Abstract
Objective
To assess the initial features and evolution of neurologic Postacute Sequelae of SARS‐CoV‐2 infection (neuro‐PASC) in patients with and without prior neurologic disease.
Methods
Participants with neurologic symptoms following acute SARS‐CoV‐2 infection were recruited from October 9, 2020 to October 11, 2021. Clinical data included a SARS‐CoV‐2 infection history, neurologic review of systems, neurologic exam, Montreal cognitive assessment (MoCA), and symptom‐based self‐reported surveys at baseline (conducted after acute infection) and 6‐month follow‐up assessments.
Results
Fifty‐six participants (69% female, mean age 50 years, 29% with prior neurologic disease such as multiple sclerosis) were enrolled, of which 27 had completed the 6‐month follow‐up visit in this ongoing study. SARS‐CoV‐2 infection severity was largely described as mild (39.3%) or moderate (42.9%). At baseline, following acute infection, the most common neurologic symptoms were fatigue (89.3%) and headaches (80.4%). At the 6‐month follow‐up, memory impairment (68.8%) and decreased concentration (61.5%) were the most prevalent, though on average all symptoms showed a reduction in reported severity score at the follow‐up. Complete symptom resolution was reported in 33.3% of participants by 6 months. From baseline to 6 months, average MoCA scores improved overall though 26.3% of participants’ scores decreased. A syndrome consisting of tremor, ataxia, and cognitive dysfunction (PASC‐TAC) was observed in 7.1% of patients.
Interpretation
Early in the neuro‐PASC syndrome, fatigue and headache are the most commonly reported symptoms. At 6 months, memory impairment and decreased concentration were most prominent. Only one‐third of participants had completed resolution of neuro‐PASC at 6 months, although persistent symptoms trended toward improvement at follow‐up.
Keywords: COVID‐19, postacute sequelae of COVID‐19 infection
Introduction
The neurologic manifestations of the novel SARS‐CoV‐2 virus are not fully understood. Long‐haul COVID, or Postacute Sequelae of SARS‐CoV‐2 infection (PASC), constitutes the continuation or emergence of persistent SARS‐CoV‐2 (COVID‐19)‐related symptoms past the time of acute infection with a wide range of symptoms in patients who experienced critically severe, moderate, or mild COVID‐19 infections. 1 , 2 In a study of both hospitalized and nonhospitalized patients, persistent symptoms 3 months after acute COVID‐19 infection were found in nearly all patients. 3 Most common persistent symptoms were fatigue and dyspnea, and only 0.7% of patients reported no symptoms at all. 3 Recent studies report common acute and long‐term neurologic sequelae of COVID‐19 including fatigue, anxiety, sleep disturbance, myalgia, and memory impairment. 4 , 5 Although most studies report sequelae of critically ill COVID‐19 infection, 6 , 7 , 8 few foci on those with mild infection or the long‐term evolution of neurologic complications. 9 , 10 Additionally, there have been case reports of ataxia syndrome, seizures, encephalopathy, vasculitis, and acute inflammatory demyelinating polyneuropathy following acute SARS‐CoV‐2 infection. 11 , 12 However, these syndromes were not studied in the context of a larger cohort, and there is a compelling need for longitudinal studies to determine their long‐term neurologic trajectory.
The associative risk factors for developing PASC have not been well studied. While some research links PASC to patients with severe disease, 13 most studies show that PASC is more prevalent among patients with mild or moderate COVID‐19 infection. 14 , 15 Additionally, limited data exist on the risks of developing PASC and the spectrum of PASC symptoms among patients with preexisting neurologic disorders. One study conducted a genetic enrichment analysis in SARS‐CoV‐2‐infected human lung tissue and found its viral interactome, and not those of other human coronaviruses or other respiratory viruses, overlapped significantly with protein expression in autoimmune disease. 16 This occurred most prominently in multiple sclerosis and suggests some patients may be genetically predisposed to a COVID‐19‐triggered autoimmune response. 16
In a cross‐disciplinary collaborative effort at the University of California San Diego (UCSD), we established the NeuCOVID longitudinal cohort study to capture the acute and long‐term neurologic sequelae of SARS‐CoV‐2 infection in those with and without prior neurologic disease. Symptoms are being tracked with the goal of a total 10‐year follow‐up period to better characterize long‐term consequences of infection and track the potential incidence of neurologic disease secondary to COVID‐19 exposure. Herein, we present current baseline and 6‐month follow‐up data from the ongoing cohort and report specific neurologic phenotypes following COVID‐19 infection.
Methods
Study design
We designed a prospective, single‐center cohort study at UC San Diego (UCSD) Health System evaluating the spectrum of neurologic symptoms following acute SARS‐CoV‐2 infection. We defined acute symptoms as symptoms associated with active infection and postacute symptoms as symptoms presenting after recovery from acute infection. Participants were assessed at an initial baseline time point (conducted after resolution of acute infection, either PCR‐confirmed infection or likely positive COVID‐19 infection confirmed by evaluation in Infectious Disease clinic) and 6 months after the initial visit, with plans for yearly follow‐up thereafter. Visits were offered by telemedicine or in‐person to improve adherence to follow‐up.
Participants
Participants were recruited consecutively from October 9, 2020 to October 11, 2021 from the outpatient neurology clinic, direct referrals from other UCSD departments (Infectious Diseases, Pulmonary and Neuropsychology clinics), and several self‐referrals from outside of the UCSD system. Participants were divided into two cohorts.
Cohort 1 consisted of patients without preexisting neurologic conditions prior to COVID‐19 infection. Inclusion criteria included a positive COVID‐19 test or suspected COVID‐19 case who met rigorous clinical criteria with documented development of neurologic symptoms/diseases. Patients were excluded if they (1) did not have a recorded positive COVID‐19 test result or clinically likely COVID‐19 infection, (2) did not have documented development of neurologic symptoms/diseases following COVID‐19 infection, or (3) did not provide informed consent.
Cohort 2 consisted of patients with preexisting neurologic conditions prior to COVID‐19 infection. Inclusion criteria included patients with a diagnosed neurologic disease/condition and a positive test result or suspected case of COVID‐19. Patients were excluded if they (1) did not have recorded prior neurologic disease or (2) did not provide informed consent.
Standard protocol approvals, registration, and patient consents
This study was approved by the UCSD Institutional Research Board Committee on Human Subjects Research (IRB #200634). Written informed consent was obtained from every participant.
Demographic data and clinical assessments
Participant demographics consisting of medical history (including chronic conditions or autoimmune disease history), tobacco use, socioeconomic status, and education were collected at visits. Demographics and study measurements were recorded and stored through a shared REDCap database.
Each measurement was completed at baseline and follow‐up time points. Trained team members and neurologists completed COVID‐19 assessments, neurologic review of systems, and neurologic exam, while participants completed additional questionnaires independently.
UCSD NeuCOVID assessment form
The COVID‐19 assessment form collected detailed information about participants’ COVID‐19 diagnosis and disease course through interviews with clinically trained personnel. Data included COVID‐19 test results and methodology (anterior nasal swab vs nasopharyngeal, antigen vs PCR). This questionnaire included information on specific symptoms, disease severity, hospitalization, and information regarding recovery. Due to a lack of published criteria early in the pandemic, COVID‐19 infection severity was assessed by patient report, given the options of asymptomatic, mild, moderate, or severe. Patient self‐assessment was cross‐referenced with reported acute viral symptoms and duration of symptoms. Patients were explicitly queried about hospitalization or treatments required during acute infection. Following the availability of vaccines in December 2020, we added a query on vaccination status: a repeating measure that assessed participants’ COVID‐19 vaccination status. Most infections were captured before the widespread availability of COVID‐19‐specific treatments (i.e., monoclonal antibody treatments) and all were before the spread of the Omicron variant.
Neurologic review of systems
Medically trained staff completed a detailed neurologic review of systems with participants at baseline and follow‐up visits. Participants were screened for the presence of the following symptoms: encephalopathy, memory difficulties, trouble concentrating, insomnia, mood disorder, fatigue, headaches, seizures, vision and hearing difficulties, facial weakness, speech abnormality, swallowing difficulty, difficulty chewing, limb weakness, muscle stiffness, numbness/tingling, difficulty walking, falls, poor limb coordination, tremors, slowness of movement, and imbalance, as well as the impact on quality of life of these symptoms. If a symptom was present, participants indicated symptom severity on a numeric rating scale (NRS) of 1 (minimal) to 10 (severe). At the baseline visit, symptom severity was trended per symptom at preinfection baseline, most severe point during infection, and current postacute infection at the time of visit. Baseline severity indicated symptom severity prior to having COVID‐19. The most severe measure indicated symptom severity at its worst since contracting COVID‐19. The current measure indicated symptom severity at the time of visit. For every follow‐up visit, the assessment only included the current level of severity at the time of visit.
Neurologic exam
Trained team members performed a standard, complete neurologic exam on participants including assessment of cranial nerves, motor function, reflexes, sensation, coordination, and gait at each visit. If the visit was telemedicine, extraocular movements, facial sensation, hearing, palate evaluation, tongue protrusion, shoulder shrug, and lateral head turns were assessed for cranial nerve function. Motor function and gait were assessed by observing patients’ ability to stand from a chair with crossed arms, perform a single squat, antigravity limb movements, single‐leg hops, finger and foot‐tapping movements, and observation of casual gait and toe/heel walking. The training was provided to medical personnel on appropriate telemedicine examination techniques to be compatible with clinically delivered telemedicine exams during the pandemic. Coordination was assessed by directing patients to perform finger‐to‐nose and repetitive finger and foot‐tapping maneuvers, and tandem gait. Reflex and sensory examinations were deferred for telemedicine visits.
Cognitive assessments
Trained team members conducted a Montreal Cognitive Assessment (MoCA) Version 7.1 at each visit. The MoCA is an approximately 10‐minute cognitive screening tool that assesses executive functioning, abstraction, short‐term memory, language, orientation, and visuospatial skills. The MoCA exam was adapted for telemedicine visits by utilizing a copy of the MoCA (examiner), blank scratch paper (participant), and conveying all visual and drawing test components (trail‐maker task, cube drawing, clock drawing, and object naming) via camera. Patients with abnormal scores (<26) were referred for formal clinical neuropsychologic assessments. This exam included measures of intellectual functioning (Test of Premorbid Functioning, TOPF and Wechsler Abbreviated Scale of Intelligence‐2nd Edition, WASI‐2), attention and working memory (Digit Span subtest from the Wechsler Adult Intelligence Scale‐4th Edition, WAIS‐IV and Paced Auditory Serial Addition Test, PASAT), processing speed (Trail Making Test – Part A, Symbol Digit Modality Test, SDMT), executive functioning (Stroop Test, Trail Making Test – Part B), language (Controlled Oral Word Association Test, COWAT, Animal Fluency, and Boston Naming Test, BNT), memory (California Verbal Learning Test‐2nd Edition, CVLT‐II and Brief Visuospatial Memory Test‐Revised, BVMT‐R), and olfaction (Brief Smell Identification Test). Participants also completed self‐report questionnaires assessing depression (Beck Depression Inventory‐2nd Edition, BDI), anxiety (Beck Anxiety Inventory, BAI), posttraumatic stress disorder (PTSD Checklist for DSM‐5), sleep disturbance (Pittsburgh Sleep Quality Index, PSQI), and subjective cognitive dysfunction (Measurement of Everyday Cognition, ECog). Raw scores for neuropsychological tests were converted to demographically adjusted T scores using published normative data and corrected for age, education, ethnicity, and gender (when available).
Self‐reported questionnaires
Six self‐reported scales were made available online through REDCap for participants to complete independently with curation by built‐in features of REDCap and study coordinators to ensure completion. These included the Revised Impact of Event Scale (IES‐R), 17 the Promis‐29 Profile v2.1, 18 Katz Index of Independence in Activities of Daily Living (ADLs), 19 Lawton–Brody Instrumental Activities of Daily Living Scale (IADLs), 20 Modified Fatigue Impact Scale (MFIS), 21 and the Epworth Sleepiness Scale (ESS). 22 Details about these questionnaires are summarized in Table S1.
Statistical analysis
Descriptive data analyses included assessments of means, medians, and frequencies as appropriate. Multivariable linear regression models were employed to evaluate associations of demographic and clinical factors with measured outcomes. Analyses were performed in SPSS and STATA 15.0 and p values <0.05 were considered significant. As an exploratory pilot study, correction for multiple comparisons was not performed.
Results
Demographics
Fifty‐six participants (mean age 50 years, SD 13.6 years) completed baseline assessments including 40 participants in Cohort 1 (no prior neurologic disease) and 16 participants in Cohort 2 (known prior neurologic disease). Table 1 summarizes demographic characteristics including sex, age, race, ethnicity, tobacco use history, body mass index (BMI), COVID‐19 infection history, and time from COVID‐19 infection to baseline assessment. Fifty‐five of 56 participants had their the baseline visits conducted at least 28 days after onset of COVID‐19 symptoms, with 36 of 56 conducted at least 84 days from symptoms onset. Patients in Cohort 2 had prior neurologic diseases including multiple sclerosis (n = 6), migraines (n = 6), optic neuritis (n = 1), narcolepsy (n = 1), traumatic brain injury (n = 1), and Guillain‐Barre syndrome (n = 1). Of those recruited, 27 participants (74% female, mean 52.5 years), 19 in Cohort 1 and 8 in Cohort 2, completed 6‐month follow‐up visits by the time of data‐lock for initial pilot analysis.
Table 1.
Baseline demographics and clinical characteristics.
| Cohort 1, n = 40 | Cohort 2, n = 16 | Overall, n = 56 | |
|---|---|---|---|
| Demographics | |||
| Mean age at infection, years (IQR) | 50.5 (26.25) | 48.8 (15.5) | 50.0 (23.25) |
| Female (%) | 68 | 81 | 69 |
| White (%) | 76.9 | 64.3 | 73.6 |
| Hispanic ethnicity (%) | 10.3 | 14.3 | 11.3 |
| Mean body mass index (kg/m2) | 28 | 26.1 | 27.5 |
| Prior tobacco use (%) | 36.8 | 35.7 | 36.5 |
| COVID infection history | |||
| PCR‐Confirmed COVID infection (n) | 39 | 15 | 54 |
| Suspected COVID infection (n) | 1 | 1 | 2 |
| Median time from acute COVID infection, weeks (IQR) | 16.1 (20.2) | 11.7 (21.6) | 15.6 (20.9) |
| Median duration of acute COVID infection, days (IQR) | 14.0 (13.5) | 14.0 (11.5) | 14.0 (14.0) |
| Infection Severity | |||
| Asymptomatic (%) | 0 | 0 | 0 |
| Mild (%) | 41 | 37.5 | 39.3 |
| Moderate (%) | 38.5 | 56.3 | 42.9 |
| Severe (%) | 20.5 | 6.3 | 16.1 |
| Requiring hospitalization (n) | 3 | 2 | 5 |
| Vaccination status (at time of COVID infection) | |||
| Zero vaccine doses (n) | 38 | 15 | 53 |
| Single vaccine dose (n) | 2 a | 0 | 2 a |
| Two or more vaccine doses (n) | 0 | 1 | 1 |
| Neurologic review of systems | |||
| Fatigue, n (%) | 36 (90.0) | 14 (87.5) | 50 (89.3) |
| Headache, n (%) | 33 (82.5) | 12 (75.0) | 45 (80.4) |
| Insomnia, n (%) | 28 (70.0) | 9 (56.3) | 37 (66.1) |
| Memory impairment, n (%) | 27 (67.5) | 9 (56.3) | 36 (64.3) |
| Decreased concentration, n (%) | 24 (60.0) | 11 (68.8) | 35 (62.5) |
| Impact on quality of life, n (%) | 32 (80.0) | 13 (81.3) | 45 (80.4) |
| Neurologic exam | Cohort 1, n = 30 | Cohort 2, n = 11 | Overall, n = 41 |
| Any cranial nerve dysfunction, n (%) | 2 (6.7) | 3 (27.3) | 5 (12.2) |
| Motor impairment, n (%) | 3 (10.0) | 1 (9.1) | 4 (9.8) |
| Motor function not assessed, n (%) | 15 (50.0) | 6 (54.5) | 21 (51.2) |
| Incoordination, n (%) | 4 (13.3) | 3 (27.3) | 7 (17.1) |
| Gait abnormalities, n (%) | 8 (26.7) | 2 (18.2) | 10 (24.4) |
| Gait not assessed, n (%) | 4 (13.3) | 2 (18.2) | 6 (14.6) |
| Normal neurologic exam, n (%) | 19 (63.3) | 7 (63.6) | 26 (63.4) |
Cohort 1 included patients with no known prior neurological disease and Cohort 2 included those with a prior known neurological diagnosis.
Abbreviation: IQR, interquartile range.
Two patients received a single vaccine dose, one Moderna and one Pfizer, both 12 days prior to their COVID infection.
Neurologic review of systems
Preinfection neurologic symptoms
As demonstrated in Figure 1, most participants reported either no history or less‐severe history of each neurologic symptom before COVID infection. Preinfection, participants reported experiencing fatigue (36.0%), headache (44.4%), insomnia (73.0%), memory impairment (44.4%), and decreased concentration (40.0%), with symptoms impacting their quality of life in 37.8%. Most of these participants experienced worsened symptoms after COVID infection.
Figure 1.
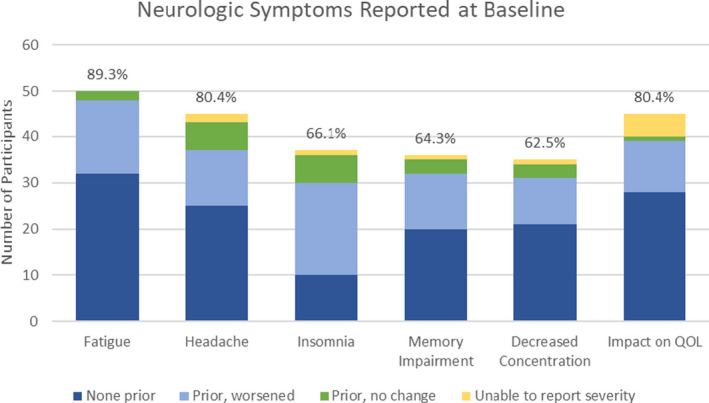
The number of participants reporting each neurologic symptom at the baseline visit, subcategorized by symptom history prior to COVID infection. “None prior” refers to the number of participants with baseline symptoms new since COVID infection; “Prior, worsened” refers to participants with baseline symptoms worse since COVID infection; “Prior, no change” refers to participants with baseline symptoms unchanged since COVID infection; “Unable to report severity” refers to participants who report the presence of symptoms at baseline visit but were unable to report severity, and therefore relative change could not be determined. Percentages represent the proportion of participants reporting each symptom out of total baseline participants (n = 56). [Colour figure can be viewed at wileyonlinelibrary.com]
Baseline assessment
The median time from onset of acute COVID‐19 infection to baseline assessment was 15.6 weeks (IQR: 10.8–31.7 weeks). Most common symptoms reported at baseline were fatigue (89.3%), headache (80.4%), insomnia (66.1%), memory impairment (64.3%), and decreased concentration (62.5%). Forty‐five participants (80.4%) reported their symptoms impacted their quality of life.
Six‐month follow‐up
For participants with 6‐month data, the median time interval from onset of acute COVID‐19 infection to baseline visit was 12.4 weeks (Cohort 1: 14.4, Cohort 2: 11.0), and from infection to the 6‐month follow‐up was 38.1 weeks (cohort 1: 40.9, cohort 2: 34.4). In this subset, the most common symptoms reported at baseline visit were fatigue (85.2%), headache (74.1%), memory impairment (59.3%), insomnia (55.6%), and decreased concentration (48.1%). Twenty participants (74.1%) reported that their symptoms impacted quality of life. Complete symptom resolution was reported at the 6‐month follow‐up in nine participants (33.3%, Cohort 1: n = 8, Cohort 2: n = 1). In remaining participants, persistent symptoms included memory impairment (68.8%), decreased concentration (61.5%), fatigue (52.2%), insomnia (46.7%), and headache (45.0%). Quality of life remained reduced compared to baseline in 16 participants (80.0%, Cohort 1: 11, Cohort 2: 5). From baseline to 6 months, symptom severity scores on average decreased (corresponding to symptom improvement) for fatigue (69.4%), headache (64.3%), insomnia (51.3%), decreased concentration (47.6%), and memory impairment (38.6%), for each patient, as seen in Figure 2;.
Figure 2.
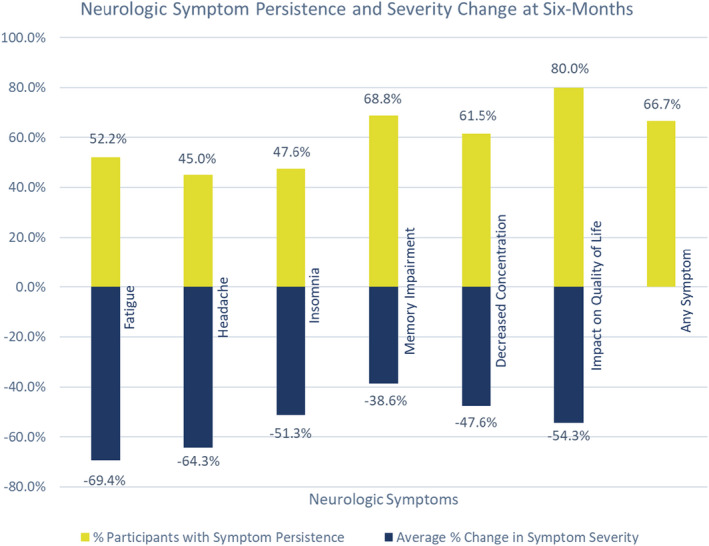
Neurologic symptom persistence and change in severity by percentage at 6 months. For each symptom, the yellow bars represent the participants with symptom persistence at 6 months as a percentage of all participants with that symptom at baseline. The blue bars represent the average percent decrease in severity score (within individual participants) from baseline to 6 months for each symptom. The final bar labeled “Any Symptom” shows the percentage of participants reporting at least one persistent symptom at 6 months. [Colour figure can be viewed at wileyonlinelibrary.com]
Neurologic exam
Baseline Assessment
Forty‐one participants completed neurologic exams, with 30 in cohort 1 and 11 in cohort 2. Fifteen participants did not complete a neurologic exam, typically due to technology‐related logistical difficulties (e.g., nonoperational video during telemedicine visit). Three baseline visits were conducted in‐person, whereas the rest were completed via telemedicine due to clinic quarantine concerns and patient accessibility. Overall, 26 participants (cohort 1: 19, cohort 2: 7) had a normal neurologic exam. Five participants (cohort 1: 2, cohort 2: 3) displayed cranial nerve dysfunction, including three with asymmetric facial sensation (CN V), two with difficulty or unequal hearing (CN VIII), one with extraocular movement abnormalities (CN III, IV, VI), and one with facial muscle weakness (CN VII). For the three patients in cohort 2 with previous neurologic disease, all cranial nerve deficits were new findings and not present at their most recent neurologic exam documented before COVID‐19 infection. Other positive neurologic findings included gait abnormalities (n = 10, 28.6%) and incoordination (n = 7, 17.1%). Eight of 10 participants with gait abnormalities, and four of seven participants with incoordination, had no prior neurologic disease.
Forty‐four participants with baseline data completed the MoCA with an overall average score of 26.0 (the normal score is 26–30). We added one point to those with less than 12 years of education. Participants most often missed points in delayed recall (n = 31, 70.5%), visuospatial/executive (n = 24, 54.5%), and language (n = 20, 45.5%) subcategories. Seventeen participants (38.6%) scored below 26 and were offered follow‐up clinical neuropsychologic testing. Many of these patients declined this. In those that participated, testing was formally scored as normal with results mainly in the average to the above average range, though some participants were in professional fields where the baseline was well above average.
Six‐month follow‐up
Of 41 participants with baseline neurologic exams, 18 completed a neurologic exam at the 6‐month follow‐up (cohort 1: 13, cohort 2: 5), consisting of 17 telemedicine and one in‐person examination. Ten participants (55.6%) had a normal neurologic exam (cohort 1: 7, cohort 2: 3). Six participants had cranial nerve dysfunction (cohort 1: 4, cohort 2: 2), including four with difficulty or unequal hearing (CN VIII), two with extraocular movement abnormalities (CN III, IV, VI), one with decreased or asymmetric facial sensation (CN V), and one with facial muscle weakness (CN VII). Gait abnormalities were present in three participants and incoordination was present in five participants. Symptom persistence, average severity score of symptoms change, and neuro exam findings are listed in Table 2.
Table 2.
Symptom persistence, average severity score change, and neuro exam findings at 6 months.
| Neurologic Review of Systems | Cohort 1, n = 19 | Cohort 2, n = 8 | Overall, n = 27 | |||
|---|---|---|---|---|---|---|
| Symptoms persistent from baseline | Persistence a | Severity b | Persistence a | Severity b | Persistence a | Severity b |
| Fatigue (%) | 43.8 | −82.4 | 71.4 | −39.7 | 52.2 | −69.4 |
| Headache (%) | 35.7 | −73.3 | 66.7 | −46.1 | 45.0 | −64.3 |
| Insomnia (%) | 40.0 | −63.3 | 60.0 | −29.6 | 47.6 | −51.3 |
| Memory impairment (%) | 54.5 | −57.2 | 100 | −1.6 | 68.8 | −38.6 |
| Decreased concentration (%) | 50.0 | −87.4 | 80.0 | +8.2 | 61.5 | −47.6 |
| None of the above symptoms (%) | 42.1 | N/A | 12.5 | N/A | 33.3 | N/A |
| Impact on quality of life (%) | 84.6 | −61.3 | 71.4 | −41.4 | 80.0 | −54.3 |
| Neurologic Exam | Cohort 1, n = 13 | Cohort 2, n = 5 | Overall, n = 18 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | New c | Persistent d | Total | New c | Persistent d | Total | New c | Persistent d | |
| Any cranial nerve dysfunction (n) | 4 | 2 | 1 e | 2 | 0 | 2 | 6 | 2 | 3 e |
| Motor impairment (n) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Motor function not assessed (n) | 2 | N/A | N/A | 0 | N/A | N/A | 2 | N/A | N/A |
| Incoordination (n) | 4 | 4 | 0 | 1 | 0 | 1 | 5 | 4 | 1 |
| Gait abnormalities (n) | 1 | 0 | 1 | 2 | 1 | 1 | 3 | 1 | 2 |
| Gait not assessed (n) | 2 | N/A | N/A | 0 | N/A | N/A | 2 | N/A | N/A |
| Normal neurologic exam (n) | 7 | N/A | N/A | 3 | N/A | N/A | 10 | N/A | N/A |
Percentage of participants with symptoms at baseline who report symptom persistence at 6 months.
Average percentage change (“+” for increase, “‐” for decrease) in participants’ symptom severity score (reported from 0 to 10) from baseline to 6 months.
Number of participants (n) with new (i.e., not present at baseline assessment) neurologic exam finding at 6 months.
Number of participants (n) with persistent (i.e., present at baseline assessment) neurologic exam finding at 6 months.
Participants (n) with “new” and “persistent” symptoms do not sum to “total” as one participant with CN VIII dysfunction at 6 months did not complete CN exam at baseline (whether neuro exam finding was “new” or “persistent” could not be determined).
At six‐month follow‐up, 19 of 44 participants completed the MoCA. Of these, 14 (73.7%) showed improvement or no change, 9 (75%) from cohort 1, and 5 (71.4%) from cohort 2. Five participants (26.3%) had decreased scores compared with baseline. Average MoCA scores improved from baseline (26.4, SD 2.1) to 6 months (28.0, SD 2.0). Participants most often missed points in delayed recall (n = 10, 52.6%), language (n = 7, 36.8%), and attention (n = 6, 31.6%) subcategories.
Self‐report questionnaires
Baseline assessment
Self‐report questionnaires, including Revised Impact of Events Scale (IESR), PROMIS‐29 profile, Katz Index, Lawton–Brody Scale, Modified Fatigue Impact Scale (MFIS), and Epworth Sleepiness Scale (ESS), were completed by 44 participants (cohort 1: 33, cohort 2: 11) at baseline assessment. Average scores and subscores are listed in Table 3. The average participant score for the IESR was 18.5 out of 88, with 79.5% (35 of 44) of participants scoring below the PTSD screening cutoff of 33. Participants indicated that their ability to complete ADLs and IADLs was largely intact in the Katz Index and Lawton–Brody measures. Participants’ levels of daytime sleepiness were within normal limits on average, with 81% (36 of 44) of participants scoring below the ESS cutoff of 10. The average total MFIS score was 36.8 of 84 (cohort 1: 36.3, cohort 2: 38.2), indicating moderate fatigue.
Table 3.
Baseline MoCA and self‐report clinical questionnaire average score.
| MoCA | Cohort 1, n = 31 | Cohort 2, n = 13 | Overall, n = 44 |
|---|---|---|---|
| Average score | 25.8 | 26.3 | 26 |
| Participants with missed points in: | Participants, n (%) | Participants, n (%) | Participants, n (%) |
| Visuospatial/Executive | 16 (51.6) | 8 (61.5) | 24 (54.5) |
| Naming | 1 (3.2) | 0 (0) | 1 (2.3) |
| Attention | 10 (32.2) | 6 (46.2) | 16 (36.4) |
| Language | 11 (35.5) | 9 (69.2) | 20 (45.5) |
| Abstraction | 5 (16.1) | 1 (7.7) | 6 (13.6) |
| Delayed Recall | 24 (77.4) | 7 (53.8) | 31 (70.5) |
| Orientation | 5 (16.1) | 1 (7.7) | 6 (13.6) |
| Self‐report questionnaires | Cohort 1, n = 33 | Cohort 2, n = 11 | Overall, n = 44 |
|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | |
| Revised impact of events scale | |||
| Intrusion | 8.0 (6.7) | 4.7 (5.3) | 7.2 (6.5) |
| Avoidance | 7.0 (6.8) | 5.2 (7.7) | 6.5 (7.0) |
| Hyperarousal | 5.1 (5.4) | 3.7 (4.5) | 4.7 (5.1) |
| Total | 20.1 (16.8) | 13.6 (15.0) | 18.5 (16.4) |
| PROMIS‐29 Profile | |||
| Physical function | 16.8 (3.6) | 16.7 (4.1) | 16.8 (3.7) |
| Anxiety | 8.5 (4.0) | 8.5 (5.3) | 8.5 (4.3) |
| Depression | 6.8 (3.1) | 7.7 (4.5) | 7.1 (3.5) |
| Fatigue | 11.7 (4.8) | 11.5 (5.8) | 11.7 (5.0) |
| Sleep disturbance | 11.5 (4.9) | 10.4 (5.6) | 11.2 (5.0) |
| Ability to participate in social roles and activities | 13.9 (4.8) | 14.5 (5.0) | 14.0 (4.8) |
| Pain interference | 7.9 (4.2) | 7.9 (6.2) | 7.9 (4.7) |
| Pain intensity | 3.0 (2.3) | 3.0 (2.8) | 3.0 (2.4) |
| Katz Index of Independence for ADLs | |||
| Total | 6.0 (0.0) | 5.8 (0.6) | 6.0 (0.3) |
| Lawton–Brody IADLs Scale | |||
| Total | 7.6 (1.1) | 7.6 (0.8) | 7.6 (1.0) |
| Modified Fatigue Impact Scale | |||
| Physical | 15.9 (9.8) | 17.4 (12.6) | 16.3 (10.4) |
| Cognitive | 17.0 (10.2) | 17.9 (13.1) | 17.2 (10.8) |
| Psychosocial | 3.2 (2.5) | 2.9 (2.7) | 3.2 (2.5) |
| Total | 36.3 (21.1) | 38.2 (25.7) | 36.8 (22.1) |
| Epworth Sleepiness Scale | |||
| Total | 6.7 (4.3) | 6.4 (6.5) | 6.6 (4.8) |
MoCA section lists average MoCA scores (0–30) and the number of participants (n, with %) with any number of missed points in each MoCA category by individual cohort and overall. The Revised Impact of Events Scale is a PTSD screening tool that measures stress levels and is scored from 0 to 88. PROMIS‐29 Profile measures eight domains of patients’ health with pain intensity scored from 0 to 10 and all other domains scored from 4 to 20 (raw scores). Katz Index measures the ability to perform ADLs, scored from 0 to 6. The Lawton–Brody scale measures the ability to perform IADLs scored from 0 to 8. The Modified Fatigue Impact Scale measures the level of disturbance caused by fatigue in physical, cognitive, and psychosocial domains and is scored from 0 to 84. The Epworth Sleepiness Scale measures daytime sleepiness levels from 0 to 24. For all measures, a higher score indicates a higher degree of that being measured. Abbreviations: MoCA, Montreal Cognitive Assessment; SD, standard deviation; ADL, activities of daily living; IADL, instrumental activities of daily living.
Six‐month follow‐up
Self‐reported questionnaire results of the 6‐month assessment are listed in Table 4. Most pronounced changes occurred in IESR scores with a decrease in the mean score to 9.9 (SD 17.8) and a corresponding 48.4% decrease in each participant’s score on average. PROMIS‐29 profile scores changed notably in categories of pain (+27.8%), pain intensity (+20.6%), and anxiety (+12.5%), reflecting an increase in pain intensity and pain interference with daily life. Scores in physical function (+0.8%), depression (+8.0%), fatigue (+4.3%), sleep disturbance (−0.7%), and ability to participate in social roles and activities (+0.1%) remained consistent from baseline. Average overall scores on Katz Index (5.9, SD 0.2) and Lawton–Brody Scale (7.6, SD 0.9) also remained consistent, representing a respective 1.3% increase and 1.7% decrease from baseline scores on average. On the MFIS, participants on average showed decreases from baseline in physical (−19.2%) and cognitive (−22.3%) subscores and the total score (−17.8%) but showed an average increase in psychosocial subscore (+36.3%). This reflects participants’ reduction in fatigue overall, despite increased fatigue in the psychosocial subcategory, which was largely consistent between both cohorts. The average score on the ESS at 6 months was 6.8, representing an average increase in daytime sleepiness by 29.9% for each participant from baseline.
Table 4.
Six‐month MoCA and self‐report questionnaire average scores.
| MoCA | Cohort 1, n = 12 | Cohort 2, n = 7 | Overall, n = 19 | |||
|---|---|---|---|---|---|---|
| Average score and average score change (%) from baseline | 28.2 | +8.9% | 27.7 | +5.0% | 28.0 | +6.4% |
| Participants (n) with missed points in a : | Baseline b | 6‐Month | Baseline b | 6‐Month | Baseline b | 6‐Month |
| Visuospatial/Executive | 4 | 3 | 4 | 2 | 8 | 5 |
| Naming | 0 | 1 | 0 | 1 | 0 | 2 |
| Attention | 3 | 3 | 4 | 3 | 7 | 6 |
| Language | 5 | 4 | 5 | 3 | 10 | 7 |
| Abstraction | 1 | 0 | 1 | 1 | 2 | 1 |
| Delayed Recall | 9 | 7 | 4 | 3 | 13 | 10 |
| Orientation | 1 | 0 | 1 | 0 | 2 | 0 |
| Self‐report questionnaires | Mean (SD) | % Change c | Mean (SD) | % Change c | Mean (SD) | % Change c |
|---|---|---|---|---|---|---|
| Revised Impact of Events Scale | ||||||
| Intrusion | 2.8 (4.1) | −57.6 | 5.5 (9.3) | −22.5 | 3.7 (6.2) | −47.6 |
| Avoidance | 2.3 (3.8) | −10.7 | 6.8 (10.2) | −18.9 | 3.8 (6.7) | −13.7 |
| Hyperarousal | 1.0 (2.3) | −48.2 | 5.2 (8.2) | −13.2 | 2.4 (5.2) | −36.5 |
| Total | 6.2 (9.8) | −59.2 | 17.5 (27.5) | −21.5 | 9.9 (17.8) | −48.4 |
| PROMIS‐29 Profile | ||||||
| Physical function | 19.3 (1.4) | +1.9 | 15.0 (5.6) | −1.4 | 17.8 (3.8) | +0.8 |
| Anxiety | 5.8 (2.0) | +14.5 | 9.7 (5.9) | +8.6 | 7.1 (4.0) | +12.5 |
| Depression | 5.2 (1.8) | +10.4 | 8.8 (5.9) | +3.3 | 6.4 (3.9) | +8.0 |
| Fatigue | 8.6 (3.8) | +10.2 | 12.8 (6.3) | −7.3 | 10.0 (5.1) | +4.3 |
| Sleep disturbance | 6.9 (2.1) | +1.5 | 10.7 (5.7) | −5.0 | 8.2 (4.0) | −0.7 |
| Ability to participate in social roles and activities | 18.1 (2.9) | +7.7 | 12.0 (7.3) | −14.1 | 16.1 (5.5) | +0.1 |
| Pain interference | 6.9 (4.2) | +22.6 | 9.3 (6.0) | +38.1 | 7.7 (4.8) | +27.8 |
| Pain intensity | 2.2 (2.7) | +4.4 | 2.8 (3.0) | +40.0 | 2.4 (2.7) | +20.6 |
| Katz Index of Independence for ADLs | ||||||
| Total | 6.0 (0.0) | 0.0 | 5.9 (0.4) | +3.6 | 5.9 (0.2) | +1.3 |
| Lawton–Brody IADLs Scale | ||||||
| Total | 8.0 (0.0) | 0.0 | 7.1 (1.2) | −4.2 | 7.6 (0.9) | −1.7 |
| Modified Fatigue Impact Scale | ||||||
| Physical | 8.7 (6.9) | −15.6 | 17.8 (13.7) | −26.4 | 11.9 (10.4) | −19.2 |
| Cognitive | 10.1 (8.0) | −25.7 | 18.7 (15.8) | −16.2 | 13.1 (11.7) | −22.3 |
| Psychosocial | 1.7 (1.5) | +40.5 | 4.0 (3.1) | +30.5 | 2.5 (2.4) | +36.3 |
| Total | 20.5 (15.1) | −16.7 | 40.5 (31.9) | −20.0 | 27.6 (23.6) | −17.8 |
| Epworth Sleepiness Scale | ||||||
| Total | 6.3 (4.0) | +22.3 | 7.8 (5.4) | +46.5 | 6.8 (4.4) | +29.9 |
The Revised Impact of Events Scale is a PTSD screening tool that measures stress levels and is scored from 0 to 88. PROMIS‐29 Profile measures eight domains of patients’ health with pain intensity scored from 0 to 10 and all other domains scored from 4 to 20 (raw scores). Katz Index measures the ability to perform ADLs, scored from 0 to 6. The Lawton–Brody scale measures the ability to perform IADLs, scored from 0 to 8. The Modified Fatigue Impact Scale measures the level of disturbance caused by fatigue in physical, cognitive, and psychosocial domains and is scored from 0 to 84. The Epworth Sleepiness Scale measures daytime sleepiness levels from 0 to 24. For all measures, a higher score indicates a higher degree of that being measured.
Abbreviations: MoCA, Montreal Cognitive Assessment; SD, standard deviation; ADL, activities of daily living; IADL, instrumental activities of daily living.
Number of participants (n) with any number of missed points in each category of the MoCA.
Of participants who completed the 6‐month assessment, number of participants (n) with any number of missed points in each category at baseline assessment.
Average percentage change (“+” for increase, “‐” for decrease) in the raw score for each participant from baseline to 6 months.
Demographic and clinical factors associated with outcomes
In a multivariable regression model with mutual adjustment, there was no association of age or gender with IESR score, MoCA cognitive screening score, or MFIS fatigue scale score in those without a history of prior neurologic disease. Age and gender were also not associated with subjective memory or concentration symptoms. There was similarly no association of BMI with any outcomes measured. The duration of initial COVID‐19 symptoms (in days) was not associated with the outcomes. Infection severity (on an ordinal scale of mild, moderate, or severe) was associated with MFIS fatigue score (beta coefficient 12.6 higher MFIS score, 95% CI 2.9–22.2, p = 0.013) at baseline after adjustment for age, but not with any other measured outcomes.
Selected phenotypes observed
In this pilot analysis of the cohort data, we preplanned to evaluate for emergent phenotypes, which would motivate iterative protocol measurement additions to the 10‐year cohort study protocol. While rare, a notable neurologic phenotype of PASC with a characteristic combination of tremor, ataxia, and cognitive deficits (PASC‐TAC) was observed in ~7% of participants without a prior neurologic history of these symptoms. PASC‐TAC (Postacute Sequelae of COVID‐19 infection with Tremor, Ataxia, and Cognitive deficit) was defined by clinical symptoms of both incoordination and cognitive change and neurological findings with the following criteria: (1) at least one of the following: tremor, dysmetria, truncal ataxia, gait ataxia dysdiadochokinesia and (2) at least one of the following on objective testing (MoCA): visuospatial difficulty, memory impairment, language impairment, executive function difficulty, or other change in cognitive function from baseline. Table 5 describes PASC‐TAC case examples.
Table 5.
Cases illustrating phenotype of interest: Neuro‐PASC‐TAC.
| Patient | 1 a | 2 a | 3 a | 4 a |
|---|---|---|---|---|
| Age | 43 | 60 | 49 | 79 |
| Baseline Data | ||||
| Disease symptoms | Fever, cough, anosmia, ageusia, congestion, fatigue, headache | Fever, chills, chest tightness, myalgia, headache, fatigue, SOB | Fever, chills, cough, SOB, severe diarrhea, fatigue, myalgia | Fever, chills, sough, SOB, chest tightness, sore throat, myalgia, fatigue, LOA, bradycardia |
| Disease Duration | 14 days | 10 days | 4 days | 14 days |
| Time interval from infection to baseline assessment | 5 months | 2 months | 4 months | 2 months |
| MoCA score | 23 | 26 | 12 | 28 |
| Points lost |
‐Visuospatial/executive ‐Attention ‐ Language ‐ Orientation |
‐ Language ‐ Delayed recall |
‐ Visuospatial/executive ‐ Attention ‐ Language ‐ Abstraction ‐ Delayed recall ‐ Orientation |
‐ Delayed recall |
| Symptoms b |
‐ Memory impairment ‐ Headache ‐ Tremor (myoclonic jerking movements in the lower extremity) ‐ Speech abnormality (word mispronunciation) |
‐ Encephalopathy ‐ Memory impairment ‐ Fatigue ‐ OSA ‐ Palpitations (racing pulse) |
‐ Encephalopathy ‐ Memory impairment ‐ Headache ‐ Trouble concentrating ‐ Insomnia ‐ Numbness/tingling ‐ Tremor ‐ Difficulty walking ‐ Speech abnormality (stuttering) ‐ Hearing difficulties ‐ Fatigue |
‐ Memory impairment ‐ Headache ‐ Trouble concentrating ‐ Muscle weakness ‐ Poor coordination ‐ Tremor ‐ Imbalance ‐ Fatigue |
|
Abnormal Neuro Exam Findings |
‐ Normal exam | ‐ Ataxia on tandem gait |
‐ Decreased light touch sensation ‐ Pallhypesthesia ‐ Positive Romberg ‐ Intention tremor in all extremities ‐ Ataxic gait |
‐ Asymmetric weakness on toe walking ‐ Difficulty with heel walking |
| 6‐month follow‐up | ||||
| MoCA Score | 26 | 29 | 23 * | 24 |
| Points lost |
‐ Visuospatial/executive ‐ Attention ‐ Language ‐ Delayed recall |
‐ Delayed recall |
‐ Visuospatial/executive ‐ Attention ‐ Language ‐ Abstraction ‐ Delayed recall ‐ Orientation |
‐ Visuospatial/executive ‐ Attention ‐ Delayed recall |
| Symptoms b |
‐ Memory impairment ‐ Headache ‐ Poor coordination ‐ Tremor (myoclonic jerking movements in the lower extremity) ‐ Fatigue |
‐ Trouble concentrating ‐ Insomnia ‐ Memory impairment ‐ Speech abnormality ‐ OSA ‐ Palpitations (racing pulse) |
‐ Memory impairment ‐ Headache ‐ Trouble concentrating ‐ Insomnia ‐ Limb weakness ‐ Muscle stiffness ‐ Difficulty walking ‐ Vision difficulties ‐ Hearing difficulties |
‐ Headache ‐ Difficulty walking ‐ Poor coordination ‐ Speech abnormality (dysarthria) ‐ Fatigue |
| Abnormal Neuro Exam Findings | ‐ Incoordination on heel‐shin test | ‐ Normal exam |
‐ Abnormal motor (unable to squat) ‐ Abnormal coordination ‐ Slow foot taps with AFOs ‐ Mild tremor with touching nose to target bilaterally |
‐ Abnormal extraocular movements (dysconjugate gaze) ‐ Dysdiadochokinesia (with right finger tapping) ‐ Ataxia on tandem gait |
| Neuro Exam change from baseline to 6 months |
MoCA improved Neuro exam worsened |
Improved MOCA and neuro exam | Improved MOCA and neuro exam | MoCA and neuro exam worsened |
| MRI findings | N/A | Nonspecific changes | Normal | N/A |
Abbreviations: OSA, Obstructive Sleep Apnea; MoCA, Montreal Cognitive Assessment; SOB, shortness of breath; PLC, poor limb coordination; BLE, bilateral lower extremities; BUE, bilateral upper extremities, AFOs, Ankle foot orthoses, LOA, loss of appetite; N/A, not available.
All four patients were from cohort 1, had no prior neurologic history or hospitalization, and had education of 12+ years.
Symptoms were ascertained through a standard neurologic review of systems administered by study team members.
Patient 3 follow‐up information is from 3 months after baseline (when the patient was able to be seen clinically).
Discussion
Our pilot data from a longitudinal cohort of neuro‐PASC suggest that while the symptom severity decreased for most participants, two‐thirds had persistent neurologic symptoms at the 6‐month follow‐up with over half reporting continued impact on quality of life. The most prominent persistent symptoms were memory impairment and decreased concentration. On the MoCA, the most affected areas of cognition at the 6‐month follow‐up were delayed recall, language, and attention. We observed a rare but concerning phenotype of tremor, ataxia, and cognitive dysfunction in a subset of patients without any known prior neurologic disease. Imaging in these patients was unremarkable, but this phenotype warrants further investigation, including serological and CSF characterization and advanced imaging modalities, especially given recent findings on the association of certain alleles (APOE4) and presenting with severe COVID‐19 or post‐COVID fatigue. 23
In our study, most patients reported mild or moderate COVID‐19 infection, with only 8.9% requiring hospitalization. This is consistent with previous data showing that nonhospitalized patients, many with a mild infection, still experience long‐haul neuro‐PASC symptoms months after infection. 15 , 24 Although some studies suggest that PASC is most prevalent in those with severe infection, 25 , 26 our data suggest that neuro‐PASC commonly presents in patients with milder infections. Our cohort largely represented those who had mild to moderate infections without acute neurovascular complications. Interestingly, while we advertised our study to ICU and stroke providers and patients, those patients with COVID‐19‐associated strokes or hemorrhages rarely presented to long‐haul COVID‐19 Infectious Disease or Neurology clinics. This may be due to COVID‐19 infection resulting in severe symptoms that obscured observation of neurocognitive and neuropsychiatric symptoms or that acute neurologic complications of COVID‐19 have a distinct pathology from postacute neurologic syndromes. Because few participants in our study were hospitalized, our cohort is largely unaffected by concerns for nonspecific neurocognitive or psychiatric effects from ICU stays or complications.
Prior to COVID‐19 infection, most participants did not experience any symptoms. However, if they had previously experienced symptoms, they worsened postinfection. The most prominent early symptoms of neuro‐PASC were fatigue and headache, which were associated with a significant impact on participants’ quality of life for 80% of those queried. Participants’ reported symptoms are consistent with those found in other studies. In a self‐reported online survey answered by 507 patients from Italy, many reported persistent fatigue (74%), myalgia (61%), and articular pains (59%) over 3 months after COVID‐19 infection. 5
At the 6‐month follow‐up, predominant symptoms shifted from headache and fatigue to memory impairment and decreased concentration. This trend has not been previously reported longitudinally in neuro‐PASC. One study utilizing online surveys noted that fatigue, postexertional malaise, and cognitive dysfunction were the most prevalent symptoms reported 6‐month postacute COVID‐19 infection. However, this cross‐sectional study did not trend these symptoms for each patient. 27
Encouragingly, average baseline scores on the Katz Index and Lawton–Brody Scale demonstrated high levels of independence in ADLs and IADLs at baseline that remained consistent at the 6‐month follow‐up. While participants’ IESR impact of event score at baseline was concerning, these scores decreased by almost 50% on average at the 6‐month follow‐up. Participant PROMIS‐29 scores of pain and anxiety increased slightly over time. Even at the 6‐month follow‐up, COVID‐19 had a prominent psychological impact on participants, consistent with the growing literature on this topic. In one prior study, 79.6% of patients reported moderate to severe levels of pain and discomfort 3‐month postinfection and 50% reported moderate levels of anxiety and depression 3‐month postinfection. 5
In our cohort, daytime sleepiness worsened, whereas most aspects of fatigue (excluding psychosocial fatigue) improved at the 6‐month follow‐up. Contrastingly, a prior cross‐sectional study demonstrated that in patients recovering from a mild COVID‐19 infection, 81.5% had impairment due to fatigue, an average of 6 months after infection onset (measured by the MFIS), whereas 35.7% of patients reported moderate–severe daytime sleepiness (measured by ESS). 28 For our data, we hypothesize that the discrepancy between fatigue and daytime sleepiness trends results from the fact that they are distinct, though related, symptoms. As described by researchers, sleepiness results from an impaired arousal mechanism and is more consistent with decreased cognition and mood. This is consistent with our data from PROMIS‐29 scores. Fatigue, on the other hand, is an overpowering feeling of exhaustion and decreased energy. 29 Additionally, the discrepancy in sleepiness and fatigue numbers may also be due to the complex aspects of fatigue that are not captured by daytime sleepiness scores. It will be important to further understand if there are relationships between the increase in anxiety and pain and persistent daytime sleepiness as well as a more detailed assessment of sleep quality in future data collections in Neuro‐PASC.
We reported four patients with no prior neurologic disease who demonstrated a pattern of incoordination coupled with cognitive dysfunction (PASC‐TAC). Longitudinal follow‐up of these patients demonstrated slow recovery though some symptoms still require medications and supportive management. One cross‐sectional study noted ataxia as a neurologic manifestation of COVID‐19 in 2 of 214 patients, following a severe infection of COVID‐19. 30 , 31 The underlying mechanism may be a postinfectious inflammatory process, though a recent study raises the possibility of cerebellar microhemorrhages. 23 Postinfectious cerebellitis and acute cerebellar ataxia in children are well‐recognized entities but have presented more rarely in adults 32 with one reported case of anti‐GAD65‐associated cerebellitis following SARS‐CoV‐2 infection. 33 It is important to recognize this as a rare complication from even mild COVID‐19 infections and further characterization is required including serological, CSF, and advanced blood‐sensitive imaging assessments, which are currently being pursued.
Additionally, a small number of participants from both cohorts exhibited cranial nerve deficits at baseline visit or newly acquired at the 6‐month visit. We hypothesize that the etiology of these newly developed cranial nerve deficits is related to neuro‐PASC, possibly due to an upregulated inflammatory response triggered by the SARS‐CoV‐2 virus.
There are several potential explanations for the pathophysiology of neuro‐PASC, including direct pathogenic invasion, upregulation of intrinsic microglial activity, and microvascular compromise. 34 However, these mechanisms have become less convincing to explain long‐term post‐COVID symptom persistence given the lack of strong evidence for direct SARS‐CoV‐2 CNS invasion. 35 , 36 , 37 Autoimmune mechanisms may provide a stronger basis for understanding neuro‐PASC symptoms. One study found anti‐SARS‐CoV‐2 antibodies to exhibit antineuronal activity and display a CSF‐specific, compartmentalized immune response. 34 While this has not been shown with perfect consistency, 38 it does suggest an autoimmunologic etiology of neuro‐PASC symptoms. The occurrence of neuro‐PASC after mild COVID‐19 infection and in younger patients could indicate a robust immune response to infection. A strong immune response may paradoxically result in less‐severe COVID‐19 infection but risk‐developing autoimmune‐mediated neuro‐PASC symptoms. Additionally, we observed more women with symptoms consistent with previously reported data. 12 Whether sex difference may be explained by an underlying autoimmune etiology is not yet clear. Given the preliminary nature of our findings, the pathophysiologic mechanisms of neuro‐PASC described are speculative but built upon other observational studies from COVID‐19 animal studies, human cohort studies, and our experiences with other postinfectious and chronic neuroinflammatory disorders.
Study strengths include the prospective, longitudinal cohort design. We applied a standardized, comprehensive, and curated data collection procedure including a detailed query of the COVID‐19 infection, neurologic review of systems administered by clinically trained personnel, neurologic examination, validated questionnaires, and cognitive screening test. Those with deficits were offered imaging and detailed neurocognitive assessment and treatment. This preliminary analysis provides insights for iterative protocol improvements and continued study of our and other ongoing cohorts of neuro‐PASC. The primary limitation of our study is the modest sample size, but nonetheless, important observations have been made to guide the next steps in neuro‐PASC research including justification of longer time points in cohort studies and directing additional analyses of the emerging neuro‐PASC‐TAC phenotype. Other limitations include the lack of imaging on all individuals, though even those with the most extreme phenotypes had normal imaging in our cohort. As an exploratory cohort launched early in the pandemic, a systematic collection of CSF samples was not available. However, based on initial results, CSF collection is planned for the ongoing cohort. Other limitations include the potential for referral bias, given that our participants were recruited from Infectious Disease or Neurology clinics after presenting with new or newly worsened neurologic symptoms. This likely selected for participants with more prominent symptoms and introduced a variable latency period between acute COVID‐19 infection and baseline measurement. Another limitation is the insensitivity of the MoCA exam to pick up on subtle cognitive problems. The improvement between baseline and 6‐month MoCA scores may mean no actual change, given the limitation of practice effect. Most participants were evaluated by telemedicine at their discretion, which may have been less sensitive in detecting subtle ataxic syndromes or other exam findings. Bias also exists in that neurologic exam and cognitive assessments were not blinded to patient‐reported symptoms as most participants had their neurologic review of systems, MoCA exam, and neurologic exam conducted in a single sitting by the same interviewer. Additionally, although the surveys employed were validated for other medical or psychiatric conditions, validated instruments specific for neuro‐PASC are yet to be established. Finally, many PASC symptoms are difficult to differentiate from general neurologic symptoms from previous neurologic disorders. We accounted for this in part by separating participants with and without preexisting neurologic disorders into distinct cohorts. However, our sample size was too small to have matching controls for all possible confounding variables.
Conclusion
Limited data exist on the long‐term neurologic sequelae from COVID‐19 infection. Our study provides longitudinal data on Postacute Sequelae of COVID focusing on neurologic symptoms in patients with and without preexisting neurologic disorders. Symptoms involving cognition and memory persist despite improvement in other domains. Continued monitoring and supportive management are needed for patients with mild to severe COVID‐19 infections.
Conflict of Interest
Jacqueline Shanley, Andrew Valenciano, Garrett Timmons, Visesha Kakarla, Annalise Miner, Amanda Gooding, Lucy Horton, Ronald Ellis, Marc Norman, and Torge Rempe do not have anything to disclose. Unrelated to this manuscript, Jennifer Yang has participated in a sponsored talk, Neurology Live MS Medications 2021. Unrelated to the current work, Sarah Banks have consulted with Boston University on NINDS funded grant and participated in the data safety monitoring board for Cleveland Clinic Foundation (NIA funded RO1). Last, unrelated to the current work, Jennifer Graves over the past year has grant/contract research support from the National MS Society, Biogen, and Octave Biosciences. She serves on a steering committee for a trial supported by Novartis. She has received honoraria for a nonpromotional, educational activity for Sanofi‐Genzyme. She has received speaker fees from Alexion and BMS and served on an advisory board for Genentech.
Authors’ Contribution
Jacqueline Shanley and Andrew Valenciano are shared first authors who conducted data collection, analysis, and the article’s first draft. Garrett Timmons contributed to the bulk of all initial data collection. Annalise Miner is responsible for project creation, data collection, and contributing to the draft. Visesha Kakarla and Torge Rempe both are responsible for substantial data collection. Jennifer Yang is also responsible for data collection as well as manuscript drafting. Marc Norman contributed to project concept codevelopment, referrals, and neuropsychological battery measures. Amanda Gooding and Sarah Banks are responsible for project concept codevelopment and referrals. Ron Ellis, Michelle Ritter, and Lucy Horton contributed to referrals and general collaboration. Our NeuCovid team is responsible for collaboration on the study and manuscript. Jennifer Graves is the PI of this project and is responsible for the project conception, study design, data collection, analysis plan, and drafting.
Supporting information
Table S1. The self‐reported outcome measure questionnaires. Participants completed these self‐reported questionnaires at baseline and 6‐month follow‐up visits.
Acknowledgments
We would like to acknowledge and thank our participants, who dedicated their time and effort to this study.
Appendix A.
A.1 NeuCovid Team
| Name, Degree | Role in Study | |
|---|---|---|
| David J. Moore, PhD | djmoore@ucsd.edu | Referrals, study collaboration |
| Emilie T. Reas, PhD | ereas@ucsd.edu | Study collaboration |
| Irene Litvan, MD | ilitvan@ucsd.edu | Study collaboration |
| Susan J. Lee, MD | s2lee@ucsd.edu | Referrals, study collaboration |
| Angela C. Wang, MD | acwang@ucsd.edu | Referrals, study collaboration |
| Mariana Cherner, PhD | mcherner@ucsd.edu | Study collaboration |
| Gabriel C. Leger, MD | gleger@ucsd.edu | Study collaboration |
| Ajay R. Bharti, MD | abhartu@ucsd.edu | Study collaboration |
References
- 1. Collins F. NIH Director's Blog. “Post‐Acute Sequelae of COVID‐19.” Accessed December 11, 2021. https://directorsblog.nih.gov/tag/post‐acute‐sequelae‐of‐covid‐19/
- 2. Amenta EM, Spallone A, Rodriguez‐Barradas MC, el Sahly HM, Atmar RL, Kulkarni PA. Postacute COVID‐19: an overview and approach to classification. Open Forum Infect Dis. 2020;7(12):ofaa509. doi: 10.1093/ofid/ofaa509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goërtz YMJ, Van Herck M, Delbressine JM, et al. Persistent symptoms 3 months after a SARS‐CoV‐2 infection: the post‐COVID‐19 syndrome? ERJ Open Res. 2020;6(4):542‐2020. doi: 10.1183/23120541.00542-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sykes DL, Holdsworth L, Jawad N, Gunasekera P, Morice AH, Crooks MG. Post‐COVID‐19 symptom burden: what is long‐COVID and how should we manage it? Lung. 2021;199(2):113‐119. doi: 10.1007/s00408-021-00423-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Orrù G, Bertelloni D, Diolaiuti F, et al. Long‐COVID syndrome? A study on the persistence of neurological, psychological and physiological symptoms. Healthcare (Basel). 2021;9(5):575. doi: 10.3390/healthcare9050575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carod‐Artal FJ. Neurological complications of coronavirus and COVID‐19. Rev Neurol. 2020;70(9):311–322. English, Spanish. doi: 10.33588/rn.7009.2020179 [DOI] [PubMed] [Google Scholar]
- 7. Abboud H, Abboud FZ, Kharbouch H, Arkha Y, el Abbadi N, el Ouahabi A. COVID‐19 and SARS‐Cov‐2 infection: pathophysiology and clinical effects on the nervous system. World Neurosurg. 2020;140:49‐53. doi: 10.1016/j.wneu.2020.05.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Berger JR. COVID‐19 and the nervous system. J Neurovirol. 2020;26(2):143‐148. doi: 10.1007/s13365-020-00840-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Higgins V, Sohaei D, Diamandis EP, Prassas I. COVID‐19: from an acute to chronic disease? Potential long‐term health consequences. Crit Rev Clin Lab Sci. 2021;58(5):297‐310. doi: 10.1080/10408363.2020.1860895 [DOI] [PubMed] [Google Scholar]
- 10. Wang F, Kream RM, Stefano GB. Long‐term respiratory and neurological sequelae of COVID‐19. Med Sci Monit. 2020;1(26):e928996. doi: 10.12659/MSM.928996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Berlit P, Bösel J, Gahn G, et al. “Neurological manifestations of COVID‐19” ‐ guideline of the German society of neurology. Neurol Res Pract. 2020;2(1):51. doi: 10.1186/s42466-020-00097-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Timmons GM, Rempe T, Bevins EA, et al. CNS lymphocytic Vasculitis in a young woman with COVID‐19 infection. Neurol Neuroimmunol Neuroinflamm. 2021;8(5):e1048. doi: 10.1212/NXI.0000000000001048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med. 2021;27(4):626‐631. doi: 10.1038/s41591-021-01292-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tenforde MW, Kim SS, Lindsell CJ, et al. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID‐19 in a multistate health care systems network — United States, march–June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(30):993‐998. doi: 10.15585/mmwr.mm6930e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Graham EL, Clark JR, Orban ZS, et al. Persistent neurologic symptoms and cognitive dysfunction in non‐hospitalized Covid‐19 “long haulers”. Ann Clin Transl Neurol. 2021;8(5):1073‐1085. doi: 10.1002/acn3.51350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bellucci G, Ballerini C, Mechelli R, et al. SARS‐CoV‐2 meta‐interactome suggests disease‐specific, autoimmune pathophysiologies and therapeutic targets. F1000Res. 2020;9:992. doi: 10.12688/f1000research.25593.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weiss DS. The impact of event scale: revised. In: Wilson JP, Tang C S‐k, eds. Cross‐Cultural Assessment of Psychological Trauma and PTSD. International and Cultural Psychology Series. Springer US; 2007:219‐238. doi: 10.1007/978-0-387-70990-1_10 [DOI] [Google Scholar]
- 18. Cella D, Riley W, Stone A, et al. The patient‐reported outcomes measurement information system (PROMIS) developed and tested its first wave of adult self‐reported health outcome item banks: 2005‐2008. J Clin Epidemiol. 2010;63(11):1179‐1194. doi: 10.1016/j.jclinepi.2010.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Katz S, Downs TD, Cash HR, Grotz RC. Progress in development of the index of ADL. Gerontologist. 1970;10(1):20‐30. doi: 10.1093/geront/10.1_part_1.20 [DOI] [PubMed] [Google Scholar]
- 20. Lawton MP, Brody EM. Assessment of older people: self‐maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179‐186. [PubMed] [Google Scholar]
- 21. Krupp LB, LaRocca NG, Muir‐Nash J, et al. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46(10):1121‐1123. doi: 10.1001/archneur.1989.00520460115022 [DOI] [PubMed] [Google Scholar]
- 22. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540‐545. doi: 10.1093/sleep/14.6.540 [DOI] [PubMed] [Google Scholar]
- 23. Kurki SN, Kantonen J, Kaivola K, Hokkanen L, Mäyränpää MI, Puttonen H, FinnGen , Martola J, Pöyhönen M, Kero M, Tuimala J, Carpén O, Kantele A, Vapalahti O, Tiainen M, Tienari PJ, Kaila K, Hästbacka J, Myllykangas L APOE ε4 associates with increased risk of severe COVID‐19, cerebral microhaemorrhages and post‐COVID mental fatigue: a Finnish biobank, autopsy and clinical study. Acta Neuropathol Commun 2021. Dec 23;9(1):199. doi: 10.1186/s40478-021-01302-7. PMID: 34949230; PMCID: PMC8696243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Apple AC, Oddi A, Peluso MJ, et al. Risk factors and abnormal cerebrospinal fluid associate with cognitive symptoms after mild COVID‐19. Ann Clin Transl Neurol. 2022;9(2):221‐226. doi: 10.1002/acn3.51498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. LaVergne SM, Stromberg S, Baxter BA, et al. A longitudinal SARS‐CoV‐2 biorepository for COVID‐19 survivors with and without post‐acute sequelae. BMC Infect Dis. 2021;21(1):677. doi: 10.1186/s12879-021-06359-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Taquet M, Geddes JR, Husain M, Luciano S, Harrison PJ. 6‐month neurological and psychiatric outcomes in 236 379 survivors of COVID‐19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8(5):416‐427. doi: 10.1016/S2215-0366(21)00084-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021. Aug;38:101019. doi: 10.1016/j.eclinm.2021.101019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Boesl F, Audebert H, Endres M, Prüss H, Franke C. A neurological outpatient Clinic for Patients with Post‐COVID‐19 syndrome ‐ a report on the clinical presentations of the first 100 patients. Front Neurol. 2021;12:738405. doi: 10.3389/fneur.2021.738405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shen J, Barbera J, Shapiro CM. Distinguishing sleepiness and fatigue: focus on definition and measurement. Sleep Med Rev 2006;10(1):63–76. doi: 10.1016/j.smrv.2005.05.004. Epub 2005 Dec 22. PMID: 16376590. [DOI] [PubMed] [Google Scholar]
- 30. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683‐690. doi: 10.1001/jamaneurol.2020.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Özdağ Acarli AN, Samanci B, Ekizoğlu E, et al. Coronavirus disease 2019 (COVID‐19) from the point of view of neurologists: observation of neurological findings and symptoms during the combat against a pandemic. Noro Psikiyatr Ars. 2020;57(2):154‐159. doi: 10.29399/npa.26148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Van Samkar A, Poulsen MNF, Bienfait HP, et al. Acute cerebellitis in adults: a case report and review of the literature. BMC Res Notes. 2017;10(1):610. doi: 10.1186/s13104-017-2935-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Emekli AS, Parlak A, Göcen NY, Kürtüncü M. Anti‐GAD associated post‐infectious cerebellitis after COVID‐19 infection. Neurol Sci. 2021;42(10):3995‐4002. doi: 10.1007/s10072-021-05506-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Song E, Bartley CM, Chow RD, et al. Divergent and self‐reactive immune responses in the CNS of COVID‐19 patients with neurological symptoms. Cell Rep Med. 2021;2(5):100288. doi: 10.1016/j.xcrm.2021.100288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bernard‐Valnet R, Perriot S, Canales M, et al. Encephalopathies associated with severe COVID‐19 present neurovascular unit alterations without evidence for strong neuroinflammation. Neurol Neuroimmunol Neuroinflamm. 2021;8(5):e1029. doi: 10.1212/NXI.0000000000001029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Solomon IH, Normandin E, Bhattacharyya S, et al. Neuropathological features of Covid‐19. N Engl J Med. 2020;383(10):989‐992. doi: 10.1056/NEJMc2019373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Iadecola C, Anrather J, Kamel H. Effects of COVID‐19 on the nervous system. Cell. 2020;183(1):16‐27.e1. doi: 10.1016/j.cell.2020.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Valencia Sanchez C, Theel E, Binnicker M, Toledano M, McKeon A. Autoimmune encephalitis after SARS‐CoV‐2 infection: case frequency, findings, and outcomes. Neurology. 2021;97(23):e2262‐e2268. doi: 10.1212/WNL.0000000000012931 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. The self‐reported outcome measure questionnaires. Participants completed these self‐reported questionnaires at baseline and 6‐month follow‐up visits.


