Abstract
Introduction:
Endothelial nitric oxide synthase (eNOS) genes have been implicated in renal hemodynamics as potent regulators of vascular tone and blood pressure. It has been linked to a reduction in plasma nitric oxide levels. Several studies have recently been conducted to investigate the role of NOS3 gene polymorphisms and end-stage renal disease (ESRD). However, the results are still unclear and the mechanisms are not fully defined. As a result, we conducted a meta-analysis to examine the relationship between NOS3 gene polymorphism and ESRD in autosomal polycystic kidney disease (ADPKD) patients.
Methods:
To assess the relationship between NOS3 gene polymorphism and ESRD, relevant studies published between September 2002 and December 2020 were retrieved from the PubMed (Medline), EMBASE, Google Scholar, and Web of Science databases. The pooled odds ratio (OR) and 95 % confidence interval (CI) were calculated using a fixed-effect model. To assess the heterogeneity of studies, we used Cochrane’s Q test and the Higgins and Thompson I2 statistics.
Results:
Our meta-analysis of 13 studies showed that the presence of the two NOS3 gene polymorphisms significantly increased ESRD risk in ADPKD patients with 4a/b gene polymorphism (aa+ab vs. bb: OR=1.95, 95% CI=1.24-3.09, p=0.004). In addition, no significant association was found between the NOS3 894G>T (Glu298Asp) polymorphism and the risk of ESRD in ADPKD patients (GT+TT vs. GG: OR=1.21, 95% CI=0.93-1.58, p=0.157). There was no evidence of publication bias.
Conclusions:
The findings of the current meta-analysis suggest that NOS3 intron 4a/b polymorphism plays a vital role in the increasing risk of ESRD in ADPKD patients.
Keywords: Polycystic Kidney, Autosomal Dominant, Polymorphism, Genetic, Kidney Failure, Chronic
Resumo
Introdução:
Genes da óxido nítrico sintase endotelial (eNOS) têm sido implicados na hemodinâmica renal como potentes reguladores do tônus vascular e pressão arterial. Tem sido vinculado a uma redução nos níveis plasmáticos de óxido nítrico. Realizou-se recentemente vários estudos para investigar o papel de polimorfismos do gene NOS3 e doença renal em estágio terminal (DRET). Entretanto, os resultados ainda não são claros e os mecanismos não estão totalmente definidos. Como resultado, realizamos meta-análise para examinar a relação entre polimorfismo do gene NOS3 e DRET em pacientes com doença renal policística autossômica dominante (DRPAD).
Métodos:
Para avaliar a relação entre polimorfismo do gene NOS3 e DRET, recuperou-se estudos relevantes publicados entre Setembro-2002 e Dezembro-2020 dos bancos de dados PubMed (Medline), EMBASE, Google Scholar, Web of Science. Calculamos odds ratio (OR) e intervalo de confiança (IC) de 95% utilizando modelo de efeitos fixos. Para avaliar a heterogeneidade dos estudos, utilizamos teste Q de Cochrane e estatísticas I2 de Higgins e Thompson.
Resultados:
Nossa meta-análise de 13 estudos mostrou que a presença dos dois polimorfismos do gene NOS3 aumentou significativamente o risco de DRET em pacientes com DRPAD com polimorfismo do gene 4a/b (aa+ab vs. bb: OR=1,95; IC 95%=1,24-3,09; p=0,004). Ademais, não encontramos associação significativa entre polimorfismo 894G>T NOS3 (Glu298Asp) e risco de DRET em pacientes com DRPAD (GT+TT vs. GG: OR=1,21; IC 95%=0,93-1,58; p=0,157). Não houve evidência de viés de publicação.
Conclusões:
Achados da meta-análise atual sugerem que o polimorfismo intron 4a/b do NOS3 desempenha papel vital no aumento do risco de DRET em pacientes com DRPAD.
Descritores: Rim Policístico Autossômico Dominante, Polimorfismo Genético, Falência Renal Crônica
Introduction
Renal cysts can have various etiologies, broadly classified into genetic and non-genetic disorders. The most common and widely accepted genetic cause of renal cystic disease in humans is autosomal dominant polycystic kidney disease (ADPKD), autosomal recessive polycystic kidney disease (ARPKD), and juvenile nephronophthisis, for which genes or chromosomal locations have been identified 1 . Among all renal cystic diseases, ADPKD is genetically heterogeneous and affects all racial groups worldwide, associated with liver, cardiovascular, gastrointestinal and genital abnormalities, with an estimated frequency between 1:400 and 1:1,000 2 . ADPKD may be caused by mutations in one of the two genes, namely polycystic kidney disease 1 (PKD1), mapped to 16p13.3, and polycystic kidney disease 2 (PKD2) gene on chromosome 4q21 3 . The frequency of mutations of PKD1gene is much higher and the gene is responsible for 85% of ADPKD cases, while 15% is caused by the PKD2 gene. Additionally, elderly patients have more cases of PKD2 mutations than of PKD1 mutations. Renal disease involves hypertension, urinary tract infections (UTI), hematuria, renal pain, and renal insufficiency, and end-stage renal disease (ESRD) occurs in approximately 50% of ADPKD patients in their late forties 4 .
ESRD is a multifactorial disease and has been shown to be more significant in many aspects of this genetic factor. Previous evidence has proven that impaired nitric oxide synthase 3 (NOS3) contributes to vascular endothelial dysfunction, and blood endothelial cells are suggested to play a vital role in the pathogenesis of ESRD 5 . NOS3 is a dimeric cellular signaling molecule with significant regulatory activities such as glomerular vasodilatation, which is essential for controlling the glomerular filtration rate (GFR). In this perspective, NOS3 in ADPKD was considered a possible candidate gene for ESRD 6 . The NOS3 gene was mapped to a chromosome of 7q36 consisting of 26 exons. Several polymorphic variants have been associated to modified NO synthesis, including promoter-786 T > C, 894 G > T, and intron 4 variable tandem repeats a / b (VNTR) polymorphisms. Among these, the 27-base pair (bp) (VNTR) intron-4 of NOS3 is known to alter eNOS expression and cause impaired NO synthesis 7 . The genotypes and haplotypes of NOS3 tagSNPs were not associated with the disease 6 .
Although many researchers examined the association between NOS3 gene polymorphisms and ESRD in ADPKD, the findings were inconsistent 7-12 . In view of the clinical heterogeneity of ADPKD, this study aimed to quantitatively summarize the association between NOS3 polymorphisms (894G>T intron 4 VNTR a / b polymorphism) and ESRD risk in ADPKD by conducting a comprehensive meta-analysis of all eligible case-control studies.
Materials and methods
Identification of eligibility studies
Articles published between September 2002 to December 2020 on the associations of the NOS3 gene polymorphism and ESRD were identified. All case-control studies considering the association in ADPKD patients published in English languages were selected and organized according to the PRISMA guidelines 13 .
A comprehensive search was conducted in electronic databases including PubMed (Medline), EMBASE, Google Scholar, and Web of Science with the combination of the following keywords and subheadings: “endothelial nitric oxide synthase”, “eNOS”, “NOS3”, “ESRD”, “intron 4 VNTR”, “meta-analysis”, “case-control”, “894G>T (Glu298Asp; rs1799983)”. The last search was carried out on 30 February 2021.
Inclusion and exclusion criteria
To conduct a more robust meta-analysis, two authors collected data from all relevant articles independently. Our selection criteria were: (1) case-control studies on the association between NOS3 polymorphisms and risk of ESRD in ADPKD, (2) available full-text articles, (3) written in English, and (4) original data and complete genotype allele count for both the case and control groups were available. Studies were excluded if they (1) had overlapping/duplicate data, (2) had no control group, (3) did not have clear genotype data, and (4) were case reports and review articles. All information about the selection of studies in ADPKD patients with or without ESRD was arranged.
Statistical analysis
The genotype data of case and control groups were recorded. The comparison group already included a selection, but NOS3 genotypes were not tested for Hardy-Weinberg equilibrium (HWE). The meta-analysis was carried out using the web tool MetaGenyo. For each study, the strength of association between ESRD risk in ADPKD and NOS3 gene polymorphisms (894G>T and intron 4 VNTR) was assessed by the summary odds ratio (OR) and the 95% confidence interval (CI) in the dominant model. To assess the between-study heterogeneity, we used Q and I2 statistics in all studies. To assess the robustness of findings, sensitivity analysis was performed using a “leave-one-out” meta-analysis. Egger’s test and Begg’s funnel plot were used to assess publication bias. All statistical analyses were done using the Comprehensive Meta-analysis software. A P-value of <0.05 was considered statistically significant.
Results
Characteristics of the studies
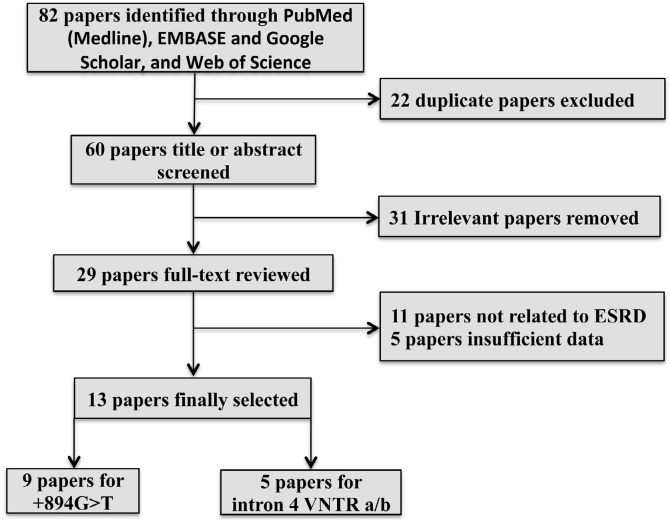
Using the aforementioned search strategy, 82 articles were identified. From these, 22 duplicate and irrelevant articles were excluded. After reading the abstracts and titles, 31 articles that did not assess the association between NOS3 894G>T and intron 4a/b polymorphisms and ESRD risk were excluded. Twenty-nine articles were fully reviewed, of which 9 papers with 520 ADPKD patients with ESRD and 563 ADPKD patients without ESRD for the NOS3 894G>T polymorphism 8,10-12,14-18 and 5 papers with 185 ADPKD patients with ESRD and 223 ADPKD patients without ESRD for NOS3 intron 4a/b polymorphism 7,9,11,19,20 that had sufficient data were included in the present meta-analysis. The process of selecting papers is depicted in Figure 1. The characteristics of all included studies are listed in Table 1.
Figure 1. Flow diagram depicting the detailed process of literature search.
TABLE 1. Characteristics of the studies on the 894G>T and intron 4 VNTR A/B polymorphisms included in the meta-analysis.
| 894G>T (Glu298Asp) Polymorphism | ||||||||
|---|---|---|---|---|---|---|---|---|
| Author | Ethnicity | Genotyping | ESRD | No-ESRD | ||||
| GG | GT | TT | GG | GT | TT | |||
| Lee et al. (2002) 14 | Asian | PCR-RFLP | 20 | 8 | 0 | 65 | 19 | 0 |
| Walker et al. (2003) 15 | Caucasian | PCR-RFLP | 39 | 50 | 7 | 47 | 56 | 16 |
| Reiterová et al. (2004) 16 | Caucasian | PCR-RFLP | 37 | 29 | 7 | 61 | 22 | 8 |
| Stefanakis et al. (2008) 8 | Caucasian | PCR-RFLP | 39 | 32 | 9 | 9 | 6 | 5 |
| Dasar et al. (2012) 10 | Caucasian | PCR-RFLP | 28 | 14 | 0 | 33 | 8 | 1 |
| Ramanathan et al. (2014) 6 | Caucasian | FRET | 34 | 13 | 1 | 39 | 14 | 1 |
| Pandita et al. (2017) 12 | Asian | ARMS-PCR | 27 | 14 | 1 | 63 | 17 | 1 |
| Kocyigit et al. (2018) 17 | Caucasian | PCR-RFLP | 43 | 30 | 5 | 12 | 12 | 6 |
| Malakoutian et al. (2020) 18 | Caucasian | PCR-RFLP | 18 | 10 | 5 | 24 | 16 | 2 |
| Intron 4 VNTR a/b | ||||||||
| bb | aa+ab | bb | aa+ab | |||||
| Reiterová et al. (2002) 20 | Caucasian | PCR-RFLP | 30 | 20 | 72 | 33 | ||
| Merta et al. (2002) 19 | Caucasian | PCR-Electrophoresis | 21 | 9 | 22 | 8 | ||
| Lamnissou et al. (2004) 9 | Caucasian | PCR- Electrophoresis | 10 | 11 | 21 | 7 | ||
| Ramanathan et al. (2014) 11 | Asian | PCR- Electrophoresis | 31 | 17 | 45 | 9 | ||
| Elumalai et al. (2014) 7 | Asian | PCR- Electro-phoresis | 25 | 12 | 16 | 0 | ||
Meta-analysis of NOS3 gene polymorphisms and esrd risk in ADPKD
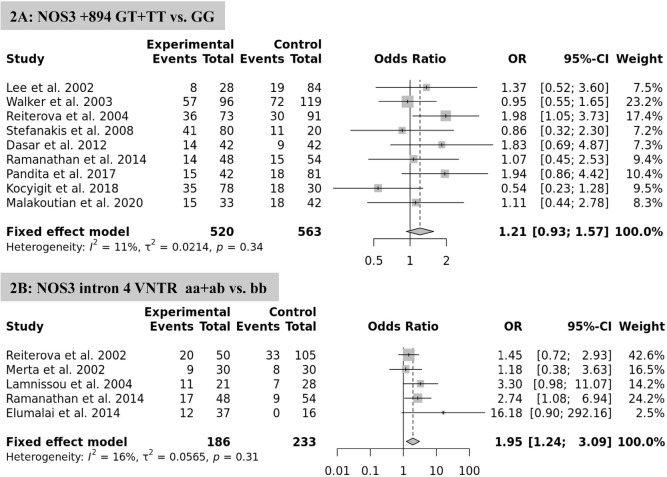
The association between NOS3 polymorphism variants and ESRD risk in ADPKD was assessed in a dominant model (Figure 2). Overall, the pooled analyses showed that the eNOS 4a/b polymorphism is significantly associated with increased risk of ESRD in the fixed-effect model (aa+ab vs. bb: OR=1.95, 95% CI=1.24-3.09, p=0.004) (Figure 2B). However, there was no significant association between NOS3 894G>T polymorphism and the risk of ESRD in ADPKD patients (GT+TT vs. GG: OR=1.21, 95% CI=0.93-1.58, p=0.157, fixed-effect model) (Figure 2A).
Figure 2. Forest plot of the meta-analysis for the association between NOS3 gene polymorphisms and risk of ESRD in ADPKD patients.
Test of heterogeneity, sensitivity, and publication BIAS
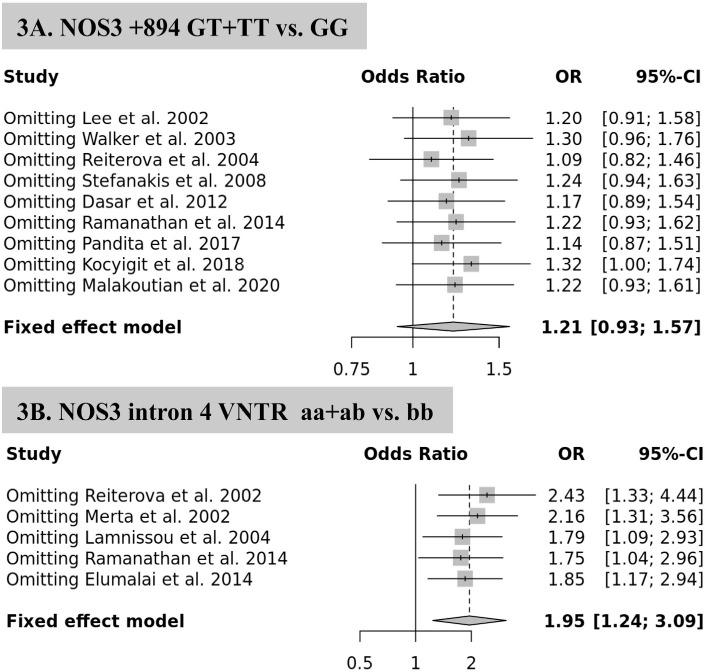
The estimated effect sizes for both NOS3 gene polymorphisms (894G>T: I2 = 11.3 %, p-heterogeneity =0.341; and intron 4a/b polymorphism: I2 = 15.6 %, p-heterogeneity =0.315) showed significant heterogeneity. Sensitivity analyses were performed by excluding studies one at a time and conducted the analysis after each omission. There were no statistically significant differences in polymorphism data, indicating that the analysis was statistically reliable and consistent (Figure 3A).
Figure 3. Sensitivity analysis of the association between NOS3 gene polymorphisms and risk of ESRD in ADPKD patients.
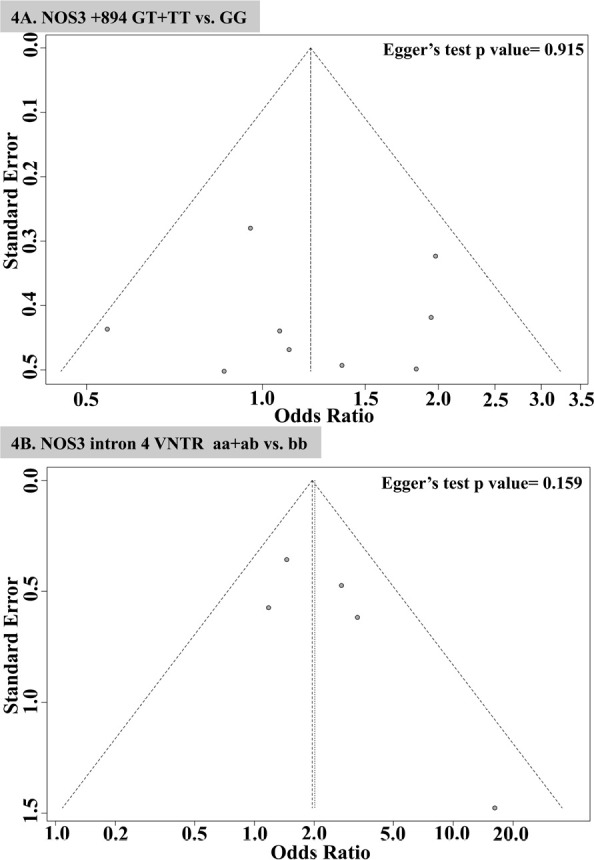
The analysis of the intron 4a/b polymorphism in two studies 19,20 demonstrated that the pooled ORs increased when each study was omitted (Figure 3B). Begg’s funnel plot and Egger’s test were used to assess the publication bias of the literature. The shape of the funnel plot was asymmetric for both the 894G>T and intron 4a/b polymorphisms. In support of this, the Egger test revealed no evidence of significant publication bias (Figure 4A and 4B). Furthermore, Egger’s linear regression test also revealed no publication bias for studies on the 894G>T (P = 0.915) and intron 4 a/b polymorphisms (P = 0.159).
Figure 4. Egger’s funnel plot of publication bias for the NOS3 gene polymorphisms and risk of ESRD in ADPKD patients.
Discussion
We included 13 published studies in this meta-analysis that revealed that NOS3 4a/b polymorphisms were significantly associated with various vascular complications, which are a cause of ESRD in ADPKD patients. Our study also showed that there is no heterogeneity or publication bias in the included studies. However, the results of the sensitivity analysis in each study group indicated that the pooled OR estimates were not changed quantitatively after each omission. Although the study suggests that nitric oxide may play a role in ADPKD pathophysiology, the NOS3 894G>T polymorphism failed to demonstrate an association with susceptibility to ESRD in ADPKD patients. The findings are consistent with a previous meta-analysis that found that the NOS3 intron 4a/b polymorphism increased the risk of ESRD in ADPKD patients 21 .
The NO synthases (NOS) are a family of enzymes that catalyze the production of nitric oxide (NO) from L-arginine in vascular endothelial cells 22 . It is well known that NO is highly reactive due to its short half-life and potent regulator of vascular tone and hemorheology via the activation of the cyclic guanosine monophosphate (cGMP)-dependent pathway 23 . Besides, it has directly involved in the vascular endothelium, complex cellular interactions, and global inflammation-mediated cell activation 24 . Generally, NO inhibits NaCl absorption along the nephron. Several studies have shown that NOS inhibitors such as nitro-L-arginine and NG-nitro-L-arginine methyl ester (L-NAME) have a tonic influence, especially on medullary circulation 25 . Despite this, renal NO synthesis is involved in the acute and chronic regulation of sodium balance.
Hypertension is the most frequent complication in ADPKD patients, occurring in approximately 60% of the patients. Miyamoto et al. discovered that the 894G>T (Glu298Asp) missense variant was significantly associated with essential hypertension, suggesting a genetic susceptibility for essential hypertension 26 . Another study found that eNOS expression and eGFR were significantly higher in ADPKD patients without hypertension than in those with hypertension. This demonstrates that eNOS gene expression is independently predictive of hypertension in the ADPKD population 27 .
In kidney disease, NO production is reduced by either a decrease in the enzyme substrate (L-arginine) or an increase in the bioavailability of the enzyme inhibitor asymmetric dimethylarginine (ADMA), which in turn reduces NO synthesis via a feedback mechanism 28 . This mechanism has been shown to accelerate the progression of pre-existing kidney disease. Various studies have shown that NO negatively regulates the renin-angiotensin system by inhibiting ACE activity and AT1 receptors 29 . The release of NO by endothelial cells plays a major role in regulating the local hemodynamics and systematic blood pressure 30 . Decreased production of NO plays a major role in the progression of renal disease 28 . A significant decrease in different isoforms of NOS in the cystic epithelium was observed during the growth of a renal cyst in Han: Sprague-Dawley (SPRD) polycystic rats 31 . Several lines of evidence suggest that ADPKD is characterized by endothelial dysfunction caused by impaired NO release 32,33 .
Animal models and clinical trials have demonstrated the importance of NOS in polycystic kidney disease 34 . ADPKD patients with the 4a allele progressed to ESRD more slowly in Belgium and France, whereas Hellen’s patients from Greece and Cyprus progressed faster 35,36 . However, some studies suggested that this locus was not linked with ESRD of different etiologies 36,37 . The substitution of aspartic acid for glutamate affects the domain of the oxidase enzyme that serves as a binding site for BH4 and the amino acid L-arginine. The change causes an enzyme variation, making it more susceptible to proteolytic cleavage in position D238-P239. Further, it produces a shorter form of the enzyme, resulting in less NO production 38 . However, the relationship between 894G>T polymorphism and the age of onset of ESRD in ADPKD patients also yielded inconsistent results 15,16,37 . While the-786T>C (rs2070744) polymorphism has a functional effect, it is linked to the replication protein A1 (RPA1), which binds to the NOS3 promoter with high affinity when the C allele is present, resulting in reduced NOS3 transcription 39 .
No significant findings were observed regarding the promoter-786 T>C polymorphism with ESRD progression in patients with type 1 ADPKD 35,40 . Our findings were inconclusive in terms of ethnicity-specific associations between NOS3 gene polymorphisms and ESRD in ADPKD patients. This is possibly due to differences in the allele frequencies of the NOS3 gene among populations. Individual studies investigating the link between NOS3 polymorphisms and ESRD complications have also been conducted. Still, these studies may have been imbalanced due to the inability of individual components to identify the desired impact of these polymorphisms.
Certain limitations and biases of the study have to be considered and resutls should be interpreted with caution. Our meta-analysis indicated that the NOS3 intron 4a/b polymorphism, but not the G894T polymorphism, seems to increase the risk of ESRD in ADPKD patients. More high-quality studies are needed to investigate the complexities of the associations between NOS3 gene polymorphisms and the therapeutic implications of ESRD in ADPKD patients.
References
- 1.Bergmann C. Genetics of autosomal recessive polycystic kidney disease and its differential diagnoses. Front Pediatr. 2018 Feb;5:221. doi: 10.3389/fped.2017.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergmann C, Guay-Woodford LM, Harris PC, Horie S, Peters DJM, Torres VE. Polycystic kidney disease. Nat Rev Dis Primers. 2018 Dec;4(1):50. doi: 10.1038/s41572-018-0047-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bitarafan F, Garshasbi M. Molecular genetic analysis of polycystic kidney disease 1 and polycystic kidney disease 2 mutations in pedigrees with autosomal dominant polycystic kidney disease. J Res Med Sci. 2019 May;24:44. doi: 10.4103/jrms.JRMS_835_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lanktree MB, Haghighi A, Di Bari I, Song X, Pei Y. Insights into autosomal dominant polycystic kidney disease from genetic studies. Clin J Am Soc Nephrol. 2021 May;16(5):790–9. doi: 10.2215/CJN.02320220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuricová K, Tanhäuserová V, Pácal L, Bartáková V, Brožová L, Jarkovský J, et al. NOS3 894G>T polymorphism is associated with progression of kidney disease and cardiovascular morbidity in type 2 diabetic patients: NOS3 as a modifier gene for diabetic nephropathy? Kidney Blood Press Res. 2013;38(1):92–8. doi: 10.1159/000355757. [DOI] [PubMed] [Google Scholar]
- 6.Ramanathan G, Elumalai R, Periyasamy S, Lakkakula B. Role of renin-angiotensin-aldosterone system gene polymorphisms and hypertension-induced end-stage renal disease in autosomal dominant polycystic kidney disease. Iran J Kidney Dis. 2014 Jul;8(4):265–77. [PubMed] [Google Scholar]
- 7.Elumalai R, Periasamy S, Ramanathan G, Lakkakula BVKS. Role of endothelial nitric oxide synthase VNTR (intron 4 a/b) polymorphism on the progression of renal disease in autosomal dominant polycystic kidney disease. J Renal Inj Prev. 2014;3(3):69–73. doi: 10.12861/jrip.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stefanakis N, Ziroyiannis P, Trygonis S, Lamnissou K. Modifier effect of the Glu298Asp polymorphism of endothelial nitric oxide synthase gene in autosomal-dominant polycystic kidney disease. Nephron Clin Pract. 2008;110(2):c101–6. doi: 10.1159/000157623. [DOI] [PubMed] [Google Scholar]
- 9.Lamnissou K, Zirogiannis P, Trygonis S, Demetriou K, Pierides A, Koptides M, et al. Evidence for association of endothelial cell nitric oxide synthase gene polymorphism with earlier progression to end-stage renal disease in a cohort of Hellens from Greece and Cyprus. Genet Test. 2004;8(3):319–24. doi: 10.1089/gte.2004.8.319. [DOI] [PubMed] [Google Scholar]
- 10.Dasar N, Ghaderian SMH, Azargashb E. Human evaluation of the Glu298Asp polymorphism in NOS3 gene and its relationship with onset age of ESRD in Iranian patients suffering from ADPKD. Int J Mol Cell Med. 2012;1(2):105–12. [PMC free article] [PubMed] [Google Scholar]
- 11.Ramanathan G, Periyasamy S, Lakkakula BV. NOS3 tagSNPs does not modify the chronic kidney disease progression in autosomal dominant polycystic kidney disease. Nephrology (Carlton) 2014 Sep;19(9):537–41. doi: 10.1111/nep.12278. [DOI] [PubMed] [Google Scholar]
- 12.Pandita S, Ramachandran V, Verma J, Kohli S, Saxena R, Verma IC. NOS3 gene Glu298Asp polymorphism and severity of disease in patients of ADPKD from North India. Meta Gene. 2017;11:75–80. [Google Scholar]
- 13.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009 Jul;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JG, Ahn C, Yoon SC, Park JH, No JJ, Moon CS, et al. The Glu/Asp polymorphism of ecNOS gene and the renal progression in Korean autosomal dominant polycystic kidney disease (ADPKD) patients. Korean J Genet. 2002 Sep;24(3):241–6. [Google Scholar]
- 15.Walker D, Consugar M, Slezak J, Rossetti S, Torres VE, Winearls CG, et al. The ENOS polymorphism is not associated with severity of renal disease in polycystic kidney disease 1. Am J Kidney Dis. 2003 Jan;41(1):90–4. doi: 10.1053/ajkd.2003.50027. [DOI] [PubMed] [Google Scholar]
- 16.Reiterová J, Miroslav M, Stekrová J, Kohoutová M, Tesar V, Kmentová D, et al. The influence of G-protein beta3-subunit gene and endothelial nitric oxide synthase gene in exon 7 polymorphisms on progression of autosomal dominant polycystic kidney disease. Ren Fail. 2004;26(2):119–25. doi: 10.1081/jdi-120038485. [DOI] [PubMed] [Google Scholar]
- 17.Kocyigit I, Eroglu E, Gungor O. Clinical problems in hemodialysis patients with autosomal dominant polycystic kidney disease. Semin Dial. 2018 May;31(3):268–77. doi: 10.1111/sdi.12696. [DOI] [PubMed] [Google Scholar]
- 18.Malakoutian T, Madadi B, Ebrahimi A. Evaluation of common polymorphisms of eNOS gene and ACE gene in autosomal dominant polycystic kidney disease patients and their association with HTN and renal failure. J Renal Inj Prev. 2021;10(1):e04. [Google Scholar]
- 19.Merta M, Reiterová J, Tesař V, Štekrová J, Viklický O. Influence of the endothelial nitric oxide synthase polymorphism on the progression of autosomal dominant polycystic kidney disease and IgA nephropathy. Ren Fail. 2002 Jul;24(4):467–75. doi: 10.1081/jdi-120006773. [DOI] [PubMed] [Google Scholar]
- 20.Reiterová J, Merta M, Tesař V, Štekrová J. Endothelial nitric oxide synthase affects the progression of autosomal dominant polycystic kidney disease. Kidney Blood Press Res. 2002;25(2):87–90. doi: 10.1159/000063513. [DOI] [PubMed] [Google Scholar]
- 21.Xue C, Zhou CC, Sun LJ, He LL, Xu CG, Dai B, et al. Effects of endothelial nitric oxide synthase gene on end stage renal disease progression in autosomal dominant polycystic kidney disease. Nephrology. 2014 Oct;19(10):630–7. doi: 10.1111/nep.12310. [DOI] [PubMed] [Google Scholar]
- 22.Förstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012 Apr;33(7):829–37d. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghanta M, Panchanathan E, Lakkakula BV. Cyclic guanosine monophosphate-dependent protein kinase i stimulators and activators are therapeutic alternatives for sickle cell disease. Turk J Haematol. 2018 Mar;35(1):77–8. doi: 10.4274/tjh.2017.0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verma H, Mishra H, Khodiar PK, Patra PK, Bhaskar LV. NOS3 27-bp and IL4 70-bp VNTR polymorphisms do not contribute to the risk of sickle cell crisis. Turk J Haematol. 2016 Dec;33(4):365–6. doi: 10.4274/tjh.2016.0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rath M, Müller I, Kropf P, Closs EI, Munder M. Metabolism via arginase or nitric oxide synthase: two competing arginine pathways in macrophages. Front Immunol. 2014 Oct;5:532. doi: 10.3389/fimmu.2014.00532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyamoto Y, Saito Y, Kajiyama N, Yoshimura M, Shimasaki Y, Nakayama M, et al. Endothelial nitric oxide synthase gene is positively associated with essential hypertension. Hypertension. 1998 Jul;32(1):3–8. doi: 10.1161/01.hyp.32.1.3. [DOI] [PubMed] [Google Scholar]
- 27.Kocyigit I, Taheri S, Sener EF, Unal A, Eroglu E, Öztürk F, et al. Endothelial nitric oxide synthase gene expression is associated with hypertension in autosomal dominant polycystic kidney disease. Cardiorenal Med. 2014 Dec;4(3-4):269–79. doi: 10.1159/000369105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amador-Martínez I, Pérez-Villalva R, Uribe N, Cortés-González C, Bobadilla NA, Barrera-Chimal J. Reduced endothelial nitric oxide synthase activation contributes to cardiovascular injury during chronic kidney disease progression. Am J Physiol Renal Physiol. 2019 Aug;317(2):F275–F85. doi: 10.1152/ajprenal.00020.2019. [DOI] [PubMed] [Google Scholar]
- 29.Neubauer B, Schrankl J, Steppan D, Neubauer K, Sequeira-Lopez ML, Pan L, et al. Angiotensin II short-loop feedback: is there a role of ang ii for the regulation of the renin system in vivo? Hypertension. 2018 Jun;71(6):1075–82. doi: 10.1161/HYPERTENSIONAHA.117.10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahmad A, Dempsey SK, Daneva Z, Azam M, Li N, Li PL, et al. Role of nitric oxide in the cardiovascular and renal systems. Int J Mol Sci. 2018 Sep;19(9):2605. doi: 10.3390/ijms19092605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kahveci AS, Barnatan TT, Kahveci A, Adrian AE, Arroyo J, Eirin A, et al. Oxidative stress and mitochondrial abnormalities contribute to decreased endothelial nitric oxide synthase expression and renal disease progression in early experimental polycystic kidney disease. Int J Mol Sci. 2020 Mar;21(6):1994. doi: 10.3390/ijms21061994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ratnam S, Nauli SM. Hypertension in autosomal dominant polycystic kidney disease: a clinical and basic science perspective. Int J Nephrol Urol. 2010;2(2):294–308. [PMC free article] [PubMed] [Google Scholar]
- 33.Klawitter J, Reed-Gitomer BY, McFann K, Pennington A, Klawitter J, Abebe KZ, et al. Endothelial dysfunction and oxidative stress in polycystic kidney disease. Am J Physiol Renal Physiol. 2014 Dec;307(11):F1198–F206. doi: 10.1152/ajprenal.00327.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wiwanitkit V. Nitric oxide synthase and polycystic kidney disease. J Nephropharmacol. 2015;4(2):85–7. [PMC free article] [PubMed] [Google Scholar]
- 35.Persu A, Stoenoiu MS, Messiaen T, Davila S, Robino C, El-Khattabi O, et al. Modifier effect of ENOS in autosomal dominant polycystic kidney disease. Hum Mol Genet. 2002 Feb;11(3):229–41. doi: 10.1093/hmg/11.3.229. [DOI] [PubMed] [Google Scholar]
- 36.Lamnissou K, Zirogiannis P, Trygonis S, Demetriou K, Pierides A, Koptides M, et al. Evidence for association of endothelial cell nitric oxide synthase gene polymorphism with earlier progression to end-stage renal disease in a cohort of Hellens from Greece and Cyprus. Genet Test. 2004;8(3):319–24. doi: 10.1089/gte.2004.8.319. [DOI] [PubMed] [Google Scholar]
- 37.Noiri E, Satoh H, Taguchi J, Brodsky SV, Nakao A, Ogawa Y, et al. Association of eNOS Glu298Asp polymorphism with end-stage renal disease. Hypertension. 2002 Oct;40(4):535–40. doi: 10.1161/01.hyp.0000033974.57407.82. [DOI] [PubMed] [Google Scholar]
- 38.Dias RG, Negrão CE, Krieger MH. Nitric oxide and the cardiovascular system: cell activation, vascular reactivity and genetic variant. Arq Bras Cardiol. 2011 Jan;96(1):68–75. [PubMed] [Google Scholar]
- 39.Miyamoto Y, Saito Y, Nakayama M, Shimasaki Y, Yoshimura T, Yoshimura M, et al. Replication protein A1 reduces transcription of the endothelial nitric oxide synthase gene containing a-786T→ C mutation associated with coronary spastic angina. Hum Mol Genet. 2000 Nov;9(18):2629–37. doi: 10.1093/hmg/9.18.2629. [DOI] [PubMed] [Google Scholar]
- 40.Tazón-Vega B, Vilardell M, Perez-Oller L, Ars E, Ruiz P, Devuyst O, et al. Study of candidate genes affecting the progression of renal disease in autosomal dominant polycystic kidney disease type 1. Nephrol Dial Transplant. 2007 Jun;22(6):1567–77. doi: 10.1093/ndt/gfm036. [DOI] [PubMed] [Google Scholar]