Abstract
AKI is a syndrome, not a disease. It results from many different primary and/or secondary etiologies and is often multifactorial, especially in the hospitalized patient. This review discusses the pathophysiology of three etiologies that cause AKI, those being kidney hypoperfusion, abdominal compartment syndrome, and urinary tract obstruction. The pathophysiology of these three causes of AKI differs but is overlapping. They all lead to a low urine flow rate and low urine sodium initially. In all three cases, with early recognition and correction of the underlying process, the resulting functional AKI can be rapidly reversed. However, with continued duration and/or increased severity, cell injury occurs within the kidney, resulting in structural AKI and a longer and more severe disease state with increased morbidity and mortality. This is why early recognition and reversal are critical.
Keywords: Critical Care Nephrology and Acute Kidney Injury Series, renal hypoperfusion, intraabdominal hypertension, glomerulus, proximal tubule, renal hemodynamics, acute kidney injury
Introduction
AKI is a heterogeneous syndrome defined by the rapid (hours to days) decline in the GFR resulting in serious morbidity, mortality, and increased hospital costs (1–3). AKI is defined by the retention of the metabolic waste product, creatinine, and/or a reduction in urine production, and it is associated with alterations in fluid, electrolyte, and acid-base homeostasis (2). AKI is often multifactorial, resulting from a large group of etiologies and pathophysiologic mechanisms, and it can be primary or secondary to a systemic process. For diagnostic purposes, AKI is classically divided into prerenal, intrarenal (intrinsic), and postrenal (extrinsic) etiologies. These processes include hypoperfusion of the kidney, termed prerenal azotemia, with the reduction in GFR resulting from reduced kidney perfusion without parenchymal injury. Intrinsic causes include processes inducing glomerular, interstitial, tubular, or endothelial cell injury. Postrenal AKI results from partial or complete obstruction of venous outflow or urinary flow.
A rapid diagnosis of AKI is critically important for several causes of AKI, termed “functional” AKI, as effective therapy can result in rapid reversal by correction of the underlying pathophysiologic process. These processes include hypoperfusion of the kidney, reductions in venous outflow from the kidney, and obstruction of urine flow. I have termed these three processes low-flow AKI as they result from a primary pathophysiologic mechanism of low arterial, venous, or urinary flow, respectively. The resulting AKI is rapidly reversible if diagnosed early; it occurs from events external to the kidney and results initially in a low urinary sodium. In all three conditions, sepsis can be a contributing factor. These etiologies of AKI, as their severity and/or duration increase, can lead to intrinsic AKI secondary to cellular injury. This results in subsequent organ dysfunction and, finally, overt organ failure, causing increased morbidity and mortality (Figure 1). Intrinsic or “structural” AKI occurs when there is cellular injury, and it is diagnosed by positive urinary cell injury biomarkers. Structural AKI can occur without a measured increase in serum creatinine as creatinine is an insensitive marker of GFR reductions, especially in patients with normal or near-normal baseline GFR (4–6). There are two main reasons for this insensitivity. First, kidneys have a reserve capacity, like the heart, that is called into action during stress situations. In a person with normal kidney function, this reserve can be up to 50% of the baseline GFR. When GFR is lost during injury, this reserve kicks in and “hides” the loss in baseline GFR. As baseline GFR is lost, as in CKD, the amount of GFR compensation available from kidney reserve decreases, reducing the likelihood of subclinical AKI. The second reason has to do with the exponential nature of GFR versus serum creatinine contributing to its insensitivity at normal GFRs and hypersensitivity at low GFRs (5).
Figure 1.
Acute kidney injury divided into prerenal azotemia (functional AKI) and acute tubular necrosis (structural AKI) on the basis of serum creatinine (sCr) and urinary biomarkers. Prerenal azotemia has negative urinary cell injury biomarkers, whereas acute tubular necrosis has positive urinary cell injury biomarkers, indicating proximal tubule cell injury or dysfunction. (A) An image of normal human cortex, and prerenal azotemia appears the same. Courtesy of Jim Hasbargen. (B) A human kidney biopsy specimen 24 hours after injury. Note the flattened proximal tubule cells and shed brush border membrane in the lumen. The peritubular capillaries are filled with white blood cells and rouleauxs, and a mitotic cell is visible. Reprinted from ref. 67, with permission.
Patients with positive urinary biomarkers and no change in serum creatinine are referred to as having “subclinical AKI” as there is known tubular cell injury but the serum creatinine definition for clinical AKI is not met. Clinical outcomes in this group of patients are poorer than in patients with prerenal azotemia but without positive urinary cell injury biomarkers (7,8). The use of urinary biomarkers has mostly been limited to research studies as the lack of an effective therapeutic agent for AKI has made the rapid diagnosis of AKI without therapeutic consequences. Also, the urinary biomarker studies are population studies, and there is wide overlap in individual values, making their use difficult. In some centers, urinary biomarkers are used to separate patients into high-risk and lower-risk patient care areas. The utility of this has not been established. As the extent of injury progresses, serum creatinine rises further, and clinical outcomes deteriorate. The one clinical area where clinically utility may be important is in drug nephrotoxicity, where early detection could limit injury.
Prerenal AKI
Prerenal azotemia, also known as functional AKI, results from many different pathophysiologic processes (Table 1) and is the most common cause of AKI, accounting for up to 50% of all AKI cases (9–11). Prerenal azotemia is diagnosed by an elevation of serum creatinine or low urine flow rate without positive urinary cell injury biomarkers. Kidney hypoperfusion results from reductions in the effective arterial blood volume, the volume of blood effectively perfusing the body organs with or without true vascular or total body volume depletion (Figure 2). True intravascular hypovolemia results from a loss of blood volume through hemorrhage, gastrointestinal volume losses, renal volume losses, and third spacing. Hypoperfusion of the kidney also results from disease processes with normal or even increased vascular volume status but reduced effective arterial blood volume. These include cardiogenic shock, septic shock, cirrhosis, pancreatitis, and abdominal compartment syndrome. If kidney perfusion is restored, prerenal azotemia reverses rapidly within 24 hours because, by definition, the integrity of the kidney parenchyma has remained intact. However, severe and/or prolonged hypoperfusion can result in tubular epithelial cell injury, leading to intrinsic (structural) AKI. The spectrum of injury and the importance of a rapid diagnosis and reversal of the underlying process are shown in Figure 1.
Table 1.
Causes of prerenal AKI
| Different Pathophysiologic Mechanisms of Kidney Hypoperfusion |
|---|
| Intravascular volume depletion |
| Hemorrhage—trauma, surgery, postpartum, gastrointestinal |
| Gastrointestinal losses—diarrhea, vomiting, NG loss |
| Kidney losses—diuretics, osmotic diuresis, diabetes insipidus |
| Skin and mucous membrane losses—burns, hyperthermia |
| Nephrotic syndrome |
| Cirrhosis |
| Capillary leak |
| Reduced cardiac output |
| Cardiogenic shock |
| Pericardial diseases—restrictive/constrictive/tamponade |
| Congestive heart failure |
| Valvular diseases |
| Pulmonary diseases—pulmonary hypertension, pulmonary embolism |
| Reduced systemic vascular resistance |
| Sepsis |
| Hepatorenal syndrome |
| Anaphylaxis |
| Renal vasoconstriction |
| Early sepsis |
| Hepatorenal syndrome |
| Acute hypercalcemia |
| Drugs—norepinephrine, vasopressin, nonsteroidals, renin-angiotensin system inhibitors |
| Calcineurin inhibitors |
| Iodinated contrast agents |
| Increased intra-abdominal pressure and/or reduced venous flow |
| Abdominal compartment syndrome |
| Venous outflow obstruction |
NG, nasogastric.
Figure 2.
Pathophysiology of prerenal azotemia. Numerous systemic and intrarenal factors predispose to the development of prerenal azotemia. Systemic factors lead to a reduced blood volume, resulting in a reduced effective arterial blood volume. This is the effective blood volume circulating through organs. A reduced systemic vascular resistance or a decreased cardiac output, even in the setting of normal or increased blood volume, can lead to hypoperfusion of the kidneys. Intrinsic factors within the kidney can also result in hypoperfusion, including that inhibition of prostaglandin synthesis by nonsteroidal anti-inflammatory drugs results in afferent arteriole vasoconstriction. Also, a reduction in efferent arteriole due to renin-angiotensin system inhibition (angiotensin-converting enzyme inhibitors, angiotensis receptor blockers, and renin inhibitors) can result in normal to increased perfusion but a reduced GFR due to a reduced pressure gradient for filtration. Finally, renal artery stenosis, vasoconstrictive drugs, and hypercalcemia can cause renal vasoconstriction and hypoperfusion. ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; GI, gastrointestinal; NSAIDs, nonsteroidal anti-inflammatory drugs.
Prerenal azotemia has also been divided into volume responsive and volume nonresponsive. The former is easy to comprehend, whereas the latter is less straightforward. In volume-nonresponsive forms, additional intravenous volume is of no help in restoring kidney perfusion and function. Disease processes such as congestive heart failure, hepatorenal syndrome, abdominal compartment syndrome, and sepsis may not respond to intravenous fluids as markedly reduced cardiac output or decreased systemic vascular resistance prevents improved kidney perfusion (Table 1).
Reductions in effective arterial blood volume lead to activation of aortic and cardiac baroreceptors and initiate a cascade of neural and humoral responses in an attempt to minimize any reduction in renal blood flow and GFR (Figure 3). Activation of the sympathetic nervous system increases production of catecholamines, especially norepinephrine. Sympathetic activation has ionotropic and chronotropic effects on the heart, stimulates systemic vasoconstriction in musculocutaneous and splanchnic circulations, inhibits salt loss through sweat, and stimulates thirst, resulting in retention of salt and water. Increased release of antidiuretic hormone mediates additional systemic vasoconstriction, water retention, and urea reabsorption. Angiotensin II also causes systemic arteriole vasoconstriction to preserve BP.
Figure 3.
Systemic and intrarenal compensatory mechanisms respond to a reduced effective arterial blood volume. Systemic and intrarenal neurohormonal activation occurs to counteract systemic and kidney hypoperfusion in a compensatory fashion in an attempt to normalize renal blood flow (RBF) and GFR. Catecholamines increase heart rate and vasoconstriction to increase BP, antidiuretic hormone (ADH) release increases to induce vasoconstriction, and angiotensin II production also causes vasoconstriction. This results in an increase in cardiac output and BP. Within the kidney, macula densa (MD)–mediated angiotensin II release increases efferent arteriole resistance, leading to stabilization of filtration pressure and GFR, and production of aldosterone, resulting in higher sodium (Na) reabsorption to increase plasma volume. ADH also increases water reabsorption to increase plasma volume in conjunction with the increased Na reabsorption. This results in an increase in plasma volume and maintenance of GFR. The lower graph shows autoregulation of RBF and GFR mediated by the intrarenal measures in response to reduced RBF. Autoregulation of RBF and GFR occurs between a mean arterial pressure (MAP) of 70 and 140 mm Hg. EA, efferent arteriole.
Concomitantly, there are various compensatory mechanisms within the kidney to compensate for the reductions in renal blood flow in an attempt to preserve glomerular perfusion and filtration (Figure 3) (12). Autoregulation, activated by stretch receptors in afferent arterioles in response to reduced perfusion pressure, causes vasodilation to increase renal blood flow. Autoregulation functions well until a mean systemic arterial BP of 70 mm Hg. Below this, the glomerular ultrafiltration pressure and GFR decline. Production of prostaglandins, kallikrein, and kinins by the kidneys as well as nitric oxide is increased, contributing to the vasodilation (13,14). Increased prostaglandin synthesis results in afferent arteriole vasodilation, and inhibition of this response by nonsteroidal anti-inflammatory drugs decreases afferent arteriole vasodilation, resulting in a lack of compensation in renal blood flow and GFR. Intrarenal angiotensin II activity is increased by activation of the renin-angiotensin-aldosterone system. It minimizes GFR reductions by maintaining glomerular hydrostatic pressure by preferentially increasing efferent arteriolar resistance. Renin-angiotensin system blockade, by angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, or direct renin inhibitors, reduces intrarenal angiotensin II effects, minimizing efferent arteriole tone and leading to reductions in glomerular hydrostatic pressure and GFR. Thus, this homeostatic protective GFR mechanism in patients with severe reductions in effective arterial blood volume is lost, and GFR is reduced.
Tubular glomerular feedback is another regulatory pathway important in controlling glomerular perfusion. A reduction in distal tubule delivery of Na and Cl, as would be seen in prerenal azotemia, is sensed by the Na/K/2Cl cotransporter and leads to reductions in adenosine and ATP release by macula densa cells of the juxtaglomerular apparatus. This results in relaxation of afferent arteriole tone, increasing glomerular flow. Sodium reabsorption by proximal tubules results from increased angiotensin II by activating the sodium-hydrogen exchangers. This leads to reduced distal sodium delivery and activation of the Na/K/2Cl exchanger, mediating increased glomerular flow (15). In intrinsic AKI with proximal tubule dysfunction, reduced proximal tubule Na reabsorption results in high distal delivery of Na and Cl, mediating increased macula densa afferent arteriole contraction and reductions in glomerular blood flow and GFR. This also occurs with both the acute and chronic use of sodium glucose transport inhibitors as Na and Cl delivery to the macula densa increases with reduced proximal tubule reabsorption of glucose (16).
Clinical risk factors for developing prerenal azotemia at lesser levels of hypotension include renovascular disease, hypertensive nephrosclerosis, diabetic kidney disease, and older age and CKD (10,11). Prerenal azotemia also predisposes patients to developing AKI in clinical situations, such as exposure to radiocontrast, other nephrotoxins such as cisplatin and aminoglycoside antibiotics, general anesthesia, and surgery. Therefore, it is clinically important to consider the possibility of prerenal azotemia promptly and initiate effective treatment to reverse this condition, minimizing the potential for ischemic acute tubular necrosis and/or nephrotoxic AKI. Also, in patients who develop intrinsic AKI, it is essential to first replete the intravascular volume to avoid worsening intrinsic AKI from continued hypoperfusion of the kidney. This is often overlooked, especially in patients with baseline CKD when the clinician is worried about volume overload and the potential need for dialysis to remove fluid (17).
Prerenal azotemia, with its rapid recovery of GFR, has always been considered a clinically insignificant event following resolution. Is this true, or are we missing important underlying issues, such as reductions in total GFR and baseline GFR plus kidney reserve, that cannot be detected simply using serum creatinine? The numerous risk factors shown in Figure 2 and listed above all indicate a delicate situation with enhanced likelihood of recurrence. Because serum creatinine is so insensitive to reductions in GFR, are we missing reductions in GFR with each prerenal event? An important contributor to this possibility is the kidney reserve component of total GFR (4,5,18,19). The kidney, like the heart, has reserve functional capacity, kidney reserve, to increase GFR during stress and following protein ingestion. Early studies highlighted the importance of kidney reserve and showed that it is lost with loss of baseline GFR but not in a predictable way (5,20,21). Thus, two patients could have the same baseline GFR and differ in the amount of kidney reserve and therefore total GFR. The patient with the lower kidney reserve would likely progress faster to advanced kidney failure, all other things being equal, as the patient’s total GFR is lower (5). The importance of quantifying an individual’s total GFR to show loss of GFR without apparent change in baseline serum creatinine had been previously postulated and has now been shown in clinical studies (7,8,22).
Debate of the clinical importance and existence of prerenal azotemia without tubular cell injury has occurred. In one study, some, but not all, urinary biomarkers were positive in patients with “prerenal azotemia.” However, the definition of prerenal azotemia included patients recovering to baseline serum creatinine for up to 48 hours, not the usual definition of 24 hours (23). This well may have included patients with mild intrinsic AKI.
Abdominal Compartment Syndrome
Abdominal compartment syndrome occurs when the intra-abdominal pressure is high enough to result in any abdominal organ dysfunction. The kidney is a primary target of abdominal compartment syndrome as it is so dependent on high blood flow, and AKI from this syndrome is primarily mediated by reductions in renal blood flow, resulting in prerenal azotemia. The normal intra-abdominal pressure is approximately 6 mm Hg, is dependent on body mass, and can reach as high as 10–15 mm Hg chronically in morbidly obese individuals without causing abdominal compartment syndrome (24,25). Therefore, the level of intra-abdominal pressure cannot be used for a strict definition for abdominal compartment syndrome. However, if the intra-abdominal pressure is <10 mm Hg, abdominal compartment syndrome does not exist, whereas if it is >25 mm Hg, abdominal compartment syndrome is highly likely (Figure 4) (26,27). This wide range of possible pressures resulting in abdominal compartment syndrome is due to a number of associated variables that will be discussed later. Abdominal compartment syndrome is classified as either primary if resulting from an intrabdominal process or secondary if resulting from a process outside of the abdomen.
Figure 4.
The spectrum of intra-abdominal pressure (IAP) and abdominal compartment syndrome (ACS) as intra-abdominal pressure rises. Normal IAP is about 6 mm Hg. Intra-abdominal hypertension (IAH) occurs when IAP reaches 12 mm Hg. ACS occurs when the IAP is elevated and there are signs of any abdominal organ hypoperfusion. This can occur at any pressure above 12 mm Hg but is more likely as IAP increases to 20 mm Hg. The research definition of ACS is IAP of 20 mm Hg, even without signs of organ hypoperfusion. Above IAP level of 25 mm Hg, ACS is almost always present.
The etiology of abdominal compartment syndrome includes a wide range of both medical and surgical conditions that often occur only after large amounts of volume administration and/or intra-abdominal hemorrhage (28). These causes include shock from sepsis, trauma, burns, pancreatitis, or liver transplantation, resulting in abdominal third spacing; massive ascites; and intra-abdominal bleeding resulting from a ruptured abdominal aortic aneurysm, pelvic fracture, or retroperitoneal bleed (24,28–32). The incidence of abdominal compartment syndrome is related to the extent of the underlying disease process and the requirement for massive volume administration for control of mean arterial pressure (24,33,34). Both the total volume and the rate of volume administration have been shown to be critical factors in the development of abdominal compartment syndrome (35). Other individual clinical factors include abdominal wall compliance and the mean arterial pressure. This latter factor determines the abdominal perfusion pressure, the difference between mean arterial pressure and intra-abdominal pressure, and maintaining it above 60 mm Hg has been shown to improve outcomes (24,35,36). Many other clinical risk factors associated with abdominal compartment syndrome have been identified and relate to reducing abdominal compliance, such as pneumonia; mechanical ventilation with positive end expiratory pressure; abdominal surgery; increased intra-abdominal contents, such as ascites, pregnancy, or morbid obesity; and increased capillary leak syndrome as occurs in abdominal infections, acute pancreatitis, trauma, sepsis, and burns (24,34–36).
Intra-abdominal hypertension is the term used to describe intra-abdominal pressure ≥12 mm Hg (37). It is graded one to four according to the measured pressure and from hyperacute to chronic according to the rate of development (Table 2). With more rapid development of intra-abdominal hypertension, the abdominal wall is less compliant, resulting in a higher rate of rise in intra-abdominal pressure and the likelihood of abdominal compartment syndrome occurring. For clinical trials, an intra-abdominal pressure of >20 mm Hg is used to define abdominal compartment syndrome when associated with new organ dysfunction (37).
Table 2.
Intra-abdominal hypertension classification
| Grades of Intra-Abdominal Hypertension | Pressures, mm Hg | Development | Time | Examples |
|---|---|---|---|---|
| I | 12–15 | Hyperacute | Seconds | Cough |
| II | 16–20 | Acute | Hours | Hemorrhage |
| III | 21–25 | Subacute | Days | Pancreatitis |
| IV | ≥25 | Chronic | Months | Ascites |
| Years | Morbid obesity |
The clinical signs of abdominal compartment syndrome include reduced urine output, increased airway pressure, and a tense abdomen. These signs and symptoms are nonspecific, making surveillance and diagnosis difficult. For this reason, measurement of intra-abdominal pressure has become a necessary and reliable method. Although direct measurement of intra-abdominal pressure is possible, the preferred technique is to measure intrabladder pressure. This correlates highly with intra-abdominal pressure and is currently the preferred technique as it is simple, reliable, reproducible, and low cost and has minimal complications (38). The technique involves draining the bladder with a foley, instilling 25 ml of normal saline, and measuring the pressure in the mid axillary line, in millimeters of mercury, with the patient in the supine position. Pressure can be measured using a monometer or transducer intermittently or continuously (38).
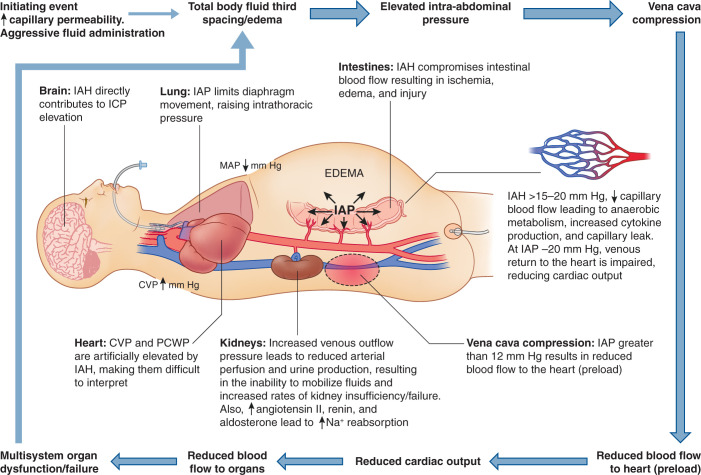
The pathophysiology of abdominal compartment syndrome includes all intra-abdominal organs, and its consequences reach outside the abdomen to affect cardiac, pulmonary, and central nervous system function (Figure 5). Many of these extra-abdominal effects result in part from the pathophysiology of AKI during abdominal compartment syndrome. Increasing intra-abdominal hypertension reduces cardiac function in several ways. First, the increase in intra-abdominal pressure reduces inferior vena cava venous return and promotes lower extremity edema (38,39), resulting in a reduced plasma volume. Second, it reduces right ventricular compliance and contractility via an elevation of the diaphragm (39). Furthermore, it can also increase central venous pressure and pulmonary capillary pressure, both further reducing right ventricular output and thus cardiac output (40). In ventilated patients, positive end expiratory pressure further reduces cardiac output (41). The elevated central venous pressure leads to higher intracranial pressure and reduced cerebral blood flow (42). Therefore, the pathophysiology of AKI from abdominal compartment syndrome is multidimensional, primarily involving increased renal venous resistance and a reduction in cardiac output, both leading to reduced kidney perfusion and a prerenal state (Figure 6).
Figure 5.
The complex multiorgan pathophysiology of abdominal compartment syndrome. Following an initiating event that leads to inflammation and increased capillary permeability, especially if associated with aggressive volume administration, intra-abdominal pressure (IAP) increases, resulting in intra-abdominal hypertension (IAH) at 12 mm Hg. Above this level, there is diaphragm elevation, resulting in increased intrathoracic pressure, abdominal venous pressure, central venous pressure (CVP), and pulmonary capillary wedge pressure (PCWP), all leading to a reduction in right ventricular function and lower extremity edema. Cerebral blood flow is also reduced by an increase in intracranial pressure (ICP) due to the high thoracic pressure. The high IAP also reduces venous return to the heart by increasing venous pressure within the abdomen, leading to reduced renal and inferior vena cava flows. This leads to a reduction in cardiac output, reducing renal blood flow. Gastrointestinal capillary perfusion is reduced, leading to tissue ischemia, cytokine production, and increased capillary permeability. Within the kidney, this results in a prerenal state as shown in Figure 2, with reductions in GFR and increases in renin, angiotensin II, and aldosterone. MAP, mean arterial pressure; Na, sodium. Adapted from LearnPICU (http://www.learnpicu.com/gi/abdominal-compartment-syndrome), with permission.
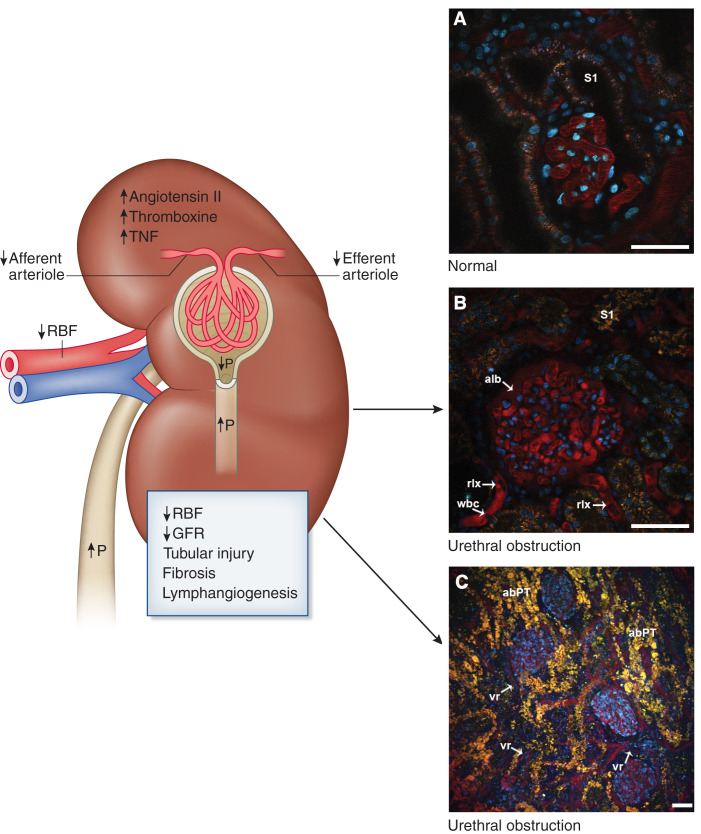
Figure 6.
The pathophysiology of urinary tract obstruction causing AKI seen using two-photon microscopy. Urinary tract obstruction results in increased pressure within the collecting system. This increased pressure is transmitted in a retrograde fashion, resulting in high tubule luminal pressure. This high luminal pressure reduces the difference between tubular and glomerular filtration pressure, leading to reduced single-nephron and total GFR. Thereafter, angiotensin II and thromboxane A2 increase further, dramatically reducing renal blood flow (RBF) by afferent and efferent arteriole vasoconstriction. TNF is released, and fibrosis is initiated rapidly. Although glomerular filtration still occurs at a low level, there is no net filtration as it cannot reach the urine. The lymphatic system increases to handle the increased interstitial accumulation of fluid following filtration. (A) A intravital two-photon image of the outer cortex of a Munich Wistar rat that has surface glomeruli. The glomerular capillaries are easily seen within Bowman’s space; nuclei are labeled in blue with an intravital nuclear dye, and the S1 portion of the proximal tubule is labeled. Blood flow through the glomerular capillaries is rapid as shown by long streaking RBCs appearing as dark lines as they do not take up the red intravascular dye. (B) The cortex of a Munich Wistar Fromter rat after 6 weeks of unilateral obstruction. Within the glomerular capillaries, many WBCs can be seen, RBCs move very slowly as RBF has been reduced to about 20% of normal, and most of the capillary space is filled with plasma and rouleauxs (rlx). The peritubular capillaries also have increased white blood cells and numerous rouleauxs, leading to reduced flow, ischemia and cortical tubular injury, and destruction. (C) The abnormal tubular structures. Not shown is the rapidly increasing fibrosis leading to nonreversible loss of kidney function. abPT, abnormal proximal tubule; alb, filtered albumin; P, pressure; RBC, red blood cell; S1, S1 segment of proximal tubule; vr, vascular rarefaction; WBC, white blood cell. Scale bars shown in A and B are 30 µm and in C 50 µm.
Abdominal compartment syndrome is also a syndrome requiring input and care from multiple medical specialties (43,44). Mortality usually ranges from 25% to 50%, but without treatment, it can reach 90% (44). Opening the abdomen is often indicated to relieve the high intra-abdominal pressure and, thus, reverse the overall pathophysiologic process (43,44). Finally, abdominal compartment syndrome is often a silent disease, especially in the intensive care setting (35). It requires consideration in multiple clinical settings, surveillance, and verification measurement of intra-abdominal pressure via the bladder using the modified Kron technique (35,45).
Obstructive Uropathy and AKI
Obstructive uropathy is a frequent cause of AKI, accounting for up to 10% of all AKI cases (46) in the general population and an even higher percentage in the elderly (46,47). The wide variety of causes of obstructive uropathy, both within and outside of the urinary track, are shown in Table 3. Obstructive uropathy differs from acute urinary retention in that it is a more severe form and associated with reduced kidney function. Acute urinary retention has an expanded list of causes, including many drugs that will not be discussed here. It is important to recognize acute urinary retention as it can be a presenting symptom and deteriorate into obstructive uropathy. The degree of injury resulting from obstructive uropathy depends on the extent of the obstruction and its duration. Most of the information regarding obstructive uropathy comes from different observations made in complete acute obstructive uropathy. This is also true of the postobstructive phase following release of the complete obstruction.
Table 3.
Etiology of obstructive AKI by site
| Anatomic Locations and Etiologies of Urinary Tract Obstuction |
|---|
| Kidney pelvis |
| Kidney stones, papillary necrosis |
| Ureter |
| Bilateral obstruction (patients with CKD, unilateral obstruction with solitary kidney) |
| Kidney stones, cancer, retroperitoneal fibrosis, severe benign prostatic hypertrophy, neurogenic bladder, stenosis, abscess, ureteral valves |
| Posterior to bladder |
| Benign prostatic hypertrophy, cancer, clots |
| Extrinsic to urinary system |
| Abdominal and pelvic malignancies, aortic aneurism, pelvic fractures, atrophic vaginitis, vulvovaginitis, pelvic organ prolapse |
Obstructive uropathy is initiated by a buildup of pressure within the collecting system that is transferred in a retrograde fashion to Bowman’s space of the glomerulus. The increased pressure results in decreased net glomerular filtration pressure across the glomerular capillary wall due to Starling forces (48–50). In the early phase of acute obstructive uropathy (the first 2–3 hours), total renal blood flow actually increases due to enhanced local vasodilatory prostaglandin synthesis (51), and then returns to baseline within 5 hours mediated by the myogenic changes in the afferent artery. Over the first 24 hours, intrarenal production of thromboxane A2 and angiotensin II leads to reductions in intraglomerular blood flow (48,49,51) by causing vasoconstriction of afferent and efferent arterioles. The overall glomerular surface area for filtration is also reduced from mesangial contraction, resulting in a further decrease in glomerular filtration (49). After 24–48 hours, renal blood flow decreases up to 60% as a result of the increase of thromboxane A2 (52) and increased intrarenal pressure. Glomerular filtration decreases even more than renal blood flow, up to an 80% reduction (48,53). Thereafter, the hemodynamic changes remain unchanged in unilateral obstruction with reduced renal blood flow, glomerular plasma flow, tubular pressure, and GFR. Bilateral obstruction only differs from unilateral obstruction in that glomerular plasma flow and glomerular capillary pressure return to normal. Interestingly but unexplained, in unilateral obstruction, the predominant site of glomerular vasoconstriction is the afferent arteriole, and in bilateral obstruction, it is the efferent arteriole. The central role of angiotensin II can be demonstrated by the ability of angiotensin-converting enzyme and other renin-angiotensin system inhibitors to minimize the decline in renal plasma flow and GFR (54). The increased level of angiotensin II also stimulates secretion of TNF-α, resulting in fibrosis and tubular cell apoptosis.
With continued obstruction, there is proximal tubule cell injury, including apical membrane shedding, cell injury, and apoptosis. Although there is an early increase in proximal tubule Na and H2O reabsorption, this is short lived, and Na and H2O reabsorption falls. This is accompanied by renal arteriole and microvascular rarefaction and further reductions in renal blood flow (55). More distal aspects of the nephron basically shut down transport activities with reductions in Na, H2O, Ca, and Mg reabsorption; Na-K-H exchange; and ammonia synthesis and excretion. Hyperchloremic metabolic acidosis occurs as renal acidification is reduced by the inability to excrete ammonium, potassium, and hydrogen, resulting in a distal renal tubular acidosis with hyperkalemia (56,57). This is in large part due to aldosterone deficiency, leading to a reduction in epithelial voltage and the inability to excrete K and H and reabsorb Na.
Interestingly, even with complete obstruction and no urine flow, glomerular filtration continues. Proximal tubule Na and H2O reabsorption actually increases early on, resulting in net fluid removal, and later, decreases. In addition, the lymphatics increase movement of interstitial fluid out of the kidney, lessening the intrarenal pressure resulting from obstruction. This does not result in net glomerular filtration as the filtered material is completely reabsorbed, and so, there is no net loss of filtered material.
Studies in rodents have shown complete unilateral ureteral obstruction results in progressive reduction in renal blood flow and GFR, tubular injury with cell death, severe interstitial fibrosis, cortical destruction, and atrophy resulting in glomeruli rising to the surface (58,59). Because mice after the age of 4 weeks very rarely have surface glomeruli, this approach has been used to study glomerular processes in normal and transgenic mice with micropuncture or intravital microscopy (60). After 6–12 weeks of complete unilateral ureteral obstruction, numerous glomeruli are seen at the surface due to cortical atrophy. Unfortunately, these are highly inflamed glomeruli with dysfunctional or nonfunctioning tubules attached (58–61). Total separation of tubules from glomeruli has also been seen, leading to atubular glomeruli (61,62).
Release of the obstruction results in a reversal of the events described above. The rate of reversal depends on the severity and duration of the obstruction, with a prolonged obstruction resulting in a slow and only partial recovery. In a dog model of unilateral ureteral obstruction for 7 days, maximal recovery of GFR took 2–4 weeks and was only two thirds of baseline values. If the obstruction was left in place for 1 month, the final GFR was only one fifth of preobstruction levels (63).
Release in patients with severe bilateral obstruction can result in a postobstructive diuresis. This is rare and requires bilateral obstruction or obstruction of a solitary kidney. There can be massive release of water and solutes secondary to reduced tubular absorptive capacity (46,64) and lost concentrating ability, resulting in nephrogenic diabetes insipidus (65). This is due to a lack of response to antidiuretic hormone and an inner medullary gradient that has been reduced. Aquaporin-2 has been found to be reduced in collecting duct principal cells following either unilateral ureteral obstruction or bilateral obstruction (66). This postobstructive diuresis can result in hypovolemia and hypernatremia. However, the diuresis, especially the later phase, can also be secondary to accumulated solute and water occurring during the obstructive period and/or too aggressive volume repletion postrelease of the obstruction.
In summary, the pathophysiology of three forms of reversible AKI resulting from factors external to the kidney is discussed. Although AKI is a syndrome and often multifactorial, these three forms result in low urine flow and low urine sodium and have overlapping pathophysiologic events. Both systemic and intrarenal neurohormonal events mediate systemic and local adaptive responses that become pathologic and fail with continued stresses placed on the body and kidney. All three lead to intrinsic AKI in an intensity- and duration-mediated fashion, making rapid diagnosis and treatment critical.
Disclosures
B.A. Molitoris reports consultancy agreements with Akebia, AM Pharma, Astellas, CSL Behring, FAST BioMedical, Ionis, Janssen, Rayze Bio, Seagan, and Tamarix; ownership interest in FAST BioMedical; research funding from FAST BioMedical, Ionis, National Institutes of Health (NIH) research grants, Razebio, and Segen; patents and inventions with FAST BioMedical for GFR and plasma volume measurement, Indiana University for C1 and C2 gentamicin and soluble thrombomodulin, and the Veterans Administration for small interfering ribonucleic acids (siRNA) for CKD; and serving as a scientific advisor or member of Akebia scientific advisory board, AM Pharma data safety and monitoring board, CSL Behring data safety and monitoring board, FAST BioMedical, Ionisdata safety and monitoring board, and NIH study section.
Funding
This work was supported by National Institute of Health National Institute of Diabetes and Digestive and Kidney Diseases grant DK 091623 and National Institute of Health grant DK079312.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Author Contributions
B.A. Molitoris wrote the original draft and reviewed and edited the manuscript.
References
- 1.Hsu CY, McCulloch CE, Fan D, Ordoñez JD, Chertow GM, Go AS: Community-based incidence of acute renal failure. Kidney Int 72: 208–212, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group : KDIGO clinical practice guideline for acute kidney injury. Available at: https://kdigo.org/wp-content/uploads/2016/10/KDIGO-2012-AKI-Guideline-English.pdf. Accessed May 9, 2022 [Google Scholar]
- 3.Lameire N, Van Biesen W, Vanholder R: Acute renal failure. Lancet 365: 417–430, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Armenta A, Madero M, Rodriguez-Iturbe B: Functional reserve of the kidney. Clin J Am Soc Nephrol 17: 458–466, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Molitoris BA: Rethinking CKD evaluation: Should we be quantifying basal or stimulated GFR to maximize precision and sensitivity? Am J Kidney Dis 69: 675–683, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porrini E, Ruggenenti P, Luis-Lima S, Carrara F, Jiménez A, de Vries APJ, Torres A, Gaspari F, Remuzzi G: Estimated GFR: Time for a critical appraisal. Nat Rev Nephrol 15: 177–190, 2019 [DOI] [PubMed] [Google Scholar]
- 7.Haase M, Kellum JA, Ronco C: Subclinical AKI—An emerging syndrome with important consequences. Nat Rev Nephrol 8: 735–739, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Ronco C, Kellum JA, Haase M: Subclinical AKI is still AKI. Crit Care 16: 313, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liaño F, Pascual J; Madrid Acute Renal Failure Study Group : Epidemiology of acute renal failure: A prospective, multicenter, community-based study. Kidney Int 50: 811–818, 1996 [DOI] [PubMed] [Google Scholar]
- 10.Nash K, Hafeez A, Hou S: Hospital-acquired renal insufficiency. Am J Kidney Dis 39: 930–936, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Sesso R, Roque A, Vicioso B, Stella S: Prognosis of ARF in hospitalized elderly patients. Am J Kidney Dis 44: 410–419, 2004 [PubMed] [Google Scholar]
- 12.Badr KF, Ichikawa I: Prerenal failure: A deleterious shift from renal compensation to decompensation. N Engl J Med 319: 623–629, 1988 [DOI] [PubMed] [Google Scholar]
- 13.Oliver JA, Sciacca RR, Cannon PJ: Renal vasodilation by converting enzyme inhibition. Role of renal prostaglandins. Hypertension 5: 166–171, 1983 [DOI] [PubMed] [Google Scholar]
- 14.Yared A, Kon V, Ichikawa I: Mechanism of preservation of glomerular perfusion and filtration during acute extracellular fluid volume depletion. Importance of intrarenal vasopressin-prostaglandin interaction for protecting kidneys from constrictor action of vasopressin. J Clin Invest 75: 1477–1487, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Persson AE, Lai EY, Gao X, Carlström M, Patzak A: Interactions between adenosine, angiotensin II and nitric oxide on the afferent arteriole influence sensitivity of the tubuloglomerular feedback. Front Physiol 18: 187, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nespoux J, Vallon V: SGLT2 inhibition and kidney protection. Clin Sci (Lond) 132: 1329–1339, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prowle JR, Kirwan CJ, Bellomo R: Fluid management for the prevention and attenuation of acute kidney injury. Nat Rev Nephrol 10: 37–47, 2014 [DOI] [PubMed] [Google Scholar]
- 18.De Moor B, Vanwalleghem JF, Swennen Q, Stas KJ, Meijers BKI: Haemodynamic or metabolic stimulation tests to reveal the renal functional response: Requiem or revival? Clin Kidney J 11: 623–654, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palsson R, Waikar SS: Renal functional reserve revisited. Adv Chronic Kidney Dis 25: e1–e8, 2018. 10.1053/j.ackd.2018.03.001 [DOI] [PubMed] [Google Scholar]
- 20.Bosch JP, Lauer A, Glabman S: Short-term protein loading in assessment of patients with renal disease. Am J Med 77: 873–879, 1984 [DOI] [PubMed] [Google Scholar]
- 21.Bosch JP, Saccaggi A, Lauer A, Ronco C, Belledonne M, Glabman S: Renal functional reserve in humans. Effect of protein intake on glomerular filtration rate. Am J Med 75: 943–950, 1983 [DOI] [PubMed] [Google Scholar]
- 22.Sharma A, Mucino MJ, Ronco C: Renal functional reserve and renal recovery after acute kidney injury. Nephron Clin Pract 127: 94–100, 2014 [DOI] [PubMed] [Google Scholar]
- 23.Nejat M, Pickering JW, Devarajan P, Bonventre JV, Edelstein CL, Walker RJ, Endre ZH: Some biomarkers of acute kidney injury are increased in pre-renal acute injury. Kidney Int 81: 1254–1262, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malbrain ML, Cheatham ML, Kirkpatrick A, Sugrue M, Parr M, De Waele J, Balogh Z, Leppäniemi A, Olvera C, Ivatury R, D’Amours S, Wendon J, Hillman K, Johansson K, Kolkman K, Wilmer A: Results from the International Conference of Experts on Intra-abdominal Hypertension and Abdominal Compartment Syndrome. I. Definitions. Intensive Care Med 32: 1722–1732, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Sanchez NC, Tenofsky PL, Dort JM, Shen LY, Helmer SD, Smith RS: What is normal intra-abdominal pressure? Am Surg 67: 243–248, 2001 [PubMed] [Google Scholar]
- 26.Ivatury RR, Diebel L, Porter JM, Simon RJ: Intra-abdominal hypertension and the abdominal compartment syndrome. Surg Clin North Am 77: 783–800, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Schein M, Ivatury R: Intra-abdominal hypertension and the abdominal compartment syndrome. Br J Surg 85: 1027–1028, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Kirkpatrick AW, Ball CG, Nickerson D, D’Amours SK: Intraabdominal hypertension and the abdominal compartment syndrome in burn patients. World J Surg 33: 1142–1149, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Karkos CD, Menexes GC, Patelis N, Kalogirou TE, Giagtzidis IT, Harkin DW: A systematic review and meta-analysis of abdominal compartment syndrome after endovascular repair of ruptured abdominal aortic aneurysms. J Vasc Surg 59: 829–842, 2014 [DOI] [PubMed] [Google Scholar]
- 30.Morken J, West MA: Abdominal compartment syndrome in the intensive care unit. Curr Opin Crit Care 7: 268–274, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Regueira T, Bruhn A, Hasbun P, Aguirre M, Romero C, Llanos O, Castro R, Bugedo G, Hernandez G: Intra-abdominal hypertension: Incidence and association with organ dysfunction during early septic shock. J Crit Care 23: 461–467, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Saggi BH, Sugerman HJ, Ivatury RR, Bloomfield GL: Abdominal compartment syndrome. J Trauma 45: 597–609, 1998 [DOI] [PubMed] [Google Scholar]
- 33.Balogh Z, McKinley BA, Cocanour CS, Kozar RA, Holcomb JB, Ware DN, Moore FA: Secondary abdominal compartment syndrome is an elusive early complication of traumatic shock resuscitation. Am J Surg 184: 538–543, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Malbrain ML, Chiumello D, Pelosi P, Wilmer A, Brienza N, Malcangi V, Bihari D, Innes R, Cohen J, Singer P, Japiassu A, Kurtop E, De Keulenaer BL, Daelemans R, Del Turco M, Cosimini P, Ranieri M, Jacquet L, Laterre PF, Gattinoni L: Prevalence of intra-abdominal hypertension in critically ill patients: A multicentre epidemiological study. Intensive Care Med 30: 822–829, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Hunt L, Frost SA, Hillman K, Newton PJ, Davidson PM: Management of intra-abdominal hypertension and abdominal compartment syndrome: A review. J Trauma Manag Outcomes 8: 2, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim IB, Prowle J, Baldwin I, Bellomo R: Incidence, risk factors and outcome associations of intra-abdominal hypertension in critically ill patients. Anaesth Intensive Care 40: 79–89, 2012 [DOI] [PubMed] [Google Scholar]
- 37.Caldwell CB, Ricotta JJ: Changes in visceral blood flow with elevated intraabdominal pressure. J Surg Res 43: 14–20, 1987 [DOI] [PubMed] [Google Scholar]
- 38.Milanesi R, Caregnato RC: Intra-abdominal pressure: An integrative review. Einstein (Sao Paulo) 14: 423–430, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cullen DJ, Coyle JP, Teplick R, Long MC: Cardiovascular, pulmonary, and renal effects of massively increased intra-abdominal pressure in critically ill patients. Crit Care Med 17: 118–121, 1989 [DOI] [PubMed] [Google Scholar]
- 40.Malbrain ML, Cheatham ML: Definitions and pathophysiological implications of intra-abdominal hypertension and abdominal compartment syndrome. Am Surg 77[Suppl 1]: S6–S11, 2011 [PubMed] [Google Scholar]
- 41.Ridings PC, Bloomfield GL, Blocher CR, Sugerman HJ: Cardiopulmonary effects of raised intra-abdominal pressure before and after intravascular volume expansion. J Trauma 39: 1071–1075, 1995 [DOI] [PubMed] [Google Scholar]
- 42.Citerio G, Vascotto E, Villa F, Celotti S, Pesenti A: Induced abdominal compartment syndrome increases intracranial pressure in neurotrauma patients: A prospective study. Crit Care Med 29: 1466–1471, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Padar M, Reintam Blaser A, Talving P, Lipping E, Starkopf J: Abdominal compartment syndrome: Improving outcomes with a multidisciplinary approach - A narrative review. J Multidiscip Healthc 12: 1061–1074, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Popescu GA, Bara T, Rad P: Abdominal compartment syndrome as a multidisciplinary challenge. A literature review. J Crit Care Med (Targu Mures) 4: 114–119, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hunt L, Frost SA, Alexandrou E, Hillman K, Newton PJ, Davidson PM: Reliability of intra-abdominal pressure measurements using the modified Kron technique. Acta Clin Belg 70: 116–120, 2015 [DOI] [PubMed] [Google Scholar]
- 46.Hamdi A, Hajage D, Van Glabeke E, Belenfant X, Vincent F, Gonzalez F, Ciroldi M, Obadia E, Chelha R, Pallot JL, Das V: Severe post-renal acute kidney injury, post-obstructive diuresis and renal recovery. BJU Int 110[11 Pt C]: E1027–E1034, 2012 [DOI] [PubMed] [Google Scholar]
- 47.Chávez-Iñiguez JS, Navarro-Gallardo GJ, Medina-González R, Alcantar-Vallin L, García-García G: Acute kidney injury caused by obstructive nephropathy. Int J Nephrol 2020: 8846622, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dal Canton A, Corradi A, Stanziale R, Maruccio G, Migone L: Glomerular hemodynamics before and after release of 24-hour bilateral ureteral obstruction. Kidney Int 17: 491–496, 1980 [DOI] [PubMed] [Google Scholar]
- 49.Klahr S, Harris K, Purkerson ML: Effects of obstruction on renal functions. Pediatr Nephrol 2: 34–42, 1988 [DOI] [PubMed] [Google Scholar]
- 50.Wright S: Effects of urinary tract obstruction on glomerular filtration rate and renal blood flow. Semin Nephrol 2: 5–16, 1982 [Google Scholar]
- 51.Moody TE, Vaughn ED Jr., Gillenwater JY: Relationship between renal blood flow and ureteral pressure during 18 hours of total unilateral uretheral occlusion. Implications for changing sites of increased renal resistance. Invest Urol 13: 246–251, 1975 [PubMed] [Google Scholar]
- 52.McGiff JC, Crowshaw K, Terragno NA, Lonigro AJ: Release of a prostaglandin-like substance into renal venous blood in response to angiotensin II. Circ Res 27[Suppl 1]: 121–130, 1970 [PubMed] [Google Scholar]
- 53.Dal Canton A, Corradi A, Stanziale R, Maruccio G, Migone L: Effects of 24-hour unilateral ureteral obstruction on glomerular hemodynamics in rat kidney. Kidney Int 15: 457–462, 1979 [DOI] [PubMed] [Google Scholar]
- 54.Purkerson ML, Klahr S: Prior inhibition of vasoconstrictors normalizes GFR in postobstructed kidneys. Kidney Int 35: 1305–1314, 1989 [DOI] [PubMed] [Google Scholar]
- 55.Nagalakshmi VK, Li M, Shah S, Gigliotti JC, Klibanov AL, Epstein FH, Chevalier RL, Gomez RA, Sequeira-Lopez MLS: Changes in cell fate determine the regenerative and functional capacity of the developing kidney before and after release of obstruction. Clin Sci (Lond) 132: 2519–2545, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Batlle DC, Sehy JT, Roseman MK, Arruda JA, Kurtzman NA: Clinical and pathophysiologic spectrum of acquired distal renal tubular acidosis. Kidney Int 20: 389–396, 1981 [DOI] [PubMed] [Google Scholar]
- 57.Weidmann P, Beretta-Piccoli C, Ziegler WH, Keusch G, Glück Z, Reubi FC: Age versus urinary sodium for judging renin, aldosterone, and catecholamine levels: Studies in normal subjects and patients with essential hypertension. Kidney Int 14: 619–628, 1978 [DOI] [PubMed] [Google Scholar]
- 58.Chevalier RL, Forbes MS, Thornhill BA: Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy. Kidney Int 75: 1145–1152, 2009 [DOI] [PubMed] [Google Scholar]
- 59.Yang HC, Zuo Y, Fogo AB: Models of chronic kidney disease. Drug Discov Today Dis Models 7: 13–19, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schießl IM, Bardehle S, Castrop H: Superficial nephrons in BALB/c and C57BL/6 mice facilitate in vivo multiphoton microscopy of the kidney. PLoS One 8: e52499, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Galarreta CI, Grantham JJ, Forbes MS, Maser RL, Wallace DP, Chevalier RL: Tubular obstruction leads to progressive proximal tubular injury and atubular glomeruli in polycystic kidney disease. Am J Pathol 184: 1957–1966, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Forbes MS, Thornhill BA, Chevalier RL: Proximal tubular injury and rapid formation of atubular glomeruli in mice with unilateral ureteral obstruction: A new look at an old model. Am J Physiol Renal Physiol 301: F110–F117, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vaughan ED Jr., Gillenwater JY: Recovery following complete chronic unilateral ureteral occlusion: Functional, radiographic and pathologic alterations. J Urol 106: 27–35, 1971 [DOI] [PubMed] [Google Scholar]
- 64.Witte MH, Short FA, Hollander W Jr.: Massive polyuria and natruresis following relief of urinary tract obstruction. Am J Med 37: 320–326, 1964 [DOI] [PubMed] [Google Scholar]
- 65.Roussak NJ, Oleesky S: Waterlosing nephritis, a syndrome simulating diabetes insipidus. Q J Med 23: 147–164, 1954 [PubMed] [Google Scholar]
- 66.Berlyne GM, Macken A: On the mechanism of renal inability to produce a concentrated urine in chronic hydronephrosis. Clin Sci 22: 315–324, 1962 [PubMed] [Google Scholar]
- 67.Molitoris BA: Actin cytoskeleton in ischemic acute renal failure Kidney Int 66: 871–883, 2004 [DOI] [PubMed] [Google Scholar]