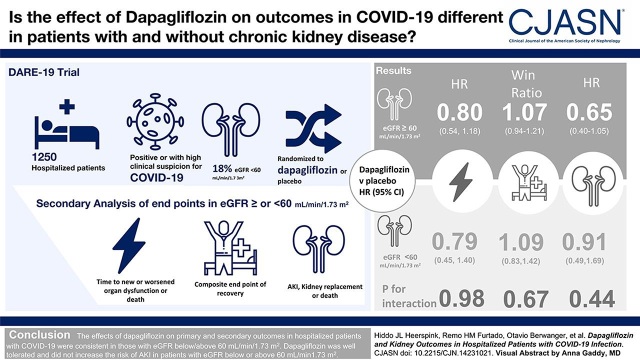
Visual Abstract
Keywords: acute kidney injury, chronic kidney disease, COVID-19, diabetes, heart failure, hospitalization, mortality risk, outcomes, randomized controlled trials, cardiovascular disease
Abstract
Background and objectives
Patients who were hospitalized with coronavirus disease 2019 (COVID-19) infection are at high risk of AKI and KRT, especially in the presence of CKD. The Dapagliflozin in Respiratory Failure in Patients with COVID-19 (DARE-19) trial showed that in patients hospitalized with COVID-19, treatment with dapagliflozin versus placebo resulted in numerically fewer participants who experienced organ failure or death, although these differences were not statistically significant. We performed a secondary analysis of the DARE-19 trial to determine the efficacy and safety of dapagliflozin on kidney outcomes in the overall population and in prespecified subgroups of participants defined by baseline eGFR.
Design, setting, participants, & measurements
The DARE-19 trial randomized 1250 patients who were hospitalized (231 [18%] had eGFR <60 ml/min per 1.73 m2) with COVID-19 and cardiometabolic risk factors to dapagliflozin or placebo. Dual primary outcomes (time to new or worsened organ dysfunction or death, and a hierarchical composite end point of recovery [change in clinical status by day 30]), and the key secondary kidney outcome (composite of AKI, KRT, or death), and safety were assessed in participants with baseline eGFR <60 and ≥60 ml/min per 1.73 m2.
Results
The effect of dapagliflozin versus placebo on the primary prevention outcome (hazard ratio, 0.80; 95% confidence interval, 0.58 to 1.10), primary recovery outcome (win ratio, 1.09; 95% confidence interval, 0.97 to 1.22), and the composite kidney outcome (hazard ratio, 0.74; 95% confidence interval, 0.50 to 1.07) were consistent across eGFR subgroups (P for interaction: 0.98, 0.67, and 0.44, respectively). The effects of dapagliflozin on AKI were also similar in participants with eGFR <60 ml/min per 1.73 m2 (hazard ratio, 0.71; 95% confidence interval, 0.29 to 1.77) and ≥60 ml/min per 1.73 m2 (hazard ratio, 0.69; 95% confidence interval, 0.37 to 1.29). Dapagliflozin was well tolerated in participants with eGFR <60 and ≥60 ml/min per 1.73 m2.
Conclusions
The effects of dapagliflozin on primary and secondary outcomes in hospitalized participants with COVID-19 were consistent in those with eGFR below/above 60 ml/min per 1.73 m2. Dapagliflozin was well tolerated and did not increase the risk of AKI in participants with eGFR below or above 60 ml/min per 1.73 m2.
Introduction
Although coronavirus disease 2019 (COVID-19) is primarily a respiratory infection, it frequently manifests as a systemic illness, affecting multiple organ systems. COVID-19 frequently results in disease progression and organ damage, especially in patients with underlying cardiometabolic risk factors, such as diabetes, heart failure, and CKD (1–3). More specifically, AKI is one of the common complications of COVID-19, especially among patients who are acutely hospitalized with cardiometabolic risk factors (4). Furthermore, when AKI occurs in the setting of COVID-19, it is associated with high mortality. Two studies report in-hospital mortality rates of approximately 60% in patients who were hospitalized with COVID-19 who required AKI-related KRT (5,6). To date, few (if any) therapies have been specifically developed and tested in COVID-19 randomized controlled trials in terms of their effects on kidney outcomes, including AKI.
Sodium-glucose cotransporter 2 (SGLT2) inhibitors have been shown in large cardiovascular and kidney outcome trials to reduce the risk of kidney failure in patients who are mostly ambulatory and have type 2 diabetes, and in those with CKD and heart failure, regardless of diabetes status (7–9). Systematic reviews and meta-analyses from these large clinical trials have suggested protective effects against AKI (10). SGLT2 inhibitors decrease proinflammatory and oxidative stress pathways, suppress cytokines, and improve endothelial function and oxygen carrying capacity, which may prevent organ damage and improve AKI outcomes after COVID-19 infection (11,12). Inflammation, oxidative stress, and endothelial dysfunction are frequently present in patients with CKD and are implicated in the progressive decline of kidney function in these patients, suggesting that the relative and absolute benefits of SGLT2 inhibitors may be more pronounced in patients with COVID-19 and CKD (13,14). However, whether SGLT2 inhibitors can prevent AKI and improve kidney outcomes in patients with CKD hospitalized with acute illness has not been previously investigated in clinical trials. In fact, soon after the emergence of the COVID-19 pandemic, there were concerns raised about their continued use in a setting of acute hospitalization for COVID-19, due to the potential risk of volume depletion (possibly increasing the risk of AKI) and diabetic ketoacidosis (15).
The Dapagliflozin in Respiratory Failure in Patients with COVID-19 (DARE-19) trial was designed to assess the efficacy and safety of the SGLT2 inhibitor dapagliflozin in 1250 patients with cardiometabolic risk factors acutely hospitalized with COVID-19 (16). The trial demonstrated that dapagliflozin was well tolerated but did not result in a statistically significant risk reduction in the primary outcomes of organ dysfunction or death or improvement in recovery (17). We report the effects of dapagliflozin on the composite kidney outcome and AKI, a key component of the composite kidney outcome, in prespecified subgroups of participants by baseline eGFR.
Materials and Methods
Study Design
The DARE-19 trial methods and primary results were published previously (16,17). Briefly, DARE-19 was an investigator-initiated, multicenter, international trial in which patients acutely hospitalized with COVID-19 were randomly assigned (1:1) in a double-blind manner to either dapagliflozin 10 mg or matching placebo daily, in addition to local standard of care, and treated for 30 days. The trial was registered with ClinicalTrials.gov (NCT04350593) on April 17, 2020. The Research Ethics Committee of the 95 participating institutions in seven countries approved the protocol; all participants gave written informed consent, and the study adhered to the Declaration of Helsinki. The corresponding and senior authors had full access to all of the trial data and take responsibility for its integrity and the data analysis.
Study Participants
Participants were enrolled if they were ≥18 years of age, hospitalized with laboratory-confirmed or clinically suspected severe acute respiratory syndrome–related coronavirus 2 infection ≤4 days before screening, had oxygen saturation of ≥94% on supplemental oxygen (≤5 L/min), chest radiography or computed tomography findings consistent with COVID-19, and history of at least one cardiometabolic risk factor (hypertension, type 2 diabetes, atherosclerotic cardiovascular disease, heart failure [regardless of ejection fraction], and/or CKD [eGFR 25–59 ml/min per 1.73 m2]). Key exclusion criteria were evidence of critical illness at the time of screening, eGFR <25 ml/min per 1.73 m2 or acute kidney failure, type 1 diabetes, and history of diabetic ketoacidosis.
Monitoring of Kidney Function
During hospitalization, monitoring of kidney function was required at screening, day 1, day 3, and day 15 (or day of discharge). Baseline eGFR was defined as the value at screening. In addition, all other assessments of kidney function performed as a part of routine clinical care during index hospitalization were also recorded in the case report forms. After discharge from index hospitalization, no additional laboratory evaluation of kidney function was required; however, participants were monitored for serious adverse events (SAEs) of AKI via phone calls at day 15 and day 30 (as applicable); if the participant had experienced a subsequent SAE after discharge (including any hospitalization), the investigator reviewed the associated medical records (source documents) and determined if the event was an AKI.
Outcomes
The DARE-19 trial had dual primary outcomes. The first primary outcome was prevention and defined as a composite of time to new or worsened respiratory, cardiovascular, or kidney organ dysfunction during the index hospitalization, or death from any cause at any time during the 30-day treatment period. For this primary outcome, worsening kidney function was defined as doubling of serum creatinine or initiation of KRT during index hospitalization. After the original protocol was designed, the rapid change in the standard of care for treatment of COVID-19 resulted in lower event rates. Accordingly, faster and more complete recovery became an important treatment goal and a frequently used trial end point in patients hospitalized with COVID-19. The DARE-19 protocol was therefore amended by the Executive Committee (which remained fully blinded) to elevate the recovery from a secondary end point to a dual primary outcome. The recovery end point was defined as a hierarchical composite end point, which ranked participants into categories using the severity and timing of events during the 30-day treatment period: death during the 30 days of follow-up, organ dysfunction during the index hospitalization, supplemental oxygen requirement for participants hospitalized at day 30 without organ dysfunction, and hospital discharge before day 30 without in-hospital organ dysfunction event and alive at day 30.
A key secondary outcome was a composite kidney end point, which included AKI (defined as doubling of serum creatinine during index hospitalization or investigator-reported SAE of AKI after discharge), initiation of KRT, or death from any cause. For participants who experienced more than one kidney event during follow-up, survival time to the first relevant end point was used in each analysis. For the purposes of this analysis, we also performed post hoc exploratory analyses to assess the effect of dapagliflozin compared with placebo on kidney function (eGFR values) over time, using both protocol-mandated laboratory evaluations and all local standard of care eGFR assessments calculated with the CKD Epidemiology Collaboration equation (18). eGFR values after hospital discharge or dialysis initiation were not included in the analysis.
Safety outcomes were on-treatment reportable SAEs, adverse events leading to study medication discontinuation, and adverse events of interest, which included AKI and diabetic ketoacidosis.
All events were investigator reported. Rigorous measures were implemented to ensure data quality, including source data verification for reported outcome and safety events and thorough review of events to ensure compliance with the protocol definitions.
Statistical Analysis
Efficacy analyses were performed in the intention-to-treat population, including all randomized participants. A Cox proportional-hazards regression model, stratified by country and adjusted for age and sex, was used to calculate the hazard ratios (HR) and 95% confidence interval (95% CI) for the primary outcome of prevention. This outcome was then assessed within prespecified subgroups of participants by baseline eGFR (25–59 [<60] versus ≥60 ml/min per 1.73 m2) with the use of the same stratified Cox regression models, and with the interaction term (treatment assignment * eGFR subgroup) also added for the interaction test. Kaplan–Meier estimates were used for visualization purposes.
The hierarchical primary outcome of recovery was analyzed using a win ratio (19) and 95% CI estimated from a Cox regression model (stratified by country), applied to ranks (with larger ranks for worse outcomes). The P value for this analysis was calculated using a country-stratified log-rank test. This outcome was then also assessed within prespecified subgroups by baseline eGFR (<60 versus ≥60 ml/min per 1.73 m2) with the use of the same country-stratified Cox regression models, and with the interaction term (treatment assignment * eGFR subgroup) also added for the interaction test.
The key secondary kidney composite outcome (AKI, KRT, or death from any cause) was examined using a Cox proportional-hazard regression model, stratified by country, and adjusted for baseline eGFR. This outcome (and its individual components) was then assessed within prespecified subgroups of participants by baseline eGFR (<60 versus ≥60 ml/min per 1.73 m2) using the same country-stratified Cox regression model, with the interaction term (treatment assignment * eGFR subgroup) also added for the interaction test.
eGFR values over time, using both protocol-mandated laboratory assessments at baseline and days 1, 3, and discharge (or day 15) and all combined protocol-mandated laboratory assessments with available standard of care laboratory values, were plotted using least squares mean values and standard errors estimated from general linear model with repeated measures, adjusted for baseline eGFR values, visit (day in hospital), randomized treatment, and interaction of treatment and visit.
Safety analyses were performed on randomized participants who received at least one dose of study medication. All analyses were performed with SAS software, version 9.4 (SAS Institute). A P value <0.05 was considered to indicate statistical significance.
Results
Baseline Characteristics
In total, 231 (18%) of the 1250 randomized patients had an eGFR <60 ml/min per 1.73 m2 at baseline. The baseline characteristics of randomized patients stratified by eGFR (≥60 or <60 ml/min per 1.73 m2) are shown in Table 1. The proportion of women and distribution by race were similar across eGFR subgroups. Randomized participants with eGFR <60 ml/min per 1.73 m2 were older and more likely to have type 2 diabetes, heart failure, atherosclerotic cardiovascular disease, hypertension, and chronic obstructive pulmonary disease, although they were less likely to be obese compared with individuals with an eGFR ≥60 ml/min per 1.73 m2. Baseline characteristics were well balanced between the dapagliflozin and placebo groups in participants with eGFR above or below 60 ml/min per 1.73 m2.
Table 1.
Demographic, clinical characteristics, and inclusion risk factors of the participants in the Dapagliflozin in Respiratory Failure in Patients with COVID-19 trial at baseline according to baseline eGFRa
| Characteristics | eGFR <60 ml/min per 1.73 m2b | eGFR ≥60 ml/min per 1.73 m2b | ||||
|---|---|---|---|---|---|---|
| Dapagliflozin (n=115) | Placebo (n=116) | Total (n=231) | Dapagliflozin (n=498) | Placebo (n=490) | Total (n=988) | |
| Age, mean (SD), yrs | 71 (11) | 71 (11) | 71 (11) | 59 (13) | 60 (13) | 59 (13) |
| Female sex, n (%) | 51 (44) | 53 (46) | 104 (45) | 205 (41) | 214 (44) | 419 (42) |
| Race, n (%) c | ||||||
| American Indian or Alaska Native | 1 (1) | 1 (1) | 2 (1) | 6 (1) | 9 (2) | 15 (2) |
| Asian | 5 (4) | 7 (6) | 12 (5) | 30 (6) | 22 (4) | 52 (5) |
| Black | 10 (9) | 13 (11) | 23 (10) | 74 (15) | 69 (14) | 143 (14) |
| Native Hawaiian or other Pacific Islander | 0 | 0 | 0 | 1 (0.2) | 0 | 1 (0.1) |
| White | 96 (83) | 86 (74) | 182 (79) | 349 (70) | 363 (74) | 712 (72) |
| Other | 3 (3) | 9 (8) | 12 (5) | 38 (8) | 27 (6) | 65 (7) |
| Ethnicity, n (%) c | ||||||
| Hispanic or Latino | 63 (56) | 58 (50) | 121 (53) | 323 (65) | 296 (60) | 619 (63) |
| Not Hispanic or Latino | 36 (32) | 42 (36) | 78 (34) | 130 (26) | 135 (28) | 265 (27) |
| Not reported/unknown | 16 (14) | 16 (14) | 32 (14) | 45 (9) | 59 (12) | 104 (11) |
| Inclusion risk factors, n (%) | ||||||
| Type 2 diabetes | 65 (57) | 62 (53) | 127 (55) | 240 (48) | 253 (52) | 493 (50) |
| Heart failure | 19 (17) | 13 (11) | 32 (14) | 24 (5) | 29 (6) | 53 (5) |
| Hypertension | 106 (92) | 103 (89) | 209 (90) | 409 (82) | 417 (85) | 826 (84) |
| Atherosclerotic cardiovascular disease | 28 (24) | 27 (23) | 55 (24) | 64 (13) | 73 (15) | 137 (14) |
| Known CKD (eGFR 25–60 ml/min per 1.73 m2)d | 25 (22) | 37 (32) | 62 (27) | 12 (2) | 7 (1) | 19 (2) |
| Participants with two or more inclusion risk factors, n (%) | 82 (71) | 80 (69) | 162 (70) | 203 (41) | 229 (47) | 432 (48) |
| Other risk factors, n (%) | ||||||
| Age ≥60 years | 102 (89) | 98 (84) | 200 (87) | 231 (46) | 257 (52) | 488 (49) |
| BMI ≥30 | 51 (44) | 45 (39) | 96 (42) | 240 (48) | 251 (51) | 491 (50) |
| Chronic obstructive pulmonary disease | 9 (8) | 6 (5) | 15 (6) | 15 (3) | 24 (5) | 39 (4) |
| Current smoker | 3 (3) | 5 (4) | 8 (3) | 26 (5) | 15 (3) | 41 (4) |
| Vitals signs, mean (SD) | ||||||
| Heart rate, beats/min | 80 (17) | 78 (14) | 79 (15) | 79 (13) | 80 (13) | 80 (13) |
| Blood pressure, mm Hg | ||||||
| Systolic | 127 (19) | 130 (22) | 128 (20) | 127 (15) | 126 (15) | 126 (15) |
| Diastolic | 76 (14) | 77 (13) | 77 (13) | 77 (10) | 76 (10) | 76 (10) |
| Temperature, °C | 36 (1) | 36 (1) | 36 (1) | 36 (1) | 36 (1) | 36 (1) |
| Oxygen saturation, %e | 95 (2) | 95 (2) | 95 (2) | 96 (2) | 95 (2) | 95 (2) |
| Laboratory values at baseline, mean (SD) | ||||||
| eGFR (ml/min per 1.73 m2) | 46 (11) | 47 (10) | 47 (10) | 93 (18) | 92 (18) | 93 (18) |
| Severe acute respiratory syndrome–related coronavirus 2 test result at baseline, n (%) | ||||||
| Positive | 103 (93) | 112 (97) | 215 (95) | 469 (96) | 448 (94) | 917 (95) |
| Negative | 8 (7) | 4 (3) | 12 (5) | 22 (4) | 29 (6) | 51 (5) |
| Medication at screening, n (%) | ||||||
| ACE inhibitor and/or ARB | 46 (40) | 45 (39) | 91 (39) | 175 (35) | 165 (34) | 340 (34) |
| Beta blocker | 37 (32) | 27 (23) | 64 (28) | 56 (11) | 68 (14) | 124 (13) |
| Calcium blocker | 21 (18) | 22 (19) | 43 (19) | 61 (12) | 65 (13) | 126 (13) |
| Loop diuretic | 25 (22) | 22 (19) | 47 (20) | 24 (5) | 40 (8) | 64 (6) |
| Statin | 30 (26) | 33 (28) | 63 (27) | 91 (18) | 104 (21) | 195 (20) |
| Glucose-lowering medication | ||||||
| Biguanide | 15 (13) | 14 (12) | 29 (13) | 66 (13) | 60 (12) | 126 (13) |
| Sulfonylurea | 8 (7) | 3 (3) | 11 (5) | 16 (3) | 18 (4) | 34 (3) |
| DPP-4 inhibitor | 4 (3) | 1 (0.9) | 5 (2) | 13 (3) | 9 (2) | 22 (2) |
| GLP-1 receptor analog | 4 (3) | 1 (0.9) | 5 (2) | 2 (0.4) | 7 (1) | 9 (0.9) |
| Insulin | 40 (35) | 43 (37) | 83 (36) | 182 (37) | 171 (35) | 353 (36) |
| Anticoagulant | 96 (83) | 99 (85) | 195 (84) | 421 (85) | 413 (84) | 834 (84) |
| Concomitant COVID-19 medication | ||||||
| Remdesivir | 23 (20) | 21 (18) | 44 (19) | 91 (18) | 90 (18) | 181 (18) |
| Systemic corticosteroids | ||||||
| Dexamethasone | 24 (21) | 22 (19) | 46 (20) | 106 (21) | 104 (21) | 210 (21) |
| Other systemic glucocorticoid | 12 (10) | 13 (11) | 25 (11) | 37 (7) | 39 (8) | 76 (8) |
BMI, body mass index; ACE, angiotensin-converting enzyme; ARB, angiotensin-receptor blocker; DPP-4, dipeptidyl-peptidase 4; GLP-1, glucagon-like peptide-1.
Numbers may differ for some parameters on the basis of data availability.
eGFR recorded at screening.
Reported by the participant.
Known history of CKD before admission. The number of participants with known history of CKD is less than those with eGFR <60 ml/min per 1.73 m2 at baseline possibly due to unknown CKD status.
Measured on supplemental oxygen.
Primary and Secondary Outcomes by Baseline eGFR
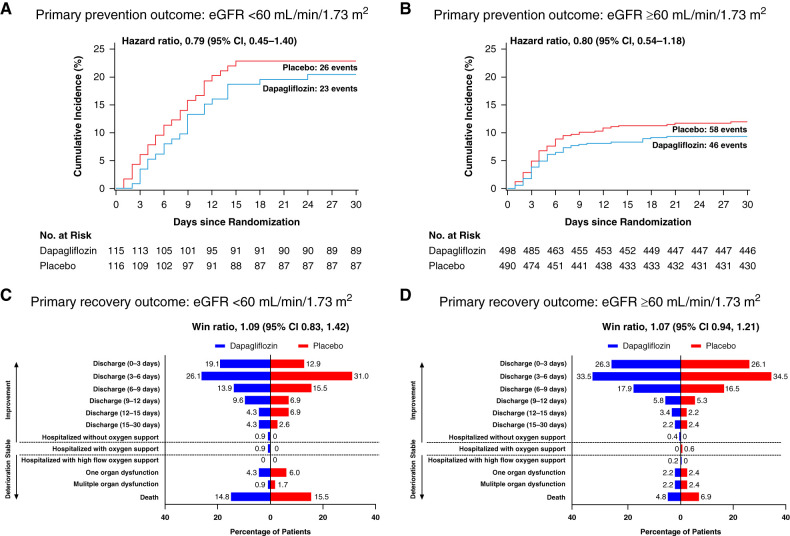
The results for both primary end points were consistent within subgroups of participants with eGFR below or above 60 ml/min per 1.73 m2. The HR for the primary outcome of prevention was 0.79 (95% CI, 0.45 to 1.40) and 0.80 (95% CI, 0.54 to 1.18; P for interaction=0.98; Figure 1, A and B) in participants with eGFR <60 and ≥60 ml/min per 1.73 m2, respectively. The win ratio for the primary outcome of recovery was 1.09 (95% CI, 0.83 to 1.42) and 1.07 (95% CI, 0.94 to 1.21; P for interaction=0.67) in participants with eGFR <60 and ≥60 ml/min per 1.73 m2, respectively (Figure 1, C and D).
Figure 1.
Effects of dapagliflozin compared with placebo on the primary outcome in participants with eGFR <60 and ≥60 ml/min per 1.73 m2. The prevention outcome is shown in participants with baseline eGFR <60 ml/min per 1.73 m2 (A) and ≥60 ml/min per 1.73 m2 (B). The recovery outcome is shown in participants with baseline eGFR <60 ml/min per 1.73 m2 (C) and ≥60 ml/min per 1.73 m2 (D). 95% CI, 95% confidence interval.
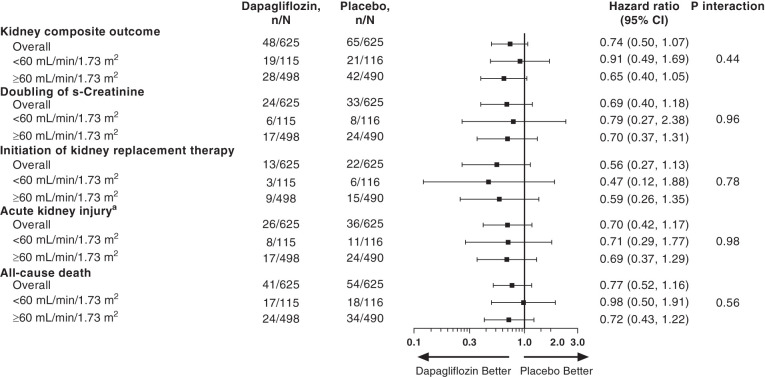
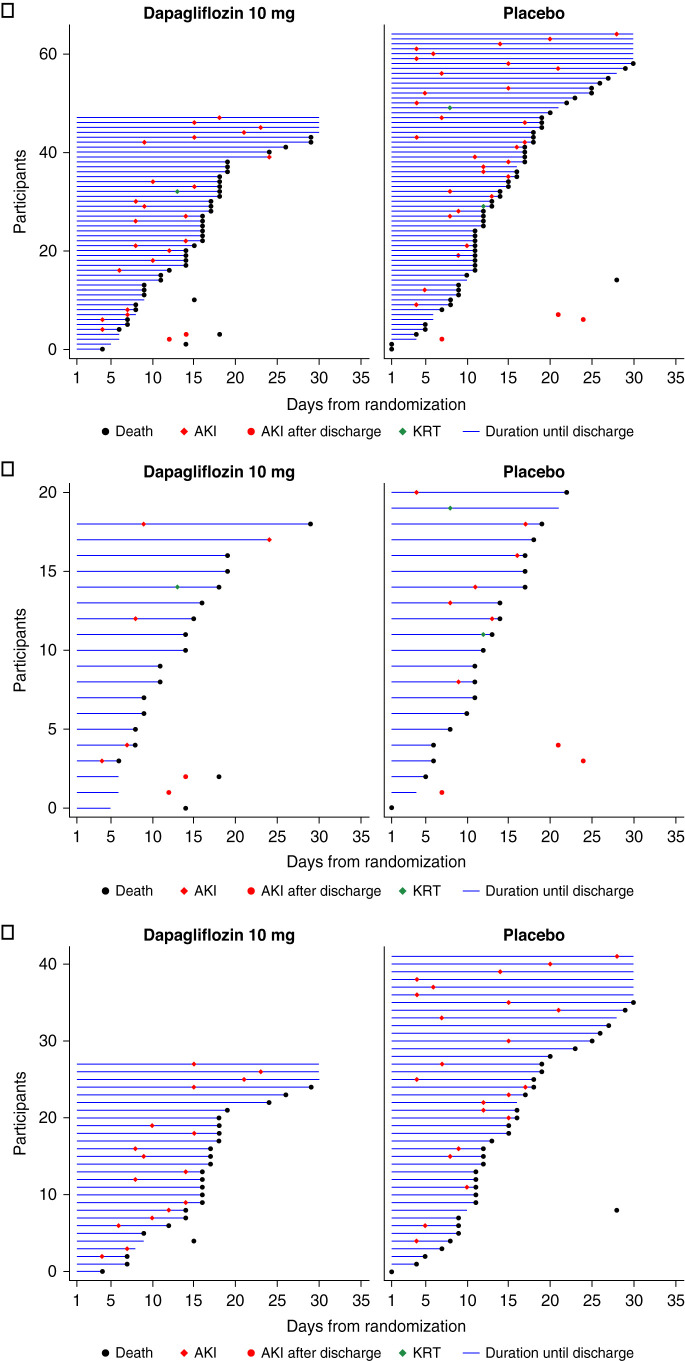
The key secondary composite kidney outcome occurred in 48 participants (8%) in the dapagliflozin group and 65 (10%) in the placebo group (HR, 0.74; 95% CI, 0.50 to 1.07; Figure 2). Of these, 23 participants in the dapagliflozin and 30 in the placebo group died without experiencing a kidney outcome. A total of 25 participants in the dapagliflozin group and 35 in the placebo group experienced a kidney outcome, of whom 18 in the dapagliflozin and 23 in the placebo group subsequently died (Figure 3). Examining the composite kidney outcome by baseline kidney function, in those with baseline eGFR <60 ml/min per 1.73 m2, 19 participants (17%) in the dapagliflozin group and 21 (18%) in the placebo group experienced this outcome (HR, 0.91; 95% CI, 0.49 to 1.69). In those with eGFR ≥60 ml/min per 1.73 m2, 28 participants (6%) in the dapagliflozin group and 42 (9%) in the placebo group experienced this outcome (HR, 0.65; 95% CI, 0.40 to 1.05; P for interaction=0.44; Figure 2). The point estimates of each individual component of the composite outcome are also shown in Figure 2. The effects of dapagliflozin versus placebo on AKI (including both doubling of serum creatinine during hospitalization or SAE of AKI postdischarge) were consistent in participants with eGFR <60 ml/min per 1.73 m2 (HR, 0.71; 95% CI, 0.29 to 1.77) and ≥60 ml/min per 1.73 m2 (HR, 0.69; 95% CI, 0.37 to 1.29; P for interaction=0.98). The majority of participants (39, 68%) who experienced AKI during the study subsequently died (Figure 3A). This was true for participants assigned to dapagliflozin and placebo and for those with eGFR <60 ml/min per 1.73 m2 and ≥60 ml/min per 1.73 m2 (Figure 3, B and C).
Figure 2.
Effects of dapagliflozin compared with placebo on the kidney composite outcome and its component in the overall population and in participants with baseline eGFR <60 and ≥60 ml/min per 1.73 m2. aDefined as either a doubling of serum creatinine during hospitalization or an adverse event of AKI after discharge through to day 30. Participants may experience multiple composite kidney outcomes. The total number of each component are reported in the forest plot. One participant in the placebo group died after withdrawing informed consent. This participant was included in the analysis of all-cause mortality, but not included in the analysis of the composite kidney outcome, because the occurrence of AKI or initiation of KRT could not be ascertained in this participant after withdrawal of consent.
Figure 3.
“Swimmer plots” of time to death or hospital discharge for participants with a composite kidney end point event. Data are shown by the overall population (A) and in participants with eGFR <60 (B) and ≥60 ml/min per 1.73 m2 (C). Plots present the first event of AKI or KRT.
When examining doubling of serum creatinine during index hospitalization, the HR was 0.79 (95% CI, 0.27 to 2.38) for participants with eGFR <60 ml/min per 1.73 m2 and 0.70 (95% CI, 0.37 to 1.31; P for interaction=0.96; Figure 2) for participants with eGFR ≥60 ml/min per 1.73 m2. Overall, 13 participants in the dapagliflozin and 22 in the placebo group initiated KRT with a corresponding HR in participants with eGFR <60 and ≥60 ml/min per 1.73 m2 of 0.47 (95% CI, 0.12 to 1.88) and 0.59 (95% CI, 0.26 to 1.35), respectively (P for interaction=0.78; Figure 2). The number of participants who died during the study was 41 (7%) in the dapagliflozin group and 54 (9%) in the placebo group, with no evidence that this effect was modified by baseline eGFR (P for interaction=0.56). There was also no heterogeneity in the effect of dapagliflozin compared with placebo on the secondary kidney outcome in other prespecified subgroups including type 2 diabetes, heart failure, atherosclerotic cardiovascular disease, hypertension, or chronic obstructive pulmonary disease status (Supplemental Figure 1).
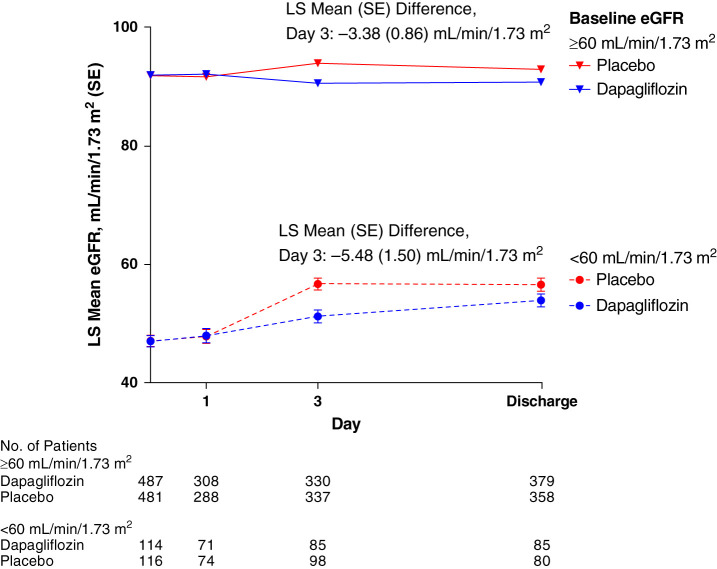
eGFR over Time
Mean (SD) baseline eGFR values were 84 (25) ml/min per 1.73 m2 in the dapagliflozin group and 83 (25) ml/min per 1.73 m2 in the placebo group. The eGFR values over time were slightly lower in participants treated with dapagliflozin compared with placebo, whether examining protocol-mandated laboratory values or all available data (Figure 4 and Supplemental Figure 2). As illustrated in Supplemental Figure 3, there was a wide variation in eGFR changes from baseline to day 3 in the dapagliflozin and placebo groups. In participants with baseline eGFR <60 ml/min per 1.73 m2, the least squares mean change from baseline in eGFR at 3 days was 4.12 ml/min per 1.73 m2 in the dapagliflozin group and 9.57 ml/min per 1.73 m2 in the placebo group (between group difference, −5.48 ml/min per 1.73 m2; 95% CI, −8.43 to −2.53) (Figure 4). In participants with baseline eGFR ≥60 ml/min per 1.73 m2, it was −1.32 ml/min per 1.73 m2 in the dapagliflozin group and 2.09 ml/min per 1.73 m2 in the placebo group (between group difference, −3.38 ml/min per 1.73 m2; 95% CI, −5.06 to −1.70) (Figure 4). Small numerical between-group differences in eGFR were sustained throughout follow-up (Supplemental Figure 2).
Figure 4.
Least squares mean eGFR (standard error) at protocol mandated timepoints in participants with eGFR <60 and ≥60 ml/min per 1.73 m2.
Safety by Baseline eGFR
Numerically fewer participants treated with dapagliflozin versus placebo experienced SAEs, adverse events that led to death, or adverse events of AKI; this was observed regardless of baseline eGFR status, although none of these differences reached statistical significance (Table 2). Diabetic ketoacidosis did not occur among participants with eGFR <60 ml/min per 1.73 m2 and was reported in two participants (0.4%) with eGFR ≥60 ml/min per 1.73 m2 who had a history of type 2 diabetes and were receiving dapagliflozin. These were SAEs but were considered nonsevere and resolved after discontinuation of the study medication.
Table 2.
Safety outcomes by baseline eGFR and treatment assignment
| eGFR <60 ml/min per 1.73 m2 | eGFR ≥60 ml/min per 1.73 m2 | |||||
|---|---|---|---|---|---|---|
| Dapagliflozin (n=114) | Placebo (n=116) | Risk Difference, % (95% Confidence Interval) | Dapagliflozin (n=487) | Placebo (n=481) | Risk Difference, % (95% Confidence Interval) | |
| Any SAEa | 22 (19.3) | 28 (24.1) | −4.8 (−15.5 to 5.9) | 42 (8.6) | 52 (10.8) | −2.2 (−6.0 to 1.6) |
| AE with the outcome of death | 13 (11.4) | 14 (12.1) | −0.7 (−9.3 to 8.0) | 19 (3.9) | 32 (6.7) | −2.8 (−5.7 to 0.1) |
| Discontinuation due to AE | 17 (14.9) | 15 (12.9) | 2.0 (−7.2 to 11.2) | 26 (5.3) | 38 (7.9) | −2.6 (−5.8 to 0.6) |
| AE of interest | ||||||
| AKIb | 7 (6.1) | 11 (9.5) | −3.3 (−10.9 to 3.9) | 14 (2.9) | 22 (4.6) | −1.7 (−4.3 to 0.7) |
| Diabetic ketoacidosis | 0 | 0 | — | 2 (0.4) | 0 | 0.4 (−0.4 to 1.5) |
Safety analyses were performed on randomized participants who received at least one dose of study medication. Risk difference for dapagliflozin versus placebo, confidence intervals, and two-sided P value are calculated from the score test for two independent proportions. SAE, serious adverse event; AE, adverse event.
Includes death.
Defined in hospital as doubling of s-creatinine compared with baseline and in an outpatient setting as a reported serious adverse event of AKI.
Discussion
In the DARE-19 trial, which evaluated patients who were hospitalized with COVID-19 and cardiometabolic risk factors, dapagliflozin compared with placebo did not result in a statistically significant reduction in the primary outcome of organ failure or death and the key secondary composite kidney outcome (AKI, KRT, or death from any cause), although numerically fewer participants treated with dapagliflozin experienced these outcomes (17). In this analysis, we demonstrate that these observations were consistent regardless of baseline eGFR (below or above 60 ml/min per 1.73 m2). Importantly, dapagliflozin was well tolerated in these acutely ill participants, with a safety profile that was also consistent in those with or without eGFR <60 ml/min per 1.73 m2.
Dapagliflozin is indicated for the treatment of type 2 diabetes, CKD, and heart failure with reduced ejection fraction according to current clinical practice guidelines from endocrinology, nephrology, and cardiology societies (20,21). In addition, SGLT2 inhibitors as a class are emerging as an integral component for the treatment of CKD and heart failure, with an increasing number of patients already using these agents or being considered for initiation in the near future. Despite their robust demonstrated outcome benefits in these patient groups under more stable, ambulatory conditions, the contemporary practice is to routinely discontinue SGLT2 inhibitors in patients who are acutely hospitalized due to potential risks of dehydration, AKI, and diabetic ketoacidosis (15). However, adequate randomized controlled clinical trial evidence to substantiate these recommendations are lacking. In DARE-19, the largest clinical trial to date examining initiation of an SGLT2 inhibitor in patients hospitalized with acute infectious illness and at high risk for complications, dapagliflozin was well tolerated even among participants with eGFR <60 ml/min per 1.73 m2, suggesting that routine discontinuation of SGLT2 inhibitors in this clinical setting may not be necessary as long as patients are adequately monitored.
Although the effect of dapagliflozin compared with placebo on the secondary kidney outcome (and its components) in the DARE-19 trial did not reach statistical significance, the direction and magnitude of the effect were generally inline and consistent with other dedicated outcome trials of SGLT2 inhibitors in patients with heart failure and CKD (with and without type 2 diabetes) (8,9). Furthermore, the mechanisms by which SGLT2 inhibitors slow the progression of kidney function decline and reduce the risks of KRT may be similar in patients who are ambulatory with CKD and those with acute illnesses. SGLT2 inhibitors exert favorable effects on proinflammatory cytokines, including those involved in COVID-19–induced cytokine release (22). Moreover, beneficial effects of SGLT2 inhibitors on oxidative stress, endothelial function, glycolysis, and lipolysis can protect the kidney in the ambulatory and acute settings. However, despite the consistency in the effects of dapagliflozin in our trial and other trials and a sound mechanistic rationale for how dapagliflozin may reduce complications in patients who are hospitalized with COVID-19, we cannot rule out a chance finding due to the lack of statistical significance.
In our trial, we defined a doubling of serum creatinine during hospitalization compared with baseline as a prespecified component of AKI. This definition is consistent with Kidney Disease Improving Global Outcomes–defined stage 2 AKI and has been associated with a high risk of KRT and mortality (23). In the DARE-19 trial, the rate of AKI events in participants with eGFR <60 ml/min per 1.73 m2 was approximately twice as high compared with those with eGFR ≥60 ml/min per 1.73 m2, consistent with previous studies (7,8). In both subgroups, the HRs for dapagliflozin versus placebo-treated participants were of similar magnitude as that observed in two other kidney and cardiovascular outcome trials with dapagliflozin using the same definition for AKI (9,24). This is further buttressed by the directionally consistent results observed with dapagliflozin for other components of the key secondary kidney outcome (including KRT and death from any cause). Of importance, when AKI occurred, and regardless of treatment allocation, the risk of mortality was high in the DARE-19 participants in accordance with previous reports (4,25). It is noteworthy that the secondary composite kidney outcome included few participants who died and was therefore not driven by the mortality outcome.
In patients who are stable with or without type 2 diabetes, SGLT2 inhibitors cause an acute decline in eGFR that is followed by a marked attenuation of kidney function loss during prolonged treatment (9,26). Among the participants in the DARE-19 trial, eGFR values in the dapagliflozin group were slightly lower compared with placebo, consistent with data from trials of stable individuals with diabetes, heart failure, and CKD. We speculate that these findings indicate that the slight initial decline in eGFR seen with SGLT2 inhibitors in the acute setting is not a harbinger of increased AKI risk, similar to what has been observed in ambulatory settings.
The results of this study should be interpreted in the context of limitations that merit consideration. First, the rates of organ dysfunction and death were lower than initially anticipated, and the study did not achieve statistical significance; therefore, the subgroup analyses should be interpreted with caution, in particular given the small number of events within the subgroups on the basis of baseline eGFR. Accordingly, no definitive conclusions can be drawn regarding the effects of dapagliflozin on kidney outcomes in patients who are hospitalized with COVID-19. Ongoing clinical trials such as Accelerating COVID-19 Therapeutic Interventions and Vaccines 4 ACUTE (ACTIV-4A) (NCT04505774) and Randomised Evaluation of COVID-19 Therapy (RECOVERY) (NCT04381936) will provide more definitive evidence about the efficacy and safety of SGLT2 inhibitors in patients who are hospitalized with COVID-19. Second, DARE-19 participants were selected on the basis of detailed inclusion and exclusion criteria and other factors that influence participation in trials. This generally leads to a more selective, lower-risk population than the all-comers population and may limit generalizability. Furthermore, a small proportion of participants (<8%) did not have a confirmatory positive test for COVID-19 due to the lack of testing supplies at the beginning of the trial. Finally, SAEs of AKI were ascertained using follow-up phone visits after hospital discharge; the associated records were then reviewed by the local investigator who determined if the event qualified as an AKI. The SAE collection via phone visits may have led to some AKI events postdischarge being missed. However, it is unlikely this has influenced the treatment effect estimates because of the double-blind design and standardized definitions.
In conclusion, the effects of dapagliflozin compared with placebo in hospitalized participants with COVID-19 on the primary and secondary outcomes were consistent in those with eGFR below or above 60 ml/min per 1.73 m2. Dapagliflozin was well tolerated and did not increase the risk of AKI in the overall population and in participants with eGFR below or above 60 ml/min per 1.73 m2.
Disclosures
A. Javaheri reports receiving a research grant from AstraZeneca to study the effects of dapagliflozin in inflammatory models; previously holding stock options in DexCom (minor financial interest, not related to this work) and Pfizer; having three pending patents (not directly related to this work) for the use of fusion protein nanodiscs for the treatment of heart failure, the use of apolipoprotein M to treat macular degeneration, and inhibition of lysosomal lipolysis to treat ischemia; having an ownership interest in AAL, Amazon, amc, Appian, Appl, AT&T, BA, BaO, bigC, Cerence, Chegg, CMG, Costco, Crwd, Csco, Datadog, Dexcom, Dis, Docn, Docusign, Domino’s, Etsy, Everbridge, Exel, Expedia, farfetch, Fastly, Fiverr, Four, fubo, Global Payments, Globus Medical, Google, IDXX, jd.com, Knsl, lmnd, lspd, mgni, Moderna, Monday, Mtch, Nflx, Now, NVDA, Pfizer, pltr, Pypl, rdfn, Rivian, Roku, Sbux, Shopify, Snap, Snow, TCA, tdoc, Trade Desk, TSLA, Twilio, Twitter, UCO, Uipath, Upstart, Versus, vygvf, Wayfair, ZM, and Zs; receiving research funding from AstraZeneca, Castle Biosciences, and Regeneron; receiving honoraria from Castle Biosciences; and reports their spouse has consultancy agreements with Castle Biosciences. A.M. Langkilde reports being an employee and stockholder of AstraZeneca. C. Morse reports a clinical trial agreement to participate as a study site in this trial; consultancy agreements with Viiv Healthcare; and receiving research funding as clinical trial investigator in COVID studies supported by Gilead, Janssen, Moderna, and Ridgeback Biotherapeutics and as clinical trial investigator in HIV trials supported by Gilead. F. Martinez reports having employment with the DAMIC Institute; consultancy agreements with AstraZeneca, Bayer, Bristol Myers Squibb (BMS), Boehringer Ingelheim, and Novartis; receiving research funding from St. Lukes University, Kansas City; receiving honoraria from AstraZeneca, Bayer, BMS, Milestone, and Novartis; receiving personal fees from AstraZeneca during the conduct of the study; serving in an advisory or leadership role for European Cardiology Reviews; and speakers bureau for AstraZeneca, Boehringer Ingelheim, and Novartis. F. Tang reports employment with Amgen and having an ownership interest in Amazon, Amgen, CRM, Facebook, Home Depot, and Lowes. G.G. Koch reports being the principal investigator for a biostatistics grant from AstraZeneca; being the principal investigator for biostatistics grants from other biopharmaceutical sponsors that have no relationship to the submitted work; having an ownership interest in IQVIA; receiving research funding from AbbVie, Acceleron, Amgen, Arena, AstraZeneca, Cytokinetics, Eli Lilly, Gilead Sciences, GlaxoSmithKline (GSK), HUYA, Johnson & Johnson, Landos Biopharma, Merck, Momentum, Novartis, Oppilan Pharma, Otsuka, Pfizer Inc., Sanofi, Union Chimique Belge (UCB), and vTv Therapeutics; and serving in an advisory or leadership role for the Journal of Biopharmaceutical Statistics, Pharmaceutical Statistics, and Statistics in Medicine. H.J.L. Heerspink reports ongoing consultancy agreements with AbbVie, AstraZeneca, Bayer, Boehringer Ingelheim, Chinook, CSL Behring, Dimerix, Fresenius, Gilead, Janssen, Merck, Mundi Pharma, Mitsubishi Tanabe, Novo Nordisk, Retrophin, and Travere Pharmaceuticals; receiving research funding from AbbVie, AstraZeneca, Boehringer Ingelheim, and Janssen research support (grant funding directed to employer); and serving on speakers bureau for AstraZeneca. J. Buenconsejo reports being an employee and stockholder of AstraZeneca and reports other interests or relationships with American Statistical Association and Drug Information Association. J. Oscarsson reports being an employee and stockholder of AstraZeneca. M.N. Kosiborod reports having consultancy agreements with Alnylam, Amgen, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Esperion Therapeutics, Janssen, Lexicon, Merck (Diabetes), Novartis, Novo Nordisk, Pharmacosmos, Sanofi, and Vifor Pharma; receiving a research grant for the conduct of this study from AstraZeneca; receiving research funding from AstraZeneca and Boehringer Ingelheim; receiving honoraria from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Janssen, Merck (Diabetes), Novartis, Novo Nordisk, Sanofi, and Vifor Pharma; and serving on the Editorial Boards of JACC and Circulation. M. Twahirwa reports serving on speakers bureau for AstraZeneca. P. Ambery reports being an employee and stockholder of AstraZeneca and having a dosing patent for Cotadutide (AstraZeneca drug). O. Berwanger reports receiving research funding from Amgen, AstraZeneca, Bayer, BMS, Boehringer Ingelheim, Novartis, Pfizer, and Servier. R. A. Maldonado reports receiving a payment/honoraria from AstraZeneca. R. Esterline reports being an employee and stockholder of AstraZeneca. R. Frankel reports consultancy agreements with AstraZeneca; stock/stock options in Boston Scientific; receiving honoraria from AstraZeneca, Boston Scientific, and Medtronic; serving in a leadership/fiduciary role in the American Medical Association, the Medical Society of the State of New York, and the Empire Foundation; and speakers bureau for AstraZeneca and Medtronic. R.H.M. Furtado reports receiving research grants from Aché, AstraZeneca, Bayer, Brazilian Ministry of Health, CytoDin, EMS Healthcare, Pfizer, Servier, University Health Network (received from his institution), and Lemann Foundation Research Fellowship; honoraria from AstraZeneca, Bayer, and Servier; serving in an advisory or leadership role for Biomm; and speakers bureau for AstraZeneca, Bayer, and Servier. S.B. Gasparyan reports being an employee and stockholder of AstraZeneca. S. Verma reports receiving research funding from Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, HLS Therapeutics, Janssen, Merck, Novo Nordisk, Pfizer, and PhaseBio; receiving speaker honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, EOCI, HLS Therapeutics, Janssen, Merck, Novartis, Novo Nordisk, Pfizer, PhaseBio, Sanofi, Sun Pharmaceuticals, and Toronto Knowlege Translation Working Group (TKTWG); has received research and/or speaking honoraria from Amarin, Amgen, AstraZeneca, Bayer, CMS, HLS Therapeutics, Janssen, Merck, Novartis, Novo Nordisk, PhaseBio, and Sanofi; serving on advisory boards for Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, HLS Therapeutics, Janssen, Merck, Novartis, Novo Nordisk, and Sanofi; holds the Tier 1 Canada Research Chair in Cardiovascular Surgery; and reports being the President of the Canadian Medical and Surgical Knowledge Translation Research Group, a federally incorporated not-for-profit physician organization. V.C. de Souza-Dantas reports receipt of drugs from AstraZeneca for the conduct of this study. All remaining authors have nothing to disclose.
Funding
This work was supported by AstraZeneca Pharmaceuticals LP.
Supplementary Material
Acknowledgments
We thank all patients, investigators, and their teams, and George Clinical for their participation in the trial and extraordinary efforts under the most difficult of circumstances due to the ongoing pandemic. We also thank Dr. Nicola Truss and Dr. Róisín O’Connor, both of inScience Communications (London, UK), for editorial assistance, funded by AstraZeneca, and Mr. Srivathsa Ravikiran, Dr. Damian Kruszewski, and Mr. Tomasz Kotecki for AstraZeneca programming support. These data were presented as a poster at the American Society of Nephrology Kidney Week, November 4–7, 2021.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Learnings from Throwing Paint at the Wall for COVID-19 with an SGLT2 Inhibitor,” on pages 628–630.
Author Contributions
P. Ambery, O. Berwanger, J. Buenconsejo, R. Esterline, S. Gasparyan, A. Javaheri, G. Koch, M. Kosiborod, A. Langkilde, F. Martinez, O. Mukhtar, J. Oscarsson, and S. Verma conceptualized the study; O. Berwanger, J. Buenconsejo, V. Cés de Souza-Dantas, M. del Sueldo, R. Frankel, R. Furtado, S. Gasparyan, A. Javaheri, G. Koch, M. Kosiborod, R. Maldonado, F. Martinez, C. Morse, M. Mota-Gomes, O. Mukhtar D. Shemin, O. Silva, F. Tang, A. Tognon, M. Twahirwa, S. Verma, and S. Windsor were responsible for the data curation; J. Buenconsejo, S. Gasparyan, and F. Tang were responsible for the formal analysis; M. Kosiborod and S. Windsor were responsible for the funding acquisition; O. Berwanger, V. Cés de Souza-Dantas, R. Furtado, M. del Sueldo, R. Frankel, A. Javaheri, G. Koch, M. Kosiborod, R. Maldonado, F. Martinez, C. Morse, M. Mota-Gomes, O. Mukhtar D. Shemin, O. Silva, A. Tognon, M. Twahirwa, S. Windsor, and S. Verma were responsible for the investigation; O. Berwanger, R. Esterline, S. Gasparyan, G. Koch, M. Kosiborod, F. Martinez, O. Mukhtar, A. Langkilde, J. Oscarsson, F. Tang, and S. Verma were responsible for the methodology; R. Esterline, S. Gasparyan, M. Kosiborod, J. Oscarsson, and S. Windsor were responsible for the project administration; J. Buenconsejo, R. Esterline, S. Gasparyan, M. Kosiborod, A. Langkilde, and S. Windsor provided supervision; S. Gasparyan was responsible for the validation; H. Heerspink and M. Kosiborod were responsible for the visualization; H. Heerspink and M. Kosiborod wrote the original draft; and P. Ambery, O. Berwanger, J. Buenconsejo, V. Cés de Souza-Dantas, M. del Sueldo, R. Esterline, R. Frankel, R. Furtado, S. Gasparyan, H. Heerspink, A. Javaheri, G. Koch, M. Kosiborod, A. Langkilde, F. Martinez, R. Maldonado, C. Morse, M. Mota-Gomes, O. Mukhtar, J. Oscarsson, D. Shemin, O. Silva, F. Tang, A. Tognon, M. Twahirwa, S. Verma, and S. Windsor reviewed and edited the manuscript. All authors gave final approval of the manuscript, made the decision to submit for publication, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Data Sharing Statement
Deidentified participant data will be made available on reasonable request 2 years after the date of publication. Requests should be directed to the senior author (mkosiborod@saint-lukes.org). Requestors will be required to sign a data access agreement to ensure the appropriate use of the study data.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.14231021/-/DCSupplemental.
Supplemental Figure 1. The secondary kidney outcome by prespecified subgroups.
Supplemental Figure 2. Least squares mean eGFR (standard error) for all recorded local standard of care assessments in participants with eGFR <60 and ≥60 ml/min per 1.73 m2.
Supplemental Figure 3. Histogram of the change in eGFR from baseline to day 3 in participants with baseline eGFR <60 ml/min per 1.73 m2 (A) and those with baseline eGFR ≥60 ml/min per 1.73 m2 (B).
References
- 1.Tajbakhsh A, Gheibi Hayat SM, Taghizadeh H, Akbari A, Inabadi M, Savardashtaki A, Johnston TP, Sahebkar A: COVID-19 and cardiac injury: Clinical manifestations, biomarkers, mechanisms, diagnosis, treatment, and follow up. Expert Rev Anti Infect Ther 19: 345–357, 2021 [DOI] [PubMed] [Google Scholar]
- 2.Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, Curtis HJ, Mehrkar A, Evans D, Inglesby P, Cockburn J, McDonald HI, MacKenna B, Tomlinson L, Douglas IJ, Rentsch CT, Mathur R, Wong AYS, Grieve R, Harrison D, Forbes H, Schultze A, Croker R, Parry J, Hester F, Harper S, Perera R, Evans SJW, Smeeth L, Goldacre B: Factors associated with COVID-19-related death using OpenSAFELY. Nature 584: 430–436, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holman N, Knighton P, Kar P, O’Keefe J, Curley M, Weaver A, Barron E, Bakhai C, Khunti K, Wareham NJ, Sattar N, Young B, Valabhji J: Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: A population-based cohort study. Lancet Diabetes Endocrinol 8: 823–833, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oliveira CB, Lima CAD, Vajgel G, Campos Coelho AV, Sandrin-Garcia P: High burden of acute kidney injury in COVID-19 pandemic: Systematic review and meta-analysis. J Clin Pathol 74: 796–803, 2021 [DOI] [PubMed] [Google Scholar]
- 5.Gupta S, Coca SG, Chan L, Melamed ML, Brenner SK, Hayek SS, Sutherland A, Puri S, Srivastava A, Leonberg-Yoo A, Shehata AM, Flythe JE, Rashidi A, Schenck EJ, Goyal N, Hedayati SS, Dy R, Bansal A, Athavale A, Nguyen HB, Vijayan A, Charytan DM, Schulze CE, Joo MJ, Friedman AN, Zhang J, Sosa MA, Judd E, Velez JCQ, Mallappallil M, Redfern RE, Bansal AD, Neyra JA, Liu KD, Renaghan AD, Christov M, Molnar MZ, Sharma S, Kamal O, Boateng JO, Short SAP, Admon AJ, Sise ME, Wang W, Parikh CR, Leaf DE; STOP-COVID Investigators : AKI treated with renal replacement therapy in critically ill patients with COVID-19. J Am Soc Nephrol 32: 161–176, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan L, Chaudhary K, Saha A, Chauhan K, Vaid A, Zhao S, Paranjpe I, Somani S, Richter F, Miotto R, Lala A, Kia A, Timsina P, Li L, Freeman R, Chen R, Narula J, Just AC, Horowitz C, Fayad Z, Cordon-Cardo C, Schadt E, Levin MA, Reich DL, Fuster V, Murphy B, He JC, Charney AW, Böttinger EP, Glicksberg BS, Coca SG, Nadkarni GN; Mount Sinai COVID Informatics Center (MSCIC) : AKI in hospitalized patients with COVID-19. J Am Soc Nephrol 32: 151–160, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde A-M, Sabatine MS; DECLARE–TIMI 58 Investigators : Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 380: 347–357, 2019 [DOI] [PubMed] [Google Scholar]
- 8.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu P-L, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW; CREDENCE Trial Investigators : Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 380: 2295–2306, 2019 [DOI] [PubMed] [Google Scholar]
- 9.Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou F-F, Mann JFE, McMurray JJV, Lindberg M, Rossing P, Sjöström CD, Toto RD, Langkilde A-M, Wheeler DC; DAPA-CKD Trial Committees and Investigators : Dapagliflozin in patients with chronic kidney disease. N Engl J Med 383: 1436–1446, 2020 [DOI] [PubMed] [Google Scholar]
- 10.Neuen BL, Young T, Heerspink HJL, Neal B, Perkovic V, Billot L, Mahaffey KW, Charytan DM, Wheeler DC, Arnott C, Bompoint S, Levin A, Jardine MJ: SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: A systematic review and meta-analysis. Lancet Diabetes Endocrinol 7: 845–854, 2019 [DOI] [PubMed] [Google Scholar]
- 11.Heerspink HJL, Perco P, Mulder S, Leierer J, Hansen MK, Heinzel A, Mayer G: Canagliflozin reduces inflammation and fibrosis biomarkers: A potential mechanism of action for beneficial effects of SGLT2 inhibitors in diabetic kidney disease. Diabetologia 62: 1154–1166, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Solini A, Giannini L, Seghieri M, Vitolo E, Taddei S, Ghiadoni L, Bruno RM: Dapagliflozin acutely improves endothelial dysfunction, reduces aortic stiffness and renal resistive index in type 2 diabetic patients: A pilot study. Cardiovasc Diabetol 16: 138, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cachofeiro V, Goicochea M, de Vinuesa SG, Oubiña P, Lahera V, Luño J: Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease. Kidney Int Suppl 74: S4–S9, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Roumeliotis S, Mallamaci F, Zoccali C: Endothelial dysfunction in chronic kidney disease, from biology to clinical outcomes: A 2020 update. J Clin Med 9: E2359, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bornstein SR, Rubino F, Khunti K, Mingrone G, Hopkins D, Birkenfeld AL, Boehm B, Amiel S, Holt RI, Skyler JS, DeVries JH, Renard E, Eckel RH, Zimmet P, Alberti KG, Vidal J, Geloneze B, Chan JC, Ji L, Ludwig B: Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol 8: 546–550, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kosiborod M, Berwanger O, Koch GG, Martinez F, Mukhtar O, Verma S, Chopra V, Javaheri A, Ambery P, Gasparyan SB, Buenconsejo J, Sjöström CD, Langkilde AM, Oscarsson J, Esterline R: Effects of dapagliflozin on prevention of major clinical events and recovery in patients with respiratory failure because of COVID-19: Design and rationale for the DARE-19 study. Diabetes Obes Metab 23: 886–896, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kosiborod MN, Esterline R, Furtado RHM, Oscarsson J, Gasparyan SB, Koch GG, Martinez F, Mukhtar O, Verma S, Chopra V, Buenconsejo J, Langkilde AM, Ambery P, Tang F, Gosch K, Windsor SL, Akin EE, Soares RVP, Moia DDF, Aboudara M, Hoffmann Filho CR, Feitosa ADM, Fonseca A, Garla V, Gordon RA, Javaheri A, Jaeger CP, Leaes PE, Nassif M, Pursley M, Silveira FS, Barroso WKS, Lazcano Soto JR, Nigro Maia L, Berwanger O: Dapagliflozin in patients with cardiometabolic risk factors hospitalised with COVID-19 (DARE-19): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol 9: 586–594, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gasparyan SB, Folkvaljon F, Bengtsson O, Buenconsejo J, Koch GG: Adjusted win ratio with stratification: Calculation methods and interpretation. Stat Methods Med Res 30: 580–611, 2021 [DOI] [PubMed] [Google Scholar]
- 20.American Diabetes Association : 9. Pharmacologic approaches to glycemic treatment: Standards of medical care in diabetes 2021. Diabetes Care 44[Suppl 1]: S111–S124, 2021 [DOI] [PubMed] [Google Scholar]
- 21.Seferović PM, Fragasso G, Petrie M, Mullens W, Ferrari R, Thum T, Bauersachs J, Anker SD, Ray R, Çavuşoğlu Y, Polovina M, Metra M, Ambrosio G, Prasad K, Seferović J, Jhund PS, Dattilo G, Čelutkiene J, Piepoli M, Moura B, Chioncel O, Ben Gal T, Heymans S, Jaarsma T, Hill L, Lopatin Y, Lyon AR, Ponikowski P, Lainščak M, Jankowska E, Mueller C, Cosentino F, Lund LH, Filippatos GS, Ruschitzka F, Coats AJS, Rosano GMC: Heart Failure Association of the European Society of Cardiology update on sodium-glucose co-transporter 2 inhibitors in heart failure. Eur J Heart Fail 22: 1984–1986, 2020 [DOI] [PubMed] [Google Scholar]
- 22.Bonnet F, Scheen AJ: Effects of SGLT2 inhibitors on systemic and tissue low-grade inflammation: The potential contribution to diabetes complications and cardiovascular disease. Diabetes Metab 44: 457–464, 2018 [DOI] [PubMed] [Google Scholar]
- 23.Kidney Disease Improving Global Outcomes (KDIGO) : KDIGO Clinical Practice Guideline for Acute Kidney Injury. 2012. Available at: https://kdigo.org/guidelines/acute-kidney-injury/. Accessed August 12, 2021
- 24.McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang C-E, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O’Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde A-M; DAPA-HF Trial Committees and Investigators : Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 381: 1995–2008, 2019 [DOI] [PubMed] [Google Scholar]
- 25.Chen YT, Shao SC, Hsu CK, Wu IW, Hung MJ, Chen YC: Incidence of acute kidney injury in COVID-19 infection: A systematic review and meta-analysis. Crit Care 24: 346, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mosenzon O, Wiviott SD, Cahn A, Rozenberg A, Yanuv I, Goodrich EL, Murphy SA, Heerspink HJL, Zelniker TA, Dwyer JP, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Kato ET, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS, Raz I: Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: An analysis from the DECLARE-TIMI 58 randomised trial. Lancet Diabetes Endocrinol 7: 606–617, 2019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.