Abstract
We used isomeric fluorotoluenes as model substrates to study the catabolism of toluene by five deuteromycete fungi and one ascomycete fungus capable of growth on toluene as the sole carbon and energy source, as well as by two fungi (Cunninghamella echinulata and Aspergillus niger) that cometabolize toluene. Whole cells were incubated with 2-, 3-, and 4-fluorotoluene, and metabolites were characterized by 19F nuclear magnetic resonance. Oxidation of fluorotoluene by C. echinulata was initiated either at the aromatic ring, resulting in fluorinated o-cresol, or at the methyl group to form fluorobenzoate. The initial conversion of the fluorotoluenes by toluene-grown fungi occurred only at the side chain and resulted in fluorinated benzoates. The latter compounds were the substrate for the ring hydroxylation and, depending on the fluorine position, were further metabolized up to catecholic intermediates. From the 19F nuclear magnetic resonance metabolic profiles, we propose that diverse fungi that grow on toluene assimilate toluene by an initial oxidation of the methyl group.
In bacteria, five different metabolic pathways for the complete aerobic degradation of toluene and its assimilation are known (9, 16, 22, 23; B. Kaphammer, J. J. Kukor, and R. H. Olsen, Abstr. 90th Annu. Meet. Am. Soc. Microbiol., abstr. K-145, p. 243, 1990). Depending upon the strain, toluene is initially oxidized either at the methyl group or at the aromatic ring. Fungi also can oxidize toluene at both molecular sites. Cultures of Mortierella isabellina converted toluene into benzyl alcohol (12). Smith and Rosazza (17) identified two zygomycetes and three deuteromycetes that hydroxylated toluene at the aromatic ring to produce o-cresol and, in some cases, p-cresol. Mineralization of toluene has been reported for the white-rot fungus Phanerochaete chrysosporium, but the metabolic pathway was not determined (25). The fungal degradation of toluene in these cases occurred only by cometabolism and, consequently, it did not support growth. We previously identified and described a Cladosporium sphaerospermum strain that can grow on toluene as the sole carbon and energy source (20). Oxygen consumption experiments with whole cells and enzyme activities in cell extracts suggest that the initial oxidation of toluene takes place at the methyl group, rather than at the aromatic ring. Recently, we identified five additional fungi that also can assimilate toluene (14).
19F nuclear magnetic resonance (19F NMR) has been used previously to characterize the degradation of fluorine-containing aromatic compounds by fungi (2, 13, 18). Fluorine, with its small size, can replace hydrogen in an organic substrate with few steric consequences. It also influences the conversion rate of many enzyme reactions (21). The 19F isotope, with a natural abundance of 100% and a broad chemical shift range, is a very sensitive NMR-active nucleus that can be advantageously used in the identification and quantification of fluorinated intermediates by 19F NMR spectroscopy.
Our objectives were to identify the initial steps for the catabolism of toluene in six previously isolated fungi (14, 20) which are capable of growth on toluene as a sole carbon and energy source: C. sphaerospermum T0, Cladophialophora sp. strains T1 and T2, Pseudeurotium zonatum T3, Exophiala sp. T4, and Leptodontium sp. strain T5. The fungi Cunninghamella echinulata CBS 596.68 and Aspergillus niger CBS 126.48 were also included in this study. These two organisms cometabolically hydroxylate the aromatic ring of toluene (1, 17). We used fluorinated toluene isomers as substrate analogs and 19F NMR spectroscopy to characterize the pattern of metabolite accumulation. In particular, we focused on the site of the initial oxidative attack, in order to determine whether fungi can assimilate toluene through pathways as diverse as those used by the aerobic bacteria.
MATERIALS AND METHODS
Chemicals.
Toluene was purchased from Labscan Ltd. (Dublin, Ireland). 2-, 3-, and 4-fluorotoluene and the reference compounds 2-, 3-, and 4-fluorobenzyl alcohol, 2-, 3-, and 4-fluorobenzaldehyde, 2-, 3-, and 4-fluorobenzoic acid, 3-fluorocatechol, and 3-fluoro-6-hydroxytoluene were from Acros Organics (Geel, Belgium). 4-Fluoro-3-hydroxytoluene was from ABCR GmbH & Co. KG (Karlsruhe, Germany). All chemicals were of analytical grade. 3-Fluoro-4-hydroxybenzoic acid was a gift of Sjef Boeren (Laboratory of Biochemistry, Wageningen University). The purity of all fluorinated compounds was verified by 19F NMR spectroscopy.
Organisms.
The fungi capable of growing on toluene used in this study were C. sphaerospermum T0, Cladophialophora sp. strains T1 and T2, P. zonatum T3, Exophiala sp. strain T4, and Leptodontium sp. strain T5. These fungi can be obtained upon request from the culture collection of the Department of Biotechnology, University of Kaiserslautern (Kaiserslautern, Germany). The strains C. echinulata CBS 596.68 and A. niger CBS 126.48 were purchased from the Centraalbureau voor Schimmelcultures (Baarn, The Netherlands). All organisms were routinely maintained at 4°C on 2% glucose mineral medium (10) agar slants.
Preparation of cell suspensions.
Toluene-grown mycelium was obtained as previously described (14). C. echinulata and A. niger were grown at 30°C as shake cultures (120 rpm) in cotton-plugged 5-liter Erlenmeyer flasks containing 0.5 liter of the following (per liter of demineralized water): 20 g of glucose, 5 g of mycological peptone, 2 g of yeast extract, 1 g of KH2PO4, and 0.5 g of MgSO4 · 7H2O. The medium was inoculated with 1 ml of a spore suspension (approximately 107 spores per ml) and incubated for 48 h. Toluene oxidation activity was induced by replacing the cotton plug with a rubber cap containing an insert filled with 5 ml of a 5:95 toluene-dibutylphthalate solution. The flask was then incubated for 12 additional hours. Mycelium was harvested with filter paper with a 4- to 7-μm retentivity (595 grade; Schleicher & Schuell, Dassel, Germany), washed with 500 ml of a 50 mM potassium phosphate buffer (pH 7.0), and resuspended in the same buffer solution. Cell suspensions were stored at 4°C and used within 3 days after preparation.
Incubations with whole cells.
(Fluoro)toluene was bioconverted in 250-ml Boston flasks closed with Teflon valves (Mininert; Phase Separations, Waddinxveen, The Netherlands). Cell suspensions of toluene-grown fungi (10 ml, approximately 8 g [dry wt] liter−1) were incubated with the fluorotoluenes (shaking conditions, 120 rpm and 30°C). Toluene and all three fluorinated isomers, 2-, 3-, and 4-fluorotoluene, were added individually up to 2 μl/10-ml culture. T3 and T5 had no degradation activity after harvest, so these fungi were cultured in Boston flasks containing 25 ml of mineral media (10) and 4.5 μl of toluene (static conditions, 25°C). After toluene exhaustion, the resulting cultures (approximately 5 g [dry wt] liter−1) were flushed with nonsterile air and then incubated with a fluorinated toluene (2 μl/25-ml culture). Similarly, toluene-induced and noninduced cells of C. echinulata and A. niger (25 ml, approximately 14 g [dry wt] liter−1) were incubated with 2 μl of each fluorotoluene (shaking conditions, 30°C). We followed fluorotoluene consumption via gas chromatographic analysis of the headspace. Incubations lasted no longer than 48 h and were stopped before complete substrate depletion. The cell suspension was stored at −20°C until analyzed. For metabolite determination, samples were thawed and divided into two portions. One was centrifuged (4°C, 10 min, 13,000 × g) to remove cell debris, and the other was extracted with 1 volume of ethyl acetate. Both the culture medium and the solvent extract were analyzed by 19F NMR.
Identification of fluorinated metabolites.
Products of fluorotoluene conversion were identified by comparing their 19F NMR chemical shift values with those of authentic reference compounds whenever available. For compounds not available commercially, the chemical shift was either taken from the literature or predicted by using the method of Rietjens et al. (15). Comparisons between known and predicted chemical shift values indicated that this approach provides reliable results (not shown). The presence of fluorinated intermediates at trace level (less than 1% of the total 19F resonance) was confirmed by analyzing new samples from the incubation media.
Analytical methods.
Toluene and the fluorinated analogs were measured by injecting 100-μl headspace samples into a HP 6890 gas chromatograph (Hewlett-Packard, Wilmington, Del.) with a 10% SE-30 Chromosorb WMP column (Chrompack B.V., Middelburg, The Netherlands). The carrier gas was nitrogen at a flow rate of 1.9 ml/min. The temperatures of the column and the flame ionization detector were 110 and 300°C, respectively. 19F NMR measurements were made with a Bruker DPX 400 MHz NMR spectrometer as previously described (19). The temperature of the measurement was 7°C. The sample volume was 2 ml containing 1.8 ml of culture medium and 0.2 ml of 0.5 M potassium phosphate buffer (pH 7). Ethyl acetate-extracted fractions (2 ml) were assayed directly. 4-Fluorobenzoate (80 μM) was added as an internal standard via an insert. 19F chemical shifts (expressed in parts per million with respect to CFCl3) and concentrations of the various metabolites were calculated by comparison of their 19F NMR integrals to that of the standard 4-fluorobenzoate. Cell dry weight was determined by weighing dried cell suspensions (24 h at 105°C).
RESULTS
Fungal conversion of fluorinated toluenes.
The two types of toluene-degrading fungi, those that degrade it by cometabolism and those that use it as the sole carbon source, both degraded the fluorinated analogs, although at very different rates (Table 1). The specific degradation activity for the fluorotoluenes was up to 1 order of magnitude higher in fungi that used toluene for both energy generation and biomass production. These fungi degraded 4-fluorotoluene faster than 3- and 2-fluorotoluene. The latter isomer was not degraded by Cladophialophora sp. strain T2. Recovery of the fluorine label from the consumed substrate as conversion products in the liquid medium was as high as 90% in most of cases. Due to the low specific degradation activity in the fungi cometabolizing toluene, the rates for fluorotoluene conversion were estimated on the basis of the amount of fluorinated products that accumulated in the media in relation to the biomass and the incubation time. The activities presented in Table 1 for these fungi were obtained with cells that had been exposed to toluene during growth and were about four times higher than those in noninduced cells.
TABLE 1.
Specific rates for the degradation of toluene and fluorinated toluene analogs
| Fungus | Toluene metabolisma | Rate of degradation (μmol h−1 g [dry wt]−1) of substrateb
|
|||
|---|---|---|---|---|---|
| Toluene | 2-F-toluene | 3-F-toluene | 4-F-toluene | ||
| A. niger CBS 126.48 | C | ND | 0.012 | 0.015 | 0.012 |
| C. echinulata CBS 596.68 | C | ND | 0.034 | 0.041 | 0.053 |
| C. sphaerospermum T0 | A | 74 (7) | 22 | 39 | 69 |
| Cladophialophora sp. strain T1 | A | 81 (4) | 29 | 30 | 59 |
| Cladophialophora sp. strain T2 | A | 74 (6) | —c | 35 | 60 |
| P. zonatum T3 | A | 2.0 (0.4) | 1.5 | 1.3 | 2.1 |
| Exophiala sp. strain T4 | A | 25 (2) | 13 | 8.0 | 15 |
| Leptodontium sp. strain T5 | A | 4.3 (0.9) | 3.3 | 2.1 | 3.5 |
C, toluene cometabolism by toluene-induced cells (degradation rates estimated from the amount of accumulated fluorinated products); A, assimilation of toluene by toluene-grown cells (degradation rates measured at the head space, n ≥ 5, r2 ≥ 0.98).
F, fluoro. Values for toluene are averages of three experiments; the standard deviation is given in parentheses. ND, not determined.
—, not degraded.
Identification of fluorinated metabolites.
The 19F NMR chemical shifts of the various fluorotoluene derivatives were assigned to specific metabolites (Table 2). Peak overlap was observed between the 19F NMR signals of 3-fluorobenzyl alcohol and 3-fluorobenzoate, since both compounds show the same chemical shift in the aqueous phase. The fluorine resonance of these compounds differed in an ethyl acetate solution. Consequently, the presence of 3-fluorobenzyl alcohol and/or 3-fluorobenzoate was confirmed by analyzing the solvent-extracted fractions. A small 19F NMR peak at −112.5 ppm resulting from the degradation of 2-fluorotoluene by C. sphaerospermum T0 and P. zonatum T3 was tentatively identified as a muconate derivative due to its proximity to the chemical shift of 2-fluoro-cis,cis-muconate. We suggest that this resonance results from a cis-trans isomerization product of 2-fluoro-cis,cis-muconate or from a fluorinated carboxymuconate arising from the ring opening of fluoroprotocatechuate. This unidentified metabolite had a low signal intensity and limited stability.
TABLE 2.
Fluorinated products resulting from the degradation of fluorotoluene by eight fungi
| Chemical shift (ppm)a
|
Compound | |
|---|---|---|
| 50 mM potassium phosphate buffer, pH 7 | Ethyl acetate | |
| −124.3 (R) | −128.1 (R) | 2-Fluorobenzyl alcohol |
| −117.9 (R) | −121.6 (R) | 3-Fluorobenzyl alcohol |
| −119.5 (R) | −124.3 (R) | 4-Fluorobenzyl alcohol |
| −120.3 (R) | — | 2-Fluorobenzoate |
| −118.0 (R) | −120.6 (R) | 3-Fluorobenzoate |
| −114.2 (R) | −114.9 (R) | 4-Fluorobenzoate |
| −116.4 (L) | — | 2-Fluoro-4-hydroxybenzoate |
| −141.7 (R) | — | 3-Fluoro-4-hydroxybenzoate |
| −141.2 (L) | — | 2-Fluoro-3,4-hydroxybenzoate |
| −126.2 (L) | — | 2-Fluoro-4,5-hydroxybenzoate |
| −140.2 (P) | — | 3-Fluoro-4,5-hydroxybenzoate |
| −140.4 (R) | −144.6 (R) | 3-Fluorocatechol |
| −111.8 (L) | — | 2-Fluoro-cis,cis-muconate |
| −129.1 (R) | −134.5 (R) | 3-Fluoro-6-hydroxytoluene |
| −120.7 (P) | — | 4-Fluoro-6-hydroxytoluene |
| −123.0 (R) | — | Free fluorine |
Metabolic pathway for the fluorotoluenes.
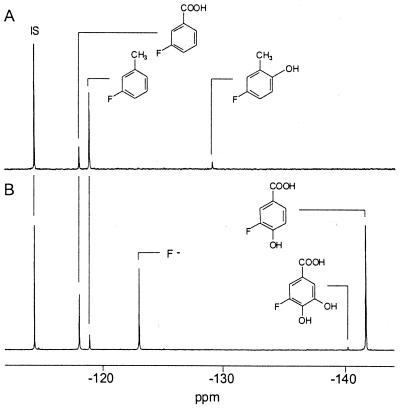
The fungi cometabolizing toluene and the toluene-grown strains differed in the nature of the metabolites accumulated (Table 3). With 3-fluorotoluene, for example, the main fluorinated product excreted by C. echinulata was 3-fluorobenzoate (Fig. 1). The phenolic metabolite 3-fluoro-6-hydroxytoluene (fluorinated o-cresol) was also detected, indicating that this fungus oxidizes toluene both at the methyl group and at the aromatic ring. No fluoride anion was observed, and consequently, the intermediates measured by 19F NMR are the ultimate accumulation products. In contrast, the metabolic profile for C. sphaerospermum T0 indicates that 3-fluorotoluene is initially oxidized only at the side chain. The resulting 3-fluorobenzoate is metabolized to 3-fluoro-4-hydroxybenzoate and 3-fluoro-protocatechuate. A relatively high concentration of free fluorine was measured, possibly as a result of the oxidative defluorination of 3-fluoro-4-hydroxybenzoate to protocatechuate.
TABLE 3.
Metabolite patterns from the fungal degradation of fluorotoluene analogs as determined by 19F NMRa
| Substrate and metabolite | Relative composition in fungus (grouped by type of toluene metabolism)b
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Cometabolism
|
Assimilation
|
|||||||
| A. niger CBS 126.48 | C. echinulata CBS 596.68 | C. sphaerospermum T0 | Cladophialophora sp. strain T1 | Cladophialophora sp. strain T2 | P. zonatum T3 | Exophiala sp. strain T4 | Leptodontium sp. strain T5 | |
| 2-F-toluene | ||||||||
| 2-F-benzyl alcohol | — | 70* | 2* | 83* | — | — | 9* | 37* |
| 2-F-benzoate | 100 | 30 | 53 | — | — | 46 | 7 | 57 |
| 2-F-4OH-benzoate | — | — | 4 | — | — | — | 2 | — |
| 2-F-3,4OH-benzoate | — | — | — | — | — | — | 12 | — |
| 2-F-4,5OH-benzoate | — | — | — | — | — | — | 2 | — |
| 3-F-catechol | — | — | — | — | — | — | 1* | — |
| 2-F-cis,cis-muconate | — | — | — | Tr | — | — | 32 | — |
| Unknownc | — | — | 2 | — | — | 6 | — | — |
| Free fluorine | — | — | 39 | 17 | — | 48 | 35 | 6 |
| 3-F-toluene | ||||||||
| 3-F-6OH-toluene | — | 29* | — | — | — | — | — | — |
| 3-F-benzyl alcohol | — | — | — | — | — | |||
| 3-F-benzoate | 83* | 71* | 18* | 51*d | 16* | 65* | 23*d | 92*d |
| 3-F-4OH-benzoate | 17 | — | 54 | 39 | 63 | 15 | 51 | — |
| 3-F-4,5OH-benzoate | — | — | Tr | Tr | 2 | 8 | — | — |
| 3-F-catechol | — | — | — | — | Tr* | — | 3* | — |
| 2-F-cis,cis-muconate | — | — | — | Tr | Tr | — | — | — |
| Free fluorine | — | — | 28 | 10 | 19 | 12 | 23 | 8 |
| 4-F-toluene | ||||||||
| 4-F-6OH-toluene | — | 7* | — | — | — | — | — | — |
| 4-F-benzyl alcohol | 4* | — | Tr* | 41* | Tr* | — | Tr* | 2* |
| 4-F-benzoate | 96* | 93* | 99* | 57* | 99* | 100* | 68* | 98* |
| Free fluorine | — | — | Tr | 2 | — | — | 31 | — |
The composition is relative to the total amount of fluorinated products other than the parent fluorotoluene, which was taken as 100%. F, fluoro.
*, identified in the ethyl acetate extracts; Tr, less than 1% of the total 19F intensity; —, not detected.
Fluorine resonance at −112.5 ppm tentatively identified the compound as a muconate derivative.
3-Fluoro-benzyl alcohol and 3-fluoro-benzoate are quantified together due to peak overlap in water solution.
FIG. 1.
19F NMR spectra (at 7°C) of the culture supernatant after incubation of whole cells of C. echinulata CBS 596.68 (A) and C. sphaerospermum T0 (B) with 3-fluorotoluene. The resonance at −114.4 ppm (IS) is from the standard 4-fluorobenzoate contained in an insert.
DISCUSSION
We characterized the initial oxidation of toluene by using fluorinated analog substrates and identifying the metabolites formed by 19F NMR. Previous attempts to measure and identify intermediates of toluene degradation by using a high-pressure liquid chromatography method were inconclusive (not shown). The fluorine substituent effectively decreased the conversion rate of specific reactions, resulting in the accumulation of intermediates that otherwise would have been rapidly metabolized further. The accumulation pattern depended primarily on the type of toluene oxidation, i.e., cometabolism versus assimilation, and the position of the fluorine.
Conversion of fluorotoluene was exceptionally low in the cometabolizing strains C. echinulata and A. niger. Aryl hydrocarbon hydroxylation by fungi is catalyzed by P-450 monooxygenases, which are usually substrate-inducible enzymes (8). But even cultures that had been exposed to toluene during growth metabolized the fluorinated analogs very slowly. Although both Cunninghamella and Aspergillus species are reported to hydroxylate the aromatic ring of several aromatic hydrocarbons (8), the two strains we tested both initiated oxidation of the fluorinated toluenes at the side chain. Only C. echinulata also hydroxylated the fluorotoluene structure at the aromatic ring to form fluorinated o-cresols. The highest yield of fluorocresol was obtained with the 3-fluorotoluene isomer, which was hydroxylated exclusively at the para position in relation to the fluorine to yield 3-fluoro-6-hydroxytoluene. A similar regioselectivity in the related species Cunninghamella elegans was found for the hydroxylation of 1-fluoronaphthalene, in which the fluorine group prevented hydroxylation at the adjacent carbons (7). Fluorinated o-cresol was also detected with 4-fluorotoluene but not with 2-fluorotoluene. As before (17), we found no evidence for cresol formation by A. niger. Taken together, our results indicate that the presence of a methyl group in the benzene ring channels the fungal oxidative attack towards the side chain. A preference for hydroxylation of alkylated aromatic hydrocarbons at the side chain has been reported for Cunninghamella species (5, 6, 11).
Fungi that used toluene for both energy generation and biomass production converted the fluorinated analogs at higher rates and to more oxidized intermediates, which all were products of the side chain metabolism (Table 3). In general, the proximity of fluorine to the methyl group had a negative effect on the degradation rate of fluorotoluene (Table 1). This steric effect might result from the change in reactivity caused by the fluorine nucleus towards the side chain monooxygenase. The extent of fluorotoluene degradation also depended on the fluorine position: 2-fluorotoluene was converted to 2-fluorobenzyl alcohol and/or 2-fluorobenzoate. A significant part of the 19F signal in the liquid media was identified as free fluorine, indicating that these metabolites accumulated transiently, with the aromatic ring being effectively defluorinated at a later stage. This pattern of substrate conversion was not seen with either Cladophialophora sp. strain T2 or Exophiala sp. strain T4. While the fluorine at the C-2 carbon center of toluene prevented the oxidative attack in the former strain, it induced accumulation of 2-fluoro-cis,cis-muconate in the latter. Similar to this, 2-fluoro-cis,cis-muconate was the main degradation product of 2-fluorophenol by phenol-grown cells of another Exophiala species (2). 3-Fluorotoluene also was metabolized to fluorinated benzoate, but in this case 3-fluoro-4-hydroxybenzoate was often the main product. Besides free fluorine, low concentrations of fluorinated protocatechuate, catechol, and cis,cis-muconate were detected with some of the fungi. Apparently, fluorine at C-3 was an important rate-limiting factor for the hydroxylation of 3-fluoro-4-hydroxybenzoate to fluorinated protocatechuate. In contrast to the 2- and 3-fluorotoluenes and with the exception of Exophiala sp. strain T4, the fungi could not cleave the carbon-fluorine bond of 4-fluorotoluene. Consequently, 4-fluorobenzoate was the end-reaction product of the degradation of 4-fluorotoluene.
In summary, the six toluene-grown fungi converted 2-, 3-, and 4-fluorotoluene to intermediates that matched the toluene metabolic pathway earlier proposed for C. sphaerospermum T0 (20). By analogy, we suggest that toluene is assimilated via an initial oxidation of the methyl group by all fungi studied. Thus, toluene is first hydroxylated to benzyl alcohol and then dehydrogenated to benzoate via a putative aldehyde intermediate. Benzoate is the substrate for the hydroxylation of the aromatic ring to 4-hydroxybenzoate and protocatechuate. Detection of fluorinated catechols and fluorinated cis,cis-muconate in the Cladophialophora sp. strains (T1 and T2) and Exophiala sp. strain T4 supports the hypothesis that protocatechuate is decarboxylated to catechol. The aromatic ring of catechol is opened at the ortho position to yield cis,cis-muconate. This ring cleavage pathway has been reported previously for fungi growing on 4-hydroxybenzoate (4). However, fungi also assimilate 4-hydroxybenzoate via two other alternative ring fission substrates: protocatechuate and hydroxyquinol (24). All three pathways converge with the formation of 3-oxoadipate. The pattern of ring cleavage for the metabolism of toluene in the strains C. sphaerospermum T0, P. zonatum T3, and Leptodontium sp. strain T5 cannot be determined from our results.
The toluene catabolic pathway is of special interest because either the benzene nucleus or the aliphatic side chain may be subject to oxidative attack. Bacteria have evolved both options, but very little is known about the fungal metabolism of toluene. The similarity of the initial oxidative steps in all these strains suggests that fungi may have less metabolic versatility than bacteria for the assimilation of toluene.
ACKNOWLEDGMENTS
This work was supported by grant BIO4 CT 972295 from the European Commission.
We acknowledge Beb van Veldhuizen for assistance in the NMR measurements.
REFERENCES
- 1.Auret B J, Boyd D R, Robinson P M, Watson C G. The NIH shift during the hydroxylation of aromatic substrates by fungi. Chem Commun. 1971;24:1585–1587. [Google Scholar]
- 2.Boersma G M, Dinarieva T Y, Middelhoven W J, van Berkel W J H, Doran J, Vervoort J, Rietjens I M C M. 19F nuclear magnetic resonance as a tool to investigate microbial degradation of fluorophenols to fluorocatechols and fluoromuconates. Appl Environ Microbiol. 1998;64:1256–1263. doi: 10.1128/aem.64.4.1256-1263.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bondar V S, Boersma M G, Golovlev E L, Vervoort J, van Berkel W J H, Finkelstein Z I, Solyanikova I P, Golovleva L A, Rietjens I M C M. 19F NMR study on the biodegradation of fluorophenols by various Rhodococcus species. Biodegradation. 1998;9:475–486. doi: 10.1023/a:1008391906885. [DOI] [PubMed] [Google Scholar]
- 4.Cain R B, Bilton R F, Darrah J A. The metabolism of aromatic acids by microorganisms. Metabolic pathways in the fungi. Biochem J. 1968;108:797–828. doi: 10.1042/bj1080797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cerniglia C E, Fu P P, Yang S K. Regio- and stero-selective metabolism of 4-methylbenz(a)anthracene by the fungus Cunninghamella elegans. Biochem J. 1983;216:377–384. doi: 10.1042/bj2160377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cerniglia C E, Lambert K J, Miller D W, Freeman J P. Transformation of 1- and 2-methylnaphthalene by Cunninghamella elegans. Appl Environ Microbiol. 1984;47:111–118. doi: 10.1128/aem.47.1.111-118.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cerniglia C E, Miller D W, Yang S K, Freeman J P. Effects of a fluoro substituent on the fungal metabolism of 1-fluoronaphthalene. Appl Environ Microbiol. 1984;48:294–300. doi: 10.1128/aem.48.2.294-300.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cerniglia C E, Sutherland J B, Crow S A. Fungal metabolism of aromatic hydrocarbons. In: Winkelmann G, editor. Microbial degradation of natural products. Weinheim, Germany: VCH Verlagsgesellschaft; 1991. p. 193. [Google Scholar]
- 9.Gibson D T, Henley M, Yoshioka H, Mabry T J. Formation of (+)-cis-2,3-dihydroxy-1-methyl-cyclohexa-4,6,-diene from toluene by Pseudomonas putida. Biochemistry. 1970;9:1626–1630. doi: 10.1021/bi00809a023. [DOI] [PubMed] [Google Scholar]
- 10.Hartmans S, van der Werf M J, de Bont J A M. Bacterial degradation of styrene involving a novel flavin adenine dinucleotide-dependent styrene monooxygenase. Appl Environ Microbiol. 1990;56:1347–1351. doi: 10.1128/aem.56.5.1347-1351.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holland H L, Bergen E J, Chenchaiah P C, Khan S H, Munoz B, Ninniss R W, Richards D. Side chain hydroxylation of aromatic compounds by fungi. 1. Products and stereochemistry. Can J Chem. 1987;65:502–507. [Google Scholar]
- 12.Holland H L, Brown F M, Munoz B, Ninniss R W. Side chain hydroxylation of aromatic hydrocarbons by fungi. Part 2: isotope effects and mechanism. J Chem Soc Perkin Trans II. 1988;1988:1557–1563. [Google Scholar]
- 13.Peelen S, Rietjens I M C M, Boersma M G, Vervoort J. Conversion of phenol derivatives to hydroxylated products by phenol hydroxylase from Trichosporon cutaneum. A comparison of regioselectivity and rate of conversion with calculated molecular orbital substrate characteristics. Eur J Biochem. 1995;227:284–291. doi: 10.1111/j.1432-1033.1995.tb20386.x. [DOI] [PubMed] [Google Scholar]
- 14.Prenafeta-Boldú, F. X., A. Kuhn, D. M. A. M. Luykx, H. Anke, J. W. van Groenestijn, and J. A. M. de Bont. Isolation and characterisation of fungi growing on volatile aromatic hydrocarbons as their sole carbon and energy source. Mycol. Res., in press.
- 15.Rietjens I C M M, Cnubben N H P, van Haandel M, Tyrakowska B, Soffers A E M F, Vervoort J. Different metabolic pathways of 2,5-difluoronitrobenzene and 2,5-difluoroaminobenzene compared to molecular orbital substrate characteristics. Chem-Biol Interact. 1995;94:49–72. doi: 10.1016/0009-2797(94)03317-2. [DOI] [PubMed] [Google Scholar]
- 16.Shields S M, Montgomery S O, Chapman P J, Cuskey S M, Pritchard P H. Novel pathway of toluene catabolism in the trichloroethylene-degrading bacterium G4. Appl Environ Microbiol. 1989;55:1624–1629. doi: 10.1128/aem.55.6.1624-1629.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith R V, Rosazza J P. Microbial models of mammalian metabolism. Aromatic hydroxylation. Arch Biochem Biophys. 1974;161:551–558. doi: 10.1016/0003-9861(74)90338-5. [DOI] [PubMed] [Google Scholar]
- 18.van Berkel W J H, Eppink M H M, Middelhoven W J, Vervoort J, Rietjens I M C M. Catabolism of 4-hydroxybenzoate in Candida parapsilosis proceeds through initial oxidative decarboxylation by a FAD-dependent 4-hydroxybenzoate 1-hydroxylase. FEMS Microbiol Lett. 1994;121:207–216. doi: 10.1111/j.1574-6968.1994.tb07100.x. [DOI] [PubMed] [Google Scholar]
- 19.Vervoort J, de Jager P A, Steenbergen J, Rietjens I M C M. Development of a 19F-NMR method for studies on the in vivo and in vitro metabolism of 2-fluoroaniline. Xenobiotica. 1990;20:657–670. doi: 10.3109/00498259009046882. [DOI] [PubMed] [Google Scholar]
- 20.Weber F J, Hage K C, de Bont J A M. Growth of the fungus Cladosporium sphaerospermum with toluene as the sole carbon and energy source. Appl Environ Microbiol. 1995;61:3562–3566. doi: 10.1128/aem.61.10.3562-3566.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whals C. Fluorinated substrate analogs: routes of metabolism and selective toxicity. Adv Enzymol. 1982;55:197–289. doi: 10.1002/9780470123010.ch3. [DOI] [PubMed] [Google Scholar]
- 22.Whited G M, Gibson D T. Separation and partial characterization of the enzymes of the toluene-4-monooxygenase catabolic pathway in Pseudomonas mendocina KR1. J Bacteriol. 1991;173:3017–3020. doi: 10.1128/jb.173.9.3017-3020.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Worsey M J, Williams P A. Metabolism of toluene and xylenes by Pseudomonas putida (arvilla) mt-2: evidence for a new function of the TOL plasmid. J Bacteriol. 1975;124:7–13. doi: 10.1128/jb.124.1.7-13.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wright J D, Ratledge C. Isolation of two Rhodotorula rubra strains showing differences in the degradation of aromatic compounds. Appl Microbiol Biotechnol. 1991;35:94–99. [Google Scholar]
- 25.Yadav J S, Reddy C A. Degradation of benzene, toluene, ethylbenzene, and xylenes (BTEX) by the lignin-degrading basidiomycete Phanerochaete chrysosporium. Appl Environ Microbiol. 1993;59:756–762. doi: 10.1128/aem.59.3.756-762.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]