Abstract
Infections of wound sites on dicot plants by Agrobacterium tumefaciens result in the formation of crown gall tumors. An early step in tumor formation is bacterial attachment to the plant cells. AttR mutants failed to attach to wound sites of both legumes and nonlegumes and were avirulent on both groups of plants. AttR mutants also failed to attach to the root epidermis and root hairs of nonlegumes and had a markedly reduced ability to colonize the roots of these plants. However, AttR mutants were able to attach to the root epidermis and root hairs of alfalfa, garden bean, and pea. The mutant showed little reduction in its ability to colonize these roots. Thus, A. tumefaciens appears to possess two systems for binding to plant cells. One system is AttR dependent and is required for virulence on all of the plants tested and for colonization of the roots of all of the plants tested except legumes. Attachment to root hairs through this system can be blocked by the acetylated capsular polysaccharide. The second system is AttR independent, is not inhibited by the acetylated capsular polysaccharide, and allows the bacteria to bind to the roots of legumes.
Infections of wound sites on dicotyledonous plants by the soil bacterium Agrobacterium tumefaciens result in the formation of crown gall tumors. The ability of the bacteria to attach to plant cells is required for virulence. All known nonattaching bacterial mutants are avirulent (1, 2, 7, 15). In previous research we have identified a region of the bacterial chromosome (att) containing a number of genes required for attachment to host cells and for virulence (10, 11). One of these genes (attR) encodes a putative transacetylase. AttR mutants lack an acetylated capsular polysaccharide found in the wild-type parent strain (14). Preparations of this polysaccharide were able to block the binding of wild-type bacteria to carrot suspension culture cells (14). This polysaccharide is believed to play a role in the virulence-associated binding of the bacteria to host cells.
Agrobacteria are capable of living in the rhizosphere as well as in plant wounds. We have previously examined the colonization by A. tumefaciens of the roots of two plants (tomato and Arabidopsis thaliana) which are susceptible to the induction of crown gall tumors (9). When nonattaching mutants of A. tumefaciens (including AttR mutants) were compared to wild-type bacteria, the mutants were found to have more than 10,000-fold-reduced ability to colonize the roots of tomato, suggesting that att genes are involved in the colonization of intact roots as well as in bacterial attachment at wound sites and in virulence.
Previous studies of nonattaching mutants of agrobacteria carried out in our laboratory and by others have used a variety of cells and organs from plants, including tobacco, tomato, carrot, zinnia, A. thaliana, and Bryophyllum daigremontiana (1, 2, 6–8, 12, 14). When we extended the study of the interaction of attachment of wild-type A. tumefaciens and att mutants to include legumes, we discovered that the roles of att genes in bacterial attachment and root colonization may differ depending on the plant host. We report here the results of examining the interactions of an AttR mutant of A. tumefaciens with various legumes.
MATERIALS AND METHODS
Bacterial strains and media.
Wild-type A. tumefaciens C58 and two AttR mutants of C58, A205 and A610, have been described previously (7, 10). All of these strains were resistant to rifampin. The A. tumefaciens strains were grown at 25°C in Luria broth. Carbenicillin and rifampin were added to the medium as needed at 50 μg/ml. The growth rates of C58 and A205 in cultures grown on a shaker at 200 rpm at room temperature (22 to 25°C) in Luria broth and in H4 minimal medium containing 0.2% glucose (7) were measured using the optical density at 600 nM and viable cell counts on Luria agar.
Plant material and microscopic studies.
Seeds of tomato (Lycopersicum esculentum cv. Marglobe) and A. thaliana ecotype Landsberg erecta were germinated as previously described (9). Alfalfa (Medicago sativa) and pea (Pisum sativa cv. Green Arrow) seeds were surface sterilized by soaking them in 0.3% sodium hypochlorite containing 1 drop of Tween 80 as a wetting agent for 20 min and washing them three times with sterile water. Seeds of garden bean (Phaseolus vulgaris cv. Great Northern) were surface sterilized by soaking them in 95% ethanol for 5 min followed by 25 min in 5.25% sodium hypochlorite and three sterile water washes of 5 min each. The seeds were germinated in sterile water. Root segments or entire roots 1 to 2 cm in length were cut from all plants except A. thaliana when the roots were about 2 to 3 cm long, and two to six segments were placed in 2 ml of sterile 0.4% sucrose–1 mM CaCl2 in a 35-mm-diameter petri dish. About 50 μl of a suspension of a stationary-phase culture of bacteria (approximately 108 bacteria) was added to the roots, and the mixture was incubated for 24 to 72 h at room temperature. The roots were then removed and placed in water in a Sedgwick-Rafter counting cell (A. H. Thomas Co.) or a probe clip press-seal incubation chamber (Sigma Chemical Co.) for observation with a Zeiss photoscope 2 using Nomarski optics. Roots of A. thaliana were incubated similarly except that they were used when they were 0.5 to 2 cm long and were cut into 0.5-cm-long pieces.
For studies of the effects of bacterial polysaccharides on attachment, the polysaccharide fraction dissolved in water was added to alfalfa or A. thaliana root segments 10 min prior to the addition of the bacteria.
Bacterial attachment.
Bacterial attachment to root segments was measured as previously described (7, 14). Briefly, 10 to 15 aseptic root segments 2 to 3 cm long were placed in 5 ml of a sterile 1/10 dilution of MS salts (Gibco BRL) containing 0.4% sucrose. Bacteria from a fresh overnight culture grown in Luria broth were diluted in phosphate-buffered saline and added to a final concentration of 3 × 103 to 6 × 103 bacteria/ml. The mixture was incubated at room temperature and sampled at the time of inoculation and after 2 h. Samples were filtered through Miracloth filters to separate free bacteria from root segments and any sloughed plant cells. The number of viable free bacteria was determined by plating the filtrate on Luria agar. The number of bound bacteria was determined by collecting the material retained on the filter, resuspending it in the original volume of MS salts, and grinding it in a Waring blender for 1 min followed by plating the grindate on Luria agar. Grinding in a Waring blender had no effect on the viability of test solutions containing known numbers of viable bacteria. Fewer than 10 bacteria/ml were recovered from the grindate of the material retained on the filter from samples taken immediately after inoculation of the bacteria. No bacterial growth was observed during the course of the incubation (2 h), presumably due to the nutritional shiftdown caused by the change in medium from Luria broth to a 1/10 dilution of MS salts plus sucrose. There was also no bacterial death observed; the total number of bacteria recovered after 2 h was not significantly different from the number of bacteria inoculated for both C58 and A205.
Root colonization.
Root colonization assays were carried out as previously described (9). Briefly, aseptic plants whose roots were 1 to 2 cm long were dipped for 1 min into a suspension of 105 bacteria per ml in 0.1 M sodium phosphate buffer (pH 7.0) containing 0.1% peptone (washing buffer) (5). A 1-min incubation time is sufficient for the formation of a water film containing bacteria on the surfaces of the roots but is not long enough to allow significant bacterial attachment to the root surface (9). The inoculated intact plants were placed in soil in conetainers (Stuewe and Sons, Inc., Corvallis, Oreg.). The soil was a Gilead loamy sand which had been pasteurized by heating it in a microwave oven in sealed bags and had been stored for more than 4 weeks before use, according to the procedure of Ferriss (9). In some experiments the seedlings were planted in sterile quartz sand which had been wetted with 15% (wt/wt) sterile 1/10 dilution of MS salts. The bottom and top of the conetainer were sealed with parafilm. The plants were grown without additional water at 22 to 24°C with a 14-hour day for up to 10 days. At various times after inoculation, the plants and soil or sand were removed from the conetainer, the soil or sand was carefully separated from the root, and the root was gently shaken. The root was placed in 5 ml of washing buffer in a vial, and the vial was sealed and sonicated in a Branson ultrasonic glassware cleaner, model B220, for 120 s. Viable cell counts of the solution were made using Luria agar containing 50 μg of rifampin/ml. The plant was then removed from the buffer, placed on a Luria agar plate containing rifampin, and covered with soft (0.7%) agar. The root length was measured, the plates were incubated until colonies were apparent, and the number of colonies per centimeter of root length was determined with a dissecting microscope. The results are expressed as CFU per centimeter of root length rather than per gram of root weight, since the drying necessary to obtain reproducible root weights interfered with the recovery of viable bacteria, which were so tightly bound to the root that they failed to be removed by sonication. The number of CFU per centimeter of root length was calculated and transformed to log10 CFU per centimeter (5) prior to calculating the mean and standard deviation. Experiments were repeated four times with a minimum of four plants per time interval.
Virulence assays.
The virulence of A205 (an AttR mutant) was determined on leaves of B. daigremontiana by inoculating toothpick wounds on the leaves with a paste of bacteria. The virulence on carrot root disks was determined as described by Klein and Tenenbaum (3). A similar assay technique was used for pea and bean root disks. For virulence assays, root disks were cut from aseptic roots of pea and bean and placed on sterile 1% water agar containing MS salts. Carrot root disks were cut from carrots purchased in a local supermarket. The carrots were washed with soap and water followed by 95% ethanol and surface sterilized by soaking them in 0.3% sodium hypochlorite for 20 min. The carrots were washed in sterile water; the epidermis was cut off, and aseptic root disks about 5 to 10 mm thick cut from the carrots were placed on 1% water agar. The root disks were inoculated with a drop of a stationary-phase culture of A. tumefaciens grown in Luria broth placed on the top of the disk and were scored after 4 to 6 weeks of incubation. Virulence on bean leaves was examined by the assay of Lippincott and Heberlein (4). Virulence on bean, alfalfa, tomato, and pea stems was examined by infecting needle wounds in the stems of greenhouse-grown plants with a paste of bacteria from a Luria agar plate mixed with 5 μl of sterile water. The inoculation sites were wrapped with small pieces of parafilm to prevent drying.
Bacterial polysaccharide preparation.
The PW/W-2 acidic acetylated capsular polysaccharide fraction prepared from the wild-type bacterial strain C58 described by Reuhs et al. (14) was added to A. thaliana and alfalfa root segments as described above. This fraction does not contain detectable lipopolysaccharide, exopolysaccharide, lipid, or protein as determined by nuclear magnetic resonance (14). It does contain KDO (ketodeoxyoctanate) or KDO-like sugars, neutral sugars, and phosphate. The estimated molecular mass of this polysaccharide is between 3 and 5 kDa. It appears to be heavily acetylated (14). The addition of 100 μg of this polysaccharide fraction/ml to A. thaliana root segments prior to the addition of wild-type bacteria inhibits the binding of the bacteria to root hairs, epidermis, and cut ends (14).
RESULTS
Virulence of wild-type and AttR mutant bacteria.
Wild-type A. tumefaciens strain C58 was virulent on tomato, pea, bean, and alfalfa stems; pea, bean, and carrot root disks; and B. daigremontiana and bean leaves. This strain had reduced virulence on alfalfa, bean, and pea stems and bean and pea root disks: between 46 and 75% of the inoculated sites formed tumors. On tomato, carrot, and B. daigremontiana, all of the inoculated sites formed tumors. The AttR mutant A205 was avirulent on all these plants and organs (Fig. 1 and 2 and Table 1). A second AttR mutant, A610, was also avirulent on B. daigremontiana and alfalfa (data not shown).
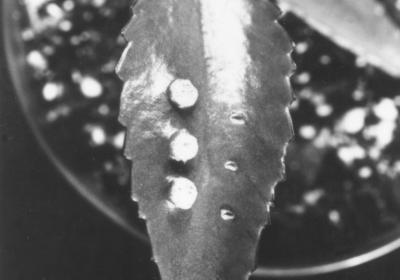
FIG. 1.
Virulence of wild-type and C58::A205 (attR) bacteria inoculated on leaves of B. daigremontiana. Bacteria were inoculated into toothpick wounds on the leaves. The leaf was photographed after 6 weeks of growth. The right side of the leaf was inoculated with the AttR mutant A205, and the left side was inoculated with the wild-type strain, C58. Tumors formed at the sites of inoculation of C58 but not at the sites of inoculation of A205.
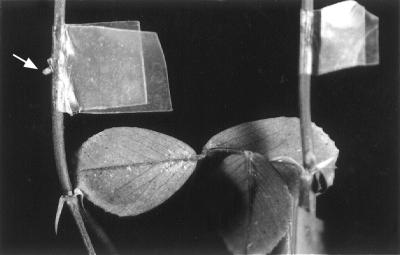
FIG. 2.
Virulence of wild-type and AttR mutant bacteria on alfalfa stems. Bacteria were inoculated into needle wounds in alfalfa stems. The sites of inoculation were wrapped with parafilm to prevent drying. The stems were photographed 5 weeks later. The stem on the left was inoculated with the wild-type strain, C58, and the stem on the right was inoculated with the AttR mutant A205. The arrow points to the small tumor formed at the site of inoculation. About 50% of the sites inoculated with C58 formed tumors. None of the sites inoculated with A205 formed tumors.
TABLE 1.
Virulence of A. tumefaciens strains on various plants
| Plant and organ | Virulencef
|
|
|---|---|---|
| C58 (wild type)b | A205 (AttR mutant)g | |
| B. daigremontiana leavesa | 100 ± 5 | 0 (80) |
| Carrot root disksc | 100 ± 10 | 0 (25) |
| Tomato stemsa | 100 ± 5 | 0 (40) |
| Bean stemsa | 75 ± 15 | 0 (10) |
| Pea stemsa | 75 ± 20 | 0 (15) |
| Alfalfa stemsc | 46 ± 10 | 0 (35) |
| Bean leavesd | 100 ± 10 | 0 (16) |
| Bean root diskse | 75 ± 20 | 0 (10) |
| Pea root diskse | 40 ± 15 | 0 (10) |
Tumors were scored 6 weeks after inoculation.
Mean ± standard deviation of a minimum of three experiments.
Tumors were scored 8 weeks after inoculation. Each disk was scored as positive or negative. The average carrot root disk inoculated with C58 had about 20 tumors.
Tumors were scored 1 week after inoculation. Each leaf was scored as positive or negative. The average leaf inoculated with C58 had about five tumors.
Tumors were scored 4 weeks after inoculation. Each root disk was scored as positive or negative. The average bean or pea root disk inoculated with C58 had about three tumors.
Percentage of inoculated sites developing tumors after inoculation with bacterial strain.
Numbers in parentheses represent the number of plants scored. No tumors were ever detected in any of these inoculations.
Growth of wild-type and AttR mutant bacteria.
In Luria broth, the mutant bacteria grew slightly faster than the wild type; the doubling time of A205 was 2.0 ± 0.1 h, and that of C58 was 2.5 ± 0.2 h. In minimal medium with 0.2% glucose, both strains had doubling times of about 4.0 ± 0.2 h.
Root colonization.
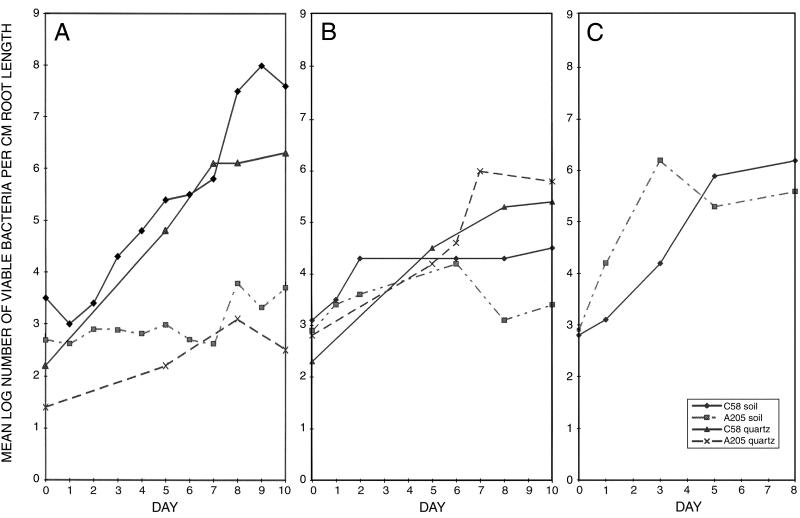
C58 was able to colonize the roots of tomato, alfalfa, and garden bean (Fig. 3) growing in microwaved soil. The differences in the number of colonizing bacteria per centimeter of root length presumably reflect differences in the size of the root and the number of root hairs, since all of the roots were observed to be densely colonized in the microscope. The AttR mutant A205 showed reduced root colonization on tomato as previously reported (9). However, root colonization by the mutant was not significantly different from that by the wild-type bacteria on alfalfa and garden bean (Fig. 3).
FIG. 3.
Bacterial colonization of roots. The mean log10 of the number of viable bacteria per centimeter of root length recovered from the roots with increasing time of growth in soil or quartz sand is shown. The wild-type strain, C58, and the AttR mutant were inoculated on tomato roots (A), alfalfa roots (B), and garden bean roots (C). For tomato and alfalfa, bacterial colonization in both microwaved soil and sterile quartz sand is shown. Note the large reduction in colonization by the AttR mutant compared to that by the parent strain on tomato roots and the lack of a significant difference between the parent and mutant strains on bean and alfalfa roots. The numbers shown are the means of four experiments. The standard deviations of all points were less than 0.5.
In order to examine the possibility that the reduced colonization of A205 on tomato roots was due to competition from residual soil organisms present in the microwaved soil, the colonization of tomato and alfalfa roots in sterile quartz sand was measured. This system contains no organisms other than those inoculated. The same results obtained with microwaved soil were observed when sterile quartz sand was used (Fig. 3); the AttR mutant (A205) showed reduced colonization of tomato, but not alfalfa, compared with its wild-type parent, C58.
Attachment of wild-type and mutant bacteria to roots examined microscopically.
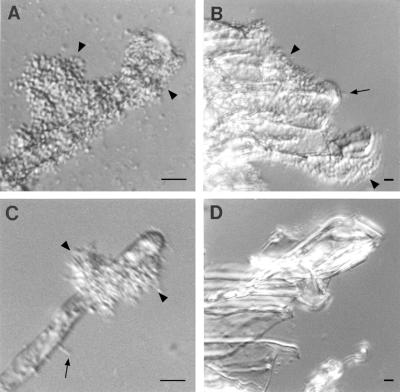
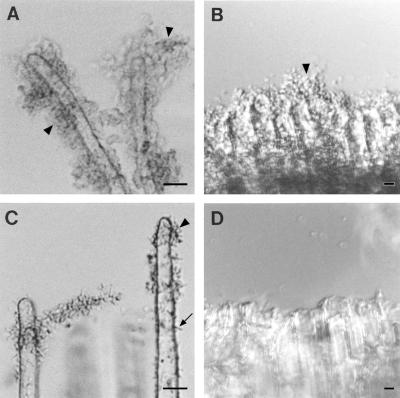
When wild-type bacteria were incubated with root segments of tomato, A. thaliana, alfalfa, garden bean, or pea and observed in the light microscope, bacteria were found to be bound to the epidermis, the root hairs, and the cut ends of the segments. Both individually attached bacteria and large clusters of bacteria were seen (Fig. 4 to 6, panels A and B). AttR mutant bacteria failed to bind to the epidermis, root hairs, or cut ends of tomato and A. thaliana roots (Fig. 4C and D). Previous studies had shown that AttR mutants fail to bind to carrot, tobacco, and B. daigremontiana (7, 14). However, AttR mutant bacteria did bind to the epidermis and root hairs of alfalfa and garden bean (Fig. 5C and 6C) and to pea roots (data not shown). AttR mutants failed to bind to the cut ends of both legume and nonlegume root segments (Fig. 4 to 6, panels D). The binding of the AttR mutant to the root hairs of alfalfa, bean, and pea appeared to be reduced compared to the binding of the parent strain. Large clusters of bacteria were more often seen on root hairs incubated with the parent strain than with the AttR mutant. However, significant attachment of AttR mutants was observed on the root hairs of all three legumes. No binding of the AttR mutants to the cut ends of roots was observed, although the parent strain was seen attached to the cut ends of both legumes and nonlegumes.
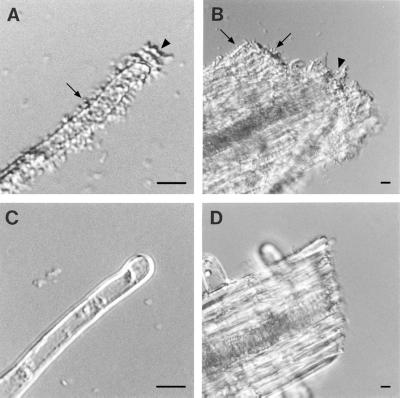
FIG. 4.
Bacterial attachment to A. thaliana root segments. (A and B) Wild-type strain C58 incubated with roots. Bacteria bound to the root hairs (A) and to the cut end of the root (B). The arrows indicate the locations of individually attached bacteria. The arrowheads indicate the locations of clusters of attached bacteria. (C and D) AttR mutant A205 bacteria incubated with roots. The mutant bacteria failed to bind to either the root hairs (C) or the cut ends of the roots (D). Equal numbers of bacteria were present in all incubations. Although not shown, wild-type bacteria also bound to the root epidermis and root cap. Mutant bacteria failed to bind to these tissues. Similar results were observed with tomato root segments. Bars, 5 μm.
FIG. 6.
Bacterial attachment to bean root segments. (A and B) Wild-type strain C58 incubated with roots. Bacteria bound to the root hairs (A) and to the cut end of the root (B). (C and D) AttR mutant A205 bacteria incubated with roots. The mutant bacteria bound to the root hairs (C) but failed to bind to the cut ends of the roots (D). The arrows indicate the locations of individually attached bacteria. The arrowheads indicate the locations of clusters of attached bacteria. Although not shown, wild-type and mutant bacteria also bound to the root epidermis and root cap. Equal numbers of bacteria were present in all incubations. Similar results were observed with pea root segments. Bars, 5 μm.
FIG. 5.
Bacterial attachment to alfalfa root segments. (A and B) Wild-type strain C58 incubated with roots. Bacteria bound to the root hairs (A) and to the cut end of the root (B). (C and D) AttR mutant A205 bacteria incubated with roots. The mutant bacteria bound to the root hairs (C) but failed to bind to the cut ends of the roots (D). The arrow indicates the location of an individually attached bacterium. The arrowheads indicate the locations of clusters of attached bacteria. Although not shown, wild-type and mutant bacteria also bound to the root epidermis and root cap. Equal numbers of bacteria were present in all incubations. Bars, 5 μm.
The binding of a second AttR mutant, A610, to carrot cells, tomato roots, and alfalfa roots was also examined. Like A205, this mutant failed to bind to carrot or tomato but was able to bind to the epidermis and root hairs of alfalfa (data not shown).
Numbers of bacteria attached to cultured cells and roots.
The numbers of viable bacteria bound to carrot suspension culture cells and root segments of tomato, A. thaliana, alfalfa, garden bean, and pea after a 2-h incubation were determined. Wild-type bacteria showed significant binding to all of these cells and organs. However, the AttR mutant (A205) was unable to bind to carrot, A. thaliana, or tomato and showed significant binding only to legumes (Table 2). No significant difference was observed between the binding of C58 and A205 to legumes.
TABLE 2.
Attachment of A. tumefaciens strains to plants
| Plant and organ or tissue | % Bacterial inoculum attacheda
|
|
|---|---|---|
| C58 | A205 | |
| Carrot suspension culture cells | 35 ± 5 | 1 ± 2 |
| Tomato root segments | 21 ± 2 | 0 ± 1 |
| A. thaliana root segments | 20 ± 3 | 0 ± 2 |
| Alfalfa root segments | 20 ± 5 | 29 ± 5 |
| Bean root segments | 20 ± 2 | 13 ± 5 |
| Pea root segments | 14 ± 2 | 12 ± 3 |
Attachment was measured after 2 h of incubation in 1/10 dilution of MS salts containing 0.4% sucrose. Shown are means ± standard deviations of a minimum of three experiments.
The effect of the capsular polysaccharide from wild-type bacteria on binding.
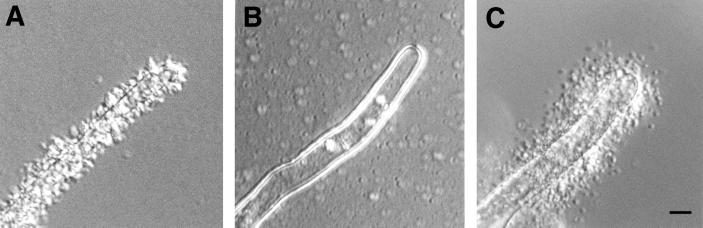
Binding of wild-type C58 bacteria to carrot cells is inhibited by the acidic fraction of the capsular polysaccharide (14). The effect of 100 μg of this polysaccharide fraction/ml purified from wild-type bacteria on bacterial binding to roots and root hairs was examined. The polysaccharide fraction inhibited binding to A. thaliana but not to alfalfa root hairs (Table 3). The polysaccharide fraction also inhibited the binding of wild-type bacteria to A. thaliana root hairs as observed in the light microscope (Fig. 7B). However, the same concentration of the polysaccharide fraction had no effect on the binding of wild-type or AttR mutant bacteria to alfalfa root hairs (Fig. 7C). This result strongly suggests that the binding of AttR mutant A. tumefaciens to alfalfa roots and root hairs is mediated by a different substance than the binding of wild-type bacteria to A. thaliana root hairs.
TABLE 3.
Effect of the PW/W polysaccharide fraction from C58 on attachment of bacteria to roots
| Bacterial strain | Root | Addition of PW/Wa | Per cent bacterial inoculum attachedb |
|---|---|---|---|
| C58 (wild type) | A. thaliana | − | 20 ± 3 |
| + | 1 ± 2 | ||
| Alfalfa | − | 20 ± 5 | |
| + | 18 ± 8 | ||
| A205 (AttR mutant) | A. thaliana | − | 0 ± 2 |
| + | 1 ± 3 | ||
| Alfalfa | − | 29 ± 5 | |
| + | 20 ± 5 |
100 μg of PW/W/ml prepared from C58 was added (+) or was not added (−) 10 min prior to the addition of the bacteria.
Mean ± standard deviation of a minimum of three experiments.
FIG. 7.
Effect of wild-type capsular polysaccharide on bacterial binding to root hairs. (A) Wild-type C58 bacteria incubated with A. thaliana roots in the absence of the polysaccharide. Numerous bacteria bound to the root hairs. (B) Wild-type C58 bacteria incubated with A. thaliana roots in the presence of 100 μg of the acetylated polysaccharide fraction/ml. Bacterial binding was strongly inhibited. (C) AttR mutant bacteria incubated with alfalfa roots in the presence of 100 μg of the acetylated polysaccharide fraction/ml. Bacterial binding was unaffected by the presence of the polysaccharide fraction. Similar results were obtained when the wild-type bacteria were used. Equal numbers of bacteria were present in all incubations. Bar, 5 μm.
DISCUSSION
Two techniques were used to examine the abilities of wild-type and AttR mutant bacteria to attach to cells and roots of nonlegumes and legumes. Microscopic studies of attachment were carried out with a large number of bacteria per milliliter (108) for 24 or more hours; under these conditions, one would expect that most plant surfaces which are capable of binding the bacteria would show the presence of some attached bacteria. Numerical studies of bacterial attachment were carried out with a smaller number of bacteria (103 to 104/ml) incubated with plant organs for a shorter time (2 h). Using both techniques, wild-type bacteria were observed to attach to all of the plants tested. However, AttR mutants failed to attach to tomato, carrot, or A. thaliana but were able to attach to legumes (alfalfa, garden bean, and pea). There was no significant difference in the attachment of wild-type bacteria and the AttR mutant to legumes when the number of bacteria which could bind in 2 h was examined (Table 2). However, when the binding of bacteria incubated with root segments for 24 h was examined, the binding of the mutant bacteria appeared to be reduced compared to that of the parent strain. No AttR mutant bacteria were seen bound to the cut end of the root, although wild-type bacteria attached well to this site. There was also an apparent reduction in the size of bacterial clusters observed on the root hairs. The difference between the results of the microscopic assay and those of the other assays may be due to the use of large numbers of bacteria in the microscopic assay as well as to differences in the surfaces of roots incubated in water and grown in soil.
The virulence of wild-type and AttR mutants was examined. Wild-type bacteria were virulent and the AttR mutant was avirulent on all plants and organs tested. This result is consistent with previous observations that all mutants of A. tumefaciens which are unable to attach to wounded surfaces (or to the surfaces of tissue culture cells) are avirulent (1, 2, 7, 15). Wild-type bacteria showed reduced virulence on legumes compared with their virulence on the other plants tested. Reduced virulence of wild-type A. tumefaciens on alfalfa has previously been reported and may be due to the production of flavonoids by the plant which are toxic to the bacteria (13).
Previous observations of the ability of nonattaching mutants to colonize the roots of tomato and A. thaliana showed that nonattaching mutants (AttB, AttD, and AttR mutants) and mutants with reduced ability to attach (CelA and CelC mutants) had markedly reduced abilities to colonize aseptic roots of tomato and A. thaliana (9). Since AttR mutants attached to the intact epidermis and root hairs of legumes, we were interested in examining the ability of this avirulent mutant to colonize legume roots. The AttR mutant was able to colonize the roots of alfalfa and garden bean to roughly the same level as the wild-type parent, suggesting that the attR gene product is not required for legume root colonization by A. tumefaciens.
The attR gene encodes a putative transacetylase (14). AttR mutants fail to make an acetylated acidic capsular polysaccharide made by wild-type bacteria (14). The purified acetylated polysaccharide from wild-type bacteria blocked the binding of the bacteria to the epidermis, root hairs, and wound sites of nonlegume roots. However, the same polysaccharide preparation failed to block the binding of wild-type or AttR mutant bacteria to the epidermis and root hairs of alfalfa.
These results suggest that A. tumefaciens has two systems for binding to roots. One system is AttR dependent and is required for binding to wound sites on both legumes and nonlegumes and for binding to the epidermis and root hairs of nonlegumes. AttR-dependent binding was required for virulence on all of the plants tested.
The second attachment system is AttR independent and is involved in the binding of the bacteria to the epidermis and root hairs of legumes. It appears to be unable to mediate bacterial binding to the roots of nonlegumes, since no binding to these roots was seen in the absence of AttR. AttR-independent binding appears to be insufficient for virulence on legumes. However, AttR-independent binding was able to mediate the colonization of legume roots at a normal level.
Agrobacteria are closely related to the rhizobia, which bind to the root hairs of legumes and colonize legume roots. Rhizobia show little ability to bind to or colonize the roots of nonlegumes (A. G. Matthysse, unpublished observations). It is interesting to speculate that the AttR-independent system for binding to legumes observed in agrobacteria might be related to the binding system of rhizobia and that this system for bacterial binding to legumes may be derived from a common ancestor of agrobacteria and rhizobia.
ACKNOWLEDGMENT
This research was supported by grant 94-37303 from the U.S. Department of Agriculture Competitive Grants Program.
REFERENCES
- 1.Cangelosi G A, Hung L, Puvanesarajah V, Stacey G, Ozaga D A, Leigh J A, Nester E W. Common loci for Agrobacterium tumefaciens and Rhizobium meliloti exopolysaccharide synthesis and their roles in plant interaction. J Bacteriol. 1987;159:2086–2091. doi: 10.1128/jb.169.5.2086-2091.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Douglas C J, Halperin W, Nester E W. Agrobacterium tumefaciens mutants affected in attachment to plants. J Bacteriol. 1982;152:1265–1275. doi: 10.1128/jb.152.3.1265-1275.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klein R M, Tenenbaum I L. A quantitative assay for crown-gall tumor formation. Am J Bot. 1955;42:709–712. [Google Scholar]
- 4.Lippincott J A, Heberlein G T. The quantitative determination of the infectivity of Agrobacterium tumefaciens. Am J Bot. 1965;52:856–863. [PubMed] [Google Scholar]
- 5.Loper J E, Suslow T V, Schroth M N. Lognormal distribution of bacterial populations in the rhizosphere. Phytopathology. 1984;74:1454–1460. [Google Scholar]
- 6.Matthysse A G. Initial interactions of Agrobacterium tumefaciens with plant host cells. Crit Rev Microbiol. 1986;13:281–307. doi: 10.3109/10408418609108740. [DOI] [PubMed] [Google Scholar]
- 7.Matthysse A G. Characterization of nonattaching mutants of Agrobacterium tumefaciens. J Bacteriol. 1987;169:313–323. doi: 10.1128/jb.169.1.313-323.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matthysse A G, Kijne J W. Attachment of Rhizobiaceae to plant cells. In: Spaink H P, Kondorosi A, Hooykaas P J J, editors. The Rhizobiaceae. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1998. pp. 235–249. [Google Scholar]
- 9.Matthysse A G, McMahan S. Root colonization by Agrobacterium tumefaciens is reduced in cel, attB, attD, and attR mutants. Appl Environ Microbiol. 1998;64:2341–2345. doi: 10.1128/aem.64.7.2341-2345.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matthysse A G, Yarnall H A, Boles S B, McMahan S. A region of the Agrobacterium tumefaciens chromosome containing genes required for virulence and attachment to host cells. Biochim Biophys Acta. 2000;1490:208–212. doi: 10.1016/s0167-4781(99)00250-x. [DOI] [PubMed] [Google Scholar]
- 11.Matthysse A G, Yarnall H A, Young N. Requirement for genes with homology to ABC transport systems for attachment and virulence of Agrobacterium tumefaciens. J Bacteriol. 1996;178:5302–5308. doi: 10.1128/jb.178.17.5302-5308.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neff N T, Binns A N, Brandt C. Inhibitory effect of a pectin-enriched tomato cell wall fraction on Agrobacterium tumefaciens binding and tumor formation. Plant Physiol. 1987;83:525–528. doi: 10.1104/pp.83.3.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palumbo J D, Kado C I, Phillips D A. An isoflavonoid-inducible efflux pump in Agrobacterium tumefaciens is involved in competitive colonization of roots. J Bacteriol. 1998;180:3107–3113. doi: 10.1128/jb.180.12.3107-3113.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reuhs B L, Kim J S, Matthysse A G. The attachment of Agrobacterium tumefaciens to carrot cells and Arabidopsis wound sites is correlated with the production of a cell-associated, acidic polysaccharide. J Bacteriol. 1997;179:5372–5379. doi: 10.1128/jb.179.17.5372-5379.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomashow M F, Karlinsky J E, Marks J R, Hurlburt R E. Identification of a new virulence locus in Agrobacterium tumefaciens that affects polysaccharide composition and plant cell attachment. J Bacteriol. 1987;169:3209–3216. doi: 10.1128/jb.169.7.3209-3216.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]