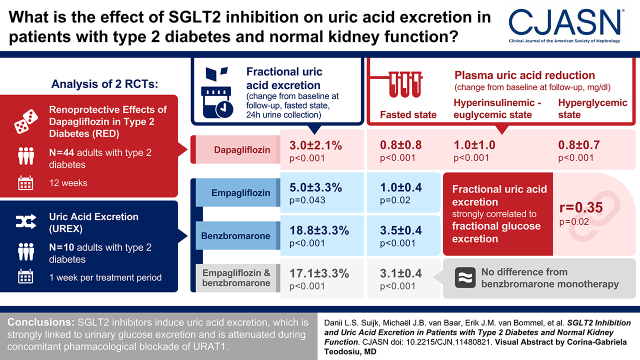
Visual Abstract
Keywords: SGLT-2 inhibition, type 2 diabetes, URAT-1, kidney, uric acid
Abstract
Background and objectives
Sodium-glucose transporter 2 (SGLT2) inhibitor–induced uric acid lowering may contribute to kidney-protective effects of the drug class in people with type 2 diabetes. This study investigates mechanisms of plasma uric acid lowering by SGLT2 inhibitors in people with type 2 diabetes with a focus on urate transporter 1.
Design, setting, participants, & measurements
We conducted an analysis of two randomized clinical trials. First, in the Renoprotective Effects of Dapagliflozin in Type 2 Diabetes study, 44 people with type 2 diabetes were randomized to dapagliflozin or gliclazide for 12 weeks. Plasma uric acid, fractional uric acid excretion, and hemodynamic kidney function were measured in the fasted state and during clamped euglycemia or hyperglycemia. Second, in the Uric Acid Excretion study, ten people with type 2 diabetes received 1 week of empagliflozin, urate transporter 1 blocker benzbromarone, or their combination in a crossover design, and effects on plasma uric acid, fractional uric acid excretion, and 24-hour uric acid excretion were measured.
Results
In the Renoprotective Effects of Dapagliflozin in Type 2 Diabetes study, compared with the fasted state (5.3±1.1 mg/dl), acute hyperinsulinemia and hyperglycemia significantly reduced plasma uric acid by 0.2±0.3 and 0.4±0.3 mg/dl (both P<0.001) while increasing fractional uric acid excretion (by 3.2%±3.1% and 8.9%±4.5%, respectively; both P<0.001). Dapagliflozin reduced plasma uric acid by 0.8±0.8 during fasting, 1.0±1.0 in hyperinsulinemic-euglycemic state, and 0.8±0.7 mg/dl during hyperglycemic conditions (P<0.001), respectively, whereas fractional uric acid excretion in 24-hour urine increased by 3.0%±2.1% (P<0.001) and 2.6%±4.5% during hyperinsulinemic-euglycemic conditions (P=0.003). Fractional uric acid excretion strongly correlated to fractional glucose excretion (r=0.35; P=0.02). In the Uric Acid Excretion study, empagliflozin and benzbromarone both significantly reduced plasma uric acid and increased fractional uric acid excretion. Effects of combination therapy did not differ from benzbromarone monotherapy.
Conclusions
In conclusion, SGLT2 inhibitors induce uric acid excretion, which is strongly linked to urinary glucose excretion and is attenuated during concomitant pharmacologic blockade of urate transporter 1.
Clinical Trial registry name and registration number:
Renoprotective Effects of Dapagliflozin in Type 2 Diabetes (RED), NCT02682563; SGLT2 Inhibition: Uric Acid Excretion Study (UREX), NCT05210517
Introduction
Elevated concentrations of plasma uric acid are frequently observed in people with type 2 diabetes and are strongly associated with diabetes-related complications such as cardiovascular disease and CKD (1). Plasma uric acid lowering has thus been explored as a potential treatment to halt the increasing prevalence of diabetic kidney disease (2). However, recent trials failed to show a benefit on kidney outcomes of lowering uric acid formation with the xanthine oxidase inhibitor allopurinol in people with either type 1 diabetes (3) or type 2 diabetes (4). Nevertheless, there are limited data on sodium-glucose cotransporter 2 (SGLT2) inhibitors, which have been ascribed kidney-protective effects in part due to plasma uric acid lowering as indicated in mediation analyses (5,6).
SGLT2 inhibitors are glucose-lowering drugs that block tubular glucose reabsorption, thereby inducing glycosuria (7). They have been shown to improve hard kidney outcomes in patients with CKD with and without diabetes (8–10). Previous studies have demonstrated that in contrast to allopurinol, SGLT2 inhibitors do not reduce uric acid production, but rather augment its excretion (11). The exact mechanisms by which SGLT2 inhibitors increase uric acid excretion are still unknown, but they are proposed to not be mediated by a direct result of SGLT2 inhibitors’ effects on tubular urate transporters (12,13). Instead, rodent studies suggest that SGLT2 inhibitor–induced glycosuria may drive uric acid excretion at least in part by inhibiting the activity of urate transporter 1 (URAT1) (13), a transporter located at the apical surface of kidney tubular cells and implicated in a significant portion of kidney uric acid reabsorption (14).
This study aims to investigate the uricosuric effect of SGLT2 inhibition in people with type 2 diabetes and preserved kidney function and to determine the relationship with the excretion of other metabolites and electrolytes. In addition, to study the role of URAT1, we conducted a mechanistic trial using SGLT2 inhibition with empagliflozin, direct URAT1 blockade with benzbromarone, and their combination.
Materials and Methods
This paper includes data from two randomized clinical trials: the Renoprotective Effects of Dapagliflozin in Type 2 Diabetes (RED) and the Uric Acid Excretion (UREX) study trials. Both studies were performed at the Amsterdam University Medical Centers, Location VU Medical Center, Amsterdam, The Netherlands. Participants were recruited from existing databases and advertisements in local newspapers. The study protocols and all protocol-specific documents were reviewed and approved by the ethics review board of the VU University Medical Center (Amsterdam, The Netherlands), and written informed consent was obtained from all participants before any trial-related activity. The studies complied with the Declaration of Helsinki and Good Clinical Practice guidelines.
The Renoprotective Effects of Dapagliflozin in Type 2 Diabetes Study
Study Design and Population.
The RED study was a randomized, double-blind, comparator-controlled intervention trial designed to assess the hemodynamic effects of 12 weeks of the SGLT2 inhibitor dapagliflozin compared with the sulfonylurea gliclazide. This analysis concerns a prespecified analysis. The trial protocol has been published previously (15) and is described in further detail in the Supplemental Appendix. Eligible participants were men or postmenopausal women aged between 35 and 75 diagnosed with type 2 diabetes with HbA1c between 7% and 9% and body mass index >25 kg/m2 (15). All participants were treated with metformin monotherapy at a stable dose for ≤3 months. BP was under control (i.e., <140/90 mm Hg), and macroalbuminuria (i.e., ACR >300 mg/g) was not allowed; in the case of previously diagnosed hypertension and/or albuminuria, treatment included at least a stable dose of a renin-angiotensin system inhibitor for ≥3 months.
Randomization, Intervention, and Outcome Measurements.
Participants were randomly assigned to dapagliflozin 10 mg daily or gliclazide 30 mg daily using encapsulated tablets (Supplemental Figure 1A); participants and investigators remained blinded until database lock. Primary end points (measured at baseline and week 12 of treatment) were iohexol-measured GFR and para-aminohippuric acid–measured effective renal plasma flow in the fasted state and during clamped euglycemia and hyperglycemia (Supplemental Figure 1B). Measurement of plasma uric acid was a prespecified end point. The complete study methods are provided in the Supplemental Appendix.
The Uric Acid Excretion Study
Study Design and Population.
The UREX study was an open-label, randomized, crossover intervention study that investigated 1-week mono- and combination therapy with the URAT1 inhibitor benzbromarone and the SGLT2 inhibitor empagliflozin on plasma uric acid and fractional excretion of uric acid (FE-UA). To investigate the role of URAT1 in SGLT2-induced glycosuria, benzbromarone was used to inhibit the activity of the URAT1 transporter. Eligible participants met the same criteria as those in the RED study with two exceptions: a combination of metformin and low-dose sulfonylurea derivative as glucose-lowering therapy was allowed, and a history of gout was added to the exclusion criteria. No participants included in the studies used other uric acid–lowering agents.
Randomization, Intervention, and Outcome Measurements.
The study started with a 4-week run-in period followed by a baseline visit. Hereafter, participants were treated for 1 week with the SGLT2 inhibitor empagliflozin 25 mg once daily, benzbromarone 100 mg once daily, or empagliflozin 25 mg once daily plus benzbromarone 100 mg once daily in a random order (Supplemental Figure 1C). Treatment periods were separated by a 4-week washout period. Primary outcome measurements were plasma uric acid and FE-UA. A detailed description of the study protocol is presented in the Supplemental Appendix.
Statistical Analyses and Post Hoc Outcome Measures.
Outcome measures of this prespecified post hoc analysis consist of plasma uric acid levels and FE-UA levels.
Data on demographics are presented as mean ± SD if normally distributed and median (interquartile range) for positively skewed variables. Continuous variables were tested for distribution and log transformed in the case of positively skewed variables.
For the RED study, different states were compared using a repeated measure ANOVA, or in the case of non-normal distribution, by using a Friedman test. Variables that correlated with plasma uric acid were included in a multivariable linear regression to adjust for potential confounders, including age, sex, body mass index, and hemodynamic kidney function. Within-group comparisons of treatment effects were analyzed using paired t tests or Wilcoxon signed rank tests. The sample size of the RED study was on the basis of the expected between-group difference in measured GFR (mGFR) using Stata version 11 (Breda, The Netherlands) as previously described (15). For the UREX study, to assess differences between groups, paired t tests or Wilcoxon signed rank tests were applied. A linear mixed model analysis was used to compare the outcome between baseline and treatments. The model included a random intercept for participants to take into account the dependency of the observations within one participant. Δ is presented as mean difference ± SEM.
Sample size for the UREX study was calculated as follows. We expected an SGLT2 inhibitor–induced reduction in fasting plasma uric acid from 5.4 to 4.5 mg/dl with an SD of 0.8 mg/dl; with α two sided at 0.05 and a power of 0.80, ten participants needed to be included given within-individuals comparisons. All analyses were performed using SPSS version 26.0, and statistical significance was defined at a two-tailed P value of 0.05. Figures were created using GraphPad Prism version 9.1.0.
Results
Population Characteristics
In this analysis, a total of 54 participants treated with SGLT2 inhibitors were included, of which 44 were RED study participants and ten were UREX study participants. Participants were predominantly overweight men with well-controlled type 2 diabetes and preserved kidney function. Detailed baseline characteristics are shown in Table 1.
Table 1.
Baseline characteristics of the Renoprotective Effects of Dapagliflozin and Uric Acid Excretion studies
| Variable | UREX, n=10 | RED, n=44 | |
|---|---|---|---|
| Dapagliflozin, n=24 | Gliclazide, n=20 | ||
| Clinical characteristics | |||
| Age, yr | 67±8 | 63±7 | 65 (8) |
| Men, N (%) | 6 (60) | 17 (71) | 17 (85) |
| Height, cm | 174±12 | 177±10 | 176±10 |
| Body mass index, kg/m2 | 27 (9) | 31±4 | 32±4 |
| eGFR, ml/min per 1.73 m2 | 88 (11) | 84 (24) | 89 (22) |
| RAS inhibitor use, N (%) | 5 (50) | 16 (67) | 16 (80) |
| ACE inhibitor, N (%) | 3 (30) | 5 (21) | 5 (25) |
| ARB, % | 2 (20) | 11 (46) | 11 (55) |
| HbA1c, % | 7.0±0.7 | 7.3 (0.8) | 7.4±0.6 |
| HbA1c, mmol/mol | 53±8 | 56 (9) | 57±7 |
| Fasting plasma glucose, mg/dl | 135 (41) | 166±27 | 157±29 |
| Fasting insulin, μIU/ml | — | 10±8 | 8±3 |
| Plasma uric acid, mg/dl | 5.3±0.9 | 5.5±1.2 | 5.2±0.9 |
| Fractional uric acid excretion, % | 5.6±0.7 | 5.5±2.3 | 4.7±2.8 |
| Systemic hemodynamic function | |||
| Heart rate, beats/min | 71±12 | 69±10 | 65±11 |
| Systolic BP, mm Hg | 137±14 | 134±11 | 137±15 |
| Diastolic BP, mm Hg | 83±10 | 83±7 | 83±4 |
| Kidney hemodynamic function | |||
| GFR, ml/min 1.73 m2 | 90±10 | 89±19 | |
| Effective blood flow, ml/min per 1.73 m2 | 1064±362 | 1161±226 | |
| Effective plasma flow, ml/min per 1.73 m2 | 654±153 | 691±119 | |
| Filtration fraction, % | 17.8±2.9 | 16.4±1.8 | |
| Vascular resistance, mm Hg/ml per min | 0.10±0.03 | 0.09±0.02 | |
| Glomerular pressure, mm Hg | 60.6±4.5 | 60.2±5.8 | |
| Afferent resistance, dyn⋅s/cm5 | 3267±1337 | 2759±964 | |
| Efferent resistance, dyn⋅s/cm5 | 2100±440 | 1926±251 | |
UREX, Uric Acid Excretion study; RED, Renoprotective Effects of Dapagliflozin study; RAS, renin-angiotensin system; ACE, angiotensin-converting enzyme; ARB, angiotensin-receptor blocker; HbA1c, hemoglobin A1c.
The Renoprotective Effects of Dapagliflozin in Type 2 Diabetes Study
Clamp Procedure.
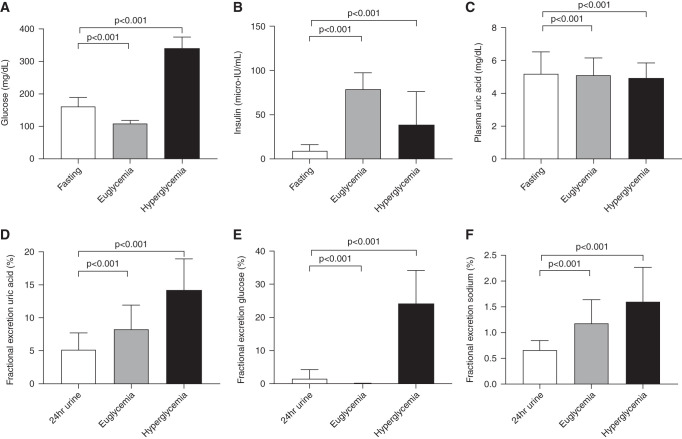
On average, participants of the RED study had fasting glucose concentrations of 162±92 g/ml, which were decreased to 110±9 mg/ml during the hyperinsulinemic-euglycemic state and increased to 339±36 mg/ml in the hyperglycemic state of the clamps (Figure 1A).
Figure 1.
Metabolic parameters and uric acid handling during clamp. Uric acid, insulin, and glucose handling at baseline. (A) Blood glucose. (B) Blood insulin. (C) Plasma uric acid. (D) Fractional excretion of uric acid. (E) Fractional excretion of glucose. (F) Fractional excretion of sodium. Data are mean ± SD.
The mean fasting insulin concentration was 9±7 μIU/ml, and it increased during steady state and urine collection to 79±19 μIU/ml during the hyperinsulinemic-euglycemic state (Figure 1B). In the hyperglycemic state, insulin concentrations averaged 40±40 μIU/ml during the urine collection periods. The mean glucose infusion rate to maintain plasma glucose concentrations at 90 mg/ml during the hyperinsulinemic-euglycemic clamp was 6±3 mg⋅kglean/min (M value).
Baseline Uric Acid Handling.
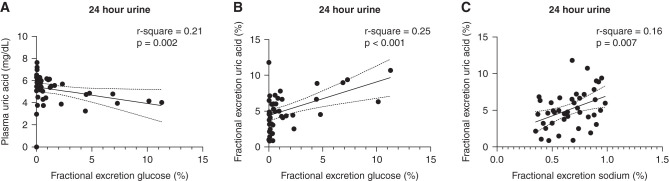
Plasma uric acid concentrations of the total population were at the higher end of the normal range in most participants and were highest during fasting conditions: 5.3±1.1 mg/dl. They were reduced during hyperinsulinemic-euglycemic (from 5.3±1.06 to 5.1±1.0 mg/dl; P<0.001) and hyperglycemic (to 4.9±0.9 mg/dl; P<0.001) conditions (Figure 1C). FE-UA was lowest during fasting conditions (5.1%±2.5%) and increased by euglycemic hyperinsulinemia (to 8.3%±3.6%; P<0.001) and by hyperglycemia (to 14.1%±4.8%; P<0.001) (Figure 1D). As depicted in Figure 1E, the fractional excretion of glucose (FE-Gluc) increased in the hyperglycemic state (24%±10%). The fractional excretion of sodium was lowest during fasting conditions (0.7%±0.2%) and increased significantly in the hyperinsulinemic-euglycemic (to 1.2%±0.6%; P<0.001) and hyperglycemic (to 1.6%±0.7%; P<0.001) states (Figure 1F). In line, plasma uric acid was inversely associated with 24-hour urinary FE-Gluc (Figure 2A). In parallel, 24-hour urinary FE-UA was positively associated with 24-hour urinary FE-Gluc (Figure 2B) as well as 24-hour fractional excretion of sodium (Figure 2C). Plasma insulin concentration or insulin sensitivity (M value) was not related to plasma uric acid or FE-UA.
Figure 2.
Determinants of uric acid handling baseline. (A) The association between plasma uric acid and fractional excretion of glucose. (B) Data on the association between fractional uric acid excretion and fraction glucose excretion. (C) Association between fractional excretion of uric acid and fractional excretion of sodium.
Effect of Dapagliflozin on Kidney Uric Acid Handling.
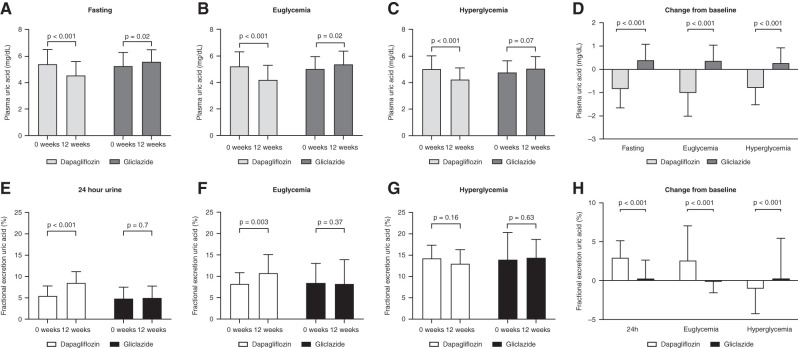
In participants treated with dapagliflozin, plasma uric acid decreased in fasting conditions from 5.5±1.1 to 4.6±1.0 mg/dl (P<0.001), during hyperinsulinemic-euglycemic condition from 5.2±1.1 to 4.2±1.1 mg/dl (P<0.001), and during hyperglycemia from 5.0±0.9 to 4.2±0.9 mg/dl (P<0.001) (Figure 3, A–C). In individuals treated with gliclazide, plasma uric acid increased significantly in the fasting state from 5.2±0.9 to 5.6±0.9 mg/dl (P=0.02) and during hyperinsulinemic-euglycemic condition from 5.0±0.9 to 5.4±0.9 mg/dl (P=0.02), whereas it did not change during hyperglycemic conditions (Figure 3, A–C). Change in plasma uric acid concentrations after 12 weeks of treatment differed significantly between dapagliflozin and gliclazide treatment during fasting, euglycemic, and hyperglycemic conditions (Figure 3D) (P<0.001 for all). The 24-hour urinary FE-UA increased significantly after treatment with dapagliflozin from 5.5%±2.3% to 8.5%±2.6% (P<0.001); it increased from 8.2%±2.6% to 10.8%±4.3% (P=0.003) during hyperinsulinemic-euglycemic conditions, whereas it did not change during hyperglycemic conditions (14.3%±3.1% to 13.0%±3.3%; P=0.16) (Figure 3, E–G). Individuals treated with gliclazide did not show any difference in FE-UA after treatment. Change in FE-UA concentrations after 12 weeks of treatment differed significantly between dapagliflozin and gliclazide under all conditions (P<0.001) (Figure 3H). During hyperglycemia, FE-UA decreased with dapagliflozin compared with gliclazide. After 12 weeks of treatment with dapagliflozin, FE-UA was positively associated with FE-Gluc (r=0.44; P<0.001).
Figure 3.
Effect of dapagliflozin on kidney uric acid handling. Uric acid levels at baseline and after 12 weeks of treatment with dapagliflozin or gliclazide. (A) Plasma uric acid levels during fasting conditions. (B) Plasma uric acid levels during hyperinsulinemic euglycemia. (C) Plasma uric acid levels during hyperglycemia. (D) Within-group change in plasma uric acid. (E) Fractional excretion of uric acid levels derived from 24-hour urine samples. (F) Fractional excretion of uric acid levels during hyperinsulinemic euglycemia. (G) Fractional excretion of uric acid levels during hyperglycemia. (H) Within-group change from baseline in fractional excretion of uric acid. Data are mean ± SD. NS, not significant.
The Uric Acid Excretion Study
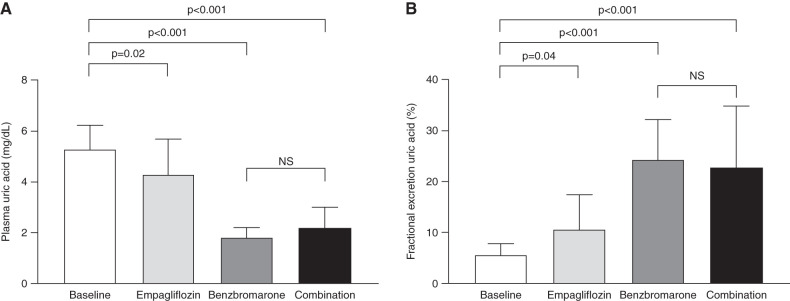
Plasma uric acid at baseline was 5.3±0.9 mg/dl and reduced by 1.0±0.4 mg/dl with empagliflozin (P=0.02), by 3.5±0.4 mg/dl with benzbromarone (P<0.001), and by 3.1±0.4 mg/dl with empagliflozin and benzbromarone combination therapy (P<0.001) (Figure 4A). In parallel, FE-UA increased from baseline 5.6%±2.5% by 5.0±3.3 with empagliflozin (P=0.04), by 18.8±3.3 with benzbromarone (P<0.001), and by 17.1±3.3 with their combination (P<0.001) (Figure 4B). No significant difference was found between benzbromarone monotherapy and combination therapy for plasma uric acid concentrations (P=0.18) and FE-UA (P=0.44).
Figure 4.
Effect of empagliflozin, benzbromarone, and combination therapy on uric acid. (A) Plasma uric acid. (B) Fractional excretion of uric acid. Data are mean ± SD. NS, not significant.
Discussion
In this paper, we demonstrate that SGLT2 inhibitors decrease plasma uric acid concentrations by accentuating urinary uric acid excretion, which is linked to urinary excretion of both glucose and sodium. Additionally, we show that the uricosuric effect of the SGLT2 inhibitor empagliflozin is no longer present when the tubular transporter URAT1 is pharmacologically blocked.
SGLT2 inhibitors were recently shown to have kidney-protective effects in people with CKD with or without type 2 diabetes (9,10,16). However, the mechanisms by which SGLT2 inhibitors improve kidney outcomes remain incompletely understood, with multiple mechanisms proposed (16,17). These potential mechanisms are not reviewed here but elsewhere (16). SGLT2 inhibitor–induced plasma uric acid reductions (5) contribute to improved kidney outcomes according to a mediation analysis of the CREDENCE trial (6). Interestingly, part of the kidney-protective effects of the angiotensin II receptor blocker losartan has previously been related to plasma uric acid reductions (18). The observation that lowering plasma uric acid contributes to kidney outcomes during SGLT2 inhibitor usage is interesting, as the clinical significance of plasma uric acid lowering was questioned recently. On one hand, plasma uric acid has been strongly associated with both incidence and progression of CKD (2). In preclinical and mechanistic studies in humans, plasma uric acid has been linked to glomerular hypertension and damage as well as tubular injury secondary to the formation of urate crystals (19–23). On the other hand, despite suggestions of positive effects in earlier and smaller trials (2), recent large-sized trials in people with type 1 diabetes (the PERL study) (3) and in people with CKD (the CKD-FIX study) (4) did not show a kidney-protective effect when plasma uric acid concentrations were reduced by blocking urate formation with allopurinol. The PERL study examined the plasma uric acid–lowering effect of allopurinol on mGFR in patients with type 1 diabetes and early diabetic kidney disease and did not find evidence for benefits on kidney outcomes, including mGFR, mGFR slopes, or urinary albumin excretion (3). The CKD-FIX study investigated the effects of allopurinol on progression of kidney disease in people with CKD stages 3 and 4 with or without diabetes, and likewise, allopurinol did not show effects on eGFR compared with placebo (4). However, the CKD-FIX study was underpowered with 369 included patients, and participants in these trials did not exhibit significant hyperuricemia. Another explanation for the neutral effects of xanthine oxidase inhibition on kidney outcomes may be that other harmful metabolites in the uric acid pathway accumulate when this enzyme is inhibited. Accumulation of these metabolites by allopurinol could be responsible for continuous kidney damage, despite lowering plasma uric acid. It is tempting to speculate that enhancing uric acid excretion could lead to different results. In line, inhibiting uric acid reabsorption with the URAT1 blocking agent verinurad, in combination with the xanthine-oxidase inhibitor febuxostat, showed a nearly 50% attenuation in albuminuria in 60 patients with type 2 diabetes (24). Larger trials are necessary to determine the kidney-protective potential of uricosuric agents.
The tubular transport of uric acid is complex and remains incompletely understood. Two tubular transporters are currently considered to play a critical role in uric acid reabsorption in the kidney: URAT1 and the glucose transporter GLUT9. URAT1 has previously been identified as a uric acid reabsorption transporter in the apical membrane of the proximal tubule. In patients with idiopathic hypouricemia, inactivation mutations of URAT1 were found (25,26), and URAT1 blockers indeed enhance uric acid excretion. On the other hand, data also suggest that in the absence of URAT1, uric acid reabsorption still occurs, implicating additional transport mechanisms (27). GLUT9, a glucose transporter expressed on both the apical and basolateral membrane of the proximal tubule, contributes to tubular uric acid reabsorption. Loss-of-function mutations in the GLUT9 gene decrease urate transport and induce hypouricemia within the kidney (27,28).
Higher tubular glucose concentrations and glucosuria have been associated with increased uric acid excretion and lowering of plasma uric acid (29,30). We demonstrate that induction of acute hyperglycemia during the hyperglycemic clamp results in an increase in urinary glucose excretion associated with an increase in uric acid excretion. This is in line with a previous study showing that induction of hyperglycemia increases urinary glucose and uric acid excretion in patients with type 1 diabetes (11). SGLT2 inhibitors may lower plasma uric acid by making use of this mechanism. Several studies have shown clear associations between urinary glucose and uric acid excretion during SGLT2 inhibition, where tubular glucose levels may compete with reabsorption of uric acid at the level of GLUT9, thereby enhancing its excretion (11,12). Despite this proposed role for GLUT9, a recent study in rodents demonstrated intact plasma uric acid–lowering effects of SGLT2 inhibitor canagliflozin in mice with kidney-specific GLUT9 knockout. Moreover, the SGLT2 inhibitor enhanced mRNA expression of GLUT9 in wild-type mice within the kidney, potentially to compensate for the uricosuric effect, but tubular GLUT9 was dispensable for the increase in FE-UA in response to canagliflozin (13). In contrast, SGLT2 inhibition did not enhance uric acid excretion in mice lacking URAT1 (13). Thus, we built upon this observation by investigating the effects of SGLT2 inhibition in the presence of pharmacologic URAT1 blockade using benzbromarone. As expected, benzbromarone monotherapy resulted in decreased plasma uric acid and increased urinary uric acid excretion. During combination therapy of empagliflozin-benzbromarone, the uric acid–lowering effects of empagliflozin were attenuated, despite tubular hyperglycemia. We cannot deduce from our data the precise role of URAT1 in this regard, which needs to be addressed in preclinical and, perhaps, rodent studies.
In addition to glucose, urinary sodium excretion has previously been related to urinary uric acid excretion (31). Vice versa, high plasma uric acid concentrations were reported to be independently associated with increased tubular sodium reabsorption (32). In this study, we demonstrate the association between sodium excretion and uric acid excretion in 24-hour urine, in line with previous findings. Insulin has earlier been described as another contributing factor in the excretion of uric acid, with hyperinsulinemia inducing uric acid retention in obese, insulin-resistant patients without diabetes (33), and insulin has been proposed to stimulate uric acid reabsorption via regulating URAT1 in rodents (34). However, in our study, insulin infusion induced a reduction in plasma uric acid by enhancing uric acid excretion. Furthermore, we did not observe a link between plasma insulin concentrations, insulin sensitivity, and plasma uric acid or urinary uric acid excretion. Therefore, the role of insulin in uric acid metabolism in people with hyperglycemia may be different than in people with insulin resistance without overt hyperglycemia.
Our studies have limitations worth mentioning. Although we had sufficient power for these end points, both studies had a relatively small sample size. Additionally, participants were well-controlled patients on oral glucose-lowering agents with preserved kidney function and uric acid concentrations in the upper end of the normal range. This limits the generalizability of our data. Another limitation includes the open-label study design of the UREX study. Additionally, we only tested one dose of both drugs, and although both drugs might have a different affinity for its receptor, higher doses might lead to different results. However, both drugs were used in their common clinical dose. Furthermore, although we show that pharmacologic URAT1 blockade mitigates the effects of tubular hyperglycemia on uric acid excretion, the precise role of URAT1 inhibition remains to be studied in dedicated animal studies. Finally, dietary intake, including purine content, was not taken into account for both studies, although excretion of uric acid was similar between patients at baseline, which suggests that these differences are likely modest.
In conclusion, in people with type 2 diabetes and preserved kidney function, SGLT2 inhibition induces a uricosuric effect linked to urinary glucose excretion attenuated by concomitant pharmacologic URAT1 regulation.
Disclosures
P. Bjornstad reports consultancy agreements with AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Horizon Pharma, Lily USA, Novo Nordisk, Sanofi, and XORTX; research funding from AstraZeneca, Horizon Pharma, and Merck; honoraria from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Horizon Pharma, and Novo Nordisk; serving on the advisory boards of AstraZeneca, Boehringer Ingelheim, Novo Nordisk, and XORTX; and serving in an advisory or leadership role for AstraZeneca, Bayer, Boehringer-Ingelheim, Horizon Pharma, and XORTX. E.J. Hoorn reports research funding from Aurinia; honoraria from UpToDate; serving on the editorial boards of American Journal of Physiology–Renal Physiology, JASN, and Journal of Nephrology; and serving as a board member of the European Renal Association Working Group on Inherited Kidney Diseases and a board member of the Dutch Federation of Nephrology. J.A. Joles reports serving on the editorial boards of Kidney International and PLoS One. M. Nieuwdorp reports employment with, consultancy agreements with, and ownership interest in Caelus Health. D. Touw reports research funding from Astellas Pharma BV and Chiesi Pharmaceuticals BV and serving as a member of the medical advisory board of Sanquin (Amsterdam, The Netherlands). V. Vallon reports consultancy agreements with Boehringer Ingelheim; research funding from AstraZeneca, Boehringer Ingelheim, Gilead, Janssen, Kyowa-Kirin, and Novo Nordisk; honoraria from Boehringer Ingelheim; and serving on the editorial boards of American Journal of Physiology–Renal Physiology, American Journal of Physiology–Regulatory, Integrative and Comparative Physiology, Nephron, and Physiological Reviews. D.H. van Raalte reports consultancy agreements with AstraZeneca, Bayer, the Boehringer Ingelheim–Eli Lilly Alliance, Merck, MSD, and Sanofi and research funding from AstraZeneca, the Boehringer Ingelheim–Eli Lilly Alliance, MSD, and Sanofi. All remaining authors have nothing to disclose.
Funding
The RED study was an investigator-initiated study funded by AstraZeneca. This work was also supported by ZonMw. M. Nieuwdorp is supported by VICI grant 09150182010020. D.H. van Raalte is supported by a fellowship from the Dutch Diabetes Foundation and is a Marie-Sklodowska Curie Fellow.
Supplementary Material
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Author Contributions
D.H. van Raalte conceptualized the study; Z. Iqbal, M.J.B. van Baar, and E.J.M. van Bommel were responsible for data curation; M.J.B. van Baar and E.J.M. van Bommel were responsible for investigation; D.L.S. Suijk was responsible for formal analysis; D.L.S. Suijk and D. Touw were responsible for methodology; D.H. van Raalte was responsible for validation; D.H. van Raalte was responsible for visualization; D.H. van Raalte provided supervision; D.L.S. Suijk, M.J.B. van Baar, and D.H. van Raalte wrote the original draft; and P. Bjornstad, E.J. Hoorn, J.A. Joles, M.M.H. Kramer, M.M. Krebber, M. Nieuwdorp, D. Touw, V. Vallon, and D.H. van Raalte reviewed and edited the manuscript.
Data Sharing Statement
All data used in this study are available in this article.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.11480821/-/DCSupplemental.
Supplemental Appendix. Additional information on methods.
Supplemental Figure 1. Study design and protocol.
References
- 1.Johnson RJ, Bakris GL, Borghi C, Chonchol MB, Feldman D, Lanaspa MA, Merriman TR, Moe OW, Mount DB, Sanchez Lozada LG, Stahl E, Weiner DE, Chertow GM: Hyperuricemia, acute and chronic kidney disease, hypertension, and cardiovascular disease: Report of a scientific workshop organized by the National Kidney Foundation. Am J Kidney Dis 71: 851–865, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sato Y, Feig DI, Stack AG, Kang DH, Lanaspa MA, Ejaz AA, Sánchez-Lozada LG, Kuwabara M, Borghi C, Johnson RJ: The case for uric acid-lowering treatment in patients with hyperuricaemia and CKD. Nat Rev Nephrol 15: 767–775, 2019 [DOI] [PubMed] [Google Scholar]
- 3.Doria A, Galecki AT, Spino C, Pop-Busui R, Cherney DZ, Lingvay I, Parsa A, Rossing P, Sigal RJ, Afkarian M, Aronson R, Caramori ML, Crandall JP, de Boer IH, Elliott TG, Goldfine AB, Haw JS, Hirsch IB, Karger AB, Maahs DM, McGill JB, Molitch ME, Perkins BA, Polsky S, Pragnell M, Robiner WN, Rosas SE, Senior P, Tuttle KR, Umpierrez GE, Wallia A, Weinstock RS, Wu C, Mauer M; PERL Study Group : Serum urate lowering with allopurinol and kidney function in type 1 diabetes. N Engl J Med 382: 2493–2503, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Badve SV, Pascoe EM, Tiku A, Boudville N, Brown FG, Cass A, Clarke P, Dalbeth N, Day RO, de Zoysa JR, Douglas B, Faull R, Harris DC, Hawley CM, Jones GRD, Kanellis J, Palmer SC, Perkovic V, Rangan GK, Reidlinger D, Robison L, Walker RJ, Walters G, Johnson DW; CKD-FIX Study Investigators : Effects of allopurinol on the progression of chronic kidney disease. N Engl J Med 382: 2504–2513, 2020 [DOI] [PubMed] [Google Scholar]
- 5.Zhao Y, Xu L, Tian D, Xia P, Zheng H, Wang L, Chen L: Effects of sodium-glucose co-transporter 2 (SGLT2) inhibitors on serum uric acid level: A meta-analysis of randomized controlled trials. Diabetes Obes Metab 20: 458–462, 2018 [DOI] [PubMed] [Google Scholar]
- 6.Li J, Neal B, Perkovic V, de Zeeuw D, Neuen BL, Arnott C, Simpson R, Oh R, Mahaffey KW, Heerspink HJL: Mediators of the effects of canagliflozin on kidney protection in patients with type 2 diabetes. Kidney Int 98: 769–777, 2020 [DOI] [PubMed] [Google Scholar]
- 7.van Bommel EJ, Muskiet MH, Tonneijck L, Kramer MH, Nieuwdorp M, van Raalte DH: SGLT2 inhibition in the diabetic kidney-from mechanisms to clinical outcome. Clin J Am Soc Nephrol 12: 700–710, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Furtado RHM, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Sabatine MS: SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet 393: 31–39, 2019 [DOI] [PubMed] [Google Scholar]
- 9.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW; CREDENCE Trial Investigators : Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 380: 2295–2306, 2019 [DOI] [PubMed] [Google Scholar]
- 10.Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, Mann JFE, McMurray JJV, Lindberg M, Rossing P, Sjöström CD, Toto RD, Langkilde AM, Wheeler DC; DAPA-CKD Trial Committees and Investigators : Dapagliflozin in patients with chronic kidney disease. N Engl J Med 383: 1436–1446, 2020 [DOI] [PubMed] [Google Scholar]
- 11.Lytvyn Y, Škrtić M, Yang GK, Yip PM, Perkins BA, Cherney DZ: Glycosuria-mediated urinary uric acid excretion in patients with uncomplicated type 1 diabetes mellitus. Am J Physiol Renal Physiol 308: F77–F83, 2015 [DOI] [PubMed] [Google Scholar]
- 12.Chino Y, Samukawa Y, Sakai S, Nakai Y, Yamaguchi J, Nakanishi T, Tamai I: SGLT2 inhibitor lowers serum uric acid through alteration of uric acid transport activity in renal tubule by increased glycosuria. Biopharm Drug Dispos 35: 391–404, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Novikov A, Fu Y, Huang W, Freeman B, Patel R, van Ginkel C, Koepsell H, Busslinger M, Onishi A, Nespoux J, Vallon V: SGLT2 inhibition and renal urate excretion: Role of luminal glucose, GLUT9, and URAT1. Am J Physiol Renal Physiol 316: F173–F185, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anzai N, Ichida K, Jutabha P, Kimura T, Babu E, Jin CJ, Srivastava S, Kitamura K, Hisatome I, Endou H, Sakurai H: Plasma urate level is directly regulated by a voltage-driven urate efflux transporter URATv1 (SLC2A9) in humans. J Biol Chem 283: 26834–26838, 2008 [DOI] [PubMed] [Google Scholar]
- 15.van Bommel EJM, Muskiet MHA, van Baar MJB, Tonneijck L, Smits MM, Emanuel AL, Bozovic A, Danser AHJ, Geurts F, Hoorn EJ, Touw DJ, Larsen EL, Poulsen HE, Kramer MHH, Nieuwdorp M, Joles JA, van Raalte DH: The renal hemodynamic effects of the SGLT2 inhibitor dapagliflozin are caused by post-glomerular vasodilatation rather than pre-glomerular vasoconstriction in metformin-treated patients with type 2 diabetes in the randomized, double-blind RED trial. Kidney Int 97: 202–212, 2020 [DOI] [PubMed] [Google Scholar]
- 16.Leoncini G, Russo E, Bussalino E, Barnini C, Viazzi F, Pontremoli R: SGLT2is and renal protection: From biological mechanisms to real-world clinical benefits. Int J Mol Sci 22: 4441, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ: Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: Cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation 134: 752–772, 2016 [DOI] [PubMed] [Google Scholar]
- 18.Russo E, Verzola D, Leoncini G, Cappadona F, Esposito P, Pontremoli R, Viazzi F: Treating hyperuricemia: The last word hasn’t been said yet. J Clin Med 10: 819, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mazzali M, Kanellis J, Han L, Feng L, Xia YY, Chen Q, Kang DH, Gordon KL, Watanabe S, Nakagawa T, Lan HY, Johnson RJ: Hyperuricemia induces a primary renal arteriolopathy in rats by a blood pressure-independent mechanism. Am J Physiol Renal Physiol 282: F991–F997, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Sánchez-Lozada LG, Tapia E, Santamaría J, Avila-Casado C, Soto V, Nepomuceno T, Rodríguez-Iturbe B, Johnson RJ, Herrera-Acosta J: Mild hyperuricemia induces vasoconstriction and maintains glomerular hypertension in normal and remnant kidney rats. Kidney Int 67: 237–247, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Johnson RJ, Segal MS, Srinivas T, Ejaz A, Mu W, Roncal C, Sánchez-Lozada LG, Gersch M, Rodriguez-Iturbe B, Kang DH, Acosta JH: Essential hypertension, progressive renal disease, and uric acid: A pathogenetic link? J Am Soc Nephrol 16: 1909–1919, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Ryu ES, Kim MJ, Shin HS, Jang YH, Choi HS, Jo I, Johnson RJ, Kang DH: Uric acid-induced phenotypic transition of renal tubular cells as a novel mechanism of chronic kidney disease. Am J Physiol Renal Physiol 304: F471–F480, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Zhou Y, Fang L, Jiang L, Wen P, Cao H, He W, Dai C, Yang J: Uric acid induces renal inflammation via activating tubular NF-κB signaling pathway. PLoS One 7: e39738, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stack AG, Dronamraju N, Parkinson J, Johansson S, Johnsson E, Erlandsson F, Terkeltaub R: Effect of intensive urate lowering with combined verinurad and febuxostat on albuminuria in patients with type 2 diabetes: A randomized trial. Am J Kidney Dis 77: 481–489, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ichida K, Hosoyamada M, Kamatani N, Kamitsuji S, Hisatome I, Shibasaki T, Hosoya T: Age and origin of the G774A mutation in SLC22A12 causing renal hypouricemia in Japanese. Clin Genet 74: 243–251, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Wakida N, Tuyen DG, Adachi M, Miyoshi T, Nonoguchi H, Oka T, Ueda O, Tazawa M, Kurihara S, Yoneta Y, Shimada H, Oda T, Kikuchi Y, Matsuo H, Hosoyamada M, Endou H, Otagiri M, Tomita K, Kitamura K: Mutations in human urate transporter 1 gene in presecretory reabsorption defect type of familial renal hypouricemia. J Clin Endocrinol Metab 90: 2169–2174, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Bobulescu IA, Moe OW: Renal transport of uric acid: Evolving concepts and uncertainties. Adv Chronic Kidney Dis 19: 358–371, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruiz A, Gautschi I, Schild L, Bonny O: Human mutations in SLC2A9 (Glut9) affect transport capacity for urate. Front Physiol 9: 476, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen J, Qiu SH, Guo HJ, Li W, Sun ZL: Increased urinary glucose excretion is associated with a reduced risk of hyperuricaemia. Diabet Med 36: 902–907, 2019 [DOI] [PubMed] [Google Scholar]
- 30.Padova J, Patchefsky A, Onesti G, Faludi G, Bendersky G: The effect of glucose loads on renal uric acid excretion in diabetic patients. Metabolism 13: 507–512, 1964 [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Hu JW, Qu PF, Wang KK, Yan Y, Chu C, Zheng WL, Xu XJ, Lv YB, Ma Q, Gao K, Yuan Y, Li H, Yuan ZY, Mu JJ: Association between urinary sodium excretion and uric acid, and its interaction on the risk of prehypertension among Chinese young adults. Sci Rep 8: 7749, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cappuccio FP, Strazzullo P, Farinaro E, Trevisan M: Uric acid metabolism and tubular sodium handling. Results from a population-based study. JAMA 270: 354–359, 1993 [PubMed] [Google Scholar]
- 33.Muscelli E, Natali A, Bianchi S, Bigazzi R, Galvan AQ, Sironi AM, Frascerra S, Ciociaro D, Ferrannini E: Effect of insulin on renal sodium and uric acid handling in essential hypertension. Am J Hypertens 9: 746–752, 1996 [DOI] [PubMed] [Google Scholar]
- 34.Toyoki D, Shibata S, Kuribayashi-Okuma E, Xu N, Ishizawa K, Hosoyamada M, Uchida S: Insulin stimulates uric acid reabsorption via regulating urate transporter 1 and ATP-binding cassette subfamily G member 2. Am J Physiol Renal Physiol 313: F826–F834, 2017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.