Abstract
The development of a non-malignant and sustainable treatment approach for eradicating mephitic organic dyes from freshwater resources is a daunting task. In a similar vein, the current work investigates the mitigation of methylene blue (MB) dye utilizing titanium dioxide nanoparticles (CS-TiO2 NPs) synthesized using cannabis sativa (bhang) leaf extract via a greener approach. The CS-TiO2 NPs are well characterized through XRD, FE-SEM, HR-TEM, UV-Vis spectroscopy, FTIR spectroscopy, and EDS spectroscopy. Microscopic studies confirm that the average particle size distribution of the individual particles was found to be in the range of 12.5 ± 1.5 nm, whereas the average size of the CS-TiO2 NPs aggregates is 24.5 ± 11.5 nm. Additionally, the synthesized CS-TiO2 NPs manifested remarkable photocatalytic degradation potential against methylene blue dye with a degradation efficiency of 98.2% and an apparent rate constant of 0.0398 min−1. As a result, this research offers a green/sustainable alternative for water purification.
Keywords: green synthesis, photocatalysis, cannabis sativa (bhang), methylene blue dye, TiO2 NPs
1. Introduction
Water is one of the most important components for all living beings on the planet to survive. In recent decades, there has been a constant rise in population, worldwide economic expansion, and reliance on certain businesses that pollute the groundwater. Due to the shortage of groundwater sources, eradication of water contaminants is among the most critical hurdles for the research world, since this process necessitates the creation of more sophisticated technologies or the improvement of presently utilized materials or procedures [1,2]. Pollutants include anything that tends to vary in chemical or physical qualities, as well as additives. Chemical, physical, radioactive, organic, or inorganic waste are examples of pollutants. Such contaminants pose a serious concern to aquatic and wildlife since they inflict severe harm to their neurological systems, leading to the development of cancerous disorders [2].
Dyes and related hydrocarbons, which are among the most major and hazardous contaminants in water, are connected to population growth owing to their industrial usage. Dyes are commonly associated with the textile, printing, and paper industries, but they are also applied to foodstuffs, healthcare, sports, and aesthetics [3]. Because dyes are organic compounds with high solubility in water, recovering colors from polluted water using traditional techniques is challenging. Given that the loss of water tainted with dyes surpasses 15%, the textile sector consumes a massive volume of water, maybe exceeding 800 thousand tons per year [4,5].
Considering the fact that dyes are extremely poisonous, there are several health risks associated with their consumption or use. Carcinogens exist in pigments. They are highly toxic to aquatic species because they restrict the accessibility of liquid to sunlight, lowering metabolic rates and oxygen saturation in the seawater [5]. Methylene blue (MB) is a cationic dye that is utilized in a wide range of chemical, biological, medicinal, and other applications. Diarrhea, discomfort, anemia, and elevated blood pressure are all common side effects of MB usage [6].
Coagulation, adsorption, biodegradation, membrane process, activated sludge treatment process (ASTP), and advanced oxidation process (AOP) are some of the methods that have been used to eliminate these harmful colors from industrial effluents. Each of these procedures has its own set of advantages and disadvantages, which are chosen based on the requirements [7,8,9]. For instance, most effluent treatment plants use the traditional ASTP because of its inexpensive cost; nevertheless, this technology is ineffective at eliminating hazardous organic dyes [10]. However, alternative physicochemical treatment procedures, such as coagulation and adsorption, need a considerable amount of chemicals and thus are not ecologically friendly [11]. Whereas membrane processes, such as membrane bioreactors, are effective, they are inefficient in terms of energy, and have a high operational cost [12].
The AOPs, for example, offer tremendous promise in the treatment of dye-based textile effluents because they can degrade soluble organic pollutants from wastewaters [13].
Heterogeneous photocatalytic degradation-based AOPs are attractive solutions for organic dye degradation because they are more successful than other AOPs owing to the inexpensive cost of semiconductor-based photocatalysts and high effectiveness in mineralizing a range of organic dyes [14]. Semiconducting photocatalysts based on nanoparticles (NPs) have a huge potential for AOP-based wastewater treatment. Nanomaterials, such as nanocrystalline transition-metal oxides, have a considerable standard of reactivity, a wide range of functionalization adaptability, a huge surface area, and other size-dependent features [15,16,17]. Numerous semiconducting NPs, such as ZnO, CuO, TiO2, NiO, SnO2, and others, have been used as photocatalysts for the removal of organic dyes [18,19,20,21,22,23]. Because of their stability, cheap cost, and optical absorption in the ultraviolet range, TiO2-based photocatalysts have been extensively used for the degradation eradication of organic dyes.
The chemical preparation of TiO2 NPs involves specific conditions; high temperature and expensive and toxic chemicals also limit TiO2 applications owing to serious eco-toxicological concerns. The green synthesis of TiO2 NPs using sustainable components such as plant extracts, microorganisms, and enzymes is now becoming increasingly prevalent due to its simplicity, cost effectiveness, greener, and minimal dangerous nature. Furthermore, the use of plant extracts to make semiconducting TiO2 NPs has piqued attention because it avoids the use of chemicals and other contaminants while also enhancing the environmental suitability of the remediation process. To date, few studies have explored the utilization of green TiO2 NPs for dye degradation [13,24,25]. Nonetheless, there is a continual impulse for an ecologically friendly TiO2 NP preparation. In this approach, organic components cap the prepared NPs to avoid agglomeration, which is known as steric stabilization.
In this regard, the current study uses cannabis sativa plant extract to synthesize TiO2 NPs in a green manner. Cannabis sativa, generally known as hemp, is a plant belonging to the cannabinaceae family that contains the pain-relieving ingredient THC (delta-9 tetrahydrocannabinol). Cannabis sativa contains medicinal benefits that have been used for hundreds of years in numerous cultures, including the treatment of pain, asthma, sleeplessness, depression, and lack of appetite [26]. In addition, the prepared CS-TiO2 NPs were used as a photocatalyst for the eradication of the methylene blue dye.
2. Materials and Methods
2.1. Materials
Parswanath Dye Stuff Industries in Ahmedabad, India, provided a methylene blue dye. All the chemicals and reagents used for the preparation of CS-TiO2 NPs were purchased from Merck India Ltd., Mumbai, India. The leaves came from a local university park.
2.2. Preparation of Extract
About 10 gm of cannabis sativa (bhang) leaves were thoroughly cleaned with water to eradicate any tainted substance before being air-dried. The air-dried leaves were then chopped into fine bits and heated for one hour in 100 mL distilled water. A light brown solution was attained using the filtration method (Whatman filters with 1.5 μm pore size) by separating from residual particulate matter.
2.3. Preparation of CS-TiO2 NPs
In total, 6 mL of 0.5 M Titanium isopropoxide [Ti{OCH(CH3)2 }4] suspension was pipetted to 40 mL of purified CS extract in a 1:1 (v/v) ratio with constant stirring at room temperature (RT) for obtaining CS-TiO2 NPs using the green technique. The reduction in metallic ions (Ti4+) in the solution caused a visible color change from translucent to whitish brown within 10 min of mixing reactant and leaves extract, revealing the synthesis of CS-TiO2 NPs. In total, 6 mL ammonia was drop-wisely introduced to the NP suspension under continuous stirring at room temperature to create the NPs in precipitate form. Filtration was used to isolate the resultant NP precipitates from the mixture. Then, the resulting solution was filtered twice or three times with filter paper before being rinsed with ethanol to confiscate ionic contaminants. After that, the precipitates were air-dried and calcined in a muffle furnace at 400 °C for 4 h to obtain the fine white powder of CS-TiO2 NPs, which was then crushed in a crystal mortar pestle.
2.4. Characterization Techniques
The crystalline nature and lattice parameters of prepared CS-TiO2 NPs were determined using PANalytical’s X’Perto Powder X-ray Diffraction (PXRD). The geometry and size of the particle were demonstrated through Hitachi HF-3300 Transmission Electron Microscope (Hitachi, Tokyo, Japan). The optical absorption spectra of the samples were recorded using a Shimadzu UV-2600 (Shimadzu, Kyoto, Japan) research-grade spectrophotometer. The carbonaceous vibrational characteristics of CS-TiO2 were determined using a Bruker Alpha Fourier Transformed Infrared (FTIR) spectrophotometer (Bruker, Billerica, MA, USA). The elemental composition of the prepared CS-TiO2 NPs was determined using Energy Dispersive Spectroscopy (EDS) analysis supplied by Oxford Instruments.
2.5. Photocatalytic Activity
In the case of a normal dye decomposition, 5 mM of methylene blue dye was prepared in 100 mL of deionized water and 1 mg of prepared CS-TiO2 NPs added to this solution. For maximum dye adsorption, the mixture was maintained in the darkness for 16 h. The solution was then subjected to UV light (UV-B, λ = 280–315 nm). After every 10 min of UV irradiation, 5 mL of the mixture was withdrawn and centrifuged to recover the photocatalyst. A UV-Vis spectrophotometer was used to check the dye content in the supernatant containing transparent dye solution. The intensity of the dye gradually decreased with irradiation time, implying that the chemical arrangement of the dye was breaking up. The photocatalytic measurements were carried out at room temperature (20 °C).
3. Results and Discussion
3.1. Optical Study
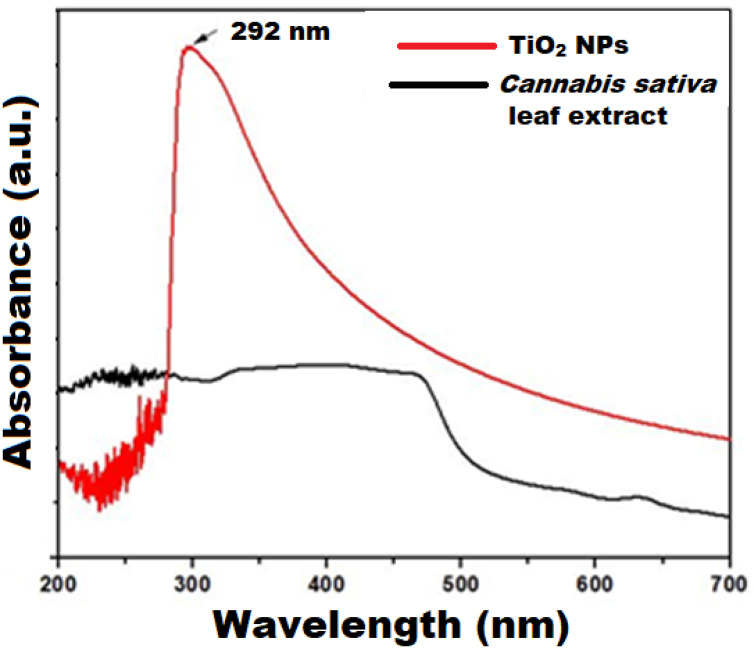
The particle size and shape are the determining factors for the optical absorption properties of NPs. To explain this behavior, UV-Vis spectra have been recorded for the cannabis sativa leaf extract and as-synthesized CS-TiO2 NPs as shown in Figure 1. For cannabis sativa leaf extract, the absorption is weak and no peaks are detected. For the as-synthesized CS-TiO2 NPs, a significant absorption peak was detected at 292 nm, providing base confirmation for the greener approach to synthesizing CS-TiO2 NPs. Nevertheless, the absorption band of the obtained biogenic CS-TiO2 NPs (292 nm) was significantly bluer than that of bulk TiO2 (380 nm), indicating the quantum confinement characteristic of NPs. Moreover, the maximum absorption band around 300 nm corresponds to the anatase phase of TiO2 NPs [27]. As the particle size is lowered, the absorption edge shifts to a lower wavelength, which can be described through the quantum confinement process. The optical band gap was obtained using the maximum absorption wavelength of CS-TiO2 NPs to be 4.24 eV, which is greater than the bulk titanium (3.2 eV).
Figure 1.
UV-vis spectra of cannabis sativa leaf extract and synthesized CS-TiO2 NPs.
3.2. FTIR Spectrum
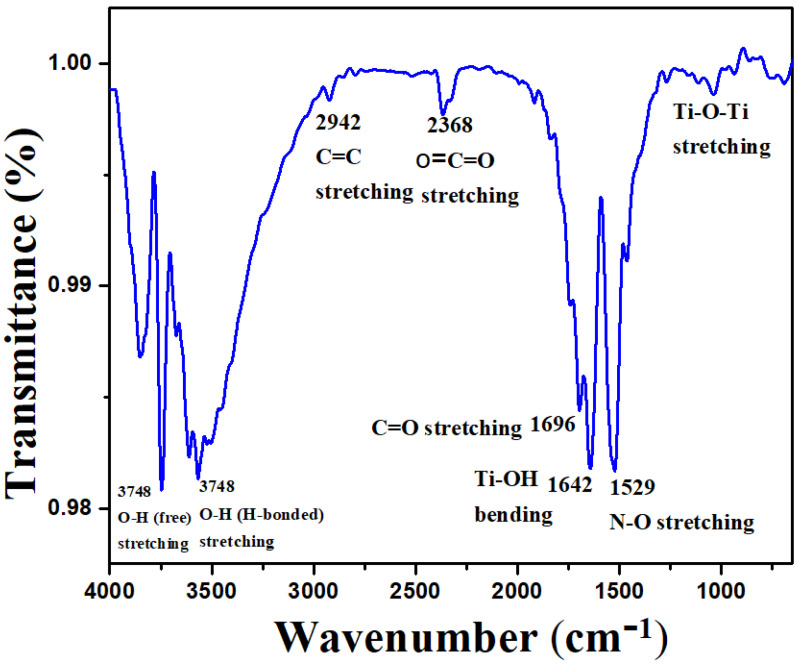
As shown in Figure 2, the FTIR spectrum of CS-TiO2 NPs derived from cannabis sativa leaf extract was investigated in the range of 4000–500 cm−1. As a result of inter-atomic vibrations, metal oxides generally have bands in the fingerprint region below 1000 cm−1 due to Ti–O and Ti–O–Ti bending vibrations [28]. The distinct band at 685 cm−1 is most usually related to the anatase phase and is attributed to the Ti–O vibration. The characteristics band of CS-TiO2 NPs at 1642 cm−1 is attributed to the stretching vibration of Ti–OH [29]. The band at 1696 cm−1 is ascribed to the C=O stretching vibration. The bending vibrations of N–O stretching are depicted by the sharp band at 1529 cm−1, whereas the bands at 2368 cm−1 and 2942 cm−1 are ascribed to O=C=O and C=C vibrations. The bands within the 4000–3500 cm−1 region inferred the H-bonded stretching and the free stretching of O-H vibrations [28,30]. According to established literature, cannabis sativa extract has a larger quantity of phenolics and flavonoids, which are essential for NP reduction, capping, and stability. It is noticeable from the FTIR spectra of TiO2 NPs that phytochemicals (phenols, flavonoids, cannabinoids, polysaccharides, water-soluble biomolecules, etc.) present in cannabis sativa extract are vital for bioreduction. As a result, proteins and other biomolecules containing -OH moiety attach to the NP’s surface and inhibit agglomeration [31,32,33].
Figure 2.
FTIR spectra of synthesized CS-TiO2 NPs by cannabis sativa leaf extract.
3.3. XRD Analysis
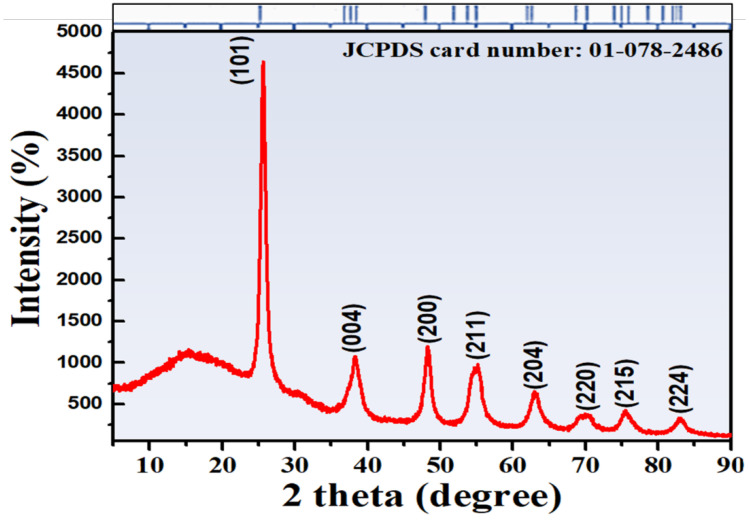
Figure 3 shows the XRD spectrum of CS-TiO2 NPs. The established XRD peaks observed at 2Ɵ values of 25.3°, 38.2°, 48.3°, 54.8°, 62.8°, 70.0°, 75.3°, and 82.1° revealed the tetragonal structure of anatase CS-TiO2 NPs with corresponding (101), (112), (200), (105), (204), (220), (215), and (224) planes, respectively. The measured peaks resemble the standard tetragonal structure of anatase TiO2 (JCPDS Card No. 01-078-2486). As shown in Figure 3, the JCPDS Card No. 01-078-2486 fits all peaks, indicating that no contaminants or mismatched peaks have been found.
Figure 3.
XRD spectrum for crystal structure investigation provided with JCPDS 01-078-2486 card.
The average crystallite size can be determined from the XRD pattern using the Debye–Scherrer formula as given in Equation (1):
| D = Kƛ/βcosθ = 0.89ƛ/βcosθ | (1) |
where D is the mean crystallite size, K is Scherrer’s constant (0.94), ƛ is the wavelength, β is the full-width half-maximum, and θ is Bragg’s angle. The average crystallite size was calculated to be 8 nm using Equation (1).
3.4. FE-SEM Analysis
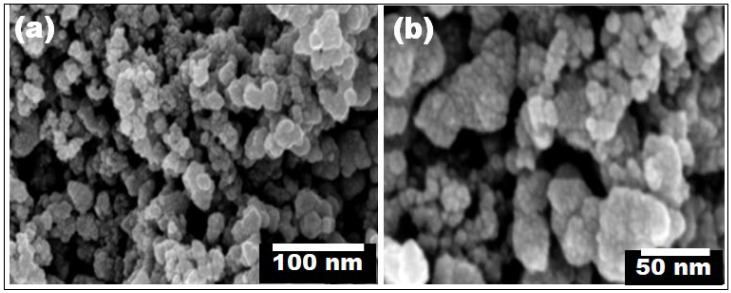
The information about the morphological characteristics of the obtained particles was provided by the FE-SEM micrographs. Figure 4 shows FE-SEM images taken at two different magnifications for the precipitated CS-TiO2 NPs after calcination at 400 °C for 4 h without ageing. It was confirmed that the particles were almost spherical in shape. As demonstrated in Figure 4, a considerable number of small and distinct CS-TiO2 NPs were found, compared to a comparatively limited number of large-sized agglomerated NPs. The average particle size of the individual CS-TiO2 NPs is 14 ± 3 nm.
Figure 4.
(a,b) SEM images of the green synthesized CS-TiO2 NPs at two different magnifications.
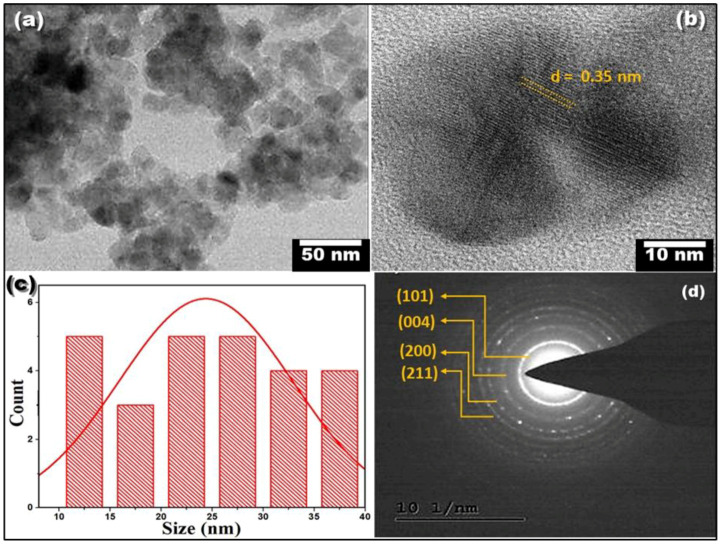
3.5. HR-TEM Analysis
Individual CS-TiO2 particle calcination at 400 °C for 4 h is shown in the HR-TEM images, Figure 5a,b at two different magnifications. CS-TiO2 NPs have a consistent spherical form. Figure 5b shows a HR-TEM micrograph of anatase TiO2 NPs at a greater magnification. The anatase form of biogenic TiO2 (101) NPs with a fringe width of 0.35 nm was verified. i.e., the d-spacing value was found to be 0.35 nm. Figure 5c shows the histogram of the particle size distribution. The average particle size distribution of the individual particles was found to be in the range of 12.5 ± 1.5 nm, whereas the average size of the aggregates is 24.5 ± 11.5 nm. The crystalline structure of biogenic CS-TiO2 NPs was further verified by a SAED pattern, Figure 5d, with bright spherical rings corresponding to planes (101), (004), (200), (105), (211), (204), (220), and (215), respectively, of the anatase crystal [34,35]. The lattice fringes clearly show that the particles are crystalline with an anatase phase, which is supported by the XRD and SAED results. Hence, the brighter spot and strong rings in the SAED pattern indicate that the green method created highly crystalline titanium dioxide NPs.
Figure 5.
Spectroscopic studies: (a,b) HR-TEM images, (c) particle size distribution histogram, and (d) SAED pattern of CS-TiO2 NPs.
3.6. Photocatalytic Activity Test of CS-TiO2
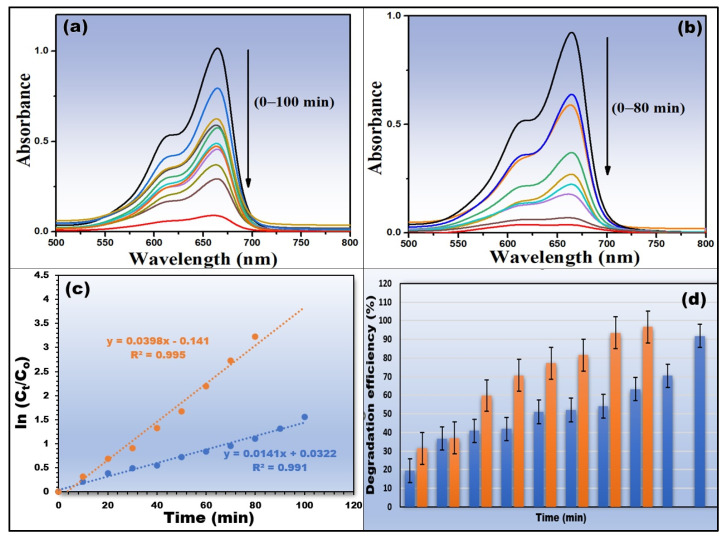
Due to its resistive nature, methylene blue dye is said to be a common hazard in the wastewater produced by textile factories, among other things. The photocatalytic capabilities of obtained CS-TiO2 NPs were tested via observing the decay of MB dye in direct sunlight. To obtain the MB dye solution, 5 mg of MB was dissolved in 100 mL DI water. The prepared CS-TiO2 was immersed in MB dye solution with a pH 9. To ensure dye adsorption/desorption equilibrium, the solution was agitated for a few hours. After exposing the system to sunlight for 20 min, a 2 mL aliquot was taken for photocatalytic analysis at the MB dye’s optimum absorbance peak of 664 nm. Figure 6a,b display the plots of photodegradation of MB dye using 5 mg and 10 mg dosages of green synthesized CS-TiO2 NPs for varying exposure times. Solar irradiation causes the chemical structure of MB dye to break down, and the MB degradation percentage reached 92.2% within a reaction period of 100 min using a catalyst dose of 5 mg (Figure 6a), whereas the observed removal percentage of MB dye is 98.2 percent within 80 min using a dose of 10 mg (Figure 6b).
Figure 6.
Photocatalysis study: (a,b) UV–Vis spectra of photocatalytic degradation of MB dye using 5 mg and 10 mg of green synthesized CS-TiO2 NPs, (c) pseudo-first-order kinetic study, and (d) histogram of degradation efficiency.
Figure 6c shows the plots of the pseudo-first-order degradation kinetics at 5 mg and 10 mg catalyst doses [36,37,38,39]. This figure reveals linear relationships with correlation coefficients > 0.99 as shown in Table 1, showing that the photocatalytic reaction has pseudo-first-order degradation kinetics. The apparent rate constants (Kapp (min−1)) for the photocatalytic activity conducted at dose values of 5 mg and 10 mg, as derived from the kinetic plots, Figure 6c, are 0.0141 min−1 and 0.0398 min−1, respectively. Figure 6d illustrates a histogram of degradation efficiency versus the exposure time, which shows an increase in the degradation efficiency when the exposure time is increased. This figure provided error bars of triplicate measurements.
Table 1.
Photo-degradation efficiency of CS-TiO2 NPs with rate constant.
| Catalyst Dose | Degradation Efficiency (%) | Time of Degradation (min) | Apparent Rate Constant (k) | R2 |
|---|---|---|---|---|
| 5 mg | 92.2 | 100 | 0.0141 min−1 | 0.991 |
| 10 mg | 98.2 | 80 | 0.0398 min−1 | 0.995 |
Table 2 shows the performance of our designed photocatalyst relative to the previously reported TiO2-based photocatalysts [35,36,37,38,39,40,41,42,43,44,45,46]. Our design showed higher photocatalytic degradation% within a short time relative to the reported data in references [36,41,43,44,46]. Additionally, using a lower catalyst dose than that reported in references [35,37,38,39,40,42,45], showed high catalytic efficiency.
Table 2.
The photocatalytic performance of CS-TiO2 NPs relative to previously reported TiO2-based photocatalysts [35,36,37,38,39,40,41,42,43,44,45,46].
| Synthesis Method | Catalyst and Dye Concentrations | Radiations | Photocatalytic Degradation | Ref. |
|---|---|---|---|---|
| Green | 10 mg/50 mL (10 mg/L, alizarin red dye) | Sunlight | 74%/180 min | [40] |
| Green | (5 mg/100 mL, corallene red dye) | Sunlight | 93%/140 min | [41] |
| Hydrothermal | 10 mg/L (1 mg/L, methylene orange) | Visible | 55%/180 min | [42] |
| Chemical | 10 mg/200 mL (methylene blue) | UV | 64%/75 min | [43] |
| Chemical | (methylene blue) | Visible | 65%/120 min | [44] |
| Bio-mediated | 100 mg (100 mL, methylene blue) | UV–Visible irradiation | 92%/120 min | [28] |
| Green | 10 mg (10 ppm, methylene blue) | UV | 96%/120 min | [45] |
| CS-TiO2 NPs | 10 mg/L (5 mM, methylene blue) | UV irradiation | 98.2%/80 min | This work |
3.7. Mechanism of Dye Degradation
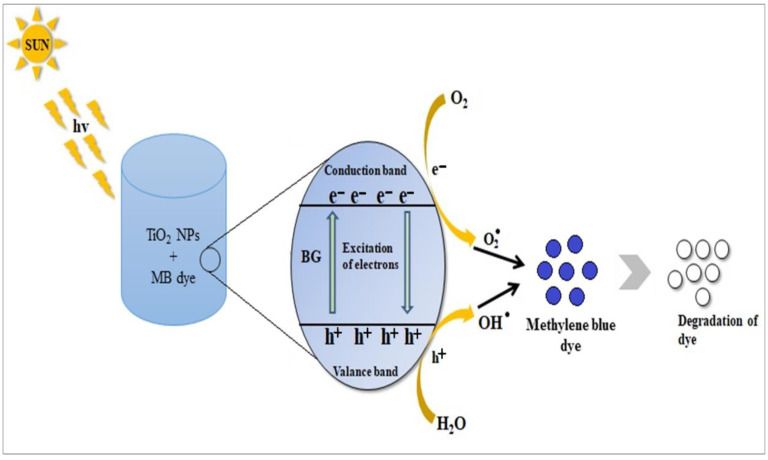
Light-dependent free radical production on the surface of CS-TiO2 NPs results in their photocatalytic activity. The photo-oxidation process begins with the light-dependent activation of CS-TiO2 NPs, which produces superoxide (O2−) ions and hydroxyl (OH) radicals, and these reactive chemicals interact with analytes to catalyze deterioration. In short, electron-hole pairs are formed when sunlight strikes the surface of CS-TiO2 NPs. Superoxide radical (O2−) anion is formed when photogenerated electrons in the conduction band react with oxygen molecules (O2). The positive holes attack the water to form hydroxyl radicals OH in the same stride. These potent oxidation reactions, O2− and OH radicals, are accountable for the photo-oxidation of dye [46]. Figure 7 depicts one probable dye degradation mechanism.
Figure 7.
The method of photocatalytic degradation of MB under continuous solar irradiation is depicted in this diagram.
Cannabis sativa (bhang)-mediated CS-TiO2 absorbs an appropriate frequency of irradiation. The electron is stimulated to the conduction band and a positive charge (hole) is produced in the lower band of CS-TiO2 [39]:
| CS-TiO2 + hv (photon)→e− (C.B.) + h+ (V.B.) | (2) |
The participation of these created charged carriers on the surface caused several reactions:
Oxidation: h+(V.B.) + H2O→H+ + OH.
Reduction: e− (C.B.) + O2 (adsorbed)→O2−.
Powerful oxidizing substances destroyed the MB dye: O2− or OH + MB)→intermediates (peroxylated or hydroxylated)→H2O + CO2 + innocuous products.
4. Conclusions
An effective green approach for the manufacturing of stable anatase CS-TiO2 NPs was established using cannabis sativa extract as a reducing/stabilizing agent. The XRD peaks of synthesized CS-TiO2 are a good match for the anatase phase, which has an average crystallite size of 8 nm. The average particle size distribution of the individual particles was found to be in the range of 12.5 ± 1.5 nm, whereas the average size of the CS-TiO2 NP aggregates is 24.5 ± 11.5 nm. The polycrystalline nature of the synthesized green CS-TiO2 is confirmed by HR-TEM. In the UV-Vis spectra, CS-TiO2 has a plasmon peak around 292 nm with an Eg of 4.2 eV. The Ti-O bond stretching at 685 cm−1, as well as a variety of functional moieties, were observed using FTIR. Under solar light, nanoparticle catalyzed photolysis of MB dye demonstrated improved degradation efficiency and apparent rate constant when the catalyst dose was increased. Furthermore, the produced CS-TiO2 NPs showed outstanding photocatalytic degradation capability against MB dye under sunlight, with a degradation efficiency of 98.2% and an apparent rate constant of 0.0398 min−1. As a result, this work adds to the advancement of green photocatalysts.
Acknowledgments
The authors gratefully acknowledge Chandigarh University, Mohali, Sri Guru Granth Sahib World University, Punjab (India), for providing necessary resources.
Author Contributions
Conceptualization, M.R. and J.S.; formal analysis, J.S., M.R. and M.S.; investigation, J.S. and M.S.; data curation, V.S., M.B., J.S. and M.S.; writing—original draft preparation, V.S., M.B. and M.S.; writing—review and editing, J.S., M.B. and M.S.; visualization, V.S., M.B. and J.S.; supervision, M.R. and J.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Raizada P., Sudhaik A., Singh P. Photocatalytic water decontamination using graphene and ZnO coupled photocatalysts: A review. Mater. Sci. Energy Technol. 2019;2:509–525. doi: 10.1016/j.mset.2019.04.007. [DOI] [Google Scholar]
- 2.Schwarzenbach R.P., Egli T., Hofstetter T.B., Von Gunten U., Wehrli B. Global water pollution and human health. Annu. Rev. Environ. Resour. 2010;35:109–136. doi: 10.1146/annurev-environ-100809-125342. [DOI] [Google Scholar]
- 3.Imran M., Crowley D.E., Khalid A., Hussain S., Mumtaz M.W., Arshad M. Microbial biotechnology for decolorization of textile wastewaters. Rev. Environ. Sci. Biotechnol. 2015;14:73–92. doi: 10.1007/s11157-014-9344-4. [DOI] [Google Scholar]
- 4.Khan S., Malik A. Toxicity evaluation of textile effluents and role of native soil bacterium in biodegradation of a textile dye. Environ. Sci. Pollut. Res. 2018;25:4446–4458. doi: 10.1007/s11356-017-0783-7. [DOI] [PubMed] [Google Scholar]
- 5.Hassan M.M., Carr C.M. A critical review on recent advancements of the removal of reactive dyes from dyehouse effluent by ion-exchange adsorbents. Chemosphere. 2018;209:201–219. doi: 10.1016/j.chemosphere.2018.06.043. [DOI] [PubMed] [Google Scholar]
- 6.Pathania D., Sharma S., Singh P. Removal of methylene blue by adsorption onto activated carbon developed from Ficus carica bast. Arab. J. Chem. 2017;10:S1445–S1451. doi: 10.1016/j.arabjc.2013.04.021. [DOI] [Google Scholar]
- 7.Pajootan E., Arami M., Mahmoodi N.M. Binary system dye removal by electrocoagulation from synthetic and real colored wastewaters. J. Taiwan Inst. Chem. Eng. 2012;43:282–290. doi: 10.1016/j.jtice.2011.10.014. [DOI] [Google Scholar]
- 8.Nassar M.Y., Moustafa M.M., Taha M.M. Hydrothermal tuning of the morphology and particle size of hydrozincite nanoparticles using different counterions to produce nanosized ZnO as an efficient adsorbent for textile dye removal. RSC Adv. 2016;6:42180–42195. doi: 10.1039/C6RA04855B. [DOI] [Google Scholar]
- 9.Nimkar U. Sustainable chemistry: A solution to the textile industry in a developing world. Curr. Opin. Green Sustain. Chem. 2018;9:13–17. doi: 10.1016/j.cogsc.2017.11.002. [DOI] [Google Scholar]
- 10.Villar-Navarro E., Baena-Nogueras R.M., Paniw M., Perales J.A., Lara-Martín P.A. Removal of pharmaceuticals in urban wastewater: High rate algae pond (HRAP) based technologies as an alternative to activated sludge based processes. Water Res. 2018;139:19–29. doi: 10.1016/j.watres.2018.03.072. [DOI] [PubMed] [Google Scholar]
- 11.Dotto J., Fagundes-Klen M.R., Veit M.T., Palácio S.M., Bergamasco R. Performance of different coagulants in the coagulation/flocculation process of textile wastewater. J. Clean. Prod. 2019;208:656–665. doi: 10.1016/j.jclepro.2018.10.112. [DOI] [Google Scholar]
- 12.Leaper S., Abdel-Karim A., Gad-Allah T.A., Gorgojo P. Air-gap membrane distillation as a one-step process for textile wastewater treatment. Chem. Eng. J. 2019;360:1330–1340. doi: 10.1016/j.cej.2018.10.209. [DOI] [Google Scholar]
- 13.Verma V., Al-dossari M., Singh J., Rawat M., Kordy M.G.M., Shaban M. A Review on Green Synthesis of TiO2 NPs: Photocatalysis and Antimicrobial Applications. Polymers. 2022;14:1444. doi: 10.3390/polym14071444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rauf M.A., Ashraf S.S. Fundamental principles and application of heterogeneous photocatalytic degradation of dyes in solution. Chem. Eng. J. 2009;151:10–18. doi: 10.1016/j.cej.2009.02.026. [DOI] [Google Scholar]
- 15.Kar P., Shukla K., Jain P., Sathiyan G., Gupta R.K. Semiconductor based photocatalysts for detoxification of emerging pharmaceutical pollutants from aquatic systems: A critical review. Nano Mater. Sci. 2021;3:25–46. doi: 10.1016/j.nanoms.2020.11.001. [DOI] [Google Scholar]
- 16.Stoilova O., Manolova N., Rashkov I. Electrospun poly(Methyl methacrylate)/TiO2 composites for photocatalytic water treatment. Polymers. 2021;13:3923. doi: 10.3390/polym13223923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blanco M., Monteserín C., Uranga N., Gómez E., Aranzabe E., García J.I. Thermal and photocatalytic performance of unsaturated polyester resins modified with TiO2 nanoparticles as panel bodies for vehicles. Polymers. 2021;13:2036. doi: 10.3390/polym13132036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh J., Kaur H., Kukkar D., Mukamia V.K., Kumar S., Rawat M. Green synthesis of SnO2 NPs for solar light induced photocatalytic applications. Mater. Res. Express. 2019;6:115007. doi: 10.1088/2053-1591/ab4412. [DOI] [Google Scholar]
- 19.Kumar M., Mehta A., Mishra A., Singh J., Rawat M., Basu S. Biosynthesis of tin oxide nanoparticles using Psidium Guajava leave extract for photocatalytic dye degradation under sunlight. Mater. Lett. 2018;215:121–124. doi: 10.1016/j.matlet.2017.12.074. [DOI] [Google Scholar]
- 20.Subash B., Krishnakumar B., Swaminathan M., Shanthi M. Highly efficient, solar active, and reusable photocatalyst: Zr-loaded Ag-ZnO for reactive red 120 dye degradation with synergistic effect and dye-sensitized mechanism. Langmuir. 2013;29:939–949. doi: 10.1021/la303842c. [DOI] [PubMed] [Google Scholar]
- 21.Hadia N.M.A., Abdelazeez A.A.A., Alzaid M., Shaban M., Mohamed S.H., Hoex B., Hajjiah A., Rabia M. Converting Sewage Water into H2 Fuel Gas Using Cu/CuO Nanoporous Photocatalytic Electrodes. Materials. 2022;15:1489. doi: 10.3390/ma15041489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Serga V., Burve R., Krumina A., Romanova M., Kotomin E.A., Popov A.I. Extraction–pyrolytic method for TiO2 polymorphs production. Crystals. 2021;11:431. doi: 10.3390/cryst11040431. [DOI] [Google Scholar]
- 23.Welter E.S., Kött S., Brandenburg F., Krömer J., Goepel M., Schmid A., Gläser R., Bahnemann W., Kowalska E., Janus M., et al. Figures of Merit for Photocatalysis: Comparison of NiO/La-NaTaO3 and Synechocystis sp. PCC 6803 as a Semiconductor and a Bio-Photocatalyst for Water Splitting. Catalysts. 2021;11:1415. doi: 10.3390/catal11111415. [DOI] [Google Scholar]
- 24.Amjad R., Mubeen B., Shahbaz Ali S., Sarim Imam S., Alshehri S., Ghoneim M.M., Alzarea S.I., Rasool R., Ullah I., Shahid Nadeem M., et al. Green Synthesis and Characterization of Copper Nanoparticles Using Fortunella margarita Leaves. Polymers. 2021;13:4364. doi: 10.3390/polym13244364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ganesh Saratale R., Dattatraya Saratale G., Ahn S., Shin H.-S. Grape Pomace Extracted Tannin for Green Synthesis of Silver Nanoparticles: Assessment of Their Antidiabetic, Antioxidant Potential and Antimicrobial Activity. Polymers. 2021;13:4355. doi: 10.3390/polym13244355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hunagund S.M., Desai V.R., Kadadevarmath J.S., Barretto D.A., Vootla S., Sidarai A.H. Biogenic and chemogenic synthesis of TiO2 NPs: Via hydrothermal route and their antibacterial activities. RSC Adv. 2016;6:97438–97444. doi: 10.1039/C6RA22163G. [DOI] [Google Scholar]
- 27.Hong S.M., Lee S., Jung H.J., Yu Y., Shin J.H., Kwon K.Y., Choi M.Y. Simple preparation of anatase Tio2 nanoparticles via pulsed laser ablation in liquid. Bull. Korean Chem. Soc. 2013;34:279–282. doi: 10.5012/bkcs.2013.34.1.279. [DOI] [Google Scholar]
- 28.Aravind M., Amalanathan M., Mary M.S.M. Synthesis of TiO2 nanoparticles by chemical and green synthesis methods and their multifaceted properties. SN Appl. Sci. 2021;3:409. doi: 10.1007/s42452-021-04281-5. [DOI] [Google Scholar]
- 29.Tsebriienko T., Popov A.I. Effect of poly(Titanium oxide) on the viscoelastic and thermophysical properties of interpenetrating polymer networks. Crystals. 2021;11:794. doi: 10.3390/cryst11070794. [DOI] [Google Scholar]
- 30.Murashkevich A.N., Lavitskaya A.S., Barannikova T.I., Zharskii I.M. Infrared absorption spectra and structure of TiO2-SiO2 composites. J. Appl. Spectrosc. 2008;75:730–734. doi: 10.1007/s10812-008-9097-3. [DOI] [Google Scholar]
- 31.Nasrullah M., Gul F.Z., Hanif S., Mannan A., Naz S., Ali J.S., Zia M. Green and Chemical Syntheses of CdO NPs: A Comparative Study for Yield Attributes, Biological Characteristics, and Toxicity Concerns. ACS Omega. 2020;5:5739–5747. doi: 10.1021/acsomega.9b03769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Csakvari A.C., Moisa C., Radu D.G., Olariu L.M., Lupitu A.I., Panda A.O., Pop G., Chambre D., Socoliuc V., Copolovici L., et al. Green synthesis, characterization, and antibacterial properties of silver nanoparticles obtained by using diverse varieties of cannabis sativa leaf extracts. Molecules. 2021;26:4041. doi: 10.3390/molecules26134041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shahzadi T., Rehman S., Riaz T., Zaib M. Eco-friendly synthesis of ZnO nanoparticles using Cannabis sativa and assessment of its activities as efficient dyes removal and antioxidant agent. Int. J. Environ. Anal. Chem. 2020:1–19. doi: 10.1080/03067319.2020.1789610. [DOI] [Google Scholar]
- 34.Bekele E.T., Gonfa B.A., Zelekew O.A., Belay H.H., Sabir F.K. Synthesis of Titanium Oxide Nanoparticles Using Root Extract of Kniphofia foliosa as a Template, Characterization, and Its Application on Drug Resistance Bacteria. J. Nanomater. 2020;2020:2817037. doi: 10.1155/2020/2817037. [DOI] [Google Scholar]
- 35.Belver C., Bedia J., Gómez-Avilés A., Peñas-Garzón M., Rodriguez J.J. Semiconductor Photocatalysis for Water Purification. Nanoscale Mater. Water Purif. 2018;108:581–651. doi: 10.1016/B978-0-12-813926-4.00028-8. [DOI] [Google Scholar]
- 36.Mohamed F., Hassaballa S., Shaban M., Ahmed A.M. Highly Efficient Photocatalyst Fabricated from the Chemical Recycling of Iron Waste and Natural Zeolite for Super Dye Degradation. Nanomaterials. 2022;12:235. doi: 10.3390/nano12020235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shaban M., Ahmed A.M., Shehata N., Betiha M.A., Rabie A.M. Ni-doped and Ni/Cr co-doped TiO2 nanotubes for enhancement of photocatalytic degradation of methylene blue. J. Colloid Interface Sci. 2019;555:31–41. doi: 10.1016/j.jcis.2019.07.070. [DOI] [PubMed] [Google Scholar]
- 38.Mohamed F., Abukhadra M.R., Shaban M. Removal of safranin dye from water using polypyrrole nanofiber/Zn-Fe layered double hydroxide nanocomposite (Ppy NF/Zn-Fe LDH) of enhanced adsorption and photocatalytic properties. Sci. Total Environ. 2018;640–641:352–363. doi: 10.1016/j.scitotenv.2018.05.316. [DOI] [PubMed] [Google Scholar]
- 39.Zayed M., Samy S., Shaban M., Altowyan A.S., Hamdy H., Ahmed A.M. Fabrication of TiO2/NiO p-n Nanocomposite for Enhancement Dye Photodegradation under Solar Radiation. Nanomaterials. 2022;12:989. doi: 10.3390/nano12060989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ganesan S., Babu I.G., Mahendran D., Arulselvi P.I., Elangovan N., Geetha N., Venkatachalam P. Green engineering of titanium dioxide nanoparticles using Ageratina altissima (L.) King & H.E. Robines. medicinal plant aqueous leaf extracts for enhanced photocatalytic activity. Ann. Phytomed. 2016;5:69–75. doi: 10.21276/ap.2016.5.2.8. [DOI] [Google Scholar]
- 41.Singh A., Goyal V., Singh J., Rawat M. Structural, morphological, optical and photocatalytic properties of green synthesized TiO2 NPs. Curr. Res. Green Sustain. Chem. 2020;3:100033. doi: 10.1016/j.crgsc.2020.100033. [DOI] [Google Scholar]
- 42.Raliya R., Avery C., Chakrabarti S., Biswas P. Photocatalytic degradation of methyl orange dye by pristine titanium dioxide, zinc oxide, and graphene oxide nanostructures and their composites under visible light irradiation. Appl. Nanosci. 2017;7:253–259. doi: 10.1007/s13204-017-0565-z. [DOI] [Google Scholar]
- 43.Senthilkumar S., Rajendran A. Biosynthesis of TiO2 nanoparticles using Justicia gendarussa leaves for photocatalytic and toxicity studies. Res. Chem. Intermed. 2018;44:5923–5940. doi: 10.1007/s11164-018-3464-3. [DOI] [Google Scholar]
- 44.Samuel J.J., Yam F.K. Photocatalytic degradation of methylene blue under visible light by dye sensitized titania. Mater. Res. Express. 2020;7:015051. doi: 10.1088/2053-1591/ab6409. [DOI] [Google Scholar]
- 45.Shimi A.K., Ahmed H.M., Wahab M., Katheria S., Wabaidur S.M., Eldesoky G.E., Islam M.A., Rane K.P. Synthesis and Applications of Green Synthesized TiO2 Nanoparticles for Photocatalytic Dye Degradation and Antibacterial Activity. J. Nanomater. 2022;2022:7060388. doi: 10.1155/2022/7060388. [DOI] [Google Scholar]
- 46.Mills A., Davies R.H., Worsley D. Water purification by semiconductor photocatalysis. Chem. Soc. Rev. 1993;22:417–425. doi: 10.1039/cs9932200417. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.