of Medicine and Health, Leeds Institute of Medical Research, University of Leeds, St James’ University Hospital, Leeds, UK; The Institute of Cancer Research/Royal Marsden, National Institute for Health Research Biomedical Research Centre, London, UK; Department of Molecular Medicine, Mayo Clinic, Rochester, Minnesota, USA.
Richard J Bram, Department of Immunology, Mayo Clinic, Rochester, Minnesota, USA; Division of Pediatric Hematology/Oncology, Mayo Clinic, Rochester, Minnesota, USA.
Funding
This work was supported by Oncolytics Biotech, Transform the Practice Award (Mayo Foundation), Shannon O’Hara Foundation.
Conflict of interest statement. Matt Coffey is president and chief executive officer of Oncolytics Biotech, the maker of the reovirus used in this study.
Authorship statement. Conception and Design: M.C., A.S., A.M., R.V., R.B.; Development of Methodology: M.S., M.C., A.S., A.M., R.V., R.B.; Data Acquisition: J.G., E.W., K.S., D.J.; Analysis and Interpretation of Data: M.S., J.G., E.W., K.S., D.J., A.S., A.M., R.V., R.B.; Writing, review, and/or revision of manuscript: M.S., J.G., M.C., E.W., K.S., D.J., A.S., A.M., R.V., R.B.; Study Supervision: R.V., R.B.; Study Chair: R.B.
References
- 1. Johnson KJ, Cullen J, Barnholtz-Sloan JS, et al. Childhood brain tumor epidemiology: a brain tumor epidemiology consortium review. Cancer Epidemiol Biomarkers Prev. 2014; 23(12):2716–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Curtin SC, Minino AM, Anderson RN. Declines in cancer death rates among children and adolescents in the United States, 1999-2014. NCHS Data Brief. 2016;. (257):1–8. [PubMed] [Google Scholar]
- 3. Coffey MC, Strong JE, Forsyth PA, Lee PW. Reovirus therapy of tumors with activated Ras pathway. Science. 1998; 282(5392):1332–1334. [DOI] [PubMed] [Google Scholar]
- 4. White CL, Twigger KR, Vidal L, et al. Characterization of the adaptive and innate immune response to intravenous oncolytic reovirus (Dearing type 3) during a phase I clinical trial. Gene Ther. 2008; 15(12):911–920. [DOI] [PubMed] [Google Scholar]
- 5. Prestwich RJ, Ilett EJ, Errington F, et al. Immune-mediated antitumor activity of reovirus is required for therapy and is independent of direct viral oncolysis and replication. Clin Cancer Res. 2009; 15(13):4374–4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Prestwich RJ, Errington F, Ilett EJ, et al. Tumor infection by oncolytic reovirus primes adaptive antitumor immunity. Clin Cancer Res. 2008; 14(22):7358–7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clements D, Helson E, Gujar SA, Lee PW. Reovirus in cancer therapy: an evidence-based review. Oncolytic Virother. 2014; 3:69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Forsyth P, Roldan G, George D, et al. A phase I trial of intratumoral administration of reovirus in patients with histologically confirmed recurrent malignant gliomas. Mol Ther. 2008; 16(3):627–632. [DOI] [PubMed] [Google Scholar]
- 9. Kicielinski KP, Chiocca EA, Yu JS, et al. Phase 1 clinical trial of intratumoral reovirus infusion for the treatment of recurrent malignant gliomas in adults. Mol Ther. 2014; 22(5):1056–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Samson A, Scott KJ, Taggart D, et al. Intravenous delivery of oncolytic reovirus to brain tumor patients immunologically primes for subsequent checkpoint blockade. Sci Transl Med. 2018; 10(422):eaam7577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kolb EA, Sampson V, Stabley D, et al. A phase I trial and viral clearance study of reovirus (Reolysin) in children with relapsed or refractory extra-cranial solid tumors: a Children’s Oncology Group Phase I Consortium report. Pediatr Blood Cancer. 2015; 62(5):751–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Adair RA, Roulstone V, Scott KJ, et al. Cell carriage, delivery, and selective replication of an oncolytic virus in tumor in patients. Sci Transl Med. 2012; 4(138):138ra–13177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ilett E, Kottke T, Donnelly O, et al. Cytokine conditioning enhances systemic delivery and therapy of an oncolytic virus. Mol Ther. 2014; 22(10):1851–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kendall J, Chalmers A, McBain C, et al. CTIM-14. pelareorep and granulocyte-macrophage colony-stimulating factor (GM-CSF) with standard chemoradiotherapy/adjuvant temozolomide for glioblastoma multiforme (GBM) patients: Reoglio Phase I Trial results. Neuro Oncol. 2020; 22(Supplement_2):ii35–ii36. [Google Scholar]
- 15. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010; 28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 16. Galanis E, Markovic SN, Suman VJ, et al. Phase II trial of intravenous administration of Reolysin((R)) (Reovirus Serotype-3-dearing Strain) in patients with metastatic melanoma. Mol Ther. 2012; 20(10):1998–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Twigger K, Vidal L, White CL, et al. Enhanced in vitro and in vivo cytotoxicity of combined reovirus and radiotherapy. Clin Cancer Res. 2008; 14(3):912–923. [DOI] [PubMed] [Google Scholar]
- 18. Gong J, Sachdev E, Mita AC, Mita MM. Clinical development of reovirus for cancer therapy: an oncolytic virus with immune-mediated antitumor activity. World J Methodol. 2016; 6(1):25–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Samson A, Bentham MJ, Scott K, et al. Oncolytic reovirus as a combined antiviral and anti-tumour agent for the treatment of liver cancer. Gut. 2018; 67(3):562–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Parrish C, Scott GB, Migneco G, et al. Oncolytic reovirus enhances rituximab-mediated antibody-dependent cellular cytotoxicity against chronic lymphocytic leukaemia. Leukemia. 2015; 29(9):1799–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Muller LME, Migneco G, Scott GB, et al. Reovirus-induced cell-mediated immunity for the treatment of multiple myeloma within the resistant bone marrow niche. J ImmunoTher Cancer. 2021; 9(3):e001803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Seyed-Khorrami SM, Soleimanjahi H, Soudi S, Habibian A. MSCs loaded with oncolytic reovirus: migration and in vivo virus delivery potential for evaluating anti-cancer effect in tumor-bearing C57BL/6 mice. Cancer Cell Int. 2021; 21(1):244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Parakrama R, Fogel E, Chandy C, et al. Immune characterization of metastatic colorectal cancer patients post reovirus administration. BMC Cancer. 2020; 20(1):569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tejada S, Diez-Valle R, Dominguez PD, et al. DNX-2401, an oncolytic virus, for the treatment of newly diagnosed diffuse intrinsic pontine gliomas: a case report. Front Oncol. 2018; 8:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kieran MW, Goumnerova L, Manley P, et al. Phase I study of gene-mediated cytotoxic immunotherapy with AdV-tk as adjuvant to surgery and radiation for pediatric malignant glioma and recurrent ependymoma. Neuro Oncol. 2019; 21(4):537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sivanandam V, LaRocca CJ, Chen NG, Fong Y, Warner SG. Oncolytic viruses and immune checkpoint inhibition: the best of both worlds. Mol Ther Oncolytics. 2019; 13:93–106. [DOI] [PMC free article] [PubMed] [Google Scholar]