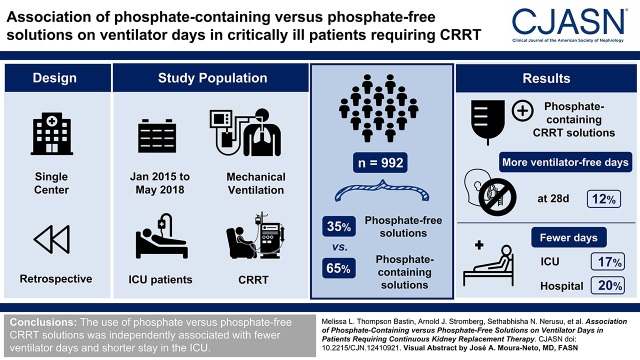
Visual Abstract
Keywords: continuous renal replacement therapy, CRRT, hypophosphatemia, critical illness, ICU, mechanical ventilation, phosphates
Abstract
Background and objectives
Hypophosphatemia is commonly observed in patients receiving continuous KRT. Patients who develop hypophosphatemia may be at risk of respiratory and neuromuscular dysfunction and therefore subject to prolongation of ventilator support. We evaluated the association of phosphate-containing versus phosphate-free continuous KRT solutions with ventilator dependence in critically ill patients receiving continuous KRT.
Design, setting, participants, & measurements
Our study was a single-center, retrospective, pre-post cohort study of adult patients receiving continuous KRT and mechanical ventilation during their intensive care unit stay. Zero-inflated negative binomial regression with and without propensity score matching was used to model our primary outcome: ventilator-free days at 28 days. Intensive care unit and hospital lengths of stay as well as hospital mortality were analyzed with a t test or a chi-squared test, as appropriate.
Results
We identified 992 eligible patients, of whom 649 (65%) received phosphate-containing solutions and 343 (35%) received phosphate-free solutions. In multivariable models, patients receiving phosphate-containing continuous KRT solutions had 12% (95% confidence interval, 0.17 to 0.47) more ventilator-free days at 28 days. Patients exposed to phosphate-containing versus phosphate-free solutions had 17% (95% confidence interval, −0.08 to −0.30) fewer days in the intensive care unit and 20% (95% confidence interval, − 0.12 to −0.32) fewer days in the hospital. Concordant results were observed for ventilator-free days at 28 days in the propensity score matched analysis. There was no difference in hospital mortality between the groups.
Conclusions
The use of phosphate-containing versus phosphate-free continuous KRT solutions was independently associated with fewer ventilator days and shorter stay in the intensive care unit.
Introduction
Phosphate is a small anion found abundantly in erythrocytes, skeletal bone, and muscle tissue, and it is an important modulator of physiologic homeostasis, including cell membrane structure, bone density, acid-base regulation, metabolism, and oxygen delivery (1–3). Critically ill patients are at risk of hypophosphatemia due to intestinal malabsorption, shock states, vomiting/nasogastric suctioning, rapid uptake due to catabolic states or infection, corticosteroids, volume overload, and extracorporeal removal with KRT (3–6).
Hypophosphatemia is a risk factor for intensive care unit (ICU) morbidity—including prolonged mechanical ventilation and receipt of tracheostomy—especially in critically ill patients with acute respiratory and kidney failure receiving both mechanical ventilation and continuous KRT (CRRT) (4,6–9). This interplay is not fully understood but could be mediated by rhabdomyolysis, anemia, impaired cardiac contractility, impaired oxygen delivery, and skeletal muscle dysfunction (10,11). Changes in intracellular phosphate concentrations may disrupt protein synthesis, contribute to mitochondrial dysfunction, and lead to skeletal muscle wasting and physical impairment (12–14).
Hypophosphatemia develops in up to 80% of patients receiving CRRT and displays a positive linear relationship with the dose of CRRT (15). CRRT-induced hypophosphatemia has been associated with prolonged respiratory failure, hospital stay, and multiple organ failure (6,8). Extended hypophosphatemia during CRRT has also been associated with higher mortality (5).
The two primary strategies for managing phosphate during CRRT include: (1) utilizing phosphate-free CRRT solutions plus protocolized phosphate replacement or (2) a preemptive strategy with phosphate-containing CRRT solutions through compounding or the use of commercially available options (16,17). The use of preemptive phosphate replacement with phosphate-containing CRRT solutions has been associated in several studies with a reduction in the severity and duration of hypophosphatemia in patients in the ICU requiring CRRT; however, none of these studies have been large enough to observe an effect on clinical outcomes (4,15,18–21). The aim of this study was to examine the relationship between the receipt of phosphate-containing (versus phosphate-free) CRRT solutions and clinical outcomes of ventilator dependence in a mixed adult patient population in the ICU receiving CRRT and mechanical ventilation.
Materials and Methods
Design and Setting
This study was a single-center, retrospective cohort study conducted at a large tertiary academic medical center with an active CRRT program (∼4000 patient-days of CRRT per year) and over 200 ICU beds, with continuous venovenous hemodiafiltration as the predominant CRRT mode. The research was approved by the institutional review board with a waiver of informed consent due to the observational nature of the investigation (institutional review board no. 17-0514-P1G). The Strengthening the Reporting of Observational Studies in Epidemiology checklist for observational studies was followed (Supplemental Material).
Study Population
The electronic health record was queried for all patients in the ICU admitted from January 1, 2015 to May 1, 2018 with an order for CRRT. Data were extracted by the study analyst. Patients were excluded from this analysis if they were age <18 or >90 years old, had a CRRT order for the administration of plasmapheresis, received both phosphate-containing and phosphate-free CRRT solutions during their course of CRRT, or had mechanical ventilation initiated >96 hours after the start of CRRT. Patient data were retrieved through discharge. Figure 1 shows the details of the study flow chart.
Figure 1.

Consort diagram. CRRT, continuous KRT; ICU, intensive care unit.
Independent Variable
The main independent variable of the study was exposure to phosphate-containing versus phosphate-free CRRT solutions during the course of CRRT. Phosphate-containing solutions were commercially available and contained 1 mmol/L phosphate. We were able to identify two well-defined exposure groups following an institution-wide adoption of phosphate-containing CRRT solutions in 2016. The time frames of the two groups are from January 2015 to February 2016 for exposure to phosphate-free solutions and from March 2016 to May 2018 for exposure to phosphate-containing solutions. Other than the change in CRRT solutions, core elements of CRRT delivery, such as devices, prescription, electrolyte replacement, and vascular access insertion protocols, did not change throughout the study period.
Study Outcomes
The primary outcome of this study was ventilator-free days at 28 days. Ventilator-free days at 28 days were computed from initiation of CRRT through 28 days (days alive and free from ventilator, assigning zero to death). Time on the ventilator was calculated in hours and converted to days by dividing by 24. Any time (in hours) the ventilator was ordered for a patient was counted. This outcome was chosen as we hypothesized that the use of phosphate-containing CRRT solutions will reduce episodes of hypophosphatemia and improve muscle function, ultimately leading to greater ventilator independence. This end point has been extensively validated as a clinically relevant outcome in clinical trials involving patients with acute respiratory distress syndrome (22,23).
Secondary outcomes included total ICU length of stay (days), total hospital length of stay (days), all-cause hospital mortality, need of tracheostomy, incidence of hypophosphatemia (<2.5 mg/dl), and trajectory of serum phosphate levels during the CRRT period.
Statistical Analyses
Multivariable models of ventilator-free days at 28 days were fit by the generalized linear mixed models using the template model builder (glmmTMB) package in R using zero-inflated negative binomial regression, where the zero inflation was fit with the same explanatory variables as the main model. Variables from Table 1 were considered for inclusion in the final model according to univariable analysis and clinical rationale. Final models were adjusted for age, sex, Charlson index, prevalent CKD, sequential organ failure assessment (SOFA) score at ICU admission, total CRRT effluent rate, time from ICU admission to CRRT start, ventilator days prior to CRRT start, CRRT duration, and admission to medical ICU. The SOFA score was calculated automatically at the time of data extraction from the electronic health record. A similar approach was used for secondary outcomes of ICU length of stay and hospital length of stay. Missing data were imputed for sensitivity analyses using the multiple imputation by chained equations approach implemented in R. Five imputed datasets were generated. Means and 95% confidence intervals (95% CIs) for parameter estimates are reported. For evaluation of the trajectory of serum phosphate levels according to solution type, we fitted unadjusted natural cubic splines to display the average of each patient’s 6-hour mean of serum phosphate measurements. Observations were restricted to patients will full data available through days 5 and 10. Knots were placed at the start and end of the observation day of CRRT, and extreme values (≥97.5 and ≤2.5 percentiles) were excluded to avoid implausible extrapolation of trajectories. The serum phosphate trajectories of the two CRRT solution groups were evaluated individually by autoregressive integrated moving average, and autoregressive integrated moving average model parameters were compared by F test. Statistical analyses were done using R version 3.6.2 (24,25).
Table 1.
Baseline characteristics of unmatched and matched cohorts
| Patient Characteristics | Entire Cohort, n=992 | Phosphate-Free Continuous KRT Solution, n=343 | Phosphate-Containing Continuous KRT Solution, n=649 | Propensity Score Matched Phosphate-Free Continuous KRT Solution, n=303 | Propensity Score Matched Phosphate-Containing Continuous KRT Solution, n=303 |
|---|---|---|---|---|---|
| Study periods | — | 1/2015–2/2016 | 3/2016–5/2018 | 1/2015–2/2016 | 3/2016–5/2018 |
| Demographics | |||||
| Age, yr, median (IQR) | 56 (13) | 56 (13) | 57 (14) | 58 (48–65) | 59 (49–66) |
| Men, n (%)a | 579 (58) | 218 (64) | 361 (56) | 182 (60) | 176 (58) |
| Race, n (%)a | White 912 (92), Black 76 (8), other 3 (0.4) | White 313 (91), Black 28 (8), other 2 (1) | White 599 (92), Black 48 (7), other 2 (1) | White 278 (92), other 25 (8) | White 278 (92), other 25 (8) |
| BMI, median (IQR)a | 33 (12) | 33 (10) | 33 (13) | 31 (26–38) | 30 (25–38) |
| Comorbidity | |||||
| Charlson index, mean (SD)a | 2.2 (1.8) | 2.3 (1.9) | 2.1 (1.7) | 2.1 (1.7) | 2.2 (1.7) |
| Elixhauser index, mean (SD) | 4.6 (1.8) | 4.5 (1.6) | 4.6 (1.8) | 4.4 (1.6) | 4.7 (1.8) |
| Prevalent CKD (ICD-10 diagnosis), n (%)a | 452 (46) | 170 (50) | 282 (43) | 144 (48) | 142 (47) |
| Acuity of illness | |||||
| Admission to MICU, n (%)a | 664 (67) | 211 (62) | 453 (70) | 197 (65) | 192 (63) |
| Admission ICU, n (%) | |||||
| Medical/pulmonary | 664 (67) | 211 (62) | 453 (70) | ||
| Surgical/trauma | 75 (8) | 27 (8) | 48 (7) | ||
| Cardiothoracic | 92 (9) | 43 (13) | 49 (8) | ||
| Cardiac/nonsurgical | 87 (9) | 40 (12) | 47 (7) | ||
| Transplant | 38 (4) | 12 (4) | 26 (4) | ||
| Surgical/general | 27 (3) | 9 (3) | 18 (3) | ||
| Neurosurgery/ neurology | 9 (1) | 1 (0) | 8 (1) | ||
| SOFA at ICU start, mean (SD)a | 13 (3) | 13 (4) | 13 (3) | 12.6 (3.5) | 12.6 (3.3) |
| Vizient expected LOS | 14 (8–19) | 15 (10–23) | 14 (8–19) | ||
| Total effluent CRRT dose, ml/kg per h, median (IQR)a | 36 (11) | 36 (13) | 37 (10) | 33 (28–43) | 35 (31–40) |
| Cumulative fluid balance from ICU admission to CRRT start, ml, median (IQR)a | 680 (0–3007) | 1179 (0–3546) | 545 (0–2520) | 1050 (0–3376) | 793 (0–2952) |
| Cumulative fluid balance from ICU admission to CRRT stop, ml, median (IQR) | 622 (–15–2254) | 635 (–165–2343) | 662 (0–2197) | 614 (–91–2306) | 614 (0–1780) |
| Cumulative fluid balance from ICU admission to ICU discharge, ml, median (IQR) | 382 (–52–2155) | 369 (–65–2024) | 402 (−52–2230) | ||
| Days from ICU admission to CRRT start, median (IQR) | 1.5 (0.5–3.8) | 1.7 (0.5–4.6) | 1.4 (0.5–3.6) | 1.6 (0.5–4.1) | 1.8 (0.6–4.2) |
| Days on CRRT, median (IQR)a | 7 (3–12) | 7 (2–12) | 7 (3–11) | 7 (2–11) | 7 (3–12) |
| Ventilator days prior to CRRT | 0.31 (9.4) | 0.02 (6.1) | 0.47 (3.3) | ||
IQR, interquartile range; BMI, body mass index; ICD-10, International Classification of Diseases 10th revision; MICU, medical intensive care unit; ICU, intensive care unit; SOFA, sequential organ failure score; LOS, length of stay; CRRT, continuous KRT.
Variables included in the propensity score matching and variables considered for the primary multivariable model.
Sensitivity Analyses
Propensity Score Matching.
A propensity score was estimated by fitting a logistic regression model to predict the individual’s probability of receiving a phosphate-free CRRT solution with a set of a priori–selected clinical parameters as the model covariates. These variables included age, sex, race, body mass index, Charlson index, CKD, SOFA score at ICU admission, cumulative fluid balance from ICU admission to CRRT start, time from ICU admission to CRRT start (days), CRRT duration (days), and admission to medical ICU. Missing data were imputed with median values for continuous variables. Individuals in the phosphate-containing and phosphate-free CRRT solution groups were matched by a caliper distance equal to 0.30 times the SD of the logit of the propensity score. Ventilator-free days at 28 days were modeled on the matched dataset using a zero-inflated negative binomial model, with adjustment for the same covariates as the primary analysis.
Addressing Intensive Care Unit Practice Variations.
To evaluate possible changes in ICU practice during the two study periods, which may have influenced the primary outcome, we compared ICU and hospital length of stay in all critically ill adult patients in the ICU who did not receive CRRT within the two CRRT solutions periods: from January 2015 to February 2016, which was the period of phosphate-free solutions use, and from March 2016 to May 2018, which was the period of phosphate-containing solutions use.
Results
Clinical Characteristics
A total of 992 critically ill adult patients were included in the analysis: 343 patients (10 patient-days of CRRT) in the phosphate-free CRRT solution group and 649 patients (9 patient-days of CRRT) in the phosphate-containing CRRT solution group. The patients were primarily men (58%) and White participants (92%). The majority of patients (667 of 992; 67%) were admitted to the medical ICU. The mean (SD) SOFA score at ICU admission was 13 (3), and the Charlson comorbidity index was 2.2 (1.8). Patients were on CRRT for a median of 7 (interquartile range [IQR], 3–11.5) days. When compared with patients in the phosphate-containing CRRT solution group, patients in the phosphate-free CRRT solution group were more frequently men (64% versus 56%), were less frequently admitted to the medical ICU (62% versus 70%), and had higher cumulative fluid balance from ICU admission to CRRT start (median of 1179 ml; IQR, 0–3546 versus 545 ml; IQR, 0–2520). When cumulative fluid balance was calculated from ICU admission to the end of CRRT, it was not different between the two groups (median of 635 ml; IQR, −165–2343 in the phosphate-free versus 662 ml; IQR, 0–2197 in the phosphate groups). There were no statistically significant differences in SOFA scores, comorbidity scores, time from ICU admission to CRRT start, CRRT dose, or duration of CRRT between the two groups (Table 1).
Serum Phosphate Trajectories According to the Continuous Kidney Replacement Therapy Solution Group
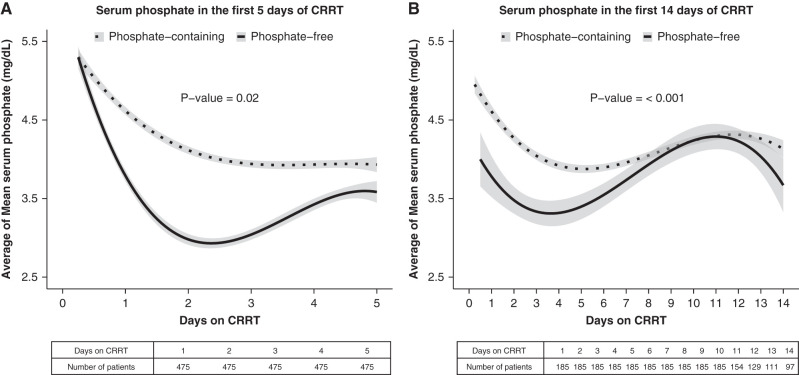
As depicted in Figure 2, trajectories of patients’ serum phosphate concentrations exhibited overall lower levels of serum phosphate in patients exposed to phosphate-free versus phosphate-containing CRRT solutions groups (P=0.05 and P=0.004) when evaluating the first 5 and 14 days of CRRT, respectively. Further, incident hypophosphatemia was more frequent in the phosphate-free CRRT solution group when compared with the phosphate-containing CRRT solution group (Table 2).
Figure 2.
Serum phosphate trajectory over 5 and 14 days of therapy. Unadjusted natural cubic splines of serum phosphate trajectories generated by averaging each patient’s 6-hour mean of measurements. (A) Represents the first 5 days of CRRT and (B) represents the first 14 days of CRRT in the whole cohort. The trajectories of serum phosphate levels of the two CRRT solution types were evaluated individually by autoregressive integrated moving average (ARIMA). The P value denotes the F test comparison of ARIMA model parameters between the two groups. The shaded area on either side of the line denotes 95% confidence intervals.
Table 2.
Unadjusted study outcomes according to the continuous KRT solution group
| Study Outcomes | Phosphate-Free Continuous KRT Solution, n=343 | Phosphate-Containing Continuous KRT Solution, n=649 | P Value |
|---|---|---|---|
| Primary outcome | |||
| Ventilator-free days at 28 d, median (IQR) | 21 (16–25) | 22 (17–25) | 0.20a |
| Secondary outcomes | |||
| Total hospital LOS, d, median (IQR) | 16 (5–25) | 12 (5–22) | 0.01a |
| Total ICU LOS, d, median (IQR) | 10 (3–23) | 8 (3–15) | 0.01a |
| Hospital mortality, n (%) | 213 (62) | 426 (66) | 0.23b |
| Other outcomes | |||
| Total days on ventilator, median (IQR) | 6 (2–12) | 4 (2–8) | 0.01a |
| Hypophosphatemia <2.5 mg/dl, n (%) | 194 (58) | 135 (22) | 0.01b |
| Severe hypophosphatemia <1 mg/dl, n (%) | 6 (2) | 79 (1) | 0.37b |
| Median phosphate level during CRRT, mg/dl, median (IQR) | 3.7 (3.1–4.7) | 4.4 (3.7–5.3) | 0.01a |
| Minimum phosphate level during CRRT, mg/dl, median (IQR) | 2.3 (1.8–3.9) | 3.4 (2.6–4.6) | 0.01a |
| Total phosphate replacement during CRRT, mmol, median (IQR) | 62 (0–302) | 0 (0–8) | 0.01a |
| Median ionized calcium | 3.15 (1.16) | 3.4 (1) | 0.01c |
| Positive ketones, n (%) | 53 (43) | 90 (40) | 0.60b |
| Tracheostomy, n (%) | 25 (7) | 19 (3) | 0.01b |
| Discharge disposition, n (%) | |||
| Alive/home | 196 (32) | 186 (29) | 0.27b |
| Rehabilitation facility | 19 (6) | 33 (5) | |
| Other | 5 (2) | 3 (1) | |
Continuous data are reported as median (IQR), and categorical data are reported as counts and percentages. IQR, interquartile range; LOS, length of stay; ICU, intensive care unit; CRRT, continuous KRT.
Wilcoxon rank sum test.
Chi-squared test.
Independent samples t test.
Study Outcomes
Receipt of phosphate-containing CRRT solutions was associated with fewer days of mechanical ventilation: median of 4 (2–8) versus 6 (2–12), respectively (P<0.001). Similarly, patients exposed to phosphate-containing versus phosphate-free CRRT solutions had less requirement of tracheostomy and shorter length of stay in the ICU and the hospital (Table 2).
After multivariable adjustment, exposure to phosphate-containing versus phosphate-free CRRT solutions was associated with 12% more ventilator-free days at 28 days (95% CI, 0.05 to 0.17; P<0.001 and 95% CI, 0.03 to 0.09; P<0.001) (Table 3). Patients exposed to phosphate versus phosphate-free solutions had 17% fewer days in the ICU (95% CI, −0.08 to −0.30; P<0.001) and 20% fewer days in the hospital (95% CI, −0.12 to −0.32; P<0.001) (Table 3).
Table 3.
Multivariable models of the continuous KRT solution group (phosphate containing versus phosphate free) as the main independent variable and study outcomes as the dependent variables in the whole and matched cohort
| Total Cohort | Matched Cohort | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Estimate (% Change) | Model 95% Confidence Interval | P Value | Imputation Mean | Imputation 95% Confidence Interval | Estimate (% Change) | Model 95% Confidence Interval | P Value |
| Ventilator-free days at 28 d | ||||||||
| Phosphate versus phosphate-free group | 0.11a (12%) | 0.05 to 0.17 | <0.001b,c | 0.100 | 0.10 to 0.10 | 0.14 (15%) | 0.07 to 0.21 | <0.001b |
| ICU LOS | ||||||||
| Phosphate versus phosphate-free group | −0.19 (–17%) | −0.08 to –0.30 | <0.001b,c | −0.142 | 0.14 to –0.14 | — | — | — |
| Hospital LOS | ||||||||
| Phosphate versus phosphate-free group | −0.22 (–20%) | −0.12 to –0.32 | <0.001b,c | −0.148 | −0.15 to 0.15 | — | — | — |
Estimates are shown for nonimputed and imputed models. ICU, intensive care unit; LOS, length of stay.
Model interpretation: exponentiating the phosphate coefficient exp(0.11)=1.12; thus, the phosphate treatment is associated with more ventilator-free days (at 28 days) by 12% on average after adjusting for everything else.
The model was adjusted for age, sex, race, Charlson index, prevalent CKD, sequential organ failure score, total continuous KRT (CRRT) effluent rate, ventilator days prior to CRRT, CRRT duration, and admission to medical ICU.
The model was constructed with zero-inflated negative binomial logistic regression, with multiple imputation of missing data included in parentheses.
Hospital mortality was similar in both groups: 66% in those receiving phosphate-containing CRRT solutions versus 62% in those receiving phosphate-free CRRT solutions (P=0.23) (Table 2).
Sensitivity Analyses
The results of the zero-inflated negative binomial regression models (ventilator-free days at 28 days, ICU length of stay, and total hospital length of stay) with imputation of the full dataset are similar to the nonimputed estimates (Table 3). When the multivariable analysis was restricted to a propensity matched cohort of 606 critically ill adult patients (n=303 in each CRRT solution group), the use of phosphate-containing versus phosphate-free CRRT solutions was associated with 15% more ventilator-free days at 28 days (log mean 0.14; 95% CI, 0.07 to 0.21; P<0.001) (Table 3). The covariate balance plot with standardized mean differences is reported in Supplemental Figure 1. Unadjusted study outcomes according to the CRRT solution group of the matched cohort are found in Supplemental Table 1.
There were no indicators of ICU practice variations influencing the primary outcome during the two study periods as determined by length of stay in non-CRRT critically ill adults. Median hospital length of stay was 7 (3–14) versus 7 (3–13) days (P=0.10) and median ICU length of stay was 2 (1–5) versus 2 (1–5) days (P=0.80) in the periods of phosphate-free and phosphate-containing CRRT solutions usage in the ICU, respectively.
Discussion
This study found an independent association between the receipt of phosphate-containing CRRT solutions and a higher number of ventilator-free days at 28 days when compared with the receipt of phosphate-free CRRT solutions in critically ill adult patients requiring CRRT and mechanical ventilation. We also found a reduction in ICU and hospital lengths of stay in patients who received CRRT with phosphate-containing solutions. Patients receiving phosphate-free CRRT solutions exhibited more incident hypophosphatemia and overall lower serum phosphate levels during CRRT when compared with those receiving phosphate-containing CRRT solutions. Our findings are supported by prior work in this area describing the notably higher incidence of hypophosphatemia with the use of phosphate-free (versus phosphate-containing) CRRT solutions in critically ill patients in the ICU (4,15,19–21).
Our findings complement prior studies, which demonstrated a relationship between incident hypophosphatemia and adverse clinical outcomes. A prospective observational study by Demirjian et al. (6) evaluating patients with AKI receiving CRRT found an association between hypophosphatemia and prolonged respiratory failure requiring tracheostomy (odds ratio, 1.81; 95% CI, 1.07 to 3.08). This observation was more pronounced when CRRT and hypophosphatemia duration were prolonged, suggesting that the interplay of severity and duration of hypophosphatemia may be especially detrimental (6). Two additional studies found that incident hypophosphatemia during CRRT was associated with 7 additional days of mechanical ventilation (8) and independently associated with mortality (5). Our results suggest that these outcomes can be mitigated through the continuous repletion of phosphate via phosphate-containing CRRT solutions; however, our study, like others, was nonrandomized and therefore subject to bias. A prospective, blinded, randomized controlled trial would provide definitive evidence of this relationship.
Prior studies have found an association between hypophosphatemia during CRRT and higher mortality (26–28). Although our study did not show a difference in hospital mortality between the two phosphate solutions groups, we did find a higher number of days alive and free from ventilator in the phosphate-containing CRRT solution group as compared with the phosphate-free CRRT solution group. Patients receiving CRRT with phosphate-containing solutions were able to be liberated from mechanical ventilation earlier, which may have a desirable effect on organ recovery and rehabilitation. There are some options to clinicians to achieve a phosphate-containing CRRT solution, including compounding individual bags and using stock solutions. Another option to mitigate hypophosphatemia during CRRT involves careful monitoring and preemptive replacement of phosphate before significant hypophosphatemia occurs (16). In our study, the trajectory analysis of serum phosphate levels during CRRT revealed overall lower levels of serum phosphate during the course of CRRT with the use of phosphate-free (versus phosphate) CRRT solutions. Effective preemptive thresholds for phosphate replacement should be carefully determined to favor a “proactive” approach rather than a “reactive” (replace as needed) approach, which could be subject to delays in phosphate replacement in some cases. Despite many patients requiring CRRT for AKI and having a presumably elevated phosphate initially, hypophosphatemia is common after a patient starts therapy. A study of the relationship between the prevention of hypophosphatemia during CRRT by the use of phosphate-containing CRRT solutions and/or proactive phosphate replacement protocols on clinical and patient-centered outcomes should be a research priority in critical care nephrology.
A possible explanation of our finding of reduced mechanical ventilation days may be related to the interaction of hypophosphatemia and diaphragmatic muscle weakness (29,30), which has been associated with longer duration of mechanical ventilation and death in patients in the ICU (31–33). Hypophosphatemia decreases oxygen delivery to skeletal muscle, reducing contractility (34). In a formative experiment, Aubier et al. (35) demonstrated that hypophosphatemia was strongly associated with transdiaphragmatic pressure (diaphragm contraction) measured during electrical stimulation of the phrenic nerve (34). It is reasonable to hypothesize that hypophosphatemia-induced diaphragm weakness impairs respiratory function, possibly prolonging mechanical ventilation and increasing the need for tracheostomy and the risk of mortality as some studies have shown (32). Importantly, this is a modifiable risk factor in critically ill patients requiring CRRT (18,19). Differing trajectories of serum phosphate levels by patients in the two CRRT solutions groups, as depicted in Figure 2, further support this hypothesis.
In addition to the effects on contractility, phosphate is an important regulator of oxygen delivery. Hypophosphatemia decreases erythrocyte 2,3-diphosphoglycerate, which, in turn, can decrease the dissociation of oxygen from hemoglobin by altering the oxygen-hemoglobin affinity curve (36). Tightly bound oxygen to hemoglobin is unavailable for delivery to the peripheral tissues, thus blunting this beneficial adaptive response to tissue hypoxia and acidemia, which is crucial to the mitigation of tissue necrosis during critical illness and has been implicated in the causal pathway for greater morbidity in critically ill patients (3,34). Sharma et al. (34) found a significant association between the duration of CRRT and the reduction of mean 2,3-diphosphoglycerate levels, which was observed independent of serum phosphate levels. Finally, animal models of hypophosphatemia have shown significant reduction in muscle ATP synthesis by oxidative phosphorylation in the mitochondria, suggesting that hypophosphatemia may have a profound effect on diaphragm and skeletal muscle function (12).
Strengths of our study are the inclusion of consecutive patients receiving CRRT who were also receiving mechanical ventilation, thus reflecting a population with multiorgan failure in need of extracorporeal organ support. This enhances external validity to a heterogeneous ICU population that would frequently receive CRRT. By excluding those who received both solution types, we controlled for a treatment crossover effect. Adopting the use of phosphate-containing CRRT solutions as a standard practice at our institution limited the indication bias inherent to observational studies comparing different exposures. We tested widely available commercial CRRT solutions, which can be readily applied to clinical practice and are a real-world intervention for patients on CRRT. Further, this work supports the hypothesis that hypophosphatemia is causally related to adverse outcomes and sets the groundwork for future prospective trials.
Our study has several limitations. First, observational studies are prone to confounding, which can often be unmeasured. Our exposure variable—CRRT solution type—was evaluated in two different periods of time in the ICU, which could be subject to practice variations. We attempted to account for this by demonstrating that the length of ICU stay was similar in non-CRRT patients treated in these two periods of time. Second, we were unable to control or perform subgroup analyses according to ICU diagnoses, CRRT indications, or degree of baseline lung injury. However, our CRRT protocol did not significantly change during the course of the study, including indications, dose, or access. Importantly, we controlled for multiple patient-specific and acute illness parameters in the presented multivariable models and matched cohort analysis. Third, retrospective data are subject to systematic biases, including documentation or extraction errors. Lastly, the nonexperimental design of this study limits the conclusions and should be viewed as hypothesis generating. In addition, the study design prohibits conclusions to be drawn on the effect of phosphate-containing solutions in a nonmechanically ventilated population or in those in whom mechanical ventilation was initiated outside our study exposure window.
In critically ill adult patients requiring CRRT and mechanical ventilation, the utilization of 1 mmol/L phosphate-containing CRRT solutions was associated with a significant reduction in days on the ventilator and in the ICU. Patients exposed to phosphate-free CRRT solutions exhibited more incident hypophosphatemia and overall lower levels of serum phosphate during CRRT. These results highlight the importance of mitigating hypophosphatemia and maintaining optimal serum phosphate levels during CRRT as potential interventions that can affect respiratory function and clinical outcomes in this debilitated population. These findings should be validated with randomized clinical trials to establish the effect of preventing hypophosphatemia during CRRT on relevant clinical and patient-centered outcomes.
Disclosures
S.M. Bagshaw reports consultancy agreements with Baxter Healthcare Corp., BioPorto Inc., and CNA Diagnostics Inc.; ownership interest in CNA Diagnostics Inc.; research funding from Baxter Healthcare Corp.; honoraria from Baxter Healthcare Corp.; serving in an advisory or leadership role for Baxter Healthcare Corp. (advisory), BioPorto (adjudication committee), CNA Diagnostics Inc. (advisory), and Critical Care (editorial board); and speaker fees from Baxter Healthcare Corp. S.M. Bagshaw is supported by a Canada Research Chair in Critical Care Nephrology. K.D. Liu reports consultancy agreements with AM Pharma, Biomerieux, BOA Medical, and Seastar Medical; holds stock in Amgen; serving on the editorial boards of American Journal of Kidney Diseases, American Journal of Respiratory and Critical Care Medicine, and CJASN; serving in an advisory or leadership role for the American Thoracic Society and the National Kidney Foundation Scientific Advisory Board; and other interests or relationships with UpToDate. K.D. Liu is currently supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant K24 DK1133381. P.E. Morris reports consultancy agreements with Dompe and Medtronic. J.A. Neyra reports consultancy agreements with Baxter Healthcare Inc., Biomedical Insights, and Leadiant Biosciences; speaker fees from Baxter; serving as a section editor for Clinical Nephrology and a guest editor for Critical Care Nephrology in Advances in Chronic Kidney Disease; and serving on the editorial boards of Advances in Chronic Kidney Disease and Kidney360. J.A. Neyra is currently supported by National Heart, Lung, and Blood Institute grant R01 HL148448-01 and NIDDK grants R56 DK126930 and P30 DK079337. A.J. Stromberg reports consultancy agreements with Doorn Corporation (Louisville, KY), Golden Law Office PLLC (Lexington, KY), and VRTogether (Lexington, KY). M.L. Thompson Bastin reports research funding from the ASHP Foundation and the (Canadian) University Hospital Foundation, serving in an advisory or leadership role at Lediant Biosciences, and speaker fees from Baxter Healthcare. R. Wald reports research funding from Baxter; serving on the editorial boards of CJASN, Kidney360, and Kidney Medicine; and other interests or relationships as a contributor to UpToDate. All remaining authors have nothing to disclose.
Funding
None.
Supplementary Material
Acknowledgments
The authors thank the University of Kentucky CRRT Quality Assurance team for the constructive feedback provided on this work. This work was performed at the University of Kentucky Medical Center.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “CRRT Fluid Choices: A Solution for a Common Problem?,” on pages 631–633.
Author Contributions
J.A. Neyra, A.J. Stromberg, and M.L. Thompson Bastin conceptualized the study; S.N. Nerusu and M.L. Thompson Bastin were responsible for data curation; S.N. Nerusu and M.L. Thompson Bastin were responsible for investigation; L.J. Liu and A.J. Stromberg were responsible for formal analysis; J.A. Neyra, A.J. Stromberg, and M.L. Thompson Bastin were responsible for methodology; J.A. Neyra and M.L. Thompson Bastin wrote the original draft; and S.M. Bagshaw, K.D. Liu, L.J. Liu, K.P. Mayer, P.E. Morris, S.N. Nerusu, J.A. Neyra, A.J. Stromberg, M.L. Thompson Bastin, and R. Wald reviewed and edited the manuscript.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.12410921/-/DCSupplemental.
Supplemental Material. STROBE checklist.
Supplemental Table 1. Unadjusted study outcomes on the matched cohort according to the CRRT solution group.
Supplemental Table 2. CRRT solutions used.
Supplemental Table 3. Phosphate replacement protocols throughout the study period.
Supplemental Figure 1. Plot representing the covariate balance of propensity score matching between critically ill adults exposed to phosphate- versus nonphosphate-containing CRRT solutions.
References
- 1.Uribarri J: Phosphorus homeostasis in normal health and in chronic kidney disease patients with special emphasis on dietary phosphorus intake. Semin Dial 20: 295–301, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Padelli M, Leven C, Sakka M, Plée-Gautier E, Carré JL: [Causes, consequences and treatment of hypophosphatemia: A systematic review]. Presse Med 46: 987–999, 2017 [DOI] [PubMed] [Google Scholar]
- 3.Geerse DA, Bindels AJ, Kuiper MA, Roos AN, Spronk PE, Schultz MJ: Treatment of hypophosphatemia in the intensive care unit: A review. Crit Care 14: R147, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pistolesi V, Zeppilli L, Fiaccadori E, Regolisti G, Tritapepe L, Morabito S: Hypophosphatemia in critically ill patients with acute kidney injury on renal replacement therapies. J Nephrol 32: 895–908, 2019 [DOI] [PubMed] [Google Scholar]
- 5.Yang Y, Zhang P, Cui Y, Lang X, Yuan J, Jiang H, Lei W, Lv R, Zhu Y, Lai E, Chen J: Hypophosphatemia during continuous veno-venous hemofiltration is associated with mortality in critically ill patients with acute kidney injury. Crit Care 17: R205, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demirjian S, Teo BW, Guzman JA, Heyka RJ, Paganini EP, Fissell WH, Schold JD, Schreiber MJ: Hypophosphatemia during continuous hemodialysis is associated with prolonged respiratory failure in patients with acute kidney injury. Nephrol Dial Transplant 26: 3508–3514, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Agusti AG, Torres A, Estopa R, Agustividal A: Hypophosphatemia as a cause of failed weaning: The importance of metabolic factors. Crit Care Med 12: 142–143, 1984 [DOI] [PubMed] [Google Scholar]
- 8.Lim C, Tan HK, Kaushik M: Hypophosphatemia in critically ill patients with acute kidney injury treated with hemodialysis is associated with adverse events. Clin Kidney J 10: 341–347, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alsumrain MH, Jawad SA, Imran NB, Riar S, DeBari VA, Adelman M: Association of hypophosphatemia with failure-to-wean from mechanical ventilation. Ann Clin Lab Sci 40: 144–148, 2010 [PubMed] [Google Scholar]
- 10.Brunelli SM, Goldfarb S: Hypophosphatemia: Clinical consequences and management. J Am Soc Nephrol 18: 1999–2003, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Sharma SHM, Castro D: Hypophosphatemia, Treasure Island, FL, StatPearls, 2018 [Google Scholar]
- 12.Pesta DH, Tsirigotis DN, Befroy DE, Caballero D, Jurczak MJ, Rahimi Y, Cline GW, Dufour S, Birkenfeld AL, Rothman DL, Carpenter TO, Insogna K, Petersen KF, Bergwitz C, Shulman GI: Hypophosphatemia promotes lower rates of muscle ATP synthesis. FASEB J 30: 3378–3387, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schubert L, DeLuca HF: Hypophosphatemia is responsible for skeletal muscle weakness of vitamin D deficiency. Arch Biochem Biophys 500: 157–161, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Knochel JP, Barcenas C, Cotton JR, Fuller TJ, Haller R, Carter NW: Hypophosphatemia and rhabdomyolysis. J Clin Invest 62: 1240–1246, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Broman M, Carlsson O, Friberg H, Wieslander A, Godaly G: Phosphate-containing dialysis solution prevents hypophosphatemia during continuous renal replacement therapy. Acta Anaesthesiol Scand 55: 39–45, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heung M, Mueller BA: Prevention of hypophosphatemia during continuous renal replacement therapy: An overlooked problem. Semin Dial 31: 213–218, 2018 [DOI] [PubMed] [Google Scholar]
- 17.Shaw AR, Chaijamorn W, Clark JS, Mueller BA: Preparation times and costs for various solutions used for continuous renal replacement therapy. Am J Health Syst Pharm 75: 808–815, 2018 [DOI] [PubMed] [Google Scholar]
- 18.Thompson Bastin ML, Adams PM, Nerusu S, Morris PE, Mayer KP, Neyra JA: Association of phosphate containing solutions with incident hypophosphatemia in critically ill patients requiring continuous renal replacement therapy. Blood Purif 51: 122–129, 2022 [DOI] [PubMed] [Google Scholar]
- 19.Crowley KE, DeGrado JR, Charytan DM: Serum glucose and phosphorus concentrations during continuous renal replacement therapy using commercial replacement solutions with or without phosphorus. Hemodial Int 24: 330–334, 2020 [DOI] [PubMed] [Google Scholar]
- 20.Godaly G, Carlsson O, Broman M: Phoxilium(®) reduces hypophosphataemia and magnesium supplementation during continuous renal replacement therapy. Clin Kidney J 9: 205–210, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Besnard N, Serveaux M, Machado S, Daubin D, Brunot V, Amigues L, Landreau L, Jonquet O, Klouche K: Electrolytes-enriched hemodiafiltration solutions for continuous renal replacement therapy in acute kidney injury: A crossover study. Blood Purif 42: 18–26, 2016 [DOI] [PubMed] [Google Scholar]
- 22.Schoenfeld DA, Bernard GR; ARDS Network : Statistical evaluation of ventilator-free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Crit Care Med 30: 1772–1777, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Blackwood B, Ringrow S, Clarke M, Marshall JC, Connolly B, Rose L, McAuley DF: A core outcome set for critical care ventilation trials. Crit Care Med 47: 1324–1331, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Navarro D: Learning Statistics with R: A Tutorial for Psychology Students and Other Beginners, 4th Ed., Adelaide, Australia, University of Adelaide, 2014 [Google Scholar]
- 25.Wilson GT: Time Series Analysis: Forecasting and Control, 5th Edition, by George E. P. Box, Gwilym M. Jenkins, Gregory C. Reinsel and Greta M. Ljung, 2015. Published by John Wiley and Sons Inc., Hoboken, New Jersey, pp. 712. ISBN: 978-1-118-67502-1. J Time Ser Anal 37: 709–711, 2016 [Google Scholar]
- 26.Shor R, Halabe A, Rishver S, Tilis Y, Matas Z, Fux A, Boaz M, Weinstein J: Severe hypophosphatemia in sepsis as a mortality predictor. Ann Clin Lab Sci 36: 67–72, 2006 [PubMed] [Google Scholar]
- 27.Amanzadeh J, Reilly RF Jr.: Hypophosphatemia: An evidence-based approach to its clinical consequences and management. Nat Clin Pract Nephrol 2: 136–148, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Camp MA, Allon M: Severe hypophosphatemia in hospitalized patients. Miner Electrolyte Metab 16: 365–368, 1990 [PubMed] [Google Scholar]
- 29.Gravelyn TR, Brophy N, Siegert C, Peters-Golden M: Hypophosphatemia-associated respiratory muscle weakness in a general inpatient population. Am J Med 84: 870–876, 1988 [DOI] [PubMed] [Google Scholar]
- 30.Newman JHNT, Neff TA, Ziporin P: Acute respiratory failure associated with hypophosphatemia. N Engl J Med 296: 1101–1103, 1977 [DOI] [PubMed] [Google Scholar]
- 31.Jung B, Moury PH, Mahul M, de Jong A, Galia F, Prades A, Albaladejo P, Chanques G, Molinari N, Jaber S: Diaphragmatic dysfunction in patients with ICU-acquired weakness and its impact on extubation failure. Intensive Care Med 42: 853–861, 2016 [DOI] [PubMed] [Google Scholar]
- 32.Goligher EC, Dres M, Fan E, Rubenfeld GD, Scales DC, Herridge MS, Vorona S, Sklar MC, Rittayamai N, Lanys A, Murray A, Brace D, Urrea C, Reid WD, Tomlinson G, Slutsky AS, Kavanagh BP, Brochard LJ, Ferguson ND: Mechanical ventilation-induced diaphragm atrophy strongly impacts clinical outcomes. Am J Respir Crit Care Med 197: 204–213, 2018 [DOI] [PubMed] [Google Scholar]
- 33.Supinski GS, Callahan LA: Diaphragm weakness in mechanically ventilated critically ill patients. Crit Care 17: R120, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharma S, Brugnara C, Betensky RA, Waikar SS: Reductions in red blood cell 2,3-diphosphoglycerate concentration during continuous renal replacment therapy. Clin J Am Soc Nephrol 10: 74–79, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aubier M, Murciano D, Lecocguic Y, Viires N, Jacquens Y, Squara P, Pariente R: Effect of hypophosphatemia on diaphragmatic contractility in patients with acute respiratory failure. N Engl J Med 313: 420–424, 1985 [DOI] [PubMed] [Google Scholar]
- 36.Chanutin A, Hermann E: The interaction of organic and inorganic phosphates with hemoglobin. Arch Biochem Biophys 131: 180–184, 1969 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.